Abstract
Recent studies have indicated that bone marrow stem cells are capable of generating muscle, cardiac, hepatic, renal, and bone cells. Purified hematopoietic stem cells have generated cardiac and hepatic cells and reversed disease manifestations in these tissues. Hematopoietic stem cells also alter phenotype with cell cycle transit or circadian phase. During a cytokine stimulated cell cycle transit, reversible alterations of differentiation and engraftment occur. Primitive hematopoietic stem cells express a wide variety of adhesion and cytokine receptors and respond quickly with migration and podia extensions on exposure to cytokines. These data suggest an "Open Chromatin" model of stem cell regulation in which there is a fluctuating continuum in the stem cell/progenitor cell compartments, rather than a hierarchical relationship. These observations, along with progress in using low dose treatments and tolerization approaches, suggest many new therapeutic strategies involving stem cells and the creation of a new medical specialty; stemology.
Full text
PDF












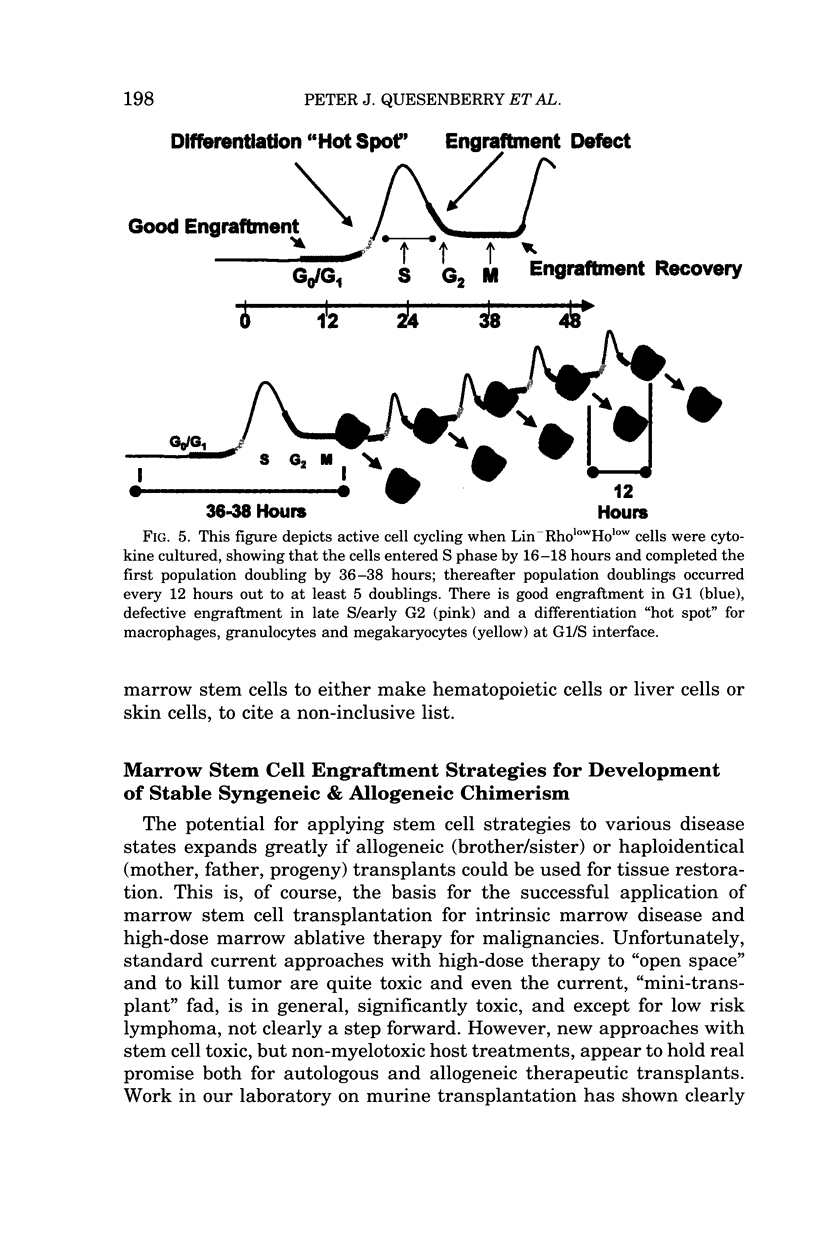
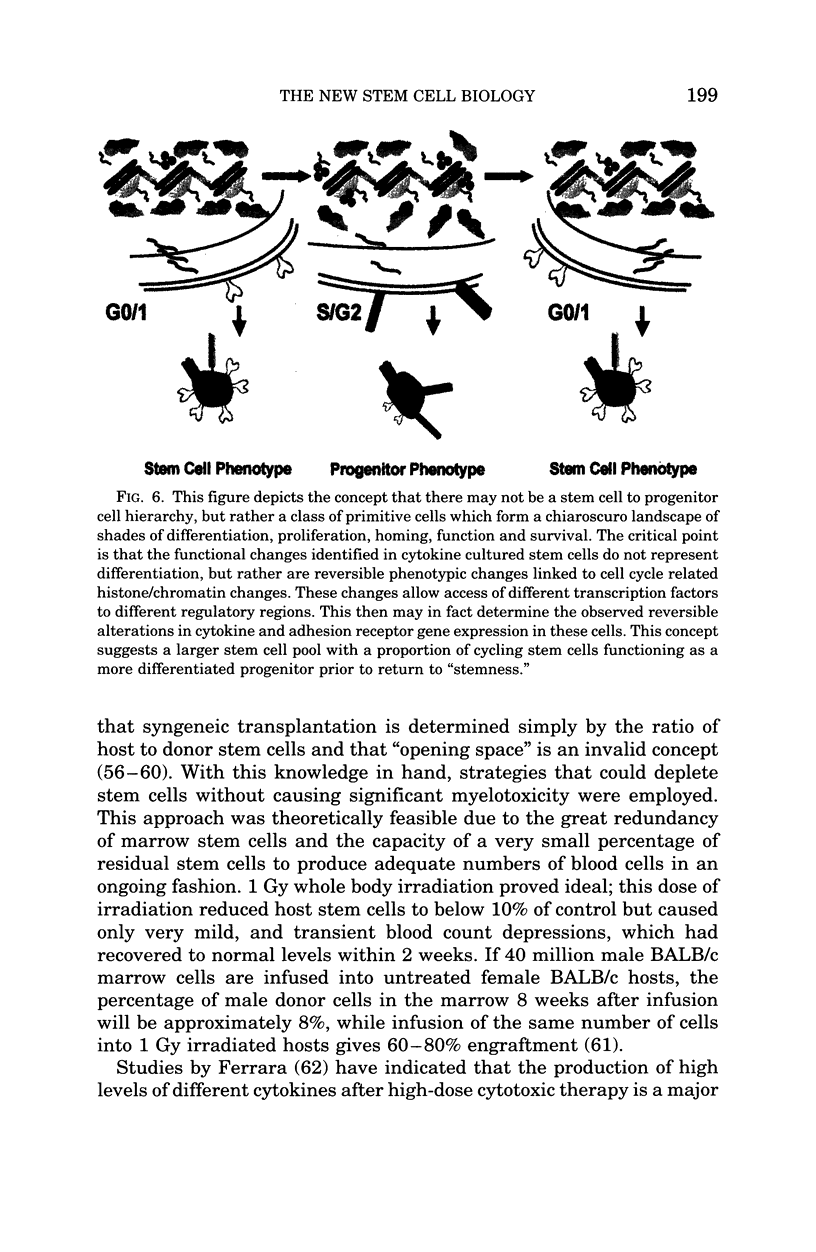








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baines P., Visser J. W. Analysis and separation of murine bone marrow stem cells by H33342 fluorescence-activated cell sorting. Exp Hematol. 1983 Sep;11(8):701–708. [PubMed] [Google Scholar]
- Baines P., Visser J. W. Analysis and separation of murine bone marrow stem cells by H33342 fluorescence-activated cell sorting. Exp Hematol. 1983 Sep;11(8):701–708. [PubMed] [Google Scholar]
- Becker P. S., Nilsson S. K., Li Z., Berrios V. M., Dooner M. S., Cooper C. L., Hsieh C. C., Quesenberry P. J. Adhesion receptor expression by hematopoietic cell lines and murine progenitors: modulation by cytokines and cell cycle status. Exp Hematol. 1999 Mar;27(3):533–541. doi: 10.1016/s0301-472x(98)00037-x. [DOI] [PubMed] [Google Scholar]
- Bertoncello I., Hodgson G. S., Bradley T. R. Multiparameter analysis of transplantable hemopoietic stem cells: I. The separation and enrichment of stem cells homing to marrow and spleen on the basis of rhodamine-123 fluorescence. Exp Hematol. 1985 Nov;13(10):999–1006. [PubMed] [Google Scholar]
- Bertoncello I., Hodgson G. S., Bradley T. R. Multiparameter analysis of transplantable hemopoietic stem cells: I. The separation and enrichment of stem cells homing to marrow and spleen on the basis of rhodamine-123 fluorescence. Exp Hematol. 1985 Nov;13(10):999–1006. [PubMed] [Google Scholar]
- Bjornson C. R., Rietze R. L., Reynolds B. A., Magli M. C., Vescovi A. L. Turning brain into blood: a hematopoietic fate adopted by adult neural stem cells in vivo. Science. 1999 Jan 22;283(5401):534–537. doi: 10.1126/science.283.5401.534. [DOI] [PubMed] [Google Scholar]
- Blomberg M., Rao S., Reilly J., Tiarks C., Peters S., Kittler E., Quesenberry P. Repetitive bone marrow transplantation in nonmyeloablated recipients. Exp Hematol. 1998 Apr;26(4):320–324. [PubMed] [Google Scholar]
- Boswell H. S., Wade P. M., Jr, Quesenberry P. J. Thy-1 antigen expression by murine high-proliferative capacity hematopoietic progenitor cells. I. Relation between sensitivity to depletion by Thy-1 antibody and stem cell generation potential. J Immunol. 1984 Dec;133(6):2940–2949. [PubMed] [Google Scholar]
- Bradford G. B., Williams B., Rossi R., Bertoncello I. Quiescence, cycling, and turnover in the primitive hematopoietic stem cell compartment. Exp Hematol. 1997 May;25(5):445–453. [PubMed] [Google Scholar]
- Cheshier S. H., Morrison S. J., Liao X., Weissman I. L. In vivo proliferation and cell cycle kinetics of long-term self-renewing hematopoietic stem cells. Proc Natl Acad Sci U S A. 1999 Mar 16;96(6):3120–3125. doi: 10.1073/pnas.96.6.3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delassus S., Titley I., Enver T. Functional and molecular analysis of hematopoietic progenitors derived from the aorta-gonad-mesonephros region of the mouse embryo. Blood. 1999 Sep 1;94(5):1495–1503. [PubMed] [Google Scholar]
- Ferrara J. L. Cytokine dysregulation as a mechanism of graft versus host disease. Curr Opin Immunol. 1993 Oct;5(5):794–799. doi: 10.1016/0952-7915(93)90139-j. [DOI] [PubMed] [Google Scholar]
- Ferrari G., Cusella-De Angelis G., Coletta M., Paolucci E., Stornaiuolo A., Cossu G., Mavilio F. Muscle regeneration by bone marrow-derived myogenic progenitors. Science. 1998 Mar 6;279(5356):1528–1530. doi: 10.1126/science.279.5356.1528. [DOI] [PubMed] [Google Scholar]
- Ford A. M., Bennett C. A., Healy L. E., Navarro E., Spooncer E., Greaves M. F. Immunoglobulin heavy-chain and CD3 delta-chain gene enhancers are DNase I-hypersensitive in hemopoietic progenitor cells. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3424–3428. doi: 10.1073/pnas.89.8.3424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis K., Ramakrishna R., Holloway W., Palsson B. O. Two new pseudopod morphologies displayed by the human hematopoietic KG1a progenitor cell line and by primary human CD34(+) cells. Blood. 1998 Nov 15;92(10):3616–3623. [PubMed] [Google Scholar]
- Frimberger A. E., McAuliffe C. I., Werme K. A., Tuft R. A., Fogarty K. E., Benoit B. O., Dooner M. S., Quesenberry P. J. The fleet feet of haematopoietic stem cells: rapid motility, interaction and proteopodia. Br J Haematol. 2001 Mar;112(3):644–654. doi: 10.1046/j.1365-2141.2001.02542.x. [DOI] [PubMed] [Google Scholar]
- Frimberger A. E., Stering A. I., Quesenberry P. J. An in vitro model of hematopoietic stem cell homing demonstrates rapid homing and maintenance of engraftable stem cells. Blood. 2001 Aug 15;98(4):1012–1018. doi: 10.1182/blood.v98.4.1012. [DOI] [PubMed] [Google Scholar]
- Gale R. P., Champlin R. E. How does bone-marrow transplantation cure leukaemia? Lancet. 1984 Jul 7;2(8393):28–30. doi: 10.1016/s0140-6736(84)92009-9. [DOI] [PubMed] [Google Scholar]
- Gololobov G. V., Chernova E. A., Schourov D. V., Smirnov I. V., Kudelina I. A., Gabibov A. G. Cleavage of supercoiled plasmid DNA by autoantibody Fab fragment: application of the flow linear dichroism technique. Proc Natl Acad Sci U S A. 1995 Jan 3;92(1):254–257. doi: 10.1073/pnas.92.1.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gussoni E., Soneoka Y., Strickland C. D., Buzney E. A., Khan M. K., Flint A. F., Kunkel L. M., Mulligan R. C. Dystrophin expression in the mdx mouse restored by stem cell transplantation. Nature. 1999 Sep 23;401(6751):390–394. doi: 10.1038/43919. [DOI] [PubMed] [Google Scholar]
- Habibian H. K., Peters S. O., Hsieh C. C., Wuu J., Vergilis K., Grimaldi C. I., Reilly J., Carlson J. E., Frimberger A. E., Stewart F. M. The fluctuating phenotype of the lymphohematopoietic stem cell with cell cycle transit. J Exp Med. 1998 Jul 20;188(2):393–398. doi: 10.1084/jem.188.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins A. L., Jones R. J., Zehnbauer B. A., Zicha M. S., Collector M. J., Sharkis S. J., Griffin C. A. Fluorescence in situ hybridization to determine engraftment status after murine bone marrow transplant. Cancer Genet Cytogenet. 1992 Dec;64(2):145–148. doi: 10.1016/0165-4608(92)90345-9. [DOI] [PubMed] [Google Scholar]
- Holloway W., Martinez A. R., Oh D. J., Francis K., Ramakrishna R., Palsson B. O. Key adhesion molecules are present on long podia extended by hematopoietic cells. Cytometry. 1999 Nov 1;37(3):171–177. doi: 10.1002/(sici)1097-0320(19991101)37:3<171::aid-cyto2>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Jackson K. A., Mi T., Goodell M. A. Hematopoietic potential of stem cells isolated from murine skeletal muscle. Proc Natl Acad Sci U S A. 1999 Dec 7;96(25):14482–14486. doi: 10.1073/pnas.96.25.14482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez G., Griffiths S. D., Ford A. M., Greaves M. F., Enver T. Activation of the beta-globin locus control region precedes commitment to the erythroid lineage. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):10618–10622. doi: 10.1073/pnas.89.22.10618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawada H., Ogawa M. Bone marrow origin of hematopoietic progenitors and stem cells in murine muscle. Blood. 2001 Oct 1;98(7):2008–2013. doi: 10.1182/blood.v98.7.2008. [DOI] [PubMed] [Google Scholar]
- Kolb H. J., Schattenberg A., Goldman J. M., Hertenstein B., Jacobsen N., Arcese W., Ljungman P., Ferrant A., Verdonck L., Niederwieser D. Graft-versus-leukemia effect of donor lymphocyte transfusions in marrow grafted patients. Blood. 1995 Sep 1;86(5):2041–2050. [PubMed] [Google Scholar]
- Krause D. S., Theise N. D., Collector M. I., Henegariu O., Hwang S., Gardner R., Neutzel S., Sharkis S. J. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell. 2001 May 4;105(3):369–377. doi: 10.1016/s0092-8674(01)00328-2. [DOI] [PubMed] [Google Scholar]
- Lagasse E., Connors H., Al-Dhalimy M., Reitsma M., Dohse M., Osborne L., Wang X., Finegold M., Weissman I. L., Grompe M. Purified hematopoietic stem cells can differentiate into hepatocytes in vivo. Nat Med. 2000 Nov;6(11):1229–1234. doi: 10.1038/81326. [DOI] [PubMed] [Google Scholar]
- Lamar E. E., Palmer E. Y-encoded, species-specific DNA in mice: evidence that the Y chromosome exists in two polymorphic forms in inbred strains. Cell. 1984 May;37(1):171–177. doi: 10.1016/0092-8674(84)90312-x. [DOI] [PubMed] [Google Scholar]
- Miyatake S., Yokota T., Lee F., Arai K. Structure of the chromosomal gene for murine interleukin 3. Proc Natl Acad Sci U S A. 1985 Jan;82(2):316–320. doi: 10.1073/pnas.82.2.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson S. K., Dooner M. S., Quesenberry P. J. Synchronized cell-cycle induction of engrafting long-term repopulating stem cells. Blood. 1997 Dec 1;90(11):4646–4650. [PubMed] [Google Scholar]
- Nilsson S. K., Dooner M. S., Tiarks C. Y., Weier H. U., Quesenberry P. J. Potential and distribution of transplanted hematopoietic stem cells in a nonablated mouse model. Blood. 1997 Jun 1;89(11):4013–4020. [PubMed] [Google Scholar]
- Nilsson S. K., Dooner M. S., Tiarks C. Y., Weier H. U., Quesenberry P. J. Potential and distribution of transplanted hematopoietic stem cells in a nonablated mouse model. Blood. 1997 Jun 1;89(11):4013–4020. [PubMed] [Google Scholar]
- Nilsson S. K., Dooner M. S., Tiarks C. Y., Weier H. U., Quesenberry P. J. Potential and distribution of transplanted hematopoietic stem cells in a nonablated mouse model. Blood. 1997 Jun 1;89(11):4013–4020. [PubMed] [Google Scholar]
- Nilsson S. K., Dooner M. S., Weier H. U., Frenkel B., Lian J. B., Stein G. S., Quesenberry P. J. Cells capable of bone production engraft from whole bone marrow transplants in nonablated mice. J Exp Med. 1999 Feb 15;189(4):729–734. doi: 10.1084/jem.189.4.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson S. K., Hulspas R., Weier H. U., Quesenberry P. J. In situ detection of individual transplanted bone marrow cells using FISH on sections of paraffin-embedded whole murine femurs. J Histochem Cytochem. 1996 Sep;44(9):1069–1074. doi: 10.1177/44.9.8773573. [DOI] [PubMed] [Google Scholar]
- Nilsson S. K., Johnston H. M., Coverdale J. A. Spatial localization of transplanted hemopoietic stem cells: inferences for the localization of stem cell niches. Blood. 2001 Apr 15;97(8):2293–2299. doi: 10.1182/blood.v97.8.2293. [DOI] [PubMed] [Google Scholar]
- Papayannopoulou T., Priestley G. V., Rohde A., Peterson K. R., Nakamoto B. Hemopoietic lineage commitment decisions: in vivo evidence from a transgenic mouse model harboring micro LCR-betapro-LacZ as a transgene. Blood. 2000 Feb 15;95(4):1274–1282. [PubMed] [Google Scholar]
- Peters S. O., Habibian H. K., Vergilis K., Quesenberry P. J. Effects of cytokines on stem cell engraftment depends on time of evaluation post-marrow-infusion. Int J Hematol. 1999 Aug;70(2):112–118. [PubMed] [Google Scholar]
- Peters S. O., Kittler E. L., Ramshaw H. S., Quesenberry P. J. Ex vivo expansion of murine marrow cells with interleukin-3 (IL-3), IL-6, IL-11, and stem cell factor leads to impaired engraftment in irradiated hosts. Blood. 1996 Jan 1;87(1):30–37. [PubMed] [Google Scholar]
- Peters S. O., Kittler E. L., Ramshaw H. S., Quesenberry P. J. Murine marrow cells expanded in culture with IL-3, IL-6, IL-11, and SCF acquire an engraftment defect in normal hosts. Exp Hematol. 1995 May;23(5):461–469. [PubMed] [Google Scholar]
- Petersen B. E., Bowen W. C., Patrene K. D., Mars W. M., Sullivan A. K., Murase N., Boggs S. S., Greenberger J. S., Goff J. P. Bone marrow as a potential source of hepatic oval cells. Science. 1999 May 14;284(5417):1168–1170. doi: 10.1126/science.284.5417.1168. [DOI] [PubMed] [Google Scholar]
- Porter D. L., Roth M. S., McGarigle C., Ferrara J. L., Antin J. H. Induction of graft-versus-host disease as immunotherapy for relapsed chronic myeloid leukemia. N Engl J Med. 1994 Jan 13;330(2):100–106. doi: 10.1056/NEJM199401133300204. [DOI] [PubMed] [Google Scholar]
- Quesenberry P. J., Zhong S., Wang H., Stewart M. Allogeneic chimerism with low-dose irradiation, antigen presensitization, and costimulator blockade in H-2 mismatched mice. Blood. 2001 Jan 15;97(2):557–564. doi: 10.1182/blood.v97.2.557. [DOI] [PubMed] [Google Scholar]
- Ramshaw H. S., Crittenden R. B., Dooner M., Peters S. O., Rao S. S., Quesenberry P. J. High levels of engraftment with a single infusion of bone marrow cells into normal unprepared mice. Biol Blood Marrow Transplant. 1995 Dec;1(2):74–80. [PubMed] [Google Scholar]
- Ramshaw H. S., Rao S. S., Crittenden R. B., Peters S. O., Weier H. U., Quesenberry P. J. Engraftment of bone marrow cells into normal unprepared hosts: effects of 5-fluorouracil and cell cycle status. Blood. 1995 Aug 1;86(3):924–929. [PubMed] [Google Scholar]
- Rao S. S., Peters S. O., Crittenden R. B., Stewart F. M., Ramshaw H. S., Quesenberry P. J. Stem cell transplantation in the normal nonmyeloablated host: relationship between cell dose, schedule, and engraftment. Exp Hematol. 1997 Feb;25(2):114–121. [PubMed] [Google Scholar]
- Reddy G. P., Tiarks C. Y., Pang L., Wuu J., Hsieh C. C., Quesenberry P. J. Cell cycle analysis and synchronization of pluripotent hematopoietic progenitor stem cells. Blood. 1997 Sep 15;90(6):2293–2299. [PubMed] [Google Scholar]
- Rolink A. G., Schaniel C., Busslinger M., Nutt S. L., Melchers F. Fidelity and infidelity in commitment to B-lymphocyte lineage development. Immunol Rev. 2000 Jun;175:104–111. [PubMed] [Google Scholar]
- Stewart F. M., Crittenden R. B., Lowry P. A., Pearson-White S., Quesenberry P. J. Long-term engraftment of normal and post-5-fluorouracil murine marrow into normal nonmyeloablated mice. Blood. 1993 May 15;81(10):2566–2571. [PubMed] [Google Scholar]
- Stewart F. M., Zhong S., Wuu J., Hsieh C., Nilsson S. K., Quesenberry P. J. Lymphohematopoietic engraftment in minimally myeloablated hosts. Blood. 1998 May 15;91(10):3681–3687. [PubMed] [Google Scholar]
- Sullivan K. M., Storb R., Buckner C. D., Fefer A., Fisher L., Weiden P. L., Witherspoon R. P., Appelbaum F. R., Banaji M., Hansen J. Graft-versus-host disease as adoptive immunotherapy in patients with advanced hematologic neoplasms. N Engl J Med. 1989 Mar 30;320(13):828–834. doi: 10.1056/NEJM198903303201303. [DOI] [PubMed] [Google Scholar]
- Theise N. D., Badve S., Saxena R., Henegariu O., Sell S., Crawford J. M., Krause D. S. Derivation of hepatocytes from bone marrow cells in mice after radiation-induced myeloablation. Hepatology. 2000 Jan;31(1):235–240. doi: 10.1002/hep.510310135. [DOI] [PubMed] [Google Scholar]
- Thomson J. A., Itskovitz-Eldor J., Shapiro S. S., Waknitz M. A., Swiergiel J. J., Marshall V. S., Jones J. M. Embryonic stem cell lines derived from human blastocysts. Science. 1998 Nov 6;282(5391):1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- Valtz N. L., Hayes T. E., Norregaard T., Liu S. M., McKay R. D. An embryonic origin for medulloblastoma. New Biol. 1991 Apr;3(4):364–371. [PubMed] [Google Scholar]
- Wakitani S., Saito T., Caplan A. I. Myogenic cells derived from rat bone marrow mesenchymal stem cells exposed to 5-azacytidine. Muscle Nerve. 1995 Dec;18(12):1417–1426. doi: 10.1002/mus.880181212. [DOI] [PubMed] [Google Scholar]
- Weiden P. L., Flournoy N., Sanders J. E., Sullivan K. M., Thomas E. D. Antileukemic effect of graft-versus-host disease contributes to improved survival after allogeneic marrow transplantation. Transplant Proc. 1981 Mar;13(1 Pt 1):248–251. [PubMed] [Google Scholar]
- Weier H. U., Polikoff D., Fawcett J. J., Greulich K. M., Lee K. H., Cram S., Chapman V. M., Gray J. W. Generation of five high-complexity painting probe libraries from flow-sorted mouse chromosomes. Genomics. 1994 Jun;21(3):641–644. doi: 10.1006/geno.1994.1326. [DOI] [PubMed] [Google Scholar]
- Weston S. A., Parish C. R. New fluorescent dyes for lymphocyte migration studies. Analysis by flow cytometry and fluorescence microscopy. J Immunol Methods. 1990 Oct 4;133(1):87–97. doi: 10.1016/0022-1759(90)90322-m. [DOI] [PubMed] [Google Scholar]