Abstract
Excessive production of reactive oxygen species (ROS) occurs in many diseases and oxidation may be a common disease mechanism generally. The original "oxidation hypothesis" concerning the pathogenesis of atherosclerosis was posited in the context of the putative central role of oxidized LDL in the process. Atherosclerosis has three major characteristic features: inflammation with accumulation of T-cells and, in particular, monocytes, which become lipid rich foam cells; remodeling of the arterial wall; and the non-random localization of lesions to areas of disturbed flow or of low shear stress. The evidence is reviewed that each of these characteristics can be attributed to excessive ROS, which are derived from cellular oxidases, especially, the NAD(P)H oxidases. This expanded concept of the central role of oxidation in the pathogenesis of atherosclerosis has led to a renewed and intense interest in the potential role of antioxidants in therapy. The vascular protective effects of existing drugs such as statins and ACE inhibitors that are not related to serum lipid alterations are attributed to their indirect but effective roles as antioxidants. These data as well as evidence that newly developed antioxidant drugs show promise, not only in experimental animals but also clinically, are reviewed.
Full text
PDF


























Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aikawa M., Rabkin E., Okada Y., Voglic S. J., Clinton S. K., Brinckerhoff C. E., Sukhova G. K., Libby P. Lipid lowering by diet reduces matrix metalloproteinase activity and increases collagen content of rabbit atheroma: a potential mechanism of lesion stabilization. Circulation. 1998 Jun 23;97(24):2433–2444. doi: 10.1161/01.cir.97.24.2433. [DOI] [PubMed] [Google Scholar]
- Aikawa M., Rabkin E., Sugiyama S., Voglic S. J., Fukumoto Y., Furukawa Y., Shiomi M., Schoen F. J., Libby P. An HMG-CoA reductase inhibitor, cerivastatin, suppresses growth of macrophages expressing matrix metalloproteinases and tissue factor in vivo and in vitro. Circulation. 2001 Jan 16;103(2):276–283. doi: 10.1161/01.cir.103.2.276. [DOI] [PubMed] [Google Scholar]
- Aikawa M., Voglic S. J., Sugiyama S., Rabkin E., Taubman M. B., Fallon J. T., Libby P. Dietary lipid lowering reduces tissue factor expression in rabbit atheroma. Circulation. 1999 Sep 14;100(11):1215–1222. doi: 10.1161/01.cir.100.11.1215. [DOI] [PubMed] [Google Scholar]
- Aikawa Masanori, Sugiyama Seigo, Hill Christopher C., Voglic Sami J., Rabkin Elena, Fukumoto Yoshihiro, Schoen Frederick J., Witztum Joseph L., Libby Peter. Lipid lowering reduces oxidative stress and endothelial cell activation in rabbit atheroma. Circulation. 2002 Sep 10;106(11):1390–1396. doi: 10.1161/01.cir.0000028465.52694.9b. [DOI] [PubMed] [Google Scholar]
- Alderman M. H., Madhavan S., Ooi W. L., Cohen H., Sealey J. E., Laragh J. H. Association of the renin-sodium profile with the risk of myocardial infarction in patients with hypertension. N Engl J Med. 1991 Apr 18;324(16):1098–1104. doi: 10.1056/NEJM199104183241605. [DOI] [PubMed] [Google Scholar]
- Alexander R. W. Atherosclerosis as disease of redox-sensitive genes. Trans Am Clin Climatol Assoc. 1998;109:129–146. [PMC free article] [PubMed] [Google Scholar]
- Alexander R. W. Theodore Cooper Memorial Lecture. Hypertension and the pathogenesis of atherosclerosis. Oxidative stress and the mediation of arterial inflammatory response: a new perspective. Hypertension. 1995 Feb;25(2):155–161. doi: 10.1161/01.hyp.25.2.155. [DOI] [PubMed] [Google Scholar]
- Anderson T. J., Elstein E., Haber H., Charbonneau F. Comparative study of ACE-inhibition, angiotensin II antagonism, and calcium channel blockade on flow-mediated vasodilation in patients with coronary disease (BANFF study) J Am Coll Cardiol. 2000 Jan;35(1):60–66. doi: 10.1016/s0735-1097(99)00537-9. [DOI] [PubMed] [Google Scholar]
- Babior B. M. NADPH oxidase: an update. Blood. 1999 Mar 1;93(5):1464–1476. [PubMed] [Google Scholar]
- Berlett B. S., Stadtman E. R. Protein oxidation in aging, disease, and oxidative stress. J Biol Chem. 1997 Aug 15;272(33):20313–20316. doi: 10.1074/jbc.272.33.20313. [DOI] [PubMed] [Google Scholar]
- Brown G., Albers J. J., Fisher L. D., Schaefer S. M., Lin J. T., Kaplan C., Zhao X. Q., Bisson B. D., Fitzpatrick V. F., Dodge H. T. Regression of coronary artery disease as a result of intensive lipid-lowering therapy in men with high levels of apolipoprotein B. N Engl J Med. 1990 Nov 8;323(19):1289–1298. doi: 10.1056/NEJM199011083231901. [DOI] [PubMed] [Google Scholar]
- Brunner H. R., Laragh J. H., Baer L., Newton M. A., Goodwin F. T., Krakoff L. R., Bard R. H., Bühler F. R. Essential hypertension: renin and aldosterone, heart attack and stroke. N Engl J Med. 1972 Mar 2;286(9):441–449. doi: 10.1056/NEJM197203022860901. [DOI] [PubMed] [Google Scholar]
- Brunner H. R., Sealey J. E., Laragh J. H. Renin as a risk factor in essential hypertension: more evidence. Am J Med. 1973 Sep;55(3):295–302. doi: 10.1016/0002-9343(73)90131-9. [DOI] [PubMed] [Google Scholar]
- Bush E., Maeda N., Kuziel W. A., Dawson T. C., Wilcox J. N., DeLeon H., Taylor W. R. CC chemokine receptor 2 is required for macrophage infiltration and vascular hypertrophy in angiotensin II-induced hypertension. Hypertension. 2000 Sep;36(3):360–363. doi: 10.1161/01.hyp.36.3.360. [DOI] [PubMed] [Google Scholar]
- Chappell D. C., Varner S. E., Nerem R. M., Medford R. M., Alexander R. W. Oscillatory shear stress stimulates adhesion molecule expression in cultured human endothelium. Circ Res. 1998 Mar 23;82(5):532–539. doi: 10.1161/01.res.82.5.532. [DOI] [PubMed] [Google Scholar]
- Corti Roberto, Fuster Valentin, Fayad Zahi A., Worthley Stephen G., Helft Gerard, Smith Donald, Weinberger Jesse, Wentzel Jolanda, Mizsei Gabor, Mercuri Michele. Lipid lowering by simvastatin induces regression of human atherosclerotic lesions: two years' follow-up by high-resolution noninvasive magnetic resonance imaging. Circulation. 2002 Dec 3;106(23):2884–2887. doi: 10.1161/01.cir.0000041255.88750.f0. [DOI] [PubMed] [Google Scholar]
- Cybulsky M. I., Gimbrone M. A., Jr Endothelial expression of a mononuclear leukocyte adhesion molecule during atherogenesis. Science. 1991 Feb 15;251(4995):788–791. doi: 10.1126/science.1990440. [DOI] [PubMed] [Google Scholar]
- Cybulsky M. I., Iiyama K., Li H., Zhu S., Chen M., Iiyama M., Davis V., Gutierrez-Ramos J. C., Connelly P. W., Milstone D. S. A major role for VCAM-1, but not ICAM-1, in early atherosclerosis. J Clin Invest. 2001 May;107(10):1255–1262. doi: 10.1172/JCI11871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Keulenaer G. W., Chappell D. C., Ishizaka N., Nerem R. M., Alexander R. W., Griendling K. K. Oscillatory and steady laminar shear stress differentially affect human endothelial redox state: role of a superoxide-producing NADH oxidase. Circ Res. 1998 Jun 1;82(10):1094–1101. doi: 10.1161/01.res.82.10.1094. [DOI] [PubMed] [Google Scholar]
- Faggiotto A., Ross R., Harker L. Studies of hypercholesterolemia in the nonhuman primate. I. Changes that lead to fatty streak formation. Arteriosclerosis. 1984 Jul-Aug;4(4):323–340. doi: 10.1161/01.atv.4.4.323. [DOI] [PubMed] [Google Scholar]
- Fukai T., Galis Z. S., Meng X. P., Parthasarathy S., Harrison D. G. Vascular expression of extracellular superoxide dismutase in atherosclerosis. J Clin Invest. 1998 May 15;101(10):2101–2111. doi: 10.1172/JCI2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukai T., Siegfried M. R., Ushio-Fukai M., Cheng Y., Kojda G., Harrison D. G. Regulation of the vascular extracellular superoxide dismutase by nitric oxide and exercise training. J Clin Invest. 2000 Jun;105(11):1631–1639. doi: 10.1172/JCI9551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Galis Z. S., Sukhova G. K., Kranzhöfer R., Clark S., Libby P. Macrophage foam cells from experimental atheroma constitutively produce matrix-degrading proteinases. Proc Natl Acad Sci U S A. 1995 Jan 17;92(2):402–406. doi: 10.1073/pnas.92.2.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galis Z. S., Sukhova G. K., Lark M. W., Libby P. Increased expression of matrix metalloproteinases and matrix degrading activity in vulnerable regions of human atherosclerotic plaques. J Clin Invest. 1994 Dec;94(6):2493–2503. doi: 10.1172/JCI117619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galis Zorina S., Khatri Jaikirshan J. Matrix metalloproteinases in vascular remodeling and atherogenesis: the good, the bad, and the ugly. Circ Res. 2002 Feb 22;90(3):251–262. [PubMed] [Google Scholar]
- Gerrity R. G. The role of the monocyte in atherogenesis: II. Migration of foam cells from atherosclerotic lesions. Am J Pathol. 1981 May;103(2):191–200. [PMC free article] [PubMed] [Google Scholar]
- Gimbrone M. A., Jr, Bevilacqua M. P., Cybulsky M. I. Endothelial-dependent mechanisms of leukocyte adhesion in inflammation and atherosclerosis. Ann N Y Acad Sci. 1990;598:77–85. doi: 10.1111/j.1749-6632.1990.tb42279.x. [DOI] [PubMed] [Google Scholar]
- Griendling K. K., Minieri C. A., Ollerenshaw J. D., Alexander R. W. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ Res. 1994 Jun;74(6):1141–1148. doi: 10.1161/01.res.74.6.1141. [DOI] [PubMed] [Google Scholar]
- Hajra L., Evans A. I., Chen M., Hyduk S. J., Collins T., Cybulsky M. I. The NF-kappa B signal transduction pathway in aortic endothelial cells is primed for activation in regions predisposed to atherosclerotic lesion formation. Proc Natl Acad Sci U S A. 2000 Aug 1;97(16):9052–9057. doi: 10.1073/pnas.97.16.9052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitzer T., Schlinzig T., Krohn K., Meinertz T., Münzel T. Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation. 2001 Nov 27;104(22):2673–2678. doi: 10.1161/hc4601.099485. [DOI] [PubMed] [Google Scholar]
- Hermann C., Zeiher A. M., Dimmeler S. Shear stress inhibits H2O2-induced apoptosis of human endothelial cells by modulation of the glutathione redox cycle and nitric oxide synthase. Arterioscler Thromb Vasc Biol. 1997 Dec;17(12):3588–3592. doi: 10.1161/01.atv.17.12.3588. [DOI] [PubMed] [Google Scholar]
- Hodis Howard N., Mack Wendy J., LaBree Laurie, Mahrer Peter R., Sevanian Alex, Liu Chao-ran, Liu Ci-hua, Hwang Juliana, Selzer Robert H., Azen Stanley P. Alpha-tocopherol supplementation in healthy individuals reduces low-density lipoprotein oxidation but not atherosclerosis: the Vitamin E Atherosclerosis Prevention Study (VEAPS). Circulation. 2002 Sep 17;106(12):1453–1459. doi: 10.1161/01.cir.0000029092.99946.08. [DOI] [PubMed] [Google Scholar]
- Huang Y., Mironova M., Lopes-Virella M. F. Oxidized LDL stimulates matrix metalloproteinase-1 expression in human vascular endothelial cells. Arterioscler Thromb Vasc Biol. 1999 Nov;19(11):2640–2647. doi: 10.1161/01.atv.19.11.2640. [DOI] [PubMed] [Google Scholar]
- Iiyama K., Hajra L., Iiyama M., Li H., DiChiara M., Medoff B. D., Cybulsky M. I. Patterns of vascular cell adhesion molecule-1 and intercellular adhesion molecule-1 expression in rabbit and mouse atherosclerotic lesions and at sites predisposed to lesion formation. Circ Res. 1999 Jul 23;85(2):199–207. doi: 10.1161/01.res.85.2.199. [DOI] [PubMed] [Google Scholar]
- Inoue N., Ramasamy S., Fukai T., Nerem R. M., Harrison D. G. Shear stress modulates expression of Cu/Zn superoxide dismutase in human aortic endothelial cells. Circ Res. 1996 Jul;79(1):32–37. doi: 10.1161/01.res.79.1.32. [DOI] [PubMed] [Google Scholar]
- Kaplan N. M. Primary hypertension. From pathophysiology to prevention. Arch Intern Med. 1996 Sep 23;156(17):1919–1920. doi: 10.1001/archinte.156.17.1919. [DOI] [PubMed] [Google Scholar]
- Khan B. V., Navalkar S., Khan Q. A., Rahman S. T., Parthasarathy S. Irbesartan, an angiotensin type 1 receptor inhibitor, regulates the vascular oxidative state in patients with coronary artery disease. J Am Coll Cardiol. 2001 Nov 15;38(6):1662–1667. doi: 10.1016/s0735-1097(01)01615-1. [DOI] [PubMed] [Google Scholar]
- Ku D. N., Giddens D. P., Zarins C. K., Glagov S. Pulsatile flow and atherosclerosis in the human carotid bifurcation. Positive correlation between plaque location and low oscillating shear stress. Arteriosclerosis. 1985 May-Jun;5(3):293–302. doi: 10.1161/01.atv.5.3.293. [DOI] [PubMed] [Google Scholar]
- Ku D. N., Giddens D. P., Zarins C. K., Glagov S. Pulsatile flow and atherosclerosis in the human carotid bifurcation. Positive correlation between plaque location and low oscillating shear stress. Arteriosclerosis. 1985 May-Jun;5(3):293–302. doi: 10.1161/01.atv.5.3.293. [DOI] [PubMed] [Google Scholar]
- Laufs U., La Fata V., Plutzky J., Liao J. K. Upregulation of endothelial nitric oxide synthase by HMG CoA reductase inhibitors. Circulation. 1998 Mar 31;97(12):1129–1135. doi: 10.1161/01.cir.97.12.1129. [DOI] [PubMed] [Google Scholar]
- Laursen J. B., Rajagopalan S., Galis Z., Tarpey M., Freeman B. A., Harrison D. G. Role of superoxide in angiotensin II-induced but not catecholamine-induced hypertension. Circulation. 1997 Feb 4;95(3):588–593. doi: 10.1161/01.cir.95.3.588. [DOI] [PubMed] [Google Scholar]
- Libby P. Atheroma: more than mush. Lancet. 1996 Nov;348 (Suppl 1):s4–s7. doi: 10.1016/s0140-6736(96)98002-2. [DOI] [PubMed] [Google Scholar]
- Libby Peter, Ridker Paul M., Maseri Attilio. Inflammation and atherosclerosis. Circulation. 2002 Mar 5;105(9):1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- Ludmer P. L., Selwyn A. P., Shook T. L., Wayne R. R., Mudge G. H., Alexander R. W., Ganz P. Paradoxical vasoconstriction induced by acetylcholine in atherosclerotic coronary arteries. N Engl J Med. 1986 Oct 23;315(17):1046–1051. doi: 10.1056/NEJM198610233151702. [DOI] [PubMed] [Google Scholar]
- Marui N., Offermann M. K., Swerlick R., Kunsch C., Rosen C. A., Ahmad M., Alexander R. W., Medford R. M. Vascular cell adhesion molecule-1 (VCAM-1) gene transcription and expression are regulated through an antioxidant-sensitive mechanism in human vascular endothelial cells. J Clin Invest. 1993 Oct;92(4):1866–1874. doi: 10.1172/JCI116778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore J. E., Jr, Xu C., Glagov S., Zarins C. K., Ku D. N. Fluid wall shear stress measurements in a model of the human abdominal aorta: oscillatory behavior and relationship to atherosclerosis. Atherosclerosis. 1994 Oct;110(2):225–240. doi: 10.1016/0021-9150(94)90207-0. [DOI] [PubMed] [Google Scholar]
- Munro J. M., Cotran R. S. The pathogenesis of atherosclerosis: atherogenesis and inflammation. Lab Invest. 1988 Mar;58(3):249–261. [PubMed] [Google Scholar]
- Mügge A., Elwell J. H., Peterson T. E., Hofmeyer T. G., Heistad D. D., Harrison D. G. Chronic treatment with polyethylene-glycolated superoxide dismutase partially restores endothelium-dependent vascular relaxations in cholesterol-fed rabbits. Circ Res. 1991 Nov;69(5):1293–1300. doi: 10.1161/01.res.69.5.1293. [DOI] [PubMed] [Google Scholar]
- Münzel T., Keaney J. F., Jr Are ACE inhibitors a "magic bullet" against oxidative stress? Circulation. 2001 Sep 25;104(13):1571–1574. doi: 10.1161/hc3801.095585. [DOI] [PubMed] [Google Scholar]
- Nishida K., Harrison D. G., Navas J. P., Fisher A. A., Dockery S. P., Uematsu M., Nerem R. M., Alexander R. W., Murphy T. J. Molecular cloning and characterization of the constitutive bovine aortic endothelial cell nitric oxide synthase. J Clin Invest. 1992 Nov;90(5):2092–2096. doi: 10.1172/JCI116092. [DOI] [PMC free article] [PubMed] [Google Scholar]
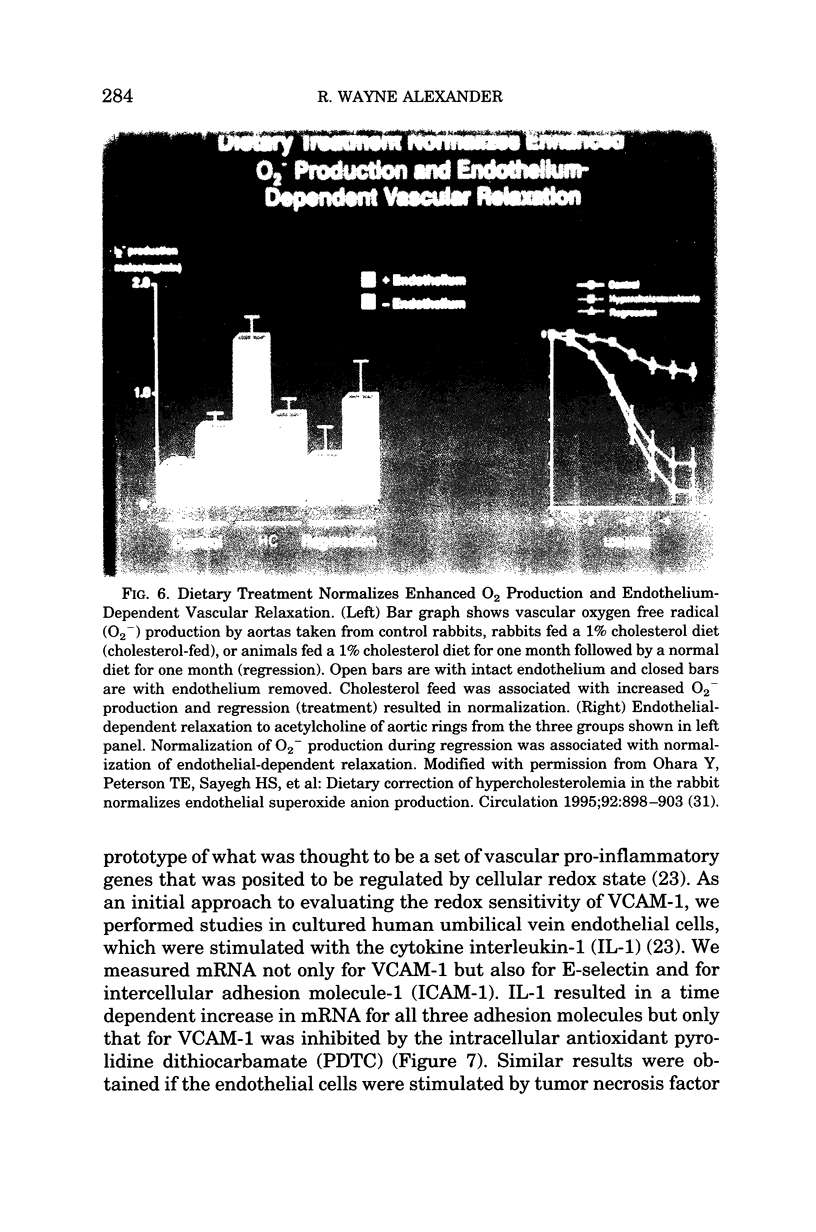
- Ohara Y., Peterson T. E., Harrison D. G. Hypercholesterolemia increases endothelial superoxide anion production. J Clin Invest. 1993 Jun;91(6):2546–2551. doi: 10.1172/JCI116491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohara Y., Peterson T. E., Sayegh H. S., Subramanian R. R., Wilcox J. N., Harrison D. G. Dietary correction of hypercholesterolemia in the rabbit normalizes endothelial superoxide anion production. Circulation. 1995 Aug 15;92(4):898–903. doi: 10.1161/01.cir.92.4.898. [DOI] [PubMed] [Google Scholar]
- Parthasarathy S., Khan-Merchant N., Penumetcha M., Khan B. V., Santanam N. Did the antioxidant trials fail to validate the oxidation hypothesis? Curr Atheroscler Rep. 2001 Sep;3(5):392–398. doi: 10.1007/s11883-001-0077-9. [DOI] [PubMed] [Google Scholar]
- Pfeffer M. A., Braunwald E., Moyé L. A., Basta L., Brown E. J., Jr, Cuddy T. E., Davis B. R., Geltman E. M., Goldman S., Flaker G. C. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. Results of the survival and ventricular enlargement trial. The SAVE Investigators. N Engl J Med. 1992 Sep 3;327(10):669–677. doi: 10.1056/NEJM199209033271001. [DOI] [PubMed] [Google Scholar]
- Pfuetze K. D., Dujovne C. A. Probucol. Curr Atheroscler Rep. 2000 Jan;2(1):47–57. doi: 10.1007/s11883-000-0094-0. [DOI] [PubMed] [Google Scholar]
- Rahman Syed T., Lauten Wright B., Khan Qamar A., Navalkar Sushant, Parthasarathy Sampath, Khan Bobby V. Effects of eprosartan versus hydrochlorothiazide on markers of vascular oxidation and inflammation and blood pressure (renin-angiotensin system antagonists, oxidation, and inflammation). Am J Cardiol. 2002 Mar 15;89(6):686–690. doi: 10.1016/s0002-9149(01)02340-2. [DOI] [PubMed] [Google Scholar]
- Rajagopalan S., Kurz S., Münzel T., Tarpey M., Freeman B. A., Griendling K. K., Harrison D. G. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J Clin Invest. 1996 Apr 15;97(8):1916–1923. doi: 10.1172/JCI118623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopalan S., Meng X. P., Ramasamy S., Harrison D. G., Galis Z. S. Reactive oxygen species produced by macrophage-derived foam cells regulate the activity of vascular matrix metalloproteinases in vitro. Implications for atherosclerotic plaque stability. J Clin Invest. 1996 Dec 1;98(11):2572–2579. doi: 10.1172/JCI119076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajavashisth T. B., Xu X. P., Jovinge S., Meisel S., Xu X. O., Chai N. N., Fishbein M. C., Kaul S., Cercek B., Sharifi B. Membrane type 1 matrix metalloproteinase expression in human atherosclerotic plaques: evidence for activation by proinflammatory mediators. Circulation. 1999 Jun 22;99(24):3103–3109. doi: 10.1161/01.cir.99.24.3103. [DOI] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993 Apr 29;362(6423):801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- Satriano J. A., Shuldiner M., Hora K., Xing Y., Shan Z., Schlondorff D. Oxygen radicals as second messengers for expression of the monocyte chemoattractant protein, JE/MCP-1, and the monocyte colony-stimulating factor, CSF-1, in response to tumor necrosis factor-alpha and immunoglobulin G. Evidence for involvement of reduced nicotinamide adenine dinucleotide phosphate (NADPH)-dependent oxidase. J Clin Invest. 1993 Sep;92(3):1564–1571. doi: 10.1172/JCI116737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawayama Yasunori, Shimizu Chie, Maeda Naoyasu, Tatsukawa Masafumi, Kinukawa Naoko, Koyanagi Samon, Kashiwagi Seizaburo, Hayashi Jun. Effects of probucol and pravastatin on common carotid atherosclerosis in patients with asymptomatic hypercholesterolemia. Fukuoka Atherosclerosis Trial (FAST). J Am Coll Cardiol. 2002 Feb 20;39(4):610–616. doi: 10.1016/s0735-1097(01)01783-1. [DOI] [PubMed] [Google Scholar]
- Schmidt A. M., Hori O., Chen J. X., Li J. F., Crandall J., Zhang J., Cao R., Yan S. D., Brett J., Stern D. Advanced glycation endproducts interacting with their endothelial receptor induce expression of vascular cell adhesion molecule-1 (VCAM-1) in cultured human endothelial cells and in mice. A potential mechanism for the accelerated vasculopathy of diabetes. J Clin Invest. 1995 Sep;96(3):1395–1403. doi: 10.1172/JCI118175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg D., Parthasarathy S., Carew T. E., Khoo J. C., Witztum J. L. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N Engl J Med. 1989 Apr 6;320(14):915–924. doi: 10.1056/NEJM198904063201407. [DOI] [PubMed] [Google Scholar]
- Stemme S., Holm J., Hansson G. K. T lymphocytes in human atherosclerotic plaques are memory cells expressing CD45RO and the integrin VLA-1. Arterioscler Thromb. 1992 Feb;12(2):206–211. doi: 10.1161/01.atv.12.2.206. [DOI] [PubMed] [Google Scholar]
- Sukhova Galina K., Williams J. Koudy, Libby Peter. Statins reduce inflammation in atheroma of nonhuman primates independent of effects on serum cholesterol. Arterioscler Thromb Vasc Biol. 2002 Sep 1;22(9):1452–1458. doi: 10.1161/01.atv.0000030360.72503.56. [DOI] [PubMed] [Google Scholar]
- Tanimoto Tatsuo, Jin Zheng-Gen, Berk Bradford C. Transactivation of vascular endothelial growth factor (VEGF) receptor Flk-1/KDR is involved in sphingosine 1-phosphate-stimulated phosphorylation of Akt and endothelial nitric-oxide synthase (eNOS). J Biol Chem. 2002 Sep 10;277(45):42997–43001. doi: 10.1074/jbc.M204764200. [DOI] [PubMed] [Google Scholar]
- Tardif J. C., Cöté G., Lespérance J., Bourassa M., Lambert J., Doucet S., Bilodeau L., Nattel S., de Guise P. Probucol and multivitamins in the prevention of restenosis after coronary angioplasty. Multivitamins and Probucol Study Group. N Engl J Med. 1997 Aug 7;337(6):365–372. doi: 10.1056/NEJM199708073370601. [DOI] [PubMed] [Google Scholar]
- Treasure C. B., Klein J. L., Weintraub W. S., Talley J. D., Stillabower M. E., Kosinski A. S., Zhang J., Boccuzzi S. J., Cedarholm J. C., Alexander R. W. Beneficial effects of cholesterol-lowering therapy on the coronary endothelium in patients with coronary artery disease. N Engl J Med. 1995 Feb 23;332(8):481–487. doi: 10.1056/NEJM199502233320801. [DOI] [PubMed] [Google Scholar]
- Ushio-Fukai M., Hilenski L., Santanam N., Becker P. L., Ma Y., Griendling K. K., Alexander R. W. Cholesterol depletion inhibits epidermal growth factor receptor transactivation by angiotensin II in vascular smooth muscle cells: role of cholesterol-rich microdomains and focal adhesions in angiotensin II signaling. J Biol Chem. 2001 Oct 3;276(51):48269–48275. doi: 10.1074/jbc.M105901200. [DOI] [PubMed] [Google Scholar]
- Vecchione Carmine, Brandes Ralf P. Withdrawal of 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors elicits oxidative stress and induces endothelial dysfunction in mice. Circ Res. 2002 Jul 26;91(2):173–179. doi: 10.1161/01.res.0000028004.76218.b8. [DOI] [PubMed] [Google Scholar]
- Walldius G., Erikson U., Olsson A. G., Bergstrand L., Hådell K., Johansson J., Kaijser L., Lassvik C., Mölgaard J., Nilsson S. The effect of probucol on femoral atherosclerosis: the Probucol Quantitative Regression Swedish Trial (PQRST). Am J Cardiol. 1994 Nov 1;74(9):875–883. doi: 10.1016/0002-9149(94)90579-7. [DOI] [PubMed] [Google Scholar]
- Xu X. P., Meisel S. R., Ong J. M., Kaul S., Cercek B., Rajavashisth T. B., Sharifi B., Shah P. K. Oxidized low-density lipoprotein regulates matrix metalloproteinase-9 and its tissue inhibitor in human monocyte-derived macrophages. Circulation. 1999 Mar 2;99(8):993–998. doi: 10.1161/01.cir.99.8.993. [DOI] [PubMed] [Google Scholar]
- Ylä-Herttuala S., Palinski W., Butler S. W., Picard S., Steinberg D., Witztum J. L. Rabbit and human atherosclerotic lesions contain IgG that recognizes epitopes of oxidized LDL. Arterioscler Thromb. 1994 Jan;14(1):32–40. doi: 10.1161/01.atv.14.1.32. [DOI] [PubMed] [Google Scholar]
- Ylä-Herttuala S., Palinski W., Rosenfeld M. E., Parthasarathy S., Carew T. E., Butler S., Witztum J. L., Steinberg D. Evidence for the presence of oxidatively modified low density lipoprotein in atherosclerotic lesions of rabbit and man. J Clin Invest. 1989 Oct;84(4):1086–1095. doi: 10.1172/JCI114271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoi H., Daida H., Kuwabara Y., Nishikawa H., Takatsu F., Tomihara H., Nakata Y., Kutsumi Y., Ohshima S., Nishiyama S. Effectiveness of an antioxidant in preventing restenosis after percutaneous transluminal coronary angioplasty: the Probucol Angioplasty Restenosis Trial. J Am Coll Cardiol. 1997 Oct;30(4):855–862. doi: 10.1016/s0735-1097(97)00270-2. [DOI] [PubMed] [Google Scholar]
- van der Wal A. C., Becker A. E., van der Loos C. M., Das P. K. Site of intimal rupture or erosion of thrombosed coronary atherosclerotic plaques is characterized by an inflammatory process irrespective of the dominant plaque morphology. Circulation. 1994 Jan;89(1):36–44. doi: 10.1161/01.cir.89.1.36. [DOI] [PubMed] [Google Scholar]