Abstract
The purpose of this study was to enhance our understanding of the mechanisms of neuronal death after focal cerebral ischemia and the neuroprotective effects of tamoxifen (TMX). The phosphorylation state of 31 protein kinases/signaling proteins and superoxide anion (O2−) production in the contralateral and ipsilateral cortex was measured after permanent middle cerebral artery occlusion (pMCAO) in ovariectomized rats treated with placebo or TMX. The study revealed that pMCAO modulated the phosphorylation of a number of kinases/proteins in the penumbra at 2 h after pMCAO. Of significant interest, phospho-ERK1/2 (pERK1/2) was elevated significantly after pMCAO. TMX attenuated the elevation of pERK1/2, an effect correlated with reduced infarct size. In situ detection of O2− production showed a significant elevation at 1–2 h after pMCAO in the ischemic cortex with enhanced oxidative damage detected at 24 h. ERK activation may be downstream of free radicals, a suggestion supported by the findings that cells positive for O2− had high pERK activation and that a superoxide dismutase (SOD) mimetic, tempol, significantly attenuated pERK activation after MCAO. TMX treatment significantly reduced the MCAO-induced elevation of O2− production, oxidative damage, and proapoptotic caspase-3 activation. Additionally, pMCAO induced a significant reduction in the levels of manganese SOD (MnSOD), which scavenge O2−, an effect largely prevented by TMX treatment, thus providing a potential mechanistic basis for the antioxidant effects of TMX. As a whole, these studies suggest that TMX neuroprotection may be achieved via an antioxidant mechanism that involves enhancement of primarily MnSOD levels, with a corresponding reduction of O2− production, and downstream kinase and caspase-3 activation.
PREVIOUS WORK HAS shown that estrogen can exert neuroprotective effects in animal models of stroke (1,2,3,4,5; see Ref. 6 for review). However, estrogen can have undesired stimulatory effects on the breast and uterus, which raises concern for a potential increased risk of developing breast and uterine cancers. These potential limitations have kindled interest in the development and therapeutic use of nonsteroidal selective estrogen receptor modulators. Along these lines, work by our laboratory and others has shown that the selective estrogen receptor modulator tamoxifen can significantly reduce infarct size in both transient and permanent occlusion/reperfusion models of cerebral ischemia (7,8,9,10). Kimelberg and colleagues (7) were the first to report a neuroprotective action of tamoxifen after stroke in male animals, an effect later extended to female animals by our laboratory (9). The effect of tamoxifen was shown to be independent of cerebral blood flow changes, indicating a potential direct neuroprotective effect in the brain by tamoxifen. In line with this suggestion is previous work showing that tamoxifen readily crosses the blood-brain barrier and accumulates in the brain (11). Tamoxifen has also been implicated to be neuroprotective in animal models of Parkinson’s disease, where it has been shown to protect the striatum against methamphetamine-induced toxicity and prevent striatal dopamine depletion in male and female animals (12,13,14,15).
The mechanism of how tamoxifen exerts neuroprotection is unclear. Kimelberg and colleagues (7,8) have shown that tamoxifen can inhibit excitatory amino acid release and nitric oxide synthase activity after temporary cerebral ischemia in male rodents, which may be important for its neuroprotective effects. Interestingly, a number of studies have suggested that tamoxifen, or its active metabolite 4-OH-tamoxifen, possesses free radical-scavenging and antioxidant activity in vitro and in vivo (15,16,17,18,19). Tamoxifen has also been shown recently to improve mitochondrial respiratory function and enhance superoxide-scavenging activity of mitochondria in the heart (20). Based on these findings, the present study was designed to examine whether tamoxifen modulates superoxide anion (O2−) production in the brain after cerebral ischemia in the ovariectomized female rat as a potential mechanism of neuroprotection. Furthermore, a kinase phosphoprotein array was performed to examine the activation state of 31 different kinases in the ipsilateral and contralateral cortex of sham-, placebo-, and tamoxifen-treated rats after permanent middle cerebral artery occlusion (pMCAO) so as to determine kinase signaling pathways affected by cerebral ischemia and/or regulated by tamoxifen. The results of the study revealed that tamoxifen significantly attenuates O2− production, reduces oxidative protein/DNA damage and caspase-3 activation, and attenuates activation of ERKs in the ipsilateral cortex after pMCAO. The study also provides evidence that O2− is responsible, at least in part, for early ERK activation after cerebral ischemia and that tamoxifen enhances manganese superoxide dismutase (MnSOD) expression as a potential mechanism for reduction of O2−.
Materials and Methods
Animal model and drug treatment
All experiments were conducted in compliance with the National Institutes of Health guidelines for the care and use of experimental animals and were approved by the Institutional Animal Care and Use Committee at the Medical College of Georgia. Sixty-day-old Holtzman Sprague Dawley female rats (Harlan, IN) were used for the study. The animals were housed in individual cages and water and rat chow were provided ad libitum. The animals were bilaterally ovariectomized and implanted sc in the mid-upper back region with pellets that contained tamoxifen (15-mg pellets, releases ∼1 mg/kg·d) or vehicle (placebo). One week later, animals underwent pMCAO for various durations as described below.
MCAO
pMCAO was used in all experiments, as described previously by our laboratory (9). Briefly, rats were anesthetized by im injection of ketamine/xylazine (60 mg/ml and 8 mg/ml, respectively). A thermal blanket was used to maintain body temperature at 37 C. The skin of the neck was shaved and swabbed with betadine, followed by making an incision directly on top of the right common carotid artery region. The fascia was then blunt dissected until the bifurcation of the external common carotid artery and internal common carotid artery is isolated. A small incision was made in the external common carotid arteryand then a 4-0 monofilament suture pretreated with poly-l-lysine (18.5–19.5 mm long with a round tip) was threaded into the internal common carotid artery via the external common carotid artery. The suture was then advanced toward the middle cerebral artery to create cerebral ischemia. The site of MCAO was confirmed by the location of suture in middle cerebral artery at the time of death. Animals were killed at different time intervals after pMCAO as described in the figure legends.
Measurement of infarct size and histopathology
To assess the ischemic damage caused by pMCAO, we measured the infarct volume and histopathology using 2,3,5-triphenyltetrazolium chloride (TTC) and Nissl/crystal violet staining after 24 and 96 h pMCAO. Animals were anesthetized with ketamine/xylazine and transcardially perfused with PBS. Brains were removed and sectioned coronally at 2-mm intervals using a brain matrix (Braintree Scientific Inc., Braintree, MA). Brain slices were placed in a Petri dish in TTC using a 2% wt/vol solution in PBS. TTC stains the viable brain tissue as red, whereas the infarcted area fails to take up the stain and remains white. The brain slices were then fixed by immersion in 2% paraformaldehyde solution. The volume of infarct was calculated by integrating the area of injury on the sections of each brain. Total infarct volumes were quantified using a computerized image analysis system, and infarct size was expressed as percent hemisphere infarcted (Scion image β). Histopathological examination of the ischemic brain was also performed by the Nissl staining method on sections obtained 2 and 24 h after pMCAO. Animals were processed as described under Immunohistochemistry, and 40-μm coronal sections were cut on a cryostat microtome. Sections were washed for 10 min in PBS followed by PBS-Triton X-100 (0.1%) for an additional 10 min. This step permeabilizes the tissue and is required for optimal staining. Sections were again washed in PBS twice for 5 min each and then incubated for about 20 min with fluorescent Nissl stain (NeuroTrace647/660; Invitrogen, Carlsbad, CA) diluted in PBS as recommended by the manufacturer. Afterward, staining solution was removed, and sections were washed first with PBS-Triton X-100 for 10 min followed by PBS for about 2 h at room temperature. After that, sections were washed briefly in water and mounted using water-based mounting medium containing antifading agents (Biomeda, Foster City, CA). Images were captured on a confocal laser microscope as described below. For crystal violet staining, sections were stained with aqueous 0.1% crystal violet solution (pH 3.8) for 5 min and analyzed by light microscopy.
Kinetworks phosphoprotein profiling
In the phosphoprotein Kinetworks profiling studies, animals were anesthetized with ketamine/xylazine and perfused with saline 2 h after pMCAO. The brains were removed and sectioned, and tissue punches (2 mm diameter) were collected from the penumbra region of the ipsilateral cortex. Punches were also collected from the noninjured contralateral cortex and from sham animals in anatomically corresponding regions to the punches collected from the ipsilateral cortex. Protein lysate (300–600 mg) from tissue punches was used for Kinetworks Phospho-Site Screen (KPSS-1.3). The KPSS-1.3 has been used and published extensively by others (21,22,23,24). It allows simultaneous detection/semiquantitative analysis of the levels of 31 different phospho-protein kinases and signaling proteins using ECL detection in Western miniblot gels (Kinexus Bioinformatics Corp., Vancouver, Canada).
Western blot analysis
Tissue was collected for Western blot analysis in the same way as described above for the phosphoprotein profiling study. The tissue was homogenized with a Polytron homogenizer in a lysis buffer containing protease inhibitors. The insoluble portion of the tissue punch lysate was removed by centrifugation at 10,000 × g for10 min. Protein concentration was determined by a Lowry protein assay kit. Western blot analysis (50 μg/lane) was carried out as described in detail previously by our laboratory (25). Protein samples were denatured in sample buffer containing β-mercaptoethanol in 25 mm Tris-glycine buffer and separated on either 10% or 4–20% gradient SDS-polyacrylamide gel after loading an equal amount of protein in each lane. Separated proteins were transferred to Immobilon P membrane (Millipore, Bedford, MA) at 27 V for 15 h in 25 mm Tris-glycine buffer (pH 8.3) with 10% methanol using a Mini Transblot apparatus (Bio-Rad Laboratories, Inc., Hercules, CA). After the transfer, the membranes were rinsed twice with Tris-buffered saline with Tween 20 (20 mm Tris, 137 mm NaCl, 0.1% Tween 20) for 5 min each rinse and then incubated with 5% nonfat dry milk for 1 h at room temperature to block nonspecific/unbound surface. The membrane was incubated overnight with a well-characterized, commercially available polyclonal rabbit anti-phospho-ERK1/2 (anti-pERK1/2) antibody (1:2500; Biosource, Camarillo, CA), or monoclonal mouse anti-MnSOD (1:1000, clone MnS-1; Chemicon, Temecula, CA). The membrane was then washed with Tris-buffered saline with Tween 20 to remove unbound antibody, followed by incubation with secondary horseradish peroxidase-conjugated goat antirabbit or antimouse IgG (Transduction Laboratories, San Diego, CA) for 1–2 h at room temperature. The signal was detected using an ECL detection kit (Pierce Biotechnology, Rockford, IL), and the membranes were exposed to Kodak Biomax MR film.
Immunohistochemistry
Perfusion and fixation.
For immunohistochemistry studies, the animals were anesthetized with ketamine/xylazine and perfused with 0.9% saline containing (containing 10 U/ml heparin), followed by fixation with cold 4% paraformaldehyde in 0.1 m phosphate buffer (pH 7.4). Brains were postfixed in the same fixative overnight at 4 C and cryoprotected with 30% sucrose in 0.1 m phosphate buffer (pH 7.4) for 24–36 h or until the brain sank. Coronal sections at a thickness of 40 μm were cut on a cryostat microtome (Leica, Wetzlar, Germany) and stored in cryoprotection (FD Neurotechnology Inc., Baltimore, MD) solution for immunohistochemistry.
Diaminobenzidine (DAB) staining.
For DAB staining, sections were incubated with 10% normal goat/horse serum in PBS containing 0.1% Triton X-100 and 0.3% H2O2 for 1 h at room temperature to block nonspecific surfaces. Sections were then incubated with the primary antibodies overnight at 4 C in PBS containing 0.1% Triton X-100. The antibodies used were as follows: polyclonal rabbit anti-pERK1/2 (1:500; Biosource), rabbit anti-active caspase-3 (1:1000; Abcam, Cambridge, MA), monoclonal mouse anti-MnSOD (1:500, clone MnS-1; Chemicon), mouse anti-4-hydroxy-2-nonenal (anti-4-HNE) (1:750, JaICA, Shizuoka, Japan), and mouse anti-8-hydroxy-2′-deoxyguanosine (anti-8-OHdG) (1:100; JaICA, Japan). Afterward, sections were washed with the same buffer, followed by incubation with secondary biotinylated goat antirabbit or horse antimouse antibodies (Vector Laboratories, Inc., Burlingame, CA) at a dilution of 1:200 in PBS containing 0.1% Triton X-100 for 1 h at room temperature. Sections were then washed, followed by incubation with ABC reagents for 1 h at room temperature in the same buffer. Sections were rinsed in the same buffer and incubated with DAB reagent according to the manufacturer’s instructions (Vector) for 2–10 min. After DAB incubation, sections were washed briefly with distilled water and dehydrated in graded alcohols, cleared in xylene, and mounted using xylene-based mounting medium. Images were captured on an Axiophot-2 visible/fluorescence microscope using an AxioVision4Ac software system (Carl Zeiss, Oberkochen, Germany) using either ×20 water or ×40 oil immersion Neofluor objective (NA 1.3). The number of DAB-stained cells and the intensity of staining was analyzed using Volocity 4.0 analytical software (Improvision Inc., Lexington, MA).
Double-immunofluorescence staining.
Coronal sections were incubated with 10% normal donkey serum for 1 h at room temperature in PBS containing 0.1% Triton X-100, followed by incubation with appropriate primary antibodies overnight at 4 C in the same buffer. The following primary antibodies were used in different combinations: polyclonal rabbit anti-pERK1/2 (1:500), rabbit anti-caspase-3 (1:500), mouse anti-pERK1/2 (1:300), monoclonal mouse anti-MnSOD (1:1000), mouse anti-NeuN (1:1000; Chemicon), and mouse anti-glial fibrillary acidic protein (1:2000; Sigma Chemical Co., St. Louis, MO). The source and origin of some of these antibodies have been mentioned above. After primary antibody incubation, sections were washed for four times for 10 min each at room temperature, followed by incubation with Alexa Fluor 488 donkey antimouse and Alexa Fluor 594 donkey antirabbit (1:500; Invitrogen) for 1 h at room temperature. Sections were then washed with PBS containing 0.1% Triton X-100 four times for 10 min each, followed by three 5-min washes with PBS and briefly with water and then mounted with water-based mounting medium containing antifading agents (Biomeda, Fischer Scientific, Pittsburgh, PA). A simultaneous examination of negative controls (omission of primary antibody) confirmed the absence of nonspecific immunofluorescent staining, cross-immunostaining, or fluorescence bleed-through.
Measurement of superoxide anion production
The production of superoxide anion (O2−) was investigated using hydroethidine (HEt) as described previously by our group and others (6,26,27,28). HEt is diffusible into the central nervous system parenchyma and rapidly taken up by the neuronal perikarya after an iv injection. After transport inside the cell, HEt is selectively oxidized to ethidium by O2− and thus provides a direct measurement of O2− production (27,28). In the present study, HEt (1 mg/ml in 200 μl PBS) was administered iv into the femoral vein 15 min before pMCAO, and the animals were kill 1 and 2 h after pMCAO. Fluorescent intensity of the oxidized HEt was measured on a confocal laser microscope using an excitation wavelength of 543 nm, and the emission was recorded at wavelength of more than 580 nm. The intensity of ethidium was analyzed using LSM 510 image examiner software, and the number of ethidium-positive cells per unit area was determined using Volocity 4.0 imaging software (Improvision).
Confocal microscopy and image analysis
All of the double- and triple-labeled images were captured on an LSM510 Meta confocal laser microscope (Carl Zeiss) in XYZ (Z-stacks) mode using either a ×40 or ×63 oil immersion Neofluor objective (NA 1.3) with the image size set at 512 × 512 pixels. The following excitation lasers/emission filters settings were used for various chromophores: argon2 laser was used for Alexa Fluor 488, with excitation maxima at 490 and emission in the range 505–530 nm, HeNe1 laser was used for Alexa Fluor 594 with excitation maxima at 543 nm and emission in the range 568–615 nm, and HeNe2 laser was used for Alexa Fluor 647 with excitation maxima at 633 nm and emission in the range 650–800 nm. The Z-stacks (15–20 optical slices) were collected at optimal pinhole diameter at 12-bit pixel depth. The Z-stacks were then converted into three-dimensional projection image using LSM510 Meta imaging software. Confocal images were also analyzed using Volocity 4.0 imaging software (Improvision)
Cell counting and statistical analysis
The light microscopy images captured from DAB-stained slides were analyzed using Volocity 4.0 or NIH ImageJ software. To measure the staining intensity, a fixed intensity threshold was set on a gray value scale (0–255), and all the images were background subtracted. A background value was determined by measuring DAB intensity on the representative sections stained without incubating with the primary antibodies. To semiquantitatively measure the cell density/number of cells per unit area, a fixed-diameter threshold was set for all the images. Confocal images were also analyzed using the same diameter threshold. Statistical analysis between ipsilateral and contralateral hemispheres or between placebo, sham, and tamoxifen treatment groups was made using one way ANOVA followed by post hoc Student-Newman Keuls test. Data are expressed as means ± se. All the experiments were repeated twice for verification of results unless otherwise stated.
Results
Effect of tamoxifen on brain infarct size and histopathology after pMCAO
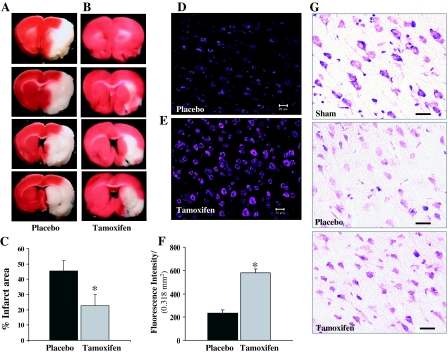
Tamoxifen has been reported by a number of laboratories, including our own, to reduce infarct damage after transient and permanent cerebral ischemia (7,8,9,10). In the current study, infarct area was determined 24 h after pMCAO using TTC staining to confirm tamoxifen reduction of infarct damage (Fig. 1). A large cortical and subcortical infract was demonstrated in placebo-treated rats at 24 h after pMCAO (Fig. 1, A and C). Tamoxifen treatment caused a significant reduction (50%) in infarct size after pMCAO (Fig. 1, B and C). We next examined histopathology using fluorescent Nissl stain. Nissl substance is abundant in neuronal cells in the endoplasmic reticulum and reflects the unusually high protein synthesis capacity of neuronal cells. In injured or regenerating neurons, Nissl substance breaks apart and redistributes around the periphery of the cell body. Therefore, the Nissl stain represents a useful marker for the physiological state of the cells. As can be seen from Fig. 1, D and F, placebo-treated animals showed a marked reduction (P > 0.001) in Nissl staining intensity analyzed in the ipsilateral cortex 24 h after pMCAO compared with tamoxifen-treated animals (Fig. 1, E and F). A similar increase in the Nissl staining intensity by tamoxifen was also observed at 96 h after pMCAO (data not shown). Figure 1G shows crystal violet staining of coronal sections from sham-, placebo-, and tamoxifen-treated animals after 96 h pMCAO. As shown in Fig. 1G, tamoxifen-treated animals (lower panel) showed a marked preservation of the neuronal morphology in the ischemic cortex at 96 h after pMCAO compared with the placebo-treated animals (middle panel).
Figure 1.
Development of infarct in the corticostriatal region of the rat brain after 24 h pMCAO. A–C, Compared with placebo-treated animals (A and C), tamoxifen-treated animals (B and C) show a significantly reduced infarct area at 24 h after pMCAO (n = 9 animals per group). D–F, Nissl staining performed after 24 h pMCAO showed a significant increase in Nissl granule intensity in tamoxifen-treated animals (E and F) compared with placebo-treated animals (D and F) (n = 4 animals per group). *, Significance was determined by one-way ANOVA with post hoc analysis using Student-Newman-Keuls test; P < 0.05. G, Crystal violet staining performed on the coronal sections from sham (upper panel), placebo (middle panel), and tamoxifen (lower panel) after 96 h pMCAO. Scale bars, 20 μm.
Effect of tamoxifen on kinase activation in the penumbra region of the cortex after pMCAO
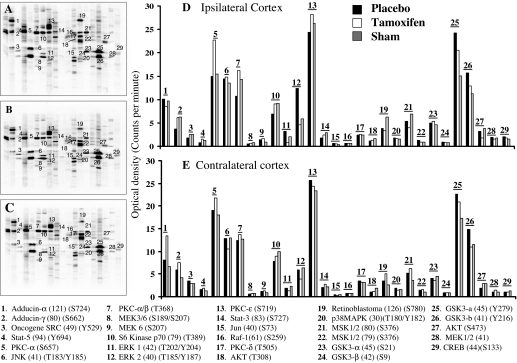
Regulation of protein kinases has been suggested to be a key determinant in the survival or death of cells after cerebral ischemia. To gain a better understanding of kinase activation after cerebral ischemia and the potential regulatory role of tamoxifen, we used the KPSS-1.3 to examine the phosphorylation state of 31 different protein kinases in tissue punches from sham and ipsilateral penumbra cortex 2 h after pMCAO in ovariectomized placebo-treated (cholesterol) and tamoxifen-treated adult female rats (Fig. 2). Punches were also collected and analyzed from the contralateral cortex.
Figure 2.
Effect of tamoxifen on protein kinase activation in the ischemic cortex after pMCAO. A–C, Kinetworks Western blot results of various phosphoprotein kinases from the ischemic cortex of sham-, placebo-, and tamoxifen-treated animals (n = 6), respectively, after 2 h pMCAO; D and E, statistical bar diagrams of the OD for various protein kinase activation after 2 h pMCAO from placebo-treated (black bars), tamoxifen-treated (empty bars), and sham-treated (gray bars) animals. Shown at the bottom are abbreviated names of various protein kinases as depicted by numbers in A–D, respectively. A change in OD of at least 25% was considered significant.
Figure 2, A–C, shows representative mini-Western blot KPSS-1.3 analysis in sham-treated (A), placebo-treated (B), and tamoxifen-treated (C) rats at 2 h after pMCAO. As shown in Fig. 2, B and D, phosphorylation of a number of protein kinases, including ERK1/2, MAP/ERK kinase 1 (MEK1), and c-Jun, significantly increased in the ischemic cortex penumbra of placebo-treated animals after pMCAO compared with sham control animals (Fig. 2, A and D). This phosphorylation-enhancing effect was significantly prevented in tamoxifen-treated animals (Fig. 2, C and D). In contrast, phosphorylation of other kinases, such as p70 S6 kinase, protein kinase C (PKC)-α/β, γ-adducin, retinoblastoma, signal transducer and activator of transcription (STAT)-3 and STAT-5, were significantly decreased (Fig. 2, B and D) at 2 h after cerebral ischemia compared with the sham control, an effect prevented by tamoxifen (except for the decrease in retinoblastoma phosphorylation) (Fig. 2, A and D). For the most part, phosphorylation of protein kinases in the contralateral cortex (Fig. 2E) showed only minor fluctuations between different treatment groups, suggesting that major changes in the phosphorylation state of the protein kinases was restricted to the injured ipsilateral cortex.
One of the most pronounced effects of tamoxifen in the injured ipsilateral cortex was the marked suppression of the phosphorylation of ERK1/2 (60–70% reduction) (Fig. 2D). Interestingly, tamoxifen also suppressed the pMCAO-induced activation of the downstream ERK effectors, c-Jun and cAMP response element-binding protein, with the effect on c-Jun the strongest (Fig. 2D). Because, ERK1/2 has been implicated in cell death after cerebral ischemia, we further verified the effects of tamoxifen on the activation of ERK1/2 and its expression in neurons in the ischemic penumbra after pMCAO using immunohistochemistry and Western blot analysis and explored its potential upstream activation by superoxide anion.
Effect of tamoxifen on ERK1/2 activation after pMCAO
Western blot analysis.
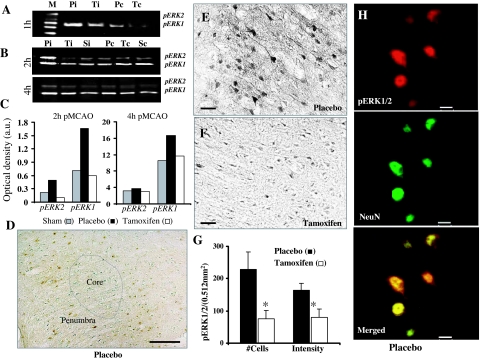
ERK activation was assessed using Western blot analysis performed on tissue punches collected from ischemic cortex penumbra at 1, 2, and 4 h after pMCAO in placebo- and tamoxifen-treated animals. Sham animal tissue samples were also collected as a control. For comparison, tissue punches from contralateral cortex were also simultaneously analyzed. Figure 3A shows that after 1 h, pMCAO pERK1/2 levels were significantly elevated in the ischemic penumbra (Pi) cortex compared with contralateral cortex (Pc) of placebo-treated animals. This elevation was significantly reduced in the ischemic cortex of tamoxifen-treated (Ti) animals. Furthermore, as shown in Fig. 3B, similar results were obtained after 2 h pMCAO where pERK1/2 levels were significantly elevated in the ischemic penumbra cortex of placebo-treated (Pi) animals compared with sham-operated (Si) animals and placebo contralateral controls. Tamoxifen treatment (Ti) significantly attenuated the elevation of pERK1/2 levels in the ipsilateral cortex at 2 h after pMCAO compared with the placebo group. No significant difference in the pERK1/2 levels was observed in the contralateral cortex of placebo (Pc), tamoxifen-treated (Tc), or sham-operated (Sc) animals after 2 h pMCAO. Also shown in Fig. 3B is that pERK1/2 levels are elevated as long as 4 h after pMCAO in the ischemic penumbra cortex of placebo (Pi) treated animals compared with sham operated (Si) animals, and that tamoxifen treatment (Ti) again significantly attenuated this elevation in pERK1/2 levels. Figure 3C shows statistical analysis of pERK elevation after correction with total ERK levels from the ischemic cortex of sham (gray bars), placebo (black bars), and tamoxifen-treated (white bars) animals after 2 and 4 h pMCAO. The corrected data confirm the strong elevation of pERK1/2 levels at 2 and 4 h after pMCAO compared with sham animals, and the marked attenuation by tamoxifen of the pMCAO-induced pERK1/2 elevation. In other studies, tamoxifen attenuation of the pMCAO elevation of pERK1/2 levels in the ipsilateral cortex was observed as early as 1 h after pMCAO, with the attenuating effect of tamoxifen on pERK1/2 lost by 24 h after pMCAO (data not shown).
Figure 3.
Effect of tamoxifen on ERK1/2 activation in the ischemic cortex after pMCAO. A and B, ERK1/2 activation in the ischemic penumbra of placebo-treated (Pi) animals was greatly increased after 1 h (A) and 2 and 4 h (B) pMCAO compared with tamoxifen-treated (Ti)-treated and sham-operated (Si) animals (n = 4–5 per group). Also shown in B, ERK1/2 activation in the contralateral cortex of the placebo-treated (Pc), tamoxifen-treated (Tc), and sham-operated (Sc) animals was not different among the groups. C, Bar diagram of pERK elevation after correction with total ERK levels from the ischemic cortex of sham-operated (gray bars), placebo-treated (black bars), and tamoxifen-treated (white bars) animals at 2 and 4 h after pMCAO. D, ERK1/2 activation (as measured by pERK1/2 DAB immunoreactivity) occurs primarily in the ischemic penumbra close to the ischemic core (dotted lines) at 2 h after pMCAO in the placebo-treated animal. E and F, High-power photographs of pERK1/2 immunoreactivity from the ischemic penumbra regions of placebo- and tamoxifen-treated animals, respectively, after 2 h pMCAO. G, Statistical analysis of pERK1/2-positive cells and pERK1/2 staining intensity per 10,7580 mm2 from the ischemic cortex of placebo- and tamoxifen-treated animals (n = 6) after 2 h pMCAO. *, Significance was determined by one-way ANOVA with post hoc analysis using Student-Newman-Keuls test; P < 0.05. H, Confocal images double-labeled for pERK1/2 (red) and NeuN (green), which demonstrates that ERK1/2 activation primarily occurs in the neurons (merged) after 2 h pMCAO but not in the astrocytes/glia (which was also verified using 1-h pMCAO animals as discussed in Fig. 4). Scale bars, 50 μm (D), 20 μm (E and F), and 10 μm (H).
Immunohistochemistry.
Immunohistochemistry was used to further confirm and extend the Western blot data. Figure 3, D–G, shows light microscopy results of DAB staining for pERK1/2 on brain sections from sham-, placebo-, and tamoxifen-treated animals at 2 h after pMCAO. As shown in Fig. 3D, ERK1/2 activation at 2 h after pMCAO mainly occurs in the penumbra around the ischemic core (dotted line) but not in the core itself, which is most often characterized as loose, fragile dead tissue. Additionally, only light pERK1/2 immunoreactivity was observed in the sham-operated animals or in the contralateral cortex of placebo-treated animals at 2 h after pMCAO (data not shown). Furthermore, as can be seen in Fig. 3, E and F, pERK1/2 immunoreactivity was strongly induced in the ischemic cortex penumbra of placebo-treated (Fig. 3E) animals, but tamoxifen-treated animals had a marked reduction in pERK1/2 immunoreactivity after pMCAO (Fig. 3F). The strong pERK1/2 immunoreactivity in placebo-treated animals at 2 h after pMCAO was localized in all the three major compartments of the cells, i.e. soma, nucleus, and dendritic fibers (Fig. 3E). Statistical analysis showed a significant suppressive effect of tamoxifen on pERK1/2 immunoreactivity both at the level of staining intensity and the number of pERK1/2-positive cells in the ischemic cortex at 2 h after pMCAO (P < 0.03 vs. placebo) (Fig. 3G).
We next examined in which cell type the induction of pERK1/2 occurs after pMCAO. As shown in Fig. 3H, confocal microscopy performed on double-immunofluorescent stained sections for pERK1/2 (red) and a neuronal marker, NeuN (green), revealed that pERK1/2 is primarily induced in neurons (merged) in the ischemic cortex at 2 h after pMCAO. The distribution of pERK1/2 staining was confined to both the soma and nucleus with some staining also detected in the apical dendrite, a pattern similar to that observed with DAB staining. Further description of pERK expression in neurons and astrocytes in the ischemic penumbra is provided below.
Effect of tamoxifen on superoxide anion production after pMCAO
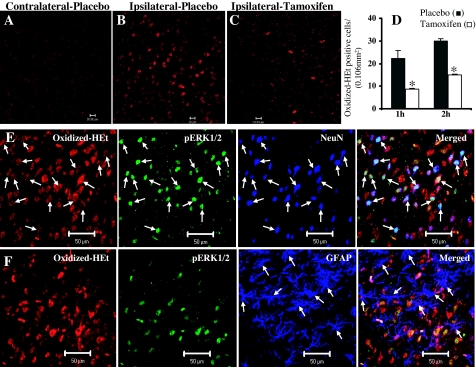
It has been previously demonstrated that production of superoxide anion (O2−) is an early event in cerebral ischemia and initiates various signaling pathways (including ERK and c-Jun) that can lead to neuronal death. We therefore measured O2− production in the ipsilateral cortex penumbra at 1 h after pMCAO and examined whether tamoxifen exerted any regulatory effects. O2− production was assessed using the in situ oxidized HEt method, in which HEt, a marker of O2− production, is selectively taken up by cells and oxidized by O2− into ethidium, which provides a red fluorescence signal at a wavelength of more than 560 nm in the visible spectrum. As shown in Fig. 4, no significant O2− production was observed in the contralateral cortex (Fig. 4A), whereas in the ipsilateral cortex, O2− production was dramatically increased in the placebo-treated animals after 1 h pMCAO (Fig. 4B). Interestingly, tamoxifen-treated animals showed significantly reduced O2− production in the ischemic cortex after pMCAO compared with the placebo-treated animals (compare Fig. 4, B and C). To verify these results, O2− production was also measured after 2 h pMCAO under identical conditions in placebo- and tamoxifen-treated animals. As shown in Fig. 4D, statistical results obtained from 2-h pMCAO experiments were comparable to those observed after 1 h pMCAO where tamoxifen-treated animals (white bars) showed a significant reduction in O2− production compared with placebo-treated animals (black bars), further verifying the elevation of O2− after pMCAO in the ipsilateral cortical penumbra and the attenuation of this elevation in tamoxifen-treated animals.
Figure 4.
Effect of tamoxifen on superoxide anion (O2−) production in the ischemic cortex after pMCAO. A–C, Confocal images showing oxidized-HEt-positive cells from contralateral cortex (A) and ischemic-penumbra cortex of placebo-treated (B) and tamoxifen-treated animals (C) at 2 h after pMCAO. Note that oxidized-HEt-positive cells are low in contralateral cortex of placebo-treated animals, high in ipsilateral cortex of placebo-treated animals, and attenuated in tamoxifen-treated ipsilateral cortex. D, Statistical analysis of HEt-positive cells (using Volocity 4.0 imaging software) at 1 and 2 h after pMCAO from ischemic penumbra of placebo-treated (black bars) and tamoxifen-treated (white bars) animals (n = 6 per group). *, Significance was determined by one-way ANOVA with post hoc analysis using Student-Newman-Keuls test; P < 0.05. E, Confical laser microscopy, performed on coronal sections triple labeled for oxidized HEt (red), pERK1/2 (green), and NeuN (blue) suggest that pERK1/2 is highly expressed in the neurons with high oxidized-Het signal (arrows) in the ischemic penumbra cortex after 1 h pMCAO. However, as shown in F, oxidized HEt (red) and pERK1/2 (green) are not significantly expressed (arrows) in the astrocytes (blue) after 1 h pMCAO. Scale bars, 20 μm (A–C) and 50 μm (E and F).
Because it has been suggested that ERK1/2 activation may be downstream of free radical production, we therefore examined O2− production and ERK1/2 activation in neurons and glia after cerebral ischemia using triple-immunofluorescence staining and confocal microscopy. As shown in Fig. 4E, 1 h pMCAO induced a massive production of O2− and activation of ERK1/2 in neurons of the ischemic penumbra cortex from placebo-treated animals. Furthermore, as also shown in Fig. 4E, O2− production occurred mainly in neurons (blue), and many cells that had high O2− production also had high pERK1/2 (green) immunoreactivity. Interestingly, as can be seen from Fig. 4F, no significant oxidized-HEt signal or pERK1/2 immunoreactivity was detected in the astrocytes (as shown by glial fibrillary acidic protein staining) after 1 h pMCAO. These results suggest ERK1/2 activation in response to O2− production after cerebral ischemia occurs primarily in the neurons and not in the astrocytes during the early period of ischemic injury in female rats.
Effect of tamoxifen on lipid peroxidation and oxidative DNA damage
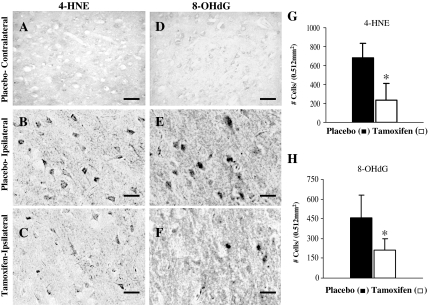
Free radical production after MCAO has been shown to induce oxidative DNA damage and lipid peroxidation in the cortex penumbra. We therefore sought to assess whether tamoxifen was capable of reducing this oxidative DNA damage and lipid peroxidation after pMCAO. Toward this end, we used immunohistochemical measurement of markers of oxidative DNA damage and lipid peroxidation (8-OHdG and 4-HNE, respectively) to assess pMCAO-induced oxidative damage in the cortex penumbra and examine the effect of tamoxifen. Figure 5, A and D, shows the immunostaining pattern of 4-HNE and 8-OHdG, respectively, in the contralateral cortex of placebo-treated animals after 24 h pMCAO. As can be seen from Fig. 5, A and D, no 4-HNE or 8-OHdG immunoreactivity, respectively, was detected in the contralateral cortex of placebo-treated animals after pMCAO. In contrast, strong 4-HNE and 8-OHdG immunoreactivity was induced in the ischemic penumbra cortex of placebo-treated (Fig. 5, B and E, for 4-HNE and 8-OHdG, respectively). Of significant interest, tamoxifen-treated animals showed a marked reduction in 4-HNE and 8-OHdG immunoreactivity in the ischemic penumbra cortex compared with placebo-treated animals at 24 h pMCAO (Fig. 5, compare C and F to B and E). Statistical analysis of 4-HNE- and 8-OHdG-positive cells in all animals from placebo-treated (n = 4) and tamoxifen-treated (n = 5) groups is shown in Fig. 5, G and H, respectively. A significant reduction (P < 0.05) in the number of 4-HNE- and 8-OHdG-positive cells was observed in the ischemic penumbra cortex of tamoxifen-treated animals (white bars) compared with placebo-treated animals (black bars). These results suggest that tamoxifen significantly attenuates oxidative DNA damage and lipid peroxidation in the ischemic penumbra after pMCAO.
Figure 5.
Effect of tamoxifen on markers of lipid peroxidation (4-HNE) and oxidative DNA damage (8-OHdG) in the ischemic cortex after pMCAO. A, DAB staining using 4-HNE antibodies on coronal sections from the contralateral cortex of placebo-treated animals shows no 4-HNE-specific staining. B and C, Intense 4-HNE staining associated with membrane was observed in the ischemic cortex of placebo-treated (B) animals, which was significantly reduced in the tamoxifen-treated (C) animals after 24 h pMCAO. D, DAB staining using 8-OHdG antibodies on the coronal sections from placebo-treated animals shows no 8-OHdG-specific staining. E and F, Intense 8-OHdG staining was observed in the ischemic cortex of placebo-treated animals (E), which was significantly reduced in the tamoxifen-treated animals (F) after 24 h pMCAO. G and H, Statistical analysis of 4-HNE- and 8-OHdG-positive cells, respectively, from placebo-treated (black bars, n = 4) and tamoxifen-treated (white bars, n = 5) animals after 24 h pMCAO. *, Significance was determined by one-way ANOVA with post hoc analysis using Student-Newman-Keuls test; P < 0.05. Scale bars, 20 μm.
Effect of tamoxifen on MnSOD and Cu/ZnSOD expression and caspase-3 activation after pMCAO
MnSOD and Cu/ZnSOD expression.
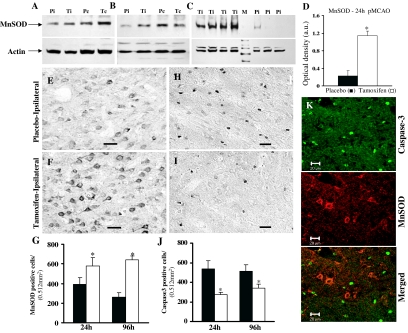
MnSOD is the primary antioxidant enzyme in the mitochondria that protects cells from oxidative injury by catalyzing dismutation of superoxide (O2−) to hydrogen peroxide and oxygen in the mitochondria of eukaryotic cells. We therefore measured protein levels for MnSOD in the ischemic penumbra cortex after 1, 4, and 24 h pMCAO in placebo- and tamoxifen-treated animals to determine whether tamoxifen exerted a regulatory effect on MnSOD levels after cerebral ischemia. As shown in Fig. 6, A and B, Western blot analysis of placebo-treated animals revealed a significant reduction in MnSOD protein expression in the ischemic penumbra cortex (Pi) compared with contralateral cortex (Pc) after 1 and 4 h pMCAO, respectively. Tamoxifen treatment (Ti) attenuated the pMCAO-induced reduction of MnSOD levels in the ischemic cortex at 1 and 4 h after pMCAO. Similar results were obtained for Cu/ZnSOD after pMCAO (data not shown). Figure 6C shows MnSOD protein levels 24 h after pMCAO in individual animals from placebo- and tamoxifen-treated groups. As shown in Fig. 6C, MnSOD protein levels were significantly enhanced in the ischemic penumbra cortex of tamoxifen-treated (Ti) animals compared with placebo-treated (Pi) animals. Figure 6D depicts a statistical bar diagram of MnSOD protein levels after correction with the housekeeping protein actin, which showed an approximately 395% increase (P < 0.001) in MnSOD protein levels in the ischemic cortex by tamoxifen treatment vs. placebo-treated animals at 24 h after pMCAO (P < 0.05). However, there was no significant difference in the levels of Cu/ZnSOD between placebo and tamoxifen-treated animals after 24 h pMCAO (data not shown).
Figure 6.
Effect of tamoxifen on MnSOD expression and caspase-3 activation in the ischemic cortex after pMCAO. A and B, Western blot analysis reveals that MnSOD protein levels are greatly reduced in the ischemic cortex (Pi) compared with contralateral cortex (Pc) of placebo-treated or sham-operated animals (not shown) after 1 h (A) and 4 h (B) pMCAO. This decrease in MnSOD expression was largely prevented in the ischemic cortex of tamoxifen-treated (Ti) animals (n = 4–5 per group). B, A similar reduction in the MnSOD protein levels was observed in the ischemic cortex of placebo-treated (Pi, represents three individual animals) but not in tamoxifen-treated (Ti, represents four individual animals) animals after 24 h pMCAO. D, Statistical bar diagram of MnSOD protein levels (as shown in panel C) after correction with the housekeeping protein β-actin after 24 h pMCAO. E and F, MnSOD-DAB staining performed on coronal sections confirmed a significant reduction in MnSOD-immunoreactive cells in the ischemic cortex of placebo-treated (E) compared with tamoxifen-treated (F) animals after 24 pMCAO (n = 4–5 per group). G, Statistical analysis of MnSOD-positive cells performed after 24 and 96 h pMCAO shows a significant (P < 0.05) reduction in placebo-treated (black bars) compared with tamoxifen-treated (white bars) animals. H and I, Caspase-3-DAB staining performed on coronal sections showed a significantly higher number of active caspase-3-immunoreactive cells in the ischemic penumbra of placebo-treated animals (H) compared with tamoxifen-treated animals (I) at 24 h pMCAO (n = 4–5 per group). J, Statistical analysis of caspase-3-positive cells performed after 24 and 96 h pMCAO shows a significant reduction in placebo (black bars) compared with tamoxifen-treated (white bars) animals. *, Significance was determined by one-way ANOVA with post hoc analysis using Student-Newman-Keuls test; P < 0.05. K, Confocal laser microscopy performed on coronal sections double-labeled for active caspase-3 (green) and MnSOD (red) indicate that these two proteins do not colocalize. Scale bars, 20 μm.
The tamoxifen enhancement of MnSOD levels after pMCAO was further verified using immunohistochemistry on coronal sections obtained from ischemic cortex of placebo- and tamoxifen-treated animals at 24 and 96 h after pMCAO. DAB staining results for MnSOD showed reduced MnSOD immunoreactivity in the ischemic penumbra cortex of placebo-treated (Fig. 6C) compared with tamoxifen-treated (Fig. 6D) animals after 24 and 96 h (not shown) pMCAO. Statistical analysis based on the DAB staining results showed a significant increase (P < 0.05) in both MnSOD-positive cells (Fig. 6F) and MnSOD immunoreactivity intensity (not shown) in tamoxifen-treated compared with placebo-treated animals after 24 and 96 h pMCAO.
Caspase-3 activation.
Continuous production of reactive oxygen species (ROS) leads to apoptosis, a form of programmed cell death that occurs during cerebral ischemia. A family of proteases known as caspases has been implicated to play a major initiator and executor role in apoptotic cell death after cerebral ischemia. Caspase-8 and caspase-9 have been shown to activate caspase-3, which in turn cleaves cytoskeletal proteins, kinases, and DNA repair enzymes, leading to apoptotic cell death. We therefore sought to determine whether tamoxifen regulates caspase-3 activation in the ischemic penumbra after pMCAO as a potential mechanism for reducing apoptotic cell death. Active caspase-3 immunoreactivity in the ischemic penumbra was thus measured at 24 h after pMCAO in placebo- and tamoxifen-treated animals using immunohistochemistry. As shown in Fig. 6, H–J, strong active caspase-3 immunoreactivity was present in the ischemic penumbra of placebo-treated animals (Fig. 6H), whereas tamoxifen-treated animals (Fig. 6I) had a marked reduction in caspase-3 immunoreactivity. We did not detect any significant caspase-3 immunoreactivity at 2 h pMCAO (data not shown). Figure 6J shows statistical analysis of results from all animals for caspase-3-positive cells at 24 and 96 h pMCAO in placebo (n = 4–5) and tamoxifen-treated (n = 5) animals. As shown in Fig. 6J, tamoxifen-treated animals (white bars) had a significantly (P < 0.05) reduced number of caspase-3-positive cells in the ischemic penumbra cortex compared with the placebo-treated (black bars) animals at 24 and 96 h after pMCAO. Colocalization studies using double-immunofluorescence labeling and confocal microscopy revealed that both caspase-3 (green) and MnSOD (red) are expressed in the ischemic penumbra cortex of placebo-treated animals after pMCAO; however, these two proteins do not colocalize with each other (Fig. 6J), which suggests that MnSOD-positive cells are healthy neurons. This observation further supports a potential protective role of tamoxifen by enhancing MnSOD levels in cerebral ischemia.
Effect of the SOD-mimetic tempol on superoxide production and ERK1/2 activation after pMCAO
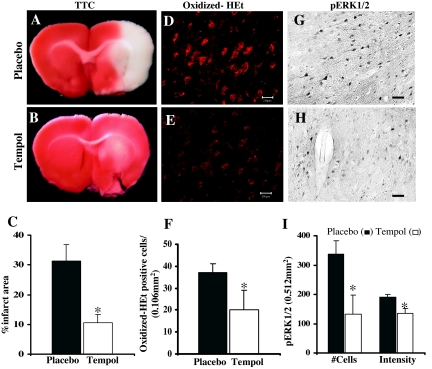
To test our hypothesis that ROS such as superoxide anion may be an upstream activator of kinases such as ERK1/2, we examined the effect of an SOD mimetic compound, tempol, on infarct volume, superoxide anion production, and ERK1/2 activation after pMCAO. The results depicted in Fig. 7 show that tempol administration 20 min before MCAO significantly prevented the development of infarct volume/ischemic damage compared with placebo-treated animals when determined at 24 h after pMCAO (Fig. 7, A–C). Tempol-treated animals also showed a marked reduction (P < 0.05) in the production of O2− compared with placebo-treated animals when examined at 1 h after pMCAO (Fig. 7, D–F). Furthermore, ERK/1/2 activation was also significantly reduced (P < 0.001) in tempol-treated animals (Fig. 7, H and I) compared with placebo-treated animals (Fig. 7, G and I) when examined at 2 h after pMCAO. Statistical analysis further revealed that the reduction in ERK1/2 activation occurs both at the level of total immunoreactivity and number of immunopositive cells (Fig. 7I).
Figure 7.
Effect of tempol on infarct volume, O2− production, and ERK1/2 activation after pMCAO. Compared with placebo-treated animals (A and C), tempol-treated animals (B and C) show a significantly reduced infarct area at 24 h pMCAO. D–F, O2− production, as measured by oxidized-HET signal, was significantly reduced (P < 0.05) in ischemic penumbra cortex of tempol-treated animals (E and F) compared with placebo-treated animals (D and F) after 2 h pMCAO (n = 6 in each group). Tempol treatment (H and I) also markedly reduced ERK1/2 activation in the penumbra compared with placebo-treated animals (G and I) at 2 h after pMCAO. *, Significance was determined by one-way ANOVA with post hoc analysis using Student-Newman-Keuls test; P < 0.05. Scale bars, 20 μm.
Discussion
Recent work has implicated ROS, particularly superoxide anion, as playing a key role in neuronal cell death after cerebral ischemia (26,28,29,30,31,32). The superoxide anion radical (O2−) is the product of a one electron reduction of oxygen and is the precursor of most ROS and a mediator in oxidative chain reactions [33, for review]. O2− combines spontaneously or is dismutated with H+ to produce H2O2, which readily breaks down in the presence of metals Fe2+ and Cu+ or O2 to form hydroxyl radicals. Hydroxyl radicals are one of the strongest oxidants in nature and are highly injurious to adjacent structures, including lipid membranes, DNA, and proteins (30). In both permanent and transient cerebral ischemia, ROS have been shown to increase significantly after onset of cerebral ischemia (31,32,33,34). Along these lines, online in vivo ROS chemiluminescence measurement shows a marked steady elevation of ROS in the penumbra (infarct border) of the parietal cortex during a 3-h measurement period after ischemia in permanent cerebral ischemia (31). Our study, using a marker of O2− production, oxidized HEt, yielded a similar pattern of rapid increase in O2− production in the ipsilateral (injured) cortex of the female rat 1–2 h after pMCAO. A similar finding of rapid superoxide production after cerebral ischemia (as determined by in situ oxidized HEt measurement) has also been reported in male mice (33,34). The contralateral (noninjured) cortex showed little oxidized HEt signal in our study, demonstrating that elevated O2− production was primarily restricted to the injured ipsilateral cortex. Furthermore, we observed that the majority of the O2− detected was in neurons at the early time points (1–2 h) after cerebral ischemia examined in our study.
Of significant interest, tamoxifen treatment significantly attenuated superoxide anion production, as measured by oxidized HEt, in the ipsilateral cortex at 1 and 2 h after pMCAO. ROS are known to damage cell membranes by inducing lipid peroxidation, which results in the formation of 4-hydroxynonenal (4-HNE), a product toxic to neurons and oligodendrocytes (35,36). Furthermore, guanine is the most oxidized base in DNA and formation of 8-hydroxyl-2′-deoxyguanosine (8-OHdG) is a well-characterized marker of oxidative DNA damage (37,38). Using these markers of oxidative damage, we found that the number of cells immunopositive for 4-HNE and 8-OHdG in the injured cortex was markedly elevated compared with the contralateral cortex at 24 h post pMCAO. Furthermore, tamoxifen-treated animals had a significant attenuation in the number of cells immunopositive for 4-HNE and 8-OHdG in the ipsilateral cortex. These findings provide evidence that tamoxifen neuroprotection after cerebral ischemia involves an antioxidant mechanism that reduces superoxide production and oxidative damage in the ischemic cortex penumbra region. Our work suggesting an antioxidant neuroprotective effect of tamoxifen in cerebral ischemia is in agreement with a recent report in male animals in which post-stroke treatment with a high dose of tamoxifen (5–10 mg/kg) reduced production of isoprostanes in the ischemic cortex, which are markers of lipid peroxidation and free radical damage (39). Our study provides a mechanistic underpinning for the antioxidant action of tamoxifen by demonstrating that it reduces superoxide anion production in the ischemic cortex, reduces lipid peroxidation and DNA damage due to free radicals after cerebral ischemia, and attenuates proapoptotic caspase-3 activation. Activation of caspase-3 has been shown to lead to apoptotic cell death, and caspase-3 activation is markedly increased after cerebral ischemia (40,41). The ability of tamoxifen to significantly attenuate caspase-3 activation in the ischemic cortex likely plays a key role in the ability of tamoxifen to reduce neuronal cell death after cerebral ischemia and may be secondary to its antioxidant actions to reduce superoxide anion production and oxidant damage. Indeed, previous work has shown that there is strong linkage between oxidative stress and caspase-3 activation in the cortex after cerebral ischemia and that oxidative stress in neurons can induce caspase-3 activation (29,42).
With respect to possible mechanisms of the antioxidant action of tamoxifen, previous work has shown that tamoxifen can decrease membrane permeability and inhibit lipid peroxidation in liposomes, which suggests a direct antioxidant action of tamoxifen (17,43,44). In further support of a direct antioxidant action, tamoxifen has been shown to have direct superoxide scavenging ability in vitro (15). Thus, inherent ROS scavenging activity of tamoxifen could explain the tamoxifen-induced attenuation of superoxide anions after cerebral ischemia that we observed in our study. Tamoxifen could also act to enhance antioxidant mitochondrial functions in cells to exert its antioxidant effects. Along these lines, tamoxifen has been reported to be a potent inhibitor of the mitochondrial permeability transition in rat liver mitochondria (18,19) and has been shown to improve mitochondrial respiratory function in cells and enhance superoxide scavenging activity of mitochondria (20). Our study provides evidence that tamoxifen can enhance the endogenous antioxidant defense mechanisms in cells in the ischemic cortex, because we observed that tamoxifen markedly increased mitochondrial MnSOD immunoreactivity levels in the ipsilateral cortex penumbra at 24 and 96 h after cerebral ischemia. MnSOD is a mitochondrial superoxide dismutase that functions to scavenge superoxide anions. Mutant mice with MnSOD deficiency have a prominent increase in superoxide anion production in normal physiological situations and especially after cerebral ischemia (28). Furthermore, MnSOD-deficient mutant mice have been shown to have increased infarct size, more severe neurological deficits, enhanced cytochromes c translocation, caspase activation, and DNA fragmentation after cerebral ischemia (26). The ability of tamoxifen to enhance MnSOD in the cerebral cortex after cerebral ischemia thus may be an important effect underlying its antioxidant and neuroprotective actions after cerebral ischemia.
Using a phosphokinase array, our study further demonstrated the activation state of multiple kinases changes significantly at 2 h after permanent cerebral ischemia in the ovariectomized female rat. Kinases that showed significantly decreased phosphorylation in the ipsilateral cortex penumbra at 2 h after permanent cerebral ischemia included p70 S6 kinase, PKC-α/β, γ-adducin, retinoblastoma, STAT-3, and STAT-5. Interestingly, tamoxifen prevented the cerebral ischemia-induced down-regulation of all these phosphokinases except for retinoblastoma. Of these kinases, p70 S6 kinase has a clear role implicated in cerebral ischemia. It is known to be an important regulator of protein synthesis, and its down-regulation after cerebral ischemia has been suggested to underlie, in part, the suppression of protein synthesis that occurs after cerebral ischemia (45,46). Tamoxifen prevention of the down-regulation of phospho-p70 S6 kinase levels could suggest that the fall in protein synthesis that occurs after cerebral ischemia may be prevented by tamoxifen. However, before any definitive conclusions can be drawn, more work is needed to confirm and clarify the temporal pattern of tamoxifen regulation of p70 S6 kinase and measure protein synthesis rates after cerebral ischemia in tamoxifen-treated animals. More work is also needed to confirm the tamoxifen effect on the other kinases (PKC-α/β, γ-adducin, retinoblastoma, STAT-3, and STAT-5) in the phosphokinase array and to determine the significance of the regulatory effects.
Kinases that showed significantly enhanced phosphorylation in the ipsilateral cortex penumbra after permanent cerebral ischemia (compared with sham animals) included ERK-1, ERK-2, c-Jun, glycogen synthase kinase-3α and -3βY216, and p38-MAPK. The elevation of these kinases in the ipsilateral cortex after transient and permanent cerebral ischemia has been reported by a number of investigators in previous studies using male rats and mice (47,48,49,50,51,52,53,54). For instance, ERKs have been previously reported to be activated in the ipsilateral penumbra of male rats and mice within minutes of permanent or transient cerebral ischemia induction, with peak elevation reported to be from 1–4 h and lower but still elevated pERK levels persisting to 12–72 h after ischemia (47,48,49,50,51,52,53). A similar elevation of activated ERKs has been shown in the penumbra of postmortem human brains after acute ischemic stroke (55). It has been proposed that the activation of ERKs plays an important role in the neuronal cell death that follows cerebral ischemia. In support of this contention, administration of specific inhibitors for ERK activation (MEK inhibitors) has been shown to be neuroprotective in cerebral ischemia (51,52,53,56,57,58). Use of Western blot analysis and immunohistochemical analysis in our study confirmed pERK1/2 elevation in the ipsilateral cortex at 1, 2, and 4 h after pMCAO. Of considerable interest, tamoxifen treatment induced a significant attenuation of pERK1/2 levels in the cerebral cortex penumbra at these time points. Because ERK activation has been implicated to be critical for neuronal cells after cerebral ischemia, tamoxifen-induced reduction of pERK levels in the cortical penumbra region could play an important role in the neuroprotective actions of tamoxifen.
Additional work using colocalization techniques revealed that pERK and oxidized-HEt signal were present in the same cells, suggesting that cells that had high superoxide anion induction also had high pERK activation. The coinduction of superoxide anion production and pERK in neurons could suggest that a causative relationship exists between the two factors (e.g. that superoxide anions may act to induce pERK activation). To further explore this possibility, we examined the effect of the SOD-mimetic compound tempol on various indexes after cerebral ischemia. Tempol significantly reduced infarct size at 24 h after pMCAO, a finding in agreement with previous reports in the literature (34,59). Of significant interest, tempol reduced superoxide anion production and pERK activation in the cortex penumbra after cerebral ischemia. This finding suggests that superoxide anions may lie upstream and be responsible for induction of pERK activation. In further support of this possibility, Chan and co-workers (53) found that transgenic mice with SOD overexpression have reduced infarct size, reduced superoxide production, and significant attenuation of pERK activation after cerebral ischemia. To activate ERK1/2, superoxide anions may act on Ras or Raf-1, which are upstream of the MEK/ERK pathway. In support of this possibility, oxidative stress-induced ERK activation has been shown to be attenuated by use of dominant-negative Raf or Ras constructs in cells (60). Thus, tamoxifen reduction of superoxide anion production may underlie the reduced pro-death ERK signaling observed after cerebral ischemia in tamoxifen-treated animals.
Finally, it is unclear whether the tamoxifen effects observed in our study involve activation of the estrogen receptor. In previous work, we demonstrated that proestrous levels of estrogen do attenuate superoxide anion production after cerebral ischemia, which could suggest estrogen receptor mediation (6). However, in a recent study in male animals, it was found that the estrogen receptor antagonist ICI782,780 administered via either iv or intracerebral ventricular routes, did not block the effect of high-dose tamoxifen on infarct size, which suggests that tamoxifen may act in an estrogen receptor-independent manner (39). More work is needed on this interesting question.
In conclusion, the current study provides evidence that tamoxifen protects the brain against cerebral ischemia via an antioxidant mechanism that involves enhancement of MnSOD expression, with correlated reduction of superoxide anion production, oxidative damage, and caspase-3 activation in the injured cortex. It is further proposed that suppression of oxidative stress by tamoxifen leads to attenuation, at least in part, of pro-death ERK signaling in the cortex penumbra, thereby facilitating neuronal survival.
Footnotes
This research was supported by a research grant from the National Institute of Neurological Disorder and Stroke, National Institutes of Health (NS050730).
Disclosure Statement: The authors have nothing to disclose.
First Published Online September 27, 2007
Abbreviations: DAB, Diaminobenzidine; HEt, hydroethidine; 4-HNE, 4-hydroxy-2-nonenal; KPSS-1.3, Kinetworks Phospho-Site Screen; MEK1, MAPK kinase 1; 8-OHdG, 8-hydroxy-2′-deoxyguanosine; pERK1/2, phospho-ERK1/2; PKC, protein kinase C; pMCAO, permanent middle cerebral artery occlusion; ROS, reactive oxygen species; SOD, superoxide dismutase; STAT, signal transducer and activator of transcription; TTC, 2,3,5-triphenyltetrazolium chloride.
References
- Simpkins JW, Rajakumar G, Zhang YQ, Simpkins CE, Greenwald D, Yu CJ, Bodor N, Day AL 1997 Estrogens may reduce mortality and ischemic damage caused by middle cerebral artery occlusion in the female rat. J Neurosurg 87:724–730 [DOI] [PubMed] [Google Scholar]
- Dubal DB, Kashon ML, Pettigrew LC, Ren JM, Finklestein SP, Rau SW, Wise PM 1998 Estrogen protects against ischemic injury. J Cereb Blood Flow Metab 18:1253–1258 [DOI] [PubMed] [Google Scholar]
- Zhang YQ, Shi J, Rajakumar G, Day AL, Simpkins JW 1998 Effects of gender and estrogen treatment on focal brain ischemia. Brain Res 784:321–324 [DOI] [PubMed] [Google Scholar]
- Rusa R, Alkayed NJ, Crain BJ, Traystman RJ, Kimes AS, London ED, Klaus JA, Hurn PD 1999 17β-Estrogen reduces stroke injury in estrogen-deficient animals. Stroke 30:1665–1670 [DOI] [PubMed] [Google Scholar]
- Shi J, Panickar KS, Yang SH, Rabbani O, Day AL, Simpkins JW 1998 Estrogen attenuates over-expression of β-amyloid precursor protein messenger RNA in an animal model of focal ischemia. Brain Res 810:87–92 [DOI] [PubMed] [Google Scholar]
- Brann DW, Dhandapani K, Wakade C, Mahesh VB, Khan MM 2007 Neurotrophic and neuroprotective effects of estrogen: Basic mechanisms and clinical implications. Steroids 72:381–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimelberg H, Feurstel P, Jin Y, Paquette J, Boulos A, Keller RW, Trammer BI 2000 Acute treatment with tamoxifen reduces ischemic damage following middle cerebral artery occlusion. Neuroreport 11:2675–2679 [DOI] [PubMed] [Google Scholar]
- Osuka K, Feustel PJ, Mongin AA, Tranmer BI, Kimelberg HL 2001 Tamoxifen inhibits nitrotyrosine formation after reversible middle cerebral artery occlusion in the rat. J Neurochem 76:1842–1850 [DOI] [PubMed] [Google Scholar]
- Mehta SH, Dhandapani KM, De Sevilla LM, Webb RC, Mahesh VB, Brann DW 2003 Tamoxifen, a selective estrogen receptor modulator, reduces ischemic damage caused by middle cerebral artery occlusion in the ovariectomized female rat. Neuroendocrinology 77:44–50 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Jin Y, Behr MJ, Feustel PJ, Morrison JP, Kimelberg HK 2005 Behavioral and histological neuroprotection by tamoxifen after reversible focal cerebral ischemia. Exp Neurol 196:41–46 [DOI] [PubMed] [Google Scholar]
- Biegon A, Brewster M, Degani H, Pop E, Somjen D, Kaye AM 1996 A permanently charged tamoxifen derivative displays anticancer activity and improved tissue selectivity in rodents. Cancer Res 56:4328–4331 [PubMed] [Google Scholar]
- Dluzen DE, Mcdermott JL, Andersen LI 2001 Tamoxifen diminishes methamphetamine-induced striatal dopamine depletion in intact female and male mice. J Neuroendocrinol 13:618–624 [DOI] [PubMed] [Google Scholar]
- D’Astous M, Mickley KR, Dluzen DE, Di Paolo T 2005 Differential protective properties of estrogen and tamoxifen against methamphetamine-induced nigrostriatal dopaminergic toxicity in mice. Neuroendocrinology 82:111–120 [DOI] [PubMed] [Google Scholar]
- Dluzen E, Mickley KR 2005 Gender differences in modulatory effects of tamoxifen upon the nigrostriatal dopaminergic system. Pharmacol Biochem Behav 80:27–33 [DOI] [PubMed] [Google Scholar]
- Kuo YM, Chen HH, Shieh CC, Chuang Cherng CG, Yu L 2003 4-Hydroxytamoxifen attenuates methamphetamine-induced nigrostriatal dopaminergic toxicity in intact and gonadectomized mice. J Neurochem 87:436–443 [DOI] [PubMed] [Google Scholar]
- Obata T 2006 Tamoxifen protect against hydroxyl radical generation induced by phenelzine in rat striatum. Toxicology 222:46–52 [DOI] [PubMed] [Google Scholar]
- Wiseman H, Quinn P, Halliwell B 1993 Tamoxifen and related compounds decrease membrane fluidity in liposomes. Mechanism for the antioxidant action of tamoxifen and relevance to its anticancer and cardioprotective actions? FEBS Lett 330:53–56 [DOI] [PubMed] [Google Scholar]
- Cardoso CM, Almeida LM, Custodio JB 2002 4-Hydroxytamoxifen is a potent inhibitor of the mitochondrial permeability transition. Mitochondrion 1:485–495 [DOI] [PubMed] [Google Scholar]
- Cardoso CM, Almeida LM, Custodio JB 2004 Protection of tamoxifen against oxidation of mitochondrial thiols and NAD(P)H underlying the permeability transition induced by prooxidants. Chem Biol Interact 148:149–161 [DOI] [PubMed] [Google Scholar]
- Zhao Y, Wang LM, Chaiswing L, Yen HC, Oberley TD, Lien YC, Lin SM, Mattson MP, St Clair D 2006 Tamoxifen protects against acute tumor necrosis factor α-induced cardiac injury via improving mitochondrial functions. Free Radic Biol Med 40:1234–1241 [DOI] [PubMed] [Google Scholar]
- Sakariassen PØ, Prestegarden L, Wang J, Skaftnesmo KO, Mahesparan R, Molthoff C, Sminia P, Sundlisaeter E, Misra A, Tysnes BB, Chekenya M, Peters H, Lende G, Kalland KH, Øyan AM, Petersen K, Jonassen I, van der Kogel A, Feuerstein BG, Terzis AJ, Bjerkvig R, Enger PØ 2006 Angiogeneis-independent tumor growth mediated by stem-like cancer cells. Proc Natl Acad Sci USA 103:16466–16471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolov BP, Cadet JL 2006 Methamphetamine causes alterations in the MAP-kinase-related pathways in brains of mice that display increased aggressiveness. Neuropsychopharmacology 31:956–966 [DOI] [PubMed] [Google Scholar]
- Avissar NE, Toia L, Sax HC 2005 Epidermal growth factor and/or growth hormone induce differential, side-specific signal transduction protein phosphorylation in enterocytes. J Parenter Enteral Nutr 29:322–336 [DOI] [PubMed] [Google Scholar]
- Wang X, Li H, De Leo D, Guo W, Koshkin V, Fantus IG, Giacca A, Chan CB, Der S, Wheeler MB 2004 Gene and protein kinase expression profiling of reactive oxygen species-associated lipotoxicity in the pancreatic β-cell line MIN6. Diabetes 53:129–140 [DOI] [PubMed] [Google Scholar]
- Dhandapani KM, Wade FM, Mahesh VB, Brann DW 2005 Astrocyte-derived transforming growth factor-β mediates the neuroprotective effects of 17β-estrogen: involvement of nonclassical genomic signaling pathways. Endocrinology 146:2749–2759 [DOI] [PubMed] [Google Scholar]
- Fujimura M., Morita-Fujimura Y, Kawase M, Copin J, Calagui B, Epstein CJ, Chan PH 1999 Manganese superoxide dismutase mediates the early release of mitochondrial cytochrome c and subsequent DNA fragmentation after persistent focal cerebral ischemia in mice. J Neurosci 19:3414–3422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bindokas VP, Jordan J, Lee CC, Miller RJ 1996 Superoxide production in rat hippocampal neurons: selective imaging with hydroethidine. J Neurosci 16:1324–1336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami K, Kondo T, Kawase M, Li Y, Sato S, Chen SF, Chan PH 1998 Mitochondrial susceptibility to oxidative stress exacerbates cerebral infarction that follows permanent focal cerebral ischemia in mutant mice with manganese superoxide dismutase deficiency. J Neurosci 18:205–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawara T, Noshita N, Lewen A, Gasche Y, Ferrand-Drake M, Fujimura M, Morita-Fujimura Y, Chan PH 2002 Overexpression of copper/zinc superoxide dismutase in transgenic rats protects vulnerable neurons against ischemic damage by blocking the mitochondrial pathway of caspase activation. J Neurosci 22:209–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan PH 1996 Role of oxidants in ischemic brain damage. Stroke 27:1124–1129 [DOI] [PubMed] [Google Scholar]
- Peters O, Back T, Lindauer U, Busch C, Megow D, Dreier J, Dirnagl U 1998 Increased formation of reactive oxygen species after permanent and reversible middle cerebral artery occlusion in the rat. J Cereb Blood Flow Metab 18:196–205 [DOI] [PubMed] [Google Scholar]
- Nagayama T, Lan J, Henshall DC, Chen D, O’Horo C, Simon RP, Chen J 2000 Induction of oxidative DNA damage in the peri-infarct region after permanent focal cerebral ischemia. J Neurochem 75:1716–1728 [DOI] [PubMed] [Google Scholar]
- Matttson MP, Culmsee C, Yu ZF 2000 Apoptotic and antiapoptotic mechanisms in stroke. Cell Tissue Res 301:173–187 [DOI] [PubMed] [Google Scholar]
- Mehta SH, Webb RC, Ergul A, Tawfik A, Dorrance AM 2004 Neuroprotection by tempol in a model of iron-induced oxidative stress in acute ischemic stroke. Am J Physiol Regul Integr Comp Physiol 286:R283–R288 [DOI] [PubMed] [Google Scholar]
- Zhang N, Komine-Kobayashi M, Tanaka R, Liu M, Mizuno Y, Urabe T 2005 Edaravone reduces early accumulation of oxidative products and sequential inflammatory responses after transient focal ischemia in mice brain. Stroke 36:2220–2225 [DOI] [PubMed] [Google Scholar]
- Lee EJ, Chen HY, Lee MY, Chen TY, Hsu YS, Hu YL, Chang GL, Wu TS 2005 Cinnamophilin reduces oxidative damage and protects against transient focal cerebral ischemia in mice. Free Radic Biol Med 39:495–510 [DOI] [PubMed] [Google Scholar]
- Won MH, Kang TC, Jeon GS, Lee JC, Kim DY, Choi EM, Lee KH, Choi CD, Chung MH, Cho SS 1999 Immunohistochemical detection of oxidative DNA damage induced by ischemia-reperfusion insults in gerbil hippocampus in vivo. Brain Res 836:70–78 [DOI] [PubMed] [Google Scholar]
- Park EM, Choi JH, Park JS, Han MY, Park YM 2000 Measurement of glutathione oxidation and 8-hydroxy-2′-deoxyguanosine accumulation in the gerbil hippocampus following global ischemia. Brain Res Brain Res Protoc 6:25–32 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Milatovic D, Aschner M, Feustel PJ, Kimelberg HK 2007 Neuroprotection by tamoxifen in focal cerebral ischemia is not mediated by an agonist action at estrogen receptors but is associated with antioxidant activity. Exp Neurol 204:819–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guegan C, Sola B 2000 Early and sequential recruitment of apoptotic effectors after permanent ischemia in mice. Brain Res 856:93–100 [DOI] [PubMed] [Google Scholar]
- Ferrer I, Planas AM 2003 Signaling of cell death and cell survival following focal cerebral ischemia: life and death struggle in the penumbra. J Neuropathol Exp Neurol 62:329–339 [DOI] [PubMed] [Google Scholar]
- Kim GW, Sugawara T, Chan PH 2000 Involvement of oxidative stress and caspase-3 in cortical infarction after photothrombotic ischemia in mice. J Cereb Blood Flow Metab 20:1690–1701 [DOI] [PubMed] [Google Scholar]
- Wiseman H, Halliwell B 1994 Tamoxifen and related compounds protect against lipid peroxidation in isolated nuclei: relevance to the potential anticarcinogenic benefits of breast cancer prevention and therapy with tamoxifen? Free Radic Biol Med 17:485–488 [DOI] [PubMed] [Google Scholar]
- Wiseman H, Cannon M, Armstein HR, Halliwell B 1990 Mechanism of inhibition of lipid peroxidation by tamoxifen and 4-hydroxytamoxifen introduced into liposomes. Similarity to cholesterol and ergosterol. FEBS Lett 274:107–110 [DOI] [PubMed] [Google Scholar]
- Janelidze S, Hu BR, Siesjo P, Siesjo BK 2001 Alterations of Akt1 (PKBα) and p70(S6K) in transient focal ischemia. Neurobiol Dis 8:147–154 [DOI] [PubMed] [Google Scholar]
- Mengesdorf T, Proud CG, Mies G, Paschen W 2002 Mechanisms underlying suppression of protein synthesis induced by transient focal cerebral ischemia in mouse brain. Exp Neurol 177:538–546 [DOI] [PubMed] [Google Scholar]
- Ferrer I, Friguls B, Dalfo E, Planas A 2003 Early modifications in the expression of MAPK/ERK, SAPK/JNK and p38, and their phosphorylated substrates following cerebral ischemia. Acta Neuropathol 105:425–437 [DOI] [PubMed] [Google Scholar]
- Wu D, Ye W, Che X, Yang G 2000 Activation of MAPKs after permanent cerebral artery occlusion in mouse brain. J Cereb Blood Flow Metab 20:1320–1330 [DOI] [PubMed] [Google Scholar]
- Krupinski J, Slevin M, Marti E, Catena E, Rubio F, Gaffney J 2003 Time-course phosphorylation of the MAPK group of signaling proteins and related molecules following MCAO in rats. Neuropathol Appl Neurobiol 29:144–158 [DOI] [PubMed] [Google Scholar]
- Wang X, Hagberg H, Sandberg M, Blongon K 2003 Activation of ERK1/2 after neonatal rat cerebral hypoxia-ischemia. J Neurochem 86:351–362 [DOI] [PubMed] [Google Scholar]
- Wang Z, Wu D, Huang F, Yang G 2004 Inhibition of MEK/ERK1/2 pathway reduces pro-inflammatory cytokine interleukin expression in focal cerebral ischemia. Brain Res 996:55–66 [DOI] [PubMed] [Google Scholar]
- Wang Z, Chen X, Zhou L, Wu D, Che X, Yang G 2003 Effects of extracellular signal-regulated kinase (ERK) on focal cerebral ischemia. Chin Med J 116:1497–1503 [PubMed] [Google Scholar]
- Noshita N, Sugawara T, Hayashi T, Lewen A, Omar G, Chan PH 2002 Copper/zinc superoxide dismutase attenuates neuronal cell death by preventing extracellular signal-regulated kinase activation after transient focal cerebral ischemia in mice. J Neurosci 22:7923–7930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi Y., Nozaki K, Sugino T, Hattori I, Hashimoto N 2000 Phosphorylation of c-Jun NH2-terminal kinase and p38 mitogen-activated protein kinase after transient forebrain ischemia in mice. Neurosci Lett 294:117–120 [DOI] [PubMed] [Google Scholar]
- Slevin M, Krupinski J, Slowik A, Rubio F, Szczudlik A, Gaffney J 2000 Activation of MAP kinase (ERK-1/ERK-2), tyrosine kinase and VEGF in the human brain following acute ischaemic stroke. Neuroreport 11:2759–2764 [DOI] [PubMed] [Google Scholar]
- Alessandro A, Namuro S, Moskowitz M, Bonventre J 1999 MEK1 protein kinase inhibition protects against damage resulting from focal cerebral ischemia. Proc Natl Acad Sci USA 96:12866–12869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namura S, Iihara K, Takami S, Nagata I, Kikuchi H, Matsushita K, Moskowitz MA, Bonventre JV, Alessandrini A 2001 Intravenous administration of MEK inhibitor U0126 affords brain protection against forebrain ischemia and focal cerebral ischemia. Proc Natl Acad Sci USA 98:11569–11574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Wang H, Xu L, Rozanski DJ, Sugawara T, Chan PH, Trzaskos JM, Feuerstein GZ 2003 Significant neuroprotection against ischemic brain injury by inhibition of the MEK1 protein kinase in mice: exploration of potential mechanisms associated with apoptosis. J Pharm Exp Toxicol 304:172–178 [DOI] [PubMed] [Google Scholar]
- Rak R, Chao DL, Pluta RM, Mitchell JB, Oldfield EH, Watson JC 2000 Neuroprotection by the stable nitroxide tempol during reperfusion in a rat model of transient focal ischemia. J Neurosurg 92:646–651 [DOI] [PubMed] [Google Scholar]
- Aikawa R, Komuro I, Yamazaki T, Zou Y, Kudoh S, Tanaka M, Shiojima I, Hiroi Y, Yazaki Y 1997 Oxidative stress activates extracellular signal-regulated kinases through Src and Ras in cultured cardiac myocytes of neonatal rats. J Clin Invest 100:1813–1821 [DOI] [PMC free article] [PubMed] [Google Scholar]