Abstract
Estrogens play a central role in regulating female reproduction throughout the reproductive axis, and the pituitary is one of the major targets of estrogen action. We hypothesized that estrogen receptor α (ERα) mediates estrogen action in the pituitary gonadotroph. To test this hypothesis, we generated a mouse line with a selective ERα deletion in the gonadotropin α-subunit (αGSU)-expressing pituitary cells (pituitary-specific ERα knockout; ERαflox/flox αGSUcre). Although the ERαflox/flox αGSUcre female mice maintain a basal level of serum LH and FSH and their ovulatory capacity is comparable to that in controls, they do not display regular estrous cycles and are infertile, indicating a potential disorder in regulating LH and/or FSH secretion. The ERαflox/flox αGSUcre female mice express equivalent levels of LHβ and αGSU mRNA compared with wild-type mice as determined by microarray analysis. Taken together, these findings indicate that pituitary gonadotroph ERα carries out the effects of estrogens with regard to estrous cyclicity and ultimately fertility.
ESTROGEN FEEDBACK to the hypothalamus and pituitary is the major factor regulating LH secretion, including the LH surge (1,2,3). Estrogens regulate LH secretion by controlling both the basal secretion of GnRH from the hypothalamus and the GnRH surge that stimulates tonic LH secretion and the LH surge, respectively (3). In addition, estrogens have also been shown to exert their feedback actions on LH secretion at the level of the pituitary (4,5) and are believed, by a mechanism yet to be fully delineated, to prime the pituitary for the LH surge.
The nuclear receptor transcription factors, estrogen receptor (ER)α and ERβ, mediate estrogen action by targeting transcription of genes whose products will ultimately alter the physiology of the cell. Both ERα and ERβ are expressed in the pituitary gonadotroph (6), yet diverse lines of evidence indicate that ERα is the major mediator of estrogen action in the pituitary. Agonists for ERα but not ERβ are capable of inducing LH secretion in estrogen-primed pituitaries in vitro (7,8). ERα is suggested to be the primary mediator of estrogen-induced reversal of hypertrophied gonadotrophs after ovariectomy (9). In support of these findings, ERα−/− female mice are completely infertile, have elevated levels of LH, and do not ovulate, whereas ERβ−/− mice can ovulate, although they are subfertile (10,11). These findings provide strong evidence indicating that ERα mediates estrogen action in the pituitary; however, the in vivo studies in particular cannot distinguish between the role of ERα in the pituitary and hypothalamus in reproductive function. Therefore, it remains to be established that ERα mediates estrogen action in the pituitary gonadotroph by isolating the estrogen-ERα system in the pituitary gonadotroph in vivo.
In this study, we investigated whether or not ERα is the mediator of estrogen action in the pituitary gonadotroph in vivo. To accomplish this, we used a genetic approach by deleting ERα in the pituitary gonadotroph. The results demonstrate that ERα in the gonadotroph is critical for estrous cyclicity and fertility in females.
Materials and Methods
Reagents
Antibody for ERα was purchased from Novocastra (RTU-ER-6F11; Newcastle upon Tyne, UK). Polyclonal antiserum for LHβ and TSHβ was acquired from the National Hormone and Peptide Program (Harbor-UCLA Medical Center, Torrance, CA). Pregnant mare serum gonadotropin (PMSG), human chorionic gonadotropin (hCG), and 17β-estradiol were purchased from Sigma Chemical Co. (St. Louis, MO). Molecular reagents were purchased from Invitrogen Life Technologies, Inc. (Carlsbad, CA).
Generation of ERαflox/flox αGSUcre and ERα−/− mice
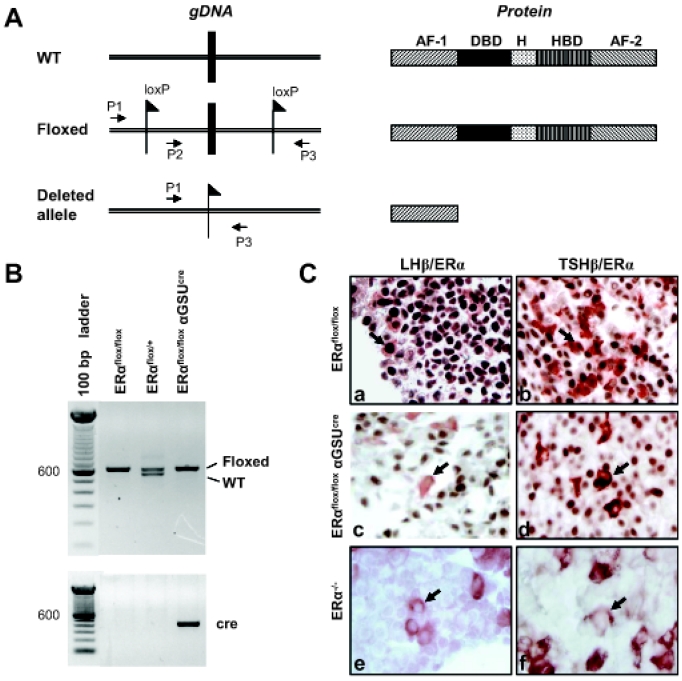
The ERαflox/flox αGSUcre mice were created using the cre/loxP approach (12). The ERαflox/flox mouse was created by a targeting strategy used to generate ERα−/− mice (10). As previously described, the floxed allele L2 was produced as a consequence of partial Cre-mediated deletion of the floxed cassette (10). Inbreeding of ERαL2/+ mice produced the conditional floxed ERαL2/L2 mice, referred to as ERαflox/flox mice in this paper. ERαflox/flox mice possess two loxP sites flanking exon 3 of the ERα gene (10). To generate ERαflox/flox αGSUcre mice, an ERαflox/flox male was crossed with an αGSUcre female in which the 4.6-kb promoter of the glycoprotein hormone α-subunit (αGSU) gene drives the expression of Cre recombinase in the αGSU-expressing cells (13). The F1 heterozygote (ERαflox/+ αGSUcre) mice were then bred with ERαflox/flox mice, which gave four genotypes: ERαflox/flox αGSUcre, ERαflox/+ αGSUcre, ERαflox/flox, and ERαflox/+. Genotyping was performed by PCR using ear-biopsy DNA. Genomic DNA was isolated from ear using the Easy-DNA Kit (Invitrogen). The primer combination of ERαP2F (5′-gtg tca gaa aga gac aat-3′) plus ERαP3 (5′-ggc att acc act tct cct ggg agt ct-3′) was used to determine the presence or absence of loxP sequences (flox or wild type) (Fig. 1A). The presence of αGSUcre recombinase was determined using the primers Cga (5′-aca ttg ttc ccc tca gat cg-3′) and Cre (5′-ata gtt ttt act gcc aga cc-3′). Examples of banding patterns used to determine the genotypes for three of the possibilities are shown (Fig. 1B). The generation of ERα−/− resulted from a cross of male ERαflox/flox with female Zp3cre, a line expressing cre recombinase in the oocyte specifically. The F1 heterozygote ERαflox/+ Zp3cre was then bred with ERαflox/flox to produce ERαflox/flox Zp3cre. Females that are ERαflox/flox Zp3cre produce oocytes that are ERα−. Oocytes fertilized by sperm from ERαflox/flox males result in progeny that are ERαflox/−. The breeding of two ERαflox/− mice produces one fourth of progeny that are ERα−/−. The primer set ERαP1 (5′-ttg ccc gat aac aat aac at-3′) plus ERαP3 was used to determine whether or not exon 3 had been deleted (ERα−). Presence of Zp3 Cre recombinase was determined using primers Cre-P1 (5′-gga cat gtt cag gga tcg cca ggc g-3′) and Cre-P85 (5′-gtg aaa cag cat tgc tgt cac tt-3′).
Figure 1.
Generation of ERαflox/flox αGSUcre mice. A, Schematic diagram showing targeted deletion of exon 3 of ERα at the level of genome (left) and the resulting translated product (right). The resulting protein product lacks both the DNA-binding domain (DBD) and the hormone-binding domain (HBD). AF-1 and AF-2, Transactivation domains; H, hinge region; P1, P2, and P3, primer binding sites used for genotyping; WT, wild type. B, Representative gel of PCR banding patterns showing three possible genotypes for F2 progeny. Amplification using ERα-P2F and ERα-P3 primers were used to detect ERα+ (543 bp) or ERαflox (607 bp). Primers Cga and Cre were used to determine the presence or absence of Cre recombinase. C, ERα protein expression in relation to the gonadotrophs and thyrotrophs was examined in the pituitary of ERαflox/flox, ERαflox/flox αGSUcre, and ERα−/− by double immunostaining with anti-ERα and anti-LHβ or anti-ERα and anti-TSHβ. In ERαflox/flox, cells positive for both LHβ (red-brown, cytoplasm) and ERα (brown, nuclear) or TSHβ (red-brown) and ERα (brown) are present as well as cells positive for ERα only. Note that cells staining positive for LHβ or TSHβ in the ERαflox/flox αGSUcre are devoid of ERα (indicated by arrows). ERα−/− shows no staining for ERα.
Animals and treatments
Animal procedures were carried out in accordance with the University of Kentucky Animal Care and Use Committee. All of the mice used in this study had C57BL/6 and SJL genetic backgrounds. For superovulation treatment, prepubertal mice were injected with 5 IU PMSG, then 48 h later with 5 IU hCG, according to the gonadotropin-primed superovulation model (14). For the microarray, the following groups of animals were used: 1) naturally cycling C57BL/6 mice collected in either metestrus or proestrus, 2) ovariectomized (OVX) ERαflox/flox, 3) OVX ERα−/−, and 4) OVX ERαflox/flox αGSUcre. Mice from groups 2, 3, and 4 were OVX between 1.5 and 4 months of age. Three weeks later, the mice were injected with 10 μg 17β-estradiol or 100 μl sesame oil at 0900 h for 2 consecutive days. On the second day, the mice were euthanized at 1500 h by carbon dioxide inhalation and the pituitaries harvested, snap frozen on dry ice, and stored at −80 C for later RNA isolation.
Fertility assay
The 45- to 50-d-old mice were individually housed with control mates for either 3 continuous months or in three consecutive matings. In the control group, ERαflox/flox females were mated with ERαflox/flox males. In the experimental group, ERαflox/flox αGSUcre females were paired with ERαflox/flox males. Females were monitored for pregnancy, and after giving birth, the number of pups was counted.
Determination of estrous cyclicity
Using vaginal lavage techniques (15), the pattern of estrous cycles was determined in 45- to 50-d-old female mice for 15 d. Vaginal lavage was performed daily, at the same time each day, by flushing the vagina with 0.9% sodium chloride. The cell samples were then examined under the microscope and scored. Estrus was determined by the presence of cornified cells and a very dense number of cells overall. Metestrus was scored by the presence of large round cells with an irregular border. A high density of leukocytes indicated the stage of diestrus, whereas small nucleated cells indicated proestrus (15).
Tissue collection, histology, and immunohistochemistry
Before animals were killed, the stage of the estrous cycle was determined and recorded. Animals were deeply anesthetized and perfused intracardially with PBS, followed by 4% paraformaldehyde at 1500 h. The pituitary, ovaries with attached oviducts, and uterus were collected in 10% buffered formalin and then embedded in paraffin blocks. For histology, sections were cut at 7 μm, mounted on slides, and stained with hematoxylin and eosin. Ovaries were cut in serial sections. Immunohistochemistry was performed as described previously (16). Briefly, sections were deparaffinized by treatment with xylenes and rehydrated through a graded alcohol series. Antigen unmasking was performed by boiling the sections in sodium citrate buffer (10 mm sodium citrate; and 0.05% Tween 20, pH 6.0) for 10 min in a microwave oven. After the sections were allowed to cool to room temperature, they were rinsed briefly in PBS and treated with 0.3% hydrogen peroxide (Sigma) in water for 30 min to quench endogenous peroxidase activity. Next, staining was performed by the ABC method using the Vectastain Elite ABC Kit (Vector Laboratories, Burlingame, CA). For double immunostaining, slides were first labeled for the nuclear antigen ERα (prediluted), followed by the cytoplasmic LHβ (1:1000) or TSHβ (1:1000); the slides were incubated for 20 min with normal blocking serum at room temperature and then incubated for 1 h at room temperature with primary antibody. Biotinylated secondary antibody was applied for 30 min, and then the slides were incubated for 30 min with ABC reagent. Slides were developed with diaminobenzidine-Ni substrate (Vector) for the ERα antigen and aminoethylcarbazole (Vector) for the LHβ or TSHβ antigen until the stain was evident.
Measurement of serum LH and FSH levels
Blood samples for hormone assay were obtained by cardiac puncture at 1500 h on the day of diestrus. Plasma LH concentrations were determined in 20- and 5-μl duplicate aliquots of serum using a modification of a previously described method (National Institutes of Health National Hormone and Peptide Program). Primary antibody (rat LH antiserum-rabbit; NIDDK anti-rLH-S-11) was diluted 1:200,000 and incubated for 48 h before adding 20,000 cpm/100 μl of trace (07-C65102; MP Biomedicals, Irvine, CA) to each tube. After an overnight incubation, secondary antibody at 1:50 (anti-RGG; B37I) was added, and the reaction was incubated for 6 h before washing, pouring off supernatant, and counting the precipitates. All incubations were performed at room temperature. The sensitivity (100% to 2 sd of maximum binding) averaged 0.03 ng/tube. RIA for FSH was performed by the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core (17).
Measurement of gonadotropin mRNA expression
DNA microarray was performed to measure the gonadotropin subunit mRNA as previously described (14). Pituitaries were collected as described in Animals and treatments, and total RNA was extracted using Trizol reagent (Invitrogen) and then purified using an RNeasy kit (QIAGEN Inc., Valencia, CA). For the primary pituitary cell culture, anterior pituitary cells were isolated from 10-wk-old female C57BL/6 mice as previously described (18) with minor modification. Isolated cells were counted and plated on poly-l-lysine-coated culture dishes containing medium (20 mm HEPES and 0.3% BSA in DMEM) supplemented with 10% fetal bovine serum. Cells were incubated in a humidified incubator at 37 C with 5% CO2. After 2 d of culture, the incubation medium was changed with medium supplemented with 10% charcoal-treated fetal bovine serum and cultured for an additional 2 d. The cells were then treated with charcoal-treated serum containing either 0.00001% ethanol or 1 nm 17β-estradiol in 0.00001% ethanol for 2 d. Two days after estrogen treatment, cells were harvested and total RNA was extracted. To verify RNA integrity, RNA was separated on a 1.5% agarose gel with ethidium bromide and the 28S rRNA and 18S rRNA bands visualized. The total RNA used for DNA microarray was pooled from at least five mice per group for the wild-type and at least two mice per group for the ERα−/− and ERαflox/flox αGSUcre, and the assay was done in duplicate. DNA microarray was performed at the DNA Microarray Core Facility of the University of Kentucky (Lexington, KY) using the Affymetrix Mouse 430 2.0 oligonucleotide array set.
Results
Targeting of ERα in αGSU-expressing cells
In this study, the Cre recombinase expression was driven by the αGSU promoter, which is the common subunit shared by LH and FSH of the gonadotrophs and TSH of the thyrotrophs, so that ERα could be removed only in these cell types. The excision of exon 3 of ERα results in a protein product lacking the DNA-binding and hormone-binding domains as well as the AF-2 transactivation domain (Fig. 1A). The successful removal of ERα in the gonadotroph and thyrotroph was confirmed by the lack of ERα protein expression in the gonadotrophs and thyrotrophs of the ERαflox/flox αGSUcre, in contrast to the strong ERα immunostaining that was apparent in the wild-type counterparts (Fig. 1C).
Pituitary gonadotroph ERα is critical for female fertility
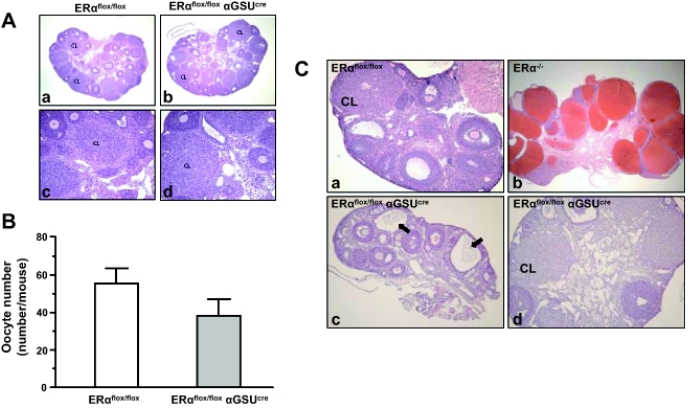
To determine whether pituitary gonadotroph ERα is necessary for female fertility, a mating assay was performed. Matings between female ERαflox/flox αGSUcre mice and proven males did not result in any pups over a 3-month period or three consecutive matings, indicating that female ERαflox/flox αGSUcre mice are infertile (Table 1). Vaginal plugs were observed in ERαflox/flox αGSUcre females, proving that the female ERαflox/flox αGSUcre are responsive to attempts to mate. In contrast, male ERαflox/flox αGSUcre mice are fertile (data not shown). To determine whether the ERαflox/flox αGSUcre maintain ovulatory capacity, immature mice were treated with exogenous gonadotropins PMSG and hCG, oocytes counted, and ovarian histology examined. Upon examination of the ovaries, it was evident that both ERαflox/flox and ERαflox/flox αGSUcre produced corpora lutea (Fig. 2A). The number of oocytes released after the superovulation regimen was not significantly different in ERαflox/flox αGSUcre compared with ERαflox/flox (Fig. 2B). Next, ovaries from mature 1.5- to 7-month-old ERαflox/flox αGSUcre mice were examined. Ovaries of the ERαflox/flox αGSUcre mice contained primary, secondary, and preovulatory follicles. The granulosa and theca cell layer of these follicles did not show any remarkable abnormality compared with controls. Furthermore, ovaries of the ERαflox/flox αGSUcre mice contained corpora lutea (Fig. 2C). Meanwhile, no hemorrhagic cysts were observed in ERαflox/flox αGSUcre ovaries in contrast to ERα−/− mouse ovaries (Fig. 2C, b and d). However, follicular cysts were occasionally observed in the ERαflox/flox αGSUcre ovaries (Fig. 2Cc).
Table 1.
Fertility of ERαflox/flox αGSUcre in mating assay
| Genotype | n | Litters | Pups | Pups/litter | Litters/female |
|---|---|---|---|---|---|
| ERαflox/flox | 7 | 18 | 126 | 7.0 ± 2.8 | 2.6 ± 0.8 |
| ERαflox/+ αGSUcre | 6 | 9 | 63 | 6.9 ± 3.1 | 1.5 ± 1.7 |
| ERαflox/flox αGSUcre | 8 | 0 | 0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
Figure 2.
Ovaries of ERαflox/flox αGSUcre mice are capable of releasing oocytes and producing corpora lutea. A, Immature 24-d-old mice were primed with PMSG (5 IU) and hCG (5 IU) and oocytes counted at 20 h. Ovaries were formalin fixed, paraffin embedded, and stained with hematoxylin and eosin. Note the presence of corpora lutea in both genotypes. B, ERαflox/flox (n = 2) produced an average of 55 oocytes per mouse, and ERαflox/flox αGSUcre (n = 3) produced 38 oocytes per animal on average. C, Ovaries from 1.5- to 7-month-old adult mice were examined in three genotypes: ERαflox/flox, ERαflox/flox αGSUcre, and ERα−/−. Corpora lutea are present in both ERαflox/flox (a) and ERαflox/flox αGSUcre (d). Some ERαflox/flox αGSUcre display cysts (c, indicated by arrows), although not hemorrhagic bloody cysts like those of ERα−/− (b). CL, Corpora lutea.
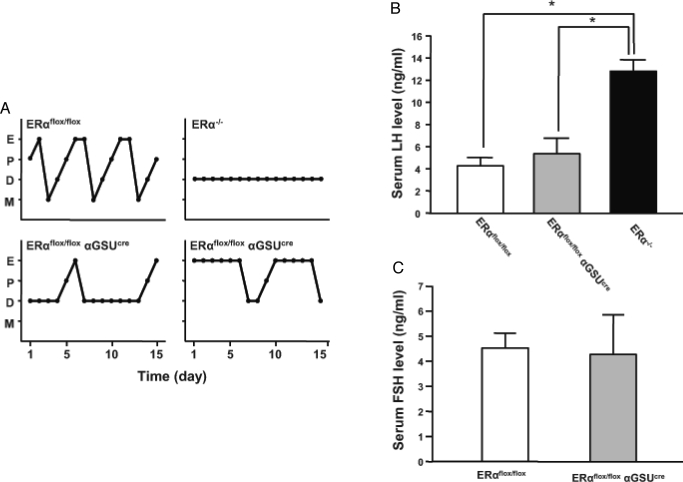
ERα in the pituitary gonadotroph is required for estrous cyclicity but not basal LH and FSH secretion
To further characterize the reproductive physiology of the ERαflox/flox αGSUcre mice, estrous cyclicity was determined by performing daily vaginal lavage and examining cytology in ERαflox/flox and ERαflox/flox αGSUcre mice. The majority of ERαflox/flox mice had regular estrous cycles. Although all cell types (cornified, leukocytic, and nucleated) could be observed in ERαflox/flox αGSUcre, the pattern was irregular (Fig. 3A). The profiles could generally be placed in one of two groups; some mice showed many consecutive days of leukocytic cells (13 of 22), whereas others showed several consecutive days of cornified cells (six of 22). The remaining mice (three of 22) displayed an equal number of days of cornified and leukocytic cells. Because the ERαflox/flox αGSUcre mice were not cycling regularly, we could not accurately predict the day of proestrus to assess LH surges. To determine whether or not pituitary gonadotroph ERα is critical for basal LH or FSH secretion, we determined serum LH and FSH concentrations in ERαflox/flox and ERαflox/flox αGSUcre mice that were in the stage of diestrus when LH and FSH secretion is basal. The serum level of LH in ERαflox/flox αGSUcre mice is comparable to that of the controls (Fig. 3B). The basal secretion of FSH during diestrus in both groups of mice was similar as well (Fig. 3C).
Figure 3.
ERαflox/flox αGSUcre females have basal levels of LH and FSH secretion but irregular estrous cyclicity. A, Vaginal lavage was performed on ERαflox/flox, ERαflox/flox αGSUcre, and ERα−/− on a daily basis for 15 d. ERαflox/flox females cycle every 4–5 d, whereas ERαflox/flox αGSUcre display a dichotomous pattern; some mice have many consecutive days of diestrus interspersed with cornified and nucleated cells, whereas other mice show long periods of cornified cells. ERα−/− show constant diestrus. All profiles are representative. D, Diestrus; E, estrus; M, metestrus; P, proestrus. B and C, Serum LH and FSH were measured in mice during diestrus. Basal LH and FSH levels in the ERαflox/flox αGSUcre female are comparable to those in ERαflox/flox mice. This is in contrast to elevated levels of LH in the ERα−/−. Significance was determined by t tests comparing ERαflox/flox and ERα−/− or ERαflox/flox αGSUcre and ERα−/−. Error bars represent sem.
Expression of LHβ, FSHβ, or αGSU mRNA is not regulated by pituitary gonadotroph ERα
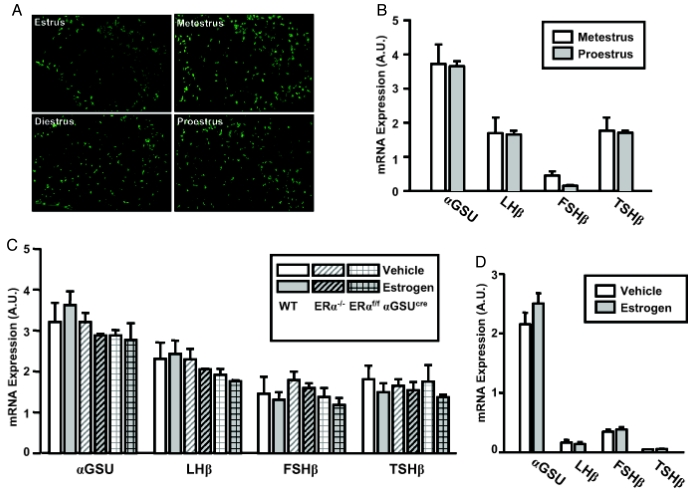
We investigated whether pituitary gonadotroph ERα regulated the expression of hormone subunits. We first determined whether pituitary content of LHβ protein changes during the estrous cycle by looking at the expression of LHβ protein in regularly cycling control mice on the day of estrus, metestrus, diestrus, and proestrus. No difference in LHβ protein expression was observed on the days of metestrus and diestrus compared with the day of proestrus as determined by immunostaining (Fig. 4A). We then determined whether or not 17β-estradiol regulates gonadotropin subunit genes as well as the genes of other pituitary hormones at the level of transcription. For this purpose, microarray analysis was performed on whole pituitary mRNA in regularly cycling mice in metestrus (low estrogen) and proestrus (high estrogen). Only FSHβ showed a decrease in expression, albeit nonsignificant, during proestrus compared with metestrus (Fig. 4B). In addition, the following groups were OVX and treated 3 wk later with either 17β-estradiol or sesame oil: wild-type, ERαflox/flox αGSUcre, and ERα−/−. On the second day of 17β-estradiol administration, mice were killed at 1500 h for pituitary harvest, and mRNA expression level was measured by microarray. There was no significant change in the transcription of αGSU, LHβ, or TSHβ in the 17β-estradiol-treated groups compared with oil-treated (Fig. 4C). In addition, the mRNA transcript of these three subunits does not differ in the ERα−/− or ERαflox/flox αGSUcre. Thus, these data show that the mRNA transcription of αGSU, LHβ, and TSHβ is not regulated by 17β-estradiol, specifically via ERα. FSHβ transcript expression is lower on proestrus than metestrus, indicating the difference in regulation of FSHβ and LHβ. However, the transcription of FSHβ is not affected by 17β-estradiol directly in this OVX model. Primary pituitary cell culture also demonstrates that 17β-estradiol alone does not alter transcription of the hormone subunits (Fig. 4D).
Figure 4.
17β-Estradiol does not increase transcription of αGSU, LHβ, FSHβ, and TSHβ. A, Immunostaining for LHβ was performed on pituitaries from regularly cycling mice. Wild-type cycling mice were monitored for estrous cyclicity for 2 wk and then killed on the afternoon of estrus, metestrus, diestrus 1, or proestrus. B, Vaginal lavage was performed on 45-d-old wild-type mice twice daily at 0900 and 1500 h for at least 10 d. Mice which showed a 4- to 5-d cycle were used and killed at 1700 h on the day of metestrus or proestrus. C, Mice were OVX at 1.5–4 months of age. Three weeks later, mice were injected with either 10 μg 17β-estradiol or oil (vehicle) at 0900 h of d 1 and 0900 h of d 2. Pituitaries were collected at 1500 h on d 2 from wild-type (WT) OVX plus vehicle, WT OVX plus 17β-estradiol, ERα−/− OVX plus vehicle, ERα−/− OVX plus 17β-estradiol, ERαflox/flox αGSUcre OVX plus vehicle, and ERαflox/flox αGSUcre OVXplus 17β-estradiol. D, Primary pituitary cells plus vehicle (n = 2), primary pituitary cells plus 17β-estradiol (n = 2). The total RNA used for DNA microarray was pooled from at least five mice for WT metestrus, WT proestrus, and WT OVX and at least two mice for ERα−/− OVX and ERαflox/flox αGSUcre OVX. The array was done in duplicate. Data are represented by mean plus sem from two independent array results.
Discussion
Estrogens act on both the hypothalamus and pituitary to control the basal and surge aspects of LH secretion. Although there is much evidence suggesting that action of estrogens in the control of reproductive aspects in the pituitary is carried out by ERα, it has not been demonstrated in vivo that ERα is the mediator of estrogen action at the pituitary level. Our results show that ERα in the gonadotroph is critical for fertility and estrous cyclicity, yet not for basal LH and FSH secretion. This indicates that estrogens via ERα do not exert their negative feedback in the control of hormone secretion at the level of pituitary. This may not be surprising because much evidence in other models supports the notion that the negative-feedback effect of estrogens at the pituitary are minor at best, because the major regulator of basal secretion is GnRH released by the hypothalamus. For example, in the OVX hypothalamo-pituitary disconnected ewe model, it was found that estrogens have only a short-term negative feedback effect. Chronic treatment of estrogens combined with pulsatile GnRH does not change LHβ mRNA levels or number of GnRH receptors, and amplitude of LH pulses decreases by only 20% (reviewed in Ref. 1).
Disruption of ERα in the pituitary gonadotroph alone produces several notable differences compared with mice in which ERα is globally deleted. ERα−/− females have elevated levels of serum LH as well as blood-filled hemorrhagic cysts, which form by 2 months of age, and absence of corpus lutea (10,19,20). Basal LH and FSH levels are not elevated in the ERαflox/flox αGSUcre. This finding in comparison with the ERα−/− phenotype provides support that in mice, the pituitary is not the primary target for the negative feedback of estrogens, but rather the hypothalamus, as has been concluded in other species (1). The normal level of serum FSH and LH secretion supports the observation of primary and secondary follicles in the ovaries of the ERαflox/flox αGSUcre mice. PMSG/hCG-induced ovulation resulted in release of a comparable number of oocytes from the ERαflox/flox αGSUcre ovary, demonstrating that the ovarian mechanisms necessary for ovulation are intact. Although a problem with the ovary cannot be ruled out, this result implies that the loss of the estrogen/ERα pathway in the gonadotroph is responsible for the infertile phenotype of the ERαflox/flox αGSUcre female mice. Furthermore, corpora lutea are present in adult ERαflox/flox αGSUcre mouse ovaries, indicating that spontaneous ovulation may occur in the intact ERαflox/flox αGSUcre female mice. It is important to consider that corpora lutea can form in the absence of ovulation (21), and normal numbers of corpora lutea can form despite a reduced LH surge (22). However, the irregular estrous cycle of these mice implies that the LH surge is mistimed, attenuated, or absent, so it is probable that the ovulation could be caused by irregular elevations of LH in the ERαflox/flox αGSUcre mice.
ERα is also absent in the thyrotrophs of ERαflox/flox αGSUcre mice; thus, it is possible that TSH could be affected in these mice. Changes in TSH and thyroid hormone can impact reproduction. Hypothyroid rats have irregular estrous cycles, specifically a prolonged diestrus (23). Although some ERαflox/flox αGSUcre mice display a prolonged diestrus, others display prolonged days of cornified cells. Additionally, hypothyroid female rats have a decreased basal serum LH (23). In contrast, the basal serum LH of ERαflox/flox αGSUcre mice is not decreased. Hypothyroid female rats that were given an hCG challenge on the day of diestrus showed a significant decrease in the number of oocytes released compared with controls (23). A challenge of PMSG and hCG to immature ERαflox/flox αGSUcre mice did not result in a significant decreased release of oocytes. This evidence suggests that the reproductive problems of the ERαflox/flox αGSUcre mice are not due to decreases in serum TSH.
Based on our evidence that ERα is indeed the mediator of estrogen action in the gonadotroph, we examined the role of ERα in gene expression of hormone subunits. First we tested whether 17β-estradiol induced the transcription of αGSU, LHβ, FSHβ, and TSHβ. In neither regularly cycling wild-type mice nor the OVX model did 17β-estradiol increase the transcription of αGSU, LHβ, or TSHβ. Thus, it is not surprising that absence of ERα did not serve to reduce the amount of mRNA transcript for these three subunits (Fig. 4C). It was surprising to find that 17β-estradiol did not increase transcription of LHβ, given the fact that an estrogen-responsive element has been identified in the rat LHβ gene (24) and that estrogen directly increases transcription of the rat gene in vitro (25). However, it has been suggested that the positive-feedback action of estrogens that generate the LH surge regulates LH secretion, not synthesis (26), and our model was designed to mimic the long exposure of estrogens that occurs before the surge. It is possible that there may have been a small immediate increase in transcription after 17β-estradiol injection, which later returned to basal. There is also evidence that LHβ mRNA increases before the LH surge in cycling rats (27). This increase, however, does not point to a direct effect of estrogens and may be explained by increased secretion of GnRH. It has also been shown that the loss of ERα in the total ERα−/− results in increases in transcript of gonadotropin subunits (20), suggesting that ERα regulates this transcription. Once again, the total ERα−/− does not isolate effects in the pituitary because ERα is absent in all tissues, and this increase may likely be due to some disruption of estrogen’s regulation of GnRH, and consequently GnRH’s control of gonadotrophin transcription, and may explain the difference in the ERαflox/flox αGSUcre that did not show an increase and are presumed to have normal GnRH pulsatility.
Estrogens have been shown to have positive feedback effect at the level of pituitary and pituitary gonadotroph in sheep and rats (1,5). Recently, neuron-specific ERα−/− mice were found to be infertile and unable to respond to the positive-feedback action of estradiol, providing evidence that the brain is important for positive estrogen feedback as well (28). The lack of ERα in the pituitary gonadotroph may affect positive feedback in the ERαflox/flox αGSUcre mice, which has yet to be tested. In fact, the ERα agonist propylpyrazole-triol elicits LH secretion from propylpyrazole-triol-primed rat pituitaries in response to consecutive GnRH challenges in vitro, comparable to that induced by 17β-estradiol; the ERβ agonist diarylpropionitrile gave no such response (7). In addition, tamoxifen-treated rats induced ERα expression in gonadotrophs, eliciting LH secretion, presumably through ERα (8). Finally, estradiol treatment does not increase LH secretion in ERα−/− gonadotroph cells in vitro (29). Our results show that pituitary gonadotroph ERα does not affect basal LH secretion; this result in combination with the above data leads us to speculate that gonadotroph ERα does have a role in the positive feedback of estrogens. Interestingly, males do not have the unique positive-feedback system that governs the LH surge, and ERαflox/flox αGSUcre males are fertile. However, it has yet to be determined whether the cause of infertility in the ERαflox/flox αGSUcre female mice is due to a problem with ovarian function, oocyte health, maintenance of pregnancy, or disruption of positive feedback and the LH surge.
In conclusion, we have shown for the first time direct in vivo evidence that ERα is the mediator of estrogen action in the pituitary gonadotroph. In particular, a direct action of estrogen/ERα in the pituitary gonadotroph is required for fertility and estrous cyclicity, but not the regulation of basal LH and FSH secretion. We speculate that estrogen/ERα is regulating positive feedback, the LH surge, and estrous cyclicity and that the genes under this control are involved in regulation of LH secretion itself, rather than expression of hormone subunits. Future studies will further investigate the role of ERα and induced genes in LH secretion during the LH surge.
Acknowledgments
We thank Dr. Phillip Bridges for his critical review and comments in the preparation of this manuscript and Dr. Dong-Wook Kang for consultation on immunostaining.
Footnotes
This work was supported by Grants P20 RR15592 (C.K.) and 1RO1HD052694-01 (C.K.) from the National Institutes of Health. RIA for FSH was performed by the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core [NICHD (SCCPRR) Grant U54-HD28934, University of Virginia, Charlottesville, VA].
Disclosure Summary: The authors have nothing to disclose.
First Published Online October 18, 2007
Abbreviations: ER, Estrogen receptor; αGSU, glycoprotein hormone α-subunit; hCG, human chorionic gonadotropin; PMSG, pregnant mare serum gonadotropin; OVX, ovariectomized.
References
- Clarke IJ 2002 Multifarious effects of estrogen on the pituitary gonadotrope with special emphasis on studies in the ovine species. Arch Physiol Biochem 110:62–73 [DOI] [PubMed] [Google Scholar]
- Levine JE 1997 New concepts of the neuroendocrine regulation of gonadotropin surges in rats. Biol Reprod 56:293–302 [DOI] [PubMed] [Google Scholar]
- Knobil E, Neill JD 1988 The physiology of reproduction. New York: Raven Press [Google Scholar]
- Nett TM, Turzillo AM, Baratta M, Rispoli LA 2002 Pituitary effects of steroid hormones on secretion of follicle-stimulating hormone and luteinizing hormone. Domest Anim Endocrinol 23:33–42 [DOI] [PubMed] [Google Scholar]
- Yin P, Kawashima K, Arita J 2002 Direct actions of estradiol on the anterior pituitary gland are required for hypothalamus-dependent lactotrope proliferation and secretory surges of luteinizing hormone but not of prolactin in female rats. Neuroendocrinology 75:392–401 [DOI] [PubMed] [Google Scholar]
- Mitchner NA, Garlick C, Ben-Jonathan N 1998 Cellular distribution and gene regulation of estrogen receptors α and β in the rat pituitary gland. Endocrinology 139:3976–3983 [DOI] [PubMed] [Google Scholar]
- Sanchez-Criado JE, Martin De Las Mulas J, Bellido C, Tena-Sempere M, Aguilar R, Blanco A 2004 Biological role of pituitary estrogen receptors ERα and ERβ on progesterone receptor expression and action and on gonadotropin and prolactin secretion in the rat. Neuroendocrinology 79:247–258 [DOI] [PubMed] [Google Scholar]
- Sanchez-Criado JE, de Las Mulas JM, Bellido C, Aguilar R, Garrido-Gracia JC 2005 Gonadotrope oestrogen receptor-α and -β and progesterone receptor immunoreactivity after ovariectomy and exposure to oestradiol benzoate, tamoxifen or raloxifene in the rat: correlation with LH secretion. J Endocrinol 184:59–68 [DOI] [PubMed] [Google Scholar]
- Sanchez-Criado JE, de Las Mulas JM, Bellido C, Navarro VM, Aguilar R, Garrido-Gracia JC, Malagon MM, Tena-Sempere M, Blanco A 2006 Gonadotropin-secreting cells in ovariectomized rats treated with different oestrogen receptor ligands: a modulatory role for ERβ in the gonadotrope? J Endocrinol 188:167–177 [DOI] [PubMed] [Google Scholar]
- Dupont S, Krust A, Gansmuller A, Dierich A, Chambon P, Mark M 2000 Effect of single and compound knockouts of estrogen receptors α (ERα) and β (ERβ) on mouse reproductive phenotypes. Development 127:4277–4291 [DOI] [PubMed] [Google Scholar]
- Hewitt SC, Korach KS 2003 Oestrogen receptor knockout mice: roles for oestrogen receptors α and β in reproductive tissues. Reproduction 125:143–149 [DOI] [PubMed] [Google Scholar]
- Sauer B 1998 Inducible gene targeting in mice using the Cre/lox system. Methods 14:381–392 [DOI] [PubMed] [Google Scholar]
- Cushman LJ, Burrows HL, Seasholtz AF, Lewandoski M, Muzyczka N, Camper SA 2000 Cre-mediated recombination in the pituitary gland. Genesis 28:167–174 [DOI] [PubMed] [Google Scholar]
- Jo M, Gieske MC, Payne CE, Wheeler-Price SE, Gieske JB, Ignatius IV, Curry Jr TE, Ko C 2004 Development and application of a rat ovarian gene expression database. Endocrinology 145:5384–5396 [DOI] [PubMed] [Google Scholar]
- Becker JB, Arnold AP, Berkley KJ, Blaustein JD, Eckel LA, Hampson E, Herman JP, Marts S, Sadee W, Steiner M, Taylor J, Young E 2005 Strategies and methods for research on sex differences in brain and behavior. Endocrinology 146:1650–1673 [DOI] [PubMed] [Google Scholar]
- Ko C, Gieske MC, Al-Alem L, Hahn Y, Su W, Gong MC, Iglarz M, Koo Y 2006 Endothelin-2 in ovarian follicle rupture. Endocrinology 147:1770–1779 [DOI] [PubMed] [Google Scholar]
- Gay VL, Midgley Jr AR, Niswender GD 1970 Patterns of gonadotrophin secretion associated with ovulation. Fed Proc 29:1880–1887 [PubMed] [Google Scholar]
- Kim HJ, Hwang IT, Lee HK, Yoo YB, Lee SK, Hwang DH, Lee BL 2000 Reconstituted basement membrane induces glandular-like morphogenesis but no difference in ACTH synthesis of anterior pituitary cells. Endocr J 47:771–776 [DOI] [PubMed] [Google Scholar]
- Schomberg DW, Couse JF, Mukherjee A, Lubahn DB, Sar M, Mayo KE, Korach KS 1999 Targeted disruption of the estrogen receptor-α gene in female mice: characterization of ovarian responses and phenotype in the adult. Endocrinology 140:2733–2744 [DOI] [PubMed] [Google Scholar]
- Couse JF, Yates MM, Walker VR, Korach KS 2003 Characterization of the hypothalamic-pituitary-gonadal axis in estrogen receptor (ER) Null mice reveals hypergonadism and endocrine sex reversal in females lacking ERα but not ERβ. Mol Endocrinol 17:1039–1053 [DOI] [PubMed] [Google Scholar]
- White R, Leonardsson G, Rosewell I, Ann Jacobs M, Milligan S, Parker M 2000 The nuclear receptor co-repressor nrip1 (RIP140) is essential for female fertility. Nat Med 6:1368–1374 [DOI] [PubMed] [Google Scholar]
- Xu M, Hill JW, Levine JE 2000 Attenuation of luteinizing hormone surges in neuropeptide Y knockout mice. Neuroendocrinology 72:263–271 [DOI] [PubMed] [Google Scholar]
- Tohei A, Imai A, Watanabe G, Taya K 1998 Influence of thiouracil-induced hypothyroidism on adrenal and gonadal functions in adult female rats. J Vet Med Sci 60:439–446 [DOI] [PubMed] [Google Scholar]
- Shupnik MA, Rosenzweig BA 1991 Identification of an estrogen-responsive element in the rat LHβ gene. DNA-estrogen receptor interactions and functional analysis. J Biol Chem 266:17084–17091 [PubMed] [Google Scholar]
- Shupnik MA 1996 Gonadotropin gene modulation by steroids and gonadotropin-releasing hormone. Biol Reprod 54:279–286 [DOI] [PubMed] [Google Scholar]
- Brown P, McNeilly AS 1999 Transcriptional regulation of pituitary gonadotrophin subunit genes. Rev Reprod 4:117–124 [DOI] [PubMed] [Google Scholar]
- Burger LL, Haisenleder DJ, Dalkin AC, Marshall JC 2004 Regulation of gonadotropin subunit gene transcription. J Mol Endocrinol 33:559–584 [DOI] [PubMed] [Google Scholar]
- Wintermantel TM, Campbell RE, Porteous R, Bock D, Grone HJ, Todman MG, Korach KS, Greiner E, Perez CA, Schutz G, Herbison AE 2006 Definition of estrogen receptor pathway critical for estrogen positive feedback to gonadotropin-releasing hormone neurons and fertility. Neuron 52:271–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindzey J, Jayes FL, Yates MM, Couse JF, Korach KS 2006 The bi-modal effects of estradiol on gonadotropin synthesis and secretion in female mice are dependent on estrogen receptor-α. J Endocrinol 191:309–317 [DOI] [PubMed] [Google Scholar]