Abstract
Reduced apoptosis of crypt stem/progenitor cells and elevated insulin and IGFs are linked to colon cancer risk. Insulin receptor substrate-1 (IRS-1) mediates the actions of insulin, IGF-I, and IGF-II, but the role of endogenous IRS-1 in crypt apoptosis and cancer is undefined. Using IRS-1−/−, IRS-1+/−, and IRS-1+/+ mice, we tested the hypothesis that reduced IRS-1 expression increases apoptosis of intestinal crypt cells and protects against Apcmin/+ (Min)/β-catenin-driven intestinal tumors. Expression of Sox9, a transcriptional target of Tcf/β-catenin and putative biomarker of crypt stem cells, was assessed in intestine of different IRS-1 genotypes and cell lines. Irradiation-induced apoptosis was significantly increased in the crypts and crypt stem cell region of IRS-1-deficient mice. Tumor load was significantly reduced by 31.2 ± 14.6% in IRS-1+/−/Min and by 64.1 ± 7.6% in IRS-1−/−/Min mice, with more prominent reductions in tumor number than size. Compared with IRS-1+/+/Min, IRS-1−/−/Min mice had fewer Sox9-positive cells in intestinal crypts and reduced Sox9 mRNA in intestine. IRS-1 overexpression increased Sox9 expression in an intestinal epithelial cell line. We conclude that even small reductions in endogenous IRS-1 increase apoptosis of crypt stem or progenitor cells, protect against β-catenin-driven intestinal tumors, and reduce Sox9, a Tcf/β-catenin target and putative stem/progenitor cell biomarker.
THE EPITHELIAL LINING of the small and large intestine is constantly renewed as a result of continuous proliferation of putative intestinal stem cells and progenitor cells within the crypts (1,2). Although the identity of intestinal stem cells is not conclusively defined, current evidence suggests that they reside near the base of the crypts (1,2). As well as continuous proliferation, there are small but significant levels of spontaneous apoptosis in the crypts. Genotoxins such as γ-irradiation lead to major increases in crypt apoptosis, and this effect is particularly pronounced in the region of the putative crypt stem cells and progenitors (3). The radiosensitivity of intestinal stem cells is thought to protect against tumor development by reducing the probability of clonal expansion of genetically damaged stem cells (3,4). A better understanding of the key modulators of irradiation-induced apoptosis could provide critical insight into potential cancer risk factors or point to new therapies. In animal models, IGFs reduce spontaneous or irradiation-induced crypt apoptosis, with effects being especially potent in the putative crypt stem cell region (5). Human studies show that low rates of spontaneous apoptosis in normal intestinal crypts predict increased risk of precancerous adenomas in the colon (6,7). Multiple studies have linked increased plasma or tissue IGFs to increased risk of intestinal cancer in humans (8,9,10,11). Our recent studies indicate that high, but within the normal range, levels of insulin in humans correlate strongly with both low apoptosis in intestinal crypts and increased adenoma risk (7,12).
This study focuses on insulin receptor substrate-1 (IRS-1), because it is a key mediator of the actions of both insulin and the IGFs and lies downstream of the insulin and IGF-I receptors (13). Considerable evidence indicates that IRS-1 is a primary mediator of the antiapoptotic or trophic actions of insulin and the IGFs (13,14). IGFs and insulin can also activate IRS-2, but findings in IRS-1 and IRS-2 null mice suggest preferential roles of IRS-1 in growth and IRS-2 in metabolism. IRS-1 null mice exhibit reduced body growth and reduced growth of several organs including the intestine (15), whereas IRS-2 null mice are normal size but develop type 2 diabetes (16,17).
Accumulating evidence links IRS-1 to cancer. A G972R polymorphism in the IRS-1 gene significantly increased risk of colorectal cancer (18). IRS-1 is overexpressed or constitutively active in a number of cancers and cancer cell lines, including colon cancer cell lines (19,20,21). Recent studies demonstrate that mice with mammary gland-specific IRS-1 overexpression develop spontaneous metastatic mammary tumors (22). Whether reduced expression of endogenous IRS-1 can protect against cancer of any organ, including colon cancer, remains unknown. The present study tested the hypothesis that reduced levels of endogenous IRS-1 promote apoptosis of genetically damaged crypt stem or progenitor cells in the intestine and protect against spontaneous intestinal adenoma. Our studies focused on the role of IRS-1 in spontaneous intestinal tumors driven by excessive activation of β-catenin-mediated transcription, because accumulating evidence suggests that the IGF-I receptor activates β-catenin via IRS-1-dependent pathways (22,23,24,25).
Apcmin/+ mice have a truncation in Apc (adenomatous polyposis coli), a gene mutated in many human intestinal cancers (26). Apcmin/+ mice spontaneously develop tens to hundreds of adenomas in small intestine and some adenomas in colon (27). Adenomas have aberrant intracellular and nuclear accumulation of β-catenin, which is normally degraded by an APC-containing complex (28). β-Catenin is a coactivator of growth and survival-regulatory genes in concert with T-cell factor/lymphoid enhancer factor transcription factors (29). By crossbreeding, we developed Apcmin/+ mice with IRS-1+/+, IRS-1+/−, and IRS-1−/− genotypes to determine whether partial or absolute IRS-1 deficiency reduces susceptibility to spontaneous intestinal tumors driven by β-catenin.
Defining IGF-I receptor/IRS-1-regulated biomarkers of intestinal stem cells could be relevant to mechanisms or biomarkers for increased intestinal cancer risk due to elevated levels of IGF or insulin. The Sry-related high-mobility group (HMG)-box DNA-binding protein (Sox9) is a β-catenin/Tcf gene target implicated in fate determination of stem cells in the pancreas, neural ectoderm, cartilage, and gonads (30,31,32,33,34,35). In intestine, recent studies demonstrate that Sox9 localizes to the nucleus of proliferating crypt cells, particularly in regions where stem or progenitor cells reside (32). Sox9 also represses expression of intestinal differentiation markers Muc2 and Cdx2, suggesting a role for Sox9 in maintenance of an undifferentiated stem or progenitor phenotype (32). Sox9 is highly expressed in human colon cancer cell lines and in intestinal tumors (32), indicating a possible role in tumorigenesis. Studies in chondrocytes indicate that Sox9 expression is induced by IGF-I (36), but whether this occurs in other cell types or is IRS-1 dependent is unknown. Our studies therefore explored whether changes in the number of Sox9-positive cells or Sox9 expression levels in crypts were associated with the effects of IRS-1 genotype on adenoma susceptibility in the Apcmin/+ mice and whether increased IRS-1 expression in intestinal epithelial cells alters Sox9 expression in vitro.
Materials and Methods
Mouse models
Mice heterozygous for targeted disruption of the IRS-1 gene (IRS-1+/−) on a purebred C57BL/6 background were previously described (16) and provided by Dr. Ronald Kahn. IRS-1+/− males and females were bred to derive sex-matched littermates with two (IRS-1+/+), one (IRS-1+/−), or zero (IRS-1−/−) functional IRS-1 alleles for studies of spontaneous and irradiation-induced apoptosis. Apcmin/+ male mice on the C57BL/6 background were obtained from Jackson Laboratories (Bar Harbor, ME) and crossed with female IRS-1+/− mice. IRS-1+/− males with the Apcmin/+ mutation were then crossbred with IRS-1+/− females. This two-step crossbreeding yielded Apcmin/+ and wild-type (WT) mice with two, one, or zero functional IRS-1 alleles. Genotyping was performed on tail DNA using primers described previously (15,37). Studies in Apcmin/+ mice were largely confined to females because males were used primarily for breeding. All animal studies were approved by the Institutional Animal Care and Use Committee of the University of North Carolina. Study protocols were in compliance with the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health.
Irradiation and tissue collection
Mice (50–75 d old) received 5 Gy whole-body irradiation delivered at 1 Gy/min with a 137Cs source and were killed 4 h after irradiation, a time of peak irradiation-induced apoptosis (38). Nonirradiated, genotype- and sex-matched littermates were used as controls. Mice were anesthetized using sodium pentobarbital (200 μg/g body weight), and the abdomen was opened by midline incision. Two pieces of jejunum (each 0.5 cm long) were fixed in 10% formalin for 4 h, dehydrated in 70% ethanol, and embedded in paraffin.
Analysis of apoptosis in irradiated mice
Apoptosis was quantified in hematoxylin- and eosin-stained 4-μm sections of jejunum as previously described (5). Briefly, morphological identification of apoptotic crypt cells was based on nuclear margination, chromatin and cytoplasmic condensation, shrinkage from neighboring cells, and the formation of apoptotic bodies due to nuclear and cytoplasmic fragmentation. Apoptosis in well-oriented crypts was recorded as previously described (39), with cells at the base designated as position 1. All scoring was performed twice by a single investigator unaware of the mouse genotype or treatment. Apoptosis was expressed as the mean number of apoptotic cells per crypt or the percentage of apoptotic cells at each position from the crypt base relative to the total number of cells counted.
Tissue collection and evaluation of tumors in Apcmin/+ mice
Mice were studied at 18–20 wk of age, when they were anesthetized, and blood was collected by cardiac puncture. Because Apcmin/+ mice develop severe anemia as disease progresses (40), hematocrit was measured as an indirect marker for tumor load and disease severity. Hematocrit was assayed by the Animal Clinical Chemistry Facility in the Pathology Department, University of North Carolina at Chapel Hill. The entire small intestine and colon were dissected, and tumor load was assessed as in previous studies (37). Briefly, tumor number was counted under a Leica dissecting scope for the entire small intestine and colon, and tumor diameter was measured using an in-lens micrometer. Tumor load was calculated by multiplying the total tumor number and mean size for small intestine and colon of each mouse. Intestinal segments were rolled into Swiss rolls, paraffin-embedded, and sectioned at 7 μm. The presence and morphology of adenomas were then confirmed by hematoxylin and eosin staining and β-catenin immunostaining. Elevated cytoplasmic and nuclear β-catenin is considered a reliable hallmark of adenomatous lesions in Apcmin/+ mice (37).
Localization of β-catenin and Sox9
β-Catenin immunostaining was performed as previously described (37). A primary mouse monocolonal β-catenin antibody (no. 610154; BD Transduction Laboratories, Franklin Lakes, NJ) was used together with a biotinylated antimouse IgG from the MOM kit using a mouse on mouse blocking protocol (Vector Laboratories, Burlingame, CA). Peroxidase was visualized under bright field using a Nikon Microphot FXA microscope. For Sox9, a rabbit polyclonal antibody (AB35535; Chemicon, Temecula, CA) was used at 1:100, followed by peroxidase or a Cy3-labeled secondary antibody at 1:100. Fluorescent images were taken under a Zeiss 510 laser-scanning confocal microscope.
Northern blot hybridization of Sox9 mRNA
Total mRNA was extracted from 2 cm of proximal ileum using TriZol reagent (Invitrogen, Carlsbad, CA) and manufacturer’s instructions. Northern blot hybridization was performed as previously described (41), using a [32P]CTP-labeled Sox9 DNA template from pCMV-SPORT6 (Open Biosystems, Huntsville, AL). Blots were reprobed for constitutively expressed 18S rRNA, and hybridization signals were visualized using a PhosphorImager (Typhoon; GE Healthcare, Piscataway, NJ) as previously described (42).
Effects of IRS-1 on Sox9 expression
Primary cultures of intestinal epithelial cells do not remain viable for sufficient times after isolation to study the role of IRS-1 in Sox9 expression. We therefore used the nontransformed intestinal epithelial cell line, IEC-6, to assess whether IRS-1 overexpression altered Sox9 expression. IEC-6 cells were grown as previously described (42). IEC-6 cells were infected with either IRS-1 adenovirus (Adex1CAIRS-1wt) (43) or an empty virus (Gene Therapy Center Virus Vector Core, University of North Carolina at Chapel Hill) at 108 particles/ml 18 h before harvesting. Total cell lysates were prepared, and equal amounts of protein were subjected to SDS-PAGE as described (44). Blots were incubated in Sox9 antibody (1:1000), followed by an IRDye 800CW goat antirabbit secondary antibody (1:5000, no. 926-32211; LI-COR Biosciences, Lincoln, NE). β-Actin was used as a loading control. Blots were imaged using the Odyssey infrared imaging system (LI-COR).
Statistical analyses
Values are expressed as mean ± sem. ANOVA was used to compare levels of apoptosis in irradiated IRS-1+/+ vs. IRS-1+/− or IRS-1−/− littermates, and post hoc analyses between each genotype were performed by Fisher’s protected least significant difference comparisons. Wilcoxon signed-rank test was used to compare tumor number, size, and load in IRS-1+/+/Min and IRS-1+/−/Min littermates. IRS-1−/−/Min mice were born at lower than expected Mendelian frequency, and it was not possible to restrict comparisons to littermates. Therefore, a Student’s t test was used to compare tumor number, size, and load in IRS-1−/−/Min mice vs. age- and sex-matched IRS-1+/+/Min or IRS-1+/−/Min mice. Two-way ANOVA was used to determine whether there was significant effect of IRS-1 or Min genotype on Sox9 expression and whether IRS-1 and Min genotype show a significant interaction. P < 0.05 was considered to be statistically significant.
Results
IRS-1 gene-dosage effects on irradiation-induced apoptosis of crypt stem cells
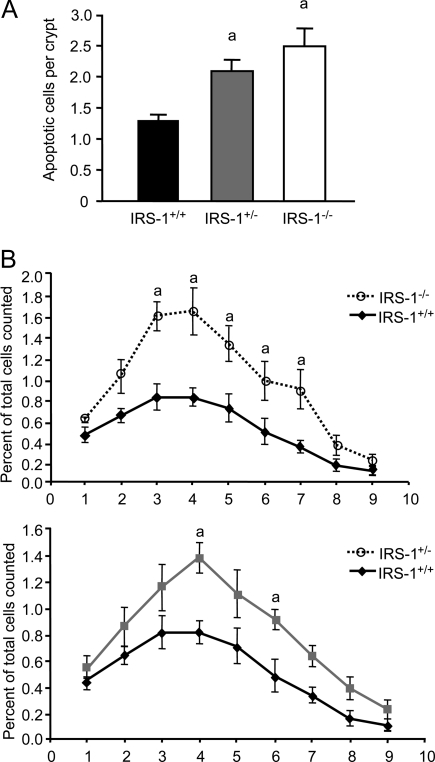
γ-Irradiation induces genetic damage and apoptosis in the intestinal crypts (5). Disruption of one or both IRS-1 alleles significantly increased irradiation-induced apoptosis (Fig. 1A). After irradiation, IRS-1+/− mice had significantly higher crypt apoptosis (2.09 ± 0.17) compared with IRS-1+/+ littermates (1.30 ± 0.09, P = 0.019), and IRS-1−/− mice had even higher rates of apoptosis (2.49 ± 0.30, P = 0.002).
Figure 1.
Irradiation-induced apoptosis in jejunal crypts of IRS-1+/+, IRS-1+/−, and IRS-1−/− mice. A, Histogram shows the mean number of apoptotic cells per crypt for each genotype. a, P < 0.05 vs. IRS-1+/+; n = 5 for each genotype. B, Graph shows the percentage of apoptotic cells at each cell position along the jejunal crypt for irradiated IRS-1+/+, IRS-1+/−, and IRS-1−/− mice. Data are expressed as percentage of apoptotic cells per total cells counted at each location among at least 40 crypts for five animals of each genotype. The increase in apoptosis in IRS-1+/− and IRS-1−/− compared with IRS-1+/+ is most prominent in the putative stem or progenitor cell region. a, P < 0.05 vs. IRS-1+/+.
Available evidence indicates that small intestinal crypt stem and progenitor cells are located primarily at cell positions 3–5 from the base of the crypts, otherwise known as the stem cell region (3,5). This region lies immediately above Paneth cells that are located at the crypt base (cells 1 and 2 relative to the base of the crypts). In IRS-1−/− mice, irradiation-induced apoptosis was significantly increased at positions 2–7 from the crypt base. In IRS-1+/− mice, apoptosis was increased significantly at positions 4 and 6 (Fig. 1B). Thus, partial or absolute IRS-1 deficiency promotes apoptosis of genetically damaged crypt cells, with most dramatic effects within the putative stem/progenitor cell region.
IRS-1 gene-dosage effects on tumor number in Apcmin/+ mice
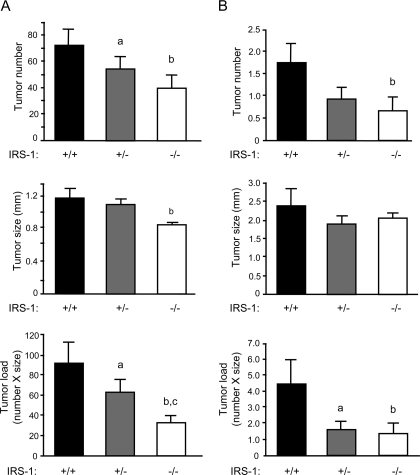
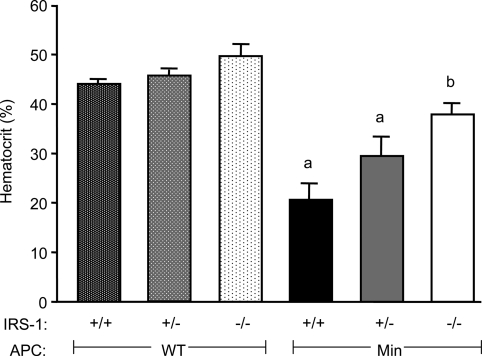
Apcmin/+ mice with disruption of just one IRS-1 allele (IRS-1+/−/Min) had a significant 24.5 ± 13.0% reduction in the number of small intestinal adenomas compared with sex-matched IRS-1+/+/Min littermates (P = 0.017, Figs. 2 and 3). This is despite the fact that loss of one IRS-1 allele has no discernible effect on growth or size of small intestine (15). IRS-1−/−/Min mice had a 55.5 ± 13.2% reduction in the number of small intestinal adenomas compared with IRS-1+/+/Min mice, an effect that is greater than the 25% reduction in small intestine mass observed in IRS-1−/− mice (15). In colon, IRS-1+/−/Min showed a 46.9 ± 15.2% decrease, and IRS-1−/−/Min a 61.9 + 19.0% decrease in adenoma number compared with IRS-1+/+/Min mice (Fig. 3). There was a small but significant decrease in adenoma size in small intestine of IRS-1−/−/Min vs. IRS-1+/+/Min, but adenoma size did not differ between IRS-1+/− and IRS-1+/+ mice. Partial and absolute IRS-1 deficiency significantly decreased tumor load (number × size) in both small intestine and colon (Fig. 3). Hematocrit is considered a useful indirect measure of tumor load in Apcmin/+ mice (45,46). Hematocrit was reduced in IRS-1+/+/Min and IRS-1+/−/Min mice compared with mice without the Apcmin/+ mutation (Fig. 4). In IRS-1−/−/Min mice, hematocrit was near normal and significantly higher than in IRS-1+/+/Min mice.
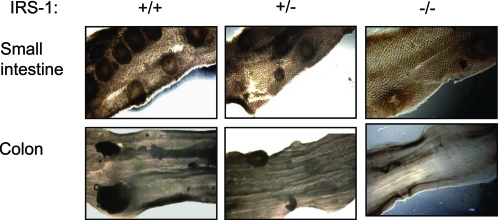
Figure 2.
Representative images of adenomas in small and large intestine of Apcmin/+ mice. Adenomas in small intestine (top) and colon (bottom) of Apcmin/+ mice with IRS-1+/+, IRS-1+/−, and IRS-1−/− genotypes are shown. Photographs were taken at ×12 under a dissecting scope with white-light optics underneath the specimen. Note the decrease in adenomas in IRS-1-deficient mice.
Figure 3.
Effects of IRS-1 gene disruption on tumor number, size, and load in Apcmin/+ mice. Histograms show mean tumor number, size, and load (number × size) in small intestine (A) and colon (B). n = 10 for IRS-1+/+/Min and IRS-1+/−/Min littermate pairs; n = 3 for IRS-1−/−/Min age-matched with IRS-1+/+/Min or IRS-1+/−/Min mice. a, P < 0.05 for IRS-1+/−/Min vs. IRS-1+/+/Min littermates; b and c, P < 0.05 for IRS-1−/−/Min vs. age-matched IRS-1+/+/Min or IRS-1+/−/Min mice, respectively.
Figure 4.
Effects of IRS-1 gene disruption on hematocrit in WT or Apcmin/+ mice. Histogram of whole-blood hematocrit from IRS-1+/+, IRS-1+/−, and IRS-1−/− mice with and without the Min mutation as indicated. All hematocrit values were at or near normal range in mice lacking the Min mutation (32.8–48.0% hematocrit). IRS-1+/+/Min and IRS-1+/−/Min mice had significantly decreased percent hematocrit levels, whereas IRS-1−/−/Min mice were within the normal range. a, P < 0.05 vs. all non-Min genotypes; b, P < 0.05 vs. IRS-1+/+/Min.
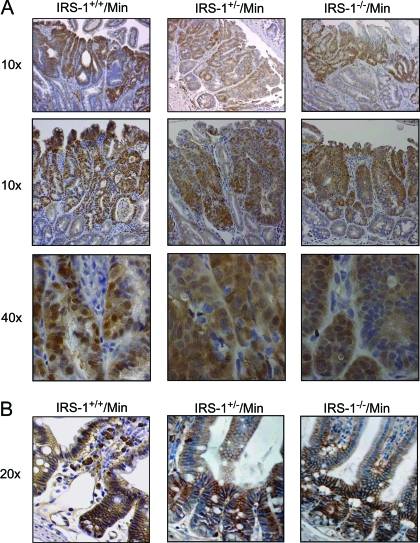
β-Catenin accumulation is a hallmark of adenomas in Apcmin/+ mice because loss of the second WT Apc allele leads to aberrant accumulation of β-catenin in the cytosol and nucleus. β-Catenin immunostaining revealed that β-catenin was localized to the intercellular junctions of normal crypts as previously reported (37) (Fig. 5). Tumor staining revealed accumulation of β-catenin in the cytoplasm and nucleus of adenomas of all genotypes (Fig. 5), confirming adenomatous phenotype.
Figure 5.
β-Catenin immunostaining of adenomas and normal mucosa. A, Images show small intestinal adenomas (low magnification at ×10 and higher magnification at ×40) in IRS-1+/+/Min, IRS-1+/−/Min, and IRS-1−/−/Min small intestine. Note the accumulated nuclear and cytoplasmic β-catenin in adenomas from all three genotypes, which is a hallmark of adenomas in the Apcmin/+ model. B, The normal mucosa (×20 magnification) shows prominent localization of β-catenin to lateral membranes, with little or no detectable immunostaining in the cytoplasm or nucleus.
Reductions in Sox9-positive cells and Sox9 expression in IRS-1-deficient mice
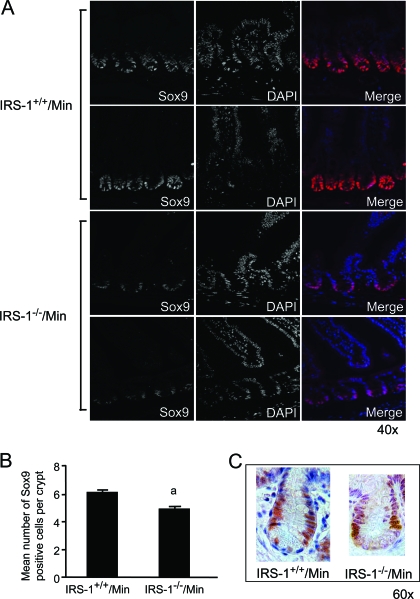
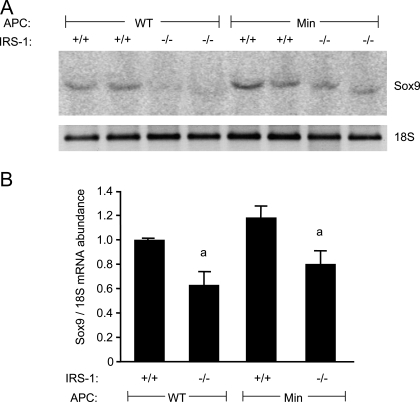
Sox9 is a reported biomarker of crypt stem or progenitor cells, is a target of β-catenin/TCF-activated transcription, and is expanded in human tumors. We therefore examined whether IRS-1 deficiency in Apcmin/+ mice reduced the number of Sox9-positive cells or Sox9 mRNA. As shown in Fig. 6A, Sox9 expression was restricted to cells at the base of normal crypts. In IRS-1−/−/Min crypts, we observed an obvious reduction in both the number and staining intensity of Sox9-positive cells, and cell number is quantified in Fig. 6B. Immunohistochemical Sox9 staining was examined to better visualize the location of Sox9-positive cells, which were found near the base of the crypts and do not appear to colocalize with granular Paneth cells at the crypt base (Fig. 6C). Evaluation of Sox9 mRNA in small intestine of IRS-1+/+ and IRS-1−/− mice with or without the Apcmin/+ mutation provided quantitative evidence that IRS-1 deficiency leads to significant reductions in Sox9 expression (Fig. 7, A and B).
Figure 6.
Decreased Sox9 expression with IRS-1 deficiency. A, Confocal images showing Sox9 (left) and 4′,6-diamino-2-phenylindole (DAPI) (middle) immunofluorescence and the merged images (right) in IRS-1+/+/Min and IRS-1−/−/Min small intestine (×40). Representative images are from two animals per genotype. B, Histogram shows the mean number of Sox9-positive stained cells in IRS-1+/+/Min vs. IRS-1−/−/Min small intestinal crypts. a, P < 0.05 vs. IRS-1+/+/Min. C, High-power bright-field images of IRS-1+/+/Min crypts (×60), showing the localization of Sox9 relative to the crypt base. Note that cells with obvious Sox9-positive nuclei appear distinct from the Paneth cells.
Figure 7.
Sox9 mRNA expression. A, Representative Northern blot of Sox9 and 18S RNAs in ileum of IRS-1+/+, IRS-1+/−, and IRS-1−/− with and without the Apcmin/+ mutation. B, Histograms show mean relative abundance of Sox9 mRNA normalized to the 18S loading control. a, P < 0.05 IRS-1−/− vs. IRS-1+/+ mice; n ≥ 4 for each genotype.
Sox9 expression correlates with IRS-1 levels in cultured cells
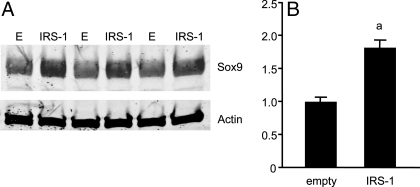
To examine more directly whether IRS-1 regulates Sox9 expression, Sox9 levels were examined in IEC-6 cells after infection with empty or IRS-1-expressing adenovirus. IEC-6 cells show constitutive Sox9 expression, and IRS-1 adenovirus significantly up-regulated Sox9 protein levels by 1.8 ± 0.1-fold (Fig. 8).
Figure 8.
Sox9 protein levels are regulated by IRS-1. A, Western immunoblot for Sox9 or β-actin in IEC-6 cells infected with empty virus (E) or IRS-1 adenovirus. B, Histogram shows Sox9 protein abundance for empty vs. IRS-1-infected IEC-6 cells normalized to actin levels. a, P < 0.05 vs. empty control; n = 6 for each adenovirus.
Discussion
Our studies provide novel evidence that intestinal crypt epithelial cells show gene dosage dependence on IRS-1 for protection from irradiation-induced apoptosis. This finding is noteworthy in light of recent evidence in humans that decreased levels of crypt apoptosis are associated with increased risk of intestinal adenoma (6), that high, but within the normal range, insulin levels correlate with both low apoptosis and adenoma risk (7), and that IRS-1 polymorphisms predict colon cancer risk (18). IRS-1+/− mice exhibit only about a 50% reduction in IRS-1 expression, and loss of one IRS-1 allele has little or no effect on body or intestinal growth (15,47). Thus, our findings that IRS-1+/− mice show significant increases in irradiation-induced apoptosis, with effects most prominent in the putative stem cell region, indicate that even small variations in levels of endogenous IRS-1 significantly impact on survival of genetically damaged stem cells.
Survival of genetically damaged stem or progenitor cells is considered a key early event in the development of intestinal tumors. Consistent with this concept, partial or absolute IRS-1 deficiency in mice carrying the Apcmin/+ mutation led to reduced tumor number relative to IRS-1+/+/Apcmin/+ mice. The more dramatic effect of IRS-1 deficiency on tumor number than size suggests a predominant role of IRS-1 in initiation or survival of early-stage tumors than rate of tumor growth. Our demonstration that reductions in endogenous IRS-1 limit tumor development is significant because most evidence linking IRS-1 and cancer involves overexpression systems and, with a few exceptions, largely in vitro studies. Given our findings in mouse models, it could be of considerable interest to establish whether the IRS-1 polymorphism linked to colon cancer risk (18) affects IRS-1 expression levels or whether patients with increased adenoma risk show high IRS-1 expression. Such studies to translate our basic science findings to the human population have been initiated in our laboratory.
Tumorigenesis mediated by the Apcmin/+ mutation is initiated by loss of the second functional Apc allele, leading to cytosolic and nuclear accumulation of β-catenin and activation of β-catenin-driven oncogenes (37). Emerging evidence based in colon cancer cell lines or based on overexpression of IRS-1 in mouse embryonic fibroblasts or mammary gland in vivo indicate that IGF/IRS-1 pathways stabilize or activate β-catenin and that there may be direct interactions between IRS-1 and β-catenin (22,23,24). Our findings that IRS-1 deficiency limits β-catenin-driven intestinal tumorigenesis in vivo provide, to our knowledge, the first evidence that endogenous IRS-1 impacts on tumor-promoting activity of β-catenin. Qualitatively, we noted that some intestinal adenomas of IRS-1−/−/Min mice appeared to show less intense or obvious nuclear β-catenin immunostaining than in IRS-1+/+/Min adenomas. This provides evidence that loss of endogenous IRS-1 may limit nuclear accumulation of β-catenin. Attempts to verify this by more quantitative biochemical assays proved problematic due to variability in total and nuclear β-catenin, even within genotypes.
More direct evidence for effects of IRS-1 on β-catenin transcription pathways stems from findings that expression of Sox9, a newly recognized β-catenin/Tcf target, is reduced in crypts of IRS-1-deficient Apcmin/+ mice in vivo and is induced in an IEC cell line by IRS-1 overexpression. These findings also provide novel evidence linking IRS-1 to Sox9. Sox9 is emerging as a potentially important biomarker of crypt stem or progenitor cells and belongs to a family of proteins known to maintain stem cells in an undifferentiated state (32). Recent studies show that inactivating the Sox9 gene at a developmental time point before enterocyte differentiation ablates Paneth cells and, to a lesser extent, goblet cells (48,49). These studies suggest that Sox9 may play an important role in stem or progenitor cell potency. Sox9 is also highly expressed in cells within human colon adenocarcinoma (32). Thus, our evidence that IRS-1 regulates Sox9 expression has important ramifications for how IGF/insulin signaling may regulate stem cell survival and cancer risk. The function of Sox9 in cancer is unknown, but one speculation is that Sox9 may mark cancer-associated stem cells. Thus, our findings indicate that the link between IRS-1 regulation of Sox9 and genetically damaged or cancer-associated stem cells will be an interesting avenue of future investigation. Furthermore, examining whether Sox9 expression correlates with apoptosis, adenoma risk, or alterations in insulin/IGFs and IRS-1 that predict adenoma risk in humans may provide new insights into the etiology of early-stage intestinal cancer.
Acknowledgments
We thank Dr. Kohjiro Ueki for providing the IRS-1 adenovirus and Dr. C. Ronald Kahn for provision of the IRS-1 heterozygote breeder pairs. We thank C. Randall Fuller for assistance with apoptosis assays. We acknowledge useful comments on this study from Dr. David Threadgill and Dr. Reade Roberts.
Footnotes
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-40247 (to P.K.L.), the National Cancer Institute Predoctoral Training Grant CA-72319 (to N.M.R.), the Seeding Postdoctoral Innovators in Research and Education Postdoctoral Grant GM000678 (to H.R.W.), the Center for Gastrointestinal Biology and Disease molecular histopathology core (P30-DK-34987), and the DNA synthesis core of the Lineberger Cancer Center (CA-16086).
All animal experimentation was conducted in accord with U.S. Public Health Service policy on humane care and use of laboratory animals and with approval from the Institutional Animal Care and Use Committee.
Disclosure Statement: N.M.R., H.R.W., S.T.M., G.H.L., J.G.S., B.P.S., and K.K.M. have nothing to declare. P.K.L. has previously consulted for Johns Hopkins University and consults for and has stock options with Saegis Pharmaceuticals.
First Published Online October 4, 2007
Abbreviations: IRS-1, Insulin receptor substrate-1; Tcf, T-cell factor; WT, wild type.
References
- Schier S, Wright NA 2005 Stem cell relationships and the origin of gastrointestinal cancer. Oncology 69(Suppl 1):9–13 [DOI] [PubMed] [Google Scholar]
- Bjerknes M, Cheng H 2005 Gastrointestinal stem cells. II. Intestinal stem cells. Am J Physiol Gastrointest Liver Physiol 289:G381–G387 [DOI] [PubMed] [Google Scholar]
- Potten CS 2004 Radiation, the ideal cytotoxic agent for studying the cell biology of tissues such as the small intestine. Radiat Res 161:123–136 [DOI] [PubMed] [Google Scholar]
- Bach SP, Renehan AG, Potten CS 2000 Stem cells: the intestinal stem cell as a paradigm. Carcinogenesis 21:469–476 [DOI] [PubMed] [Google Scholar]
- Wilkins HR, Ohneda K, Keku TO, D’Ercole AJ, Fuller CR, Williams KL, Lund PK 2002 Reduction of spontaneous and irradiation-induced apoptosis in small intestine of IGF-I transgenic mice. Am J Physiol Gastrointest Liver Physiol 283:G457–G464 [DOI] [PubMed] [Google Scholar]
- Martin C, Connelly A, Keku TO, Mountcastle SB, Galanko J, Woosley JT, Schliebe B, Lund PK, Sandler RS 2002 Nonsteroidal anti-inflammatory drugs, apoptosis, and colorectal adenomas. Gastroenterology 123:1770–1777 [DOI] [PubMed] [Google Scholar]
- Keku TO, Lund PK, Galanko J, Simmons JG, Woosley JT, Sandler RS 2005 Insulin resistance, apoptosis, and colorectal adenoma risk. Cancer Epidemiol Biomarkers Prev 14:2076–2081 [DOI] [PubMed] [Google Scholar]
- Woodson K, Flood A, Green L, Tangrea JA, Hanson J, Cash B, Schatzkin A, Schoenfeld P 2004 Loss of insulin-like growth factor-II imprinting and the presence of screen-detected colorectal adenomas in women. J Natl Cancer Inst 96:407–410 [DOI] [PubMed] [Google Scholar]
- Sakatani T, Kaneda A, Iacobuzio-Donahue CA, Carter MG, de Boom Witzel S, Okano H, Ko MS, Ohlsson R, Longo DL, Feinberg AP 2005 Loss of imprinting of Igf2 alters intestinal maturation and tumorigenesis in mice. Science 307:1976–1978 [DOI] [PubMed] [Google Scholar]
- Giovannucci E 2001 Insulin, insulin-like growth factors and colon cancer: a review of the evidence. J Nutr 131:3109S–3120S [DOI] [PubMed] [Google Scholar]
- Duran B 2005 The effects of long-term total parenteral nutrition on gut mucosal immunity in children with short bowel syndrome: a systematic review. BMC Nurs 4:2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogazzi F, Russo D, Locci MT, Chifenti B, Ultimieri F, Raggi F, Cosci C, Sardella C, Costa A, Gasperi M, Bartalena L, Martino E 2005 Apoptosis is reduced in the colonic mucosa of patients with acromegaly. Clin Endocrinol (Oxf) 63:683–688 [DOI] [PubMed] [Google Scholar]
- Riedemann J, Macaulay VM 2006 IGF1R signalling and its inhibition. Endocr Relat Cancer 13(Suppl 1):S33–S43 [DOI] [PubMed] [Google Scholar]
- White MF 2002 IRS proteins and the common path to diabetes. Am J Physiol Endocrinol Metab 283:E413–E422 [DOI] [PubMed] [Google Scholar]
- Pete G, Fuller CR, Oldham JM, Smith DR, D’Ercole AJ, Kahn CR, Lund PK 1999 Postnatal growth responses to insulin-like growth factor I in insulin receptor substrate-1-deficient mice. Endocrinology 140:5478–5487 [DOI] [PubMed] [Google Scholar]
- Araki E, Lipes MA, Patti ME, Bruning JC, Haag 3rd B, Johnson RS, Kahn CR 1994 Alternative pathway of insulin signalling in mice with targeted disruption of the IRS-1 gene. Nature 372:186–190 [DOI] [PubMed] [Google Scholar]
- White MF 2003 Insulin signaling in health and disease. Science 302:1710–1711 [DOI] [PubMed] [Google Scholar]
- Slattery ML, Samowitz W, Curtin K, Ma KN, Hoffman M, Caan B, Neuhausen S 2004 Associations among IRS1, IRS2, IGF1, and IGFBP3 genetic polymorphisms and colorectal cancer. Cancer Epidemiol Biomarkers Prev 13:1206–1214 [PubMed] [Google Scholar]
- Chang Q, Li Y, White MF, Fletcher JA, Xiao S 2002 Constitutive activation of insulin receptor substrate 1 is a frequent event in human tumors: therapeutic implications. Cancer Res 62:6035–6038 [PubMed] [Google Scholar]
- Nishiyama M, Wands JR 1992 Cloning and increased expression of an insulin receptor substrate-1-like gene in human hepatocellular carcinoma. Biochem Biophys Res Commun 183:280–285 [DOI] [PubMed] [Google Scholar]
- Koda M, Sulkowska M, Kanczuga-Koda L, Sulkowski S 2005 Expression of insulin receptor substrate 1 in primary breast cancer and lymph node metastases. J Clin Pathol 58:645–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dearth RK, Cui X, Kim HJ, Kuiatse I, Lawrence NA, Zhang X, Divisova J, Britton OL, Mohsin S, Allred DC, Hadsell DL, Lee AV 2006 Mammary tumorigenesis and metastasis caused by overexpression of insulin receptor substrate 1 (IRS-1) or IRS-2. Mol Cell Biol 26:9302–9314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Playford MP, Bicknell D, Bodmer WF, Macaulay VM 2000 Insulin-like growth factor 1 regulates the location, stability, and transcriptional activity of β-catenin. Proc Natl Acad Sci USA 97:12103–12108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Wu A, Sun H, Drakas R, Garofalo C, Cascio S, Surmacz E, Baserga R 2005 Functional significance of type 1 insulin-like growth factor-mediated nuclear translocation of the insulin receptor substrate-1 and β-catenin. J Biol Chem 280:29912–29920 [DOI] [PubMed] [Google Scholar]
- Wu A, Chen J, Baserga R 2007 Nuclear insulin receptor substrate-1 activates promoters of cell cycle progression genes. Oncogene [DOI] [PubMed] [Google Scholar]
- Yamada Y, Mori H 2003 Pre-cancerous lesions for colorectal cancers in rodents: a new concept. Carcinogenesis 24:1015–1019 [DOI] [PubMed] [Google Scholar]
- Moser AR, Hegge LF, Cardiff RD 2001 Genetic background affects susceptibility to mammary hyperplasias and carcinomas in Apcmin/+ mice. Cancer Res 61:3480–3485 [PubMed] [Google Scholar]
- Yang J, Zhang W, Evans PM, Chen X, He X, Liu C 2006 Adenomatous polyposis coli (APC) differentially regulates β-catenin phosphorylation and ubiquitination in colon cancer cells. J Biol Chem 281:17751–17757 [DOI] [PubMed] [Google Scholar]
- Hu R, Khor TO, Shen G, Jeong WS, Hebbar V, Chen C, Xu C, Reddy B, Chada K, Kong AN 2006 Cancer chemoprevention of intestinal polyposis in ApcMin/+ mice by sulforaphane, a natural product derived from cruciferous vegetable. Carcinogenesis 27:2038–2046 [DOI] [PubMed] [Google Scholar]
- Lynn FC, Smith SB, Wilson ME, Yang KY, Nekrep N, German MS 2007 Sox9 coordinates a transcriptional network in pancreatic progenitor cells. Proc Natl Acad Sci USA 104:10500–10505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymour PA, Freude KK, Tran MN, Mayes EE, Jensen J, Kist R, Scherer G, Sander M 2007 SOX9 is required for maintenance of the pancreatic progenitor cell pool. Proc Natl Acad Sci USA 104:1865–1870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blache P, van de Wetering M, Duluc I, Domon C, Berta P, Freund JN, Clevers H, Jay P 2004 SOX9 is an intestine crypt transcription factor, is regulated by the Wnt pathway, and represses the CDX2 and MUC2 genes. J Cell Biol 166:37–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung M, Briscoe J 2003 Neural crest development is regulated by the transcription factor Sox9. Development 130:5681–5693 [DOI] [PubMed] [Google Scholar]
- Akiyama Ddagger H, Kim Ddagger JE, Nakashima K, Balmes G, Iwai N, Deng JM, Zhang Z, Martin JF, Behringer RR, Nakamura T, de Crombrugghe B 2005 Osteo-chondroprogenitor cells are derived from Sox9 expressing precursors. Proc Natl Acad Sci USA 102:14665–14670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaboissier MC, Kobayashi A, Vidal VI, Lutzkendorf S, van de Kant HJ, Wegner M, de Rooij DG, Behringer RR, Schedl A 2004 Functional analysis of Sox8 and Sox9 during sex determination in the mouse. Development 131:1891–1901 [DOI] [PubMed] [Google Scholar]
- Shakibaei M, Seifarth C, John T, Rahmanzadeh M, Mobasheri A 2006 Igf-I extends the chondrogenic potential of human articular chondrocytes in vitro: molecular association between Sox9 and Erk1/2. Biochem Pharmacol 72:1382–1395 [DOI] [PubMed] [Google Scholar]
- Roberts RB, Min L, Washington MK, Olsen SJ, Settle SH, Coffey RJ, Threadgill DW 2002 Importance of epidermal growth factor receptor signaling in establishment of adenomas and maintenance of carcinomas during intestinal tumorigenesis. Proc Natl Acad Sci USA 99:1521–1526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potten CS 1992 The significance of spontaneous and induced apoptosis in the gastrointestinal tract of mice. Cancer Metastasis Rev 11:179–195 [DOI] [PubMed] [Google Scholar]
- Potten CS 1996 Protection of the small intestinal clonogenic stem cells from radiation-induced damage by pretreatment with interleukin 11 also increases murine survival time. Stem Cells 14:452–459 [DOI] [PubMed] [Google Scholar]
- Hassan AB, Howell JA 2000 Insulin-like growth factor II supply modifies growth of intestinal adenoma in ApcMin/+ mice. Cancer Res 60:1070–1076 [PubMed] [Google Scholar]
- Ohneda K, Ulshen MH, Fuller CR, D’Ercole AJ, Lund PK 1997 Enhanced growth of small bowel in transgenic mice expressing human insulin-like growth factor I. Gastroenterology 112:444–454 [DOI] [PubMed] [Google Scholar]
- Miller ME, Michaylira CZ, Simmons JG, Ney DM, Dahly EM, Heath JK, Lund PK 2004 Suppressor of cytokine signaling-2: a growth hormone-inducible inhibitor of intestinal epithelial cell proliferation. Gastroenterology 127:570–581 [DOI] [PubMed] [Google Scholar]
- Ueki K, Yamauchi T, Tamemoto H, Tobe K, Yamamoto-Honda R, Kaburagi Y, Akanuma Y, Yazaki Y, Aizawa S, Nagai R, Kadowaki T 2000 Restored insulin-sensitivity in IRS-1-deficient mice treated by adenovirus-mediated gene therapy. J Clin Invest 105:1437–1445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons JG, Pucilowska JB, Keku TO, Lund PK 2002 IGF-I and TGF-β1 have distinct effects on phenotype and proliferation of intestinal fibroblasts. Am J Physiol Gastrointest Liver Physiol 283:G809–G818 [DOI] [PubMed] [Google Scholar]
- Perkins S, Verschoyle RD, Hill K, Parveen I, Threadgill MD, Sharma RA, Williams ML, Steward WP, Gescher AJ 2002 Chemopreventive efficacy and pharmacokinetics of curcumin in the min/+ mouse, a model of familial adenomatous polyposis. Cancer Epidemiol Biomarkers Prev 11:535–540 [PubMed] [Google Scholar]
- Wechter WJ, Murray Jr ED, Kantoci D, Quiggle DD, Leipold DD, Gibson KM, McCracken JD 2000 Treatment and survival study in the C57BL/6J-APCMin/+(Min) mouse with R-flurbiprofen. Life Sci 66:745–753 [DOI] [PubMed] [Google Scholar]
- Shirakami A, Toyonaga T, Tsuruzoe K, Shirotani T, Matsumoto K, Yoshizato K, Kawashima J, Hirashima Y, Miyamura N, Kahn CR, Araki E 2002 Heterozygous knockout of the IRS-1 gene in mice enhances obesity-linked insulin resistance: a possible model for the development of type 2 diabetes. J Endocrinol 174:309–319 [DOI] [PubMed] [Google Scholar]
- Bastide P, Darido C, Pannequin J, Kist R, Robine S, Marty-Double C, Bibeau F, Scherer G, Joubert D, Hollande F, Blache P, Jay P 2007 Sox9 regulates cell proliferation and is required for Paneth cell differentiation in the intestinal epithelium. J Cell Biol 178:635–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori-Akiyama Y, van den Born M, van Es JH, Hamilton SR, Adams HP, Zhang J, Clevers H, de Crombrugghe B 2007 SOX9 is required for the differentiation of Paneth cells in the intestinal epithelium. Gastroenterology 133:539–546 [DOI] [PubMed] [Google Scholar]