Abstract
We previously described a colocalization between arginine vasopressin (AVP) and the chemokine stromal cell-derived factor-1α (SDF-1) in the magnocellular neurons of both the hypothalamic supraoptic and paraventricular nucleus as well as the posterior pituitary. SDF-1 physiologically affects the electrophysiological properties of AVP neurons and consequently AVP release. In the present study, we confirm by confocal and electron microscopy that AVP and SDF-1 have a similar cellular distribution inside the neuronal cell and can be found in dense core vesicles in the nerve terminals in the posterior pituitary. Because the Brattleboro rats represent a good model of AVP deficiency, we tested in these animals the fate of SDF-1 and its receptor CXCR4. We identified by immunohistochemistry that both SDF-1 and CXCR4 immunoreactivity were strongly decreased in Brattleboro rats and were strictly correlated with the expression of AVP protein in supraoptic nucleus, paraventricular nucleus, and the posterior pituitary. We observed by real-time PCR an increase in SDF-1 mRNA in both heterozygous and homozygous rats. The effect on the SDF-1/CXCR4 system was not linked to peripheral modifications of kidney water balance because it could not be restored by chronic infusion of deamino-8D-ariginine-vasopressin, an AVP V2-receptor agonist. These original data further suggest that SDF-1 may play an essential role in the regulation of water balance.
THE NONAPEPTIDE ARGININE vasopressin (AVP), also known as antidiuretic hormone, is synthesized by the magnocellular neurons of the hypothalamic supraoptic nucleus (SON) and paraventricular nucleus (PVN) and transported via the median eminence to the posterior lobe of the pituitary gland, in which it is secreted into the general blood circulation (1). AVP plays a major role in the regulation of body fluid homeostasis. Thus, AVP is responsible for regulating water absorption in the distal nephron of the kidney. The absence of AVP leads to diabetes insipidus characterized by an excretion of large volumes of dilute urine and consequently to an increased fluid intake. This human disease can be studied in an animal model, the Brattleboro rat (2).
The homozygous Brattleboro rat (DI) is a mutant form of the Long Evans rat (LE) strain that suffers from a polyuropolydipsic syndrome (3). This is due to a genetic inability to produce cerebral functional AVP because of a single base deletion in the gene encoding AVP (4). The DI rat hypothalamus contains a large quantity of the AVP gene product, but the normal mRNA translation is blocked by a frame shift in the C-terminal region of the AVP precursor (5). The AVP-deficient neurons of the hypothalamo-neurohypophyseal system of DI rats are continuously stimulated (6). On the other hand, the heterozygous Brattleboro rat (HZ) has a partial deficit in the synthesis (3) and release of AVP (7,8).
Chemokines are small secreted proteins (7–14 kDa) with chemoattractant properties whose main accepted role is leukocyte recruitment in inflammatory sites (9,10). Recent investigations into the possible involvement of chemokines in a number of neurological disorders associated with neuroinflammation have led to the possibility that many chemokines and their respective receptors can be expressed in the brain (11,12). Recent data demonstrated that they are not only observed in neuroinflammatory conditions but also can be constitutively expressed by different cell types, including neurons, in the normal brain (12) in which they act as modulators of neuronal and neuroendocrine functions (13). Thus, we recently demonstrated that the chemokine stromal cell-derived factor-1 (SDF-1/CXCL12) and its receptor CXCR4 are selectively colocalized with AVP-expressing neurons in both SON and PVN as well as in the median eminence and the AVP-positive nerve terminals in the posterior pituitary (14,15). We reported that SDF-1 via its receptor CXCR4 affects the electrical activity of AVP neurons and inhibits plasma AVP release induced by angiotensin II or osmotic shock in vivo (15). Finally, a change in water balance by long-term salt loading induces a decrease in both SDF-1 and CXCR4 immunoreactivity parallel to that of AVP immunostaining in both SON and PVN (15).
The aim of the present work was first to further study the colocalization of SDF-1 and AVP at the subcellular level in the rat hypothalamus and posterior pituitary and second, to investigate the expression of the chemokine SDF-1 and its receptor CXCR4 in the hypothalamo-neurohypophyseal system of the DI and HZ rats in an attempt to clarify the interaction between AVP and SDF-1 in the regulation of body fluid homeostasis.
Materials and Methods
Animals
Male LE rats were provided by CERJ (Le Genest, France) and male HZ and DI rats were bred in the laboratory of A. Burlet (Nancy, France). The rats were bred in the local animal facilities and maintained on a 12-h dark, 12-h light cycle (0700/1900 h) with food and water ad libitum. All the protocols were carried out in accordance with French standard ethical guidelines for laboratory animals (Agreement 75-178, 05/16/2000).
Drugs and antibodies
The polyclonal goat antibody against the SDF-1 was obtained from Santa Cruz Biotechnology (catalog no. sc-6193; Santa Cruz, CA). This affinity-purified antibody, raised against a peptide mapping at the carboxyl terminus (C-19) of human SDF-1, cross-reacts totally with murine and rat SDF-1; its specificity for immunohistochemistry on rat brain tissues was previously described (14). The polyclonal goat antibody against CXCR4 was obtained from Santa Cruz Biotechnology (catalog no. sc-6190). This affinity-purified antibody, raised against a peptide mapping at the carboxyl terminus (C-20) of human CXCR4 cross-reacts totally with murine and rat CXCR4; its specificity for immunohistochemistry on rat brain tissues was previously tested (16). Murine anti-AVP antibody for immunohistochemistry was a gift from A. Burlet (Centre National de la Recherche Scientifique, Nancy, France). Rabbit anti-AVP antibody used for electron microscopy was a gift from G. Alonso (Montpellier, France). The deamino-8D-arginine-vasopressin (dDAVP; Minirin) was purchased from Sandoz (Basel, Switzerland).
Tissue preparation and immunohistochemistry
Rats were anesthetized with sodium pentobarbital (70 mg/kg, ip; Sanofi, Libourne, France) and perfused transcardially with heparin (1000 U/ml) in physiological saline, followed by 400–500 ml of a freshly prepared solution of 4% (wt/vol) paraformaldehyde (PFA) in PBS-saturated picric acid (1 vol/2 vol). The brains and pituitaries were rapidly removed and postfixed overnight in 4% PFA-picric acid at 4 C. Pituitaries were then embedded in 30% (wt/vol) gelatin. Forty-micrometer-thick coronal sections of both anterior hypothalamus and pituitaries were cut with a Vibratome (VT 1000S; Leica Microsystems, Nussloch, Germany). Pituitary sections were mounted on glass slices, whereas brain free-floating sections were collected in cold PBS.
Briefly, sections from both brain and pituitary tissues were pretreated for 20 min with 3% hydrogen peroxide in PBS to quench endogenous peroxidase. They were then washed with PBS (3 × 10 min) and preincubated for 90 min at room temperature in PBS containing 0.1% Triton X-100, 3% normal horse serum, and 3% donkey serum. Then sections were incubated 36 h at 4 C simultaneously with goat anti-SDF-1 antibody (1:100 dilution) or goat anti-CXCR4 antibody (1:100 dilution) and mouse anti-AVP antibody (1:5000 dilution). After incubation, sections were rinsed extensively (six times for 10 min each) with PBS and incubated at room temperature for 90 min in a 1:100 dilution of biotin-conjugated horse antigoat antibody (Vector, Burlingame, CA) used against the anti-SDF-1 or the anti-CXCR4 antibody. Texas Red-conjugated donkey antimouse antibody (1:200 dilution; Jackson ImmunoResearch, West Grove, PA) was used as secondary antibody against the anti-AVP antibody. Then, sections were washed with PBS (three times for 10 min each). For signal amplification, a tyramide signal amplification fluorescence procedure (PerkinElmer-NEN Life Science Products, Boston, MA) was used. Briefly, sections were incubated for 30 min in a 1:100 dilution of streptavidin-horseradish peroxidase-conjugated (Amersham Pharmacia Biotech, Little Chalfont, UK). They were then washed extensively with PBS and incubated in a 1:50 dilution of fluorescein isothiocyanate-conjugated Tyramide (PerkinElmer-NEN Life Science Products) for 5 min. After washing, free-floating sections were mounted onto gelatin-coated slices. All sections were mounted in Vectashield (Vector) and observed on a fluorescent microscope (BX 61; Olympus, Melville, NY). Pictures were taken by using a digital camera (DP 50; Olympus) and a connected image-acquisition software (Analysis, Münster, Germany) was used. Quantitation of neuronal immunostaining was carried out using Image J program (National Institutes of Health, Bethesda, MD)
Some tissues obtained as described above were examined by confocal microscopy to obtain a cellular localization of the staining. Sections of the SON, PVN, and posterior pituitary were examined using a TCS SP Leica confocal 3-laser scanning microscope (Lasertechnik GmbH, Goexe, Germany). For each optical section, dual fluorescence was acquired simultaneously using a krypton-argon mixed-gas laser adjusted to 488 nm for fluorescein isothiocyanate and to 568 nm for Texas Red.
Subcellular fractionation
Anesthetized rats were perfused transcardially with chilled saline solution. Pituitaries were rapidly removed. Nine posterior pituitaries were homogenized in homogeneization buffer [4 mm HEPES with 0.32 m sucrose (pH 7.4) containing aprotinin (10 ng/μl) and phenylmethylsulfonyl fluoride (100 ng/μl)]. The homogenate was centrifuged 10 min at 1000 × g to separate a pellet P1 containing nuclei, myelin, blood cells, and unlysed fragments and a supernatant S1 containing cell components (microsomes, cytosol, mitochondria, synaptosomes, and membranes). The supernatant S1 was centrifuged 20 min at 10,000 × g to individualize S2 (microsomes and cytosol) from P2 (synaptosomes and mitochondria). The crude synaptosomal pellet P2 was resuspended in homogeneization buffer and completed with an equal volume of distilled water. The resulting suspension was centrifuged on a linear sucrose gradient according to Navone et al. (17) with minor modifications. Gradient consisted of nine layers of sucrose between 0.6 and 2.5 m, and centrifugation was performed for 3 h at 65,000 × g in an ultracentrifuge (L-70, rotor SW 55Ti; Beckman, Palo Alto, CA). All sucrose solutions were prepared in 4 mm HEPES buffer (pH 7.4). After centrifugation, nine fractions of 300 μl were collected and stored at −20 C until use. Before ELISA, Triton X-100 (0.5% final) was added in each fraction and fractions were sonicated 5 min in a bath. All steps of fractionation were performed at 4 C.
SDF-1 was quantified using a DuoSet ELISA development kit (R&D Systems, Minneapolis, MN) according to the manufacturer-recommended procedure. AVP was measured by means of an Arg8-vasopressin ELISA kit (Assay Designs, Ann Arbor, MI) according to advised protocol. Proteins in each fraction were determined by a DC protein assay kit (Bio-Rad, Marnes la Coquette, France).
Electron microscopy of posterior pituitaries
Anesthetized male rats were perfused transcardially with heparin in physiological saline, followed by a freshly prepared solution of 4% (wt/vol) PFA. The posterior pituitaries were immediately removed and postfixed overnight in 4% PFA at 4 C. The neuronal lobe was dehydrated at room temperature in absolute ethanol for 1 h and then in 1:1 LRW hard grade acrylic resin (Pelanne Instruments, Toulouse, France) in absolute ethanol for 2 h, 2:1 LRW/absolute ethanol during 1 h, and LRW overnight at 4 C. Finally the posterior pituitaries was embedded in resin LRW and polymerized at 50 C. Ultrathin sections (70–80 nm) from LRW embedded blocks were collected onto precleaned uncoated nickel or gold grids (200 mesh) and then proceeded for immunogold immunohistochemistry as previously described (18,19).
All solutions were filtered with disposable filters (0.22 μm; Millipore, Bedford, MA). Grids were incubated on drops (20 μl) of solutions. For double-immunogold immunohistochemistry, grids were incubated separately for each antibody on the same side. Grids were floated on drops of a NH4Cl (50 mm) in Tris-buffered saline (TBS) buffer [Tris-HCl 50 mm (pH 7.4) with NaCl 150 mm] 15 min at room temperature. Grids were incubated for 1 h at room temperature in TBS/1% BSA/0.1% Triton X-100. They were transferred onto drops of primary rabbit antiserum anti-AVP diluted 1:1000 in TBS/1% BSA/0.1% Triton X-100 and incubated overnight in a wet chamber at room temperature. The grids were thoroughly washed in TBS/1% BSA (three times for 5 min each) and incubated at room temperature for 2 h with a secondary goat antirabbit antiserum coupled with 10 nm gold particles diluted 1:100 in TBS/1% BSA. Grids were washed in TBS/1% BSA (three times for 5 min each) and fixed with PFA 4% in PBS buffer 0.1 m for 2 min and rinsed in TBS/1%BSA (three times for 5 min each). Grids were then incubated for 1 h in TBS/1% BSA/0.1% Triton X-100. The second primary antiserum anti-SDF-1, as described above, was diluted 1:25 in TBS/1% BSA/0.1% Triton X-100 and incubated overnight in a wet chamber at room temperature. Grids were thoroughly washed in TBS/1% BSA (three times for 5 min each) and incubated at room temperature for 2 h with the secondary antiserum Protein A Gold 20 nm diluted 1:40 in TBS/1% BSA. Grids were washed in TBS/1% BSA (three times for 5 min each) and then fixed with PFA 4% in PBS 0.1 m for 2 min. Grids were then rinsed in TBS/1% BSA (three times for 5 min each) and 5 min in double-distilled water and then allowed to dry protected from dust. Grids were counterstained with 1:1 uranyl acetate/absolute ethanol for 5 min and washed again in 1:1 ethanol/ double-distilled water and dry protected from dust before observation at 80 KV with a JEM-×100 electron microscope (Jeol, Peabody, MA). Control grids without primary antisera do not show any gold particles.
RNA extraction, reverse transcription (RT), and quantitative real-time PCR
Under sodium pentobarbital anesthesia, rats were perfused transcardially with chilled saline solution. Tissues were rapidly removed and immediately frozen on dry ice and stored at −80 C. RNA was isolated from punched hypothalamic PVN, SON (20), and the posterior pituitary. RNA was purified using an RNA II extraction kit (Macherey-Nagel, Hoerdt, France) according to the advised procedure. RNA concentration was measured spectrophotometrically at the wavelength of 260 nm. RT and quantitative PCR were performed using Absolute Max 2-Step QRT-PCR kit (catalog no. AB-1269; ABgene, Surrey, UK) according to the manufacturer’s protocol. RNA was reverse transcribed (250 ng) in a 20-μl RT reaction. Reaction mixtures were incubated at 42 C for 60 min and 10 min at 75 C. Quantitative real-time PCR was carried out in the Applied Biosystems 7300 Real-Time PCR system (Applied Biosystems, Courtaboeuf, France) using TaqMan gene expression assays for SDF-1 (assay ID Rn00573260_m1), CXCR4 (assay ID Rn01483207_m1), AVP (assay ID Rn00566449_m1), and 18S (assay ID Rn01428915_g1). The 18S rRNA was used to normalize cDNA concentration. The quantitative PCR thermal cycling consisted of a preincubation step at 95 C for 15 min, followed by 40 cycles of denaturation (95 C, 15 sec) and annealing and extension (60 C, 1 min). To determine the copy number of cDNA of each sample, the RT samples were amplified and the each signal compared with that of standard curve run on the same plate. Standard series consisted of 100, 101, 102, 103, and 104 copies of a sample cDNA.
Homozygous Brattleboro treatment with dDAVP
DI rats were isolated in metabolic cages to measure fluid intake and to collect urine, 5 d before treatment. They received, under anesthesia, one minipump (Alzet, Cupertino, CA; delivering 0.25 μl/h) loaded with dDAVP (20 μg/ml, Minirin; Sandoz) diluted in isotonic saline and placed under the neck skin. Controls received one minipump loaded with isotonic saline only. Measurements of diuresis and fluid intake were performed daily, and urine osmolality was measured in urine collected for 5 h during the light period. Ten days after pump implantation, the rats were perfused transcardially with heparin (1000 U/ml) in physiological saline, followed by PFA 4% in PBS (pH 7.4) as described previously in tissue preparation and immunohistochemistry section.
Statistical analysis
Data are given as mean ± sem. For the treatment of DI rats with dDAVP, values from control (DI rats) and experimental animals (DI treated with dDAVP) were compared using an unpaired Student’s t test. Differences of P < 0.05 were considered significant.
For quantitative real-time PCR and quantitation of immunostaining, a one-way ANOVA test was used to analyze the differences between groups, followed by a protected least significant difference Fisher post hoc test with a threshold of significance of P < 0.05, P < 0.01, and P < 0.001 using a statistical software package (Statview 5 software; Abacus Concepts, Palo Alto, CA).
Results
Expression of SDF-1 in control rats
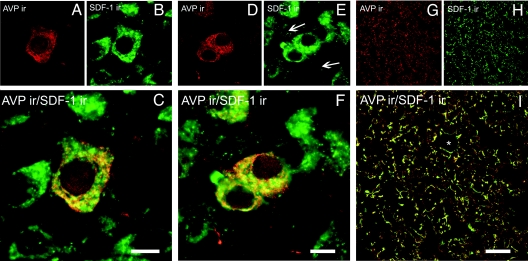
Confocal microscopic images show that in control rats, SDF-1 is constitutively expressed in the magnocellular neurons of the PVN (Fig. 1B) and SON (Fig. 1E). In both nuclei SDF-1 staining is observed in several AVP-immunoreactive perikarya (Fig. 1, A and D) and presents a punctuate labeling. The SDF-1 fluorescent clusters are preferentially distributed inside the cytoplasm mainly in a perinuclear localization, whereas AVP immunoreactivity is homogeneously concentrated as small dots throughout the cytoplasm (Fig. 1, C and F). The majority of SDF-1 staining overlaps with AVP, suggesting a very similar subcellular distribution. In the posterior pituitary, the localization of both SDF-1 (Fig. 1H) and AVP (Fig. 1G) staining is discontinuous all along fiber-like processes and swellings, which in some cases surrounded blood vessels. Here, too, both SDF-1 and AVP staining overlap (Fig. 1I).
Figure 1.
Confocal microscopic images of sections from the PVN (A–C), SON (D–F), and posterior pituitary (G–I) of control (LE) rats immunolabeled with SDF-1 (green) and vasopressin (red). Dually labeled neurons stand out in yellow when images are merged. *, Blood vessels; ←, dendrite processes. Scale bars, 10 μm (PVN and SON); 50 μm (posterior pituitary).
Subcellular localization of SDF-1 in the posterior pituitary
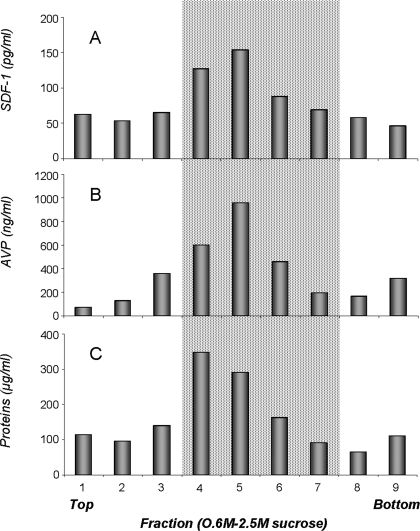
According to the confocal analysis, it was of interest to further investigate the relationship between SDF-1 and AVP. For that purpose, we carried out subcellular localization by means of subcellular fractionation and electron microscopic examination of posterior pituitaries from normal rats. Figure 2A shows that SDF-1 is differentially distributed in the various sucrose density fractions. The highest amount of SDF-1 is detected in fractions 4–7 corresponding to sucrose concentrations from 1.2 to 1.8 m. These fractions are associated with vesicular neurosecretory granules (17) and the dense protein-rich peak (Fig. 2C). The relative distribution of SDF-1 is similar to that of AVP (Fig. 2B). Because it is known that AVP is stored in posterior pituitary in large dense core vesicles, it suggests that SDF-1 can be localized in such vesicles.
Figure 2.
Subcellular fractionation from posterior pituitaries in an equilibrium sucrose gradient. ELISAs are used to detect the subcellular distribution of SDF-1 (A) and AVP (B). The highest amount of SDF-1 is found in fractions corresponding to large dense core vesicles (gray area fraction 4: 1.2 m sucrose to fraction 7: 1.8 m sucrose), identified by both AVP (B) and protein quantification (C).
To answer that question, we carried out electron microscope immunohistochemistry of posterior pituitaries. As shown in Fig. 3, immunohistochemical labeling demonstrates a profuse distribution of small gold particles corresponding to AVP on neurosecretory granules in nerve terminals. Fibers abut on basal lamina in the vicinity of blood vessels. Double-immunohistochemical labeling shows, together with numerous small 10-nm gold particles corresponding to AVP, scarce large 20-nm gold particles also localized on dense-core vesicles. Furthermore, whereas AVP small gold particles are randomly distributed on the whole granule, the SDF-1 gold particles, although rare in number, are invariably localized on the periphery of the AVP dense core vesicles.
Figure 3.
Electron microscopic examination of ultrathin section of rat posterior pituitary treated with antibodies against AVP and SDF-1 revealed by secondary antibodies coupled to 10- and 20-nm gold particles, respectively. Arrows indicate double-labeled secretory granules, which are enlarged in insets. Neurosecretory axon terminals abut on a perivascular space (PVS). Magnification, ×28,000. Scale bar, 1.4 cm = 0.5 μm.
Expression of SDF-1 and CXCR4 receptor in HZ and DI rats
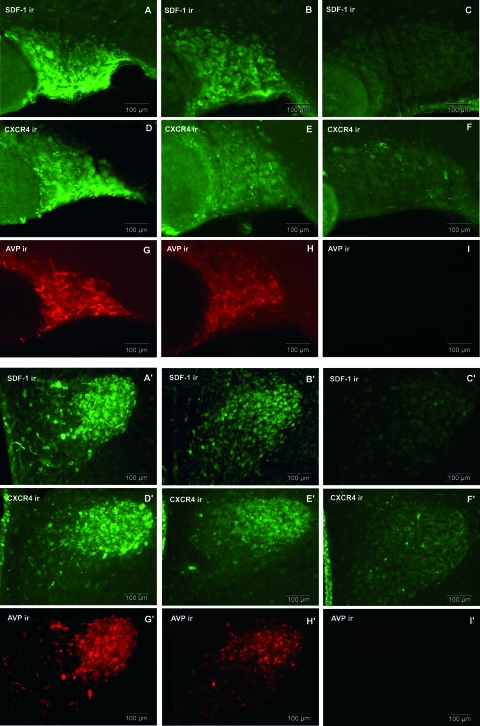
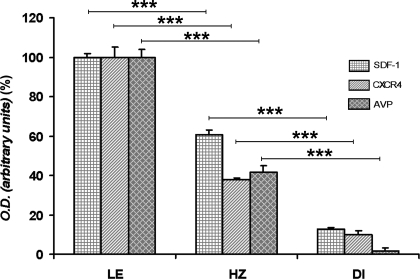
If SDF-1 and AVP are found in the same vesicles, it is possible that both substances can be regulated in the same manner. In HZ rat that presents a partial deficit in the synthesis of AVP, SDF-1 immunoreactivity is partially decreased in SON and PVN, compared with their expression in LE rats (Fig. 4, A and B and A′ and B′). This decrease in SDF-1 immunoreactivity in both SON and PVN is proportional to the decrease of AVP immunostaining in HZ animals as compared with LE rats (Fig. 4, G and H and G′ and H′). Quantitative analysis of the intensity of SDF-1 immunoreactivity carried out within SON revealed a 40% decrease for SDF-1 immunostaining in HZ rats, compared with the LE rats (Fig. 5).
Figure 4.
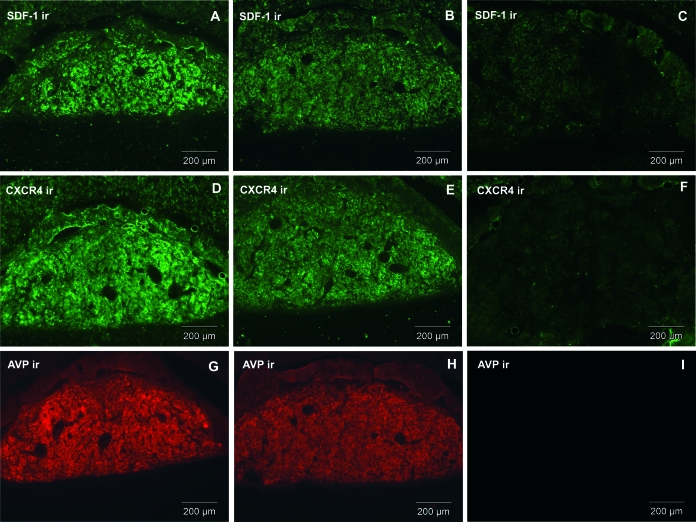
Upper panel, Expression of SDF-1 and CXCR4 in SON of HZ and DI rats, compared with LE rats. SDF-1 and CXCR4, which are colocalized with AVP in control SON, partially decrease in HZ animal as does AVP (B, E, and H). SDF-1, CXCR4, and AVP in DI rats are dramatically decreased (C, F, and I), compared with control LE (A, D, and G). LE, HZ, and DI pictures were taken at the same intensity. Bottom panel, Expression of SDF-1 and CXCR4 in PVN of HZ and DI rats, compared with LE rats. SDF-1 and CXCR4, which are colocalized with AVP in control PVN, partially decrease in HZ animals as does AVP (B′, E′, and H′). SDF-1, CXCR4, and AVP in DI rats are dramatically decreased (C′, F′, and I′), compared with control LE (A′, D′, and G′). LE, HZ, and DI pictures were taken at the same intensity. Similar data were obtained with the other animals tested (n = 6 rats/group). ir, Immunoreactivity. Scale bars, 100 μm.
Figure 5.
Quantitative analysis of SDF-1, CXCR4, and AVP immunoreactivity in the SON of LE, HZ, and DI rats. Values are mean ± sem of four values for each group. ***, P < 0.001.
Similarly to what we observed for SDF-1, the CXCR4 receptor immunoreactivity is decreased by 60% in SON (Figs. 4, D and E, and 5) and PVN (Fig. 4, D′ and E′) in HZ rats.
As for HZ animals, the level of SDF-1 and CXCR4 proteins is strongly decreased by 90% in SON (Fig. 5) and PVN of DI rat as compared with LE rats (Fig. 4, A and D, for LE vs. Fig. 4, C and F, for DI in SON and Fig. 4, A′ and D′, for LE vs. Fig. 4, C′ and F′, for DI in PVN). Furthermore, this dramatic decrease in SDF-1 and CXCR4 immunostaining is strictly correlated with the disappearance of AVP in DI animals (Figs. 4, I and I′, and 5).
Similar decrease in SDF-1 and CXCR4 immunostaining was observed in the posterior pituitary of Brattleboro rats (Fig. 6). Indeed, Fig. 6, B and E, shows that in HZ animals, SDF-1 and CXCR4 immunostaining is partially decreased as compared with LE rats (Fig. 6, A and D) and correlates with AVP level (Fig 6, G and H). A more significant decrease (90%) of SDF-1 and CXCR4 can be observed in the posterior pituitary of DI rats (Fig. 6, C and F). This decrease is, once again, in strict correlation with that of AVP immunostaining in the posterior pituitary of DI animals (Fig. 6I).
Figure 6.
Expression of SDF-1, CXCR4, and AVP in posterior pituitary of HZ (B, E, and H) and DI (C, F, and I) rats, compared with LE (A, D, and G) rats. ir, Immunoreactivity. Scale bars, 200 μm.
Quantitation of SDF-1 mRNA levels in the hypothalamus of LE, HZ, and DI rats
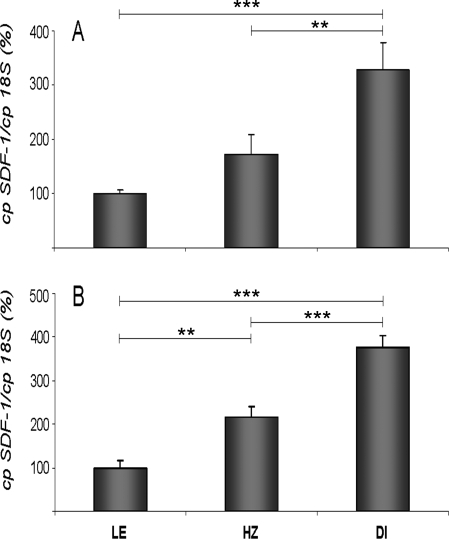
To check whether the decrease in SDF-1 immunoreactivity in both HZ and DI rats can be linked to changes in SDF-1 synthesis, SDF-1 mRNA levels were determined in both SON and PVN using quantitative real-time PCR. Figure 7, A and B, shows that SDF-1 mRNA levels in SON and PVN are strongly increased in HZ and DI rats, compared with control LE rats. The increase is more important in DI rats than HZ rats (3- to 3.5-fold and 2- to 2.5-fold, respectively).
Figure 7.
Hypothalamic SDF-1 mRNA levels of HZ and DI rats, compared with LE rat. SDF-1 mRNA levels of LE, HZ, and DI rats in the SON (A) and PVN (B). Normalized number of copies vs. 18S represents the mean ± sem (five animals/group) and are expressed as percent of LE (control) rats (set at 100%). **, P < 0.01, ***, P < 0.001 vs. control group (LE) or HZ group.
Effect of dDAVP treatment on SDF-1 and CXCR4 expression
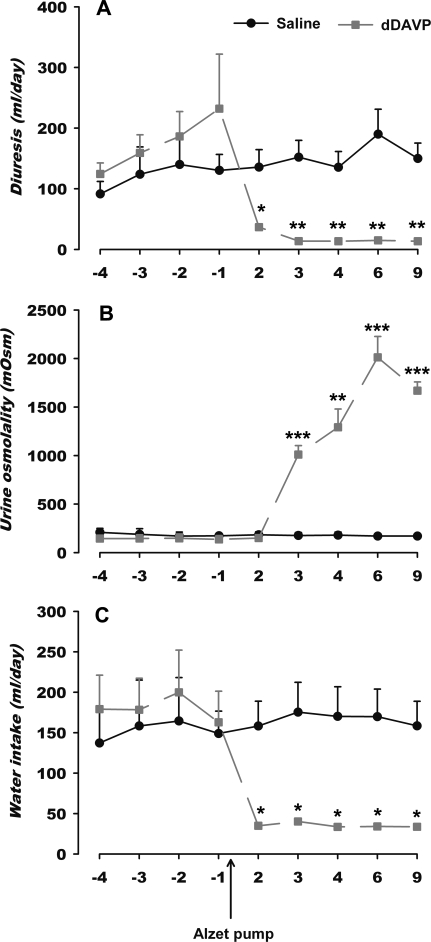
To test whether the decrease in SDF-1 and CXCR4 immunoreactivity in SON and PVN of DI rats was dependent on a peripheral normal hydroosmotic balance, DI rats were treated with dDAVP to restore the AVP renal function in these animals. Chronic infusion of dDAVP in Alzet pump normalizes the hydroosmotic parameters in DI rats. The treatment significantly reduces the daily diuresis of DI rats (Fig. 8A) to the level observed in control. Such effect on diuresis results in an increase in urine osmolality (Fig. 8B). Both effects restore daily water intake (Fig. 8C) observed in the LE rat.
Figure 8.
Effect of dDAVP infusion in Alzet pump on hydroosmotic parameters of DI rats. Each result is expressed as the mean ± sem of values of daily diuresis (A), urine osmolality (B), and water intake (C). *, P < 0.05; **, P < 0.01, and ***, P < 0.001 vs. untreated DI in respective time (n = 3 rats/group).
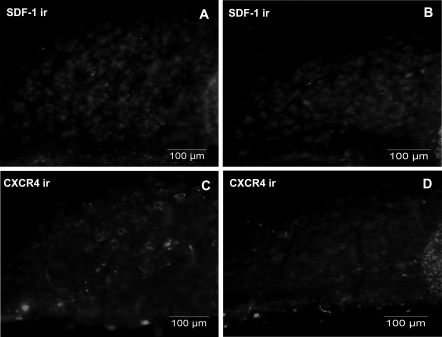
Under these conditions of restoration of peripheral AVP function, Fig. 9, A and B, shows that the chronic treatment with dDAVP does not restore SDF-1 immunostaining in SON in DI rats. As for SDF-1, the immunolabeling for the receptor CXCR4 remained low in the SON of the DI rats after treatment with dDAVP (Fig. 9, C and D). The same data can also be obtained in the PVN (data not shown) of DI rat having received a chronic infusion of dDAVP, suggesting that the decrease in SDF-1 and CXCR4 protein content is not due to a peripheral but a central effect on SDF-1 and CXCR4 expression in the DI rat.
Figure 9.
Effect of dDAVP treatment on SDF-1 and CXCR4 immunoreactivity (ir) in the DI rat SON. A and C, Untreated DI rats. B and D, dDAVP-treated DI rats. Scale bars, 100 μm.
Discussion
The present data further support a close relationship between SDF-1 and AVP in magnocellular hypothalamo-neurohypophysial system. They demonstrate for the first time that: 1) the chemokine SDF-1 and its receptor CXCR4 colocalize in SON and PVN and in nerve terminals in the posterior pituitary in which SDF-1 is stored in AVP neurosecretory granules; 2) the levels of SDF-1 and CXCR4 proteins are dramatically decreased in the SON, PVN, and posterior pituitary of the HZ and DI rats, in strict correlation with the amount of AVP expression; 3) in contrast to the depletion of SDF-1 in the SON and PVN, the expression of SDF-1 mRNA was increased in HZ and DI rats; 4) the low expression of SDF-1 and CXCR4 in the DI rats is linked to a central regulation; and 5) finally, in relation to their cellular localization, both AVP and SDF-1 may be concomitantly centrally regulated in response to modification of the water balance.
We recently provided evidence that, in normal rats, SDF-1 and CXCR4 are found in the magnocellular nuclei of the PVN and SON in which they colocalize with AVP as well as in the AVP pathway and the AVP-positive nerve terminals in the posterior pituitary (14,15). In the magnocellular neurons the proportion of SDF-1 and CXCR4 colocalized with AVP represented 80 and 90%, respectively. The present data obtained by confocal microscopy further demonstrate that although AVP is homogeneously distributed as scattered cytoplasmic vesicles throughout the cytoplasm as already reported (21,22), SDF-1 presents a more perinuclear localization. Such differential distribution has been observed for other neuropeptides colocalized with AVP in PVN and SON such as apelin (23) and galanin (22). It has been suggested that such distribution can be linked to a differential routing of these coexisting neuropeptides in AVP neurons (22,23).
Subcellular fractionation in various sucrose gradient concentrations obtained from posterior pituitaries of control rats further confirms a similar distribution pattern between SDF-1 and AVP. The highest concentrations of SDF-1 are found in fractions in which AVP is highly expressed. Because AVP is known to be located in dense core vesicles in the posterior pituitary (24), it suggests that SDF-1 can be also stored in such vesicles. Immunogold electron microscope analysis further supports such interpretation because gold particles for SDF-1 can be found in AVP-labeled dense core vesicles in nerve endings in the posterior pituitary (Fig. 3). Another chemokine, CCL21, was found by electron microscopy to be localized in vesicles and transported along axons of cultured cortical neurons (25). The present data further demonstrate that chemokines can be costored with neuropeptides in dense core secretory vesicles and strengthen the concept that chemokines may represent a new class of neuromodulators in the central nervous system.
The number of gold particles for SDF-1 is very low, compared with that of AVP. Indeed, the amount of SDF-1 measured in the various sucrose fractions represent around 850 pg/ml in contrast to 3200 ng/ml of AVP. Considering that the molecular weight of AVP is 7-fold smaller than that of SDF-1, the ratio between SDF-1 and AVP represents around 1:30,000. It is thus interesting to note that the electron microscope data are coherent with such calculation, enhancing the significance of the SDF-1/AVP interaction. Furthermore, we observed that inside the neurosecretory granules, SDF-1 gold particles are always seen on the periphery of the AVP dense core vesicles. It is possible that such localization may result in a differential association with neurosecretory proteins such as soluble N-ethylmaleimide factor attachment protein receptor SNARE proteins as already reported for the chemokine CX3CL1 (26), resulting in a differential release of AVP and SDF-1. Further work has to be carried out to test this hypothesis.
Due to the low ratio between SDF-1 and AVP observed in the nerve endings (one or two molecules of SDF-1 per AVP vesicle), it was not possible to examine the distribution of both peptides as well as CXCR4 at the electron microscopic level in the magnocellular neurons. However, as shown in Fig. 1E, few SDF-1-labeled dots can be observed on some proximal processes corresponding to dendritic localization, which may be the site of release for autocrine effects. This point deserves some consideration in the future. Evidence exists on hippocampal neurons that SDF-1 can activate CXCR4 in dendrites as well as axons, resulting in an increase in calcium mobilization (27). It is thus possible that a similar dendritic localization of CXCR4 can be found in SON and PVN as previously observed for several G-protein coupled receptors. Finally, it is known that AVP can be released from dendrites in hypothalamic neurons, and increased somatodendritic release of AVP has been shown to facilitate AVP release (28).
We previously observed that SDF1 was able to modulate induced AVP release without affecting basal AVP (15). Thus, we found that under long-term dehydration, local hypothalamic SDF-1 and CXCR4 protein levels were dramatically decreased, facilitating AVP release (13,15). It was thus of great interest to test the SDF-1/CXCR4 system in conditions in which central AVP production is strongly modified. Such situation can be seen in Brattleboro rats.
Similar to salt-loading or dehydration conditions, in which AVP is strongly released by nerve endings in the posterior pituitary, in diabetes insipidus as observed in the present work in DI rats, SDF-1 and CXCR4 are drastically decreased. Furthermore, the decrease in SDF-1 seems to be correlated with an increase in SDF-1 mRNA as measured by RT-PCR in both the SON and PVN. It was previously reported that the synthesis of several neuropeptides coexisting with AVP such as galanin, cholecystokinin, dynorphin, and the chemokine cytokine-induced neutrophil chemoattractant mRNA are increased under long-term osmotic stimulation and in DI rats (29,30,31,32). Such enhancement in mRNA has been related to an increase release of the peptides resulting in a massive discharge from neurosecretory granules under such experimental conditions (32,33). Thus, the present data suggest that the decrease in SDF-1 immunoreactivity in the SON and PVN of HZ and DI rats is not due to a lack of SDF-1 synthesis but rather to an increase in release both at the hypothalamic level and from the nerve terminals in the posterior pituitary. Because SDF-1 molecules seem to be copackaged in the same granules as AVP, as shown by electron microscopic examination of the posterior pituitary, it is also possible that, as already observed for AVP in the Brattleboro rat, SDF-1 packaging may be also disrupted, explaining the decrease in SDF-1 protein expression. Due to the low amount of SDF-1, it was not possible to directly measure in vivo extracellular concentrations of SDF-1 in the SON and PVN with the techniques available. Furthermore, plasma concentrations of SDF-1 mainly represent SDF-1 produced by the immune cells rather than SDF-1 released from the posterior pituitary and cannot be used as an appropriate marker for SDF-1 release from brain structures. In connection with an enhanced release of SDF-1, the decrease in CXCR4 observed in HZ and DI may be due to change in synthesis or internalization linked to the increase of SDF-1. Indeed, it is well known that such effects take place for several G-protein coupled receptors with high doses of ligands, in particular for CXCR4 in response to SDF-1 (34).
This study also reveals that the low expression of SDF-1 in the SON and PVN of DI is not due to a peripheral effect that induces a chronic stimulation of hypothalamo-neurohypophysial system of the DI rat. This low expression is demonstrated in not only DI rats maintained under basal conditions but also the DI rats treated with dDAVP, a vasopressin 2 receptor agonist. In the DI rat, the increased urine diuresis is known to be directly related to the lack of AVP binding to renal V2 receptors. Functional V2 receptors have been identified in the DI kidney medulla (35,36) and their sensitivity to dDAVP demonstrated (35,37). The dDAVP infusion restores normal hydroosmotic parameters (Fig. 8) such as daily diuresis, urine osmolality, and water intake. The biological parameters, diuresis and urine osmolality, which are directly regulated by AVP, are consistent with measurements made in Brattleboro rats in other studies (30,38). The dDAVP infusion corrects the diabetes insipidus of the Brattleboro rat by a peripheral effect on the kidney. It reduces the chronic stimulation of the hypothalamo-neurohypophysial system of the DI rats, as demonstrated by the restoration of a normal level of oxytocin (OT) (39,40), the normal size of hypertrophied OT neurons (41), and the decrease of OT mRNA in SON and PVN neurons (30). It may be surprising that dDAVP does not restore the decrease in SDF-1 and CXCR4 immunostaining in both SON and PVN, suggesting that the low expression of SDF-1 and CXCR4 in the SON and PVN is independent of the chronic peripheral stimulation of the hypothalamo-neurohypophysial system of DI rats but related to a central regulation. However, it was already shown that such treatment by dDAVP does not restore galanin overexpression observed in PVN of DI rats (30). Further work is needed to understand the possible brain mechanisms involved. DI rats are known to represent a model of central diabetes insipidus because hypothalamic injection of AVP mRNA or AVP-encoding adenoviral or lentiviral vectors transiently corrected diabetes insipidus in DI rats (42,43,44,45). Finally, the present results with dDAVP further suggest that the central interaction between AVP and SDF-1 may be mediated by V1 receptors present in the brain rather than peripheral V2 receptors (46,47).
Altogether these original results show that brain SDF-1 may play a key role in the regulation of water balance by modulating central AVP and strengthen the novel concept that chemokines may be implicated in neuroendocrine functions (13).
Acknowledgments
The authors thank Philippe Fontanges (IFR 65) for his precious help in confocal microscopy, Stéphanie Blanchard for taking care of the Brattleboro rats, and Jean-Claude Beauvillain for his help in electron microscopy.
Footnotes
This work was supported by Institut National de la Santé et de la Recherche Médicale and Centre National de la Recherche Scientifique. C.C. is recipient of a French government fellowship and from the Fondation pour la Recherche Médicale.
Disclosure Statement: All authors have nothing to declare.
First Published Online September 27, 2007
Abbreviations: AVP, Arginine vasopressin; DI, homozygous Brattleboro rat; dDAVP, deamino-8d-arginine-vasopressin; HZ, heterozygous Brattleboro rat; LE, Long Evans; OT, oxytocin; PFA, paraformaldehyde; PVN, hypothalamic paraventricular nucleus; RT, reverse transcription; SDF-1, stromal cell-derived factor 1α; SON, hypothalamic supraoptic nucleus; TBS, Tris-buffered saline.
References
- Brownstein MJ, Russell JT, Gainer H 1980 Synthesis, transport, and release of posterior pituitary hormones. Science 207:373–378 [DOI] [PubMed] [Google Scholar]
- Grant FD 2000 Genetic models of vasopressin deficiency. Exp Physiol 85(Spec No):203S–209S [DOI] [PubMed] [Google Scholar]
- Valtin H 1992 Genetic models of diabetes insipidus. In: Windhager EE, ed. Handbook of physiology. New York: Oxford University Press; 1281–1315 [Google Scholar]
- Schmale H, Richter D 1984 Single base deletion in the vasopressin gene is the cause of diabetes insipidus in Brattleboro rats. Nature 308:705–709 [DOI] [PubMed] [Google Scholar]
- Ivell R, Schmale H, Krisch B, Nahke P, Richter D 1986 Expression of a mutant vasopressin gene: differential polyadenylation and read-through of the mRNA 3′ end in a frame-shift mutant. EMBO J 5:971–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JF 1982 The Brattleboro magnocellular neurosecretory system: a model for the study of peptidergic neurons. Ann NY Acad Sci 394:54–71 [DOI] [PubMed] [Google Scholar]
- Moses AM, Miller M 1970 Accumulation and release of pituitary vasopressin in rats heterozygous for hypothalamic diabetes insipidus. Endocrinology 86:34–41 [DOI] [PubMed] [Google Scholar]
- Miller M, Moses AM 1971 Radioimmunoassay of urinary antidiuretic hormone with application to study of the Brattleboro rat. Endocrinology 88:1389–1396 [DOI] [PubMed] [Google Scholar]
- Rossi D, Zlotnik A 2000 The biology of chemokines and their receptors. Annu Rev Immunol 18:217–242 [DOI] [PubMed] [Google Scholar]
- Proudfoot AE 2002 Chemokine receptors: multifaceted therapeutic targets. Nat Rev Immunol 2:106–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White FA, Bhangoo SK, Miller RJ 2005 Chemokines: integrators of pain and inflammation. Nat Rev Drug Discov 4:834–844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banisadr G, Rostene W, Kitabgi P, Parsadaniantz SM 2005 Chemokines and brain functions. Curr Drug Targets Inflamm Allergy 4:387–399 [DOI] [PubMed] [Google Scholar]
- Callewaere C, Banisadr G, Rostene W, Parsadaniantz SM 2007 Chemokines and chemokine receptors in the brain: implication in neuroendocrine regulation. J Mol Endocrinol 38:355–363 [DOI] [PubMed] [Google Scholar]
- Banisadr G, Skrzydelski D, Kitabgi P, Rostene W, Parsadaniantz SM 2003 Highly regionalized distribution of stromal cell-derived factor-1/CXCL12 in adult rat brain: constitutive expression in cholinergic, dopaminergic and vasopressinergic neurons. Eur J Neurosci 18:1593–1606 [DOI] [PubMed] [Google Scholar]
- Callewaere C, Banisadr G, Desarmenien MG, Mechighel P, Kitabgi P, Rostene WH, Melik Parsadaniantz S 2006 The chemokine SDF-1/CXCL12 modulates the firing pattern of vasopressin neurons and counteracts induced vasopressin release through CXCR4. Proc Natl Acad Sci USA 103:8221–8226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banisadr G, Fontanges P, Haour F, Kitabgi P, Rostene W, Melik Parsadaniantz S 2002 Neuroanatomical distribution of CXCR4 in adult rat brain and its localization in cholinergic and dopaminergic neurons. Eur J Neurosci 16:1661–1671 [DOI] [PubMed] [Google Scholar]
- Navone F, Di Gioia G, Jahn R, Browning M, Greengard P, De Camilli P 1989 Microvesicles of the neurohypophysis are biochemically related to small synaptic vesicles of presynaptic nerve terminals. J Cell Biol 109:3425–3433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landry M, Vila-Porcile E, Calas A 2004 Immunogold detection of co-localized neuropeptides: methodological aspects. J Histochem Cytochem 52:617–627 [DOI] [PubMed] [Google Scholar]
- Varndell IM, Polak JM 1984 Double immunostaining procedures: techniques and applications. Immunolabelling for electron microscopy. Amsterdam: Elsevier; 155–177 [Google Scholar]
- Paxinos G, Watson C, eds. 1998 The rat brain in stereotaxic coordinates. 4th ed. San Diego, CA: Academic Press [Google Scholar]
- Morris JF, Budd TC, Epton MJ, Ma D, Pow DV, Wang H 1998 Functions of the perikaryon and dendrites in magnocellular vasopressin-secreting neurons: new insights from ultrastructural studies. Prog Brain Res 119:21–30 [DOI] [PubMed] [Google Scholar]
- Landry M, Vila-Porcile E, Hokfelt T, Calas A 2003 Differential routing of coexisting neuropeptides in vasopressin neurons. Eur J Neurosci 17:579–589 [PubMed] [Google Scholar]
- Reaux-Le Goazigo A, Morinville A, Burlet A, Llorens-Cortes C, Beaudet A 2004 Dehydration-induced cross-regulation of apelin and vasopressin immunoreactivity levels in magnocellular hypothalamic neurons. Endocrinology 145:4392–4400 [DOI] [PubMed] [Google Scholar]
- Alonso G, Bloch B, Lutz-Bucher B, Bugnon G, Assenmacher I 1981 Light- and electron-microscope differentiation of axons containing vasopressin and oxytocin in the neurohypophyseal lobe of the rat. Neurosci Lett 25:113–118 [DOI] [PubMed] [Google Scholar]
- de Jong EK, Dijkstra IM, Hensens M, Brouwer N, van Amerongen M, Liem RS, Boddeke HW, Biber K 2005 Vesicle-mediated transport and release of CCL21 in endangered neurons: a possible explanation for microglia activation remote from a primary lesion. J Neurosci 25:7548–7557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu GY, Kulasingam V, Alexander RT, Touret N, Fong AM, Patel DD, Robinson LA 2005 Recycling of the membrane-anchored chemokine, CX3CL1. J Biol Chem 280:19858–19866 [DOI] [PubMed] [Google Scholar]
- Pujol F, Kitabgi P, Boudin H 2005 The chemokine SDF-1 differentially regulates axonal elongation and branching in hippocampal neurons. J Cell Sci 118:1071–1080 [DOI] [PubMed] [Google Scholar]
- Ludwig M 1998 Dendritic release of vasopressin and oxytocin. J Neuroendocrinol 10:881–895 [DOI] [PubMed] [Google Scholar]
- Meister B, Cortes R, Villar MJ, Schalling M, Hokfelt T 1990 Peptides and transmitter enzymes in hypothalamic magnocellular neurons after administration of hyperosmotic stimuli: comparison between messenger RNA and peptide/protein levels. Cell Tissue Res 260:279–297 [DOI] [PubMed] [Google Scholar]
- Odorizzi M, Max JP, Tankosic P, Burlet C, Burlet A 1999 Dietary preferences of Brattleboro rats correlated with an overexpression of galanin in the hypothalamus. Eur J Neurosci 11:3005–3014 [DOI] [PubMed] [Google Scholar]
- Koike K, Sakamoto Y, Kiyama H, Masuhara K, Miyake A, Inoue M 1997 Cytokine-induced neutrophil chemoattractant gene expression in the rat hypothalamus by osmotic stimulation. Brain Res Mol Brain Res 52:326–329 [DOI] [PubMed] [Google Scholar]
- van Leeuwen FW 1992 Mutant vasopressin precursor producing cells of the homozygous Brattleboro rat as a model for co-expression of neuropeptides. Prog Brain Res 92:149–155 [DOI] [PubMed] [Google Scholar]
- Landry M, Roche D, Calas A 1995 Short-term effects of centrally administered galanin on the hyperosmotically stimulated expression of vasopressin in the rat hypothalamus. An in situ hybridization and immunohistochemistry study. Neuroendocrinology 61:393–404 [DOI] [PubMed] [Google Scholar]
- Baudouin SJ, Pujol F, Nicot A, Kitabgi P, Boudin H 2006 Dendrite-selective redistribution of the chemokine receptor CXCR4 following agonist stimulation. Mol Cell Neurosci 33:160–169 [DOI] [PubMed] [Google Scholar]
- Cornett LE, Breckinridge SM, Koike TI 1989 Induction of V2 receptors in renal medulla of homozygous Brattleboro rats by arginine vasopressin. Peptides 10:985–991 [DOI] [PubMed] [Google Scholar]
- Shen T, Laycock J, Suzuki Y, Defer N, Hanoune J 1998 Stability of the vasopressin V2 receptor-adenylyl cyclase system in rat kidney. Eur J Pharmacol 341:87–94 [DOI] [PubMed] [Google Scholar]
- Kinter LB 1982 Water balance in the Brattleboro rat: considerations for hormone replacement therapy. Ann NY Acad Sci 394:448–463 [DOI] [PubMed] [Google Scholar]
- Burlet A, Desor D, Max JP, Nicolas JP, Krafft B, Burlet C 1990 Ingestive behaviors of the rat deficient in vasopressin synthesis (Brattleboro strain). Effect of chronic treatment by dDAVP. Physiol Behav 48:813–819 [DOI] [PubMed] [Google Scholar]
- Cheng SW, North WG 1986 Exogenous vasopressin modulates activity of oxytocin neurons in homozygous Brattleboro rats. Am J Physiol 251:E556–E562 [DOI] [PubMed] [Google Scholar]
- Balment RJ, Henderson IW, Oliver JA 1975 The effects of vasopressin on pituitary oxytocin content and plasma renin activity in rats with hypothalamic diabetes insipidus (Brattleboro strain). Gen Comp Endocrinol 26:468–477 [DOI] [PubMed] [Google Scholar]
- Orkand PM, Palay SL 1967 Effect of treatment with exogenus vasopressin on the structural alterations in the hypothalamo-neurohypophysial system of rats with hereditary diabetes insipidus. Anat Rec 157:295–303 [Google Scholar]
- Jirikowski GF, Sanna PP, Maciejewski-Lenoir D, Bloom FE 1992 Reversal of diabetes insipidus in Brattleboro rats: intrahypothalamic injection of vasopressin mRNA. Science 255:996–998 [DOI] [PubMed] [Google Scholar]
- Geddes BJ, Harding TC, Lightman SL, Uney JB 1997 Long-term gene therapy in the CNS: reversal of hypothalamic diabetes insipidus in the Brattleboro rat by using an adenovirus expressing arginine vasopressin. Nat Med 3:1402–1404 [DOI] [PubMed] [Google Scholar]
- Ideno J, Mizukami H, Honda K, Okada T, Hanazono Y, Kume A, Saito T, Ishibashi S, Ozawa K 2003 Persistent phenotypic correction of central diabetes insipidus using adeno-associated virus vector expressing arginine-vasopressin in Brattleboro rats. Mol Ther 8:895–902 [DOI] [PubMed] [Google Scholar]
- Bienemann AS, Martin-Rendon E, Cosgrave AS, Glover CP, Wong LF, Kingsman SM, Mitrophanous KA, Mazarakis ND, Uney JB 2003 Long-term replacement of a mutated nonfunctional CNS gene: reversal of hypothalamic diabetes insipidus using an EIAV-based lentiviral vector expressing arginine vasopressin. Mol Ther 7:588–596 [DOI] [PubMed] [Google Scholar]
- Barberis C, Tribollet E 1996 Vasopressin and oxytocin receptors in the central nervous system. Crit Rev Neurobiol 10:119–154 [DOI] [PubMed] [Google Scholar]
- Pouzet B, Serradeil-Le Gal C, Bouby N, Maffrand JP, Le Fur G, Bankir L 2001 Selective blockade of vasopressin V2 receptors reveals significant V2-mediated water reabsorption in Brattleboro rats with diabetes insipidus. Nephrol Dial Transplant 16:725–734 [DOI] [PubMed] [Google Scholar]