Abstract
Corticotropin-releasing factor (CRF) activates locus coeruleus (LC)-norepinephrine neurons during stress. Previous stress or CRF administration attenuates the magnitude of this response by decreasing postsynaptic sensitivity to CRF. Here we describe the fate of CRF receptors (CRFr) in LC neurons after stress. Rats were exposed to swim stress or handling and perfused 1 or 24 h later. Sections through the LC were processed for immunogold-silver labeling of CRFr. CRFr in LC dendrites was present on the plasma membrane and within the cytoplasm. In control rats, the ratio of cytoplasmic to total dendritic labeling was 0.55 ± 0.01. Swim stress increased this ratio to 0.77 ± 0.01 and 0.80 ± 0.02 at 1 and 24 h after stress, respectively. Internalized CRFr was associated with different organelles at different times after stress. At 1 h after stress, CRFr was often associated with early endosomes in dendrites and perikarya. By 24 h, more CRFr was associated with multivesicular bodies, suggesting that some of the internalized receptor is targeted for degradation. In perikarya, more internalized CRFr was associated with Golgi apparatus 24 vs. 1 h after stress. This is suggestive of changes in CRFr synthesis. Alternatively, this may indicate communication between multivesicular bodies and Golgi apparatus in the process of recycling. Administration of the selective CRF1 antagonist, antalarmin, before swim stress attenuated CRFr internalization. The present demonstration of stress-induced internalization of CRFr in LC neurons provides evidence that CRF is released in the LC during swim stress to activate this system and initiate cellular trafficking of the receptor that determines subsequent sensitivity of LC neurons to CRF.
CORTICOTROPIN-RELEASING FACTOR (CRF), the hypothalamic neurohormone that mediates stress-induced release of ACTH (1), also acts as a brain neurotransmitter. This is supported by the distribution of CRF-immunoreactive neuronal processes and receptors in extrahypophyseal regions and the behavioral and autonomic effects produced by central CRF administration (2,3,4,5). The noradrenergic nucleus, locus coeruleus (LC), is a putative target of CRF neurotransmission (6). CRF-immunoreactive axon terminals synapse with catecholaminergic LC dendrites (7,8). Intracoerulear CRF microinfusion increases LC discharge rate, norepinephrine (NE) levels in prefrontal cortex and produces cortical electroencephalographic activation (9,10). Moreover, LC activation elicited by certain stimuli is abated by microinfusion of a CRF antagonist into the LC, suggesting that CRF neurotransmission in the LC mediates stress-induced LC activation (11,12,13). Given the role of the LC-NE system in arousal and attention, this may be part of a cognitive limb of the stress response (14).
LC sensitivity to CRF is affected by many conditions. Previous CRF administration decreases the subsequent response of LC neurons to CRF for up to a week (15). Cross-desensitization has been demonstrated between CRF and stressors (16). In contrast, certain conditions increase LC sensitivity to CRF, including chronic morphine administration (17). Swim stress, which produces relatively long-term changes in behavior, shifts the CRF dose-response curve for LC activation in a complex manner, increasing LC sensitivity to low doses of CRF, but with a lower plateau (18). Because LC sensitivity to CRF determines the magnitude of the arousal and attentional response to stress, it is important to understand cellular mechanisms regulating this response.
Agonist-induced internalization of G protein-coupled receptors, such as CRFr, is a common mechanism for modulating cellular sensitivity to neurotransmitters (19). Evidence for CRF-induced trafficking of CRFr has been demonstrated in cultured neurons (20,21,22,23). Recently, we provided evidence for agonist-induced internalization of CRFr in LC neurons in vivo (24). This phenomenon may underlie acute desensitization of the LC-NE system to CRF.
Although pharmacologically induced receptor internalization is of interest, it is important to determine whether receptor internalization occurs under physiological conditions. To this end, the present study used electron microscopic analysis to examine cellular trafficking of the CRFr within LC neurons at different times after swim stress. An additional set of studies examined the ability of the selective CRF1 receptor antagonist antalarmin to alter stress-induced receptor trafficking.
Materials and Methods
Subjects
Eighteen adult male Sprague Dawley rats (Taconic, Germantown, NY) housed three to a cage (20 C, 12-h light, 12-h dark cycle, lights on 0700 h) were used in this study. Food and water were freely available. Rats were housed in the animal facility for at least 5 d before experimentation. The care and use of animals were approved by the Children’s Hospital of Philadelphia Institutional Animal Care and Use Committee and in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Only the minimum numbers of animals necessary to produce reliable scientific data were used.
Swim stress
The swim stress used in the present study followed the protocols that have been previously described (18). Individual rats were placed in a cylindrical glass tank (46 cm height × 20 cm diameter) filled with water (25 ± 1 C) to a depth of 30 cm for 15 min. The 30-cm depth allowed rats to swim or float without having their tails touch the bottom of the tank. Immediately after a 15-min swim, rats were removed from the tank, towel dried, and put in a warming cage (37 C) that contained a heating pad covered with towels for 15 min. Rats were then returned to their home cage and perfused 1 or 24 h later. Control rats were brought to the same room, picked up once, and put back in their home cage. For experiments involving drug pretreatment, the selective CRF1 receptor antagonist antalarmin (20 mg/kg) or vehicle (1 ml/kg) was administered 30 min before the 15-min swim stress, and rats were killed 24 h later. Swim stress occurred between 1000 and 1400 h.
Tissue preparation
Rats were deeply anesthetized with sodium pentobarbital (70 mg/kg) and transcardially perfused through the ascending aorta with 10 ml heparinized saline, 50 ml 3.75% acrolein (Electron Microscopy Sciences, Fort Washington, PA), and 200 ml 2% formaldehyde in 0.1 m phosphate buffer (PB; pH 7.4). Immediately after perfusion fixation, the brains were removed, sectioned into coronal slices, and postfixed in the same fixative overnight at 4 C.
Immunoelectron microscopy
Every fourth section through the rostrocaudal extent of the LC was cut in the coronal plane at a setting of 40 μm using a Vibratome (Technical Product International, St. Louis, MO) and collected into 0.1 m PB. Sections were placed for 30 min in 1% sodium borohydride in 0.1 m PB and rinsed thoroughly in 0.1 m PB to remove reactive aldehydes and incubated in 0.5% BSA in 0.1 m Tris-buffered saline (TBS; pH 7.6) for 30 min. Then tissue sections were incubated 12–14 h in the rabbit anti-CRFr antisera (H215 directed against amino acids 230–444; Santa Cruz Biotechnology, Inc., Santa Cruz, CA) at 1:1000 or in a cocktail of rabbit anti-CRFr antisera (Santa Cruz Biotechnology) at 1:1000, and mouse monoclonal tyrosine hydroxylase (TH) antibody (Immunostar Inc., Hudson, WI) at 1:2000 diluted in 0.1 m TBS containing 0.1% BSA. The CRFr antiserum recognizes both CRF1 and CRF2 receptor subtypes. Sections were rinsed and incubated in appropriate secondary antisera at room temperature. For immunogold-silver localization of CRFr, sections were rinsed three times with 0.1 m TBS, followed by rinses with 0.1 m PB and 0.01 m PBS (pH 7.4). Sections were then incubated in a 0.2% gelatin-PBS and 0.8% BSA buffer for 10 min. This was followed by incubation in goat antirabbit IgG conjugate in 1-nm gold particles (1:50; Amersham Bioscience Corp., Piscataway, NJ) at room temperature for 2 h. Subsequently, sections were rinsed in buffer containing the same concentration of gelatin and BSA as above and rinsed with 0.01 m PBS. Sections were then incubated in 2% glutaraldehyde (Electron Microscopy Sciences) in 0.01 m PBS for 10 min followed by washes in 0.01 m PBS and 0.2 m sodium citrate buffer (pH 7.4), respectively. A silver enhancement kit (Amersham Bioscience Corp.) was used for silver intensification of the gold particles. After intensification, tissues were rinsed in 0.2 m citrate buffer and 0.1 m PB and incubated in 2% osmium tetroxide (Electron Microscopy Sciences) in 0.1 m PB for 1 h, washed in 0.1 m PB, dehydrated in an ascending series of ethanol followed by propylene oxide and flat embedded in Epon 812 (Electron Microscopy Sciences) (25). For tissues labeled for both CRFr and TH, TH immunoreactivity was detected using biotinylated donkey antimouse IgG (1:400; Vector Laboratories, Burlingame, CA) and avidin-biotin complex solution (1:200, Vector). The peroxidase reaction product was visualized by incubating sections in 0.02% 3,3′-diaminobenzidine (Sigma-Aldrich Inc., St. Louis, MO) containing 0.01% H2O2. CRFr immunoreactivity was detected using goat antirabbit IgG conjugate in 1-nm gold particles (Amersham) following the same protocol described above. Thin sections of approximately 50–80 nm in thickness were cut with a diamond knife (Diatome-US, Fort Washington, PA) using a Leica Ultracut (Leica Microsystems, Wetzlar, Germany). Sections were collected on copper mesh grids, examined with an electron microscope (Morgagni, Fei Co., Hillsboro, OR), and digital images were captured using the AMT advantage HR/HR-B CCD camera system (Advance Microscopy Techniques Corp., Danvers, MA). Figures were assembled and adjusted for brightness and contrast in Adobe Photoshop.
Controls and data analysis
To verify that the CRFr antibody was detecting CRF1, immunolabeling was also examined in sections from mice with a deletion of CRF1 and wild-type mice (kindly provided by Dr. Stephen C. Gammie of the University of Wisconsin, Madison, WI). After perfusion with 5% acrolein, the brains were removed and cryoprotected in 30% sucrose and frozen. Frozen 40-μm-thick sections were cut in the coronal plane using a freezing microtome (Micron HM550 cryostat; Richard-Allan Scientific, Kalamazoo, MI) and rinsed extensively in 0.1 m PB and 0.1 m TBS. Sections were placed for 30 min in 1% sodium borohydride in 0.1 m PB. The tissue sections were then incubated in 0.5% BSA and 0.25% Triton X-100 in 0.1 m TBS for 30 min. Thorough rinses in 0.1 m TBS were done after incubation. Sections were then placed in 3% H2O2 in 0.1 m PB for 30 min and rinsed in 0.1 m PB and 0.1 m TBS. Subsequently, sections were incubated in the rabbit anti-CRFr antisera (1:500; Santa Cruz Biotechnology) for 12–14 h at room temperature. The following day, tissue sections were rinsed three times in 0.1 m TBS and incubated in biotinylated donkey antirabbit (1:400; Vector) for 30 min followed by rinses in 0.1 m TBS. The sections were then incubated with avidin-biotin complex (Vector) for 30 min. For all incubations and washes, sections were continuously agitated with a rotary shaker. CRFr was visualized in 22 mg 3,3′-diaminobenzidine (Sigma-Aldrich) and 10 μl 30% H2O2 in 100 ml 0.1 m TBS. Sections were collected, dehydrated, and coverslipped for light microscopic analysis of CRFr immunoreactivity.
In addition to the above control, control sections for each experiment were run in parallel in which only the primary antibody was omitted. No detectable immunoreactivity was observed in the absence of the primary antibody.
The classification of identified cellular elements was based on the method of Peters et al. (26). Dendrites usually contained endoplasmic reticulum and were postsynaptic to axon terminals. Axon terminals contained synaptic vesicles and were at least 0.3 μm in diameter. A varicosity was considered as synaptic when it showed a junctional complex, a restricted zone of apposed parallel membranes with slight enlargement of the intercellular space, and/or associated postsynaptic thickening.
Dendrites were sampled from at least 10 grids containing five to 10 thin sections each from at least three plastic-embedded sections of the LC from each animal. Dendrites were not classified based on size because our previous investigation showed that there was no difference between groups in the type or size of dendrites that were sampled for analysis or in the ratio of cytoplasmic to total silver grains in different sized dendrites (24). Selective immunogold-silver-labeled profiles were identified by the presence, in single thin sections, of at least two to three immunogold particles within a cellular compartment, as we previously described (24). As observed in low-magnification electron micrographs, background labeling in the neuropil, deemed spurious, was not commonly encountered.
To quantify the degree of CRFr internalization after swim stress, tissue sections from three rats in each group (control, 1 h after stress, and 24 h after stress) with optimal preservation of ultrastructural morphology were used. Similarly, the effect of a CRF antagonist on CRFr internalization was examined using tissue sections with optimal preservation of ultrastructural morphology from three rats in each group (control/handled, antagonist plus swim and vehicle plus swim). Analysis was exclusively carried out on the most superficial portions of the tissue section in direct contact with the embedding plastic to minimize artificial differences based on the penetration of the antibody (25).
As previously described, CRFr internalization was measured by the ratio of cytoplasmic to total silver grains in a dendrite (24). Silver grains that measured more than 0.10 μm were included in the analysis. Large and irregularly shaped silver grains that measured more than 0.25 μm and silver grains that measured less than 0.10 μm were not included in the data analysis. Silver grains were identified as plasmalemmal if they were associated with the plasma membrane and cytoplasmic if they were not in contact with the plasma membrane. This ratio was determined for each dendrite, and the mean ratio (from at least 138 dendrites) was determined per animal. The average of the three animals was taken as the group mean, and treatment group means were statistically compared using an ANOVA followed by Tukey’s multiple comparison test. All data are expressed as mean ± sem. Statistical analyses were carried out using GraphPad Prism (GraphPad Software, Inc., San Diego, CA).
To measure the association of CRFr with particular subcellular compartments after stress, the silver grains were identified and counted in association with seven subcellular structures. The subcellular structures included the plasma membrane, endosome-like vesicles, multivesicular bodies, Golgi apparatus, and endoplasmic reticulum. Silver grains that were not associated with these defined structures were classified under unidentified subcellular structures. The endosome-like structures were small, round, or irregular shaped vesicles that measured 0.08–0.21 μm. The multivesicular bodies were large round vesicles that measured 0.175–0.550 μm and contained small round-shaped vesicles with a clear content. At least 51 dendrites and 18 perikarya per rat were analyzed for this subcellular quantification. The profiles were magnified to allow the identification of the subcellular structure showing silver grains. The results are expressed as the percentage of silver grains associated with subcellular structures. The average of the three animals was taken as the group mean, and treatment group means were statistically compared using an ANOVA followed by Tukey’s multiple comparison test.
Results
Control immunolabeling
CRFr immunolabeling was present in the LC and cerebellum of wild-type mice, consistent with studies by others using a different CRFr antibody (27,28). In contrast, this immunolabeling was absent in sections from mice with a CRF1 deletion, verifying that the antibody detects CRF1 (Fig. 1).
Figure 1.
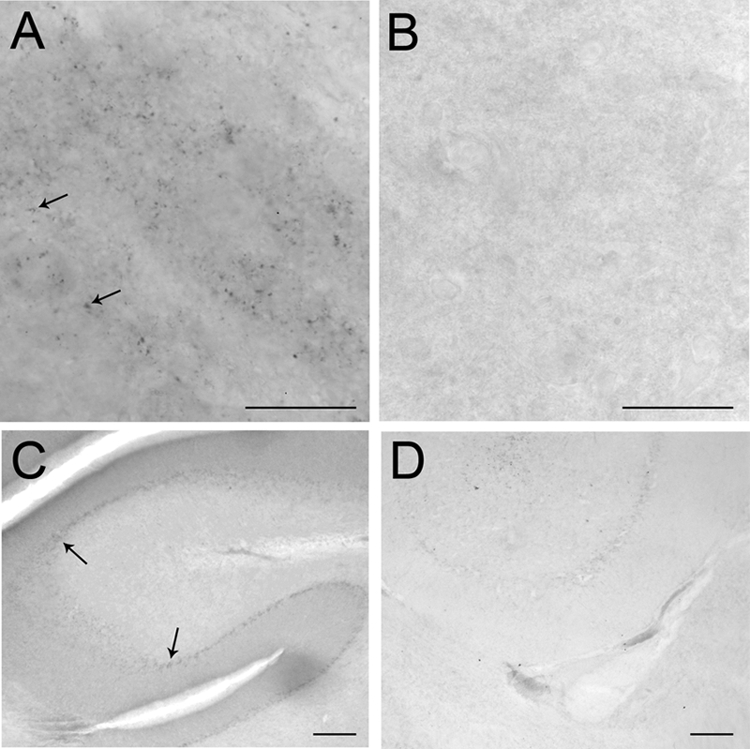
Bright-field photomicrographs showing CRFr immunoreactivity in the LC (A) and cerebellum (C) of wild-type mouse. CRFr immunoreactivity is absent in the LC (B) and cerebellum (D) of CRFr knockout mouse. Arrows point to CRFr immunolabeling. Scale bar, 100 μm.
Ultrastructural localization of CRFr in the LC
As we recently demonstrated (24), CRFr was identified in somatodendritic processes in the LC, and in control subjects, it was preferentially associated with the cytoplasmic surfaces of plasma membranes (Figs. 2A and 3A). Ultrastructural analysis has shown that labeling for neurotransmitter receptors may be either parasynaptic (along portions of the plasma membrane near the active zone) and/or extrasynaptic (along portions of the plasma membrane in which synaptic input is not observed (29). CRFr immunolabeling was often associated with parasynaptic portions of the dendritic plasma membrane, although silver grains indicative of CRFr immunolabeling were also observed extrasynaptically, (e.g. Fig. 2A). CRFr-labeled dendrites received synaptic specializations from terminals lacking immunogold-silver labeling and containing heterogeneous types of synaptic vesicles, including large dense-core vesicles and small clear vesicles (Fig. 2A). Dual labeling for CRFr and TH demonstrated CRFr immunolabeling within TH-labeled dendrites and perikarya, and here it was mostly associated with the plasma membrane in control rats (Figs. 2D and 4A).
Figure 2.
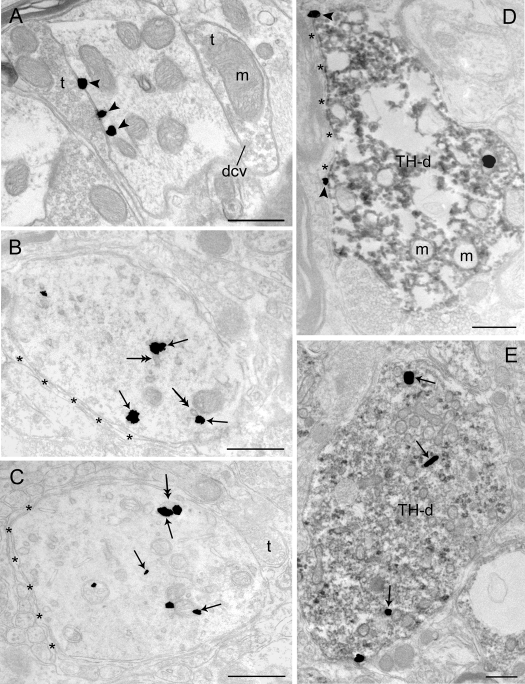
Electron microscopic visualization evidence for swim stress-induced internalization of CRFr in LC dendrites. Sections from a control rat (A) and a rat perfused 1 h (B) and a rat perfused 24 h (C) after 15-min swim stress. A, Immunogold-silver labeling for CRFr (arrowheads) can be seen along the plasmalemma in a dendritic profile. The dendrite receives synaptic contacts from axon terminals (t). B and C, CRFr labeling shifts from the plasmalemma to the cytoplasm 1 h (B) and 24 h (C) after 15-min swim stress, respectively. Arrows point to immunogold-silver labeling in the cytoplasm. Double-headed arrows point to endosome-like vesicles. Asterisks indicate astrocytic processes. D and E, Electron photomicrographs showing TH-immunoperoxidase-labeled dendrites (TH-d) containing immunogold-silver labeling for CRFr in control (D) and 24 h (E) after 15-min swim stress. CRFr labeling shifts from the plasmalemma to the cytoplasm in TH-immunoperoxidase-labeled dendrites 24 h after 15-min swim stress. Arrows point to immunogold-silver labeling in the cytoplasm, whereas arrowheads point to immunogold-silver labeling on the plasma membrane. dcv, Dense-core vesicles; m, mitochondria. Scale bar, 0.5 μm.
Figure 3.
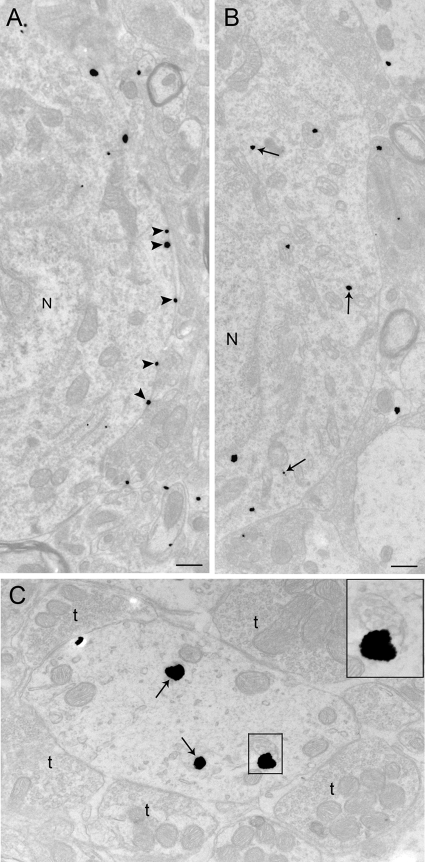
Electron microscopic evidence for stress-induced CRFr internalization in LC perikarya and dendrites. A and B, Electron photomicrographs showing CRFr cellular localization in perikarya from a control rat (A) and from a rat perfused 24 h after 15-min swim stress (B). A, Immunogold-silver labeling for CRFr (arrowheads) along the plasma membrane in a control case; B, CRFr labeling shifts from the plasma membrane to the cytoplasm 24 h after 15-min swim stress. Arrows point to immunogold-silver labeling in the cytoplasm, whereas arrowheads point to immunogold-silver labeling on the plasma membrane. N, Nucleus. C, Dendrite from a 24-h post-stress subject showing CRFr in the cytoplasm and association with a multivesicular body. The dendrite is targeted by multiple axon terminals (t). The inset shows a higher-magnification view of the area denoted by the box in C where CRFr is associated with a multivesicular body. Scale bar, 0.5 μm.
Figure 4.
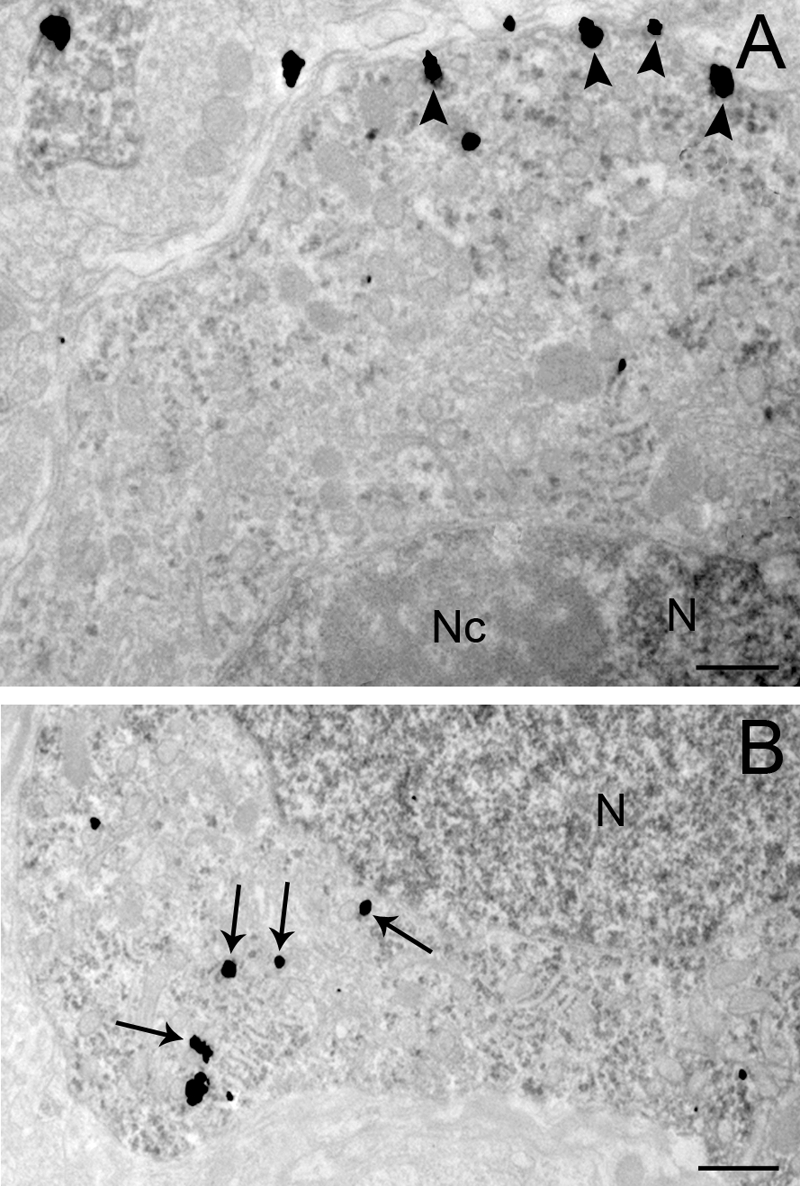
Electron microscopic evidence for stress-induced CRFr internalization in LC perikarya containing immunogold-silver labeling for CRFr and immoperoxidase labeling for TH. A, Electron photomicrograph showing immunogold-silver labeling for CRFr (arrowheads) along the plasma membrane in control rats; B, Electron photomicrograph from a rat perfused 24 h after 15-min swim stress. CRFr labeling shifts from the plasma membrane to the cytoplasm 24 h after 15-min swim stress in TH-labeled perikarya. Arrows point to immunogold-silver labeling in the cytoplasm, whereas arrowheads point to immunogold-silver labeling on the plasma membrane. Nc, Nucleolus; N, nucleus. Scale bar, 0.5 μm.
Swim-induced internalization of CRFr in LC
Exposure of rats to swim stress resulted in a shift in the predominant localization of CRFr from the plasma membrane to the cytoplasmic compartment in dendrites that was apparent at both 1 h (Fig. 2B) and 24 h (Fig. 2, C and E) after swim stress. In these cases, CRFr labeling within the cytoplasm was often associated with endosome-like structures (Fig. 2B). Swim-stress-induced CRFr internalization was also apparent in perikarya (Figs. 3, A and B, and 4, A and B). In sections that were dual labeled for CRFr and TH, swim stress-induced CRFr internalization was identified in TH-labeled dendrites (Fig. 2E) and perikarya (Fig. 4B).
The magnitude of CRFr internalization was quantified using the ratio of cytoplasmic to total silver grains and compared between groups (Table 1). An ANOVA revealed a significant treatment effect (F2,6 = 441; P < 0.0001). In control rats, the ratio of cytoplasmic to total silver grains was similar to the basal cytoplasmic to total ratio determined in a previous study (i.e. 0.57) (24). The shift from plasma membrane to cytoplasmic compartment was apparent in both the 1- and 24-h post-stress group. The ratio at 24 h was slightly but statistically elevated over the ratio at 1 h after swim (Table 1). Interestingly, this was similar to the ratio of cytoplasmic to total CRFr labeling in LC dendrites quantified after CRF microinfusion into the LC (i.e. 0.81–0.86) (24).
Table 1.
Ratio of cytoplasmic to total CRFr label
| Control | 1 h after stress | 24 h after stress | Statistics |
|---|---|---|---|
| 0.54 + 0.003
|
0.77 + 0.007a
|
0.80 + 0.009a,b
|
F2,6 = 441; P < 0.0001
|
| Control
|
Vehicle/swim
|
Antalarmin/swim
|
|
| 0.52 + 0.04 | 0.79 + 0.02a | 0.66 + 0.03a,b | F2,6 = 23; P < 0.002 |
Compared with control: a P < 0.05, b P < 0.01, c P < 0.0001.
P < 0.05 compared with alternate treatment group.
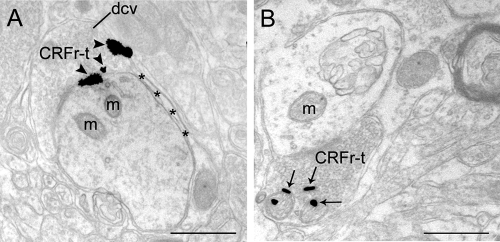
Immunogold-silver labeling for CRFr was also occasionally identified in axon terminals, as previously reported (24). CRFr was associated with the plasma membrane of axon terminals in control rats (Fig. 5A). Similar to the results obtained for somatodendritic processes, there was evidence for stress-induced CRFr internalization in axon terminals (Fig. 5B).
Figure 5.
CRFr-labeled axon terminals in LC from control and stressed rats. A, CRFr immunogold-labeling is present in an axon terminal (CRFr-t) from a control rat and is associated with the plasma membrane (arrowheads). Also shown is an astrocytic process (asterisks) apposed to the dendrite. B, CRFr immunogold labeling (arrows) is prominently associated within the intracellular compartment in a CRFr-labeled axon terminal (CRFr-t) from a case perfused 1 h after 15-min swim stress. dcv, Dense-core vesicles; m, mitochondria. Scale bar, 0.5 μm.
Targeting of CRFr to distinct cellular compartments in dendrites and perikarya
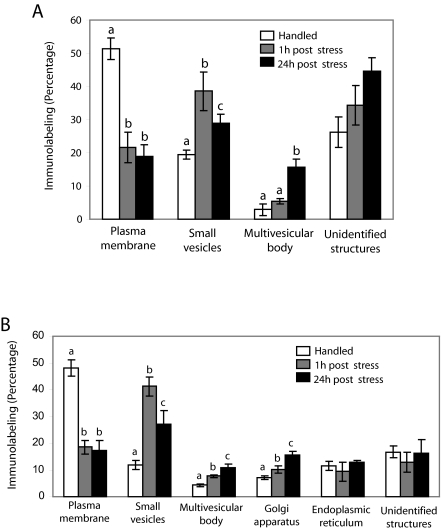
CRFr was associated with distinct cellular compartments in dendrites and perikarya that related to the duration of time that elapsed after stress (Fig. 6). Within dendrites, the CRFr silver grains in association with the plasma membrane, endosome-like vesicles, multivesicular bodies, or unidentified structures were identified and counted (Fig. 6A). In dendrites of control rats, a greater percentage of CRFr silver grains was associated with the plasma membrane compared with 1 and 24 h after stress (F2,6 = 41; P < 0.0003). At 1 h after stress, CRFr was primarily associated with endosome-like vesicles, and the percentage of these associations was significantly greater compared with 24 h after stress or the control group (F2,6 = 22; P < 0.002). By 24 h after stress, associations of CRFr with endosome-like vesicles remained above control levels (Fig. 6A). However, a distinguishing feature of this group was a greater percentage of CRFr silver grains associated with multivesicular bodies (F2,6 = 20; P < 0.005). Figure 3C shows an example of CRFr associated with a multivesicular body in a case from the 24-h post-stress group.
Figure 6.
Quantification of associations of CRFr with distinct subcellular structures in LC dendrites and perikarya in control and stressed rats. A, Bars represent the mean percentage of CRFr associated with different subcellular dendritic structures in control rats and in rats perfused 1 and 24 h after 15-min swim stress; B, Percentage of CRFr associated with different subcellular structures in the perikarya in control rats and in rats perfused 1 and 24 h after 15-min swim stress. Values are means ± sem of three rats per group. Values with different letters are significantly different (P < 0.0001) from each other in each time point studied (Tukey’s multiple comparison test after ANOVA).
In perikarya, CRFr silver grains in association with the plasma membrane, endosome-like vesicles, multivesicular bodies, Golgi apparatus, endoplasmic reticulum, or unidentified structures were identified and counted (Fig. 6B). Similar to the findings in dendrites, the percentage of CRFr silver grains associated with the plasma membrane was significantly greater in control rats compared with either group of stressed rats (F2,6 = 56; P < 0.0001). At 1 and 24 h after stress, there was a significant increase in the percentage of CRFr silver grains associated with endosome-like vesicles (F2,6 = 26; P < 0.005), multivesicular bodies (F2,6 = 22; P < 0.005), and Golgi apparatus (F2,6 = 42; P < 0.0005). Whereas associations with endosomal-like vesicles were greater at 1 vs. 24 h after stress, associations with multivesicular bodies and Golgi apparatus were greater at 24 vs. 1 h after stress (Fig. 6B). There was no significant difference in the percentage of CRFr silver grains associated with endoplasmic reticulum or unidentified structures between treatment groups examined.
Attenuation of stress-induced CRFr internalization by a CRF1 antagonist
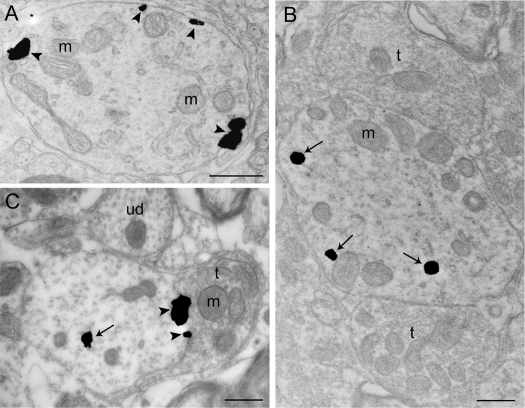
In a separate experiment, the ability of pretreatment with the selective CRF1 antagonist antalarmin to attenuate CRFr internalization induced by swim stress was determined. Similar to the previous experiment, in control rats, CRFr immunolabeling was distributed along the plasma membrane of LC dendrites (Fig. 7A). In sections from these subjects that were handled and perfused 24 h later, the ratio of cytoplasmic to total CRFr label was similar to that of controls in the experiment described above (Table 1). In rats injected with vehicle before swim and perfused 24 h later, there was clear evidence of CRFr internalization (Fig. 7B and Table 1). Notably, the ratio of cytoplasmic to total CRFr determined in this case was similar to the previous experiment using rats that did not receive vehicle but were perfused 24 h after stress. Pretreatment with antalarmin (Fig. 7C and Table 1) resulted in a statistically significant reduction in CRFr internalization, although the ratio of cytoplasmic to total CRFr was still somewhat greater in antagonist-treated rats than that determined in control rats.
Figure 7.
Attenuation of CRFr internalization by antagonist pretreatment. A, Electron photomicrograph showing immunogold-silver labeling for CRFr along the plasmalemma (arrowheads) in a dendrite of a control rat; B, CRFr labeling is more prominent in the cytoplasm in a dendrite from a subject pretreated with vehicle before swim and perfused 24 h after 15-min swim stress; C, A dendrite from an antagonist-pretreated subject exposed to swim stress and perfused 24 h later indicating a reduction in CRFr internalization. Arrows point to immunogold-silver particles distributed within the cytoplasmic compartment, whereas arrowheads point to immunogold-silver labeling on the plasma membrane. m, Mitochondria; t, terminal; ud, unlabeled dendrite. Scale bar, 0.5 μm.
Discussion
The present results provide the first evidence of stress-induced CRFr internalization in vivo. Swim stress resulted in a shift in CRFr localization from the plasma membrane to the cytoplasm that was apparent at both 1 and 24 h after the stress, with a greater effect at 24 h, underscoring the persistence of the internalization. Pretreatment with the selective CRF1 antagonist antalarmin attenuated swim stress-induced CRFr internalization. These results are consistent with the idea that swim stress releases CRF that then acts on CRF1 in LC neurons and support the concept that CRF serves as a neuromodulator to regulate LC activity during stress. The profile of organelles that CRFr was associated with changed with increasing time after stress in a manner that suggested degradation and possible new synthesis of CRFr. These cellular events are consistent with reported electrophysiological changes in LC sensitivity to CRF that are observed 24 h after swim stress (18). CRFr internalization may be an important mechanism for regulating the sensitivity of the LC-NE system to CRF and other stressors (18,30). Targeting cellular substrates involved in the trafficking of CRFr may provide a novel approach toward manipulating the sensitivity of the LC-NE system to CRF for treatment of stress-related psychiatric disorders.
Methodological considerations
Despite physiological evidence for direct effects of CRF on LC neurons (31), in situ hybridization studies have failed to detect CRFr mRNA in the LC, arguing against the presence of CRFr protein in these neurons (32). Although our group and others have demonstrated CRFr immunolabeling in LC neurons (24,28,33), it might be argued that the antibodies used are detecting another protein with strong homology to CRFr. The present study controlled for this possibility by demonstrating a lack of CRFr immunolabeling in sections from mice with a deletion of CRF1. As previously reported in studies using other CRFr antibodies, we were able to detect CRF immunolabeling in these areas in wild-type mice. This important control confirms the specificity of the antibody used in the present study. Finally, our demonstrations that CRF (24) and stress (the present study) cause cellular internalization of the immunolabeled protein and that stress does so in a manner that is sensitive to a CRF1 antagonist, further support the idea that the antibody is detecting CRF1 in LC neurons. It would be difficult to suggest an alternate scenario that would explain both the control findings and these trafficking events. The discrepancy between expression of CRFr protein and CRFr mRNA in the LC remains but may be related to an issue of detection.
The preembedding immunogold method provides distinct subcellular localization of reaction product while maintaining morphological preservation (25). Moreover, it is more suitable than postembedding methods for localization of immunoreactivity at extrasynaptic sites and for determining regional distributions (34). However, the preembedding immunogold method is likely to underestimate the CRFr levels due to limited penetration, compared with the postembedding method, which minimizes penetration problems because of the relative thickness of the sections. This limitation of the preembedding technique was minimized in the present study by collecting ultrathin tissue sections near the tissue-Epon interface to ensure that labeling was clearly detectable in sections included for analysis (35). Furthermore, experimental groups were processed in parallel; therefore, this caveat should not contribute to group differences.
Characteristics of CRFr internalization: comparison with other studies
After stimulation with agonists, most G protein-coupled receptors are internalized (36,37). Receptor internalization prevents persistent receptor signaling and allows cells to regulate sensitivity to subsequent agonist exposure on both a short and long-term basis. Receptor internalization may also play a role in cell signaling, particularly in axon terminals (38). Agonist-induced internalization of CRFr, a G protein-coupled receptor, has been characterized in vitro using cultured pituitary cells, primary cortical cells, HEK-293, and CHO-K1 cells (20,21,22,23,39,40). Our recent study provided the first in vivo evidence of agonist-induced CRFr internalization (24). This was produced in LC neurons by a physiologically relevant dose of CRF. Functional correlates of agonist-induced internalization can be seen as acute tachyphylaxis of LC neurons to cumulative local application of CRF (9). Although CRFr internalization was not examined at time points later than 30 min in that study, physiological studies showing long-term desensitization of LC neurons to CRF after a single intracerebroventricular dose suggests that this may be an enduring phenomenon that is related to receptor down-regulation (15).
The present study demonstrates that CRFr internalization is more than a pharmacological phenomenon, but occurs with a stressor, underscoring the functional relevance of this process. Taken with the attenuation of CRFr internalization by prior treatment with a CRF1 antagonist, this provides evidence that CRF is released during stress to act on CRF1 receptors in LC neurons. The inability of antalarmin to completely prevent stress-induced CRFr internalization could be related to suboptimal onset and/or duration of antagonist effect with respect to the time course of CRFr internalization. Interestingly, some CRF antagonists have been reported to induce CRFr internalization through pathways that differ from agonist-induced internalization (21). However, this requires antagonist binding at both the extracellular and juxtacellular receptor domains, and nonpeptide antagonists such as antalarmin bind solely to the juxtacellular domain and so are not likely to do this.
The consistency in the mean ratio of cytoplasmic to total silver grains in basal (^0.5) and stimulated (∼0.8) states was striking. This was seen between experiments in the present study and between studies involving CRF or swim stress-induced stimulation (24) (present study). Interestingly, a study examining cellular localization of dopamine 1 and muscarinic 2 receptors in striatum under basal and pharmacologically stimulated conditions reported similar ratios (41,42,43). This may reflect common underlying processes for internalization of G protein-coupled receptors.
Intracellular fate of CRFr
It is of great interest to identify the fate of internalized receptors, because this determines the duration of any changes in cellular sensitivity or whether the receptor plays a role in other cellular processes. For example, internalized GH receptor stimulates mitochondrial function (44). Using endosomal markers, Holmes et al. (39) suggested that internalized CRFr in HEK-293 and primary cortical cells was associated with early endosomes and transited to recycling endosomes, as opposed to degradative lysosomes, suggestive of a rapid recycling process. Consistent with this is evidence that CRFr behaves like a class A G protein-coupled receptor that interacts transiently with β-arrestin and therefore can be recycled more rapidly (40). These studies have all used in vitro preparations. In vivo, CRFr that becomes internalized in LC dendrites after either agonist stimulation or stress is also associated with endosomes (24) (present study). However, the present study shows a progression from associations with early endosomes to multivesicular bodies as time after stress increases. Because multivesicular bodies often provide a route to the lysosomal system, this path may favor degradation/down-regulation of CRFr. Such a cellular effect would be consistent with the relatively enduring electrophysiological changes in LC sensitivity to CRF that have been reported (15,16,18,30). Increased associations of CRFr with the Golgi apparatus at a later time after stress could reflect synthesis of new receptor. Alternatively, the associations with vesicular bodies and the Golgi may reflect communication between the two compartments that is involved in recycling. More studies using labeled receptor proteins would be required to make conclusions regarding the temporal aspects of new receptor formation.
Functional significance of swim stress-induced CRFr internalization
Prior exposure to swim stress changes LC sensitivity to CRF in a complex way, as indicated by the shift in the CRF dose-response curve for LC activation (30,45). Twenty-four hours after swim stress, LC neurons are sensitized to CRF in that they are activated by doses of CRF that would be ineffective in control rats. However, the CRF dose-response curve plateaus at a lower response. A similar type of shift occurs in rats that have had repeated sessions of foot shock (30). It is speculated that the sensitization to low doses of CRF is a result of altered receptor coupling, whereas the plateau reflects a loss of receptor at the plasma membrane as a result of internalization. This would be a good mechanism for blunting the response of the LC-NE system to high levels of CRF but maintaining a degree of sensitivity so that the system is still able to respond to a challenge. The temporal correlation between LC sensitivity and magnitude of internalization supports the causality of these cellular events. Interestingly, the changes in LC sensitivity to CRF observed 24 h after swim stress occur in male but not female rats (45). Rather, LC neurons of control female rats are more sensitive to CRF compared with male rats, and prior swim stress merely produces a slight shift to the right in the CRF dose-response curve of female rats. This suggests the intriguing possibility of sex differences in cellular trafficking of CRFr that are expressed as differences in the stress sensitivity of the LC-NE system.
Stress-induced CRFr internalization in axon terminals was observed sometimes as we reported previously (24). Internalization of receptors in axon terminals has been reported in cannabinoid receptors in hippocampus (38) and neurotensin in neostriatal synaptosomes (46). Retrograde transport of the receptor-endosome complex toward the soma has been shown and suggests a role for the internalized receptor in cellular signaling (38). Localization of receptors in the axon terminals may function as autoreceptors and heteroreceptors to exert presynaptic inhibition of transmitter release as shown by serotonin 1B receptor (47). Whether CRFr in the axon terminals serves as heteroreceptor or autoreceptor in the present study is not known. Nevertheless, localization of CRFr in axon terminals suggests potential synaptic effects of CRF to LC neurons.
In summary, using electron microscopy, evidence was provided for stress-induced internalization of CRFr in LC neurons. By governing the sensitivity of the LC-NE system to stress, this dynamic cellular process may regulate the magnitude of emotional arousal in response to a stressor.
Acknowledgments
We thank Dr. Stephen C. Gammie for the CRF1 knockout and wild-type mice. We thank Mr. Ronaldo Magtoto for expert technical assistance.
Footnotes
Disclosure Statement: The authors have nothing to disclose.
This work was supported by U.S. Public Health Service Grants MH40008, DA09082, and DA15395 (Research Career Award to E.J.V.) and a NARSAD Distinguished Investigator Award (R.J.V.).
First Published Online October 18, 2007
Abbreviations: CRF, Corticotropin-releasing factor; LC, locus coeruleus; NE, norepinephrine; PB, phosphate buffer; TBS, Tris-buffered saline; TH, tyrosine hydroxylse.
References
- Vale WW, Spiess J, Rivier C, Rivier J 1981 Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and β-endorphin. Science 213:1394–1397 [DOI] [PubMed] [Google Scholar]
- De Souza EB 1987 Corticotropin-releasing factor receptors in the rat central nervous system: characterization and regional distribution. J Neurosci 7:88–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens MJ, Nemeroff CB 1991 Physiology and pharmacology of corticotropin-releasing factor. Pharmacol Rev 43:425–473 [PubMed] [Google Scholar]
- Sakanaka M, Shibasaki T, Lederes K 1987 Corticotropin-releasing factor-like immunoreactivity in the rat brain as revealed by a modified cobalt-glucose oxide-diaminobenzidene method. J Comp Neurol 260:256–298 [DOI] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE, Rivier J, Vale WW 1983 Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: an immunohistochemical study. Neuroendocrinology 36:165–186 [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Foote SL, Page ME 1993 The locus coeruleus as a site for integrating corticotropin-releasing factor and noradrenergic mediation of stress responses. Ann NY Acad Sci 697:173–188 [DOI] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Colago EEO, Valentino RJ 1996 Corticotropin-releasing factor-containing axon terminals synapse onto catecholamine dendrites and may presynaptically modulate other afferents in the rostral pole of the nucleus locus coeruleus in the rat brain. J Comp Neurol 364:523–534 [DOI] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Colago EEO, Valentino RJ 1998 Amygdaloid corticotropin-releasing factor targets locus coeruleus dendrites: substrate for the co-ordination of emotional and cognitive limbs of the stress response. J Neuroendocrinol 10:743–757 [DOI] [PubMed] [Google Scholar]
- Curtis AL, Florin-Lechner SM, Pavcovich LA, Valentino RJ 1997 Activation of the locus coeruleus noradrenergic system by intracoerulear microinfusion of corticotropin-releasing factor: effects on discharge rate, cortical norepinephrine levels and cortical electroencephalographic activity. J Pharmacol Exp Ther 281:163–172 [PubMed] [Google Scholar]
- Smagin GN, Swiergiel AH, Dunn AJ 1995 Corticotropin-releasing factor administered into the locus coeruleus, but not the parabrachial nucleus, stimulates norepinephrine release in the prefrontal cortex. Brain Res Bull 36:71–76 [DOI] [PubMed] [Google Scholar]
- Curtis AL, Bello NT, Valentino RJ 2001 Evidence for functional release of endogenous opioids in the locus coeruleus during stress termination. J Neurosci 21:1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechner SM, Curtis AL, Brons R, Valentino RJ 1997 Locus coeruleus activation by colon distention: role of corticotropin-releasing factor and excitatory amino acids. Brain Res 756:114–124 [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Page ME, Curtis AL 1991 Activation of noradrenergic locus coeruleus neurons by hemodynamic stress is due to local release of corticotropin-releasing factor. Brain Res 555:25–34 [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Rajkowski J, Cohen J 1999 Role of locus coeruleus in attention and behavioral flexibility. Biol Psychiatry 46:1309–1320 [DOI] [PubMed] [Google Scholar]
- Conti LH, Foote SL 1995 Effects of pretreatment with corticotropin-releasing factor on the electrophysiological responsivity of the locus coeruleus to subsequent corticotropin-releasing factor challenge. Neuroscience 69:209–219 [DOI] [PubMed] [Google Scholar]
- Conti LH, Foote SL 1996 Reciprocal cross-desensitization of locus coeruleus electrophysiological responsivity to corticotropin-releasing factor and stress. Brain Res 722:19–29 [DOI] [PubMed] [Google Scholar]
- Xu G-P, Van Bockstaele EJ, Reyes BAS, Bethea T, Valentino RJ 2004 Chronic morphine sensitizes the brain norepinephrine system to corticotropin-releasing factor and stress. J Neurosci 24:8193–8197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis AL, Pavcovich LA, Valentino RJ 1999 Long term regulation of locus coeruleus sensitivity to corticotropin-releasing factor by swim stress. J Pharmacol Exp Ther 289:1211–1219 [PubMed] [Google Scholar]
- Segredo V, Burford NT, Lameh J, Sadee W 1997 A constitutively internalizing and recycling mutant of the μ-opioid receptor. J Neurochem 68:2395–2404 [DOI] [PubMed] [Google Scholar]
- Childs GV, Morell JL, Niendorf A, Aguilera G 1986 Cytochemical studies of corticotropin-releasing factor (CRF) receptors in anterior lobe corticotropes: binding, glucocorticoid regulation, and endocytosis of [biotinyl-Ser1]CRF. Endocrinology 119:2129–2142 [DOI] [PubMed] [Google Scholar]
- Perry SJ, Junger S, Kohout TA, Hoare SR, Struthers RS, Grigoriadis DE, Maki RA 2005 Distinct conformations of the corticotropin releasing factor type receptor adapted following agonist and antagonist binding are differentially regulated. J Biol Chem 280:11560–11568 [DOI] [PubMed] [Google Scholar]
- Rasmussen TN, Novak I, Nielsen SM 2004 Internalization of the human CRF receptor 1 is dependent of classical phosphorylation sites and of β-arrestin 1 recruitment. Eur J Biochem 271:4366–4374 [DOI] [PubMed] [Google Scholar]
- Westlund KN, Wynn PC, Chmielowiec S, Collins TJ, Childs GV 1984 Characterization of a potent biotin-conjugated CRF analog and the response of anterior pituitary corticotropes. Peptides 5:627–634 [DOI] [PubMed] [Google Scholar]
- Reyes BAS, Fox K, Valentino RJ, Van Bockstaele EJ 2006 Agonist-induced internalization of corticotropin-releasing factor receptors in noradrenergic neurons of the rat locus coeruleus. Eur J Neurosci 23:2991–2998 [DOI] [PubMed] [Google Scholar]
- Leranth C, Pickel VM 1989 Electron microscopic preembedding double-labeling methods. In: Heimer L, Zaborszky L, eds. Neuroanatomical tracing methods 2. 1st ed. New York: Plenum Press; 129–172 [Google Scholar]
- Peters A, Palay SL, Webster Hd 1991 The fine structure of the nervous system. New York: Oxford University Press [Google Scholar]
- Bishop GA, Seelandt CM, King JS 2000 Cellular localization of corticotropin-releasing factor receptors in the adult mouse cerebellum. Neuroscience 101:1083–1092 [DOI] [PubMed] [Google Scholar]
- Sauvage M, Steckler T 2001 Detection of corticotropin-releasing hormone receptor 1 immunoreactivity in cholinergic, dopaminergic and noradrenergic neurons of the murine basal forebrain and brainstem nuclei-potential implication for arousal and attention. Neuroscience 104:643–652 [DOI] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Commons KG 2001 Internalization of mu-opioid receptors produced by etorphine in the rat locus coeruleus. Neuroscience 108:466–477 [DOI] [PubMed] [Google Scholar]
- Curtis AL, Pavcovich LA, Grigoriadis DE, Valentino RJ 1995 Previous stress alters corticotropin-releasing factor neurotransmission in the locus coeruleus. Neuroscience 65:541–550 [DOI] [PubMed] [Google Scholar]
- Jedema HP, Grace AA 2004 Corticotropin-releasing hormone directly activates noradrenergic neurons of the locus coeruleus recorded in vitro. J Neurosci 24:9703–9713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Pett K, Viau V, bittencourt JC, Chan RK, Li HY, Arias C, Prins GS, Perrin M, Vale W, Sawchenko PE 2000 Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J Comp Neurol 428:191–212 [DOI] [PubMed] [Google Scholar]
- Chen Y, Brunson KL, Muller MB, Cariaga W, Baram TZ 2000 Immunocytochemical distribution of corticotropin-releasing hormone receptor type-1 (CRF(1))-like immunoreactivity in the mouse brain: light microscopy analysis using an antibody directed against the C-terminus. J Comp Neurol 420:305–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lujan R, Nusser Z, Roberts JD, Shigemoto R, Somogyi P 1996 Perisynaptic location of metabotropic glutamate receptors mGluR1 and mGluR5 on dendrites and dendritic spines in the rat hippocampus. Eur J Neurosci 8:1488–1500 [DOI] [PubMed] [Google Scholar]
- Chan J, Aoki C, Pickel VM 1990 Optimization of differential immunogold-silver and peroxidase labeling with maintenance of ultrastructure in brain sections before plastic embedding. J Neurosci Methods 33:113–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefkowitz RJ 1998 G protein-coupled receptors. III. New roles for receptor kinases and β-arrestins in receptor signaling and desensitization. J Biol Chem 273:18677–18680 [DOI] [PubMed] [Google Scholar]
- Yu SS, Lefkowitz RJ, Hausdorff WP 1993 β-Adrenergic receptor sequestration. A potential mechanism of receptor resensitization. J Biol Chem 268:337–341 [PubMed] [Google Scholar]
- Coutts AA, Anavi-Goffer S, Ross RA, MacEwan DJ, Mackie K, Pertwee RG, Irving AJ 2001 Agonist-induced internalization and trafficking of cannabinoid CB1 receptors in hippocampal neurons. J Neurosci 21:2425–2433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes KD, Babwah AV, Dale LB, Poulter MO, Ferguson SS 2006 Differential regulation of corticotropin releasing factor 1α receptor endocytosis and trafficking by β-arrestins and Rab GTPases. J Neurochemistry 96:934–949 [DOI] [PubMed] [Google Scholar]
- Oakley RH, Olivares-Reyes JA, Hudson CC, Flores-Vega F, Dautzenberg FM, Hauger RL 2007 Carboxyl-terminal and intracellular loop sites for CRF1 receptor phosphorylation and β-arrestin-2 recruitment: a mechanism regulating stress and anxiety responses. Am J Physiol Regul Integr Comp Physiol 293:R209–R222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard V, Laribi O, Levey AI, Bloch B 1998 Subcellular redistribution of m2 muscarinic acetylcholine receptors in striatal interneurons in vivo after acute cholinergic stimulation. J Neurosci 18:10207–10218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch B, Bernard V, Dumartin B 2003 “In vivo” intraneuronal trafficking of G protein coupled receptors in the striatum: regulation by dopaminergic and cholinergic environment. Biol Cell 95:477–488 [DOI] [PubMed] [Google Scholar]
- Dumartin B, Caille I, Gonon F, Bloch B 1998 Internalization of D1 dopamine receptor in striatal neurons in vivo as evidence of activation by dopamine agonists. J Neurosci 18:1650–1661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perret-Vivancos C, Abbate A, Ardail D, Raccurt M, Usson Y, Lobie PE, Morel G 2006 Growth hormone activity in mitochondria depends on GH receptor Box 1 and involves caveolar pathway targeting. Exp Cell Res 312:215–232 [DOI] [PubMed] [Google Scholar]
- Curtis AL, Bethea T, Valentino RJ 2006 Sexually dimorphic responses of the brain norepinephrine system to stress and corticotropin-releasing factor. Neuropsychopharmacology 31:544–554 [DOI] [PubMed] [Google Scholar]
- Nguyen HMK, Cahill CM, McPherson PS, Beaudet A 2002 Receptor-mediated internalization of [3H]-neurotensin in synaptosomal preparations from rat neostriatum. Neuropharmacology 42:1089–1098 [DOI] [PubMed] [Google Scholar]
- Morikawa H, Manzoni OJ, Crabbe JC, Williams JT 2000 Regulation of central synaptic transmission by 5-HT(1B) auto- and heteroreceptors. Mol Pharmacol 58:1271–1278 [DOI] [PubMed] [Google Scholar]