Abstract
Signal transducer and activator of transcription (Stat)5a is a well-established regulator of mammary gland development. Several pathways for activating Stat5a have been identified, but little is known about the mechanisms that regulate its expression in this tissue. In this report, we used immunofluorescent staining to examine Stat5a expression in mammary epithelial cells during normal development and in response to treatment with the ovarian hormones estrogen (E) and progesterone (P). Stat5a was present at very low levels in the prepubertal gland and was highly induced in a subset of luminal epithelial cells during puberty. The percentage of positive cells increased in adult virgin, pregnant, and lactating animals, dropped dramatically during involution, and then increased again after weaning. Ovariectomy ablated Stat5a expression in virgin animals, and treatment with both E and P was necessary to restore it. Double-labeling experiments in animals treated with E plus P for 3 d demonstrated that Stat5a was localized exclusively to cells containing both E and P receptors. Together, these results identify a novel role for E and P in inducing Stat5a expression in the virgin mammary gland and suggest that these hormones act at the cellular level through their cognate receptors.
SIGNAL TRANSDUCER AND activator of transcription (Stat)5 plays an important role in mammary gland development. Two isoforms of Stat5, a and b, are produced by separate genes (1) and act as signaling mediators involved in numerous cellular functions including proliferation, differentiation, and survival (2,3,4). They are members of the Stat family of proteins, which are latent transcription factors that localize to the cytoplasm until activated by a variety of cytokines, growth factors, and hormones (5,6,7). Binding of these ligands to their cognate receptors activates either an intrinsic receptor kinase domain or an associated Jak kinase, which then recruit and phosphorylate Stat proteins (8,9). Phosphorylated Stat proteins translocate to the nucleus where they bind DNA and activate responsive genes.
The majority of studies of Stat5 in the mammary gland have focused on its role in pregnancy and lactation. When both a and b isoforms were deleted, Stat5 −/− mice exhibited reduced alveolar expansion during pregnancy and a lactational defect (10). A similar phenotype was seen when only the Stat5a isoform was deleted, but Stat5b-deficient mice had a much less severe mammary gland defect (10,11,12). This indicates a specific requirement for Stat5a during mammary gland development, which is consistent with the fact that it is the predominant isoform expressed in this tissue (10).
In terms of specific functions, Stat5a has been reported to play important roles in mammary cell differentiation, proliferation, and survival. In the final stages of mammary cell differentiation, it activates expression of genes encoding milk constituents such as α-lactalbumin, β-casein, and whey acidic protein (WAP) (10,13). Further supporting a role for Stat5a in differentiation, recent studies have demonstrated that after parturition, Stat5 −/− epithelial cells lack a specific marker of alveolar cells (Npt2b) while retaining a marker of virgin-like ductal cells (NKCC1) (14,15). Stat5a may also play a role in the proliferative response to estrogen (E) and progesterone (P) during pregnancy, because 5-bromo-2-deoxyuridine (BrdU) incorporation was significantly decreased in Stat5 −/− mouse mammary epithelial cells in response to E+P treatment (14,15). Finally, conditionally deleting Stat5 (a and b) during pregnancy induced premature cell death, indicating that it is critical for cell survival at this developmental stage (14).
Developmental studies using Northern and Western blotting of whole murine mammary gland homogenates demonstrated that Stat5a is present in both the immature and mature virgin, increases during pregnancy, and reaches a maximal level during late pregnancy and lactation (16,17). However, expression in the stromal and epithelial compartments could not be discriminated using these approaches, and the increase in Stat5a might therefore reflect increased epithelial cell number rather than increased expression per epithelial cell. In addition, Western blotting does not permit one to localize expression to specific epithelial structures such as ducts, end buds, and alveoli. A more recent study used immunohistochemistry to examine total Stat5 expression in mouse mammary epithelium. They found that Stat5 was expressed in the adult virgin as well as the pregnant gland, but immature animals were not examined (18). Surprisingly, the majority of Stat5 was activated, even in virgin animals. In the mammary gland, Stat5 can be activated by GH, epidermal growth factor, or prolactin (PRL) (19,20). In lactating animals, Stat5a induces expression of milk protein genes, largely in response to PRL, and Stat5a activation in virgin animals also seems to be predominantly due to PRL (18).
Although the pathways leading to Stat5a activation have been extensively studied, little information is available regarding the regulation of its expression in mammary cells. Progestin treatment was reported to induce Stat5a transcription in the human breast cancer cell line, T47D. There are two forms of the progesterone receptor (PR), PRA and PRB (21), and Stat5a induction occurred only in cells expressing the PRB isoform (22). However, the factors regulating Stat5a expression in the normal mammary gland have not been identified. In the current work, we have used immunofluorescence to study Stat5a expression in mouse mammary epithelial cells during normal development and in response to hormone treatments. The results demonstrate that Stat5a expression increases dramatically at puberty and after ovariectomy in response to the ovarian hormones E and P. Furthermore, Stat5a extensively colocalizes with both estrogen receptors (ER) and PR, suggesting that it is a target of these receptors and may mediate some of the effects of E and P in the mammary gland.
Materials and Methods
Animal experiments
Female 18-wk-old virgin BALB/c mice from our own colony were bilaterally ovariectomized 1 wk before hormone treatment. Animals were injected sc in the dorsum of the neck with saline, 17β-estradiol (Sigma Chemical Co., St. Louis, MO) (1 μg), P (Sigma) (1 mg), or the combination of both hormones every 24 h for either 3 or 10 consecutive days. PRL (Sigma) was injected sc every 12 h (1 μg/g body weight per day). For bromocriptine (BC) experiments, 0.5 mg BC (Sigma) was suspended in 0.1 ml sesame oil (Sigma) and administered sc every 24 h for 3 d. This amount of BC is reported to be adequate for a sustained suppression of serum PRL levels for 24 h (23). Animals were injected ip with 70 μg/g body weight of BrdU (Sigma) 2 h before they were euthanized. All animal experimentation was conducted in accordance with accepted standards of humane animal care and approved by the All University Committee on Animal Use and Care at Michigan State University.
Antibodies
Rabbit polyclonal α-Stat5a (catalog item sc-1081), goat polyclonal α-phospho Stat5 (sc-11761), and rabbit polyclonal α-WAP (sc-25526) antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Mouse monoclonal α-Stat5a (catalog item 611834) antibody was obtained from BD Biosciences (San Jose, CA). Goat polyclonal α-receptor activator of nuclear factor-κB ligand (α-RANKL) (AF462) antibody was obtained from R&D Systems (Minneapolis, MN). Mouse monoclonal α-smooth muscle actin (α-SMA) antibody (clone 14A) was from Sigma. Mouse monoclonal α-human ERα antibody (NCL-ER-6F11) was obtained from Novocastra Laboratories (Newcastle, UK). Rabbit polyclonal α-PR, which detects only PRA (catalog item A0098) was obtained from Dako (Carpinteria, CA). Rabbit polyclonal α-PRB was produced commercially using a peptide corresponding to 15 amino acids unique to mouse PRB by Affinity Bioreagents (Golden, CO) and has been verified to detect only PRB (24). Mouse monoclonal α-BrdU antibody was provided in a kit from Amersham Biosciences (Piscataway, NJ). All secondary antibodies were conjugated to Alexa fluor dyes and were obtained from Invitrogen (Carlsbad, CA).
Immunohistochemistry
Inguinal mammary glands were fixed in 10% phosphate-buffered formalin overnight at 4 C, dehydrated, cleared, and embedded in paraffin. Tissue was sectioned to 5 μm, mounted on 3-aminopropyl triethoxysilane-coated coverslips, and allowed to dry for 24 h at room temperature. The tissue was then dewaxed and rehydrated through a descending gradient of ethanol. Sections were autoclaved (121 C at 15 psi) for 20 min in 0.01 m sodium citrate buffer (pH 6.0) for antigen retrieval. Specific blocking and antibody incubation protocols are detailed below. Each incubation step was followed by two 5-min washes in PBS.
For Stat5a labeling, sections were blocked for 30 min in 2% BSA in PBS, pH 7.3 (2% PBSA). Samples were first incubated with rabbit α-Stat5a antibody (in 2% PBSA, overnight at 4 C) and then incubated with goat α-rabbit antibody conjugated to Alexa 488 (green) (in PBS, 30 min). For double-labeling experiments, sections were first blocked with goat α-mouse IgG Fab fragments (Jackson ImmunoResearch Laboratories, West Grove, PA) (1:100 1% PBSA for 1 h) and then blocked with normal goat serum (Vector Laboratories, Burlingame, CA) (1:1 in PBS for 30 min). The tissue was incubated with either mouse α-BrdU for 1 h or mouse α-SMA (in PBS), α-ERα (in PBS-0.5% Triton X-100) or α-Stat5a antibody (in PBS) overnight at 4 C. This was followed by incubation with goat α-mouse Alexa 488 for Stat5a-PRA double labeling or Alexa 546 (red) for all other staining (in PBS for 30 min). Samples were re-blocked with 2% PBSA for 30 min and incubated with rabbit α-Stat5a, α-WAP, α-PRA, or α-PRB antibody in 2% PBSA overnight at 4 C. This was followed by incubation with goat α-rabbit Alexa 546 for Stat5a-PRA labeling or Alexa 488 for all other staining (in PBS for 30 min). For Stat5a-RANKL double staining, sections were incubated with normal rabbit serum (1:1 in PBS for 30 min), α-RANKL antibody (in PBS and 0.5% Triton X-100 overnight at 4 C), rabbit α-goat Alexa 488 (30 min), goat α-mouse IgG Fab (1:100 in 1% PBSA for 1 h), normal goat serum (1:1 in PBS for 30 min), mouse α-Stat5a (overnight at 4 C), and goat α-mouse Alexa 546 (30 min). For Stat5a and SMA, WAP, RANKL, ERα, PRA, or BrdU staining, nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI) (Sigma) and pictures were taken on a Nikon Eclipse fluorescent microscope. Intensity measurements were made using MetaMorph 6 software. For Stat5a and PRB double staining, nuclei were counterstained using TOPRO-3 iodide (Molecular Probes, Eugene, OR; now Invitrogen), and visualized using a Pascal laser scanning confocal microscope (Zeiss, Oberkochen, Germany). For Stat5a visualization after BC treatment, nuclei were counterstained using TOPRO-3 iodide and visualized using a FluoView laser scanning confocal microscope (Olympus, Tokyo, Japan).
To detect phosphorylated Stat5, antigen retrieval was accomplished as previously described (20). After blocking in 10% normal rabbit serum (Vector) in PBS for 30 min, the sections were incubated overnight at 4 C with goat α-phospho-Stat5 antibody (in 10% normal rabbit serum in PBS). The tissue was then incubated with rabbit α-goat Alexa 488 antibody (in PBS for 30 min). Nuclei were stained with DAPI, and samples were visualized using a Nikon Eclipse microscope and MetaMorph software.
Results
Immunofluorescent localization and quantitation of Stat5a in epithelial cells during mammary gland development
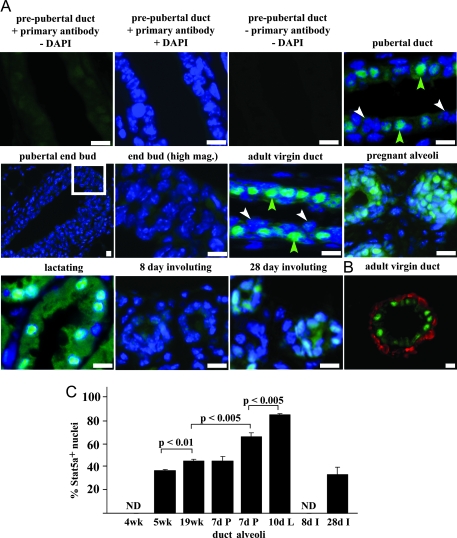
To examine Stat5a expression during mammary gland development, the level and pattern of expression was examined by immunofluorescence in mammary tissue from prepubertal (4 wk), pubertal (5 wk), adult (19 wk), pregnant (7 d), lactating (10 d), and involuting (8 and 28 d) animals (Fig. 1A). In 4-wk-old animals, a diffuse signal that was slightly above the secondary antibody alone control was observed. By 5 wk of age, intense staining was seen in a subpopulation of luminal epithelial cells that were present throughout the ducts in a punctate pattern. An exception to this punctuate pattern was noted in end buds, where only diffuse light staining was present. Most of the intense staining in ductal cells was nuclear, suggesting that the protein is activated even at this early age. In mature (19 wk) virgin animals, where the ducts have reached the limit of the fat pad and end buds are no longer present, the punctate distribution of positive cells persisted, and the staining remained predominantly nuclear.
Figure 1.
Immunofluorescent staining of Stat5a during mouse mammary gland development. A, Sections from 4-wk-old prepubertal, 5-wk-old pubertal, 19-wk-old mature adult, 7-d pregnant, 10-d lactating, and 8- and 28-d involuting mammary glands were stained with α-Stat5a antibody (green), and nuclei were labeled with DAPI (blue) as described in Materials and Methods. Control sections from 4-wk-old glands without primary antibody are also shown. Green arrowheads indicate Stat5a-positive cells and white arrowheads indicate Stat5a-negative cells. B, Mammary glands from mature (19-wk-old) mice were stained for SMA (red) and Stat5a (green). Scale bar for all images, 5 μm. C, Quantitation of nuclear Stat5a expression at different stages of mouse mammary gland development. Immunofluorescent staining using an α-Stat5a antibody was carried out as described in A on tissue sections from 4-, 5-, or 19-wk-old virgin, 7-d pregnant (P), 10-d lactating (L), and 8- or 28-d involuting (I) mice. The values represent the mean ± sem from three animals per group, with a minimum of 1000 cells per mouse analyzed, and significance was determined by Student’s t test. No nuclear staining was detected (ND) at 4 wk of age or 8 d of involution.
Because Stat5a is required for lobuloalveolar development and lactation, its expression was also examined in the pregnant, lactating, and involuting mammary gland (Fig. 1A). Intense nuclear Stat5a staining was observed in both ductal and alveolar cells in the pregnant mammary gland, and an increase in cytoplasmic staining was also observed in the Stat5a-positive alveolar cells. In sections from lactating animals, the vast majority of cells exhibited both intense nuclear and cytoplasmic Stat5a staining. Expression was dramatically decreased in the 8-d postlactational involuting gland, where staining was less intense than in the virgin gland and resembled that seen in the prepubertal gland. However, Stat5a staining was restored by 28 d after weaning and approached the level seen in the adult virgin gland. Double staining for Stat5a and the myoepithelial cell marker SMA demonstrated that Stat5a expression was limited to luminal epithelial cells (Fig. 1B).
Quantitation of the results shown in Fig. 1A revealed that 37% of luminal epithelial cells had intense nuclear Stat5a staining in 5-wk-old animals, and this increased to 46% in 19-wk-old animals (Fig. 1C). Stat5a was expressed at similar levels in ducts in the pregnant gland, where 45% of luminal epithelial cells were positive. Expression was higher in the alveoli, where 67% of cells were positive. Expression was highest during lactation, with 87% of cells staining positive. Stat5a staining was undetectable at d 8 of involution but was present 28 d after weaning, where 34% of cells were positive.
Regulation of Stat5a expression by ovarian hormones
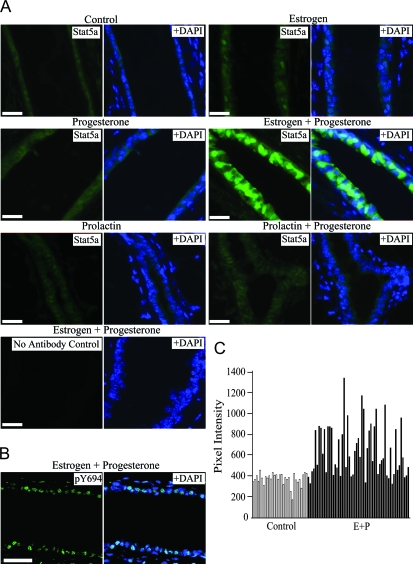
The appearance of Stat5a between 4 and 5 wk of age, when ovarian cycles typically begin, and the further increase during pregnancy suggested that its expression might be regulated by the ovarian hormones E and P. To test this possibility, 18-wk-old mice were ovariectomized and allowed to recover for 1 wk. They were then injected with E, P, E+P, or vehicle control once per day for 3 d, and their mammary glands were examined for Stat5a expression. After ovariectomy, control-treated animals had weak Stat5a staining (Fig. 2A), similar to that seen in the 4-wk-old, intact prepubertal gland (see Fig. 1A). Ovariectomized animals treated with P alone were indistinguishable from the control-treated mice. The E-treated animals had a slight increase in nuclear Stat5a staining in a few cells (Fig. 2A). In contrast, robust Stat5a expression was observed in both the nucleus and cytoplasm in E+P-treated mice, with 41 ± 1% of luminal epithelial cells being positive (Fig. 2A). Overall, both the percentage and distribution of Stat5a-positive cells in the 3-d E+P-treated animals were similar to that observed in the mammary glands of intact, mature mice (see Fig. 1A), indicating that expression of Stat5a in the mammary gland is largely dependent on ovarian hormones.
Figure 2.
Stat5a expression in hormone-treated mice. A, Virgin 18-wk-old mice were ovariectomized and then treated with vehicle control, E, P, PRL, E+P, or PRL+P for 3 d as described in Materials and Methods. Representative photomicrographs of Stat5a staining are shown. B, The 3-d E+P-treated mammary glands were stained with an α-pY694 Stat5 antibody. All images show Stat5a staining (green) in the left panel and the merged image with DAPI-stained nuclei in the right panel. Scale bar for all images, 20 μm. C, Quantitation of Stat5a staining intensity in E+P-treated mice. The total pixel intensity of every cell (nucleus plus cytoplasm) was measured from a single representative structure from the 3-d control (white bars) and E+P-treated (black bars) mice (each bar represents one cell).
Nevalainen et al. (18) previously used phosphospecific antibodies to demonstrate that Stat5 is activated in adult virgin mouse mammary epithelial cells, and the nuclear localization of Stat5a in the glands from E+P-treated animals (Fig. 2A) suggested that Stat5a is also phosphorylated and activated under these conditions. To determine whether this was the case, immunofluorescent staining was carried out with an antibody that detects both a and b Stat5 isoforms phosphorylated on tyrosine 694 (pY694) (Fig. 2B). When scored, 47 ± 7% of luminal epithelial cells from E+P-treated animals were positive for pY694, similar to the percentage of cells showing intense nuclear Stat5a staining.
Because the Stat5a observed after E+P treatment was predominantly nuclear, we considered the possibility that some or all of the apparent increase in expression was actually due to translocation of the protein into the nucleus. To address this issue, the staining intensities of individual cells (cytoplasm plus nucleus) in representative structures from the 3-d control and E+P-treated mice were quantitated and compared. Cells with a low level of staining in the E+P gland had similar intensities to the cells in the control gland (Fig. 2, A and C). However, cells with bright nuclear staining in the E+P gland had approximately twice the intensity of the other cells (Fig. 2, A and C). This increase in intensity demonstrates that total cellular Stat5a protein levels are increased in a subset of luminal epithelial cells in response to E+P treatment.
PRL treatment increased the amount of activated Stat5 in hypophysectomized animals (18), and E can induce PRL secretion (25,26). One potential role of E in these experiments could therefore be to induce PRL, which would then lead to Stat5a nuclear accumulation. To examine the possibility that PRL might increase expression as well as activation of Stat5a, ovariectomized virgin animals were treated for 3 d with PRL or PRL+P. Neither treatment increased Stat5a expression significantly compared with the ovariectomized control (Fig. 2A), indicating that E is not increasing Stat5a expression via PRL. It may, however, contribute to Stat5a activation and nuclear localization via PRL.
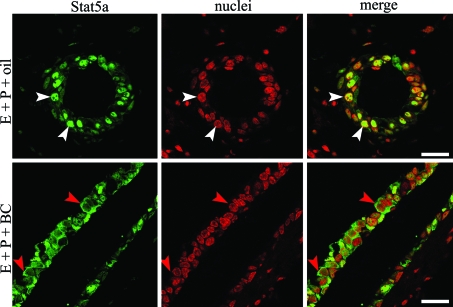
The results shown in Fig. 2 demonstrated that E+P treatment increased Stat5a expression in the mammary epithelium and that the protein was phosphorylated and localized to the nucleus. Because PRL is the major activator of Stat5a in the adult virgin gland (18), this suggested that E+P treatment in the absence of PRL would lead to increased protein without nuclear localization. To test this hypothesis, BC was used to inhibit the secretion of PRL from the pituitary gland. Ovariectomized mice were treated with E+P for 3 d as described above and were additionally given BC or vehicle control. Mammary glands were examined by confocal microscopy to assess the cellular localization of Stat5a. As expected, strong nuclear Stat5a staining was observed in the tissue from E+P+vehicle-treated mice (Fig. 3). In contrast, mammary epithelial cells from E+P+BC-treated mice had predominantly cytoplasmic Stat5a staining (Fig. 3). Based on these results, we propose a model where E+P induces the expression of Stat5a, which can subsequently be activated by PRL, leading to its nuclear localization.
Figure 3.
Cellular localization of Stat5a in BC-treated mice. The 18-wk-old virgin mice were ovariectomized and treated with E+P+vehicle control (oil) or E+P+BC for 3 d. Staining for Stat5a (green) and nuclei (red) was visualized with an Olympus laser scanning confocal microscope. White arrowheads indicate nuclear Stat5a staining, and red arrowheads indicate cytoplasmic Stat5a staining. Scale bar, 20 μm.
Colocalization of Stat5a with ER and PR
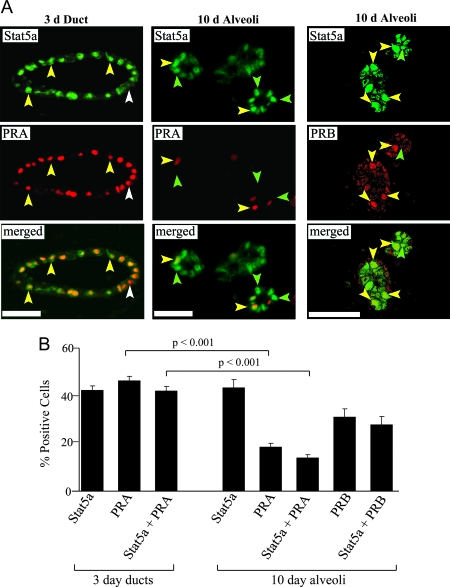
The induction of Stat5a expression by E+P suggested that the gene might be a target of the ER and/or PR. Previous studies in T47D human breast cancer cells have indicated that transcription of the Stat5a gene is induced by progestin in cells expressing PRB but not PRA (22). However, PRA is the dominant isoform observed in virgin mice, where it is present in ductal epithelial cells in a punctate pattern that is reminiscent of that seen with Stat5a (27). In contrast, PRB is not detectable in adult virgin mice, increases during pregnancy, and is predominately localized in alveoli. To determine whether Stat5a colocalizes with PRA or PRB in the mouse mammary gland, samples from the 3- and 10-d E+P-treated animals were examined by double indirect immunofluorescent staining using Stat5a and PR isoform-specific antibodies. After 3 d of E+P treatment, only ducts were scored because no alveoli were present. In these ductal structures, 42% of luminal cells were Stat5a positive, 46% were PRA positive, and 42% were both Stat5a and PRA positive (Fig. 4). Thus, after 3 ds of E+P treatment, virtually all cells expressing Stat5a contained PRA, but there was a small subset (∼9%) of PRA-positive cells that did not express Stat5a.
Figure 4.
Colocalization of Stat5a with PRA or PRB. A, Staining for Stat5a and PRA was carried out on sections from 3- and 10-d E+P-treated ovariectomized mice and was visualized with a Nikon Eclipse fluorescent microscope. Staining for Stat5a and PRB was carried out on sections from 10-d E+P-treated mice only and was visualized with a Pascal laser scanning confocal microscope. Yellow arrowheads indicate Stat5a and PRA or Stat5a and PRB colocalization (yellow nuclei). White arrowhead indicates Stat5a-negative, PRA-positive cell (red nucleus). Green arrowheads indicate Stat5a-positive, PRA- or PRB-negative cells (green nuclei). Scale bar, 20 μm. B, Quantitation of Stat5a and PR colocalization in 3- and 10-d E+P-treated animals. The values represent the mean ± sem from three animals per group, with a minimum of 800 cells per mouse analyzed. The percentage of PRA-positive cells and Stat5a/PRA-positive cells were both significantly lower in 10-d E+P alveolar structures than 3-d E+P ducts (P < 0.001) as determined by Student’s t test.
We previously observed that PRB is absent from ducts and is detectable only in alveoli during pregnancy (27) or after 5 or more days of E+P treatment (28). To determine whether Stat5a colocalizes with PRB, we examined Stat5a/PR colocalization in alveolar structures after 10 d of E+P treatment. As expected, PRB was not detected in ducts (data not shown) but was present in 31% of alveolar cells (Fig. 4). PRA was present in 18% of alveolar cells, compared with 47% of ductal cells from 3-d treated animals. When Stat5a expression was quantitated, 43% of alveolar cells were positive, which is similar to the percentage seen in the 3-d treated samples. Scoring for both Stat5a and PRA or PRB revealed that 35% of Stat5a-expressing cells were PRA positive, and 65% were PRB positive (Fig. 4). Because PRA and PRB are rarely expressed in the same cells in the mouse mammary gland (27), we infer from these data that virtually all Stat5a-positive alveolar cells are also PR positive, with roughly one third containing PRA and the other two thirds containing PRB.
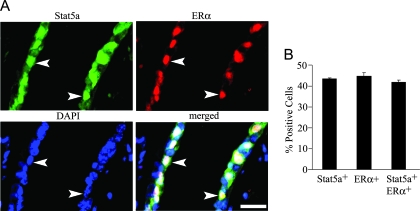
Because both E and P were required to induce Stat5a expression in ovariectomized animals, we examined whether ERα and Stat5a colocalize in ductal epithelial cells from 3-d E+P-treated mammary glands. Double staining revealed that the two proteins were highly colocalized, with 43% of cells being Stat5a positive, 45% ERα positive, and 42% both Stat5a and ERα positive (Fig. 5). Thus, virtually all Stat5a-positive cells in ducts contain ERα. Because PRA also colocalizes with Stat5a in ducts, this suggests that a subpopulation of ductal cells contain ERα, PRA, and Stat5a.
Figure 5.
Colocalization of Stat5a with ERα. A, Staining for Stat5a and ERα was carried out on sections from 3-d E+P-treated ovariectomized mice. White arrowheads indicate Stat5a- and ERα-positive cells. Scale bar, 20 μm. B, Quantitation of Stat5a and ERα colocalization in 3-d E+P-treated animals. The values represent the mean ± sem from three animals per group, with a minimum of 800 cells per mouse analyzed.
Colocalization of Stat5a and BrdU
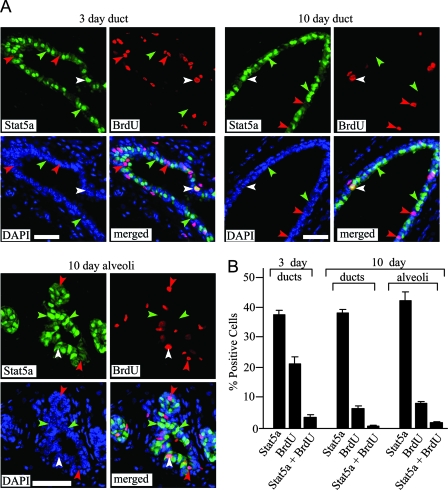
Mouse mammary glands lacking both Stat5a and -b (Stat5 −/−) display a significant reduction in epithelial cell proliferation in response to E+P treatment compared with wild-type glands, suggesting that Stat5 is a positive regulator of proliferation in response to these hormones (14,15). Because Stat5a is the predominant isoform in the mammary gland and Stat5a −/− mice display a more pronounced mammary gland developmental defect than Stat5b −/− mice, the effect on proliferation is likely due to the lack of Stat5a. To determine whether Stat5a-positive cells proliferate in response to E+P, 3- and 10-d E+P-treated animals were injected with BrdU 2 h before they were euthanized, and tissue sections were double labeled for BrdU and Stat5a. After 3 d of treatment, 38% of ductal epithelial cells were Stat5a positive, 21% were BrdU positive, and 3% were both Stat5a and BrdU positive (Fig. 6). Thus, only 9% of the cells expressing Stat5a were BrdU positive. A similar result was observed in the 10-d E+P-treated mice, where 6% of ductal cells and 8% of alveolar cells were BrdU positive, but only 2 and 4% of the Stat5a-expressing cells were BrdU positive, respectively (Fig. 6). When BrdU incorporation was compared between the three populations of cells (total, Stat5a positive and Stat5a negative), Stat5a-negative cells were three to six times more likely to incorporate BrdU than Stat5a-positive cells (P < 0.05) after either 3- or 10-d E+P treatments. Thus, although some Stat5a-positive cells incorporate BrdU in response to hormone treatment, they are underrepresented in the pool of proliferating cells.
Figure 6.
Immunodetection of Stat5a and BrdU in mammary glands of hormone-treated mice. Ovariectomized 19-wk-old mice were treated with E+P for 3 or 10 d, and animals were injected with BrdU 2 h before being euthanized as described in Materials and Methods. A, Representative structures from mammary glands showing Stat5a (green), BrdU (red), DAPI-stained nuclei (blue), and merged images. Green arrowheads indicate Stat5a-positive, BrdU-negative cells. Red arrowheads indicate BrdU-positive, Stat5a-negative cells. White arrowheads indicate BrdU-positive, Stat5a-positive cells. Scale bar, 25 μm. B, Quantitation of Stat5a and BrdU colocalization in 3- and 10-d E+P-treated animals. The values represent the mean ± sem from three animals per group, with a minimum of 800 cells per mouse analyzed.
Colocalization of Stat5a and WAP or RANKL
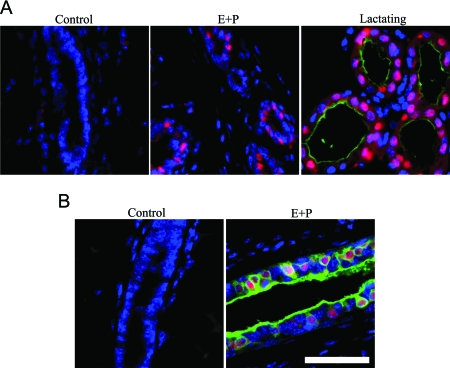
The observation that Stat5a is expressed at significant levels in the virgin gland prompted us to investigate potential target genes at this developmental stage. Stat5a is a well-established differentiation factor that induces the expression of milk protein genes including WAP in the lactating mammary gland. Immunohistochemistry was used to investigate whether this gene was also induced in Stat5a-expressing cells in E+P-treated mice. In agreement with previous studies, WAP staining was present on the luminal surface of alveoli when lactating mammary glands were examined (Fig. 7A), and a large percentage of the alveolar cells were Stat5a positive. As expected, both WAP and Stat5a were absent from mammary glands in control ovariectomized mice. Despite the induction of nuclear Stat5a in mammary glands from 3-d E+P-treated mice, no WAP staining was detected. Thus, the presence of Stat5a is not sufficient to induce the expression of the milk protein WAP in the virgin gland.
Figure 7.
Immunodetection of Stat5a and WAP or Stat5a and RANKL. Tissue sections from 3-d E+P-treated ovariectomized mice were stained for Stat5a and either WAP or RANKL. A, Representative structures showing merged images of Stat5a (red), WAP (green), and DAPI-stained nuclei (blue). B, Representative structures of merged images of Stat5a (red), RANKL (green), and DAPI-stained nuclei (blue). Scale bar, 25 μm.
In addition to its role in differentiation, Stat5a may also be a proliferation factor in the mammary gland. Because Stat5a-positive cells are less likely to proliferate than their Stat5a-negative neighbors (Fig. 6), it is likely that any proliferative activity of Stat5a is accomplished via a paracrine mechanism. One candidate paracrine factor is the TNF family member RANKL. RANKL is a secreted protein that is essential for normal mammary gland development (29). PRL induces RANKL expression in primary mammary epithelial cells, and experiments in heterologous cell culture models suggest that this is through the Jak2/Stat5a pathway (30). To investigate whether RANKL might be a Stat5a target in vivo, mammary gland sections from 3-d control or E+P-treated mice were stained for both Stat5a and RANKL. Mammary cells from control mice lacked both Stat5a and RANKL staining (Fig. 7B), and both were induced after 3 d of E+P treatment. In addition, there was a high level of colocalization of the two proteins (Fig. 7B), supporting the hypothesis that they are functionally linked.
Discussion
Despite the importance of Stat5a in mammary gland biology, very little is known about the mechanisms that regulate its expression in this tissue. To address this issue, we studied the pattern of Stat5a expression in mammary epithelial cells during normal development and in response to treatment with the ovarian hormones E and P. Immunofluorescent staining revealed that Stat5a was expressed at extremely low levels in the prepubertal gland. Expression increased at puberty, with 37% of luminal cells in ducts exhibiting bright nuclear staining. The percentage of Stat5a-positive cells increased significantly in adult virgin ducts, and increased further in alveolar cells during pregnancy. It was at its highest level during lactation when more than 80% of luminal epithelial cells had both nuclear and cytoplasmic staining, decreased dramatically during involution, and was then restored to near virgin levels. The increase in Stat5a expression at puberty suggested that ovarian steroid hormones induce its expression. This was confirmed by the finding that Stat5a levels dropped dramatically after ovariectomy, and treatment with E+P was necessary to restore it. Although pregnancy levels of hormones were used in these studies, it is likely that lower concentrations are sufficient to support Stat5a expression because it was present at high levels in the adult virgin gland. Activation and expression of Stat5 are not affected by variations in circulating hormone levels during the estrous cycle in mice (18), suggesting that relatively low levels of E and P are required for the amount of Stat5a observed in the virgin gland.
To investigate the mechanism by which E and P induce Stat5a, we examined the pattern of ERα, PR, and Stat5a expression by immunohistochemistry. After 3 or 10 d of E+P treatment, virtually all Stat5a-positive cells also contained either PRA or PRB, which is consistent with a model in which PR directly or indirectly activates the Stat5a gene. Because the two PR isoforms are rarely coexpressed in the same cell in the mouse mammary gland (27), these findings further suggest that either isoform can induce Stat5a expression in this system. This differs from the situation in human T47D breast cancer cells, where progestin induced Stat5a mRNA in cells expressing PRB but not those expressing PRA (22). One potential explanation for this difference is that we have examined Stat5a protein instead of mRNA. Our experiments do not therefore establish whether the observed increase in Stat5a levels after E+P treatment is due to increased gene transcription, mRNA stabilization, mRNA translation, protein stabilization, or a combination of the above.
The fact that combined E+P treatment was required to induce Stat5a in ovariectomized animals indicated an important role for E in regulating its expression. Because PR levels decrease significantly after ovariectomy (28,31), one role for E could be to induce PR, which would then activate the Stat5a gene. ERα is present in the almost all Stat5a/PRA-positive cells after 3 d of E+P treatment (Fig. 4), which is consistent with this hypothesis. An alternative possibility is that E and P, acting through their respective receptors, are independently required to induce Stat5a in PRA-positive cells. The situation in PRB-positive cells is more complex, because the majority of PRB-positive cells in the mammary gland do not contain ERα (28). However, E is required for high levels of PRB expression in 10-d E+P-treated animals (28) and may therefore contribute to Stat5a expression in these cells by indirectly regulating PRB levels.
We also considered a model in which E acts indirectly by increasing systemic PRL. It is well established that PRL activates Stat5 via the PRLR/Jak pathway (8,32,33), and E induces PRL secretion from the pituitary gland (25,26). To investigate whether this pathway also induces Stat5a expression, we examined glands from PRL and PRL+P-treated animals. Neither treatment reproduced the increase in Stat5a expression seen with E+P. However, when PRL secretion was suppressed by bromocriptine in E+P-treated mice, Stat5a was still induced but was localized predominantly in the cytoplasm. This leads us to propose a model in which E+P, acting through their receptors, induce Stat5a expression in luminal epithelial cells in the virgin mammary gland. The protein is then activated via the PRL/PRLR/Jak pathway, leading to its phosphorylation and nuclear accumulation. This model is consistent with the results of Nevalainen et al. (18), who demonstrated that treatment with PRL 24 h after hypophysectomy increased the level of phosphorylated Stat5 in mammary epithelial cells but did not affect the level of total cellular Stat5. It is likely that ovarian function was maintained for 24 h after hypophysectomy and that Stat5a was present but inactive. It is interesting to note that alternative mechanisms are likely to be responsible for the high levels of Stat5a expression during lactation, because E, P, and PR all decrease dramatically at parturition (34,35,36).
The importance of Stat5a during pregnancy and lactation is well established (10,11,14,15), and the presence of activated Stat5 in the adult virgin gland suggested that it also has a function before pregnancy (18). The current observation that Stat5a appears in epithelial cells at puberty, when the mammary gland is undergoing ductal development, suggests that it regulates growth or differentiation in the virgin gland. The absence of Stat5a in terminal end bud cells argues against a role in ductal elongation and suggests a possible role in differentiation or branching. The phenotype of Stat5a-deficient mice should help to provide insight into its function. Although no overt mammary phenotype was initially noted in Stat5a −/− virgin mice (10,11), one report using mammary transplants indicated that Stat5a −/− epithelium had maintained terminal end buds and reduced secondary branching at 8 wk compared with wild-type epithelium (37). The reduction in branching suggests that Stat5a is required for the differentiation of cells that are programmed to become alveolar cells during pregnancy. Such a lack of alveolar progenitor cells could then account for the stunted alveolar development seen in pregnant Stat5a −/− mice. The alveolar-like structures that do exist in Stat5 −/− mice do not express the normal alveolar differentiation marker Npt2b and retain a marker normally seen only in ducts, NKCC1 (14,15), indicating that even those alveolar structures that do arise are not fully differentiated. In addition to its potential role in differentiation in the virgin gland, Stat5 has been reported to positively regulate proliferation in the pregnant or E+P-treated mammary gland (14,15). The present results demonstrate that the majority of cells proliferating in response to E+P do not express Stat5a, indicating that this isoform could stimulate proliferation only through a paracrine mechanism.
To fully understand the role of Stat5a in the virgin mammary gland, it will be important to identify its downstream targets. The WAP gene is induced by Stat5a during lactation, but the protein is not expressed at detectable levels in Stat5a-positive cells in the virgin gland. Thus, it is likely that novel Stat5a targets remain to be identified. One potential candidate is the secreted cytokine RANKL. RANKL expression in mouse mammary cells in vivo requires E, P, and PRL (Fig. 7 and Ref. 30), and in vitro experiments indicate that PRL is acting through Stat5a (30). Furthermore, Stat5a and RANKL −/− mice exhibit similar mammary gland phenotypes (10,11,29), suggesting that the two genes may act in the same pathway. RANKL promotes cell cycle progression by binding to its receptor, RANK, and initiating a signaling cascade leading to activation of the cyclin D1 gene (38). Our results demonstrate that RANKL colocalizes with Stat5a in the mammary epithelium (Fig. 7). This is consistent with the hypothesis that Stat5a induces RANKL, which then stimulates proliferation in adjacent, Stat5a-negative cells. However, it is also possible that Stat5a and RANKL are independently induced by E+P. A second candidate Stat5a target in the virgin gland is Wnt-4. Wnt-4 is required for ductal side branching and has been implicated as a mediator of the paracrine effects of P in mammary gland development (39). Because Stat5a colocalizes with PR and is induced by P, it could be an intermediate in a PR/Stat5a/Wnt-4 pathway.
In summary, the experiments reported here identify a novel role for E and P as critical regulators of Stat5a expression in the pubertal and adult virgin mammary gland and indicate that this regulation likely occurs at the cellular level through epithelial ERα and PR. The newly synthesized Stat5a is then phosphorylated via PRL signaling, leading to accumulation of the active transcription factor in the nucleus where it can induce genes involved in differentiation and/or proliferation. Our results further indicate that any proliferative effects of Stat5a are through a paracrine mechanism and suggest that RANKL may mediate these or other effects. A detailed analysis of the developmental phenotypes of Stat5a −/− mice, and identification of Stat5a target genes, will be required to fully elucidate its functions in the virgin mammary gland.
Acknowledgments
We thank Mark Aupperlee for his help providing materials and assistance and Brenda Marrero for helping with the RANKL staining.
Footnotes
This publication was made possible by the Breast Cancer and the Environment Research Centers Grant 1-U01 ES/CA O12800 01 from the National Institute of Environmental Health Sciences (NIEHS), and the National Cancer Institute (NCI), National Institutes of Health (NIH), Department of Health and Human Services. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIEHS or NCI, NIH.
Disclosure Statement: The authors have nothing to disclose.
First Published Online September 20, 2007
Abbreviations: BC, Bromocriptine; BrdU, 5-bromo-2-deoxyuridine; DAPI, 4′,6-diamidino-2-phenylindole; E, estrogen; ER, estrogen receptor; PBSA, BSA in PBS, pH 7.3; P, progesterone; PR, progesterone receptor; PRL, prolactin; RANKL, receptor activator of nuclear factor-κB ligand; SMA, smooth muscle actin; Stat, signal transducer and activator of transcription; WAP, whey acidic protein.
References
- Liu X, Robinson GW, Gouilleux F, Groner B, Hennighausen L 1995 Cloning and expression of Stat5 and an additional homologue (Stat5b) involved in prolactin signal transduction in mouse mammary tissue. Proc Natl Acad Sci USA 92:8831–8835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard WJ, O’Shea JJ 1998 Jaks and STATs: biological implications. Annu Rev Immunol 16:293–322 [DOI] [PubMed] [Google Scholar]
- Akira S 1999 Functional roles of STAT family proteins: lessons from knockout mice. Stem Cells 17:138–146 [DOI] [PubMed] [Google Scholar]
- Buitenhuis M, Coffer PJ, Koenderman L 2004 Signal transducer and activator of transcription 5 (STAT5). Int J Biochem Cell Biol 36:2120–2124 [DOI] [PubMed] [Google Scholar]
- Darnell Jr JE 1997 STATs and gene regulation. Science 277:1630–1635 [DOI] [PubMed] [Google Scholar]
- Darnell Jr JE, Kerr IM, Stark GR 1994 Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science 264:1415–1421 [DOI] [PubMed] [Google Scholar]
- Ihle JN 1996 STATs: signal transducers and activators of transcription. Cell 84:331–334 [DOI] [PubMed] [Google Scholar]
- Ihle JN, Kerr IM 1995 Jaks and Stats in signaling by the cytokine receptor superfamily. Trends Genet 11:69–74 [DOI] [PubMed] [Google Scholar]
- Schindler C, Darnell Jr JE 1995 Transcriptional responses to polypeptide ligands: the JAK-STAT pathway. Annu Rev Biochem 64:621–651 [DOI] [PubMed] [Google Scholar]
- Teglund S, McKay C, Schuetz E, van Deursen JM, Stravopodis D, Wang D, Brown M, Bodner S, Grosveld G, Ihle JN 1998 Stat5a and Stat5b proteins have essential and nonessential, or redundant, roles in cytokine responses. Cell 93:841–850 [DOI] [PubMed] [Google Scholar]
- Liu X, Robinson GW, Wagner KU, Garrett L, Wynshaw-Boris A, Hennighausen L 1997 Stat5a is mandatory for adult mammary gland development and lactogenesis. Genes Dev 11:179–186 [DOI] [PubMed] [Google Scholar]
- Udy GB, Towers RP, Snell RG, Wilkins RJ, Park SH, Ram PA, Waxman DJ, Davey HW 1997 Requirement of STAT5b for sexual dimorphism of body growth rates and liver gene expression. Proc Natl Acad Sci USA 94:7239–7244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen JM, Wyszomierski SL, Hadsell D 1999 Regulation of milk protein gene expression. Annu Rev Nutr 19:407–436 [DOI] [PubMed] [Google Scholar]
- Cui Y, Riedlinger G, Miyoshi K, Tang W, Li C, Deng CX, Robinson GW, Hennighausen L 2004 Inactivation of Stat5 in mouse mammary epithelium during pregnancy reveals distinct functions in cell proliferation, survival, and differentiation. Mol Cell Biol 24:8037–8047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi K, Shillingford JM, Smith GH, Grimm SL, Wagner KU, Oka T, Rosen JM, Robinson GW, Hennighausen L 2001 Signal transducer and activator of transcription (Stat) 5 controls the proliferation and differentiation of mammary alveolar epithelium. J Cell Biol 155:531–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazansky AV, Raught B, Lindsey SM, Wang YF, Rosen JM 1995 Regulation of mammary gland factor/Stat5a during mammary gland development. Mol Endocrinol 9:1598–1609 [DOI] [PubMed] [Google Scholar]
- Liu X, Robinson GW, Hennighausen L 1996 Activation of Stat5a and Stat5b by tyrosine phosphorylation is tightly linked to mammary gland differentiation. Mol Endocrinol 10:1496–1506 [DOI] [PubMed] [Google Scholar]
- Nevalainen MT, Xie J, Bubendorf L, Wagner KU, Rui H 2002 Basal activation of transcription factor signal transducer and activator of transcription (Stat5) in nonpregnant mouse and human breast epithelium. Mol Endocrinol 16:1108–1124 [DOI] [PubMed] [Google Scholar]
- Gallego MI, Binart N, Robinson GW, Okagaki R, Coschigano KT, Perry J, Kopchick JJ, Oka T, Kelly PA, Hennighausen L 2001 Prolactin, growth hormone, and epidermal growth factor activate Stat5 in different compartments of mammary tissue and exert different and overlapping developmental effects. Dev Biol 229:163–175 [DOI] [PubMed] [Google Scholar]
- Jones FE, Welte T, Fu XY, Stern DF 1999 ErbB4 signaling in the mammary gland is required for lobuloalveolar development and Stat5 activation during lactation. J Cell Biol 147:77–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lydon JP, Sivaraman L, Conneely OM 2000 A reappraisal of progesterone action in the mammary gland. J Mammary Gland Biol Neoplasia 5:325–338 [DOI] [PubMed] [Google Scholar]
- Richer JK, Jacobsen BM, Manning NG, Abel MG, Wolf DM, Horwitz KB 2002 Differential gene regulation by the two progesterone receptor isoforms in human breast cancer cells. J Biol Chem 277:5209–5218 [DOI] [PubMed] [Google Scholar]
- McMurray R, Keisler D, Kanuckel K, Izui S, Walker SE 1991 Prolactin influences autoimmune disease activity in the female B/W mouse. J Immunol 147:3780–3787 [PubMed] [Google Scholar]
- Kariagina A, Aupperlee M, Haslam S 2007 Progesterone receptor isoforms and proliferation in the rat mammary gland during development. Endocrinology 148:2723–2736 [DOI] [PubMed] [Google Scholar]
- Ajika K, Krulich L, Fawcett CP, McCann SM 1972 Effects of estrogen on plasma and pituitary gonadotropins and prolactin, and on hypothalamic releasing and inhibiting factors. Neuroendocrinology 9:304–315 [DOI] [PubMed] [Google Scholar]
- Chen CL, Meites J 1970 Effects of estrogen and progesterone on serum and pituitary prolactin levels in ovariectomized rats. Endocrinology 86:503–505 [DOI] [PubMed] [Google Scholar]
- Aupperlee MD, Smith KT, Kariagina A, Haslam SZ 2005 Progesterone receptor isoforms A and B: temporal and spatial differences in expression during murine mammary gland development. Endocrinology 146:3577–3588 [DOI] [PubMed] [Google Scholar]
- Aupperlee MD, Haslam SZ 2007 Differential hormonal regulation and function of progesterone receptor isoforms in normal adult mouse mammary gland. Endocrinology 148:2290–2300 [DOI] [PubMed] [Google Scholar]
- Fata JE, Kong YY, Li J, Sasaki T, Irie-Sasaki J, Moorehead RA, Elliott R, Scully S, Voura EB, Lacey DL, Boyle WJ, Khokha R, Penninger JM 2000 The osteoclast differentiation factor osteoprotegerin-ligand is essential for mammary gland development. Cell 103:41–50 [DOI] [PubMed] [Google Scholar]
- Srivastava S, Matsuda M, Hou Z, Bailey JP, Kitazawa R, Herbst MP, Horseman ND 2003 Receptor activator of NF-κB ligand induction via Jak2 and Stat5a in mammary epithelial cells. J Biol Chem 278:46171–46178 [DOI] [PubMed] [Google Scholar]
- Shyamala G, Barcellos-Hoff MH, Toft D, Yang X 1997 In situ localization of progesterone receptors in normal mouse mammary glands: absence of receptors in the connective and adipose stroma and a heterogeneous distribution in the epithelium. J Steroid Biochem Mol Biol 63:251–259 [DOI] [PubMed] [Google Scholar]
- Ihle JN, Stravapodis D, Parganas E, Thierfelder W, Feng J, Wang D, Teglund S 1998 The roles of Jaks and Stats in cytokine signaling. Cancer J Sci Am 4(Suppl 1):S84–S91 [PubMed] [Google Scholar]
- Groner B, Gouilleux F 1995 Prolactin-mediated gene activation in mammary epithelial cells. Curr Opin Genet Dev 5:587–594 [DOI] [PubMed] [Google Scholar]
- Mizoguchi Y, Yamaguchi H, Aoki F, Enami J, Sakai S 1997 Corticosterone is required for the prolactin receptor gene expression in the late pregnant mouse mammary gland. Mol Cell Endocrinol 132:177–183 [DOI] [PubMed] [Google Scholar]
- Tucker HA 1979 Endocrinology of lactation. Semin Perinatol 3:199–223 [PubMed] [Google Scholar]
- Haslam SZ, Shyamala G 1979 Progesterone receptors in normal mammary glands of mice: characterization and relationship to development. Endocrinology 105:786–795 [DOI] [PubMed] [Google Scholar]
- Liu X, Gallego MI, Smith GH, Robinson GW, Hennighausen L 1998 Functional rescue of Stat5a-null mammary tissue through the activation of compensating signals including Stat5b. Cell Growth Differ 9:795–803 [PubMed] [Google Scholar]
- Kim NS, Kim HJ, Koo BK, Kwon MC, Kim YW, Cho Y, Yokota Y, Penninger JM, Kong YY 2006 Receptor activator of NF-κB ligand regulates the proliferation of mammary epithelial cells via Id2. Mol Cell Biol 26:1002–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisken C, Heineman A, Chavarria T, Elenbaas B, Tan J, Dey SK, McMahon JA, McMahon AP, Weinberg RA 2000 Essential function of Wnt-4 in mammary gland development downstream of progesterone signaling. Genes Dev 14:650–654 [PMC free article] [PubMed] [Google Scholar]