Abstract
Pulsatile release of GnRH-1 is essential for secretion of gonadotropin hormones. The frequency of GnRH-1 pulses is regulated during the reproductive cycle by numerous neurotransmitters. Cyclic nucleotide-gated (CNG) channels have been proposed as a mechanism to integrate the cAMP signal evoked by many neurotransmitters. This study reports the expression of the CNGA2 subunit in GnRH-1 neurons obtained from mouse nasal explants and shows the ability of GnRH-1 neurons to increase their activity in response to forskolin (activator of adenylyl cyclases), or 3-isobutyl-1-methylxanthine (inhibitor of phosphodiesterases) even after removal of γ-aminobutyric acid (A)-ergic input. Next, the endogenous activity of adenylyl cyclases was evaluated as a component of the oscillatory mechanism of GnRH-1 neurons. Inhibition of endogenous activity of adenylyl cyclases did not alter GnRH-1 activity. The potential involvement of CNGA2 subunit in basal or induced activity was tested on GnRH-1 neurons obtained from CNGA2-deficient mice. Without up-regulation of CNGA1 or CNGA3, the absence of functional CNGA2 did not alter either the endogenous GnRH-1 neuronal activity or the response to forskolin, negating CNG channels from cAMP-sensitive mechanisms leading to changes in GnRH-1 neuronal activity. In addition, the potential role of CNGA2 subunit in the synchronization of calcium oscillations previously described was evaluated in GnRH-1 neurons from CNGA2-deficient explants. Synchronized calcium oscillations persisted in CNGA2-deficient GnRH-1 neurons. Taken together, these results indicate that CNGA2 channels are not necessary for either the response of GnRH-1 neurons to cAMP increases or the basal rhythmic activity of GnRH-1 neurons.
GnRH-1 REGULATES REPRODUCTION, being an integral component of hypothalamic-pituitary-gonadal axis. The GnRH-1 neuronal population is comprised of relatively few cells (∼800 in mouse) (1) that are not contained within a single anatomical nucleus. In rodents, the GnRH-1 neurons form bilateral continuums, on either side of midline, from the olfactory bulbs caudally to the hypothalamus. Independent of location, the majority of GnRH-1 axons target the pituitary portal blood where GnRH-1 is released in a pulsatile manner (2). The pulsatility of GnRH-1 secretion is essential for maintenance of anterior pituitary gonadotropin secretion (3), preventing desensitization of gonadotrope cells. The mechanisms involved in the pulsatile release of GnRH-1 remain unclear.
Reproduction is a multisensory response being modulated by physiological status as well as environmental conditions (2). As such, GnRH-1 neurons must integrate multiple signals (4,5). Data from an immortalized GnRH-1 cell line, GT-1 cells, indicate that the secondary messenger cAMP is involved in many transduction pathways in these cells [norepinephrine, dopamine, acetylcholine, and γ-aminobutyric acid (GABA) (6); estradiol (7); and serotonin (8)]. Increasing cAMP levels with agonists (6), adenylyl cyclase (AC) activators (6,9,10), or phosphodiesterase (PDE) inhibitors (11) increases GnRH-1 release, whereas decreasing cAMP levels with PDE overexpression (12,13) decreases GnRH-1 release. Specific overexpression of PDE in GnRH-1 neurons in rats did not affect hypothalamic GnRH-1 levels but resulted in decreased amplitude of the preovulatory LH surge and impaired fertility in females and LH levels, and LH pulse frequency in ovariectomized rats were also attenuated (12), supporting the importance of cAMP-dependent modulation of GnRH-1 neuronal activity.
Transcripts for cyclic nucleotide-gated (CNG) channel subunits have been detected in GT-1 cells (6), and the involvement of CNG channels in the excitability of GT-1 cells has been evaluated. The functionality of CNG channels in GT-1 cells has been shown by cAMP-evoked microscopic as well as macroscopic currents (6). Moreover, forskolin (FSK) and cell-permeant cAMP (Sp-cAMPS) increase calcium oscillations, and pretreatment with l-cis-diltiazem (LCD), a common CNG channel blocker, inhibits Sp-cAMPS-induced oscillations (6). Because the CNGA2 subunit is essential for formation of functional CNG channels, a recent study specifically targeted this subunit in GT-1 cells with short interference RNA. A significant decrease in the interpulse interval for GnRH-1 secretion was found (14). A role of CNG channels in GnRH-1 neurons in vivo is supported by expression of CNGA2 subunit transcript in GnRH-1 neurons in rats and expression of the protein in GnRH-1 perikarya as well as in the external zone of the median eminence (15).
Native prenatal GnRH-1 neurons maintained in nasal explants have proven to be a useful model for evaluating GnRH-1 neuronal activity (16,17,18,19). In this in vitro model, GnRH-1 neurons, devoid of central nervous system cues, exhibit spontaneous electrical activity (20), are highly differentiated with respect to their electrophysiological properties, possess a wide variety of voltage- and ligand-gated ion channels (20), and exhibit synchronous calcium oscillations (21,22) and pulsatile GnRH-1 secretion (23,24,25). In two different species, the periodicity of synchronous calcium oscillations correlates with the periodicity of GnRH-1 secretion (21,22), thereby linking synchronous calcium oscillations to secretory events. The goal of this study was to investigate whether cAMP modulated GnRH-1 neuronal activity and to evaluate the potential role of CNG channels under stimulation and basal conditions, using both wild-type (WT) mice and CNGA2 subunit-deficient mice (26). This work indicates that CNGA2 channels are not necessary for either the response of GnRH-1 neurons to cAMP increases or the basal rhythmic activity of GnRH-1 neurons.
Materials and Methods
In vitro nasal explants
Nasal regions were cultured as previously described (18) (Fig. 1A). Briefly, embryos were obtained from timed pregnant animals in accordance with NIH guidelines. Nasal pits of embryonic d 11.5 (E11.5) staged NIH Swiss mice or CNGA2-deficient mice (26) were isolated under aseptic conditions and adhered onto coverslips by a plasma (Cocalico Biologicals, Reamstown, PA)/thrombin (Sigma Chemical Co., St. Louis, MO) clot. Nasal explants were maintained at 37 C in a humidified atmosphere with 5% CO2, in a defined serum-free medium (SFM) (18). On culture d 3, fresh medium containing fluorodeoxyuridine (8 × 10−5 m; Sigma) was given for 3 d to inhibit proliferation of dividing olfactory neurons and nonneuronal explant tissue. On culture d 6, and every 2 d afterward, the medium was changed with fresh SFM.
Figure 1.
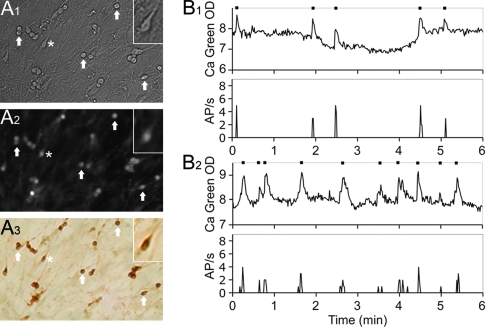
Calcium oscillations reflect electrical events in GnRH-1 neurons. A, GnRH-1 neurons in nasal explants recorded by calcium imaging: A1, GnRH-1-like cells were identified by their bipolar morphology; A2, uptake of Calcium Green-1; A3, immunolabeling confirmed the phenotype of the recorded cells as GnRH-1 neurons. Arrows indicate same cells in all fields, and the asterisk marks the cell in the insets. B, Typical simultaneous recordings of intracellular calcium levels by imaging and of electrical activity of cell’s membrane in two different cells (B1 and B2).
PCR on single GnRH-1 cells
cDNA libraries have been created from individual GnRH-1 cells maintained in nasal explants (27). Using these cDNA libraries, the presence of specific transcripts can be evaluated in individual GnRH-1 neurons and relative expression of that transcript within the GnRH-1 population determined (28,29,30). Briefly, GnRH-1-like neurons, identified in vitro by their bipolar morphology, association with outgrowing axons, and location within the explant, were removed with pulled glass capillaries. cDNA were produced, and PCR amplification was performed as previously described (27).
Based on the technique used to generate the cDNA pools, 3′-untranslated region biased primers are necessary. Primers were designed with the 5′ primer being less than 300 bases from the polyA site and the 3′ primer close to, but not into, the polyadenylation region. All designed primers were screened using BLAST to ensure specificity of binding. For each reaction, 30.5 μl H2O, 5 μl 10× PCR buffer (Applied Biosystems, Foster City, CA), 4 μl 25 mm MgCl2 (Applied Biosystems), 5 μl dNTP mix (25 μl of each 100 mm dNTP, 900 μl H2O), 2 μl 6.25 μm forward primer, 2 μl 6.25 μm reverse primer, and 0.5 μl AmpliTaq Gold (Applied Biosystems) were added to 1 μl template cDNA. PCR was performed at 94 C (10 min); 94 C (30 sec); 55, 60, or 65 C, depending on primers (30 sec); and 72C (2 min) for 40 cycles with a postelongation at 72 C for 10 min. Amplified products were run on a 1.5% agarose gel. Specific bands of the predicted size were observed in the control total brain lane, whereas no bands were seen in water. All cDNA were initially screened by PCR for GnRH-1 (correct cell phenotype), III-tubulin, and L19 (two housekeeping genes, respectively microtubule and ribosomal; primer sequences given in Ref. 31). Used in previous studies (27,32), only cells positive for all three transcripts were used in this study.
PCR were performed to determine the expression of CNGA1 (accession number NM_007723; forward primer 5′-TGG GAG AAA GAG TCG GTC TGG-3′, reverse primer 5′-TCT CCT TTT CAG GCC ACT TG-3′; product size, 248 bp; 65 C), CNGA2 (NM_007724; forward 5′-TCC CAA GGC ATG CAA GGT CT-3′, reverse 5′-CAG TAT CTC ATG CAG CAG TAG C-3′; 183 bp; 60 C), and CNGA3 (NM_009918; forward 5′-ATG GAT GTC TTA CGG GGC TG-3′, reverse 5′-TGC ACA TAT ATC CAC CTG CCC GAA G-3′; 156 bp; 65 C) pore-forming subunits of CNG channels on single GnRH-1 cell cDNA [3 days in vitro (div n = 10); 6–8 div (n = 15)]. Two immortalized GnRH-1 cell lines, GT-1 (33) and NLT (34), were screened in parallel. A positive brain lane and a negative water lane validated the experimental run.
In vitro calcium imaging
Calcium Green-1 calcium imaging was undertaken as reported previously (22). Briefly, the Calcium Green-1 AM (Molecular Probes, Eugene, OR) was diluted to 2.7 mm concentration in 20% pluronic F-127 / DMSO solution (Molecular Probes). This solution was diluted 1:200 with SFM to a final Calcium Green-1 concentration of 13.5 μm. Nasal explants, maintained at 37 C in a 5% CO2 humidified incubator, were incubated with this loading solution for 20 min and then washed twice with fresh SFM (10 min each). Explants were mounted into a perfusion chamber and were continuously perfused with medium, at a rate of about 280 μl/min, using a gravity system (Warner Instruments, Hamden, CT) as inflow and a peristaltic pump as outflow. Drugs were applied by placing the inflow catheter as close as possible to the explant (∼1–2 mm), thereby maximizing the onset of drug exposure (∼5 sec). Calcium Green-1 was visualized using an inverted microscope (Nikon) through a ×20 fluorescence objective and a digital CCD camera (Retiga, Qimaging, Burnaby, Canada) connected to a Macintosh computer. Experiments were piloted via imaging software (IPLab Spectrum, Scanalytics Inc., Rockville, MD) controlling the shutter (Uniblitz, Vincent Associates, Rochester, NY) and the acquisition. Excitation wavelengths were provided through a medium-width excitation bandpass filter at 465–495 nm, and emission was monitored through a 40-nm bandpass centered on 535 nm. Pictures were acquired each 20 sec for long-term recordings (100 min) or each second for short recordings (<20 min).
Calcium imaging recordings were performed from 6–10 div, and intracellular calcium fluctuations were analyzed a posteriori with IPLab software. Each cell, individually identified, was circled. Calcium Green-1 fluorescence intensity was plotted and analyzed with MATLAB (Mathworks, Natick, MA). When acquired at one picture/sec, a calcium fluctuation was first identified when a value was greater than the five previous and five subsequent points. Then, that calcium fluctuation had to be greater than the mean of the five previous and five next points plus a minimal value (which represented small fluctuations in baseline) to be considered as a calcium oscillation (peak). The frequency of calcium oscillations was calculated as the number of detected calcium peaks per time unit (minute). When acquired at one picture/20 sec, neuronal activity was analyzed using a floating mean and a floating sd, both calculated on five points before and five points after the point of interest. A calcium peak was defined as a point value greater than the mean plus 2 × the sd (mean + 2 sd). The synchronization of calcium events was detected using a wavelet analysis as previously described (22). In Results, n and N represent the number of cells and explants recorded, respectively. Statistical analysis was performed using paired t tests (to identify an effect of a drug on the peak frequency among a pool of cells), ANOVA (to compare the interpulse interval in WT mice and CNGA2-deficient mice), and a Wilcoxon-Mann-Whitney U test (to evaluate the shift in the cell population toward higher or lower frequencies in calcium oscillations). In all cases, a P value of 0.05 was chosen for significance.
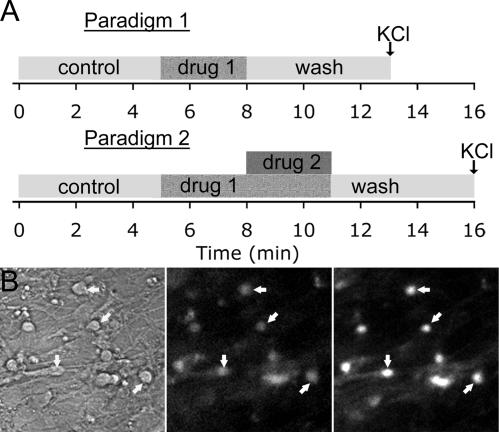
FSK, 3-isobutyl-1-methylxanthine (IBMX), dideoxyadenosine (DDA), and bicucullin (BIC) were obtained from Sigma. Rp-8-Br-cGMPS and LCD were purchased from BIOMOL International, L.P. (Plymouth Meeting, PA). All stock solutions were stored at −20 C and solutions were prepared before each experiment by diluting stock solutions (1/500 to 1/2000) into SFM (used for growing the nasal explants) (18). For short recording experiments, subdivision into three to four periods was done for analysis [i.e. for 3 periods: control period in SFM (5 min), treatment period (3 min), and washout period in SFM (5 min); Fig. 2A]. For DDA experiments, the treatment period was done over 6 min to evaluate the effects of blockade of cAMP production on GnRH-1 neuronal activity and 15 min to reach a steady state in intracellular cAMP level. All recordings were terminated by a 40 mm KCl stimulation to ensure the viability of the recorded cells (Fig. 2B).
Figure 2.
Calcium imaging experimental paradigms. A, Two different paradigms were used. The first one (upper) was used to determine an appropriate drug concentration compatible with a reversible effect, stimulating period. The second one (lower) was used to induce a perturbation, conditioning period, before the stimulation. Both paradigms were initiated after a 5-min control period to ensure the quality of the cells and followed by a 5-min washout to examine the reversibility of the effect. A KCl stimulation at the end of the experiment was performed to verify the viability of the cells included in the analysis (B1, recorded field before the experiment; B2, loaded cells during the experiment; B3, after KCl).
Electrophysiological recordings
To ensure that calcium imaging could be used to evaluate GnRH-1 neuronal activity, simultaneous electrophysiological recordings were performed during calcium imaging under SFM control conditions. Under the same conditions previously described for calcium imaging, GnRH-1 neurons were recorded using the patch clamp technique in the loose-seal patch clamp technique (35). This configuration was chosen to minimize membrane disturbance of GnRH-1 neurons and to preserve the intracellular content of the cells. Transient modifications of the membrane potential, which define action potentials, were recorded as capacitive currents (36). This elementary event, called action current, is concomitant with the action potential and reflects the voltage modification (35). Electrodes with a resistance of 5–8 ΜΩ were pulled from standard-walled borosilicate capillaries with inner filament (GC150-F; Harvard Apparatus, Edenbridge, UK) on a two-stage vertical puller (PP-830; Narishige, Tokyo, Japan). Pipettes were back-filled with a 0.22-μm filtered solution containing (in mm) 120 NaCl, 4.7 KCl, 2.6 CaCl2, 2 MgCl2, 0.7 MgSO4, 10 HEPES, and 10 glucose (pH 7.4, NaOH). The cells were visualized using an inverted Nikon microscope (Eclipse TE2000) and approached with a water hydraulic micromanipulator (MHW-3; Narishige). Signals were recorded with an Axopatch-200B (Axon Instruments, Foster City, CA) amplifier linked to a Digidata 1322A (Axon Instruments) using a 5-kHz built-in Bessel filter and digitized at 5 kHz (Axon Instruments). Experiments were performed in voltage-clamp mode with a holding potential of 0 mV. The liquid junction potentials were canceled before seal formation, and seal resistances ranged from 10–100 MΩ.
Sodium current was recorded in GnRH-1 neurons (8–10 div) in voltage-clamp mode, whole-cell configuration. Pipettes were back-filled with a 0.22-μm filtered solution containing (in mm) 20 NaCl, 100 CsCl, 1.8 MgCl2, 4 MgATP, 10 HEPES, and 10 EGTA (pH 7.3, KOH). Extracellular solution was SFM (Na, ∼124 mm) supplemented with 200 μm CdCl2 (to inhibit calcium currents) and 5 mm 4-aminopyridine (to inhibit type A and delayed-rectifier potassium currents), and intrapipette cesium was used to inhibit the inward rectifier potassium current. Whole-cell data were 2-kHz filtered and digitized at 20 kHz. Electrodes were pulled with a resistance of about 3 MΩ, and seal resistances ranged from 1–3 GΩ. From a resting potential clamped at −90 mV, activation protocol was performed from −100 to 50 mV by 10-mV steps, and inactivation protocol was run from −110 to 60 mV by 10-mV steps for the conditioning pulse with a −10-mV test pulse. All pulses lasted for 300 msec. Activation and inactivation curves were fitted with a Boltzmann function to determine the voltage of half-activation (Vact1/2) or half-inactivation (Vinac1/2).
In vitro GnRH-1 immunocytochemistry
To confirm the phenotype of recorded cells, nasal explants were immunocytochemically stained for GnRH-1 as previously described (18). Briefly, after calcium imaging recordings, explants were fixed (4% formaldehyde, 1 h), rinsed in PBS, and placed in cryoprotectant until staining. Explants were washed in PBS, blocked in 10% normal goat serum/0.3% Triton X-100 for 1 h, washed several times in PBS, and then incubated in GnRH-1 antibody (1:3000, SW-1) overnight at 4 C. The next day, explants were washed in PBS, incubated for 1 h in biotinylated secondary antibody (1:500 in PBS/0.3% Triton X-100; Vector Laboratories, Inc., Burlingame, CA), washed in PBS, and processed for avidin-biotin-horseradish peroxidase/3′3-diaminobenzidine cytochemistry (Fig. 1A).
In vivo GnRH-1 immunocytochemistry on CNGA2 knockout (KO) mice
To evaluate the GnRH-1 system in CNGA2-deficient mice, immunocytochemistry was performed on adult brain sections (KO, n = 4; WT, n = 3). Briefly, immediately after death, the brain was removed and frozen and kept at −80 C until cryostat cutting (Leica Microsystems GmbH, Wetzlar, Germany). The brains were mounted using OCT compound (Tissue-Tek Sakura Finetek, Torrance, CA), and frozen sections (14 μm) were generated (six series per animal) and stored at −80 C until immunocytochemistry. After being dried for 30 min at room temperature and then fixed in 4% formaldehyde PBS for 30 min, sections were immunocytochemically stained following the same protocol described above. The slides were examined microscopically. The number of GnRH-1 neurons was examined in each animal.
Results
Explant models of primary GnRH-1 neurons in nasal regions have been successfully used to investigate the molecular and cellular biology of the GnRH-1 system. This in vitro model minimizes many of the complexities encountered in vivo and maintains a large number of GnRH-1 neurons that differentiate and share many similarities with GnRH-1 cells in vivo (20,28,29,31,32).
Electrophysiological events correlate with calcium peaks
Calcium imaging of GnRH-1 cells in nasal explants allows one to monitor the activity of multiple GnRH-1 cells in a single experiment. This provides information on individual GnRH-1 cells as well as dynamics within the GnRH-1 neuronal population. However, to date, the relationship of electrical events and calcium peaks in GnRH-1 cells maintained in this model system has not been evaluated. Calcium imaging as a technique for evaluating GnRH-1 neuronal activity was validated by recording simultaneously the electrical activity from the neuronal membrane and the fluctuations in intracellular level of calcium from the cell soma. Here we show (Fig. 1B) that there is a direct temporal correlation (r = 0.99) between action potentials and calcium peaks in single GnRH-1 neurons (n = 5; N = 2).
cAMP-induced modulation of GnRH-1 neuronal activity
To ensure the stability of GnRH-1 neurons without exogenous perturbation, calcium imaging recordings were initially performed over the experimental paradigm periods in only SFM (Fig. 2A). No significant changes were detected: 1.35 ± 0.09 peaks/min during SFM period 1 vs. 1.46 ± 0.12 peaks/min during SFM period 2 vs. 1.54 ± 0.12 peaks/min during SFM period 3 (paired t test, P > 0.05 for all periods) (n = 64; N = 2; Fig. 3A).
Figure 3.
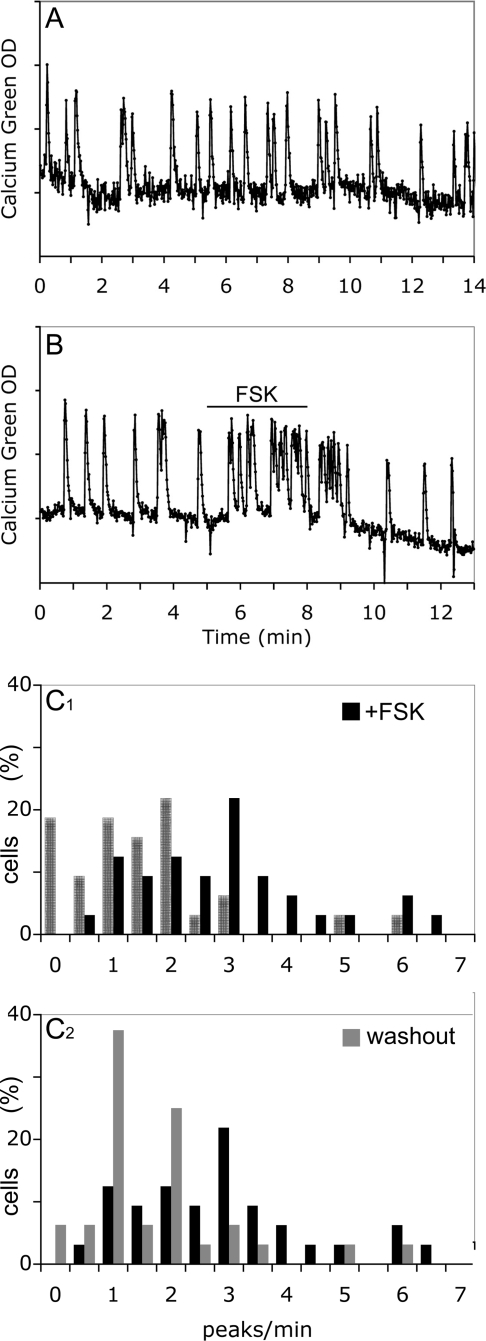
Effect of FSK on GnRH-1 neuronal activity. A, GnRH-1 neurons maintained in SFM did not show major spontaneous changes in neuronal activity over the total experimental time; B, FSK (1 μm) induced a reversible increase of calcium oscillations; C, histograms show the distribution of cells as a function of their frequency of calcium oscillations (peaks per minute: C1, a significant shift in the cell distribution (SFM, hatched bars) toward higher frequencies occurred upon exposure to FSK (black bars; Wilcoxon-Mann-Whitney U test, P < 0.05); C2, the shift induced by FSK was reversible with the cell distribution returning to lower frequencies during the washout period (gray bars; Wilcoxon-Mann-Whitney U test, P < 0.05).
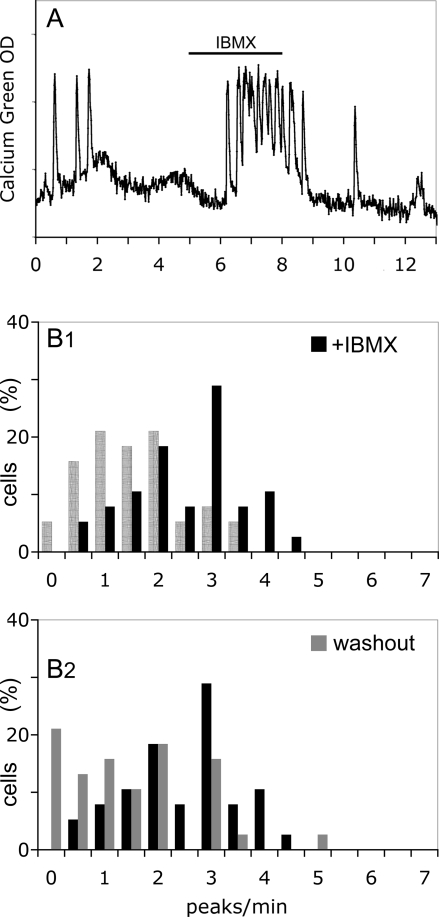
Next, FSK, activator of AC, was tested at two different concentrations (1 and 10 μm). At 1 μm, a reversible significant increase in the frequency of calcium oscillations was observed in GnRH-1 cells [1.37 ± 0.23 peaks/min in SFM vs. 2.73 ± 0.27 peaks/min in FSK (paired t test, P < 0.01); 1.56 ± 0.22 peaks/min in washout SFM (P < 0.01); n = 32; N = 3; Fig. 3B; Fig. 3C1–2 shows significant shifts in the cell population (Wilcoxon-Mann-Whitney U test, P < 0.05)]. At 10 μm, this increase turned quickly into a sustained plateau of intracellular calcium and/or a complete arrest of calcium oscillations (n = 25; N = 1; data not shown). For additional experiments, only FSK at 1 μm was used. To determine whether endogenously synthesized cAMP could be an intrinsic mechanism for modulating GnRH-1 neuronal activity, IBMX (100 μm) was applied to inhibit PDE, enzymes that hydrolyze cAMP. Inhibiting degradation of cAMP induced an increase in the frequency of calcium oscillations [1.32 ± 0.15 peaks/min in SFM vs. 2.36 ± 0.17 peaks/min in IBMX (paired t test, P < 0.01); 1.30 ± 0.19 peaks/min in washout SFM (P < 0.01); n = 38; N = 2; Fig. 4A; Fig. 4B1–2 shows significant shifts in the cell population (Wilcoxon-Mann-Whitney U test, P < 0.05)], confirming the hypothesis of a cAMP sensitive mechanism for regulating GnRH-1 neuronal activity.
Figure 4.
Activation of GnRH-1 neuronal activity by endogenous cAMP production. To determine the efficiency of endogenously synthesized cAMP to stimulate GnRH-1 neurons, blockade of PDE was induced by IBMX (100 μm). A, IBMX was able to increase the calcium oscillations; B1, a significant shift in the activity of GnRH-1 neurons (SFM, hatched bars) toward more peaks per minute occurred upon exposure to IBMX (black bars; Wilcoxon-Mann-Whitney U test, P < 0.05); B2, the shift induced by IBMX was reversible with the activity of GnRH-1 neurons returning to fewer peaks per minute during the washout period (gray bars; Wilcoxon-Mann-Whitney U test, P < 0.05).
Endogenous GnRH-1 neuronal rhythmicity and cAMP production
To evaluate whether the endogenous activity of AC contributed to GnRH-1 neuronal activity, pharmacological blockade of AC was done using DDA (40 μm) (37). The blockade of cAMP production over a 6-min period did not alter the frequency in calcium oscillations in GnRH-1 neurons [1.42 ± 0.19 peaks/min in SFM vs. 1.45 ± 015 peaks/min in DDA (paired t test, P > 0.05); n = 48; N = 3]. These results indicate that cAMP modulates rather than triggers GnRH-1 neuronal activity in nasal explants. To ensure that the cAMP remaining after the 6-min treatment was not still promoting GnRH-1 neuronal activity, some recordings were performed for 16.5 min in the presence of DDA to reach the steady-state level of inhibition of AC blockade previously described in pituitary cells (38). Over 16.5 min, DDA application still failed to modify the activity of GnRH-1 neurons [1.47 ± 0.13 peaks/min for the first period (5.5 min), 1.48 ± 0.12 peaks/min for the next period (5.5 min), and 1.65 ± 0.12 for the last period (5.5 min) (paired t test, P > 0.05); n = 34; N = 2]. In addition to increasing intracellular cAMP level, FSK (1 μm) and IBMX (100 μm) are able to increase intracellular cGMP level in pituitary cells (38). To verify that the FSK-induced increases in calcium oscillations in GnRH-1 neurons were mediated by cAMP rather than cGMP, AC were blocked before application of FSK to prevent the FSK-induced synthesis of cAMP. The FSK-induced response was blocked, showing that the response was dependent on an increase in cAMP (1.73 ± 0.25 peaks/min in DDA vs. 2.00 ± 0.41 peaks/min in DDA+FSK (paired t test, P > 0.05); n = 14; N = 1].
Direct or indirect action of cAMP on GnRH-1 neurons
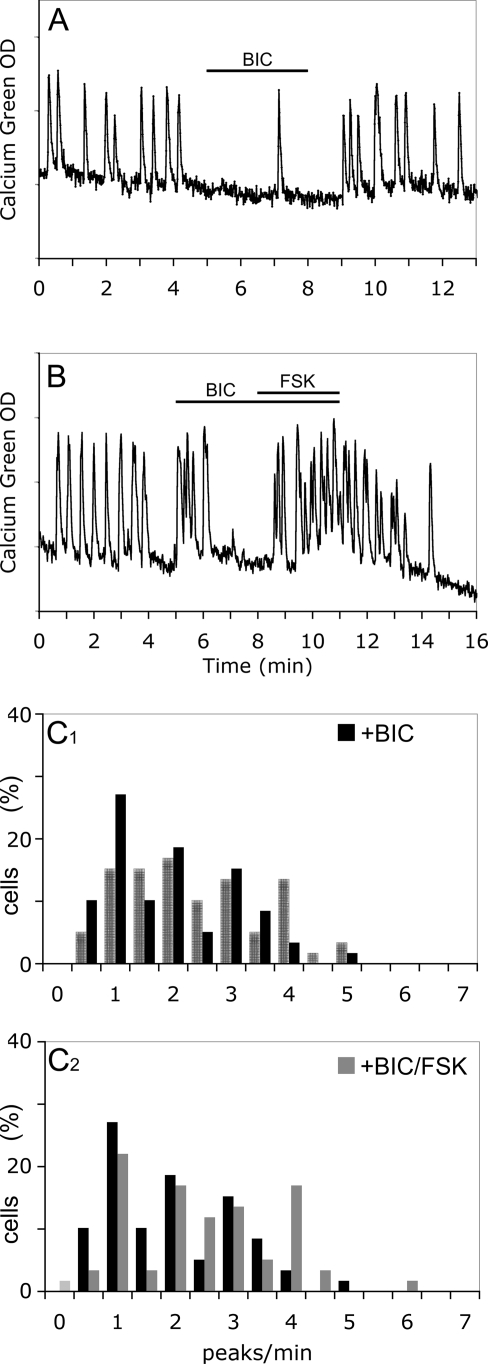
Extensive evidences indicate that glutamatergic and GABAergic neurons are important primary afferent inputs to GnRH-1 neurons in vivo (2). Although glutamatergic responses have been recorded in older explants (20), endogenous glutamatergic populations have not been shown. In addition, in vivo, tonic glutamatergic input is relatively low compared with GABAergic input (2). A subpopulation of GABAergic neurons, known to be present in nasal explants, regulates GnRH-1 neuronal activity via excitatory input (20,39) and is important for synchronized activity (22). To confirm that FSK was not activating GABAergic neurons that would subsequently stimulate GnRH-1 neurons, a GABAA receptor antagonist, BIC (20 μm), was used. Consistent with previous results, GnRH-1 neuronal activity decreased with blockade of GABAA receptors (P < 0.01; n = 59; N = 2; Fig. 5A). However, the FSK-induced increase in calcium oscillations in GnRH-1 cells still occurred after BIC pretreatment [1.78 ± 0.14 peaks/min in BIC vs. 2.30 ± 0.16 peaks/min in BIC+FSK (paired t test, P < 0.01); SFM control period (2.19 ± 0.15 peaks/min) was significantly higher than BIC treatment; n = 59; N = 2; Fig. 5B; Fig. 5C1–2 shows significant shifts in the cell population (Wilcoxon-Mann-Whitney U test, P < 0.05)]. These data suggest that cAMP acts directly on GnRH-1 neurons, increasing neuronal activity. To verify that GnRH-1 cells could respond to endogenous production of cAMP after GABAergic input removal, IBMX was applied in the presence of BIC. A significant increase in GnRH-1 calcium peaks was still observed [1.35 ± 0.18 peaks/min in BIC vs. 1.67 ± 0.20 peaks/min in BIC+IBMX (paired t test, P < 0.05); SFM control period (2.51 ± 0.85 peaks/min) was significantly higher than BIC treatment; n = 46; N = 2]. To further investigate the role of the endogenous AC activity in GnRH-1 neurons, deprived of GABAergic input, a 6- and 16.5-min blockade of AC with DDA was performed after BIC pretreatment. No significant changes in GnRH-1 neuronal activity were observed [6-min blockade: 0.80 ± 0.17 peaks/min in BIC vs. 1.02 ± 0.15 peaks/min in BIC+DDA (paired t test, P > 0.05); n = 33; N = 3; 16.5-min blockade: 1.23 ± 0.23 peaks/min for the first 5.5 min, 1.43 ± 0.20 peaks/min for the next 5.5 min, and 1.46 ± 0.19 for the last 5.5 min (paired t test, P > 0.05); n = 22; N = 2].
Figure 5.
Direct activation of GnRH-1 neuronal activity by FSK. Blockade of endogenous GABAergic input on GnRH-1 neurons by BIC (20 μm) decreased calcium oscillations. A, Example of a highly BIC-sensitive cell [SFM = 1.71 ± 0.19 peaks/min; BIC = 1.21 ± 0.16 peaks/min (paired t test, P < 0.01); washout SFM = 1.88 ± 0.16 peaks/min (P < 0.01; n = 59; N = 2)]; B, BIC did not prevent the FSK-induced stimulation of calcium oscillations; C1, a significant shift in the activity of GnRH-1 neurons (SFM, hatched bars) toward fewer peaks per minute occurred upon exposure to BIC (black bars; Wilcoxon-Mann-Whitney U test, P < 0.05) (note example in A was a cell in the 0.5- to 1-peak/min bin after BIC exposure); C2, even in presence of BIC, a significant shift in the activity of GnRH-1 neurons toward more peaks per minute still occurred upon exposure of FSK (gray bars; Wilcoxon-Mann-Whitney U test, P < 0.05).
Involvement of CNGs in the cAMP-induced modulation of GnRH-1 neuronal activity
PCR examination of cDNAs from single GnRH-1 cells from WT mice was performed for the CNG A1–3 subunits, the only subunits able to form a functional homomeric channel when expressed heterologously (40). All GnRH-1 cells examined were negative for CNGA1 and CNGA3 subunits. In contrast, CNGA2 transcript was found in GnRH-1 neurons (3 div, 60%; 6–8 div, 40%). CNGA2 channels are described to be more sensitive to cGMP than cAMP (41). Thus, a cGMP analog that activates CNG channels but not cGMP-dependent protein kinase (42), Rp-8-Br-cGMPS, was used to test the involvement of CNGA2 channels in the regulation of GnRH-1 neuronal activity. Application of Rp-8-Br-cGMPS failed to stimulate GnRH-1 neurons [1.62 ± 0.19 peaks/min in SFM vs. 1.47 ± 0.19 peaks/min in Rp-8-Br-cGMPS (paired t test, P > 0.05); n = 26; N = 2]. Examination of the response of all cells individually did not highlight a subpopulation response (22 of 26 cells did not respond to Rp-8-Br-cGMPS).
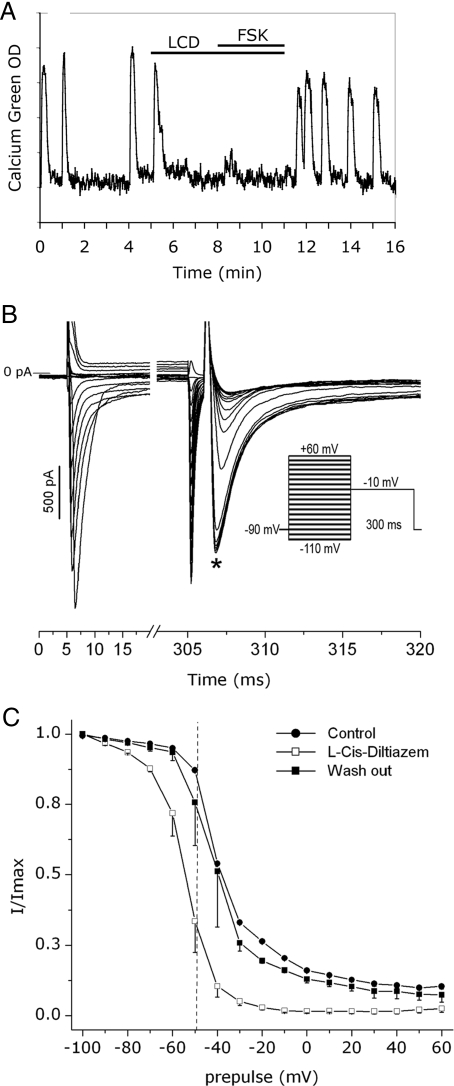
LCD (80 μm), a CNG channel blocker, was then applied on nasal explants before the addition of FSK. Surprisingly, LCD itself inhibited calcium oscillations in the majority of GnRH-1 neurons (n = 32/42; N = 2 explants; Fig. 6A). LCD has been classified as nonspecific to the CNGA2 subunit (41), and modification of sodium current by LCD has been observed in cardiomyocytes (43). Therefore, whole-cell recordings of sodium current were performed on GnRH-1 neurons in the absence of LCD, in the presence of LCD, and after washout to investigate the availability of sodium channels (Fig. 6B). Tetrodotoxin (1 μm), a sodium channel blocker, was initially applied to confirm the nature of the recorded current and (as predicted by Ref. 20), was found to abolish all the inward current. The application of LCD induced a significant and reversible shift toward lower potentials of the activation curve [Vact1/2 = −44.2 ± 0.1 mV in SFM (n = 2) vs. Vact1/2 = −55.3 ± 0.3 mV in SFM+LCD (n = 2)] and of the steady-state inactivation curve [Vinac1/2 = −39.0 ± 0.3 mV in SFM (n = 2) vs. Vinac1/2 = −56.4 ± 2.2 mV in SFM+LCD (n = 2) vs. Vinac1/2 = −41 ± 5.3 mV in washout (n = 2); Fig. 6C], indicating alteration of sodium channel properties. To our knowledge, this is the first report of LCD modifying the activation of sodium channels. Hashimoto et al. (43) also report a decrease in the amplitude of the current recorded in myocytes exposed to LCD (43). This was not found in GnRH-1 neurons and may be the result of the differences in resting potentials of these two cell types: myocytes −90 mV (44) vs. GnRH-1 neurons −50 mV (20) vs. the holding potentials used (−90 mV). Hashimoto et al. (43) did find LCD blockade to be highly dependent on the holding potential, with higher potentials facilitating the blockade of sodium current (100 μm LCD blocking 80 and 100% of the current from holding potential −90 and −80 mV, respectively. Consistent with Ref. 43, a pronounced effect on the inactivation of sodium channels was found in GnRH-1 neurons exposed to LCD. For GnRH-1 neurons whose resting potential is around −50 mV, this shift in the inactivation curve represents a decrease of available sodium channels of about 50% and might explain the loss of excitability of GnRH-1 neurons observed in calcium imaging.
Figure 6.
LCD effects on GnRH-1 neurons. A, LCD (80 μm) inhibited calcium oscillations in GnRH-1 neurons. Because the loss of excitability of GnRH-1 neurons was complete in the presence of LCD, sodium current recordings were performed to ensure the specificity of LCD blockade. B, The steady-state inactivation protocol used (inset) and the current family generated by conditioning prepulses (left) and testing pulses (right). The star indicates where the amplitude of the current was determined. The resulting steady-state inactivation curves (C) showed a reversible shift toward lower potentials in the presence of LCD (each point and vertical bar represents, respectively, mean and sem of two experiments). The dotted line represents the resting potential described in GnRH-1 neurons (−50 mV) (20). Note that the percentage of available sodium channels as this membrane potential is lowered to about 40% with LCD in comparison with control (∼90%).
GnRH-1 neuronal activity in CNGA2-deficient (KO) mice.
Because LCD altered sodium current, current that directly modulates the excitability of cells, nasal explants from CNGA2-deficient mice were generated to test the involvement of CNGs in cAMP-induced modulation of GnRH-1 neuronal activity. Before generating nasal explants from CNGA2 KO mice, the GnRH-1 system was examined in adult KOs. The distribution of GnRH-1 neurons in the hypothalamic area of CNGA2 KO was consistent with that described in WT adult mice as was the total number of GnRH-1 neurons (KO = 692 ± 143 cells, n = 4; WT = 695 ± 77 cells, n = 3; P > 0.05, no genotype difference). Timed mating of CNGA2 KO revealed subfertility [Disruption of the CNGA2-dependent signal transduction in olfactory receptor cells prevented pups from finding the dam’s nipples and led to starvation (26,45), and impaired chemoinvestigation of females and mating in CNGA2-deficient males (46) led to decreased pregnancy rate (47)]. The gene coding the CNGA2 subunit is on the X chromosome (26). Thus, to avoid maternal and/or mating behavioral problems, previous studies in CNGA2 KO mice have been performed only on males, obtained by crossing hetero- or homozygous females and WT males (26,48,49). Although time-matings were difficult to achieve, to prevent the exclusion of females from our experiments, CNGA2 KO (−/−) females were mated with CNGA2 KO (−/Y) males. GnRH-1 cells migrated into the periphery in nasal explants generated from E11.5 KO, and no obvious differences between nasal explants generated from E11.5 KO compared with WT embryos were observed. To make sure that the two other CNGA pore-forming subunits were not up regulated in KO animals, RT-PCR was performed on single GnRH-1 cells plucked from CNGA2-deficient nasal explants. PCR for both CNGA1 and CNGA3 subunits revealed no changes in comparison with GnRH-1 cells obtained from WT nasal explants. Neither subunit was expressed in CNGA2-deficient GnRH-1 cells (n = 10, 7 div).
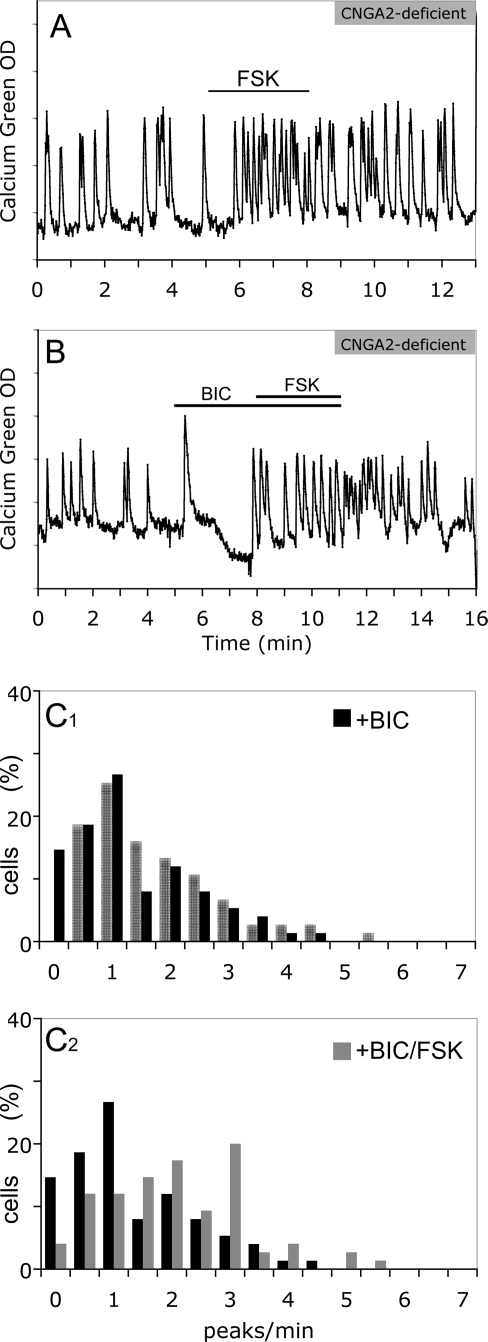
The frequency of calcium oscillations in GnRH-1 neurons was not significantly different in CNGA2-deficient explants under normal conditions [1.49 ± 0.13 peaks/min in KO/SFM (n = 75; N = 5) vs. 1.59 ± 0.07 peaks/min in WT/SFM (n = 248; N = 13); ANOVA, P > 0.05]. Production of cAMP induced by FSK still resulted in an increase in the frequency of calcium oscillations in GnRH-1 neurons from CNGA2-deficient explants [1.10 ± 0.14 peaks/min in SFM vs. 1.92 ± 0.18 peaks/min in FSK; n = 36; N = 4 (paired t test, P < 0.01); Fig. 7A], suggesting another pathway leading to the increase in the frequency of calcium oscillations.
Figure 7.
CNGA2-independent pathway for FSK-induced GnRH-1 neuronal activity. A, FSK induced an increase of calcium oscillations in CNGA2-deficient GnRH-1 neurons; B, the blockade of GABAergic input still inhibited calcium oscillations in CNGA2-deficient GnRH-1 neurons, and without GABAergic input, the absence of CNGA2 did not alter the ability of GnRH-1 neurons to respond to FSK; C1, a significant shift in the activity of GnRH-1 neurons (SFM, hatched bars) toward fewer peaks per minute occurred upon exposure to BIC (black bars; Wilcoxon-Mann-Whitney U test, P < 0.05) (note that the number of cells in the 0- to 0.5-peak/min bin was increased by BIC); C2, even in the presence of BIC, a significant shift in the activity of GnRH-1 neurons toward more peaks per min still occurred upon exposure to FSK (gray bars; Wilcoxon-Mann-Whitney U test, P < 0.05).
To evaluate whether GABAergic input was still present and excitatory in CNGA2-deficient explants, BIC was applied alone. In a manner similar to GnRH-1 cells in nasal explants from WT mice, the blockade of GABAA receptors led to an inhibition of the frequency of calcium oscillations [1.49 ± 0.13 peaks/min in KO/SFM vs. 1.18 ± 0.12 peaks/min in KO/BIC; n = 75; N = 5; (paired t test, P < 0.01)]. After removal of GABAergic inputs, the frequency of calcium oscillations in GnRH-1 neurons was still similar between CNGA2 KO and WT explants [1.18 ± 0.12 peaks/min in KO/BIC (n = 75; N = 5) vs. 1.39 ± 0.07 peaks/min in WT/BIC (n = 266; N = 9); ANOVA, P > 0.05]. In CNGA2 KO explants, GnRH-1 neurons without GABAergic input still responded to FSK stimulation [1.18 ± 0.12 peaks/min vs. 1.89 ± 0.14 peaks/min (paired t test, P < 0.01); n = 75; N = 5; Fig. 7B; Fig. 6C1–2 shows significant shifts in the cell population (Wilcoxon-Mann-Whitney U test, P < 0.05)], and this response was similar to that observed in WT (1.89 ± 0.14 peaks/min in KO/BIC+FSK vs. 2.30 ± 0.16 peaks/min in WT/BIC+FSK (n = 59; N = 2); ANOVA, P > 0.05], revealing a pathway other than CNGA2 modulating GnRH-1 neuronal activity.
Synchronization of GnRH-1 neurons in CNGA2-deficient explants.
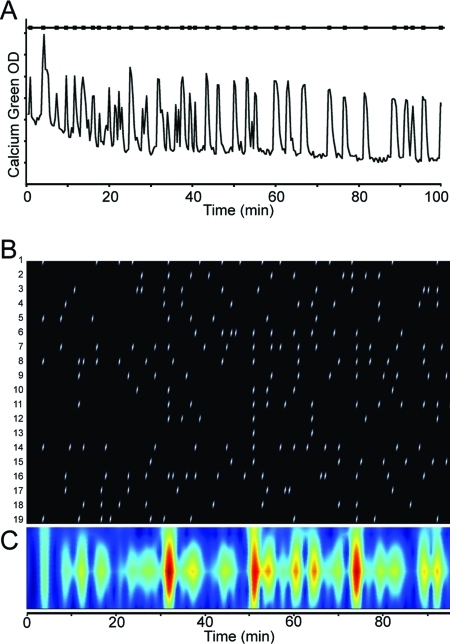
The involvement of CNGA2 in the synchronization of GnRH-1 neurons was evaluated in long-term recordings (100 min, one picture/20 sec; Fig. 8A) by comparing inter-synchronized events in WT explants and CNGA2 KO explants. As previously described (22), GnRH-1 neurons from WT explants exhibited synchronized calcium oscillations with a periodicity of about 20 min (17.2 ± 1.8 min; N = 3). In CNGA2 KO explants, the synchronization among GnRH-1 neurons was persistent, showing the same periodicity (17.0 ± 2.0 min; N = 4; Fig. 8, B and C; ANOVA, P > 0.05).
Figure 8.
GnRH-1 neurons from CNGA2-deficient mice show synchronized calcium oscillations similar to WT. A, Recording of calcium oscillations in one GnRH-1 neuron from a CNGA2-deficient mice over 100 min (one picture/20 sec) and its corresponding detected peaks (black squares on upper line); B, temporal localization of each calcium oscillation detected (white ticks) in each GnRH-1 neuron (rows) in one CNGA2-deficient explant; C, temporal localization of synchronized calcium oscillations (red) identified by wavelet analysis, revealing a periodicity of about 20 min.
Discussion
In addition to intrinsic regulatory mechanisms, a variety of neurotransmitters modulate GnRH-1 neurons in vivo (2). Hypothalamic explants have increased our understanding of the complex regulation of GnRH-1 release (2), but the fine tuning of cellular mechanisms leading to GnRH-1 secretion have been detailed in the immortalized cell line GT-1 (6,8,50,51). cAMP is a ubiquitous second messenger evoked by the binding of many neurotransmitters to their receptors. CNG channels have been localized to GnRH-1 neurons in vivo (15), and based on GT-1 cell data, have been proposed as an integrator of the cAMP signals that modulate GnRH-1 neuronal activity as well as a constitutive mechanism for basal GnRH-1 oscillatory activity (52). The goal of the present work was to evaluate the role of CNG channels in the neuronal activity of native embryonic GnRH-1 neurons obtained from mouse nasal explants. In contrast to data from GT-1 cells (6), cAMP signal and CNG channels are not essential for basal or stimulated neuronal activity of GnRH-1 neurons in this model.
To determine whether modifying the intracellular level of cAMP would alter GnRH-1 neuronal activity, the specific activator of AC, FSK, was applied. GnRH-1 calcium oscillations were stimulated. These data are consistent with observations on hypothalamic tissue from 28-d-old rats (53,54) and correlate with data from GT-1 cells in which calcium oscillations (6), calcium level (9), and GnRH-1 release (6,10) are stimulated by FSK. Interestingly, hypothalamic fragments from rats older than 30 d no longer respond to FSK (55), leading the authors to suggest that the responsiveness to FSK might be a characteristic of prepubertal animals. One neurotransmitter thought to be a potential switch to GnRH-1 neurons at puberty is GABA (56,57). GABAergic neurons, present in nasal explants (39), are a major excitatory input to GnRH-1 neurons (20,22). To determine whether GnRH-1 neurons were responding directly to FSK or responding to FSK-stimulated excitatory input, application of FSK was done after a conditioning treatment with BIC, a GABAA receptor antagonist. Under these conditions, GnRH-1 neurons still responded to FSK with an increase of calcium oscillations, suggesting their ability to integrate cAMP signals directly.
FSK pharmacologically activates AC, preventing evaluation of the endogenous activity of AC. Thus, experiments were performed with IBMX, a nonspecific inhibitor of PDE, enzymes that hydrolyze cAMP. Application of IBMX increased GnRH-1 calcium oscillations. This observation is consistent with data from GT-1 cells, showing stimulation of GnRH-1 release in the presence of IBMX correlated with an increase of intracellular cAMP (11). To verify that cAMP was endogenously synthesized by GnRH-1 neurons, rather than by GABAergic neurons, similar experiments were performed after removal of GABAergic input. An increase in GnRH-1 neuronal activity persisted. These data suggest that PDE are an essential component for regulating the basal and/or the induced activity of GnRH-1 neurons. This observation corroborates data obtained from GT-1 cells on basal (11) or stimulated (58) GnRH-1 release. To determine whether AC endogenous activity, as revealed by IBMX, was important for basal GnRH-1 neuronal activity, experiments were performed in the presence of AC inhibitor DDA. Interestingly, even if GnRH-1 neurons were modulated by FSK-induced increases in cAMP, the removal of basal cAMP production did not alter the basal GnRH-1 neuronal activity. This is in contrast to data from developing spinal neurons (37) and removes cAMP from the potential pacemaking mechanisms in GnRH-1 neurons.
A role for CNG channels in cAMP-induced responses in GnRH-1 neurons has been suggested (6). The expression of the CNG channels pore-forming subunits was examined in cDNA from single GnRH-1 neurons. Only the olfactory subunit CNGA2 was found, consistent with the detection of the CNGA2 transcript in GT-1 cells (6) and the detection of the CNGA2 protein in adult rat GnRH-1 neurons (15). At the time when GnRH-1 neurons in nasal explants are known to show synchronized calcium oscillations and GnRH-1 release, the CNGA2 subunit was detected in many but not all of the GnRH-1 neurons sampled (40%). Similar numbers of GnRH-1 cells expressing ERβ (60%) were found in a previous study (28) and produced dramatic changes in GnRH-1 neuronal activity (59).
When tested in this study, the commonly used CNG channel blocker LCD altered sodium current properties, consistent with previous data (43,60). This, together with the fact that specific blockers for CNGA2 channels are unavailable (41), led us to examine GnRH-1 neuronal activity in nasal explants obtained from CNGA2-deficient mice (26). The basal activity of GnRH-1 neurons was not altered by the absence of functional CNGA2 channels. GnRH-1 neurons from CNGA2-deficient mice still exhibited calcium oscillations with a synchronization interval of about 20 min as in controls. The ability of GnRH-1 neurons from CNGA2-deficient mice to respond to FSK remained unchanged. To ensure that the lack of CNGA2 channels did not alter other cell types such as the GABAergic neurons, BIC was applied before the application of FSK. Both the inhibition of GABAergic input and the subsequent stimulation by FSK remained unchanged in CNGA2-deficient GnRH-1 neurons. Up-regulation of other CNGA pore-forming subunits was examined in CNGA2-deficient GnRH-1 neurons. Although an early developmental compensatory mechanism cannot be excluded, the absence of CNGA1 and CNGA3 subunits in CNGA2-deficient GnRH-1 neurons strongly suggests that CNG channels are not necessary for either basal or stimulated GnRH-1 neuronal activity. In addition, a specific stimulation of CNG channels in WT nasal explants by Rp-8-Br-cGMPS failed to stimulate the majority of GnRH-1 cells when individually examined post hoc.
Consistent with the idea that CNG channels are not the integrator of FSK-induced cAMP signal, data from GT-1 cells have shown that an intracellular calcium increase induced by FSK is dependent on both extracellular sodium and calcium (9). This observation is inconsistent with the pure calcium selectivity of olfactory CNG channels at physiological extracellular calcium concentration (61). Moreover, in contrast with GT-1 cells in which a variety of neurotransmitters can induce changes in cAMP correlated with changes in GnRH-1 release (6,8), data from hypothalamic explants are not as consistent. The norepinephrine-induced GnRH-1 release, attributed to the activation of β1-adrenoreceptors in GT-1 (6), is inhibited by α1-adrenoreceptor antagonists and inhibitors of nitric oxide synthase in hypothalamic explants as well as the subsequent increase in prostaglandin E2 (2). Finally, the dopamine-induced GnRH-1 release is inhibited by α1-adrenoreceptor antagonists (62), and only hypothalamic explants from supplemented-castrated females released GnRH-1 in response to cAMP (63), neuropeptide Y (2), or serotonin (64). Differences between data from GT-1 cells and hypothalamic explants could result from species differences, the steroidal environment, cell subpopulations, and/or developmental differences. GT-1 cells are derived from mouse and might retain some embryonic properties because they were isolated from a tumor at the rostral boundary of the optic chiasm (33). In contrast, hypothalamic explants are usually dissected from rats, which are, moreover, either prepubertal or mature, intact or gonadectomized with or without supplement, males or females (53,63,65,66,67,68,69). In addition, due to the scattered distribution of GnRH-1 neurons in the brain, hypothalamic explants are made from the preoptic area (70), mediobasal hypothalamus (69). or median eminence (66) regions, and these dissections might bias GnRH-1 subpopulations involved in GnRH-1 pulses or GnRH-1 surges (71). GT-1 cells and GnRH-1 cells in mouse nasal explants exhibit many similarities including electrophysiological properties and possession of a wide variety of voltage- and ligand-gated ion channels (20). Thus, both cells exhibit characteristics of well differentiated neurons. However, data presented in this study contrast data obtained from GT-1 cells (6). One explanation for these differences could be the maintenance of interneuronal networks in nasal explants, even though minimized, which could modulate the observed response: GABAergic (22), cholecystokininergic (31), purinergic (72), and catecholaminergic (73). To date, all of the identified modulators in mouse nasal explants are also present during development in vivo and thus may shape the final characteristics of GnRH-1 neurons postnatally.
In summary, the studies in this paper indicate that basal or stimulated GnRH-1 neuronal activity is not simply driven by CNG channels. However, the response of GnRH-1 neurons to FSK-induced cAMP increases demonstrates the ability of GnRH-1 neurons to integrate cAMP signals and thus to be a component for modulation by neurotransmitters. Two potential pathways could be activated by a rise in cAMP. One could be activation of protein kinase A by cAMP (74), and protein kinase A-dependent phosphorylations are known to modulate the properties of voltage-activated channels involved in membrane excitability such as sodium channels, potassium channels, and calcium channels, all found in GnRH-1 neurons (2,20). A second pathway could be directly linked to the rise of cAMP such as hyperpolarization-activated CNG-modulated cation (HCN) channels recently shown to be in GnRH-1 neurons in vivo (75). HCN channels, sensitive to cyclic nucleotides (76) and to neurotransmitters (77), have been shown to be involved in rhythmic activity, control of the membrane potential, and neuronal responsiveness (78). Whether voltage-activated channels or HCN channels, together with cAMP signals, are involved in basal neuronal activity and/or oscillatory activity of GnRH-1 neurons remains to be determined.
Acknowledgments
We appreciate the help of Jennifer Pakiam with immunocytochemistry of adult CNGA2-deficient animals and cell counting, Andree Reuss with culturing nasal explants from CNGA2-deficient embryos, Dr. Brett Shoelson (Mathworks) and Dr. Joby Joseph (National Institute of Child Health and Human Development) with Matlab, and Dr. Eric Dubuis (University of Liverpool) for his comments. We thank Dr. Randall Reed for providing CNGA2-deficient mice. We thank the Howard Hughes Medical Institute National Institutes of Health Research Scholars Program and the Newcomb Fellowship Program for providing student interns who helped in this work, Amy Liou and Cina Karodeh, respectively.
Footnotes
This work was supported by the Intramural Research Program of the National Institutes of Health National Institutes of Neurological Disorders and Stroke.
Disclosure Statement: The authors have nothing to disclose.
First Published Online October 4, 2007
Abbreviations: AC, Adenylyl cyclase; BIC, bicucullin; CNG, cyclic nucleotide-gated; DDA, dideoxyadenosine; div, days in vitro; E11.5, embryonic d 11.5; FSK, forskolin; GABA, γ-aminobutyric acid; HCN, hyperpolarization-activated CNG-modulated cation; IBMX, 3-isobutyl-1-methylxanthine; KO, knockout; LCD, l-cis-diltiazem; PDE, phosphodiesterase; SFM, serum-free medium; Vact1/2, voltage of half-activation; Vinac1/2, voltage of half-inactivation; WT, wild type.
References
- Wray S, Grant P, Gainer H 1989 Evidence that cells expressing luteinizing hormone-releasing hormone mRNA in the mouse are derived from progenitor cells in the olfactory placode. Proc Natl Acad Sci USA 86:8132–8136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbison AE 2006 Physiology of the gonadotropin-releasing hormone neuronal network. In: Knobil E, Neill J, eds. The physiology of reproduction. 3rd ed. New York: Academic Press; 1415–1482 [Google Scholar]
- Belchetz PE, Plant TM, Nakai Y, Keogh EJ, Knobil E 1978 Hypophysial responses to continuous and intermittent delivery of hypothalamic gonadotropin-releasing hormone. Science 202:631–633 [DOI] [PubMed] [Google Scholar]
- Yoon H, Enquist LW, Dulac C 2005 Olfactory inputs to hypothalamic neurons controlling reproduction and fertility. Cell 123:669–682 [DOI] [PubMed] [Google Scholar]
- Boehm U, Zou Z, Buck LB 2005 Feedback loops link odor and pheromone signaling with reproduction. Cell 123:683–695 [DOI] [PubMed] [Google Scholar]
- Weiner RI, Charles A 2001 Regulation of gonadotropin-releasing hormone release by cyclic AMP signalling pathways. Growth Horm IGF Res 11(Suppl A):S9–S15 [DOI] [PubMed] [Google Scholar]
- Navarro CE, Saeed SA, Murdock C, Martinez-Fuentes AJ, Arora KK, Krsmanovic LZ, Catt KJ 2003 Regulation of cyclic adenosine 3′,5′-monophosphate signaling and pulsatile neurosecretion by Gi-coupled plasma membrane estrogen receptors in immortalized gonadotrophin-releasing hormone neurons. Mol Endocrinol 17:1792–1804 [DOI] [PubMed] [Google Scholar]
- Wada K, Hu L, Mores N, Navarro CE, Fuda H, Krsmanovic LZ, Catt KJ 2006 Serotonin (5-HT) receptor subtypes mediate specific modes of 5-HT-induced signaling and regulation of neurosecretion in gonadotropin-releasing hormone neurons. Mol Endocrinol 20:125–135 [DOI] [PubMed] [Google Scholar]
- Kaneishi K, Sakuma Y, Kobayashi H, Kato M 2002 3′,5′-Cyclic adenosine monophosphate augments intracellular Ca2+ concentration and gonadotropin-releasing hormone (GnRH) release in immortalized GnRH neurons in an Na+-dependent manner. Endocrinology 143:4210–4217 [DOI] [PubMed] [Google Scholar]
- Chen EC, Javors MA, Norris C, Siler-Khodr T, Schenken RS, King TS 2004 Dependence of 3′,5′-cyclic adenosine monophosphate-stimulated gonadotropin-releasing hormone release on intracellular calcium levels and L-type calcium channels in superfused GT1–7 neurons. J Soc Gynecol Investig 11:393–398 [DOI] [PubMed] [Google Scholar]
- Sakakibara H, Conti M, Weiner RI 1998 Role of phosphodiesterases in the regulation of gonadotropin-releasing hormone secretion in GT1 cells. Neuroendocrinology 68:365–373 [DOI] [PubMed] [Google Scholar]
- Paruthiyil S, El Majdoubi M, Conti M, Weiner RI 2002 Phosphodiesterase expression targeted to gonadotropin-releasing hormone neurons inhibits luteinizing hormone pulses in transgenic rats. Proc Natl Acad Sci USA 99:17191–17196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H, Beltran-Parrazal L, Butler P, Conti M, Charles AC, Weiner RI 2003 Lowering cyclic adenosine-3′,5′-monophosphate (cAMP) levels by expression of a cAMP-specific phosphodiesterase decreases intrinsic pulsatile gonadotropin-releasing hormone secretion from GT1 cells. Mol Endocrinol 17:1982–1990 [DOI] [PubMed] [Google Scholar]
- Blackman BE, Hiroshi Y, Paruthiyil S, Weiner RI, Frequency of intrinsic pulsatile GnRH secretion is regulated by the expression of cyclic nucleotide gated channels in GT1 cells. Proc 35th Annual Meeting of the Society for Neuroscience, 2005 (Abstract 126.7) [Google Scholar]
- El-Majdoubi M, Weiner RI 2002 Localization of olfactory cyclic nucleotide-gated channels in rat gonadotropin-releasing hormone neurons. Endocrinology 143:2441–2444 [DOI] [PubMed] [Google Scholar]
- Terasawa E, Quanbeck CD, Schulz CA, Burich AJ, Luchansky LL, Claude P 1993 A primary cell culture system of luteinizing hormone releasing hormone neurons derived from embryonic olfactory placode in the rhesus monkey. Endocrinology 133:2379–2390 [DOI] [PubMed] [Google Scholar]
- Daikoku S, Koide I, Chikamori-Aoyama M, Shimomura Y 1993 Migration of LHRH neurons derived from the olfactory placode in rats. Arch Histol Cytol 56:353–370 [DOI] [PubMed] [Google Scholar]
- Fueshko S, Wray S 1994 LHRH cells migrate on peripherin fibers in embryonic olfactory explant cultures: an in vitro model for neurophilic neuronal migration. Dev Biol 166:331–348 [DOI] [PubMed] [Google Scholar]
- Duittoz AH, Batailler M, Caldani M 1997 Primary cell culture of LHRH neurones from embryonic olfactory placode in the sheep (Ovis aries). J Neuroendocrinol 9:669–675 [DOI] [PubMed] [Google Scholar]
- Kusano K, Fueshko S, Gainer H, Wray S 1995 Electrical and synaptic properties of embryonic luteinizing hormone-releasing hormone neurons in explant cultures. Proc Natl Acad Sci USA 92:3918–3922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terasawa E, Schanhofer WK, Keen KL, Luchansky L 1999 Intracellular Ca2+ oscillations in luteinizing hormone-releasing hormone neurons derived from the embryonic olfactory placode of the rhesus monkey. J Neurosci 19:5898–5909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore Jr JP, Shang E, Wray S 2002 In situ GABAergic modulation of synchronous gonadotropin releasing hormone-1 neuronal activity. J Neurosci 22:8932–8941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terasawa E, Keen KL, Mogi K, Claude P 1999 Pulsatile release of luteinizing hormone-releasing hormone (LHRH) in cultured LHRH neurons derived from the embryonic olfactory placode of the rhesus monkey. Endocrinology 140:1432–1441 [DOI] [PubMed] [Google Scholar]
- Duittoz AH, Batailler M 2000 Pulsatile GnRH secretion from primary cultures of sheep olfactory placode explants. J Reprod Fertil 120:391–396 [PubMed] [Google Scholar]
- Funabashi T, Daikoku S, Shinohara K, Kimura F 2000 Pulsatile gonadotropin-releasing hormone (GnRH) secretion is an inherent function of GnRH neurons, as revealed by the culture of medial olfactory placode obtained from embryonic rats. Neuroendocrinology 71:138–144 [DOI] [PubMed] [Google Scholar]
- Zhao H, Reed RR 2001 X inactivation of the OCNC1 channel gene reveals a role for activity-dependent competition in the olfactory system. Cell 104:651–660 [DOI] [PubMed] [Google Scholar]
- Kramer PR, Krishnamurthy R, Mitchell PJ, Wray S 2000 Transcription factor activator protein-2 is required for continued luteinizing hormone-releasing hormone expression in the forebrain of developing mice. Endocrinology 141:1823–1838 [DOI] [PubMed] [Google Scholar]
- Sharifi N, Reuss AE, Wray S 2002 Prenatal LHRH neurons in nasal explant cultures express estrogen receptor β transcript. Endocrinology 143:2503–2507 [DOI] [PubMed] [Google Scholar]
- Toba Y, Pakiam JG, Wray S 2005 Voltage-gated calcium channels in developing GnRH-1 neuronal system in the mouse. Eur J Neurosci 22:79–92 [DOI] [PubMed] [Google Scholar]
- Giacobini P, Wray S 2007 Cholecystokinin directly inhibits neuronal activity of primary gonadotropin-releasing hormone cells through cholecystokinin-1 receptor. Endocrinology 148:63–71 [DOI] [PubMed] [Google Scholar]
- Giacobini P, Kopin AS, Beart PM, Mercer LD, Fasolo A, Wray S 2004 Cholecystokinin modulates migration of gonadotropin-releasing hormone-1 neurons. J Neurosci 24:4737–4748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temple JL, Wray S 2005 Developmental changes in GABA receptor subunit composition within the gonadotrophin-releasing hormone-1 neuronal system. J Neuroendocrinol 17:591–599 [DOI] [PubMed] [Google Scholar]
- Mellon PL, Windle JJ, Goldsmith PC, Padula CA, Roberts JL, Weiner RI 1990 Immortalization of hypothalamic GnRH neurons by genetically targeted tumorigenesis. Neuron 5:1–10 [DOI] [PubMed] [Google Scholar]
- Radovick S, Wray S, Lee E, Nicols DK, Nakayama Y, Weintraub BD, Westphal H, Cutler Jr GB, Wondisford FE 1991 Migratory arrest of gonadotropin-releasing hormone neurons in transgenic mice. Proc Natl Acad Sci USA 88:3402–3406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunemaker CS, DeFazio RA, Moenter SM 2003 A targeted extracellular approach for recording long-term firing patterns of excitable cells: a practical guide. Biol Proced Online 5:53–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolters H, Willemien W, Ypey DL, Boom HB 1994 Ionic currents during action potentials in mammalian skeletal muscle fibers analyzed with loose patch clamp. Am J Physiol 267:C1699–C1706 [DOI] [PubMed] [Google Scholar]
- Gorbunova YV, Spitzer NC 2002 Dynamic interactions of cyclic AMP transients and spontaneous Ca2+ spikes. Nature 418:93–96 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Iglesias AE, Jiang Y, Tomic M, Kretschmannova K, Andric SA, Zemkova H, Stojilkovic SS 2006 Dependence of electrical activity and calcium influx-controlled prolactin release on adenylyl cyclase signaling pathway in pituitary lactotrophs. Mol Endocrinol 20:2231–2246 [DOI] [PubMed] [Google Scholar]
- Wray S, Fueshko SM, Kusano K, Gainer H 1996 GABAergic neurons in the embryonic olfactory pit/vomeronasal organ: maintenance of functional GABAergic synapses in olfactory explants. Dev Biol 180:631–645 [DOI] [PubMed] [Google Scholar]
- Bradley J, Reisert J, Frings S 2005 Regulation of cyclic nucleotide-gated channels. Curr Opin Neurobiol 15:343–349 [DOI] [PubMed] [Google Scholar]
- Hofmann F, Biel M, Kaupp UB 2003 International Union of Pharmacology. XLII. Compendium of voltage-gated ion channels: cyclic nucleotide-modulated channels. Pharmacol Rev 55:587–589 [DOI] [PubMed] [Google Scholar]
- Wei JY, Cohen ED, Genieser HG, Barnstable CJ 1998 Substituted cGMP analogs can act as selective agonists of the rod photoreceptor cGMP-gated cation channel. J Mol Neurosci 10:53–64 [DOI] [PubMed] [Google Scholar]
- Hashimoto Y, Yabana H, Murata S 2000 Electrophysiological effect of l-cis-diltiazem, the stereoisomer of d-cis-diltiazem, on isolated guinea-pig left ventricular myocytes. Eur J Pharmacol 391:217–223 [DOI] [PubMed] [Google Scholar]
- Draper MH, Weidmann S 1951 Cardiac resting and action potentials recorded with an intracellular electrode. J Physiol 115:74–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker H, Cummings DM, Munger SD, Margolis JW, Franzen L, Reed RR, Margolis FL 1999 Targeted deletion of a cyclic nucleotide-gated channel subunit (OCNC1): biochemical and morphological consequences in adult mice. J Neurosci 19:9313–9321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandiyan VS, Coats JK, Shah NM 2005 Deficits in sexual and aggressive behaviors in Cnga2 mutant mice. Nat Neurosci 8:1660–1662 [DOI] [PubMed] [Google Scholar]
- Keverne EB 2005 Odor here, odor there: chemosensation and reproductive function. Nat Neurosci 8:1637–1638 [DOI] [PubMed] [Google Scholar]
- Brunet LJ, Gold GH, Ngai J 1996 General anosmia caused by a targeted disruption of the mouse olfactory cyclic nucleotide-gated cation channel. Neuron 17:681–693 [DOI] [PubMed] [Google Scholar]
- Lin DM, Wang F, Lowe G, Gold GH, Axel R, Ngai J, Brunet L 2000 Formation of precise connections in the olfactory bulb occurs in the absence of odorant-evoked neuronal activity. Neuron 26:69–80 [DOI] [PubMed] [Google Scholar]
- Van Goor F, Krsmanovic LZ, Catt KJ, Stojilkovic SS 2000 Autocrine regulation of calcium influx and gonadotropin-releasing hormone secretion in hypothalamic neurons. Biochem Cell Biol 78:359–370 [PubMed] [Google Scholar]
- Beltran-Parrazal L, Noris G, Clapp C, Martinez de la Escalera G 2001 GABA inhibition of immortalized gonadotropin-releasing hormone neuronal excitability involves GABAA receptors negatively coupled to cyclic adenosine monophosphate formation. Endocrine 14:189–195 [DOI] [PubMed] [Google Scholar]
- LeBeau AP, Van Goor F, Stojilkovic SS, Sherman A 2000 Modeling of membrane excitability in gonadotropin-releasing hormone-secreting hypothalamic neurons regulated by Ca2+-mobilizing and adenylyl cyclase-coupled receptors. J Neurosci 20:9290–9297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojeda SR, Urbanski HF, Katz KH, Costa ME 1985 Stimulation of cyclic adenosine 3′,5′-monophosphate production enhances hypothalamic luteinizing hormone-releasing hormone release without increasing prostaglandin E2 synthesis: studies in prepubertal female rats. Endocrinology 117:1175–1178 [DOI] [PubMed] [Google Scholar]
- Lee BJ, Kim K, Cho WK 1990 Activation of intracellular pathways with forskolin and phorbol ester increases LHRH mRNA level in the rat hypothalamus superfused in vitro. Brain Res Mol Brain Res 8:185–191 [DOI] [PubMed] [Google Scholar]
- Hartter DE, Ramirez VD 1985 Responsiveness of immature versus adult male rat hypothalami to dibutyryl cyclic AMP- and forskolin-induced LHRH release in vitro. Neuroendocrinology 40:476–482 [DOI] [PubMed] [Google Scholar]
- Mitsushima D, Marzban F, Luchansky LL, Burich AJ, Keen KL, Durning M, Golos TG, Terasawa E 1996 Role of glutamic acid decarboxylase in the prepubertal inhibition of the luteinizing hormone releasing hormone release in female rhesus monkeys. J Neurosci 16:2563–2573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim JA, Skynner MJ, Pape JR, Herbison AE 2000 Late postnatal reorganization of GABAA receptor signalling in native GnRH neurons. Eur J Neurosci 12:3497–3504 [DOI] [PubMed] [Google Scholar]
- Yoshida H, Paruthiyil S, Butler P, Weiner RI 2004 Role of cAMP signaling in the mediation of dopamine-induced stimulation of GnRH secretion via D1 dopamine receptors in GT1-7 Cells. Neuroendocrinology 80:2–10 [DOI] [PubMed] [Google Scholar]
- Temple JL, Laing E, Sunder A, Wray S 2004 Direct action of estradiol on gonadotropin-releasing hormone-1 neuronal activity via a transcription-dependent mechanism. J Neurosci 24:6326–6333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itogawa E, Kurosawa H, Yabana H, Murata S 1996 Protective effect of l-cis-diltiazem on hypercontracture of rat myocytes induced by veratridine. Eur J Pharmacol 317:401–406 [DOI] [PubMed] [Google Scholar]
- Frings S, Seifert R, Godde M, Kaupp UB 1995 Profoundly different calcium permeation and blockage determine the specific function of distinct cyclic nucleotide-gated channels. Neuron 15:169–179 [DOI] [PubMed] [Google Scholar]
- Jarjour LT, Handelsman DJ, Raum WJ, Swerdloff RS 1986 Mechanism of action of dopamine on the in vitro release of gonadotropin-releasing hormone. Endocrinology 119:1726–1732 [DOI] [PubMed] [Google Scholar]
- Kim K, Ramirez VD 1985 Dibutyryl cyclic adenosine monophosphate stimulates in vitro luteinizing hormone-releasing hormone release only from median eminence derived from ovariectomized, estradiol-primed rats. Brain Res 342:154–157 [DOI] [PubMed] [Google Scholar]
- Meyer DC 1989 Serotonin stimulation of the period of in vitro LHRH release is estradiol dependent. Brain Res Bull 22:525–530 [DOI] [PubMed] [Google Scholar]
- Ramirez VD, Gallardo E, Hartter D 1980 Factors altering the secretion of LHRH from superfused fragments of rat hypothalamus. J Endocrinol Invest 3:29–37 [DOI] [PubMed] [Google Scholar]
- Ojeda SR, Negro-Vilar A 1985 Prostaglandin E2-induced luteinizing hormone-releasing hormone release involves mobilization of intracellular Ca2+. Endocrinology 116:1763–1770 [DOI] [PubMed] [Google Scholar]
- Leadem CA, Crowley WR, Simpkins JW, Kalra SP 1985 Effects of naloxone on catecholamine and LHRH release from the perifused hypothalamus of the steroid-primed rat. Neuroendocrinology 40:497–500 [DOI] [PubMed] [Google Scholar]
- Ojeda SR, Urbanski HF, Katz KH, Costa ME, Conn PM 1986 Activation of two different but complementary biochemical pathways stimulates release of hypothalamic luteinizing hormone-releasing hormone. Proc Natl Acad Sci USA 83:4932–4936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rettori V, Gimeno M, Lyson K, McCann SM 1992 Nitric oxide mediates norepinephrine-induced prostaglandin E2 release from the hypothalamus. Proc Natl Acad Sci USA 89:11543–11546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourguignon JP, Franchimont P 1984 Puberty-related increase in episodic LHRH release from rat hypothalamus in vitro. Endocrinology 114:1941–1943 [DOI] [PubMed] [Google Scholar]
- Kimura F, Funabashi T 1998 Two subgroups of gonadotropin releasing hormone neurons control gonadotropin secretion in rats. News Physiol Sci 13:225–231 [DOI] [PubMed] [Google Scholar]
- Terasawa E, Keen KL, Grendell RL, Golos TG 2005 Possible role of 5′-adenosine triphosphate in synchronization of Ca2+ oscillations in primate luteinizing hormone-releasing hormone neurons. Mol Endocrinol 19:2736–2747 [DOI] [PubMed] [Google Scholar]
- Izvolskaia M, Duittoz AH, Ugrumov MV, Tillet Y 2006 Tyrosine hydroxylase expression in the olfactory/respiratory epithelium in early sheep fetuses (Ovis aries). Brain Res 1083:29–38 [DOI] [PubMed] [Google Scholar]
- Tasken K, Aandahl EM 2004 Localized effects of cAMP mediated by distinct routes of protein kinase A. Physiol Rev 84:137–167 [DOI] [PubMed] [Google Scholar]
- Arroyo A, Kim B, Rasmusson RL, Bett G, Yeh J 2006 Hyperpolarization-activated cation channels are expressed in rat hypothalamic gonadotropin-releasing hormone (GnRH) neurons and immortalized GnRH neurons. J Soc Gynecol Investig 13:442–450 [DOI] [PubMed] [Google Scholar]
- Ludwig A, Zong X, Jeglitsch M, Hofmann F, Biel M 1998 A family of hyperpolarization-activated mammalian cation channels. Nature 393:587–591 [DOI] [PubMed] [Google Scholar]
- Pape HC 1996 Queer current and pacemaker: the hyperpolarization-activated cation current in neurons. Annu Rev Physiol 58:299–327 [DOI] [PubMed] [Google Scholar]
- Luthi A, McCormick DA 1998 H-current: properties of a neuronal and network pacemaker. Neuron 21:9–12 [DOI] [PubMed] [Google Scholar]