Abstract
A substantial fraction of the noradrenergic innervation targeting the mammalian ovary is provided by neurons of the celiac ganglion. Although studies in the rat have shown that noradrenergic nerves reach the ovary near the time of birth, it is unknown how the functional capacity of this innervation unfolds during postnatal ovarian development. To address this issue, we assessed the ability of the developing ovary to incorporate and release 3H-norepinephrine. Incorporation of 3H-norepinephrine was low during the first 3 wk of postnatal life, but pharmacological inhibition of norepinephrine (NE) neuronal uptake with cocaine showed that an intact transport mechanism for NE into nerve terminals is already in place by the first week after birth. Consistent with this functional assessment, the mRNA encoding the NE transporter was also expressed in the celiac ganglion at this time. During neonatal-infantile development [postnatal (PN) d 5–20], the spontaneous, vesicle-independent outflow of recently taken up NE was high, but the NE output in response to K+-induced depolarization was low. After PN d 20, spontaneous outflow decreased and the response to K+ increased markedly, reaching maximal values by the time of puberty. Tyramine-mediated displacement of NE stored in vesicles, which displace vesicular NE, showed that vesicle-dependent NE storage becomes functional by PN d 12 and that vesicular release increases during the juvenile-peripubertal phases of sexual development. These results indicate that vesicular release of NE from ovarian noradrenergic nerves begins to operate by the third week of postnatal life, becoming fully functional near the time of puberty.
IT IS NOW WELL established that the mammalian ovary is innervated by sympathetic and sensory neurons that are extrinsic to the gland (1) and synaptically connected to the paraventricular nucleus of the hypothalamus (2,3). It is also clear that the rat ovary contains the necessary elements for noradrenergic function, including the enzymes involved in norepinephrine (NE) biosynthesis (4,5), a substantial content of NE (6), and the ability to release the catecholamine in vivo during the estrous cycle and during pathological states (7,8), and in vitro in response to depolarization of nerve terminals (9,10). Furthermore, β-noradrenergic receptors are expressed in both the thecal and granulosa cell compartments of the gland, and these receptors are positively coupled to steroidogenic responses (11,12). Whereas most of these studies have been carried out in either peripubertal or adult rats, less information is available concerning the role of the extrinsic ovarian innervation during early postnatal life, when follicular development begins.
Years ago we (13,14) demonstrated that both passive and active neonatal sympathectomy reduces follicular development in the rat ovary during adulthood, suggesting an involvement of sympathetic nerves in the control of early follicular growth. The presence of sympathetic nerves in the mammalian ovary before birth (15,16) and the ability of the neonatal ovary to respond in vitro to β-adrenergic stimulation with cAMP production and synthesis of FSH receptors led to the suggestion that neural activity might be an important factor in the regulation of follicular development before the ovary acquires responsiveness to gonadotropins (17). According to this concept, ovarian nerves may act via neurotransmitters coupled to the cAMP-generating system to influence the differentiation process by which newly formed primary follicles acquire FSH receptors and responsiveness to FSH.
Because the sympathetic innervation of the ovary also develops as the ovary approaches reproductive maturity (18), changes in sympathetic nerve activity would be expected to occur as follicular development proceeds. To address this issue, we examined the activity of ovarian sympathetic nerves in the rat ovary from neonatal development to the peripubertal period and found that although the nerves can already incorporate NE after birth, the capacity for vesicular release of the catecholamine does not fully develop until near the time of puberty.
Materials and Methods
Animals
Ovaries from neonatal-infantile (5–20 d of age), juvenile (21–30 d of age), and peripubertal (31–40 d of age) Sprague Dawley rats derived from a stock maintained at the University of Chile were used. For the incorporation and release studies and measurement of NE content, the ovaries were assigned to eight groups of the following ages (in days): 5–8 (n = 15), 9–11 (n = 10), 12–15 (n = 10), 16–20 (n = 10), 21–25 (n = 10), 26–30 (n = 10), 31–35 (n = 10), and 36–40 (postovulatory rats, n = 10). Additionally, for cocaine experiments, we used five rats/group at 7, 20, and 40 d of age. These later animals had already ovulated at the time of the experiment. For tyramine experiments we used five rats at each of the following days: 5–8, 9–11, and 21–25 and after ovulation, and for calcium dependency we used five rats at 12–15 d and five postovulatory rats. All animal procedures were approved by the Institutional Ethic Committee of the Faculty of Chemistry and Pharmaceutical Sciences, Universidad de Chile, and the experiments were conducted in accordance with the International Guiding Principles for Biomedical Research Involving Animals as promulgated by the Society for the Study of Reproduction.
After euthanizing the rats by decapitation, the ovaries were rapidly removed, cleaned of adherent tissue, immediately weighed, and used for NE release experiments. Other ovaries of the same ages were also weighed and frozen at −80 C for NE determination.
NE release
NE release was measured using a procedure previously described (10,19), with slight modifications. Each sample consisted of the two ovaries from one animal, with the exception of 5- to 8-d-old ovaries, which were pooled to have four glands per sample. The ovaries were preincubated for 20 min in Krebs-Ringer bicarbonate (KRB) buffer (pH 7.4) gassed with 95% O2-5% CO2 and then incubated for 30 min at 37 C with 2 μCi of 3H-NE (40.1 Ci/mmol, DuPont/NEN Life Science Products, Boston, MA). Radioactivity not retained by the tissue was washed out by an additional 60-min incubation period in Krebs-bicarbonate free of 3H-NE.
The tissues were then transferred to a thermoregulated perifusion chamber (1 ml capacity). The perifusion fluid was KRB and the flow rate was 1.5 ml/min. Fractions were collected every minute. After a 3-min collection period to measure spontaneous NE release, the K+ concentration in the perifusate was increased from 5 to 80 mm for 1 min. To maintain osmolarity constant, NaCl concentration was decreased accordingly. After 1 min of stimulation, the perifusion buffer was switched to normal KRB and four additional 1-min fractions were collected.
To biochemically characterize NE outflow, we challenged the ovaries using two manipulations routinely used to study basic properties of functional noradrenergic synapses: 1) dependence to extracellular calcium and 2) displacement of NE stored in vesicles by tyramine, a false neurotransmitter (20).
To study the dependence of depolarization-induced release of NE on extracellular calcium, the ovaries were first perifused for 1 min with the high K+ solution (control response), then for 4 min in normal KRB, followed by 10 min with KRB lacking calcium and containing 0.1 mm EGTA. Thereafter, three 1-min samples were collected to measure basal efflux, and the ovaries were depolarized again with 80 mm KCl in calcium-free, EGTA-containing medium.
To determine the effect of NE depletion from vesicular stores on evoked release, the ovaries were first subjected to a control 1-min pulse with high K+. Four minutes later, they were perfused for 10 min with KRB containing 10−5 m l-tyramine (Sigma Chemical Co., St. Louis, MO). After a 3 min collection period in the presence of tyramine, the tissues were challenged again with K+. The dose of tyramine we used has been shown to fully displace endogenous NE from synaptic vesicles (20).
At the end of each experiment, the ovaries were homogenized in 1.5 ml 0.4 n perchloric acid. The resulting suspension was centrifuged at 15,000 × g for 10 min, and the supernatant containing the 3H-labeled NE remaining in the tissue was collected. The radioactivity present in portions (0.6 ml) from each 1-min fraction, and from the tissue homogenates, was assessed (at 52% efficiency) in a scintillation counter. To express the results, we added the radioactivity remaining in the tissue to the radioactivity present in each of the 1-min fractions and expressed the 3H-labeled NE detected in the medium at each 1-min interval as fractional release of this value.
NE incorporation
Incorporation of 3H-labeled NE into the ovarian tissue was estimated as described earlier (10,19), i.e. by assuming that the radioactivity remaining in the tissue at the end of the experiment plus the amount of radioactivity previously released in each 1-min fraction represent the labeled NE retained in the tissue
To determine whether ovarian nerves are endowed with a functional NE transporter, ovaries from 5- to 8-, 20- to 25-, and 36- to 40-d-old rats were exposed to 10 μm cocaine (Sigma) for 10 min at 37 C, and the incorporation of 3H-NE was evaluated. Cocaine binds differentially to dopamine, serotonin, and NE transporters, preventing reuptake of these neurotransmitters into presynaptic terminals (21,22). One ovary from each animal was incubated in KRB and the contralateral gland in KRB containing cocaine. The tissues were then incubated with 2 μl/ml 3H-NE [levo-(7,8-3H)norepinephrine, 40 Ci/mmol, Amersham Biosciences, Buckinghamshire, UK] for 30 min at 37 C. Thereafter they were washed six times for 10 min each with KRB to remove nonincorporated 3H-NE. To eliminate the possibility of residual nonneuronal 3H-NE incorporation during washing, the ovaries treated with cocaine were washed two times in the presence of 10 μm cocaine. After the sixth wash, the tissues were homogenized in 1.5 ml 0.4 n perchloric acid, the resulting suspension was centrifuged at 15,000 × g for 10 min, and the supernatant containing [3H]labeled NE was used for quantization. Aliquots (0.6 ml) from each homogenate were counted for 3H (72.5% efficiency) in a scintillation counter. The radioactivity retained in the tissue was expressed as outlined above.
Measurement of endogenous tissue NE content.
The two frozen ovaries from each animal were homogenized in 200 μl 0.1 m acetic acid. Fifty microliters of the homogenate were precipitated with 180 μl 0.2 m HClO4 and centrifuged (15,000 × g, 15 min). NE was measured in the supernatant by HPLC coupled with electrochemical detection (23). A 150-μl sample was mixed with 50 mg activated alumina in 1 ml Tris 1.5 m (pH 8.3–8.5), containing 2% EDTA. Dihydroxybenzyl amine (2000 pg in 20 μl; Sigma) was added as an internal standard. The alumina was rinsed thoroughly with nanopure water and NE was eluted with 100 μl of 0.2 n perchloric acid and centrifuged; 20 μl of the resulting supernatant were injected into a Waters HPLC system equipped with a C18 reverse phase column (Lichrosphere, 60 RP-Select B; Merck, Darmstadt, Germany) and an electrochemical detector (Waters 464). The mobile phase contained 0.1 m NaH2PO4, 0.42 mm octyl-sulfate, 0.02% EDTA, and 1.5% acetonitrile (pH 2.5) with a flow rate of 0.9 ml/min. The potential of the amperometric detector was set at 0.7 V. Under these experimental conditions, the retention time was 4 min for NE and 10 min for dihydroxybenzyl amine.
RT-PCR
We used RT-PCR to determine whether the mRNA encoding NE transporter (NET) is expressed in the celiac ganglion at the time of postnatal development when pharmacological blockade of NET activity inhibits NE uptake. In addition, we measured tyrosine hydroxylase (TH) mRNA as a positive control. The celiac ganglion was dissected as described earlier (3,24). Total RNA was extracted as recommended (25). Five milligrams total RNA were subjected to reverse transcription at 42 C for 60 min, using 1.6 mm deoxynucleotide triphosphates, 10 mm dithiothreitol, 176 nm random hexamers, 25 U RNaseOUT, 125 U reverse transcriptase SuperScript II (all from Invitrogen, Carlsbad, CA), and first-strand buffer in a 30-ml volume. The reaction was terminated by heating the samples at 75 C for 10 min. Dilutions of the reverse transcription reaction were subjected to PCR amplification using 1 U of DNA Taq polymerase (Promega, Madison, WI), 1 mm deoxynucleotide triphosphates, and 0.5 μm of each gene-specific primer in a total volume of 30 μl. The PCR consisted of 32 cycles (denaturation at 94 C for 1 min, annealing at 60 C for 60 sec, and extension at 72 C for 1 min) using a DNA thermal cycler (MJ Research, Watertown, MA). The forward oligodeoxynucleotide primer used to detect TH mRNA (5′-GGT CTA CTG TCC GCC CGT GAT-3′) corresponds to nt 1244–1264 in rat TH mRNA (NM_12740); the reverse primer (5′-GCC CCC AGA GAT GCA AGT CCA ATG-3′) is complementary to nt1443–1420. The primers for NET were previously reported (26). The forward primer (5′-CAG CAC CAT CAA CTG TGT TAC C-3′) corresponds to nt 1038–1059; the reverse primer (5′-AGG ACC TGG AAG TCA TCA GC-3′) is complementary to nt 1313–1294). The primers used to detect 18s ribosomal RNA (used as constitutively expressed gene for normalization purposes) were obtained from Ambion (Wiesbaden, Germany). Amplification of 18s RNA was performed in a different tube to avoid interference with the amplification of NET and TH mRNAs. Reaction tubes lacking reverse transcriptase were used as PCR controls. The RT-PCR products were separated on 2.0% agarose gels, stained with ethidium bromide, and photographed digitally.
Statistics.
Differences between age groups were assessed by ANOVA, followed by the Student-Newman-Keuls multiple comparison test for unequal replications. Differences between two groups of the same age were analyzed using the Student’s t test. P < 0.05 was considered to be statistically significant.
Results
Incorporation of 3H-NE during postnatal ovarian development
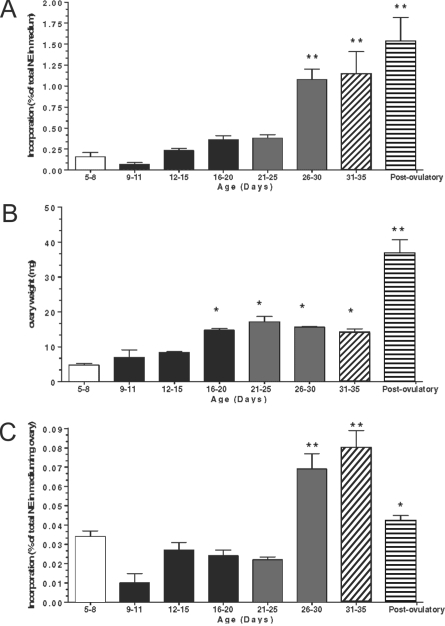
Total incorporation of 3H-NE into ovarian tissue increased moderately during the neonatal-infantile period (d 5–20) and then abruptly during the second half of juvenile development (d 26–30), remaining elevated at puberty (d 31–35), and after the first ovulation (d 40) (Fig. 1A). Because these changes may be related to the increase in ovarian weight that occurred during this developmental period (Fig. 1B), the incorporation of 3H-NE was expressed as a function of ovarian weight. As shown in Fig. 1C, incorporation of NE per milligram ovary was as high in neonatal-early infantile (5–8 d old) ovaries as in early juvenile (21–25 d old) gonads. After a transient decline at d 9–11, the uptake returned to neonatal-infantile values, remaining constant throughout the early part of juvenile development (d 20–26). By the second half of the juvenile period (d 26–30), and reproducing the pattern of NE uptake expressed per ovary, the incorporation of NE increased markedly, remaining elevated through puberty to decrease again after ovulation. This later decrease likely represents a dilution effect related to the presence of corpora lutea, which are devoid of sympathetic nerves.
Figure 1.
A, Increase in the capacity of the ovary to incorporate 3H-NE during development. Results are expressed as percentage of incorporation calculated as described in Materials and Methods. **, P < 0.01 vs. all groups between 5 and 25 d of age. B, Changes in ovarian weight during development. For both panels, each bar represents the mean ± sem of five experiments, each performed using four ovaries (two rats) for the 5- to 8-d-old group and two ovaries (one rat) for all other ages. C, Incorporation of 3H-NE per milligram ovarian tissue. The bars with different filling patterns represent the different developmental phases examined: 5–8 (late neonatal-early infantile), 9–20 (infantile), 21–30 (juvenile), 31–35 (peripubertal), and 40 (postovulatory). Bars are means and vertical bars are sem. *, P < 0.05 vs. 5–15 d old; **, P < 0.01 vs. all other groups.
Postnatal development of neuronal NE transport
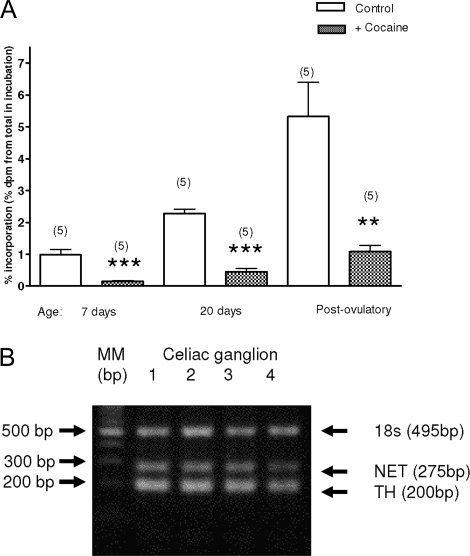
To determine whether the changes in 3H-NE incorporation observed during prepubertal development are due to the activation of a specific transport mechanism in ovarian nerve terminals, ovaries from late neonatal (7 d old), late infantile (20 d old), and postovulatory (40 d old) rats were exposed to 10 μm cocaine, a NET/dopamine transporter (DAT) blocker. This dose was previously shown to block 3H-NE incorporation into peripheral sympathetic nerves (27). At all ages examined, cocaine reduced substantially (∼80%) the incorporation of 3H-NE into ovarian tissue (Fig. 2A). Although cocaine was very effective in blocking 3H-NE incorporation into 7-d-old ovaries, subsequent experiments showed that ovaries of this age fail to respond to elevated K+ with increased NE efflux (see below). It was therefore important to determine whether noradrenergic neurons of the celiac ganglion express the gene encoding NET at this early time of postnatal development. As shown in Fig. 2B, both TH and NET mRNAs are readily detectable in the celiac ganglion of 5-d-old rats, supporting the conclusion derived from the pharmacological approach that the capacity of NET-mediated NE transport is already developed by the end of the neonatal period.
Figure 2.
A, Effect of cocaine (10 μm) on the capacity of the ovary to incorporate 3H-NE during development. Results are expressed as percent incorporation calculated as outlined in Materials and Methods in presence or absence of 10 μm cocaine. Results are mean value ± sem of five experiments, each performed using two ovaries (one rat) for each condition. **, P < 0.01; ***, P < 0.001 vs. control of the same age not exposed to cocaine. B, Presence of the mRNAs encoding TH and NET in the celiac ganglion of 5-d-old rats.
Release of recently incorporated 3H-NE from the developing rat ovary
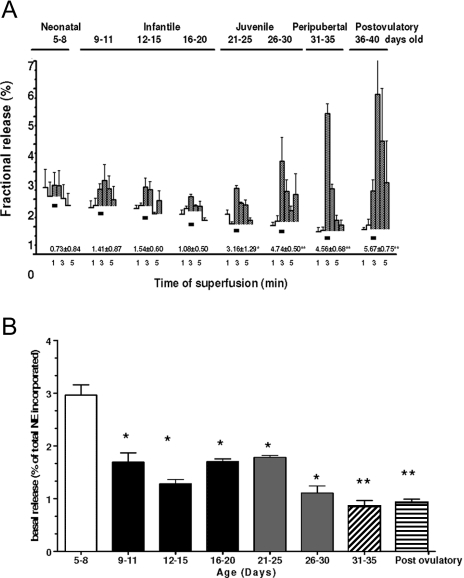
This experiment consisted of exposing ovaries preloaded with 3H-NE to a depolarizing 1-min pulse of K+ (Fig. 3, black rectangles). To quantify the total amount of 3H-NE released by the K+ stimulation, we added the 3H-NE released into each fraction after stimulation (denoted by black rectangles), and the data are presented below each release profile. As shown in Fig. 3, K+ depolarization resulted in little, if any, NE efflux from late neonatal-early infantile (5–8 d old) ovaries. A significant response was first detected in the 20- to 25-d-old group (P < 0.05 vs. all younger animals), with the NE outflow increasing markedly (P < 0.05) thereafter (d 26–40). Although the response of postovulatory 40-d-old ovaries was delayed and more variable, presumably due to the presence of corpora lutea, the total amount of NE released after stimulation was similar to the amount released from juvenile-peripubertal ovaries (see values for each group under the respective release profiles). Thus, the capacity of ovarian noradrenergic nerves to release NE appears to fully develop at the time of puberty.
Figure 3.
A, Developmental changes in the capacity of the rat ovary to release 3H-NE in response to K+-induced depolarization (black rectangles). Results are expressed as percent of 3H-NE retained in the tissue at each interval studied. Numbers below release profiles represent total 3H-NE released by the K+ stimulation. Each bar represents the mean ± sem of five experiments, each consisting of four ovaries (two rats) for 5- to 8-d-old rats and two ovaries (one rat) for each of the others ages. B, Changes in basal 3H-NE release from the ovary at different postnatal developmental stages. Results represent the radioactivity released during the first 2 min of collection (before depolarization) and are expressed as percent of the total 3H-NE present in the tissue at the end of the experiment. The bars with different filling patterns represent the different developmental phases examined: 5–8 (late neonatal-early infantile), 9–20 (infantile), 21–30 (juvenile), 31–35 (peripubertal), and 40 (postovulatory). Each bar represents the mean ± sem of five experiments, each consisting of four ovaries (two rats) for 5- to 8-d-old rats and two ovaries (one rat) for each of the others ages. *, P < 0.05 vs. 5- to 8-d-old group; *, P < 0.01 vs. all groups younger than 20–25 d of age.
Of note, these developmental increases in evoked NE efflux were accompanied by a steady age-related decrease in basal, nonvesicular NE release (Fig. 3B). The highest level of spontaneous, basal NE release was observed in 5- to 8-d-old ovaries (P < 0.05 vs. all other ages).
Developmental changes in endogenous ovarian NE content and concentration
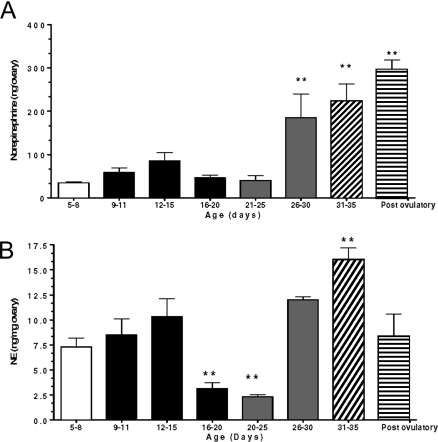
Because these changes in spontaneous NE release may merely reflect an age-related decreased capability of ovarian nerve terminals to store NE, we measured endogenous NE levels at different times of postnatal development. As seen in Fig. 4A, ovarian NE content (nanograms NE per ovary) remained relatively constant throughout neonatal-infantile/early juvenile development [postnatal (PN) d 5–8 to 21–25] but increased markedly by the end of the juvenile period (PN d 26–30), remaining elevated thereafter. Similar changes were detected when the data were expressed as nanograms NE per milligram ovary with two differences: NE concentrations decrease significantly between PN d 12–15 and 21–25 and also after ovulation. The latter change is likely due to the increase in ovary weight that occurs with the formation of corpora lutea. It is therefore clear that the developmental decrease in spontaneous, nonregulated NE release from the maturing ovary (shown in Fig. 3B) is a process tied to an increasing capacity of the ovarian nerves to retain NE and not to a reduction in tissue stores of the catecholamine.
Figure 4.
Age-related changes in NE content and concentration in the rat ovary. A, NE content. B, NE concentration. Results are expressed as nanograms of NE per ovary (content) and as nanograms per milligram ovary (concentration) as determined by HPLC with electrochemical detection. The bars with different filling patterns represent the different developmental phases examined: 5–8 (late neonatal-early infantile), 9–20 (infantile), 21–30 (juvenile), 31–35 (peripubertal), and 40 (postovulatory). Results are expressed as means ± sem of five rats (two ovaries/rat) per age. **, P < 0.01 vs. all groups younger than 25 d of age (A); **, P < 0.01 vs. all other groups (B).
Effect of depleting vesicular NE stores on depolarization-induced H3-NE release
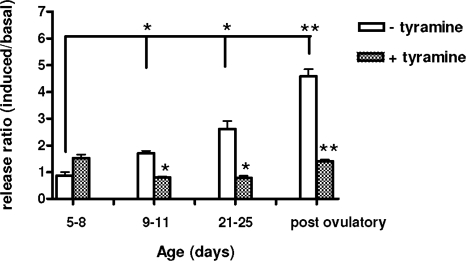
To determine whether the fraction of 3H-NE released in response to K+ derives from NE stored in vesicles or from NE remaining free in the cytosol, we exposed ovaries at different phases of development to tyramine to displace NE stored in vesicles and make the catecholamine unavailable for evoked release. The blocker failed to affect evoked NE release from neonatal-early infantile (5–8 d old) ovaries but significantly reduced the outflow from all other age groups examined, including infantile (9–11 d old) ovaries (Fig. 5). The effect of tyramine was more pronounced in juvenile and postovulatory ovaries than in the infantile gland (P < 0.01).
Figure 5.
The effect of displacing NE stored in vesicles with tyramine on basal and induced 3H-NE release from the rat ovary at different postnatal developmental stages. The ovaries were exposed twice to elevated K+. After the first stimulation, medium samples were collected from three poststimulation fractions. After collection of these fractions, tyramine (10−5 m) was added to the incubation medium and maintained throughout the second K+-induced depolarization. Results are presented as the ratio between induced and basal release, comparing the response to the first stimulation in the absence of tyramine with the second in the presence of the drug. Bars represent means ± sem from five rats (two ovaries/rat) per age. *, P < 0.05; **, P < 0.01 vs. without tyramine.
Dependence of ovarian NE release on extracellular calcium
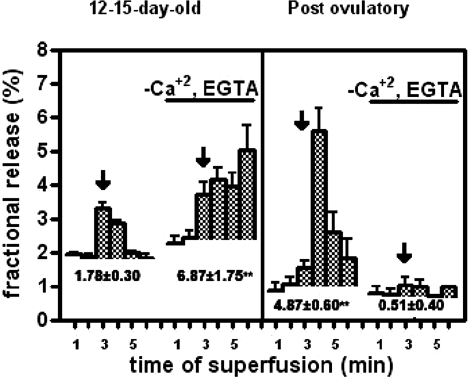
The decrease in spontaneous NE release that occurs during development coinciding with an increase in stimulated NE outflow suggests that maturation of a vesicular mode of NE release is accompanied by loss of a nonvesicular, cytoplasmic mode of catecholamine outflow. To determine the involvement of extracellular calcium in this transition, we compared the effect of removing extracellular calcium on evoked NE outflow at two developmental stages, infantile (12–15 d of age) and postovulatory (40 of age). Surprisingly, the results showed that depolarization evoked NE outflow was not only extracellular calcium independent in the infantile ovaries, but also in fact, the evoked outflow was greater in the absence than in the presence of calcium (Fig. 6). This suggests the presence of a nonvesicular overflow of radioactivity. As expected, evoked NE outflow from postovulatory ovaries was fully dependent on extracellular calcium.
Figure 6.
Evoked 3H-NE release from infantile ovaries is not dependent on extracellular calcium. The experiment was performed in two parallel incubation chambers, one containing infantile rat ovaries (two ovaries/chamber) and the other an adult gland. After an initial depolarization (arrow), three poststimulus fractions were collected, and the ovaries were incubated for 10 min in KRB without calcium and in the presence of 0.1 mm EGTA. A second depolarization protocol was performed under the same conditions. Numbers below each release profile indicate the total H3-NE released by each stimulation. Bars are means ± sem of five experiments, each consisting of two infantile or one adult ovary per chamber.
Discussion
In the present study, we found an age-dependent increase in the capacity of intraovarian nerve terminals to release NE in response to a depolarization stimulus by high extracellular levels of K+. We also show that before the third week of postnatal life, ovarian nerves have a limited capacity to release NE in response to K+. Surprisingly, this response does not require extracellular calcium. K+-induced depolarization is commonly used to study the capacity of nerve cells to release neurotransmitters. K+ does not act on voltage-dependent sodium channels but instead functions as a general depolarizing agent (28). In the case of the Sprague Dawley rat ovary, NE is likely released from noradrenergic fibers innervating the gland and not from intraovarian neurons because these cells are present in the monkey ovary and ovaries from Wistar rats but not in the Sprague Dawley rat ovary (29,30).
Extensive studies with neural tissues have shown that the ability of noradrenergic neurons to respond to depolarization, and hence to release NE, is directly related to the capacity of the cell to store the catecholamine in vesicles, which protects it from degradation by monoamine oxidases (31). Our study shows that this storing capacity, although clearly detectable in infantile 9- to 11-d-old ovaries (as evidenced by the ability of tyramine to block NE release), increases markedly during the fourth week of postnatal life, as the animals approach puberty. It appears likely that this developmental change is directly related to the functional maturation of a developing network of ovarian nerves that accompanies the onset of puberty (18).
Although ovarian nerves have been shown to first emerge in the rat ovary around the time of birth (15) and become a prominent feature of ovarian morphology in prepubertal and adult ovaries (7,32,33), it is not known whether their functional capacity changes during postnatal life. Ben-Jonathan et al. (4) measured the content of NE in developing rat ovaries and found it to be elevated before PN d 10, decreased between this time and PN d 20, to increase again in older prepubertal animals. Our results are broadly consistent with these findings because they show that the ovarian NE concentration (expressed as nanograms NE per milligram of wet weight) increases between PN d 5–8 to the end of the second week of life (d 12–15), decreases from this age to the midjuvenile period (d 21–25), and increases markedly as the animal approaches puberty (d 26–35). In addition, our study shows that, despite clear quantitative differences in comparison with juvenile-peripubertal glands, neonatal rat ovaries are able to incorporate 3H-NE, with values reaching near 30% of the content of endogenous NE.
The capacity of cocaine to inhibit 3H-NE incorporation at all ages indicates that incorporation of the catecholamine into the ovary reflects transfer of the catecholamine into nerve fibers via a catecholamine membrane transporter. Because the celiac ganglion (in which sympathetic neurons projecting to the ovary originate) of neonatal rats contains both NET and TH mRNAs, it appears evident that both biosynthesis of NE and its transport mechanism are already functional in neonatal rats. In contrast, the capacity of ovarian nerve terminals to release NE from vesicular stores in response to K+-induced depolarization appears to fully develop only after the third week of postnatal life. It is possible that the sensitivity of the method we used is not sufficient to detect small changes in NE outflow, despite its demonstrated sensitivity in both the central and peripheral nervous systems (7,34,35). A more parsimonious explanation is that the innervation of neonatal-infantile ovaries has the capacity to synthesize and incorporate NE but has not yet developed the capacity to release the catecholamine on neuronal depolarization. A similar developmental profile has been shown to occur in neurons of the rat central nervous system during the first 2 wk of postnatal development (36). These authors demonstrated that even though immature central nervous system neurons have the machinery to synthesize neurotransmitters and store vesicles at the release sites, the required docking and releasing mechanisms are not yet operative.
An initial event in the response of noradrenergic nerve terminals to action potentials is the opening of voltage-dependent calcium channels. The resulting transient increase in calcium entry then leads to NE release (28). Our results show that this mechanism is absent in infantile (12–15 d old) ovaries and fully developed in postovulatory ovaries, in agreement with previous observations showing that NE release from the adult human ovary requires extracellular calcium (37). The increase in NE outflow seen in infantile ovaries deprived of extracellular calcium is puzzling and difficult to explain. Depolarizing conditions were achieved by increasing the extracellular concentration of K+ from 5 to 80 mm. One explanation is that when osmolarity is maintained (as we did in these experiments) by decreasing the extracellular concentration of sodium from 130 to 50 mm, the Na+ gradient required for transporter-mediated uptake of catecholamines is inverted, allowing nonvesicular NE to diffuse from the nerve terminal to the medium (38). To determine whether the discrete NE response to K+-induced depolarization observed in infantile ovaries represents true vesicular release, we studied the effect of l-tyramine on both the spontaneous and K+-induced NE overflow. In noradrenergic or dopaminergic neurons, tyramine is first incorporated into nerve terminals via the NET or DAT if present and then into vesicles via the nonspecific vesicular monoamine transporter. The transmembrane proteins transporters DAT and NET mediate Na+-dependent reaccumulation of released neurotransmitters into presynaptic terminals (39). Once into the terminal, tyramine acts as a false neurotransmitter, being incorporated into storage vesicles and displacing NE from the vesicles. Displacement of the catecholamine from vesicles then decreases the availability of NE for depolarization-induced release. Our results showed that tyramine did not block NE release from neonatal-early infantile (5–8 d old) ovaries but was progressively more effective in infantile, early juvenile, and postovulatory ovaries. Thus, despite the morphological evidence for the presence of storage vesicles in nerves of neonatal rat ovaries (15), it does not appear that at this age the nerves contain a sufficient number of functional storage vesicles. As described for the central nervous system (40), it also appears that the NE storage capacity of ovarian nerve terminals develops before they acquire the capacity of vesicular NE release.
Importantly, the present study also demonstrates that the innervation of the rat ovary acquires biochemical and functional maturity near the time of puberty. This conclusion is in keeping with findings in rhesus monkeys showing that both the density and composition of the sympathetic ovarian innervation, in addition to the number of intrinsic catecholaminergic neurons present in the ovary of this species, reach a maximum near the time of puberty (16,18). It would not be unreasonable to expect that similar developmental changes in sympathetic input to the ovary occur in humans because the adult human ovary not only contains TH-positive nerve terminals but also responds to depolarization with incorporation and release of NE (37). Because NE has steroidogenic activity (11,12), our results also suggest that an enhanced activity of catecholaminergic ovarian nerves may contribute to the increase in ovarian responsiveness to gonadotropins that occur at puberty (41).
Acknowledgments
The authors declare that there is no conflict of interest that would prejudice the impartiality of this scientific work.
Footnotes
This work was supported by Fondecyt Grant 1050765 (to H.E.L.) and National Institutes of Health Grants HD-24870 and RR-00163 for the operation of the Oregon National Primate Research Center, and National Institute of Child Health and Human Development through cooperative agreement U54 HD18185 as part of the Specialized Cooperative Centers Program in Reproduction and Fertility Research (to S.R.O.).
Disclosure Statement: The authors have nothing to disclose.
First Published Online October 18, 2007
Abbreviations: DAT, Dopamine transporter; KRB, Krebs-Ringer bicarbonate; NE, norepinephrine; NET, NE transporter; PN, postnatal; TH, tyrosine hydroxylase.
References
- Dissen G, Paredes A, Romero C, Dees W, Ojeda S 2004 Neural and neurotrophic control of ovarian development. In: Leung P, Adashi E, eds. The ovary. 2nd ed. San Diego: Academic Press; 3–23 [Google Scholar]
- Gerendai I, Toth IE, Boldogkoi Z, Medveczky I, Halasz B 1998 Neuronal labeling in the rat brain and spinal cord from the ovary using viral transneuronal tracing technique. Neuroendocrinology 68:244–256 [DOI] [PubMed] [Google Scholar]
- Luza SM, Arancibia S, Venegas M, Lara HE 2003 Thyrotropin-releasing hormone as a mediator of the central autonomic pathway controlling ovarian function. Neuroendocrinology 77:273–281 [DOI] [PubMed] [Google Scholar]
- Ben-Jonathan N, Arbogast LA, Rhoades TA, Bahr JM 1984 Norepinephrine in the rat ovary: ontogeny and de novo synthesis. Endocrinology 115:1426–1431 [DOI] [PubMed] [Google Scholar]
- Morimoto K, Okamura H, Tanaka C 1982 Developmental and periovulatory changes of ovarian norepinephrine in the rat. Am J Obstet Gynecol 143:389–392 [DOI] [PubMed] [Google Scholar]
- Lawrence I, Burden H 1980 The origin of the extrinsic adrenergic innervation to the rat ovary. Anat Rec 196:51–59 [DOI] [PubMed] [Google Scholar]
- Lara HE, Dorfman M, Venegas M, Luza SM, Luna SL, Mayerhofer A, Guimaraes MA, Rosa E, Silva AA, Ramírez VD 2002 Changes in sympathetic nerve activity of the mammalian ovary during a normal estrous cycle and in polycystic ovary syndrome: studies on norepinephrine release. Microsc Res Tech 59:495–502 [DOI] [PubMed] [Google Scholar]
- Wolf R, Meier-Fleitmann A, Duker EM, Wuttke W 1986 Intraovarian secretion of catecholamines, oxytocin, β-endorphin, and γ-amino-butyric-acid in freely moving rats: development of a push-pull tubing method. Biol Reprod 35:599–607 [DOI] [PubMed] [Google Scholar]
- Lara HE, Ferruz JL, Luza S, Bustamante DA, Borges Y, Ojeda SR 1993 Activation of ovarian sympathetic nerves in polycystic ovary syndrome. Endocrinology 133:2690–2695 [DOI] [PubMed] [Google Scholar]
- Ferruz J, Barria A, Galleguillos X, Lara HE 1991 Release of norepinephrine from the rat ovary: local modulation of gonadotropins. Biol Reprod 45:592–597 [DOI] [PubMed] [Google Scholar]
- Hernandez ER, Jimenez JL, Payne DW, Adashi EY 1988 Adrenergic regulation of ovarian androgen biosynthesis is mediated via β2-adrenergic theca-interstitial cell recognition sites. Endocrinology 122:1592–1602 [DOI] [PubMed] [Google Scholar]
- Ojeda S, Aguado L 1985 Adrenergic control of the prepubertal ovary: involvement of local innervation and circulating catecholamines. New York: Serono Symposia Publications, Raven Press [Google Scholar]
- Lara HE, McDonald JK, Ojeda SR 1990 Involvement of nerve growth factor in female sexual development. Endocrinology 126:364–375 [DOI] [PubMed] [Google Scholar]
- Lara HE, McDonald JK, Ahmed CE, Ojeda SR 1990 Guanethidine-mediated destruction of ovarian sympathetic nerves disrupts ovarian development and function in rats. Endocrinology 127:2199–2209 [DOI] [PubMed] [Google Scholar]
- Malamed S, Gibney JA, Ojeda SR 1992 Ovarian innervation develops before initiation of folliculogenesis in the rat. Cell Tissue Res 270:87–93 [DOI] [PubMed] [Google Scholar]
- Dees WL, Hiney JK, McArthur NH, Johnson GA, Dissen GA, Ojeda SR 2006 Origin and ontogeny of mammalian ovarian neurons. Endocrinology 147:3789–3796 [DOI] [PubMed] [Google Scholar]
- Mayerhofer A, Dissen GA, Costa ME, Ojeda SR 1997 A role for neurotransmitters in early follicular development: induction of functional follicle-stimulating hormone receptors in newly formed follicles of the rat ovary. Endocrinology 138:3320–3329 [DOI] [PubMed] [Google Scholar]
- Schultea TD, Dees WL, Ojeda SR 1992 Postnatal development of sympathetic and sensory innervation of the rhesus monkey ovary. Biol Reprod 47:760–767 [DOI] [PubMed] [Google Scholar]
- Paredes A, Galvez A, Leyton V, Aravena G, Fiedler JL, Bustamante D, Lara HE 1998 Stress promotes development of ovarian cysts in rats: the possible role of sympathetic nerve activation. Endocrine 8:309–315 [DOI] [PubMed] [Google Scholar]
- Takauchi Y, Yamazaki T, Akiyama T 2000 Tyramine-induced endogenous noradrenaline efflux from in situ cardiac sympathetic nerve ending in cats. Acta Physiol Scand 168:287–293 [DOI] [PubMed] [Google Scholar]
- Heikkila RE, Orlansky H, Cohen G 1975 Studies on the distinction between uptake inhibition and release of (3H)dopamine in rat brain tissue slices. Biochem Pharmacol 24:847–852 [DOI] [PubMed] [Google Scholar]
- Reith ME, Meisler BE, Sershen H, Lajtha A 1986 Structural requirements for cocaine congeners to interact with dopamine and serotonin uptake sites in mouse brain and to induce stereotyped behavior. Biochem Pharmacol 35:1123–1129 [DOI] [PubMed] [Google Scholar]
- Dorfman M, Arancibia S, Fiedler JL, Lara HE 2003 Chronic intermittent cold stress activates ovarian sympathetic nerves and modifies ovarian follicular development in the rat. Biol Reprod 68:2038–2043 [DOI] [PubMed] [Google Scholar]
- Lara HE, Dissen GA, Leyton V, Paredes A, Fuenzalida H, Fiedler JL, Ojeda SR 2000 An increased intraovarian synthesis of nerve growth factor and its low affinity receptor is a principal component of steroid-induced polycystic ovary in the rat. Endocrinology 141:1059–1072 [DOI] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N 1987 Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162:156–159 [DOI] [PubMed] [Google Scholar]
- Comer AM, Qi J, Christie DL, Gibbons HM, Lipski J 1998 Noradrenaline transporter expression in the pons and medulla oblongata of the rat: localisation to noradrenergic and some C1 adrenergic neurones. Brain Res Mol Brain Res 62:65–76 [DOI] [PubMed] [Google Scholar]
- Donoso E, Sapag-Hagar M, Lara H 1992 Neurochemical evidence for the presence of sympathetic nerve terminals in the rat mammary gland: Changes during the lactogenic cycle. Mol Cell Neurosciences 3:23–28 [DOI] [PubMed] [Google Scholar]
- Douglas WW 1975 Secretomotor control of adrenal medullary secretion: synaptic, membrane, and ionic events in stimulus-secretion coupling. In: Greep RO, Atwood EB, eds. Handbook of physiology. Section 7. Washington, DC: American Physiological Society; 367–388 [Google Scholar]
- Dees WL, Hiney JK, Schultea TD, Mayerhofer A, Danilchik M, Dissen GA, Ojeda SR 1995 The primate ovary contains a population of catecholaminergic neuron-like cells expressing nerve growth factor receptors. Endocrinology 136:5760–5768 [DOI] [PubMed] [Google Scholar]
- D’Albora H, Lombide P, Ojeda SR 2000 Intrinsic neurons in the rat ovary: an immunohistochemical study. Cell Tissue Res 300:47–56 [DOI] [PubMed] [Google Scholar]
- de Champlain J, Mueller RA, Axelrod J 1969 Subcellular localization of monoamine oxidase in rat tissues. J Pharmacol Exp Ther 166:339–345 [PubMed] [Google Scholar]
- Burden HW 1985 The adrenergic innervation of mammalian ovaries. In: Ben-Jonathan N, Bahr JMA, Weiner RI, eds. Catecholamines as hormone regulators. New York: Raven Press; 261–278 [Google Scholar]
- Papka RE, Cotton JP, Traurig HH 1985 Comparative distribution of neuropeptide tyrosine-, vasoactive intestinal polypeptide-, substance P-immunoreactive, acetylcholinesterase-positive and noradrenergic nerves in the reproductive tract of the female rat. Cell Tissue Res 242:475–490 [DOI] [PubMed] [Google Scholar]
- Majewski H, Kotsonis P, Murphy TV, Barrington M 1997 Noradrenaline release and the effect of endogenous activation of the phospholipase C/protein kinase C signalling pathway in rat atria. Br J Pharmacol 121:1196–1202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Lonart G, Sanford LD 2007 Transient fear-induced alterations in evoked release of norepinephrine and GABA in amygdala slices. Brain Res 1142:46–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dresbach T, Torres V, Wittenmayer N, Altrock WD, Zamorano P, Zuschratter W, Nawrotzki R, Ziv NE, Garner CC, Gundelfinger ED 2006 Assembly of active zone precursor vesicles: obligatory trafficking of presynaptic cytomatrix proteins Bassoon and Piccolo via a trans-Golgi compartment. J Biol Chem 281:6038–6047 [DOI] [PubMed] [Google Scholar]
- Lara HE, Porcile A, Espinoza J, Romero C, Luza SM, Fuhrer J, Miranda C, Roblero L 2001 Release of norepinephrine from human ovary: coupling to steroidogenic response. Endocrine 15:187–192 [DOI] [PubMed] [Google Scholar]
- Torok TL, Salamon Z, Nguyen TT, Magyar K 1984 Spontaneous [3H]noradrenaline release from the main pulmonary artery of the rabbit induced by sodium-pump inhibition. Q J Exp Physiol 69:841–865 [DOI] [PubMed] [Google Scholar]
- Uhl GR 1992 Neurotransmitter transporters (plus): a promising new gene family. Trends Neurosci 15:265–268 [DOI] [PubMed] [Google Scholar]
- Ziv NE, Garner CC 2004 Cellular and molecular mechanisms of presynaptic assembly. Nat Rev Neurosci 5:385–399 [DOI] [PubMed] [Google Scholar]
- Ojeda S, Skinner M 2006 Puberty in the rat. In: Neill JD, ed. The physiology of reproduction. San Diego: Academic Press/Elsevier; 2061–2126 [Google Scholar]