Abstract
The recent dramatic increase in fructose consumption is tightly correlated with an equally dramatic surge in the incidence of type 2 diabetes and obesity in children, but little is known about dietary fructose metabolism and absorption in neonates. The expression of the rat intestinal fructose transporter GLUT5 [Slc2A5, a member of the glucose transporter family (GLUT)] can be specifically induced by its substrate fructose, but only after weaning begins at 14 d of age. In suckling rats younger than 14 d old, dietary fructose cannot enhance GLUT5 expression. The aim of this study was to identify the mechanisms allowing fructose to stimulate GLUT5 during weaning. After intestines were perfused with fructose or glucose (control), using microarray hybridization we showed that of 5K genes analyzed in 10-d-old pups, only 13 were fructose responsive. Previous work found approximately 50 fructose-responsive genes in 20-d-old pups. To identify fructose-responsive genes whose expression also changed with age, intestines of 10- and 20-d-old littermate pups perfused with fructose were compared by microarray. Intestines of 10- and 20-d-old pups perfused with glucose were used to segregate age- but not fructose-responsive genes. About 28 genes were up- and 22 down-regulated in 20- relative to 10-d-old pups, under conditions of fructose perfusion, and many were found, by cluster analysis, to be regulated by corticosterone. When dexamethasone was injected into suckling pups before fructose perfusion, the expression of GLUT5 but not that of the sodium glucose cotransporter (SGLT) 1 and of GLUT2, as well as the uptake of fructose but not of glucose increased dramatically. Thus, dexamethasone, which allows dietary fructose to precociously stimulate intestinal fructose absorption, can mimic the effect of age and modify developmental timing mechanisms regulating GLUT5.
THROUGHOUT THE WORLD and especially in the United States, the per capita consumption of corn syrup containing mostly fructose has increased by approximately 2100% in the last century (1), and has been tightly correlated with an increased incidence of type 2 diabetes and childhood obesity (2). Infants generally malabsorb fructose (3), but perinatal fructose transport by the small intestine of mammals is a neglected subject area of research because fructose has long been considered to be a minor contributor to metabolic diseases. The signaling mechanisms underlying dietary regulation of the intestinal fructose transporter GLUT5 [Slc2A5, a member of the glucose transporter family (GLUT)] are virtually unknown. In normal adult rats and mice, fructose transport and GLUT5 expression level are increased 2- to 3-fold by dietary fructose or fructose perfusion (4). However, in the neonates of many mammals, the regulation of GLUT5 appears to be more complicated. During the suckling period when neonatal rats, rabbits, and humans do not consume fructose, GLUT5 expression and activity are low and increase only when weaning has been completed (5). In rats, which like humans are omnivores and therefore undergo during weaning a switch to a diet likely containing fructose, GLUT5 becomes fructose responsive at more than or equal to 14 d of age, and can be stimulated by introducing fructose into the intestinal lumen (6); other transporters are not affected by fructose, and other sugars and solutes have no effect on GLUT5 (7,8). Incredibly, the rat intestine is fructose insensitive before approximately 14 d of age, when GLUT5 and fructose uptake are not inducible by luminal fructose (6). Thus, the ontogenetic development of the intestine determines when substrate regulation of GLUT5 can occur. However, the mechanisms underlying the interaction between substrate regulation of GLUT5 and small intestinal maturation have never been studied. Unraveling these mechanisms may yield information that will increase our understanding not only of neonatal fructose metabolism but also of developmental processes that control maturation of intestinal transport.
GLUT5 is localized in the apical membrane of intestinal cells (9), and cytosolic fructose is transported to the blood by the basolateral glucose/fructose transporter GLUT2 (Slc2A2), which has also been hypothesized to participate in the apical transport of sugars (10). The third major sugar transporter is the sodium-glucose cotransporter 1 (SGLT1) (Slc5A1), located in the apical membrane. Recently, another glucose/fructose transporter, GLUT7 (Slc2A7), had been identified (11) in the apical membrane. However, only GLUT5 is specifically and markedly regulated by fructose (12); in fact, its response is directly proportional to luminal concentrations of fructose. In contrast, GLUT5 expression in intestines perfused with glucose is always low and the same as those in intestines perfused with nonmetabolizable glucose or fructose analogs (6). In weaning, 20-d-old rat intestines characterized by fructose sensitivity, we have already identified by microarray analysis 50 other fructose-responsive genes, paralleling the changes in GLUT5 expression (13).
In this study we first tested, using microarray hybridization, the hypothesis that the population of fructose-responsive genes at 20 d of age is different from the population of fructose-responsive genes in the 10-d-old pups when GLUT5 is fructose insensitive. For this hypothesis, intestines of same-age (10 d old) pups were compared after either fructose or glucose perfusion. Having proven this hypothesis, we then tried to identify regulatory genes that modulate fructose sensitivity by tracking changes in expression as a function of age. For this, the intestines of 10- and 20-d-old pups perfused with fructose were compared by microarray analysis (same perfusion solution, different age groups). Intestines of 10- and 20-d-old pups perfused with glucose were used to segregate age- but not fructose-responsive genes. When the microarray results revealed that a significant number of age- and fructose-responsive genes was modulated by glucocorticoids, we then tested the hypothesis that corticosteroids play a major role in regulating intestinal GLUT5 development by injecting pups with a synthetic glucocorticoid, dexamethasone (dex).
Materials and Methods
Experimental design
Experiment 1: microarray studies.
Ten- and 20-d-old rat pups from five litters were divided into four perfusion groups. Five 10-d-old pups were perfused with high glucose solution (10G) and five with high fructose solution (10F) (Fig. 1). At 20 d old, five pups were perfused with the glucose (20G) and five with the fructose solution (20F). Glucose and fructose solutions were continuously perfused for 4 h; we have previously shown that 4-h perfusion is sufficient time to detect fructose-induced changes in GLUT5 mRNA abundance, GLUT5 protein abundance in the apical membrane, and in fructose transport rate (7). The sugar concentration (100 mm in Ringer solution) was also based on previous work indicating that peak luminal sugar concentration in rats fed high carbohydrate (14) exceeded 100 mm.
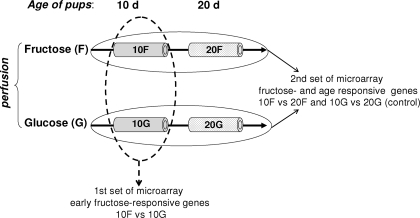
Figure 1.
Experimental design. Five 10-d-old pups were perfused with glucose solution (10G), five with fructose solution (10F), five 20-d-old pups were perfused with glucose (20G) and five with fructose solution (20F). With the first set of microarray, 10G and 10F littermates were compared with identify fructose-responsive genes in the suckling stage. With a second set of microarray, we compared the expression of genes in the intestines of 10- and 20-d-old pups perfused with fructose (10 vs. 20F) and the expression of genes in the intestines of 10- and 20-d-old pups perfused with glucose (10 vs. 20G). To segregate specific fructose- and age-responsive genes, the 10 and 20G groups were used as control to identify genes that change with age in intestines not perfused with fructose.
The intestinal transcriptome of the perfused pups was analyzed by microarrays in two different sets of experiments (Fig. 1). In the first set, the intestinal gene expression levels from 10-d-old rats perfused with glucose were compared with those perfused with fructose (10G vs. 10F, respectively) by microarray to determine fructose-responsive genes in the suckling stage. These genes are fructose responsive but age independent. With a second set of microarrays, we identified fructose-responsive genes whose expression also changes with age. For this, we compared the expression of the genes in the intestines of 10 and 20-d-old pups perfused with fructose (10 vs. 20F, respectively), to yield a list of genes that change with age under fructose perfusion. We also compared the expression of genes in the intestines of 10- and 20-d-old pups perfused with glucose. This comparison between 10- and 20-d-old pups perfused with glucose was used as control to identify genes that are not fructose responsive but whose expression changes with age.
Experiment 2: dex study.
To determine the role of glucocorticoids in GLUT5 development, we examined the effect of dex on fructose induction of GLUT5 in the 10-d-old pups. Pups received daily injections for 2-d dex (ip 50 ng/kg body weight) or vehicle (the same volume of 5% dimethyl sulfoxide, the solvent for dex, in saline solution) beginning at 8 d of age. dex and vehicle-treated animals were perfused at 10 d of age with glucose or fructose solution as described previously. The dose and time course of dex injection were chosen because preliminary work showed that a daily injection of dex for 2 d, from 40–400 ng/kg body weight (the dose for humans), is effective in inducing GLUT5 expression in the small intestine of 10-d-old fructose-perfused pups.
Animals
All the procedures conducted in this study were approved by the Institutional Animal Care and Use Committee, University of Medicine and Dentistry of New Jersey, New Jersey Medical School. Pregnant female Sprague Dawley rats purchased from Taconic (Germantown, NY) were housed in the research animal facility under a 12-h light, 12-h dark photoperiod in a temperature-controlled room (22–24 C). Dams were fed ad libitum a commercial diet (Purina Mills, Richmond, IN). After birth, rat pups were kept with their dams; age at birth was considered d 0. At 10 or 20 d old, rat pups were removed randomly from their dams and used for perfusion. The pups used for the dex study were injected once daily for 2 d beginning at 8 d of age with dex or vehicle and then removed from the dam at 10 d of age for perfusion experiments.
Intestinal perfusion
The rat intestinal perfusion procedure was conducted following the method previously described (7). Rat pups (10 and 20 d old, not starved) were initially anesthetized (0.2–0.4 ml/100 g body weight, ip, of ketamine 20 mg/ml and xylazine 2.5 mg/ml) and kept under continuous anesthesia for 4-h perfusion. Then after opening the abdominal cavity, the intestine was exposed, and a small incision was made 5-cm distal to the ligament of Treitz and 10-cm proximal from the ileocecal valve, and a catheter was inserted into the lumen. After the contents were flushed, the small intestine was continuously perfused with sugar solution (100 mm fructose or glucose in Ringer) at a rate of 30 ml/h at 37 C using a peristaltic pump. Composition of the perfusion solution was as follows (in mm): 78 NaCl, 4.7 KCl, 2.5 CaCl2 · 5H2O, 1.2 MgSO4, 19 NaHCO3, 2.2 KH2PO4, and 100 glucose or fructose (pH 7.4) (300 mOsm). After perfusion, sugar uptakes were measured in vitro, and tissues for mRNA analysis were frozen.
Glucose and fructose uptakes
Briefly, four 1-cm segments were everted and mounted on grooved steel rods and preincubated at 37 C for 5 min in Krebs ringer bicarbonate buffer [in mm: NaCl 128, KCl 4.7, NaHCO3 19, CaCl2 2.5, MgSO4 1.2, KH2PO4 1.2 (pH 7.4)] bubbled with 95% O2-5% CO2 (15). Two sleeves were then incubated under agitation (1200 rpm) at 37 C in an oxygenated solution containing either d-[14C]glucose for 1 min or d-[14C]fructose for 2 min. l-[3H]glucose was used to correct for adherent fluid and passive diffusion of glucose or fructose. The tissues were quickly rinsed (20 sec) and processed for radioactivity as previously described. The uptake rates of both d-glucose and d-fructose were determined at 50 mm and expressed as nanomoles per milligram wet weight of small intestine.
mRNA extraction, DNase treatment, and RT reaction
Total RNA was extracted from 100 mg scraped mucosa using 1 ml TRIzol reagent (Invitrogen, Carlsbad, CA). The total RNA concentration was determined by spectrophotometry (Beckman DUR640; Beckman Coulter, Inc., Fullerton, CA) and the quality analyzed by 1% agarose gel electrophoresis with ethidium bromide staining. To hydrolyze contaminating DNA in the RNA preparations, 20 μg RNA was combined with 1 μl RQ1 Rnase free DNase I (Promega, Madison, WI) and 10 μl 10×DNase buffer in a final volume of 100 μl. After Dnase treatment, RNA concentration and quality were analyzed as described previously. The cDNA was generated from 2.5 μg DNase-treated RNA using SuperScript III RNase H − Reverse Transcriptase and oligo (dT)20 (Invitrogen) in a total volume of 20 μl.
Microarray analysis
The design, analysis, and interpretation of this microarray experiment followed the same protocol used previously by us (13). We used a rat 5000-oligonucleotide array built from 4854 oligonucleotides representing 4803 independent genes, and all oligonucleotides were 60–70 nucleotides in length [Rat 5K Oligo Array (NGEL 2.0.1); Center for Applied Genomics, Public Health Research Institute, Newark, NJ]. A detailed description of array design elements is available from the following web site: http://www.cag.icph.org. The cDNA was synthesized with the 3DNA Submicro Oligo Expression Array Detection Kit (Genisphere, Hatfield, PA) and labeled with fluorescent dyes Cy3 or Cy5 following the manufacturer’s instructions (13). In the microarray experiments, 10 slides were used to compare the 10-d-old rat intestines perfused with fructose (10F) (n = 5) against those perfused with glucose (10G) (n = 5), with each pair being littermates. cDNA from 10F was then labeled with Cy5, and cDNA from 10G with Cy3. To eliminate dye bias, 10F cDNA from pups in the next experiment was labeled with Cy3, whereas 10G cDNA was labeled with Cy5. Data from this dye flip were used to correct results as described later in Data processing. Moreover, two pools of the same amount of total RNA from each of five 10F and 10G pups were made. The 10F and 10G pools were self-against-self-hybridized on two additional slides to identify potentially false-positive genes.
In the second set, 10 microarray slides were used to compare the 10-d-old rat intestines perfused with fructose for 4 h (10F) (n = 5) against 20-d-old rat intestines also perfused with fructose (20F) (n = 5). cDNA from 10F intestines was labeled alternatively with Cy5 or Cy3, whereas the paired 20F cDNAs were labeled correspondingly with Cy3 or Cy5. This random Cy3 and Cy5 labeling switch between 10 and 20F, as well as pooled self-against self-hybridizations were used to eliminate dye bias. The same procedure was used for 10- and 20-d-old rats whose intestines were perfused with glucose (10 vs. 20G comparisons).
Data processing
The data processing, normalization, and statistical analyses were done as described by previous work (13). The results (median pixel intensity of each array spot) were normalized by the locally weighed scatter smoother method (16). To eliminate genes that were considered false positives and to choose genes that might change significantly with fructose perfusion or/and age, we followed the following criteria. First, genes whose expression was so low that their average median intensity was less than 2-fold that of background intensity were eliminated. Moreover, genes whose expression changed by more than 50% in self-self hybridization experiments were also eliminated. Then, sugar-induced changes in gene expression should not be altered when a different dye was used to label the same sample, so any gene whose expression changed from a positive value to a negative value when the dye was switched was eliminated. Finally, only genes that changed by more than or equal to 50% in at least four of five comparisons in the experiment involving 10 and 20-d-old pups, and in three of five comparisons in the experiment comparing 10-d-old pups perfused in fructose and glucose, were considered. We then performed a one-sample t test to determine significance of changes in gene expression in the microarray results, following earlier work (13). Gene expression was further analyzed using Pathway Assist (Stratagene, La Jolla, CA) and Bibliosphere (Genomatix Software Inc., Ann Arbor, MI), software applications that can build and examine biological association networks, including traditional pathways, among genes selected as nodes (see Fig. 3) to reveal gene regulatory networks.
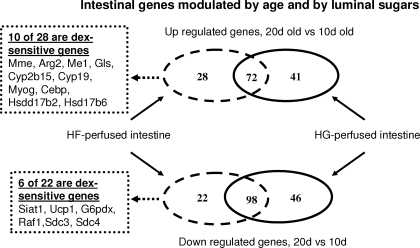
Figure 3.
Effect of development (20 and 10 d old) on small intestines perfused with glucose (HG) or fructose (HF) solutions. Only the genes that were differentially regulated (P < 0.05) with age are shown. The intersections in the middle represent the number of genes showing similar regulation under both perfusion conditions. Pathway analysis indicates a significant number of fructose- and age-responsive genes that are affected by dex.
Real-time PCR
There were 21 primers designed using primer3 software (http://frodo.wi.mit.edu/cgi-bin/primer3/primer3_www.cgi) (Table 1), and were purchased from Integrated DNA Technologies (IDT, Coralville, IA). Quantitative PCR analyses (MX 3000P; Stratagene) for candidate responsive genes were performed using the iTaq SYBR Green Supermix with Rox (Bio-Rad Laboratories, Hercules, CA) as described by Kirchner et al. (17). For each primer set (Table 1), the efficiency of the PCR reaction (linear equation: y = slope + intercept) was measured in triplicate on serial dilutions of the same cDNA sample (pool of reverse-transcribed RNA samples). Real-time PCR efficiencies (E) for each reaction were then calculated using the following equation: E = [10(1/slope)] − 1. Melting-curve analysis also was performed for each gene to check the specificity and identity of the RT-PCR products. The relative expression ratio of a target gene was calculated as follows:
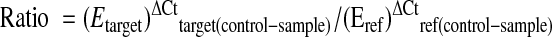 |
This calculation is based on real-time PCR efficiencies (E) of target genes and the cycle threshold (Ct) difference (Δ) of an unknown sample vs. a control (ΔCtcontrol − sample). In our experiment the control is the 10-d-old pups perfused with glucose solution. Because the expression level of elongation factor 1α (EF1α) is not affected by F and G perfusion, or by age of pups (data not shown), the target gene expression has been normalized to EF1α.
Table 1.
Primer sequences of genes whose expression level had been measured by real-time PCR
| Gene name | Symbol | Accession no. | Direction | Primer sequence (5′→3′) | Probe size (bp) | T |
|---|---|---|---|---|---|---|
| CCAAT/enhancer binding protein (C/EBP), δ | Cebpd | NM_013154 | Forward | AGCCCACACCACCCACTTC | 257 | 60 |
| Reverse | CTGCTCCACACGCTGATGC | |||||
| Insulin-like growth factor-II | Igf2 | X14834 | Forward | CATCGTGGAAGAGTGCTGCT | 133 | 58 |
| Reverse | GGGGTATCTGGGGAAGTCGT | |||||
| Murine leukemia viral (v-raf-1) oncogene homolog 1 | Raf1 | NM_012639 | Forward | CCCTACTCCCACATCAACAACC | 123 | 57 |
| Reverse | GTCAGCCACCAACCTCTTCA | |||||
| Myogenin | Myog | NM017115 | Forward | CTGGGCGTGTAAGGTGTGT | 600 | 57 |
| Reverse | CTGGGCTGGGTGTTAGTCTT | |||||
| Cyclic nucleotide-gated channel | Cnga3 | AB002801 | Forward | GGGAAAGGCAGGAAGAAGGA | 325 | 57 |
| Reverse | TGGGGATGAGAGACAGGATG | |||||
| Nucleobinding | Nucb | Z36277 | Forward | TTGGTTCCGTGCGTGCTC | 216 | 59 |
| Reverse | GCTCCTTATCTCCTCTATGTCTGCTTT | |||||
| Nuclear receptor subfamily 1, group H, member 4 | Nr1h4 | U18374 | Forward | GCAGAGAGATGGGAATGTTGG | 153 | 56 |
| Reverse | GCTTGGTCGTGGAGGTCA | |||||
| Karyopherin | Kpna2 | NM_053483 | Forward | TGTGGTGGATGGAGGTGCTA | 257 | 57 |
| Reverse | CGGGATTCTTGTTCCGACAG | |||||
| Bleomycin hydrolase | Blmh | D87336 | Forward | AGAAATGCTTCCCTGAATCG | 128 | 54 |
| Reverse | CTCTCCTTTGGTTGCTCCAC | |||||
| Arginase 2 | Arg2 | NM_019168 | Forward | CGTCTCCCGTCTCCTCCAC | 291 | 59 |
| Reverse | ACCACCTCAGCCAGTTCCTG | |||||
| Sialyltransferase 1 | Siat1 | M18769 | Forward | TGGGAACTGTGGGACATCATT | 219 | 56 |
| Reverse | GAAGAGGAGCGGGTCGTAGG | |||||
| Glucose-6-phosphatase catalytic | G6Pc | NM_013098 | Forward | GGCTCACTTTCCCCATCAGG | 146 | 59 |
| Reverse | ATCCAAGTGCGAAACCAAACAG | |||||
| Glutaminase | Gls | NM_012569 | Forward | AACGAGAAAGTGGAGACCGAAA | 233 | 56 |
| Reverse | GCAGAAACCGCCATTAGCC | |||||
| 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 4 | Pfkfb4 | NM_019333 | Forward | TGGATGAAGAACAGGACAGG | 154 | 55 |
| Reverse | CGGCAGAGGTAGATGGAG | |||||
| Phospholipase A2, group IIA | Pla2g2a | NM_031598 | Forward | ACGGTTGCCATTGTGGTGTG | 353 | 59 |
| Reverse | GGAGAGGTGTTAGAGGATGTCTGGA | |||||
| Sucrase isomaltase | SI | L25926 | Forward | GCCTTACCCTGCCTTTGA | 216 | 55 |
| Reverse | ATTTCTCTTGCCCACCACTC | |||||
| Facilitated glucose transporter | GLUT2 | NM_012879 | Forward | GTCCAGAAAGCCCCAGATACC | 279 | 57 |
| Reverse | TGCCCCTTAGTCTTTTCAAGCT | |||||
| Facilitated glucose transporter | GLUT5 | NM_031741 | Forward | TGCAGAGCAACGATGGAGAAA | 220 | 59 |
| Reverse | ACAGCAGCGTCAGGGTGAAG | |||||
| Sodium-glucose cotransporter | SGLT1 | NM_013033 | Forward | GACTGGGTTTGCTTTCCGAGA | 451 | 59 |
| Reverse | TGTTGGGTAGGCGATGTTGG | |||||
| α-Fetoprotein | Afp | NM_012493 | Forward | AAATGAGTAGCGATGCGTTGG | 219 | 57 |
| Reverse | GAAAGTGGAAGGGTGGGACA | |||||
| Membrane metallo endopeptidase | Mmed | NM_012608 | Forward | CACTTGGATGGATGCTGA | 192 | 52 |
| Reverse | TGCTTGCTTTGGCTGAAT | |||||
| Hydroxysteroid 11-β dehydrogenase 2 | Hsd11b2 | NM_017081 | Forward | TCAAGGTCAGCATCATCCAG | 196 | 56 |
| Reverse | GGGCTAAGGTCAGGCAATG | |||||
| Elongation factor 1α | EF1α | NM_1758378 | Forward | CTCCACTTGGTCGTTTTGCTGT | 165 | 59 |
| Reverse | AGACTGGGGTGGCAGGTGTT | |||||
| 17-β Hydroxysteroid dehydrogenase type 2 | Hsd17b2 | NM_024391 | Forward | TCGGTGTCCTGCTTCTTCCT | 258 | 57 |
| Reverse | TTTATCTGCTCTGGCTTGGTGA | |||||
| 17-β Hydroxysteroid dehydrogenase type 6 | Hsd17b6 | U89280 | Forward | GGAAAGAACAAACAGGGCAGAA | 317 | 56 |
| Reverse | CACGATACCAACGCAGAAGG | |||||
| Steroid sulfatase | Sts | NM_012661 | Forward | CTTCACCATCACCCAACAGC | 203 | 57 |
| Reverse | TCTCCTCCACAGCATCTCCA | |||||
| Steroid 5-α-reductase 2 | Srd5a2 | NM_022711 | Forward | TACGGGAAACACACAGAGAG | 385 | 54 |
| Reverse | TGAAAAAGGCGAAAGCGAAG |
The symbol of each gene is based on the nomenclature by the Human Gene Organisation Gene Nomenclature Committee.
Statistical analyses
Data are presented as means ± sem. A one-way ANOVA was first used to determine the difference of relative mRNA abundance and sugar uptakes among groups with different treatments. If there was a significant difference, Fisher’s paired least significant difference test was used to determine the particular effect that caused that difference (StatView, Abacus Concepts; SAS Institute Inc., Cary, NC). Differences were considered significant at P < 0.05.
Results
Effect of age on sugar transporter expression and activity
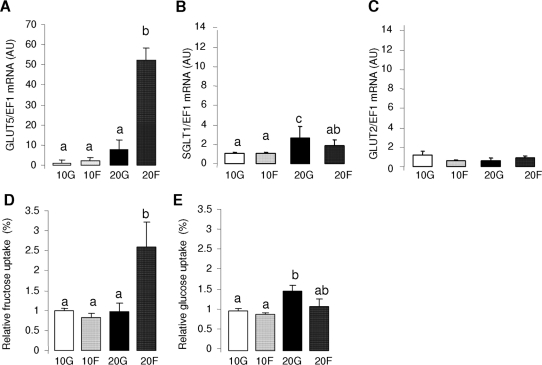
Fructose perfusion increased GLUT5 expression in the intestine of 20-d-old pups by 8-fold when compared with that in the glucose-perfused intestine of same-age pups (Fig. 2A), and by 20- to 50-fold when compared with those in the intestines of 10-d-old pups. Fructose perfusion had no effect on the GLUT5 mRNA abundance in the intestine of the 10-d-olds pups. Compared with those in GLUT5, age-related increases in SGLT1 expression were very modest (approximately two times, Fig. 2B), and were significant only for glucose-perfused intestine. Larger glucose-induced increases in SGLT1 expression are usually observed in adult intestines (18). Intestinal GLUT2 mRNA abundance was independent of age and sugar perfusion (Fig. 2C).
Figure 2.
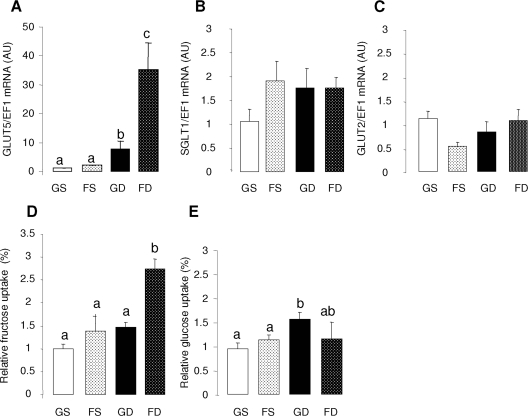
The effects of glucose and fructose perfusion on sugar transporters mRNA levels and sugar uptakes in the small intestine of the 10-d- (10G or 10F) and 20-d- (20G or 20F) old pups. The mRNA abundance was measured by real-time PCR using EF1α as a reference gene. Bars are means ± sem (n = 5). mRNA abundance and activities in 10-d-old glucose-perfused pups were designated as 100%, to normalize other groups to this value. Letters indicate significant differences (P < 0.05). Relative mRNA abundance of GLUT5 (A), SGLT1 (B), and GLUT2 (C). Relative uptake rates of fructose (D) and glucose (E). Please note that the ordinate of A differs markedly from B and C. It is clear that only older pups allow fructose to simulate GLUT5 expression and activity (compare with Fig. 2).
The effects of age and perfusion solution on fructose uptake rate paralleled their effects on GLUT5 expression level (Fig. 2D). Fructose perfusion in 20-d-old pups increased fructose uptake by 2.5 times compared with intestines of same-age pups whose intestines were perfused with glucose. In contrast, there was no effect of fructose perfusion on fructose uptake in the intestine of 10-d-old pups, whose fructose uptakes were similar to that in 20-d-old pups perfused with glucose. Glucose uptake rate increased modestly with glucose perfusion in 20-d-old pups (Fig. 2E).
Microarray results
Fructose-responsive genes in 10-d-old pups.
Using glucose-perfused intestines as control, we identified seven genes whose expression changes in the intestine of 10-d-old pups perfused with fructose for 4 h (Table 2). We found five genes that were significantly up-regulated between 1.5 and four times, and two down-regulated approximately 1.6 times with fructose perfusion. The up-regulation of glucose-6-phosphatase (G6Pc) has been confirmed by real-time PCR (2.3-fold; P < 0.05). As has previously been shown in Northern blots (6) and in Fig. 2A, there was no fructose-induced increase in intestinal GLUT5 mRNA abundance as measured by microarray at 10 d of age.
Table 2.
Intestinal genes of 10-d-old pups whose expression changed with fructose perfusion
| Access no. | Symbol | Name | Mean fold change | se | Frequency |
|---|---|---|---|---|---|
| Up-regulated genesa | |||||
| Metabolic activity | |||||
| L20869 | Pap3 | Pancreatitis-associated protein 3 | 3.79 | 1.42 | 5 |
| S43715 | Reg | Regeneration protein | 2.87 | 1.18 | 5 |
| NM_013098 | G6Pc | Glucose-6-phosphatase, catalytic | 2.14 | 1.17 | 4 |
| Development | |||||
| NM_171992 | Ccnd1 | Cyclin D1 | 1.54 | 1.14 | 4 |
| Unknown | |||||
| D78359 | Srpx | Sushi-repeat-containing protein | 1.54 | 1.20 | 3 |
| Down-regulated genesa | |||||
| Metabolic activity | |||||
| RNU42627 | rVH6 | Dual-specificity protein tyrosine phosphatase | −1.62 | 1.14 | 3 |
| Development | |||||
| K01231 | α -Fetoprotein 3′ end | −1.69 | 1.29 | 3 | |
| NM_012493 | Afp | α -Fetoprotein | −1.58 | 1.38 | 4 |
Values are mean ± se for each gene (n = 5). Negative values indicate the fold decrease in the expression of a gene in fructose compared with glucose-perfused intestine.
Genes listed were up-regulated and down-regulated by more than 1.5-fold in at least three of five samples and whose the fold change is significantly different from a fold change of 1.0 (no change) (P < 0.05).
Microarray analysis of age-related genes responding to both glucose and fructose.
In the second experiment, we compared the expression of intestinal genes from 20 d with those from 10-d-old pups perfused with either fructose or glucose. A total of 306 genes, representing 3.6% of all genes analyzed, was identified as differentially expressed with age. Among these genes, 140 (28 + 71 + 41) were up- and 166 down-regulated (Fig. 3). The complete list of genes was divided into three groups: those whose expression changed with age only under fructose perfusion (Tables 3 and 4); those whose expression changed only with age under glucose perfusion (supplemental Tables 1A and 2A, published as supplemental data on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org); and those whose expression changed with age under both fructose and glucose perfusion (Tables 5 and 6, and supplemental Tables 3A and 4A). DAVID and EASE bioinformatics resources were used to find the functional annotations [gene ontology (GO)], and the biological process of the differentially expressed genes (http://david.niaid.nih.gov/david/ease.htm).
Table 3.
Microarray results of intestinal genes up-regulated, under fructose perfusion only, in 20 d relative to those in 10-d-old pups
| Access no. | Symbol | Name | Mean fold change 20 vs. 10F | se | Frequency |
|---|---|---|---|---|---|
| Metabolic activity | |||||
| U33500 | Rdh2 | Retinol dehydrogenase type 2 | 5.10 | 2.06 | 5 |
| NM_012608 | Mmed | Membrane metallo endopeptidase | 3.12 | 1.57 | 5 |
| NM_019168 | Arg2 | Arginase 2 | 3.05 | 1.32 | 5 |
| NM_012600 | Me1 | Malic enzyme 1 | 2.07 | 0.28 | 5 |
| NM_012569 | Gls | Glutaminase | 1.98 | 0.24 | 5 |
| NM_017156 | Cyp2b15 | Cytochrome P450, 2b15 | 1.81 | 0.57 | 4 |
| NM_017085 | Cyp19 | Cytochrome P450, subfamily 19 | 1.80 | 0.51 | 4 |
| AF121345 | Phyh | Phytanoyl-CoA hydroxylase (Refsum disease) | 1.78 | 0.20 | 4 |
| U03629 | Gda | Guanine deaminase | 1.66 | 0.17 | 5 |
| Transcription regulation/transcription factor | |||||
| NM_017115 | Myog | Myogenin | 2.31 | 0.16 | 4 |
| AB026288 | Cebpd | CCAAT/enhancer binding, protein (C/EBP) δ | 1.61 | 0.10 | 5 |
| Signal transduction activity | |||||
| M92920 | Phkb | Phosphorylase kinase, β | 5.61 | 2.26 | 5 |
| AJ130946 | Kpna2 | Karyopherin (importin) α-2 | 2.33 | 0.80 | 5 |
| U32434 | Rgs3 | Regulator of G protein signaling 3 | 1.84 | 0.52 | 5 |
| Receptor activity | |||||
| AF177685 | Pcdha11 | Protocadherin α 11 | 2.31 | 0.88 | 4 |
| NM_019295 | Cd5 | CD5 antigen | 2.18 | 0.71 | 4 |
| AF016184 | LOC286985 | Putative pheromone receptor (Go-VN7) | 1.61 | 0.35 | 4 |
| AF016178 | LOC286915 | Putative pheromone receptor (Go-VN1) | 1.60 | 0.23 | 4 |
| Hormone metabolism | |||||
| X91234 | Hsd17b2 | 17-β Hydroxysteroid dehydrogenase type 2 | 2.03 | 0.63 | 5 |
| U89280 | Hsd17b6 | 17-β Hydroxysteroid dehydrogenase type 6 | 1.77 | 0.71 | 4 |
| NM_017092 | Tyro3 | TYRO3 protein tyrosine kinase 3 | 1.67 | 0.51 | 5 |
| Unclassified | |||||
| M83209 | Psp | Parotid secretory protein | 4.47 | 2.98 | 5 |
| X60661 | Potential ligand-binding protein, RYD5 | 2.81 | 1.03 | 4 | |
| S82649 | Narp | Neuronal activity regulated pentraxin | 2.65 | 0.74 | 4 |
| AF078778 | Microtubule-associated protein 1B, noncoding exon 3U | 2.56 | 0.47 | 5 | |
| AF168362 | Pass1 | Protein associating with small stress protein PASS1 | 1.85 | 0.73 | 5 |
| AJ005161 | Mitochondrial translational elongation factor | 1.82 | 0.41 | 4 | |
| M93401 | Mmsdh | Methylmalonate semialdehyde dehydrogenase gene | 1.64 | 0.14 | 4 |
Up-regulated genes must have changed by more than 1.5-fold in at least four of five samples, and the fold change must be significant (P < 0.05). Values are means ± se for each gene (n = 5). Values indicate the fold increase in the expression of a gene in 20-d-old fructose-perfused compared with 10-d-old fructose-perfused intestine.
Table 4.
Microarray results of intestinal genes down-regulated, under fructose perfusion only, in 20 d relative to those in 10-d-old pups
| Access no. | Symbol | Name and function | Mean fold change 20 vs. 10F | se | Frequency |
|---|---|---|---|---|---|
| Metabolism activity | |||||
| M18769 | Siat1 | Sialyltransferase 1 | −4.00 | 1.92 | 5 |
| U13617 | Z49858 | Plasmolipin | −3.13 | 0.59 | 5 |
| NM_012682 | Ucp1 | Uncoupling protein 1 | −1.82 | 0.79 | 4 |
| NM_19333 | Pfkfb4 | 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 4 | −1.64 | 0.40 | 4 |
| NM_017006 | G6pdx | Glucose-6-phosphate dehydrogenase | −1.56 | 0.24 | 4 |
| Transcription regulation activity/transcription factor | |||||
| NM_012639 | Raf1 | Murine leukemia viral (v-raf-1) oncogene homolog 1 (3611-MSV) | −1.92 | 0.18 | 5 |
| U56241 | Mafb | v-maf musculoaponeurotic fibrosarcoma oncogene family, protein B (avian) | −1.61 | 0.60 | 4 |
| AF286534 | Rab11b | RAB11B, member RAS oncogene family | −1.61 | 0.23 | 4 |
| Signal transduction activity | |||||
| D87336 | Blmh | Bleomycin hydrolase | −5.00 | 4.75 | 5 |
| U73184 | Sdc3 | Syndecan 3 | −2.44 | 0.59 | 5 |
| NM_012649 | Sdc4 | Syndecan 4 | −1.69 | 0.26 | 4 |
| Development/proliferation/differentiation | |||||
| U69279 | Efna5 | eph-related receptor tyrosine kinase ligand 7 | −3.85 | 1.63 | 5 |
| U61261 | Lama3 | Laminin 5 α 3 | −1.96 | 0.77 | 4 |
| X81448 | Krt1–18 | Keratin complex 1, acidic, gene 18 | −1.85 | 0.51 | 4 |
| AF017637 | Cpz | Carboxypeptidase Z | −1.75 | 0.71 | 4 |
| X77934 | Aplp2 | Amyloid β (A4) precursor-like protein 2 | −1.72 | 0.15 | 5 |
| Transport | |||||
| NM_019229 | Slc12a4 | Solute carrier family 12, member 4 | −1.96 | 0.81 | 4 |
| Immune activity | |||||
| AJ271303 | Birc1 | Baculoviral IAP repeat-containing 1 | −1.85 | 0.65 | 5 |
| AJ006070 | Rag1 | Recombination activation protein 1 | −1.59 | 0.18 | 4 |
| Unclassified | |||||
| L01122 | Ftl1 | Ferritin light chain 1 | −1.75 | 0.09 | 5 |
| NM_017188 | Uncl19 | UNC-119 homolog (C. elegans) | −1.75 | 0.25 | 5 |
| S74323 | Clone E5103, estrogen-induced gene | −1.75 | 0.28 | 4 |
Down-regulated genes must have changed by more than 1.5-fold in at least four of five samples, and the fold change must be significantly different (P < 0.05). Values are mean ± se for each gene (n = 5). Negative values indicate the fold decrease in the expression of a gene in 20-d-old compared with 10-d-old fructose-perfused intestine.
Table 5.
Microarray results of intestinal genes differentially regulated under glucose or fructose perfusion between 20- and 10-d-old pups
| Access no. | Symbol | Name and function | 20F vs. 10F
|
20G vs. 10G
|
||||
|---|---|---|---|---|---|---|---|---|
| Mean fold change | se | Freq | Mean fold change | se | Freq | |||
| Up-regulated genes | ||||||||
| Metabolism | ||||||||
| NM_013061 | Si | Sucrase isomaltase | 19.89 | 5.98 | 5 | 29.40 | 10.85 | 5 |
| NM_017013 | Gsta2 | Glutathione-S-transferase, α type2 | 7.45 | 4.67 | 5 | 10.12 | 6.86 | 5 |
| M25148 | Pla2g2a | Phospholipase A2, group IIA | 6.38 | 6.14 | 5 | 5.08 | 2.26 | 5 |
| D10354 | Gpt | Glutamic-pyruvate transaminase (alanine amino-transferase) | 4.99 | 1.72 | 5 | 5.33 | 1.37 | 5 |
| L81170 | Cyp2j4 | CYP2J4 | 4.52 | 2.97 | 5 | 7.64 | 4.43 | 5 |
| AF111160 | Gsta1 | Glutathione S-transferase, α-1 | 3.92 | 1.50 | 4 | 6.22 | 3.54 | 5 |
| Nucleotide/DNA metabolism | ||||||||
| NM_013097 | Dnase1 | Deoxyribonuclease I | 11.04 | 2.44 | 5 | 10.30 | 3.84 | 5 |
| U90888 | Ampd3 | Adenosine monophosphate deaminase 3 | 4.33 | 1.97 | 5 | 6.15 | 3.43 | 5 |
| Signal transduction activity | ||||||||
| AB002801 | Cnga3 | Cyclic nucleotide gated channel α 3 | 7.10 | 3.24 | 5 | 2.90 | 1.31 | 5 |
| U44750 | Hpgd | NAD-dependent 15-hydroxyprostaglandin dehydro-genase | 5.95 | 3.51 | 5 | 5.83 | 1.83 | 5 |
| Development/proliferation/differentiation | ||||||||
| AF205717 | LRTM4 | Tetraspan protein | 3.90 | 1.59 | 5 | 7.57 | 2.88 | 5 |
| NM_017037 | Pmp22 | Peripheral myelin protein 22 | 2.17 | 0.89 | 5 | 6.27 | 2.85 | 5 |
| Transport | ||||||||
| D13871 | Slc2a5 | Fructose transporter | 7.12 | 6.20 | 4 | 2.70 | 0.93 | 4 |
| AF058714 | SDCT1 | Sodium-dicarboxylate cotransporter | 2.38 | 1.29 | 4 | 5.36 | 2.93 | 5 |
| Immunity/inflammation | ||||||||
| U16683 | Defensin RatNP-3 precursor | 3.91 | 4.22 | 5 | 10.50 | 6.36 | 5 | |
| Unknown | ||||||||
| AF296690 | Rattus norvegicus unknown mRNA | 5.61 | 2.13 | 5 | 4.91 | 2.42 | 5 | |
| Z30584 | Zg-16p | ZG-16p protein | 5.35 | 3.37 | 5 | 5.27 | 1.12 | 5 |
| Down-regulated genes | ||||||||
| Metabolism | ||||||||
| D00680 | Gpx3 | Glutathione peroxidase 3 | −14.29 | 2.36 | 5 | −16.67 | 4.59 | 5 |
| AF154349 | Prsc1 | Protease, cysteine, 1 (legumain) | −10.00 | 3.21 | 5 | −10.00 | 3.78 | 5 |
| J02773 | Fabp3 | Fatty acid binding protein 3 | −5.88 | 2.25 | 5 | −6.25 | 3.25 | 4 |
| AB048711 | Dpp7 | Dipeptidylpeptidase 7 | −5.00 | 2.03 | 5 | −7.69 | 3.01 | 5 |
| NM_012562 | Fuca | Fucosidase, α-l-1, tissue | −4.76 | 1.89 | 5 | −16.67 | 5.30 | 5 |
| NM_013156 | Ctsl | Cathepsin L | −4.17 | 1.90 | 5 | −16.67 | 4.78 | 5 |
| NM_012939 | Ctsh | Cathepsin H | −3.57 | 2.01 | 4 | −5.56 | 2.12 | 5 |
| J00705 | Apoe | Apolipoprotein E | −3.03 | 1.20 | 5 | −5.56 | 3.01 | 4 |
| M33648 | Hmgcs2 | 3-hydroxy-3-methylglutaryl-coenzyme A synthase 2 | −2.78 | 0.98 | 4 | −5.88 | 3.20 | 4 |
| NM_012558 | Fbp1 | Fructose-1,6- biphosphatase 1 | −2.70 | 0.68 | 5 | −5.26 | 2.31 | 5 |
| Signal transduction | ||||||||
| D10233 | Renbp | Renin-binding protein | −6.25 | 1.96 | 5 | −7.14 | 2.31 | 5 |
| U24489 | Tnxa | Tenascin XA | −5.26 | 2.11 | 5 | −11.11 | 3.21 | 5 |
| AB016536 | Hnrpab | Heterogeneous nuclear ribonucleoprotein A/B | −3.45 | 1.20 | 5 | −9.09 | 3.54 | 4 |
| Development/proliferation/differentiation | ||||||||
| NM_012493 | Afp | α -Fetoprotein | −9.09 | 2.01 | 5 | −25.00 | 6.54 | 5 |
| AF045657 | Dab2 | Disabled homolog 2, mitogen-responsive phosphoprotein | −4.55 | 1.98 | 5 | −7.69 | 2.02 | 5 |
| Transport | ||||||||
| L37380 | LOC252882 | Apical early endosomal glycoprotein | −8.33 | 2.15 | 5 | −16.67 | 3.58 | 5 |
| AF219904 | Folr1 | Folate receptor 1 (adult) | −6.25 | 2.23 | 5 | −11.11 | 2.89 | 5 |
| AB003400 | Dao1 | d-amino acid oxidase | −4.55 | 1.45 | 5 | −7.69 | 3.78 | 5 |
Genes up- and down-regulated that changed by more than 5-fold in four of five samples in at least one sugar perfusion condition (fructose or glucose perfusion) and whose the fold change is significant (P < 0.05). Values are means ± se for each gene (n = 5 experiments). Values in the ″20F vs. 10F″ column indicate the fold change in the expression of a gene in 20-d-old compared with 10-d-old fructose-perfused intestine. Values in the ″20G vs. 10G″ column indicate the fold change in the expression of a gene in 20-d-old compared with the 10-d-old glucose-perfused intestine. Freq, Frequency.
Table 6.
Real-time PCR confirmation of microarray results for selected genes significantly up- or down-regulated between 20- and 10-d-old pups
| Accession no. | Symbol | Name and function | Mean fold change 20F vs. 10F
|
Mean fold change 20G vs. 10G
|
||
|---|---|---|---|---|---|---|
| Real-time PCR | Microarray | Real-time PCR | Microarray | |||
| Transcription regulation/transcription factor | ||||||
| AB026288 | Cebpd | CCAAT/enhancer binding, protein (C/EBP) δ | 1.1 | 1.6a | −1.1 | NS |
| Z36277 | Nucb | Nucleobinding | 1.3 | −1.8a | 1.1 | −2.0a |
| NM_012639 | Raf1 | Murine leukemia viral (v-raf-1) oncogene homolog 1 (3611-MSV) | 2.0 | −2.0a | −1.2 | NS |
| NM_017115 | Myog | Myogenin | −1.6 | 2.3a | −1.3 | NS |
| U18374 | Nr1h4 | Nuclear receptor subfamily 1, group H, member 4 | −3.1b | −2.3a | −2.6 | −2.6a |
| Signal transduction activity | ||||||
| AB002801 | Cnga3 | Cyclic nucleotide gated channel α 3 | 1.1 | 7.1a | −1.3 | 2.9a |
| D87336 | Blmh | Bleomycin hydrolase | 1.1 | −5.0a | −1.6 | NS |
| AJ130946 | Kpna2 | Karyopherin (importin) α-2 | 3.1b | 2.3a | 1.8 | NS |
| Carbohydrate metabolism | ||||||
| NM_019333 | Pfkfb4 | 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 4 | 1.4 | −1.9a | 1.3 | −2.3a |
| NM_013098 | G6Pc | Glucose-6-phosphatase, catalytic | −2.0b,c | −2.2a | −12.5b | −4.7a |
| M18769 | Siat1 | Siat1 | −7.1b | −4.0a | −4.1 | NS |
| Glucocorticoid-sensitive or involved in glucocorticoids metabolism | ||||||
| NM_013061 | Si | Sucrase isomaltase | 159.9b | 19.9a | 166.6b | 29.4a |
| NM_019168 | Arg2 | Arginase 2 | 45.0b | 3.0a | 50.0b | NS |
| NM_012608 | Mmed | Membrane metallo endopeptidase | 17.9b,c | 3.1a | 9.5b | NS |
| M25148 | Pla2g2a | Phospholipase A2, group IIA | 7.8b,c | 6.1a | 3.5b | 5.1a |
| NM_017081 | Hsd11b2 | Hydroxysteroid 11-β dehydrogenase 2 | 3.1b | 2.2a | 2.6 | 3.8a |
| NM_012569 | Gls | Glutaminase | 1.4 | 1.9a | 3.0b | NS |
| NM_012493 | Afp | α -Fetoprotein | −1.1.108b | −9.1a | −1.4.108b | −25.0a |
| X14834 | IGF2 | Insulin-like growth factor-II | −125b | −2a | −125.0b | −2.0a |
| Steroid metabolism | ||||||
| X91234 | Hsd17b2 | 17-β hydroxysteroid dehydrogenase type 2 | 2.4b | 2.0a | 1.9 | NS |
| U89280 | Hsd17b6 | 17-β hydroxysteroid dehydrogenase type 6 | 10.0b | 1.6a | 7.8b | NS |
| NM_012661 | Sts | Steroid sulfatase | −6.6b | −1.8a | −5.4b | −1.7a |
| NM_017154 | Srd5a2 | Steroid 5-α -reductase 2 | −66.6b | NS | −83.3b | −2.0a |
Columns ″20F vs. 10F″ indicate the fold change in expression of a gene in fructose-perfused intestine of 20-d compared with 10-d-old pups. Columns ″20G vs. 10G″ indicate the fold change in expression of a gene in glucose-perfused intestine of 20-d compared with 10-d-old pups. NS, No significant difference in fold change measured by microarray.
Values, measured by microarray, significantly different from 1.0 (P < 0.05).
Values, measured by real-time PCR, significantly (P < 0.05) different from 1.0, between 20 and 10F, or between 20 and 10G groups.
Values, measured by real-time PCR, significantly different from 1.0, between 20F and 20G groups.
Genes differentially expressed between 20- and 10-d-old intestines perfused with fructose.
We identified 28 up- and 22 down-regulated genes that changed with age under fructose conditions (Tables 3 and 4, and Fig. 3). Some up- and down-regulated genes belonged to similar categories, primarily metabolism (nine are up-regulated genes and five down-regulated), transcription regulation (two up-regulated genes and three down-regulated), and signal transduction (three up-regulated and three down-regulated). It is quite interesting to note that development/proliferation/differentiation genes were mainly down-regulated with age, whereas receptor activity and hormone metabolism genes were mainly up-regulated with age. Two genes involved in steroid hormone metabolism, 17-β hydroxysteroid dehydrogenase type 2 (Hsd17b2) and 17-β hydroxysteroid dehydrogenase type 6 (Hsd17b6), were also up-regulated. The function of seven genes was not yet known. The fold change for up-regulated genes ranged between 1.6 (putative pheromone receptor) and 5.1 [retinol dehydrogenase type 2 (Rdh2)], whereas down-regulated genes exhibited fold changes between −5 [bleomycin hydrolase (Blmh)] and −1.56 [glucose-6-phosphate dehydrogenase (G6pdx)].
Genes differentially expressed between 20 and 10-d-old intestines perfused with glucose.
A total of 41 up-regulated and 46 down-regulated genes has been identified in the intestine of 20-d-old pups perfused only with glucose, relative to those in the 10-d-old pups (supplemental Tables 1A and 2A). The majority of the up-regulated genes belonged to three categories: metabolism (14 genes), cell-to-cell communication (14), and transport (7). The main categories of down-regulated genes were metabolism (10 genes), signal transduction (10), proliferation and development (15), and transport (5). Moreover, two genes down-regulated by age and glucose were involved in steroid hormone metabolism: Steroid 5-α-reductase 2 (Srd5a2) and 3β-hydroxysteroid dehydrogenase 1 (Hsd3b1). It is interesting to note that expression of glucose-sensitive genes, like that of fructose-sensitive genes, involved in proliferation and development was being markedly down-regulated as the rat was nearing completion of weaning.
Gene differentially expressed in 20- and 10-d-old intestines perfused with either fructose or glucose.
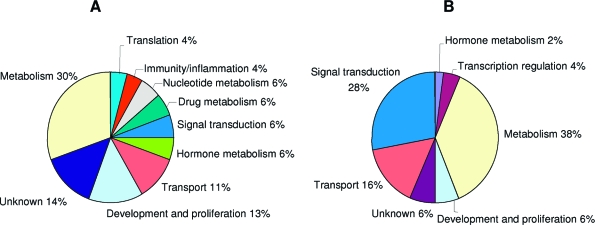
Relative to 10-d-old pups, we identified 71 genes up-regulated and 98 down-regulated in the intestine of 20-d-old pups perfused with either fructose or glucose. Therefore, these genes change in expression with age under either perfusion condition. In Tables 5 and 6 we show only the genes with more than 4-fold changes, the rest is presented in supplemental Tables 3A (for up-regulated) and 4A (down-regulated). The majority of these highly age-sensitive genes are involved in metabolism, transport, or development and differentiation activities (Fig. 4, A and B). The major difference between up- and down-regulated genes is that 28% of the down-regulated genes are involved in transduction activities, but only 6% in the up-regulated genes. Among the genes involved in carbohydrate metabolism, all of the up-regulated genes are involved in the glycolysis or Krebs cycle (enolase, pyruvate kinase, lactate dehydrogenase, phosphoglycerate kinase 1, and fumarate hydratase 1), whereas some down-regulated genes participate in gluconeogenesis (G6Pc and fructose-1,6- biphosphatase 1) (supplemental Tables 3A and 4A).
Figure 4.
GO of the genes differentially expressed in the intestine of pups after glucose or fructose perfusion. A, GO of the genes up-regulated in 20-d compared with 10-d-old pups, under glucose or fructose perfusion. B, GO of the genes differentially down-regulated between 20-d- and 10-d-old pups, under glucose or fructose perfusion.
Surprisingly, from the microarray results, GLUT5 has been sorted in this category because it was up-regulated modestly with age, even in glucose-perfused intestine (2.7 times; P < 0.05). GLUT5 up-regulation with age, also as measured by microarray, was much greater under fructose perfusion (7.12 times; P < 0.001). However, as measured by real-time PCR, GLUT5 is only significantly up-regulated under fructose perfusion (Fig. 2A).
Genes responsive to age and fructose, and those regulated by glucocorticoids
We reexamined by real-time PCR the expression of approximately 20 genes based on expression changes, GO analysis, and potential involvement in glucocorticoid metabolism or glucocorticoid sensitivity as identified using the Pathway Assist and Genomatix Bibliosphere programs (Table 6). We selected representative genes involved in the regulation of transcription and signal transduction to identify those potentially involved in GLUT5 regulation by age and fructose. The expression level of genes involved in carbohydrate metabolism (Table 6) or sugar transport (the results for GLUT5, SGLT1, and GLUT2 are illustrated in Fig. 2, A–C, respectively) were also measured by real-time RT-PCR. Finally, we analyzed eight genes identified as being glucocorticoid sensitive or involved in glucocorticoid/steroid metabolism.
By microarray analysis, the age-related changes in expression of some genes identified as transcription factors were either borderline or not significant, and these findings were in agreement with those obtained by real-time PCR (Table 6). The nuclear receptor for bile acids, nuclear receptor subfamily 1, group H, member 4 (Nr1h4), was confirmed to be down-regulated with age by two to three times under fructose perfusion conditions. The up-regulation as measured by microarray of expression of two genes involved in signal transduction, cyclic nucleotide-gated channel and Blmh, was not confirmed by real-time PCR; PCR also failed to confirm microarray determined up-regulation of cyclic nucleotide-gated channel in previous work (13). However, karyopherin (Kpna2) was confirmed to be up-regulated (P < 0.01) by age and fructose perfusion. Both microarray and real-time PCR analyses also confirmed that β-galactoside α 2,6-sialyltransferase 1 (Siat1), a member of the glycosyltransferase family of enzymes, is uniquely up-regulated with age only under fructose perfusion conditions.
All the genes affected by age and identified to be glucocorticoid sensitive by microarray were generally confirmed by real-time PCR. For example, membrane metallo endopeptidase (Mmed) and Pla2g2 were significantly up-regulated by age, and markedly so when the intestine was perfused with fructose. Hsd1b6 was up-regulated, and Sts as well as Srd5a2 were down-regulated by age under both glucose and fructose perfusion. By real-time PCR, Hsd11b2 and Hsd17b2, genes involved in steroid metabolism, appear to be specifically up-regulated by age only under fructose perfusion conditions, confirming microarray results. Glutaminase (Gls) up-regulation was borderline; it was up-regulated by microarray with age under fructose perfusion, but real-time failed to confirm this finding. Instead, real-time PCR found Gls to increase in the intestine of rats perfused with glucose.
A significant number of genes that was specifically responsive to both age and fructose was also identified by Pathway Assist to be regulated by glucocorticoids, suggesting that glucocorticoids may play an important role not only in the development of GLUT5, but also of these genes. Ten of these genes [Mmed, arginase 2 (Arg2), malic enzyme 1 (Me1), Gls, cytochrome P450 2b15 (Cyp2b15), cytochrome P450 subfamily 19 (Cyp19), Myog, Cebp, Hsdd17b2, and Hsd17b6] were up-regulated, and six were down-regulated [Siat1, uncoupling protein 1 (Ucp1), G6pdx, Raf1, syndecan 3 (Sdc3), and syndecan 4 (Sdc4)] by age and fructose perfusion. Because of this interesting link, we reevaluated the role of corticosteroids in intestinal GLUT5 development. We also determined the expression of several genes identified by microarray and Pathway Assist as either being glucocorticoid sensitive or involved in glucorticoid metabolism.
Dex effect parallels the effect of age on GLUT5 development.
Incredibly, dex at doses far below that used for humans allows fructose to enhance GLUT5 expression in 10-d-old pups (compare Fig. 5A with Fig. 2A) at magnitudes similar to those elicited by fructose perfusion of intestines in 20-d-old pups. Without dex, there is no increase in GLUT5 mRNA abundance in the intestine of fructose compared with glucose-perfused pups. In contrast, dex induces a dramatic 35-fold increase in GLUT5 mRNA abundance in fructose-perfused pups and a modest increase of 7-fold (P < 0.01) in glucose-perfused pups. SGLT1 and GLUT2 mRNA abundance each did not vary with dex treatment (P > 0.50) and sugar perfusion (P > 0.10) (Fig. 5, B and C).
Figure 5.
The effects of glucose and fructose perfusion on sugar transporter mRNA levels and sugar uptakes in the small intestine of the 10-d-old pups injected with dex (GD or FD) or salt solution (GS or FS). The mRNA abundance was measured by real-time PCR using EF1α as a reference gene. Bars are means ± sem (n = 5). mRNA abundance and activities in 10-d-old glucose perfused without dex pups (GS) were designated as 100%, to normalize other groups to this value. Letters indicate significant differences (P < 0.05). Relative mRNA abundance of GLUT5 (A), SGLT1 (B), and GLUT2 (C). Relative uptake rates of fructose (D) and glucose (E). Please note that the ordinate of A differs markedly from B and C. It is clear that dex allows fructose to simulate GLUT5 expression and activity, and that its effects mimic that of age (compare with Fig. 2).
Like the effect of age, dex also allowed luminal fructose to stimulate fructose uptake. There was an increase of 2.7-fold in fructose uptake in dex-treated, fructose-perfused pups (compare Fig. 5D with Fig. 2D). Intestinal glucose uptake increased by 1.6 times (statistically borderline; P = 0.05) in pups perfused with glucose and injected with dex, but there was no effect of dex on glucose uptake in the fructose-perfused pups (Fig. 5E). Proline uptake was independent of dex treatment (P = 0.82) and perfusion solution (P = 0.42) (data not shown), suggesting that dex affects sugar transporters and substantially affects GLUT5 more than SGLT1.
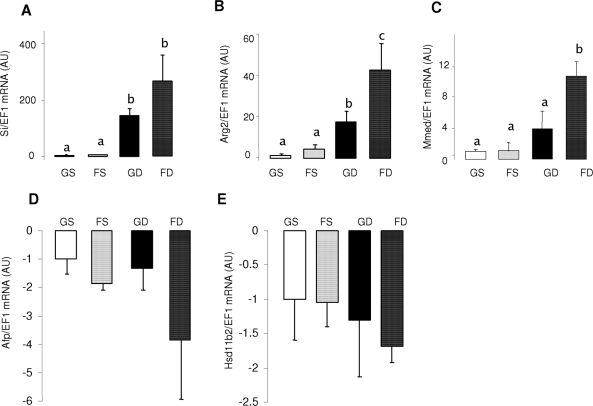
Other genes whose expression are sensitive to both age and dex.
The expression of sucrase isomaltase (Si) increased by 20–30 times with age (Table 5) and was precociously induced by dex 100–300 times. The magnitudes of age and dex effects for Si were similar in both glucose- and fructose-perfused intestines (Fig. 6A). In contrast, the magnitude of age-related increase in mRNA abundance for Arg2 and Mmed expression was greater under fructose perfusion (Table 3). The magnitude of dex-related increase in Arg2 and Mmed mRNA expression was also greater in fructose-perfused intestines (Fig. 6, B and C). Fructose magnified the effect of dex, such that Arg2 expression increased from 15 times in glucose perfused to 45 times in fructose perfused, and Mmed from three times to 10 times. Genes down-regulated with age, like α-fetoprotein (Afp) and Hsd11b2 (Table 5), were interestingly not significantly affected by dex (Fig. 6, D and E), although the mRNA abundance of Afp tended to decrease by more than three times with dex and fructose perfusion.
Figure 6.
The effects of glucose and fructose perfusion in combination with dex on mRNA levels of five highly fructose-responsive genes in the small intestine of 10-d-old pups. The mRNA abundance has been measured by real-time PCR using EF1α as a reference gene. Bars are means ± sem (n = 5). The mRNA abundance in 10-d-old glucose-perfused pups was designated as 100%, to normalize other groups to this value. Letters indicate significant differences (P < 0.05). Relative mRNA abundance of Si (A), Arg2 (B), Mmed (C), Afp (D), and Hsd11b2 (E). Arg2 and Mmed expression are regulated by dex in a similar manner as GLUT5 (compare with Fig. 5A).
Discussion
GLUT5 is a highly interesting model of developing transporter systems in the small intestine because it has sharply defined stages that are characterized by differences in the ability of its substrate to regulate GLUT5 transcription. In this study we answer two related questions “Since GLUT5 is regulatable by fructose when pups are weaning but not when pups are suckling, what are the fructose-responsive genes in the suckling stage and are they different from those in the weaning stage?” Although only a few genes were found fructose sensitive at 10 d old, we identified a large number of genes that, like GLUT5, was fructose sensitive at 20 d of age (13). Second, “What intestinal genes change in expression from suckling to weaning stage?” Here, we identified an enormous number of genes that changes with age but were able to segregate a few genes that change in expression only under conditions when the enterocytes were exposed to fructose. A significant number of these age-responsive and fructose-sensitive genes turned out to be subject to glucocorticoid regulation, and we did find that glucocorticoids can prematurely induce GLUT5 expression and fructose uptake in the 10-d-old pups perfused with fructose. Siat1 and Kpna2, two regulatory genes that specifically become more fructose sensitive with age, could play a major role in regulating GLUT5 during the suckling period.
Fructose-sensitive genes in intestine of 10-d-old rats
Among the fructose-sensitive genes identified by Cui et al. (8) in 20-d-old rat intestine, only cyclin D1 and G6Pc are also regulated by fructose at 10 d old. Interestingly, G6Pc was the only gene up-regulated by fructose after 20-min perfusion in the intestine of 20-d-old pups. The fold increase of G6Pc measured at 10 d old after 4-h fructose perfusion (2.1 times) is similar to the one measured in 20 d old after either 20-min or 4-h fructose perfusion (1.9 times), and much less than that measured at 20 d after 4-h fructose perfusion (10.5 times) (8). The rapid response after just 20-min intestinal exposure to fructose, the magnitude of the response after 4-h perfusion, and the universal response to fructose across various developmental stages indicate that G6Pc has a much higher fructose sensitivity compared with the other genes, including GLUT5. G6Pc is a major regulatory enzyme in the gluconeogenic pathway and may be involved in converting excess fructose to glucose in neonatal enterocytes. Intestinal gluconeogenesis (19) remains a highly controversial issue, with most studies suggesting it does not occur in adults (20,21) but may occur in neonates (22).
Down-regulated genes may also be important. G6pdx catalyzes the conversion of glucose-6-phosphate to 6-phosphogluconolactone, and in doing so, generates reduced nicotinamide adenine dinucleotide phosphate (NADPH) from nicotinamide adenine dinucleotide phosphate positive (NADP+). The 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase 4 (Pfkfb4) is responsible for maintaining the cellular levels of fructose-2,6-bisphosphate, which is a key regulator of glycolysis. Therefore, G6pdx and Pfkfb4 are key enzymes regulating intracellular sugar metabolism and contributing to the energy level in the cells. The role of these enzymes and the many others that are regulated differently by fructose and/or glucose would indeed be interesting studies to pursue because of their potential importance to GLUT5 regulation.
Specificity of GLUT5 induction by age and dex
The increasing sensitivity of GLUT5 to fructose with age appears very specific because only GLUT5 and not SGLT1 or GLUT2 gene expression is up-regulated (∼50 times) by fructose. Moreover, glucose perfusion does not affect GLUT5 at any age. GLUT2 expression is unchanged between the 10 and 20-d-old rat intestine perfused with either glucose or fructose. Similarly, SGLT1 expression is not affected by age and fructose perfusion. GLUT1 and GLUT8 are age sensitive but not fructose sensitive (supplemental Table 4A). Luminal fructose also increases only fructose uptake at 20 d of age; luminal glucose does not regulate fructose uptake at 10 and 20 d of age. Because glucose uptake is not regulated by fructose, it indicates that the brush border GLUT SGLT1 is not regulated by fructose. Therefore, developmental regulation of GLUT5 is markedly different from SGLT1 and other GLUTs.
Likewise, the role of dex in the fructose-induced increase in GLUT5 expression in 10-d-old pups is also highly specific because SGLT1 and GLUT2 expression is not or only modestly affected by dex, independent of the type of sugar perfused. Dex also allows fructose to only increase fructose and not glucose or proline uptakes (17). Moreover, dex does not allow glucose to increase fructose uptake. Therefore, regulation by glucocorticoids of GLUT5 is specific and markedly different from SGLT1 and other GLUTs.
The role of glucocorticoids in regulating GLUT5 expression during development
Glucocorticoids could be one of the key factors that modulate the ontogenic appearance of GLUT5 in the gut and allow its induction by fructose at 20 but not at 10 d of age. Plasma corticosterone concentration increases dramatically after 14 d of age (23) and may trigger gut maturation. In fact, before weaning in mink and other animals, cortisol generally increased hydrolytic and absorptive capacities of the entire small intestine (24). Therefore, it is important to distinguish the effect of age from the effect of corticosterone during the weaning stage. When gene expression in the small intestine of dex-treated and untreated mice was compared, genes in the development category emerged as being likely candidates for mediation of glucocorticoid-induced maturation of intestinal function (25). Glucocorticoids can modify age-induced changes in meprin β, Gls, and Arg2 expression in neonatal rats (26,27) and pigs (28), but it is not clear whether their effect is independent of age. In this study dex allowed fructose to enhance GLUT5 expression in 10-d-old pups at magnitudes (∼20–30 times) similar to those allowed by age in 20-d-old pups, suggesting that dex might mimic the specific effect of age on GLUT5 development.
A previous study by us attempted to distinguish glucocorticoid from age effects by using adrenalectomized rats (29). We showed that in 20-d-old rats adrenalectomized at 10 d old before the endogenous corticosterone surge and exhibiting low corticosterone levels in the plasma, GLUT5 expression and activity were still inducible by fructose. This suggested that fructose used glucocorticoid-independent mechanisms to induce GLUT5 at 20 d of age. However, to avoid salt and water wasting, these adrenalectomized pups received a daily injection of aldosterone. In vivo, mineralocorticoids (aldosterone) and glucocorticoids (corticosterone in rodent) can act in a complementary manner and bind to either the glucocorticoid (GR) or mineralocorticoid (MR) receptors (30). In the study by Monteiro and Ferraris (29), aldosterone may have bound to the MR and/or GR and compensated for the absence of corticosterone, therefore allowing gut maturation and subsequent GLUT5 induction by fructose. Therefore, renewed efforts must be done using adrenalectomized pups to distinguish age from corticosterone effects.
Dex, like corticosterone, is able to bind MR, GR, or the pregnane xenobiotic receptor (31), each of which is expressed in the small intestine of neonatal rats (our unpublished observations). Interestingly, among the age- and fructose-sensitive genes also up-regulated by dex, Arg2 is transcriptionally regulated by glucocorticoids through the intestinal GR (32), whereas CYP3A family members are regulated by pregnane xenobiotic receptor, also in the intestine (33). Moreover, Hsd11b2, which in vivo ensures the selective access of the aldosterone to the MR, is up-regulated by fructose and age. [Because corticosterone and aldosterone bind with equal affinity to MR, the catabolism of corticosterone into inactive 11-dehydro-corticosterone by Hsd11b2 enhances the binding of aldosterone to the MR (30)]. We also identified six potential GR response elements in the GLUT5 promoter regions 0- to 1250-bp upstream from the transcription start site (−128/−119, −306/−297, −551/−542, −1041/−1032, −1238/−1229, and −1246/−1232) using the ElDorado system (www.genomatix.de), suggesting that dex could have a direct nuclear action. However, more studies need to be done to characterize the specific nuclear receptor(s) and response element(s) modulating the effect of dex on GLUT5.
GLUT5 transports a nonessential nutrient that normally appears in the gut lumen only when rats and other omnivorous mammals are weaned, therefore, GLUT5 may require tighter regulation (presence of substrate fructose plus either age or corticosterone) to prevent premature synthesis. More vital transporter genes like SGLT1 and the proline transporter SIT1 are already significantly expressed.
Are Kpna2 and Siat1 involved in GLUT5 development
The failure of fructose to stimulate GLUT5 in intestines of 10-d-old rats could be the result of either inhibitory factors in 10-d-old pups preventing precocious GLUT5 stimulation by fructose, or stimulatory factors appearing only at 20 d of age, and allowing GLUT5 transcription. Two genes, each confirmed by microarray and real-time PCR to be simultaneously fructose responsive and age dependent, may be one of these factors. Kpna2 is overexpressed with age and could be involved in GLUT5 stimulation in 20 d old, whereas Siat1, highly expressed in 10-d-old rat intestines perfused with fructose, could act as an inhibitor of GLUT5 regulation at this age.
Kpna2 is a nuclear import receptor belonging to the importin α-family and implicated in the transport through the nuclear envelope of more than 45 kDa proteins (34). GRs enter the nucleus along with importin proteins. Kpna2 may be linked to GLUT5 regulation because its mRNA is overexpressed in normal testis and malignant breast tumors (35), tissue types characterized by high levels of GLUT5 mRNA or protein (36,37). Moreover, metabolizable sugars induced Kpna2 translocation from the hepatocyte nucleus to the cytoplasm (38,39). This glucose- and fructose-induced movement of Kpna2 is energy dependent but wortmannin insensitive (38). In neonatal rats, fructose-induced GLUT5 expression is also wortmannin insensitive (8). Kpna2 proteins are also known to be tethered to GLUT2 in hepatoma cells, which do not express GLUT5, and may be involved in transmission of the signals that regulate expression of glucose-sensitive genes (39).
Siat1 transfers sialic acid groups to cell-surface glycoproteins and glycolipids, thereby affecting their function (40). The progressive loss of sialic acids in brush-border membrane glycoproteins is one of the major changes occurring in the rat (Table 6) and mouse (41) small intestine during the transition from suckling to weaning. Reduced sialylation with intestinal maturation is the result of a lower expression of Siat1 in weaning rats. Precocious reduction of Siat1 levels could be induced earlier by glucocorticoids (41,42). Interestingly, sialylation could also affect nuclear proteins, and play a role in signal transduction and in repression or activation of gene expression (43). In fact, inhibiting glycosylation of the transcription factor SP1 decreases the expression of membrane transport proteins. Therefore, one of the hypotheses to explain the regulation of GLUT5 during development could be that the decrease in Siat1 expression with age leads to a loss of sialic acids on the nuclear factor(s) involved in GLUT5 transcription.
Caveats, relevance, and future studies
Although we found remarkable differences in expression of intestinal genes between 10- and 20-d-old pups, and between 10-d-old pups perfused with fructose and glucose, the microarray was based on a 5K chip, and, therefore, the list is incomplete. However, the primary objective of the study was not to compile a complete list of age- or fructose-responsive genes (already there are a large number) but to identify pathways involved in GLUT5 development, which can be accomplished by analyzing interactions among a smaller number of genes. For example, the important role of glucocorticoids was discovered when a significant number of fructose- and age-sensitive genes was identified to also be sensitive to glucocorticoids by computer tools that visualize biological pathways and gene regulation networks. The second concern is that the RNA was sampled from a heterogenous cell population. Cell culture approaches do provide a homogenous cell population but cannot be used to study ontogenetic development. In this in vivo study, only the intestinal epithelial layer consisting of more than 80% enterocytes was exposed to differences in luminal sugar; whereas most other cell types were not. Moreover, only the intestinal mucosa was used in this study, thereby eliminating muscle and connective tissue layers, so differences in expression as we have already shown by in situ hybridization (6) and immunocytochemistry (8) would have occurred from the differences in response by enterocytes. In future work, colocalization of age-, dex- or fructose-responsive genes with GLUT5 will confirm their expression in enterocytes. Finally, intestinal development may involve activation (e.g. phosphorylation) of some proteins (44), and not necessarily changes in mRNA expression, therefore, microarray analysis would be unable to detect those changes. However, genes downstream of these activated proteins may still change in expression and would be detected by microarray.
Although they account for less than 10% of births, preterm infants in the United States account for half of infant hospitalization costs occurring, mainly from respiratory distress and necrotizing enterocolitis (45). Because prenatal corticosteroid treatment stimulates lung surfactant synthesis in the preterm infant, prenatal mothers at risk for premature delivery and/or preterm infants receive corticosteroids (46), which as we have clearly shown in this study, also affect the maturation of the fructose transporter system in the small intestine. Biologically, the intestine’s capability to reprogram indicates developmental plasticity that allows a range of phenotypes to develop from a single genotype (47). Plasticity provides organisms with the ability to change function in response to environmental cues. When the dam is under stress, it may release high levels of glucocorticoids in the milk (48) that subsequently and precociously enable the small intestine of the pup to digest and absorb nutrients obtained from the environment, thereby enhancing its survival in case the dam becomes malnourished or dies.
In conclusion, microarray in this study permitted us to identify a pathway, involving glucocorticoids, that participates in the ontogenetic development of GLUT5. In vivo, the timing of the increases in glucocorticoids and GLUT5 fructose sensitivity suggests that GLUT5 gene expression is glucocorticoid dependent at the early stage of neonatal rats. For the first time, the role played by glucocorticoids in GLUT5 regulation in the small intestine has been clearly demonstrated. The potential interaction of signaling factors such as Kpna2 or Siat1 with the glucocorticoids needs to be explored, as well as determining the nuclear receptor involved in glucocorticoid induction of GLUT5.
Supplementary Material
Footnotes
Present address for X.-L.C.: Inotek Pharmaceuticals Corp., 100 Cummings Center, Beverly, Massachusetts 01915.
This work was supported by NIH Grant RDK075617A and NSF Grant IBN-722365 (to R.P.F.) and by the Philippe Foundation (to V.D.).
Disclosure Statement: The authors have nothing to declare.
First Published Online October 18, 2007
Abbreviations: Arg2, Arginase 2; Afp, α-fetoprotein; Blmh, bleomycin hydrolase; Cebpd, CCAAT/enhancer binding protein (C/EBP), δ; Cyp2b15, cytochrome P450, 2b15; Cyp19, cytochrome P450, subfamily 19; dex, dexamethasone; EF1α, elongation factor 1α; G6Pc, glucose-6-phosphate, catalytic; G6pdx, glucose-6-phosphate dehydrogenase; Gls, glutaminase; GLUT, glucose transporter; GLUT2 (Slc2A2), basolateral glucose/fructose transporter; GLUT5 (Slc2A5), intestinal fructose transporter; GLUT7 (Slc2A7), facilitative hexose transporter; GO, gene ontology; GR, glucocorticoid receptor; Hsd3b1, 3β-hydroxysteroid dehydrogenase 1; Hsd11b2, hydroxysteroid 11-β dehydrogenase type 2; Hsd17b2, 17-β hydroxysteroid dehydrogenase type 2; Hsd17b6, 17-β hydroxysteroid dehydrogenase type 6; 10F or 10G, intestine of 10-d-old pups perfused with fructose or glucose, respectively; 20F or 20G, intestine of 20-d-old pups perfused with fructose or glucose, respectively; Kpna2, karyopherin; Me1, malic enzyme 1; Mmed, membrane metallo endopeptidase; Myog, myogenin; MR, mineralocorticoid receptor; Nr1h4, nuclear receptor subfamily 1, group H, member 4; Nucb, nucleobinding; Pfkfb4, 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase 4; Pla2g2a, phospholipase A2, group IIA; Rdh2, retinol dehydrogenase type 2; Sdc3, syndecan 3; Sdc4, syndecan 4; Si, sucrase isomaltase; Siat1, β-galactoside α 2,6-sialyltransferase 1; SGLT1, sodium-glucose cotransporter 1; Srd5a2, steroid 5-α-reductase 2; UCP1, uncoupling protein 1.
References
- Gross LS, Li L, Ford ES, Liu S 2004 Increased consumption of refined carbohydrates and the epidemic of type 2 diabetes in the United States: an ecologic assessment. Am J Clin Nutr 79:774–779 [DOI] [PubMed] [Google Scholar]
- Morrill AC, Chinn CD 2004 The obesity epidemic in the United States. J Public Health Policy 25:353–366 [DOI] [PubMed] [Google Scholar]
- Nobigrot T, Chasalow FI, Lifshitz F 1997 Carbohydrate absorption from one serving of fruit juice in young children: age and carbohydrate composition effects. J Am Coll Nutr 16:152–158 [DOI] [PubMed] [Google Scholar]
- Shu R, David ES, Ferraris RP 1998 Luminal fructose modulates fructose transport and GLUT-5 expression in small intestine of weaning rats. Am J Physiol 274(2 Pt 1):G232–G239 [DOI] [PubMed] [Google Scholar]
- Buddington RK, Diamond JM 1989 Ontogenetic development of intestinal nutrient transporters. Annu Rev Physiol 51:601–619 [DOI] [PubMed] [Google Scholar]
- Jiang L, David ES, Espina N, Ferraris RP 2001 GLUT-5 expression in neonatal rats: crypt-villus location and age-dependent regulation. Am J Physiol Gastrointest Liver Physiol 281:G666–G674 [DOI] [PubMed] [Google Scholar]
- Jiang L, Ferraris R 2001 Developmental reprogramming of rat GLUT-5 requires de novo mRNA and protein synthesis. Am J Physiol Gastrointest Liver Physiol 280:G113–G120 [DOI] [PubMed] [Google Scholar]
- Cui XL, Schlesier AM, Fisher EL, Cerqueira C, Ferraris RP 2005 Fructose-induced increases in neonatal rat intestinal fructose transport involve the PI3-kinase/Akt signaling pathway. Am J Physiol Gastrointest Liver Physiol 288:G1310–G1320 [DOI] [PubMed] [Google Scholar]
- Davidson NO, Hausman AM, Ifkovits CA, Buse JB, Gould GW, Burant CF, Bell GI 1992 Human intestinal glucose transporter expression and localization of GLUT5. Am J Physiol 262(3 Pt 1):C795–C800 [DOI] [PubMed] [Google Scholar]
- Boudry G, Cheeseman CI, Perdue MH 2007 Psychological stress impairs Na+-dependent glucose absorption and increases GLUT2 expression in the rat jejunal brush-border membrane. Am J Physiol Regul Integr Comp Physiol 292:R862–R867 [DOI] [PubMed] [Google Scholar]
- Li Q, Manolescu A, Ritzel M, Yao S, Slugoski M, Young JD, Chen XZ, Cheeseman CI 2004 Cloning and functional characterization of the human GLUT7 isoform SLC2A7 from the small intestine. Am J Physiol Gastrointest Liver Physiol 287:G236–G242 [DOI] [PubMed] [Google Scholar]
- Shu R, David ES, Ferraris RP 1997 Dietary fructose enhances intestinal fructose transport and GLUT5 expression in weaning rats. Am J Physiol 272(3 Pt 1):G446–G453 [DOI] [PubMed] [Google Scholar]
- Cui XL, Soteropoulos P, Tolias P, Ferraris RP 2004 Fructose-responsive genes in the small intestine of neonatal rats. Physiol Genomics 18:206–217 [DOI] [PubMed] [Google Scholar]
- Ferraris RP, Yasharpour S, Lloyd KC, Mirzayan R, Diamond JM 1990 Luminal glucose concentrations in the gut under normal conditions. Am J Physiol 259(5 Pt 1):G822–G837 [DOI] [PubMed] [Google Scholar]
- Karasov W, Diamond J 1983 A simple method for measuring intestinal solute uptake in vitro. J Comp Physiol [B] 152:105–116 [Google Scholar]
- Yang YH, Dudoit S, Luu P, Lin DM, Peng V, Ngai J, Speed TP 2002 Normalization for cDNA microarray data: a robust composite method addressing single and multiple slide systematic variation. Nucleic Acids Res 30:e15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchner S, Kwon E, Muduli A, Cerqueira C, Cui XL, Ferraris RP 2006 Vanadate but not tungstate prevents the fructose-induced increase in GLUT5 expression and fructose uptake by neonatal rat intestine. J Nutr 136:2308–2313 [DOI] [PubMed] [Google Scholar]
- Dyer J, Vayro S, King TP, Shirazi-Beechey SP 2003 Glucose sensing in the intestinal epithelium. Eur J Biochem 270:3377–3388 [DOI] [PubMed] [Google Scholar]
- Croset M, Rajas F, Zitoun C, Hurot JM, Montano S, Mithieux G 2001 Rat small intestine is an insulin-sensitive gluconeogenic organ. Diabetes 50:740–746 [DOI] [PubMed] [Google Scholar]
- Azzout-Marniche D, Gaudichon C, Blouet C, Bos C, Mathe V, Huneau JF, Tome D 2007 Liver glyconeogenesis: a pathway to cope with postprandial amino acid excess in high-protein fed rats? Am J Physiol Regul Integr Comp Physiol 292:R1400–R1407 [DOI] [PubMed] [Google Scholar]
- Martin G, Ferrier B, Conjard A, Martin M, Nazaret R, Boghossian M, Saade F, Mancuso C, Durozard D, Baverel G 2007 Glutamine gluconeogenesis in the small intestine of 72 h-fasted adult rats is undetectable. Biochem J 401:465–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn P, Wei-Ning H 1986 Gluconeogenesis from lactate in the small intestinal mucosa of suckling rats. Pediatr Res 20:1321–1323 [DOI] [PubMed] [Google Scholar]
- Lebenthal A, Lebenthal E 1999 The ontogeny of the small intestinal epithelium. JPEN J Parenter Enteral Nutr 23(5 Suppl):S3–S6 [DOI] [PubMed] [Google Scholar]
- Elnif J, Buddington RK, Hansen NE, Sangild PT 2006 Cortisol increases the activities of intestinal apical membrane hydrolases and nutrient transporters before weaning in mink (Mustela vison). J Comp Physiol [B] 176:233–241 [DOI] [PubMed] [Google Scholar]
- Agbemafle BM, Oesterreicher TJ, Shaw CA, Henning SJ 2005 Immediate early genes of glucocorticoid action on the developing intestine. Am J Physiol Gastrointest Liver Physiol 288:G897–G906 [DOI] [PubMed] [Google Scholar]
- Henning SJ, Oesterreicher TJ, Osterholm DE, Lottaz D, Hahn D, Sterchi EE 1999 Meprin mRNA in rat intestine during normal and glucocorticoid-induced maturation: divergent patterns of expression of α and β subunits. FEBS Lett 462:368–372 [DOI] [PubMed] [Google Scholar]
- Meetze WH, Shenoy V, Martin G, Musy P, Neu J 1993 Ontogeny of small intestinal glutaminase and glutamine synthetase in the rat: response to dexamethasone. Biol Neonate 64:368–375 [DOI] [PubMed] [Google Scholar]
- Flynn NE, Wu G 1997 Glucocorticoids play an important role in mediating the enhanced metabolism of arginine and glutamine in enterocytes of postweaning pigs. J Nutr 127:732–737 [DOI] [PubMed] [Google Scholar]
- Monteiro IM, Ferraris RP 1997 Precocious enhancement of intestinal fructose uptake by diet in adrenalectomized rat pups. Pediatr Res 41:353–358 [DOI] [PubMed] [Google Scholar]
- Funder JW 1992 Glucocorticoid receptors. J Steroid Biochem Mol Biol 43:389–394 [DOI] [PubMed] [Google Scholar]
- Sheppard KE 2002 Nuclear receptors. II. Intestinal corticosteroid receptors. Am J Physiol Gastrointest Liver Physiol 282:G742–G746 [DOI] [PubMed] [Google Scholar]
- Flynn NE, Meininger CJ, Kelly K, Ing NH, Morris Jr SM, Wu G 1999 Glucocorticoids mediate the enhanced expression of intestinal type II arginase and argininosuccinate lyase in postweaning pigs. J Nutr 129:799–803 [DOI] [PubMed] [Google Scholar]
- Moore JT, Kliewer SA 2000 Use of the nuclear receptor PXR to predict drug interactions. Toxicology 153:1–10 [DOI] [PubMed] [Google Scholar]
- Macara IG 2001 Transport into and out of the nucleus. Microbiol Mol Biol Rev 65:570–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl E, Kristiansen G, Gottlob K, Klaman I, Ebner E, Hinzmann B, Hermann K, Pilarsky C, Durst M, Klinkhammer-Schalke M, Blaszyk H, Knuechel R, Hartmann A, Rosenthal A, Wild PJ 2006 Molecular profiling of laser-microdissected matched tumor and normal breast tissue identifies karyopherin alpha2 as a potential novel prognostic marker in breast cancer. Clin Cancer Res 12:3950–3960 [DOI] [PubMed] [Google Scholar]
- Burant CF, Takeda J, Brot-Laroche E, Bell GI, Davidson NO 1992 Fructose transporter in human spermatozoa and small intestine is GLUT5. J Biol Chem 267:14523–14526 [PubMed] [Google Scholar]
- Zamora-Leon SP, Golde DW, Concha, II, Rivas CI, Delgado-Lopez F, Baselga J, Nualart F, Vera JC 1996 Expression of the fructose transporter GLUT5 in human breast cancer. Proc Natl Acad Sci USA [Erratum (1996) 93:15522]93:1847–1852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassany A, Guillemain G, Klein C, Dalet V, Brot-Laroche E, Leturque A 2004 A karyopherin α2 nuclear transport pathway is regulated by glucose in hepatic and pancreatic cells. Traffic 5:10–19 [DOI] [PubMed] [Google Scholar]
- Guillemain G, Munoz-Alonso MJ, Cassany A, Loizeau M, Faussat AM, Burnol AF, Leturque A 2002 Karyopherin α2: a control step of glucose-sensitive gene expression in hepatic cells. Biochem J 364:201–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biol-N′garagba M C, Louisot P 2003 Regulation of the intestinal glycoprotein glycosylation during postnatal development: role of hormonal and nutritional factors. Biochimie 85:331–352 [DOI] [PubMed] [Google Scholar]
- Dai D, Nanthakumar NN, Savidge TC, Newburg DS, Walker WA 2002 Region-specific ontogeny of α-2,6-sialyltransferase during normal and cortisone-induced maturation in mouse intestine. Am J Physiol Gastrointest Liver Physiol 282:G480–G490 [DOI] [PubMed] [Google Scholar]
- Hamr A, Delannoy P, Verbert A, Kolinska J 1997 The hydrocortisone-induced transcriptional down-regulation of β-galactoside α2,6-sialyltransferase in the small intestine of suckling rats is suppressed by mifepristone (RU-38.486). J Steroid Biochem Mol Biol 60:59–66 [DOI] [PubMed] [Google Scholar]
- Kudlow JE 2006 Post-translational modification by O-GlcNAc: another way to change protein function. J Cell Biochem 98:1062–1075 [DOI] [PubMed] [Google Scholar]
- Marion J, Petersen YM, Rome V, Thomas F, Sangild PT, Le Dividich J, Le Huerou-Luron I 2005 Early weaning stimulates intestinal brush border enzyme activities in piglets, mainly at the posttranscriptional level. J Pediatr Gastroenterol Nutr 41:401–410 [DOI] [PubMed] [Google Scholar]
- Russell RB, Green NS, Steiner CA, Meikle S, Howse JL, Poschman K, Dias T, Potetz L, Davidoff MJ, Damus K, Petrini JR 2007 Cost of hospitalization for preterm and low birth weight infants in the United States. Pediatrics 120:e1–e9 [DOI] [PubMed] [Google Scholar]
- Bunt JE, Carnielli VP, Darcos Wattimena JL, Hop WC, Sauer PJ, Zimmermann LJ 2000 The effect in premature infants of prenatal corticosteroids on endogenous surfactant synthesis as measured with stable isotopes. Am J Respir Crit Care Med 162:844–849 [DOI] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA, Spencer HG 2005 Predictive adaptive responses and human evolution. Trends Ecol Evol 20:527–533 [DOI] [PubMed] [Google Scholar]
- Lesage J, Blondeau B, Grino M, Breant B, Dupouy JP 2001 Maternal undernutrition during late gestation induces fetal overexposure to glucocorticoids and intrauterine growth retardation, and disturbs the hypothalamo-pituitary adrenal axis in the newborn rat. Endocrinology 142:1692–1702 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.