Abstract
Receptor activator of nuclear factor-κB ligand (RANKL) is essential for osteoclast differentiation, and hormones and cytokines that stimulate bone resorption increase RANKL expression in stromal/osteoblastic cells. We have previously shown that PTH and 1,25-dihydroxyvitamin D3 control murine RANKL gene expression in vitro, in part, via an evolutionarily conserved transcriptional enhancer, designated the distal control region (DCR), located 76 kb upstream from the transcription start site. Herein we describe the phenotype of mice lacking this enhancer. Deletion of the DCR reduced PTH and 1,25-dihydroxyvitamin D3 stimulation of RANKL mRNA and osteoclast formation in primary bone marrow cultures as well as stimulation of RANKL mRNA in bone. DCR deletion also reduced basal RANKL mRNA levels in bone, thymus, and spleen. Moreover, mice lacking the DCR exhibited increased bone mass and strength. The increase in bone mass was due to reduced osteoclast and osteoblast formation leading to a low rate of bone remodeling similar to that observed in humans and mice with hypoparathyroidism. These findings demonstrate that hormonal control of RANKL expression via the DCR is a critical determinant of the rate of bone remodeling.
BONE REMODELING IN mammals serves to maintain the biomechanical integrity of the skeleton by continuously replacing old bone with new. This is accomplished by teams of bone-resorbing osteoclasts and bone-forming osteoblasts (1). The rate of bone remodeling is controlled, at least in part, by the circulating level of PTH. Consequently, loss or reduction of PTH lowers osteoclast and osteoblast formation in humans and rodents (2,3,4), and elevation of PTH, as in hyperparathyroidism, increases osteoclast and osteoblast formation (5). PTH also stimulates expression of receptor activator of nuclear factor-κB ligand (RANKL), a membrane-bound member of the TNF superfamily that is required for osteoclast differentiation and supports osteoclast survival and bone-resorbing activity (6,7,8,9). It has therefore been proposed that PTH controls the rate of bone remodeling via its ability to control RANKL. In addition, PTH increases production of 1,25-dihydroxyvitamin D3 [1,25(OH)2D3] via direct stimulation of renal 25-hydroxyvitamin D-1α-hydroxylase (10,11,12); 1,25(OH)2D3 is another potent stimulator of RANKL gene expression (13). Thus, changes in PTH levels can affect RANKL expression alone or in concert with 1,25(OH)2D3.
Initial attempts to identify the cis-acting elements that control RANKL transcription in response to PTH or 1,25(OH)2D3 produced inconsistent results. Kitazawa and colleagues (14,15,16) reported that RANKL promoter constructs containing up to 2 kb of 5′-flanking region were stimulated by both forskolin and 1,25(OH)2D3 in a murine stromal cell line. These results have been difficult to confirm by us and others (17,18,19) using reporter constructs containing up to 7 kb of 5′-flanking sequence. Recent studies, however, identified a distant transcriptional enhancer located 76 kb upstream of the murine RANKL transcription start site that appears to mediate responsiveness to PTH and 1,25(OH)2D3, using an approach that involved bacterial artificial chromosome-derived reporter constructs (20). In these studies, sequences were identified within the distal region of this enhancer that bind cAMP response element binding protein upon stimulation by PTH and are required for up-regulation of RANKL expression. Separate studies using the method of chromatin immunoprecipitation (ChIP)-on-chip analysis revealed that the proximal portion of this 2-kb enhancer also contained an unusual vitamin D-responsive element (VDRE) that mediated the actions of 1,25(OH)2D3 (21). Based on its striking distance from the RANKL transcription start site and its ability to integrate signals from multiple hormones, we designated this entire 2-kb enhancer as the RANKL distal control region (DCR) (20,21).
The importance of the DCR for PTH control of RANKL transcription was confirmed by targeted deletion of the enhancer from the mouse genome (20). We found that mice lacking the DCR developed normally and, in contrast to RANKL-deficient mice, were not osteopetrotic. Nonetheless and consistent with our reporter gene studies, PTH stimulation of RANKL expression was significantly blunted in bone marrow stromal cells from DCR-deficient mice cultured in vitro. However, it remained unknown whether RANKL expression was altered in DCR-null mice and, if altered, whether such changes were sufficient to impact either osteoclast formation or overall skeletal homeostasis.
In the studies presented here, we show that deletion of the DCR reduced RANKL expression in bone as well as lymphoid tissues and reduced direct stimulation of RANKL expression by both PTH and 1,25(OH)2D3 in vivo. More importantly, DCR-null mice displayed increased bone mass and strength due to reduced osteoclast formation leading to low bone remodeling. These studies demonstrate that hormonal control of RANKL expression, via the DCR, controls the rate of bone remodeling.
Materials and Methods
Materials
Human PTH(1–84) was purchased from Bachem California Inc. (Torrance, CA). Bovine PTH(1–34) was purchased from Sigma (St. Louis, MO). 1,25(OH)2D3 was purchased from Biomol (Plymouth Meeting, PA). All cells were maintained in growth medium consisting of αMEM (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (HyClone, Logan, UT) and 1% each of penicillin, streptomycin, and glutamine (Sigma).
Animal studies
Generation of DCR−/− mice was described previously (20). To obtain mice for the studies described herein, the DCR-null allele was crossed into the C57BL/6 genetic background for four to six generations, and DCR+/− mice were bred to generate DCR+/+ and DCR−/− littermates. Six-month-old DCR+/+ and DCR−/− male mice were injected ip with PBS without or with PTH(1–84) (230 ng/g) or polypropylene glycol without or with 1,25(OH)2D3 (1 ng/g). All studies involving mice were approved by the Institutional Animal Care and Use Committees of the University of Arkansas for Medical Sciences and the University of Wisconsin, Madison.
Primary cell cultures
Bone marrow cells were harvested from femurs at 2 months of age via a centrifugation method as previously described (22). Briefly, the ends of the femurs were removed and the shaft was placed into a 1.5-ml microcentrifuge tube containing 0.5 ml αMEM with Hanks’ salts, supplemented with 10% fetal bovine serum, and centrifuged at 12,000 × g for 2 min at room temperature to elute marrow cells. After centrifugation, the cells were resuspended in the above medium and debris was removed by filtering through a nylon mesh. For time-course and dose-response studies, the bone marrow cells were plated at 5 × 106 cells/well in 12-well plates containing growth medium and cultured for 10 d. Growth medium was changed completely on d 3 and 8, after which only adherent cells remained. On d 10 of culture, the cells were treated with vehicle, PTH(1–34), or 1,25(OH)2D3 at the concentrations and times indicated in the figures. For osteoclast formation assays, cells were plated at 1.5 × 106 cells/well in 24-well plates or 5 × 106 cells/well in 12-well plates. After 3 d, PTH(1–34) (10−7 m), 1,25(OH)2D3 (10−8 m), or RANKL+ macrophage colony-stimulating factor (M-CSF) (30 ng/ml each) were added, and the cultures were incubated for 6 additional days, with a change of half of the medium at d 3 and 6. On the last day, cells were fixed and stained for tartrate-resistant acid phosphatase (TRAP) activity as previously described (8) or used for RNA extraction.
RNA analysis
Total RNA was purified from cell cultures and tissues using Ultraspec reagent (Biotecx Laboratories, Houston, TX), according to the manufacturer’s directions. Taqman quantitative RT-PCR was performed as previously described (23) using the following primer probe sets from Applied Biosystems (Foster City, CA): RANKL (Mm0041908-m1); IL-6 (Mm00446190-m1); osteoprotegerin (OPG) (Mm00435451-m1); cathepsin K (Mm01255862-g1); A kinase anchor protein 11 (Mm01313936-m1), and ribosomal protein S2 (forward, 5′-CCCAGGATGGCGACGAT-3′; reverse, 5′-CCGAATGCTGTAATGGCGTAT-3′; probe, FAM-5′-TCCAGAG CAGGATCC-3′-NFQ). SYBR Green quantitative RT-PCR was performed as described (24) using the following primer set for RANKL, 5′-ATTCAGGTGTCCAACCCTTCC-3′, reverse, 5′-TGCTAATGTTCCACGAAATG-3′. Expression levels were determined using the ΔCt method (25).
Bone mineral density (BMD) determinations
Starting at 1 month of age, sequential measurements of BMD in live mice were performed by dual-energy x-ray absorptiometry with a PIXImus mouse densitometer (Lunar, Fitchburg, WI) using the manufacturer’s software as previously described (26).
Bone histomorphometry
The first through fourth lumbar vertebrae were fixed and embedded undecalcified in methyl methacrylate as previously described (27). Histomorphometric examination was done with a computer and digitizer tablet (OsteoMetrics, Decatur, GA) interfaced to a Zeiss Axioscope (Carl Zeiss, Thornwood, NY) with a drawing tube attachment. The percentage of the cancellous perimeter covered by plump, cuboidal osteoblasts lining osteoid (osteoblast perimeter) and the percentage of the cancellous perimeter bearing TRAP-positive multinucleated cells (osteoclast perimeter) were measured directly, whereas the rate of bone formation per cancellous perimeter was calculated. Terminology used was that recommended by the Histomorphometry Nomenclature Committee of the American Society for Bone and Mineral Research (28).
Biomechanical testing
The load-bearing properties of the sixth lumbar vertebrae (L6) were measured using a single column material testing machine and a calibrated tension/compression load cell (model 5542; Instron Corp., Grove City, PA), as previously described (26).
Biochemical markers
Plasma and urine were collected from 5-month-old wild-type and DCR-deficient female mice. Deoxypyridinoline (DPD) and creatinine were quantified in urine using ELISA kits (Metra Biosystems, Quidel, San Diego, CA), and DPD values were corrected by creatinine. Plasma calcium, PTH, and osteocalcin levels were quantified using a colorimetric assay (StanBio, Boerne, TX), an ELISA (Immutopics Inc., San Clemente, CA), or a RIA (Metra Biosystems), respectively.
Statistics
Data were analyzed using SigmaStat (SPSS Science, Chicago, IL). All values are reported as the mean ± sd. Differences between group means were evaluated with Student’s t test or two-way ANOVA. For experiments in which the data were not normally distributed, the Mann-Whitney rank sum test was used.
Results
Hormonal control of RANKL is blunted in cells from DCR−/− mice
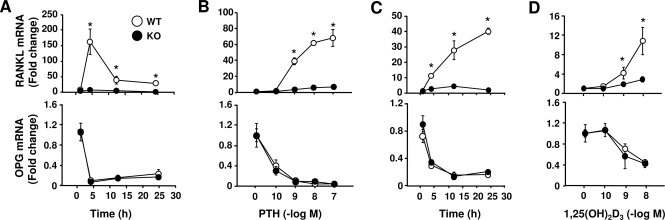
We have previously shown that PTH responsiveness of the RANKL gene was significantly blunted in primary bone marrow cultures from DCR−/− mice (20). However, these studies were performed at a single time point (24 h) with a maximal concentration of PTH. To determine whether the responsiveness to PTH was different in DCR−/− cells at earlier time points and submaximal concentrations of PTH, we performed time-course and dose-response studies in primary bone marrow cultures quantifying RANKL mRNA by real-time RT-PCR. PTH at a concentration of 10−7 m stimulated RANKL mRNA in wild-type cells as early as 1 h after treatment, with maximum response achieved after 4 h; this effect was significantly reduced in DCR−/− cells by 4 h (Fig. 1A). Deletion of the DCR had no effect on PTH suppression of OPG, a control gene. Increasing concentrations of PTH led to a progressive increase in RANKL mRNA in wild-type cells, and this effect was significantly blunted in DCR−/− cells (Fig. 1B). In contrast, inhibition of OPG was not different between wild-type and DCR−/− cells at any concentration of PTH.
Figure 1.
DCR ablation blunts RANKL stimulation by PTH and 1,25(OH)2D3 in vitro. Quantitative RT-PCR analysis of RANKL and OPG mRNA in primary bone marrow cultures treated with 10−7 m PTH (A) or 10−8 m 1,25(OH)2D3 (C) for the indicated times or treated with increasing concentrations of PTH (B) or 1,25(OH)2D3 (D) for 4 h. RANKL and OPG values were normalized to ribosomal protein S2 mRNA levels. Values represent the mean fold change relative to contemporaneous vehicle-treated cultures ± sd. All treatments were performed in triplicate. WT, Wild type; KO, knockout. *, P < 0.05 vs. WT.
Our previous mapping (20) and ChIP-on-chip analysis (21) suggested that 1,25(OH)2D3 may also use the DCR to control RANKL transcription. As shown in Fig. 1C, 1,25(OH)2D3 stimulated RANKL mRNA in wild-type cells at all time points, with a maximal response after 24 h. Deletion of the DCR, however, reduced 1,25(OH)2D3 responsiveness at 4, 12, and 24 h (Fig. 1C). In contrast, 1,25(OH)2D3 suppression of a control gene, OPG, was unaffected by loss of the DCR. A significant reduction in the stimulation of RANKL by 1,25(OH)2D3 was also apparent at the two highest concentrations, again with no difference in the response of OPG between genotypes (Fig. 1D). Taken together, these results demonstrate that deletion of the DCR blunts responsiveness of the RANKL gene to both PTH and 1,25(OH)2D3 in primary bone marrow cultures.
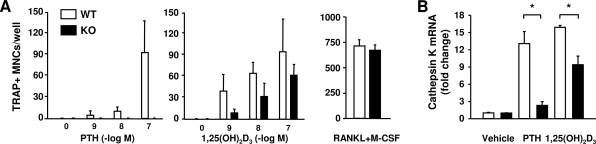
To determine whether this reduction in RANKL gene responsiveness was sufficient to alter hormone-induced osteoclastogenesis, bone marrow cultures from wild-type and DCR−/− mice were treated with PTH or 1,25(OH)2D3, and osteoclastogenesis was quantified by enumerating TRAP-positive multinucleated cells. Increasing concentrations of PTH increased osteoclast number in wild-type cultures in a dose-dependent manner (Fig. 2A). However, this response was abolished in DCR−/− cultures (Fig. 2A). DCR deletion also reduced osteoclastogenesis induced by 1,25(OH)2D3 but to a lesser extent than with PTH. The reduction in osteoclast formation was not due to reduced numbers of osteoclast precursors because soluble RANKL and M-CSF stimulated similar levels of osteoclast differentiation in bone marrow-derived mononuclear cell cultures from both genotypes. In separate cultures we quantified osteoclast formation by measuring transcripts of the osteoclast-specific gene cathepsin K. Cathepsin K mRNA in PTH-treated cultures from DCR−/− mice was reduced, compared with cultures from wild-type mice (Fig. 2B). DCR deletion also blunted the response to 1,25(OH)2D3, although this reduction was less than that seen with PTH. These results demonstrate that loss of the DCR reduces hormonal stimulation of osteoclastogenesis in vitro.
Figure 2.
Lack of DCR reduces osteoclast formation by PTH and 1,25(OH)2D3 in vitro. A, Bone marrow cells from DCR−/− and wild-type (WT) mice were treated with increasing concentrations of PTH, 1,25(OH)2D3 or with RANKL+M-CSF (30 ng/ml), and TRAP-positive cells containing at least four nuclei were counted after 6 d. B, Quantitative RT-PCR analysis of cathepsin K mRNA in bone marrow cultures after stimulation with 10−7 m PTH or 10−8 m 1,25(OH)2D3 for 6 d. Values were normalized to ribosomal protein S2 mRNA, are the mean of triplicate cultures ± sd, and are presented as fold change relative to vehicle-treated WT cells. KO, knockout. *, P < 0.05 vs. WT.
Hormonal control of RANKL is blunted in vivo
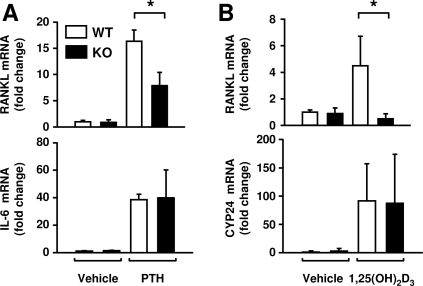
We have shown previously that the DCR is required for elevation of RANKL mRNA in bone in vivo after 7 d on a calcium-deficient diet, a maneuver that elevates both PTH and 1,25(OH)2D3 (20). However, it is unclear whether the elevated RANKL in this situation was due to direct control of RANKL transcription by PTH, 1,25(OH)2D3, or both or through downstream factors regulated by these hormones. To determine whether the DCR was required for control of RANKL by acute elevation of either hormone, mice were injected with PTH or 1,25(OH)2D3, and RANKL mRNA was quantified in bone 1 h after PTH injection or 6 h after 1,25(OH)2D3 injection. Loss of the DCR significantly blunted the response of the RANKL gene to either hormone (Fig. 3, A and B). Control genes, IL-6 for PTH and CYP24 for 1,25(OH)2D3, responded equivalently in both genotypes (Fig. 3, A and B). These results are consistent with the blunted in vitro responsiveness and suggest that the DCR is indeed required for direct control of RANKL by these two calciotropic hormones.
Figure 3.
DCR deletion blunts RANKL stimulation by calciotropic hormones in vivo. Quantitative RT-PCR analysis of RANKL, IL-6, or CYP24 mRNA in calvaria of wild-type (WT) and DCR−/− mice after ip administration of either PTH (in PBS) or 1,25(OH)2D3 (in polypropylene glycol). A, Six-month-old male mice were injected with PTH(1–84) (230 ng/g) and killed after 1 h. B, Three-month-old male and female mice were injected with 1,25(OH)2D3 (1 ng/g) 6 h before the animals were killed. Values were normalized to ribosomal protein S2 mRNA, are the mean of three to six mice/group ± sd and are presented as fold change relative to vehicle-treated WT mice. KO, Knockout. *, P < 0.05 vs. WT.
RANKL expression is reduced in bone and lymphoid tissues of DCR−/− mice
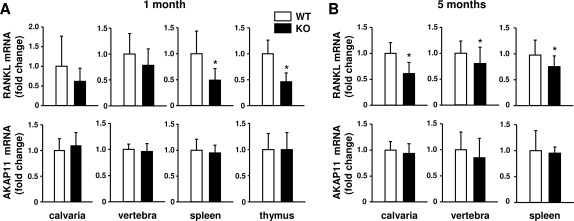
We next determined whether DCR deletion altered RANKL mRNA abundance in tissues that express the highest levels of the gene, namely bone and lymphoid tissues. At 1 month of age, RANKL expression in bone was not significantly reduced by loss of the DCR (Fig. 4A). In contrast, RANKL expression was reduced by approximately 50% in the thymus and spleen of DCR−/− mice. At 5 months of age, however, RANKL expression in DCR−/− mice was reduced by 20–40% in bone and 25% in spleen (Fig. 4B). Thymus was not examined in the 5-month-old mice because the organ had undergone atrophy in both genotypes. As a control for these experiments, we quantified expression of AKAP11, a gene located immediately upstream of RANKL on chromosome 14. Transcripts for this gene were not different between wild-type and DCR−/− mice in any of the tissues examined. These results demonstrate that the DCR contributes to RANKL expression in bone and lymphoid tissue.
Figure 4.
DCR deletion reduces RANKL mRNA levels in bone and lymphoid tissue. Quantitative RT-PCR analysis of RANKL and AKAP11 mRNA in calvaria, vertebra, spleen, and thymus of wild-type (WT) and DCR−/− animals at 1 (A) or 5 (B) months of age. Values were normalized to ribosomal protein S2 mRNA, are the mean of nine to 18 mice/group ± sd, and are presented as fold change relative to WT mice. KO, Knockout. *, P < 0.05 vs. WT.
Bone mass and strength are elevated in DCR−/− mice
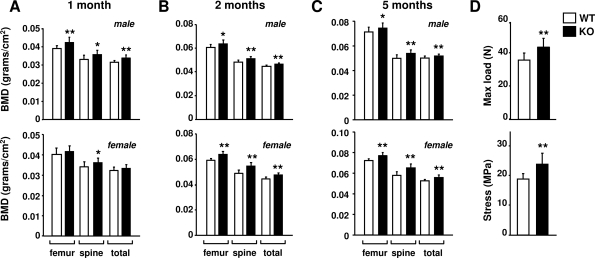
To determine whether the reduction in RANKL expression in DCR−/− mice resulted in changes in bone mass, BMD was measured. BMD was elevated in 1-month-old DCR−/− male mice at all skeletal sites but only in the spine in females (Fig. 5A). At 2 and 5 months of age, BMD was elevated at all sites in both sexes, with an increase of approximately 10% in the female spine (Fig. 5, B and C). Consistent with increased bone mass, L6 vertebra from 6-month-old mice exhibited a 26% increase in compression strength that was still present when normalized for bone size (Fig. 5D). Therefore, loss of the DCR increased not only bone mass but also the biomechanical properties of bone.
Figure 5.
BMD and bone strength are increased in mice lacking the DCR. Femoral, spinal, and total-body BMD of 1- (A), 2- (B), or 5-month-old (C) male or female wild-type (WT) and DCR−/− mice. The same cohort of animals was used for each time point. D, Load-bearing properties of the sixth lumbar vertebra (maximum load and stress) from 6-month-old male WT and DCR−/− mice. Values are mean of 10–16 mice/group ± sd. KO, Knockout. *, P < 0.05, **, P < 0.01 vs. WT.
Bone remodeling is reduced in DCR−/− mice
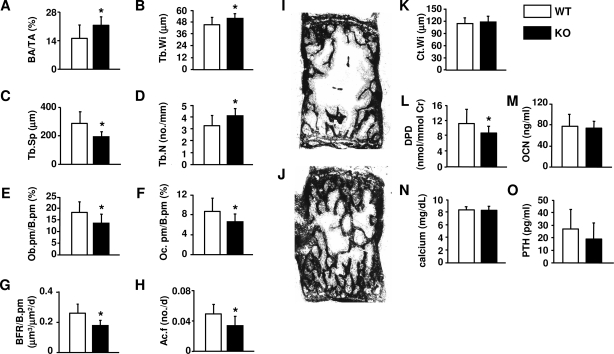
Finally, to establish the cellular basis for the elevated bone mass observed in DCR−/− mice, we performed histomorphometric studies on vertebral cancellous bone of 5-month-old mice. In agreement with the BMD analysis, cancellous bone area was significantly elevated in mice lacking the DCR (Fig. 6, A, I, and J). Trabecular width and number were increased, whereas trabecular separation was decreased (Fig. 6, B–D). Osteoclast and osteoblast perimeter were both reduced, as was the bone formation rate and activation frequency (Figs. 6, E–H). Cortical width was not different between wild-type and DCR−/− mice (Fig. 6K). Consistent with the changes in cancellous osteoclast perimeter, urinary DPD, a marker of bone resorption, was significantly reduced in 5-month-old DCR−/− mice (Fig. 6L). In contrast, no difference in circulating osteocalcin, a marker of bone formation, was detected (Fig. 6M). Calcium homeostasis was unperturbed in DCR−/− mice because serum calcium and PTH levels were comparable in both genotypes (Fig. 6, N and O). Taken together, these results demonstrate that deletion of the DCR reduced bone turnover in adult mice.
Figure 6.
DCR deletion reduces bone remodeling. Vertebral sections (L1–4) from 5-month-old female wild-type (WT) and DCR−/− mice were used to determine the following histomorphometric measurements: bone area over total tissue area (BA/TA) (A), trabecular width (Tb.Wi) (B), trabecular separation (Tb.Sp) (C), trabecular number (Tb.N) (D), osteoblast perimeter (Ob.pm/B.pm) (E), osteoclast perimeter (Oc.pm/B.pm) (F), bone formation rate per bone perimeter (BFR/B.pm) (G), activation frequency (Ac.F) (H), and cortical width (Ct.Wi) (K). Longitudinal undecalcified vertebral sections of WT (I) and DCR−/− (J) mice (unstained slides viewed without coverslips at ×25). Urinary DPD. (L), plasma osteocalcin (M), calcium (N), and PTH (O) levels. Values represent the mean of 12–18 mice/group ± sd. KO, Knockout. *, P < 0.05 vs. WT.
Discussion
The results presented here show that loss of the DCR blunts responsiveness of the RANKL gene to PTH and 1,25(OH)2D3 and results in reduced bone remodeling, an effect that is associated in turn with increased bone mass and strength. The increase in bone mass was confined to cancellous bone because cortical width was unchanged and occurred despite reductions in both resorption and formation. The increase in trabecular number and width, together with the decreased trabecular separation, indicates that bone mass was elevated in part because columns of the primary spongiosa were not resorbed as effectively as in the wild-type animals during growth. Thus, more of them persisted in the secondary spongiosa. If the increase in bone mass is due to unbalanced bone remodeling in favor of bone formation, the difference in bone mass caused by lack of the DCR should increase with time. However, the difference in BMD between wild-type and DCR−/− mice was relatively constant between 2 and 5 months of age (Fig. 5), suggesting that although both resorption and formation were reduced, bone remodeling remained balanced.
The results of our study are consistent with the idea that the DCR mediates the effects of PTH on bone remodeling. The changes in bone remodeling in the DCR−/− mice are similar to those observed in mice lacking PTH due to genetic manipulation (3) as well as to those seen in parathyroidectomized rats (4) and humans with hypoparathyroidism (2). If RANKL is reduced in DCR−/− mice because this enhancer mediates the effects of PTH, then RANKL should be reduced in these situations as well. Although RANKL expression has not been measured in humans with hypoparathyroidism, RANKL mRNA in bone was reduced after parathyroidectomy in rats (4) but was not significantly reduced in PTH-null mice (29). Maintenance of RANKL mRNA levels in PTH-null mice may have resulted from elevation of PTHrP expression because haploinsufficiency of PTHrP combined with PTH-deletion dramatically reduced RANKL mRNA (29). Because the DCR mediates some of the effects of 1,25(OH)2D3 on RANKL, it is possible that the effects of this hormone on bone remodeling are also mediated via the DCR. However, the finding that a diet that restores circulating calcium levels in vitamin D receptor (VDR)-deficient mice also restores normal numbers of osteoclasts and osteoblasts on cancellous bone (30) suggests that the VDR, and thus its action on the DCR, is not required for basal bone remodeling.
Deletion of the DCR reduced both the osteoclast and osteoblast perimeters. These results demonstrate that the normal coordination between bone resorption and bone formation, frequently referred to as coupling, remains intact in DCR−/− mice. Whereas the mechanisms that underlie this coordination remain unknown, two different explanations have been proposed. According to the first, release of factors, such as TGFβ, from the bone matrix as a consequence of osteoclast activity recruits osteoblast progenitors and promotes their differentiation (31). Thus, osteoblastogenesis in this serial pathway of coupling is a consequence of osteoclastogenesis (32). Because of evidence that osteoclastogenesis may depend on cells of the osteoblast lineage, we proposed earlier the existence of a second or parallel pathway in which coupling results in part from linkage of osteoclastogenesis to osteoblastogenesis (32). Because abundant evidence demonstrates that RANKL controls osteoclast differentiation and function but has no effect on osteoblasts (33), the decrease in osteoblasts and bone formation observed in DCR-deficient mice is most likely secondary to the decrease in osteoclast production, bone resorption, or both and thus supports the existence of the serial pathway. Alternatively, the reduction in osteoblasts may have resulted from decreased levels of an unknown factor produced by osteoclasts that promotes osteoblastogenesis (34).
Because deletion of the DCR reduced hormone-stimulated RANKL expression in bone marrow stromal cell cultures, it is likely that the reduced RANKL levels observed in bone occurred in this same cell population. However, our observation that RANKL mRNA was also reduced in lymphocyte-containing tissues, together with previous studies showing that activated T lymphocytes express significant amounts of RANKL (35), leaves open the possibility that the DCR contributes to RANKL expression in this cell type. Nonetheless, the finding that RANKL mRNA expression in bone marrow was unaffected by loss of T lymphocytes suggests that, at least under basal conditions, this cell type is not a major source of RANKL in bone (36).
Although deletion of the DCR appeared to have a similar impact on RANKL mRNA stimulation by both PTH and 1,25(OH)2D3, the magnitude of osteoclast formation in vitro in response to PTH was more blunted than in response to 1,25(OH)2D3. The reason for this difference is unclear but may be related to the different timeframes of the two end points. Specifically, the osteoclastogenesis assays involved 6 d of continuous exposure to the hormones, whereas the longest time point in our analysis of RANKL mRNA expression was 24 h. Thus, it is possible that at later time points, 1,25(OH)2D3 was more effective than PTH at stimulating RANKL expression in the absence of the DCR, possibly via the more proximal VDREs identified previously (21).
Importantly, serum calcium concentration was normal in DCR−/− mice, suggesting that PTH control of RANKL is not required for calcium homeostasis under basal conditions. Although the reduced bone resorption caused by DCR deletion must have reduced the skeletal contribution to circulating calcium levels, the contribution from intestinal absorption and renal reabsorption should not have been affected. Moreover, it is likely that calcium levels did not fall, and PTH was not elevated, in DCR−/− mice because the demand for calcium was also reduced as a result of lower bone formation, thus maintaining homeostasis, at least under basal conditions. Because lactation increases bone resorption, likely via PTHrP (37), a decrease in the ability to nurse offspring could have been anticipated in DCR−/− mice. DCR-null dams produced pups that at weaning were similar in size to pups from wild-type dams (data not shown). It should be noted, however, that pup size is not necessarily affected by a reduced ability to resorb bone in nursing dams because these end points were not altered by mammary gland-specific deletion of PTHrP, a maneuver that decreases bone resorption (37).
Bone strength was elevated in 6-month-old DCR−/− mice. Assuming that one purpose of bone remodeling is to maintain skeletal strength, one might expect that the reduced bone remodeling in DCR−/− mice would lead with age to an accumulation of fatigue damage and reduced bone strength. If increased fatigue damage was present in the 6-month-old mice analyzed in our study, it must have been more than compensated for by the increase in bone mass. Be that as it may, future studies will be required to assess the presence and extent of microdamage in DCR-null mice and whether strength does indeed decline with age.
The results presented here confirm the validity of our in vitro enhancer-mapping studies. Nonetheless, it is likely that additional response elements exist because deletion of the DCR did not completely eliminate hormonal responsiveness. Whereas such elements may include the cAMP response elements and VDREs believed to be located in the proximal promoter (14,15,16,38), it seems more likely that the multiple, highly conserved binding sites for both cAMP response element binding protein and the VDR identified more recently by ChIP-on-Chip analysis and direct ChIP studies mediate the residual hormonal responsiveness (21,39). Additional targeted deletion of these putative enhancers will be required to address this question definitively.
In conclusion, our results demonstrate that the DCR enhancer integrates signals from multiple hormones and is a key regulator of the rate of bone remodeling. The increase in bone mass and strength in DCR-deficient mice suggests that the molecular mechanisms by which the DCR functions may represent a novel therapeutic target for reducing bone turnover. However, as with other antiresorptive therapies, it will be important to determine the consequences of long-term suppression of bone remodeling on bone strength. Additional studies will also be required to determine the role of the DCR in the bone loss due to elevated PTH. Finally, it has been suggested that optimal bone anabolic responsiveness to intermittent PTH requires stimulation of bone resorption (34); DCR-deficient mice represent a unique tool with which to directly address this idea.
Acknowledgments
We thank P. E. Cazer, S. Berryhill, W. Webb, C. Wicker III, E. Hogan, T. Chambers, and J. Crawford for technical assistance and R. L. Jilka, T. Bellido, and J. J. Goellner for helpful discussions.
Footnotes
This work was supported by an institutional award from the University of Parma (to C.G.), University of Arkansas for Medical Sciences Tobacco Settlement funds, National Institutes of Health Grants R01 AR049794 (to C.A.O.), R01 DK074993 (to J.W.P.), and P01 AG13918 (to S.C.M.), and a Veterans Affairs Merit Review (to S.C.M.).
Disclosure Statement: The authors of this manuscript have nothing to declare.
First Published Online October 11, 2007
Abbreviations: BMD, Bone mineral density; ChIP, chromatin immunoprecipitation; DCR, distal control region; DPD, deoxypyridinoline; M-CSF, macrophage colony-stimulating factor; 1,25(OH)2D3, 1,25-dihydroxyvitamin D3; OPG, osteoprotegerin; RANKL, receptor activator of nuclear factor-κB ligand; TRAP, tartrate-resistant acid phosphatase; VDR, vitamin D receptor; VDRE, vitamin D-responsive element.
References
- Parfitt AM 2005 Modeling and remodeling: how bone cells work together. In: Feldman D, Pike JW, Glorieux FH, eds. Vitamin D. 2nd ed. San Diego: Academic Press Inc.; 497–513 [Google Scholar]
- Langdahl BL, Mortensen L, Vesterby A, Eriksen EF, Charles P 1996 Bone histomorphometry in hypoparathyroid patients treated with vitamin D. Bone 18:103–108 [DOI] [PubMed] [Google Scholar]
- Miao DS, He B, Lanske B, Bai XY, Tong XK, Hendy GN, Goltzman D, Karaplis AC 2004 Skeletal abnormalities in Pth-null mice are influenced by dietary calcium. Endocrinology 145:2046–2053 [DOI] [PubMed] [Google Scholar]
- Ueno Y, Shinki T, Nagai Y, Murayama H, Fujii K, Suda T 2003 In vivo administration of 1,25-dihydroxyvitamin D-3 suppresses the expression of RANKL mRNA in bone of thyroparathyroidectomized rats constantly infused with PTH. J Cell Biochem 90:267–277 [DOI] [PubMed] [Google Scholar]
- Parfitt AM 2003 Renal bone disease: a new conceptual framework for the interpretation of bone histomorphometry. Curr Opin Nephrol Hypertens 12:387–403 [DOI] [PubMed] [Google Scholar]
- Ma YL, Cain RL, Halladay DL, Yang X, Zeng Q, Miles RR, Chandrasekhar S, Martin TJ, Onyia JE 2001 Catabolic effects of continuous human PTH (1–38) in vivo is associated with sustained stimulation of RANKL and inhibition of osteoprotegerin and gene-associated bone formation. Endocrinology 142:4047–4054 [DOI] [PubMed] [Google Scholar]
- Lee SK, Lorenzo JA 1999 Parathyroid hormone stimulates TRANCE and inhibits osteoprotegerin messenger ribonucleic acid expression in murine bone marrow cultures: correlation with osteoclast-like cell formation. Endocrinology 140:3552–3561 [DOI] [PubMed] [Google Scholar]
- Fu Q, Jilka RL, Manolagas SC, O’Brien CA 2002 Parathyroid hormone stimulates receptor activator of NFκ B ligand and inhibits osteoprotegerin expression via protein kinase A activation of cAMP-response element-binding protein. J Biol Chem 277:48868–48875 [DOI] [PubMed] [Google Scholar]
- Clowes JA, Riggs BL, Khosla S 2005 The role of the immune system in the pathophysiology of osteoporosis. Immunol Rev 208:207–227 [DOI] [PubMed] [Google Scholar]
- Fraher LJ, Avram R, Watson PH, Hendy GN, Henderson JE, Chong KL, Goltzman D, Morley P, Willick GE, Whitfield JF, Hodsman AB 1999 Comparison of the biochemical responses to human parathyroid hormone-(1–31)NH2 and hPTH-(1–34) in healthy humans. J Clin Endocrinol Metab 84:2739–2743 [DOI] [PubMed] [Google Scholar]
- Horiuchi N, Suda T, Takahashi H, Shimazawa E, Ogata E 1977 In vivo evidence for the intermediary role of 3′,5′-cyclic AMP in parathyroid hormone-induced stimulation of 1α,25-dihydroxyvitamin D3 synthesis in rats. Endocrinology 101:969–974 [DOI] [PubMed] [Google Scholar]
- Shen V, Birchman R, Xu R, Lindsay R, Dempster DW 1995 Short-term changes in histomorphometric and biochemical turnover markers and bone mineral density in estrogen-and/or dietary calcium-deficient rats. Bone 16:149–156 [PubMed] [Google Scholar]
- Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S, Tomoyasu A, Yano K, Goto M, Murakami A, Tsuda E, Morinaga T, Higashio, Udagawa N, Takahashi N, Suda T 1998 Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci USA 95:3597–3602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori K, Kitazawa R, Kondo T, Maeda S, Yamaguchi A, Kitazawa S 2006 Modulation of mouse RANKL gene expression by Runx2 and PKA pathway. J Cell Biochem 98:1629–1644 [DOI] [PubMed] [Google Scholar]
- Kitazawa R, Kitazawa S 2002 Vitamin D-3 augments osteoclastogenesis via vitamin D-responsive element of mouse RANKL gene promoter. Biochem Biophys Res Commun 290:650–655 [DOI] [PubMed] [Google Scholar]
- Kitazawa S, Kajimoto K, Kondo T, Kitazawa R 2003 Vitamin D-3 supports osteoclastogenesis via functional vitamin D response element of human RANKL gene promoter. J Cell Biochem 89:771–777 [DOI] [PubMed] [Google Scholar]
- O’Brien CA, Kern B, Gubrij I, Karsenty G, Manolagas SC 2002 Cbfa1 does not regulate RANKL gene activity in stromal/osteoblastic cells. Bone 30:453–462 [DOI] [PubMed] [Google Scholar]
- Fan X, Roy EM, Murphy TC, Nanes MS, Kim ST, Pike JW, Rubin J 2004 Regulation of RANKL promoter activity is associated with histone remodeling in murine bone stromal cells. J Cell Biochem 93:807–818 [DOI] [PubMed] [Google Scholar]
- Kabe Y, Yamada J, Uga H, Yamaguchi Y, Wada T, Handa H 2005 NF-Y is essential for the recruitment of RNA polymerase II and inducible transcription of several CCAAT box-containing genes. Mol Cell Biol 25:512–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Q, Manolagas SC, O’Brien CA 2006 Parathyroid hormone controls receptor activator of NF-κB ligand gene expression via a distant transcriptional enhancer. Mol Cell Biol 26:6453–6468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Yamazaki M, Zella LA, Shevde NK, Pike JW 2006 Activation of receptor activator of NF-κB ligand gene expression by 1,25-dihydroxyvitamin D3 is mediated through multiple long-range enhancers. Mol Cell Biol 26:6469–6486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Gregorio GB, Yamamoto M, Ali AA, Abe E, Roberson P, Manolagas SC, Jilka RL 2001 Attenuation of the self-renewal of transit-amplifying osteoblast progenitors in the murine bone marrow by 17β-estradiol. J Clin Invest 107:803–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien CA, Jia D, Plotkin LI, Bellido T, Powers CC, Stewart SA, Manolagas SC, Weinstein RS 2004 Glucocorticoids act directly on osteoblasts and osteocytes to induce their apoptosis and reduce bone formation and strength. Endocrinology 145:1835–1841 [DOI] [PubMed] [Google Scholar]
- Zella LA, Kim S, Shevde NK, Pike JW 2006 Enhancers located within two introns of the vitamin D receptor gene mediate transcriptional autoregulation by 1,25-dihydroxyvitamin D3. Mol Endocrinol 20:1231–1247 [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD 2001 Analysis of relative gene expression data using real-time quantitative PCR and the 2[-ΔΔ C(T)] method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
- O’Brien CA, Jilka RL, Fu Q, Stewart S, Weinstein RS, Manolagas SC 2005 IL-6 is not required for parathyroid hormone stimulation of RANKL expression, osteoclast formation, and bone loss in mice. Am J Physiol Endocrinol Metab 289:E784–E793 [DOI] [PubMed] [Google Scholar]
- Weinstein RS, Jilka RL, Parfitt AM, Manolagas SC 1998 Inhibition of osteoblastogenesis and promotion of apoptosis of osteoblasts and osteocytes by glucocorticoids. Potential mechanisms of their deleterious effects on bone. J Clin Invest 102:274–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR 1987 Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res 2:595–610 [DOI] [PubMed] [Google Scholar]
- Miao D, Li J, Xue Y, Su H, Karaplis AC, Goltzman D 2004 Parathyroid hormone-related peptide is required for increased trabecular bone volume in parathyroid hormone-null mice. Endocrinology 145:3554–3562 [DOI] [PubMed] [Google Scholar]
- Amling M, Priemel M, Holzmann T, Chapin K, Rueger JM, Baron R, Demay MB 1999 Rescue of the skeletal phenotype of vitamin D receptor-ablated mice in the setting of normal mineral ion homeostasis: formal histomorphometric and biomechanical analyses. Endocrinology 140:4982–4987 [DOI] [PubMed] [Google Scholar]
- Bonewald LF, Mundy GR 1990 Role of transforming growth factor-beta in bone remodeling. Clin Orthop Relat Res 250:261–276 [PubMed] [Google Scholar]
- Manolagas SC, Jilka RL, Bellido T, O’Brien CA, Parfitt AM 1996 Interleukin-6-type cytokines and their receptors. In: Bilezikian JP, Raisz LG, Rodan GA, eds. Principles of bone biology. San Diego: Academic Press, Inc.; 701–713 [Google Scholar]
- Mori K, Le GB, Berreur M, Riet A, Moreau A, Blanchard F, Chevalier C, Guisle-Marsollier I, Leger J, Guicheux J, Masson M, Gouin F, Redini F, Heymann D 2007 Human osteosarcoma cells express functional receptor activator of nuclear factor-κB. J Pathol 211:555–562 [DOI] [PubMed] [Google Scholar]
- Martin TJ, Quinn JM, Gillespie MT, Ng KW, Karsdal MA, Sims NA 2006 Mechanisms involved in skeletal anabolic therapies. Ann NY Acad Sci 1068:458–470 [DOI] [PubMed] [Google Scholar]
- Kong YY, Feige U, Sarosi I, Bolon B, Tafuri A, Morony S, Capparelli C, Li J, Elliott R, McCabe S, Wong T, Campagnuolo G, Moran E, Bogoch ER, Van G, Nguyen LT, Ohashi PS, Lacey DL, Fish E, Boyle WJ, Penninger JM 1999 Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature 402:304–309 [DOI] [PubMed] [Google Scholar]
- Li Y, Toraldo G, Li A, Yang X, Zhang H, Qian WP, Weitzmann MN 2007 B cells and T cells are critical for the preservation of bone homeostasis and attainment of peak bone mass in vivo. Blood 109:3839–3848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanHouten JN, Dann P, Stewart AF, Watson CJ, Pollak M, Karaplis AC, Wysolmerski JJ 2003 Mammary-specific deletion of parathyroid hormone-related protein preserves bone mass during lactation. J Clin Invest 112:1429–1436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanematsu M, Yoshimura K, Takaoki M, Sato A 2002 Vector-averaged gravity regulates gene expression of receptor activator of NF-κB (RANK) ligand and osteoprotegerin in bone marrow stromal cells via cyclic AMP/protein kinase A pathway. Bone 30:553–558 [DOI] [PubMed] [Google Scholar]
- Kim S, Yamazaki M, Shevde NK, Pike JW 2007 Transcriptional control of receptor activator of nuclear factor-κB ligand by the protein kinase A activator forskolin and the transmembrane glycoprotein 130-activating cytokine, oncostatin M, is exerted through multiple distal enhancers. Mol Endocrinol 21:197–214 [DOI] [PubMed] [Google Scholar]