Abstract
Both the disproportionate loss of adipose tissue in the case of lipodystrophies and the disproportionate gain of adipose tissue in obesity are frequently associated with an increase in insulin resistance and its complications. Leptin replacement is a very promising therapeutic approach for the management of the complications of lipodystrophy. In contrast, leptin treatment for the reversal of obesity-related metabolic disorders has not proven to be successful. There is a need to better understand both of these phenomena. Mouse models of lipodystrophy may provide us with new pharmaceutical targets for the treatment and prevention of metabolic disturbances related to dysfunctional adipose tissue both in the context of lipodystrophy and obesity.
Etiology of Lipodystrophy in the Clinic
Lipodystrophy is characterized by abnormal or degenerative conditions of the adipose tissue and can be partial/localized or generalized. The term lipodystrophy comprises a heterogenous group of disorders that may broadly be classified into genetic or acquired types, but the detailed pathophysiology of most human lipodystrophies remains unknown. The genetic forms of lipodystrophy are very rare and include congenital generalized lipodystrophy (the Berardinelli-Seip syndrome) and several types of familial partial lipodystrophy (the Dunnigan type, the Köbberling type, the mandibuloacral dysplasia type). The acquired forms of lipodystrophy include acquired generalized lipodystrophy (the Lawrence syndrome), acquired partial lipodystrophy (the Barraquer-Simons syndrome), and lipodystrophy induced by protease inhibitors used to treat HIV. HIV-related lipodystrophies are is the most common form of lipodystrophy affecting up to 50 % of the HIV patients. (see [1] for review)
Adipose Tissue and Metabolic Disease
Why do we need lipodystrophy models?
The loss of adipose tissue in lipodystrophy, irrespective of the underlying etiology, is associated with increased prevalence of insulin resistance and its complications, e.g. dyslipidemia, hypertension, hepatic steatosis and increased predisposition to atherosclerosis. Thus, lipodystrophy is paradoxically associated with similar metabolic disturbances as obesity (Table 1). This highlights the central role for adipose tissue as an essential organ for proper metabolic regulation (see [2] for review). In particular, hepatic dysfunction driven by increased ectopic lipid disposition is an immediate reflection of dysfunctional adipose tissue.
Table 1. Comparison of obesity and lipodystrophy.
Lipodystrophy (LD) and obesity are associated with similar metabolic complications. The reasons for this may be altered levels of adipokines and reduced capacity to buffer circulating lipids. Both in obesity and lipodystrophy it is apparent that the ability of dynamic regulation of metabolism is lost i.e. the ability to respond adequately to altered metabolic demands is blunted.
| LD | Obesity | |
|---|---|---|
| Fat mass | ⇓ | ⇑ |
| Leptin | ⇓ | ⇑ |
| Adiponectin | ⇓ | ⇓ |
| Infl. Cytokines | ⇑ ⇓? | ⇑ |
| Met. Syndrome | ⇑ | ⇑ |
(Infl. = Inflammatory, Met =Metabolic)
Unless the specific gene defect causing a lipodystrophy is known, a rational therapeutic approach to handle the complications of lipodystrophy is by treating it as an endocrine disorder, i.e. to replace the dysfunctional remnant fat with the missing adipocyte-derived factors, commonly referred to as adipokine(s). Indeed, leptin replacement therapy improves both insulin sensitivity and lipid metabolism in patients with generalized lipodystrophy [3,4]. However, although leptin replacement is very promising for the treatment of lipodystrophy, adipose tissue secretes many other factors that are essential for proper regulation of metabolism. In addition, mouse models of lipodystrophy crossed into the ob/ob mice lacking functional leptin display an even worse metabolic phenotype than ob/ob mice allowed to expand their adipose tissue, suggesting that additional fad-derived factors play a role in the process [17,30].
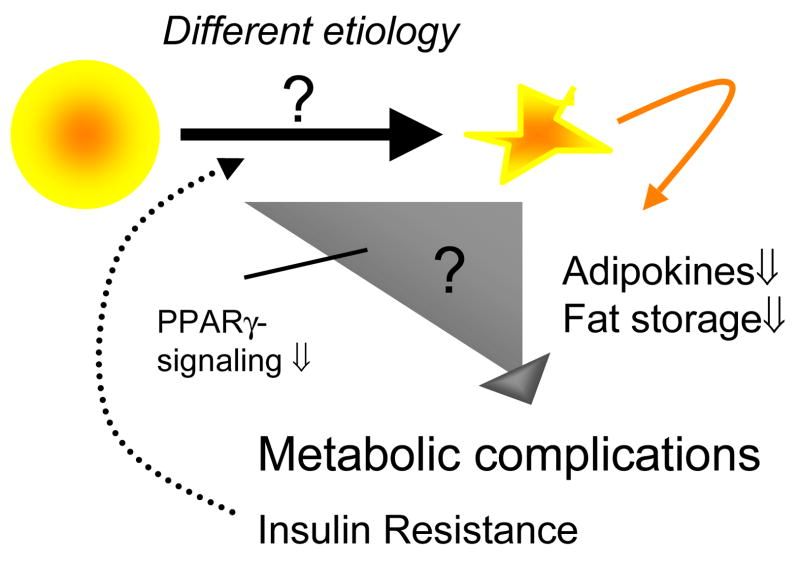
Dysfunctional adipose tissue may participate in metabolic disease in two ways; 1) diminished capacity to store triglycerides causing increased levels of circulating lipids and fat storage in non-fat tissues and 2) abnormal secretion of adipokines and hormones. These two pathways may well be connected but may also exert distinct effects (Figure 1). However, since they often occur simultaneously it is difficult to distinguish the most important players for the pathogenesis of lipodystrophy- or obesity-related metabolic disease in humans. In contrast, a well-controlled lipodystrophy model in the form of a genetically modified mouse can provide us with new perspectives. Thus, models of lipodystrophy are not only of importance towards the unraveling the mechanism behind impaired accumulation of adipose tissue, but may also reveal novel pharmaceutical targets for the treatment and prevention of metabolic dysfunction related to excess adipose tissue. Here, we will focus primarily on the latter subject and provide an overview of the currently available murine lipodystrophy models.
Figure 1. Metabolic Complications in Lipodystrophy.
The underlying causes of lipodystrophy can be of many different origins. However, the more severe the liposdystrophy, the more severe are the metabolic complications. These complications may occur both as a consequence of dysfunctional adipocytes. The ensuing insulin resistance further aggravates the lipodystrophy phenotype.
Mouse models of lipodystrophy
Mouse models of lipodystrophy can be divided into non-inducible (constitutive, early or late onset) or inducible models.
Early onset models
aP2-SREBP-1c mouse
The transgenic aP2-SREBP-1c mouse [5] expresses a constitutively active form of the SREBP-1c transcription factor in the adipose tissue. This mouse model has markedly reduced body fat and develops liver steatosis, organomegaly, profound insulin resistance, hyperglycemia and increased levels of triglycerides. Leptin treatment corrects the metabolic abnormalities in these mice [6,7].
PPARγ deficient mice rescued from embryonic lethality
Global deletion of PPARγ is lethal, but the Mox2-Cre-floxed PPARy (MORE-PG) knockout preserves PPARγ expression in the trophoblasts and is therefore somewhat viable (10 % reach adulthood) [8]. These mice display severely decreased levels of body fat, organomegaly, decreased plasma leptin and adiponectin, elevated free fatty acids (FFA:s), decreased insulin sensitivity as judged by insulin tolerance tests. Interestingly though the females display increased glucose tolerance in a glucose tolerance test potentially mediated through constitutive hyperinsulinemia. In contrast to human lipodystrophy, these mice are hypotensive and do not have liver steatosis. As these mice also lack PPARγ in the liver, this phenotype is consistent with other data showing that liver-specific PPARγ deficiency exerts potent protective effects [9,10].
Adipose-specific PPARγ knockout mice
Two similar adipose-specific PPARγ knockout mice, both using floxed PPARγ knock-in mice crossed with transgenic aP2-Cre mice, have been published with slightly different phenotypes. He and colleagues report that these mice exhibit markedly decreased and highly inflamed white and brown adipose tissue at young age, have increased FFAs and triglycerides, have decreased leptin and adiponectin, develop liver steatosis, and are more sensitive to high fat diet (HFD)-induced decrease in insulin sensitivity [11]. On the other hand, Jones and colleagues report data from their mice, demonstrating that these mice [12] are protected against HFD-induced decrease in insulin sensitivity despite increased triglycerides levels, liver steatosis, reduced adiponectin and leptin levels. The differences between these rather similar models might be due to that different aP2-cre mice were used. Since the transgenic cassette takes advantage of the aP2 promoter, it is expected that slightly different versions of the aP2-Cre give rise to different phenotypes. The aP2 promoter is a direct PPARγ target, such that PPARγ inactivation during differentiation will reduce the levels of Cre. The degree and uniformity of PPARγ inactivation may therefore be influenced by many different factors, such as strain, sex and nutritional state of the animals, hence giving rise to potentially distinct phenotypes.
PPARγ-2 knockout mice
In contrast to the early embryonic lethality of the global PPARγ knockouts, PPARγ-2 knockout mice [13] are viable, but have reduced fat mass and reduced circulating leptin and adiponectin levels. They have however normal lipid levels and no signs of liver steatosis. Males, but not females, are severely resistant to the glucose-lowering effect of exogenous insulin. However, the insulin resistance in these males could be reversed by PPARγ agonist treatment.
PPARy Hypomorphic mice
PPARγ hypomorphic mice were generated by targeting a PPARγ-2 specific exon, these mice also displayed decreased levels of PPARγ-1 [14]. About 40 % of these mice die before the age of 5 weeks. Mice that survive have very little adipose tissue throughout life. Circulating triglyceride levels are reduced and the liver appear normal but both heart and muscle have increased fat content. The levels of adiponectin and leptin were reduced and the mice display relatively mild form of insulin resistance. PPARγ agonist treatment reversed glucose intolerance, but not insulin resistance. The relatively modest degree of insulin resistance was explained on the basis of a compensatory up-regulation of β-oxidation in muscle.
PPARγ P465L mice
Human patients with the dominant negative P465L mutation in the PPARγ gene suffer from partial lipodystrophy, severe insulin resistance, impaired lipid metabolism, liver steatosis and hypertension [15]. In contrast, the mouse model with the same mutation has normal fat mass and insulin sensitivity, but impaired postprandial lipid clearance and altered fat depot distribution [16]. This indicates a distinct species difference between human and mouse. However, when the P465L mutation is expressed in an ob/ob background, the phenotype is much more similar to the metabolic state in humans with this mutation. The PPARγ P465L ob/ob mice have increased triglyceride levels, decreased adiponectin levels and develop more severe insulin resistance and liver steatosis, but less lipid deposition in muscle compared to the ob/ob control mice [17]. Gray and colleagues suggest that PPARγ facilitates the expansion of adipose tissue and thereby protects peripheral tissues from toxic lipid intermediates, and thus improving insulin sensitivity. However, a synergistic effect between PPARγ inactivation and hypoleptinemia causing the severe insulin resistance in the P465L ob/ob mice cannot be ruled out [17].
Fatty liver dystrophy (fld) mice
The fatty liver dystrophy (fld) mouse results from a spontaneous mutation in the lipin1 gene [18] and is characterized by neonatal fatty liver and hypertriglyceridemia that resolves at weaning, and neuropathy affecting peripheral nerves in adulthood. Males are infertile and females generate fewer litters than wild type mice. Moreover, these mice have impaired adipose tissue development with immature adipocytes, have reduced white and brown fat, have decreased leptin and adiponectin levels, are insulin resistant and have increased susceptibility to diet-induced atherosclerosis [19,20]. The absence of liver steatosis in the adult fld mouse may be explained by a compensatory increase in energy expenditure and fat oxidation [21].
A-ZIP/F-1 mice
The A-ZIP/F-1 mice [22] express a dominant negative version of the C/EBPα leucine zipper domain that potently interferes with adipocyte differentiation. This mouse model has essentially no white adipose tissue, reduced levels of brown fat and a very severe metabolic phenotype with reduced life span. These mice display massive hepatomegaly causing increased body weight, have liver steatosis, severe diabetes (hyperglycemia, hyperinsulinemia, hyperphagic, polydipsia, polyuria), are hypertensive [23], have increased triglycerides and FFAs levels, have alveolar foam cells, have reduced leptin levels and are unable to respond to sustain glucose levels during fasting. The insulin resistance and much of the liver steatosis in the A-ZIP/F-1 mice can be reversed by transgenic over-expression of leptin [3] or by transplanting normal adipose tissue [24]. In contrast, transplantation of adipose tissue from ob/ob mice did not reverse the phenotype of the A-ZIP/F-1 mice indicating that leptin deficiency strongly contributes to the metabolic complications in lipodystrophy [25]. Furthermore, while PPARγ agonists lower lipid levels in these mice, they also cause increased hepatic steatosis and remain ineffective with respect to lowering of glucose levels.
IRS1/IRS3 double knockout mice
The IRS1/IRS3 double knockout mice [26] have a significant growth retardation, develop early onset lipoatrophy and diabetes. The mice have decreased leptin and adiponectin levels and decreased triglyceride levels and have no signs of lipid accumulation in liver or muscle. The hyperglycemia and hyperinsulinemia can be reversed by leptin treatment, which underlines the important role that leptin plays for insulin sensitivity.
Late onset models
aP2-diphteria toxin A mice
aP2-diphteria toxin A mice [27] are born normally and initially lack any distinguished phenotypic features, but develop atrophy and necrosis of the adipose tissue at 5–6 month resulting in the complete absence of subcutaneous or intra abdominal adipose tissue at 8–9 month of age. This late onset of adipose tissue loss is associated with reduced leptin levels, increased food consumption, hyperlipidemia, hyperglycemia and insulin resistance. Initial observations suggested that many metabolic abnormalities (with the exception of reduced leptin levels and hyperphagia) could be reversed by PPARγ agonist treatment [28], an observation that is not fully consistent with observations in other mouse models.
Ribosomal S6 Kinase 2 null mice
The Ribosomal S6 Kinase 2 knockout mouse was first generated to study the Coffin-Lowry syndrome (CLS), which is characterized by mental retardation and short stature [29]. However, besides having impaired brain function and decreased body length, these mice have a partial but specific loss of adipose tissue, have decreased leptin levels, are insulin resistant and have a tendency towards hepatic steatosis. The mice are also resistant to high fat diet-induced weight gain. The size of the brown fat and all other non-adipose tissues were normal. The relative loss of adipose tissue becomes more prominent with age in these mice. It is not clear what the specific mechanistic role of ribosomal S6 kinase 2 is that leads to such a rather dramatic adipose tissue phenotype.
Inducible models
FAT-ATTAC mice
The ‘fat apoptosis through targeted activation of caspase 8′ (‘FAT-ATTAC’) mouse [30] is a transgenic mouse in which white and brown fat can be ablated within 1–2 weeks of treatment with a chemical dimerizer that forces the dimerization of a transgenically expressed caspase-8 fusion protein expressed specifically in adipocytes, thereby inducing apoptosis. Short-term treatment causes disruption of local adipose tissue structures and infiltration of macrophages and long-term treatment results in apoptosis of a majority of the adipocytes, marked reduction of adipokines, hyperphagia and glucose intolerance. However, the long-term treated mouse displays no difference in serum insulin, glucose, triglycerides, FFAs or liver triglycerides. The phenotype is reversible although glucose intolerance and reduced adiponectin levels remain 6 weeks post treatment. The fatless FAT-ATTAC mouse has reduced levels of IL-6 at baseline and reduced inflammatory response to endotoxin indicating that adipocytes directly or indirectly contribute to systemic levels of inflammatory cytokines.
The FAT-ATTAC mouse was crossed and compared with the ob/ob mouse to reveal whether the phenotype of FAT-ATTAC depends mostly on leptin [30]. After 10 weeks of dimerizer treatment, the FAT-ATTAC ob/ob mouse had nearly no visible fat, but displayed increased food intake and exacerbated glucose and lipid metabolism, including increased liver steatosis compared to littermate ob/ob mice. The adiponectin and resistin levels were markedly reduced while MCP-1 levels were increased, suggesting that cells other than adipocytes can produce significant levels of MCP-1. The FAT-ATTAC ob/ob mice had also decreased glucose-stimulated insulin secretion although there was no change of the pancreatic islet area or morphology. This indicates that the adipocytes are important for maximal glucose-stimulated insulin secretion and that hypoleptinemia is not the only factor of importance for metabolic disease in lipodystrophy.
Protease inhibitor-induced lipodystrophy
The mechanism behind protease inhibitor treatment and the pathogenesis of human lipodystrophy is not understood and only a limited number of in vivo studies in mice have been performed. To date, it has been shown that long-term treatment of mice with ritonavir reproduces the clinical features of protease inhibitor-induced lipodystrophy in HIV infected patients. Human patients that are treated with ritonavir may develop severe hypercholesterolemia and hypertriglyceridemia, surprisingly without much of an effect on glucose metabolism [31]. The treatment with equivalent doses in mice causes hypercholesterolemia, hypertriglyceridemia, liver steatosis, reduced leptin and adiponectin levels and reduction of the inguinal fat pat. Similar to the clinical studies, no impact on carbohydrate metabolism was observed [32–35]. The underlying mechanism behind the effect of ritonavir in mice seems to involve increased levels of SREBP-1c in adipose tissue and liver [33–35]. Leptin as well as adiponectin replacement treatment are able to reduce the ritonavir-induced lipid abnormalities [32,34]. Indinavir, another protease inhibitor, is known to relatively rapidly induce insulin resistance in human patients as well as in rats [36,37]. In vitro experiments show that Indinavir inhibits GLUT-4, which may be a common mechanism for protease inhibitor-induced insulin resistance although conflicting data exist in the literature [38,39].
Model Comparison
In general, the inducible mouse models make it possible to characterize the role of adipocytes and their secreted factors in health and disease without the confounding effects of secondary pathological changes of adipose tissue loss, such as long-term ectopic lipid accumulation in liver and muscle and changes in many other tissues due to insulin resistance. The study of the acute loss of adipocytes provides better mechanistic insights into the role of the adipocyte in maintaining a healthy metabolic phenotype. Earlier constitutive loss of adipocytes during development seems to have much more profound consequences on systemic energy homeostasis compared to a late onset loss, underlining the important role that adipose tissue plays during the early postnatal period. More refined time courses of fat loss are needed during development to determine the critical window during which metabolic pathways become hardwired under the influence of adipose tissue, both centrally in the brain as well as in other peripheral tissues under the influence of adipocytes (Table 2).
Table 2. Comparison Summary Table.
The murine models are divided into early-onset, late-onset and inducible models based on their lipodystrophy characteristics.
| Murine models | Early onset | Late onset | Inducible |
|---|---|---|---|
| Pros | Similar to congenital generalized lipodystrophy. | Somewhat easier to follow the pathogenesis time line. Similar to mild forms of lipodystrophy. | No risk of uncontrolled adaptations early in life. Easier to distinguish the time course of the disease. No secondary effects due to e.g. long-term insulin resistance. |
| Cons | The severe phenotype and the early onset makes it difficult to distinguish primary from secondary effects. Adaptations not relevant for the human disease progression may occur. | Adaptations not relevant for the human disease progression may occur. The onset of disease may depend on environmental factors that are hard to control for. | The abrupt onset does nor have a clinical counterpart. |
| Best use of model | Study whether the severe phenotype can be reversed. Compare with healthy lean models. What makes the healthy mice healthy? | Study disease progression. What markers may be of importance for pathogenesis of metabolic disease. | Study the acute effect of no or decreased adipose tissue on different organ systems. Distinguish what apects of the metabolic syndrome are directly dependent on adipose tissue and what may be related to secondary effects. |
| How to get access to the model | Research collaboration. Some mice are commercially available. | Research collaboration. Some mice are commercially available. | Research collaboration. |
Model Translation to Humans
It remains unclear how effectively these models replicate the etiology of human lipodystrophy, even though many common features are seen (Table 3). This relates particularly to the strong tendency towards ectopic deposition of lipids in the liver.
Table 3.
Features of the Murine models
| LD | Murine models | Ap2-SREBP-1c [5] | Adipo-PPARγ ko [11,12] | MORE-PG ko [8] | PPARy -2 ko [13] | PPARy hypo [14] | PPARy P465L [16] | Fld [18] | IRS1-IRS3 ko [26] | A-ZIP/F-1 [22] | RSK2 ko [29] | Ap2-dipthe ria toxin [27] | FAT ATTAC [30] | Ob/ob FAT ATTAC [30] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tg ↑ | yes | yes | yes | no | no6 | no | no | no6 | yes | yes | no | yes | ||
| Insulinresistance | yes | unclear | Yes1 | yes3 | yes | no | yes | yes | yes | yes | yes | no | yes | |
| Glucose ↑ | yes | no | Yes3 | no | no | no | no | yes | yes | yes | yes | no | yes | |
| Liver steatosis | yes | yes | no | no | no | no | no | yes | tend. | yes | no | yes | ||
| Hypertension | No4 | yes | yes | |||||||||||
| Food intake ↑ | Yes5 | no | no2 | yes | no2 | yes | yes | yes | ||||||
| EE ↑ | Yes5 | yes | yes | yes | ||||||||||
| Organomegaly | yes | yes | no | no | no | yes | yes | |||||||
| Risk of atherosclerosis ↑ | yes | |||||||||||||
| Leptin ↓ | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes | n/a | |||
| Adiponectin ↓ | yes | yes | yes | yes | yes | yes | yes | |||||||
Tg = Triglycerides, EE = Energy Expenditure, ko = knock-out, tend. = tendency.
= Insulin tolerance test show insulin resistance in both gender, while females have improved glucose tolerance,
= Yes, if related to body weight,
= In males,
= Hypotension,
= On high fat diet,
= Decreased
The MORE-PG mouse and the adipose-specific PPARγ knockout exhibit very complicated and unexpected phenotypes possibly making them less favorable for studies of metabolic disorders caused by lipodystrophy. On the other hand, Gray et al hypothesize that genetic manipulations of PPARγ in mice induce biochemical defects similar to those in humans but that they may only become physiologically relevant in mice challenged with an extreme positive energy balance [17]. Thus, crossing these mice (and other lipodystrophic mouse models) with the severely obese ob/ob mouse may further reveal additional phenotypes in these lipodystrophy models. Frequently, more distinct phenotypes can already be seen in these mice when challenged with a high fat diet (HFD), upon exposure to low temperature, during exercise or food deprivation.
The aP2-diphteria toxin A and the Ribosomal S6 Kinase 2 knockout mouse models represent mild lipodystrophic phenotypes and complement the more severe models very well. However, the power of these models is somewhat diminished in the Ribosomal S6 Kinase 2 knockout mouse as these mice also display other abnormalities. It is surprising that the aP2-diphteria toxin A mice take a long time to develop lipodystrophy despite the constitutive presence of the toxin in adipocytes. These adipocytes are exposed to a constitutive necrotic stimulus and may therefore induce a potent anti-necrotic program that enables them to survive for an extensive amount of time.
The A-ZIP/F-1 mice may be useful for pharmacological studies aiming to reverse the diabetic phenotype. However, due to its severe phenotype, it is difficult to separate primary from secondary effects. The potential of obtaining new insights into the direct involvement of adipocytes in the pathogenesis of the metabolic syndrome, infectious disease and cancer is limited. The lack of a “lipid phenotype” in the adult Fld, the PPARγ-2, PPARγ hypomorphic, the IRS1 IRS3 double knockout and the FAT-ATTAC mice make these models suitable for studying metabolic disease without the complications of lipotoxicity, although the severe growth retardation of the IRS1/IRS3 double knockout mice might make these mice less suitable.
As pointed out earlier the inducible FAT-ATTAC model makes it possible to study direct effect of adipose tissue on metabolic regulation without interference from secondary pathological processes. Since precursor cells are not eliminated with this method (the transgene is only expressed in mature adipocytes), the model is reversible and regenerates adipose tissue upon cessation of dimerizer treatment. This reversibility of this mouse model provides further advantages for the study of adipogenesis in an adult animal.
Studies of the metabolic consequences of ritonavir treatment show that protease inhibitor-induced lipodystrophy as seen in the clinic can be reproduced in mice. This indicates that the mouse can be a suitable model under some circumstances for the screening of different protease inhibitors in the context of a negative impact of these inhibitors on metabolism as well as investigating some aspects of the pathogenesis of lipodystrophy in general. However, rodents metabolize drugs differently than humans. Therefore, it may be difficult to distinguish unspecific toxic effects from more direct effects leading to lipodystrophy. Moreover, the mechanism behind the association between highly active antiretroviral treatment (HAART) and lipodystrophy is very complicated. The HIV infected patients are usually treated with a cocktail of several different drugs at the same time and the outcome in different individuals is likely to be affected by their genetic make-up, age and possible environmental risk factors (e.g. smoking and diet). Thus, although it is of interest to elucidate the mechanistic connection between HAART and lipodystrophy, it is even more important to learn how to modify and improve the present HIV treatment strategy to avoid metabolic complications in these patients. More recently developed protease inhibitors are indeed associated with a reduced impact on adipose tissue distribution.
Conclusions
The metabolic syndrome is often associated with either too much or too little fat. Dysfunctional adipose tissue is therefore at the very core of metabolic dysfunction. Mouse models of lipodystrophy approach this issue in similar ways that other mouse models with an excess of adipose tissue contribute to a better understanding of the specific contributions of healthy adipose tissue towards energy homeostasis. As such, these murine lipodystrophy models have assumed an important place in our arsenal of tools that we can employ to unravel the still vastly unknown mechanisms of metabolic dysregulation prevalent in vast portions of developed countries and increasingly in the third world. Further work is needed to improve these models to provide more accurate and effective ablation of adipocytes, particularly related to the ability to reversibly block adipogenesis in utero and in the early postnatal period.
Acknowledgments
The authors were supported by NIH grants R01-DK55758, R24-DK071030-01, R01-CA112023 and R21DK075887 (PES). IWA was also supported with a fellowship from the Throne-Holst Foundation and the Swedish Research Council (2006-3931), and NH is supported by a stipend from the Faculty of Health Science of the University of Copenhagen.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hegele RA, et al. Thematic review series: Adipocyte Biology. Lipodystrophies: windows on adipose biology and metabolism. J Lipid Res. 2007;48 (7):1433–1444. doi: 10.1194/jlr.R700004-JLR200. [DOI] [PubMed] [Google Scholar]
- 2.Garg A. Adipose tissue dysfunction in obesity and lipodystrophy. Clin Cornerstone. 2006;8(Suppl 4):S7–S13. doi: 10.1016/s1098-3597(06)80039-6. [DOI] [PubMed] [Google Scholar]
- 3.Ebihara K, et al. Efficacy and safety of leptin-replacement therapy and possible mechanisms of leptin actions in patients with generalized lipodystrophy. J Clin Endocrinol Metab. 2007;92 (2):532–541. doi: 10.1210/jc.2006-1546. [DOI] [PubMed] [Google Scholar]
- 4.Javor ED, et al. Long-term efficacy of leptin replacement in patients with generalized lipodystrophy. Diabetes. 2005;54 (7):1994–2002. doi: 10.2337/diabetes.54.7.1994. [DOI] [PubMed] [Google Scholar]
- 5.Shimomura I, et al. Insulin resistance and diabetes mellitus in transgenic mice expressing nuclear SREBP-1c in adipose tissue: model for congenital generalized lipodystrophy. Genes Dev. 1998;12 (20):3182–3194. doi: 10.1101/gad.12.20.3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Asilmaz E, et al. Site and mechanism of leptin action in a rodent form of congenital lipodystrophy. J Clin Invest. 2004;113 (3):414–424. doi: 10.1172/JCI19511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shimomura I, et al. Leptin reverses insulin resistance and diabetes mellitus in mice with congenital lipodystrophy. Nature. 1999;401 (6748):73–76. doi: 10.1038/43448. [DOI] [PubMed] [Google Scholar]
- 8.Duan SZ, et al. Hypotension, lipodystrophy, and insulin resistance in generalized PPARgamma-deficient mice rescued from embryonic lethality. J Clin Invest. 2007;117 (3):812–822. doi: 10.1172/JCI28859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gavrilova O, et al. Liver peroxisome proliferator-activated receptor gamma contributes to hepatic steatosis, triglyceride clearance, and regulation of body fat mass. J Biol Chem. 2003;278 (36):34268–34276. doi: 10.1074/jbc.M300043200. [DOI] [PubMed] [Google Scholar]
- 10.Matsusue K, et al. Liver-specific disruption of PPARgamma in leptin-deficient mice improves fatty liver but aggravates diabetic phenotypes. J Clin Invest. 2003;111 (5):737–747. doi: 10.1172/JCI17223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He W, et al. Adipose-specific peroxisome proliferator-activated receptor gamma knockout causes insulin resistance in fat and liver but not in muscle. Proc Natl Acad Sci U S A. 2003;100 (26):15712–15717. doi: 10.1073/pnas.2536828100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones JR, et al. Deletion of PPARgamma in adipose tissues of mice protects against high fat diet-induced obesity and insulin resistance. Proc Natl Acad Sci U S A. 2005;102 (17):6207–6212. doi: 10.1073/pnas.0306743102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang J, et al. Selective disruption of PPARgamma 2 impairs the development of adipose tissue and insulin sensitivity. Proc Natl Acad Sci U S A. 2004;101 (29):10703–10708. doi: 10.1073/pnas.0403652101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koutnikova H, et al. Compensation by the muscle limits the metabolic consequences of lipodystrophy in PPAR gamma hypomorphic mice. Proc Natl Acad Sci U S A. 2003;100 (24):14457–14462. doi: 10.1073/pnas.2336090100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barroso I, et al. Dominant negative mutations in human PPARgamma associated with severe insulin resistance, diabetes mellitus and hypertension. Nature. 1999;402 (6764):880–883. doi: 10.1038/47254. [DOI] [PubMed] [Google Scholar]
- 16.Tsai YS, et al. Hypertension and abnormal fat distribution but not insulin resistance in mice with P465L PPARgamma. J Clin Invest. 2004;114 (2):240–249. doi: 10.1172/JCI20964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gray SL, et al. Leptin deficiency unmasks the deleterious effects of impaired peroxisome proliferator-activated receptor gamma function (P465L PPARgamma) in mice. Diabetes. 2006;55 (10):2669–2677. doi: 10.2337/db06-0389. [DOI] [PubMed] [Google Scholar]
- 18.Peterfy M, et al. Lipodystrophy in the fld mouse results from mutation of a new gene encoding a nuclear protein, lipin. Nat Genet. 2001;27 (1):121–124. doi: 10.1038/83685. [DOI] [PubMed] [Google Scholar]
- 19.Phan J, et al. Lipin expression preceding peroxisome proliferator-activated receptor-gamma is critical for adipogenesis in vivo and in vitro. J Biol Chem. 2004;279 (28):29558–29564. doi: 10.1074/jbc.M403506200. [DOI] [PubMed] [Google Scholar]
- 20.Reue K, et al. Adipose tissue deficiency, glucose intolerance, and increased atherosclerosis result from mutation in the mouse fatty liver dystrophy (fld) gene. J Lipid Res. 2000;41 (7):1067–1076. [PubMed] [Google Scholar]
- 21.Phan J, Reue K. Lipin, a lipodystrophy and obesity gene. Cell Metab. 2005;1 (1):73–83. doi: 10.1016/j.cmet.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 22.Moitra J, et al. Life without white fat: a transgenic mouse. Genes Dev. 1998;12 (20):3168–3181. doi: 10.1101/gad.12.20.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takemori K, et al. Elevated blood pressure in transgenic lipoatrophic mice and altered vascular function. Hypertension. 2007;49 (2):365–372. doi: 10.1161/01.HYP.0000255576.16089.b9. [DOI] [PubMed] [Google Scholar]
- 24.Gavrilova O, et al. Surgical implantation of adipose tissue reverses diabetes in lipoatrophic mice. J Clin Invest. 2000;105 (3):271–278. doi: 10.1172/JCI7901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Colombo C, et al. Transplantation of adipose tissue lacking leptin is unable to reverse the metabolic abnormalities associated with lipoatrophy. Diabetes. 2002;51 (9):2727–2733. doi: 10.2337/diabetes.51.9.2727. [DOI] [PubMed] [Google Scholar]
- 26.Laustsen PG, et al. Lipoatrophic diabetes in Irs1(−/−)/Irs3(−/−) double knockout mice. Genes Dev. 2002;16 (24):3213–3222. doi: 10.1101/gad.1034802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ross SR, et al. Targeted expression of a toxin gene to adipose tissue: transgenic mice resistant to obesity. Genes Dev. 1993;7 (7B):1318–1324. doi: 10.1101/gad.7.7b.1318. [DOI] [PubMed] [Google Scholar]
- 28.Burant CF, et al. Troglitazone action is independent of adipose tissue. J Clin Invest. 1997;100 (11):2900–2908. doi: 10.1172/JCI119839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.El-Haschimi K, et al. Insulin resistance and lipodystrophy in mice lacking ribosomal S6 kinase 2. Diabetes. 2003;52 (6):1340–1346. doi: 10.2337/diabetes.52.6.1340. [DOI] [PubMed] [Google Scholar]
- 30.Pajvani UB, et al. Fat apoptosis through targeted activation of caspase 8: a new mouse model of inducible and reversible lipoatrophy. Nat Med. 2005;11 (7):797–803. doi: 10.1038/nm1262. [DOI] [PubMed] [Google Scholar]
- 31.Periard D, et al. Atherogenic dyslipidemia in HIV-infected individuals treated with protease inhibitors. The Swiss HIV Cohort Study. Circulation. 1999;100 (7):700–705. doi: 10.1161/01.cir.100.7.700. [DOI] [PubMed] [Google Scholar]
- 32.Xu A, et al. Adiponectin ameliorates dyslipidemia induced by the human immunodeficiency virus protease inhibitor ritonavir in mice. Endocrinology. 2004;145 (2):487–494. doi: 10.1210/en.2003-1140. [DOI] [PubMed] [Google Scholar]
- 33.Prot M, et al. Long-term treatment with lopinavir-ritonavir induces a reduction in peripheral adipose depots in mice. Antimicrob Agents Chemother. 2006;50 (12):3998–4004. doi: 10.1128/AAC.00625-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Riddle TM, et al. Leptin replacement therapy but not dietary polyunsaturated fatty acid alleviates HIV protease inhibitor-induced dyslipidemia and lipodystrophy in mice. J Acquir Immune Defic Syndr. 2003;33 (5):564–570. doi: 10.1097/00126334-200308150-00003. [DOI] [PubMed] [Google Scholar]
- 35.Riddle TM, et al. HIV protease inhibitor induces fatty acid and sterol biosynthesis in liver and adipose tissues due to the accumulation of activated sterol regulatory element-binding proteins in the nucleus. J Biol Chem. 2001;276 (40):37514–37519. doi: 10.1074/jbc.M104557200. [DOI] [PubMed] [Google Scholar]
- 36.Hruz PW, et al. Indinavir induces acute and reversible peripheral insulin resistance in rats. Diabetes. 2002;51 (4):937–942. doi: 10.2337/diabetes.51.4.937. [DOI] [PubMed] [Google Scholar]
- 37.Mulligan K, et al. Hyperlipidemia and insulin resistance are induced by protease inhibitors independent of changes in body composition in patients with HIV infection. J Acquir Immune Defic Syndr. 2000;23 (1):35–43. doi: 10.1097/00126334-200001010-00005. [DOI] [PubMed] [Google Scholar]
- 38.Dube MP, et al. Prospective, intensive study of metabolic changes associated with 48 weeks of amprenavir-based antiretroviral therapy. Clin Infect Dis. 2002;35 (4):475–481. doi: 10.1086/341489. [DOI] [PubMed] [Google Scholar]
- 39.Murata H, et al. The mechanism of insulin resistance caused by HIV protease inhibitor therapy. J Biol Chem. 2000;275 (27):20251–20254. doi: 10.1074/jbc.C000228200. [DOI] [PubMed] [Google Scholar]