Abstract
TLX is an orphan nuclear receptor that is expressed exclusively in vertebrate forebrains. Although TLX is known to be expressed in embryonic brains, the mechanism by which it influences neural development remains largely unknown. We show here that TLX is expressed specifically in periventricular neural stem cells in embryonic brains. Significant thinning of neocortex was observed in embryonic d 14.5 TLX-null brains with reduced nestin labeling and decreased cell proliferation in the germinal zone. Cell cycle analysis revealed both prolonged cell cycles and increased cell cycle exit in TLX-null embryonic brains. Increased expression of a cyclin-dependent kinase inhibitor p21 and decreased expression of cyclin D1 provide a molecular basis for the deficiency of cell cycle progression in embryonic brains of TLX-null mice. Furthermore, transient knockdown of TLX by in utero electroporation led to precocious cell cycle exit and differentiation of neural stem cells followed by outward migration. Together these results indicate that TLX plays an important role in neural development by regulating cell cycle progression and exit of neural stem cells in the developing brain.
NUCLEAR RECEPTORS ARE ligand-dependent transcription factors that have important roles in several biological processes, including cell proliferation, differentiation, and cellular homeostasis (1). TLX was initially identified as an orphan nuclear receptor that is homologous to the Drosophila tailless gene and plays an important role in vertebrate brain functions (2). Expression of TLX in the mouse starts at embryonic d 8 (E8), peaks at E13.5, and decreases by E16, with barely detectable levels at birth. TLX expression increases again after birth with high levels of expression in adult brains (3). The biphasic expression pattern of TLX suggests that TLX could play a role in both brain development and adult brain function.
We have shown that TLX is an essential regulator of neural stem cell maintenance and self-renewal in the adult brain (4). The TLX-expressing cells isolated from adult TLX-heterozygote brains can proliferate, self-renew, and differentiate into all three major neural cell types in vitro. By contrast, TLX-null cells isolated from the brains of adult TLX-mutant mice fail to proliferate. Reintroducing TLX into TLX-null cells rescued their ability to proliferate and self-renew (4).
Mature TLX knockout mice suffer from severe limbic defects, aggressiveness, decreased copulation, progressively violent behavior, and retinopathies (4,5,6,7,8,9,10,11,12). Fierce mice, a natural mutant homozygous for a deletion of TLX, show pathological aggression (13), similar to the TLX knockout mice. Introducing the human TLX gene into fierce mice ameliorates the brain and eye abnormalities (14), suggesting that the mechanisms underlying the behavioral abnormalities may be conserved in humans. Therefore, mutation of the TLX gene may contribute to human behavioral disorders.
Although the role of TLX in adult neural stem cells is unfolding, the mechanism by which it influences neural development remains unknown. In this study, we show that TLX is specifically expressed in the periventricular neural stem cells in the developing brain. Using TLX knockout mice we show that loss of TLX expression led to significant thinning of embryonic neocortex, reduced nestin labeling, and decreased cell proliferation in the ventricular zone (VZ) of E14.5 brains. Both prolonged cell cycles and reduced cell cycle reentry were observed in the VZ of TLX−/− embryonic brains. Moreover, TLX short interfering RNA (siRNA) treatment through in utero electroporation triggered cell cycle exit and neuronal differentiation of neural stem cells and outward migration of the differentiated cells in the embryonic neocortex. These results suggest that TLX is an important regulator of embryonic neural stem cell proliferation and differentiation through cell cycle control.
RESULTS
TLX-Expressing Cells Are VZ-Specific Neural Stem Cells
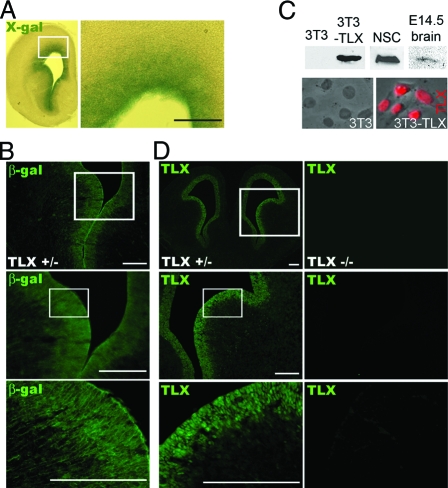
Taking advantage of a β-galactosidase (β-gal) reporter that was knocked into the TLX loci, we examined the expression pattern of TLX in embryonic brains of TLX heterozygote mice. 5-Bromo-4-chloro-3-indolyl-β-galactopyranoside (X-gal) staining revealed that TLX is specifically expressed in the VZ of embryonic brains (Fig. 1A). Immunostaining with a β-gal-specific antibody showed similar VZ-specific staining pattern (Fig. 1B).
Figure 1.
Expression of TLX in the Ventricular Zone of E14.5 Brains
A, X-gal staining of β-gal enzymatic activity in coronal brain sections from E14.5 TLX+/− mice. B, Antibody staining of β-gal in brain sections from E14.5 TLX+/− mice. Enlarged images of the boxed regions were shown below the boxed panels. C, Characterization of TLX-specific antibody. Polyclonal antibody specific for TLX was generated against mouse TLX ligand-binding domain (amino acid residues 180–385). The top panels are Western blot analyses using cell lysates from 3T3, TLX-transfected 3T3 (3T3-TLX) cells, neural stem cells (NSC), and E14.5 brains. The bottom panels are immunofluorescence analysis of 3T3 and 3T3-TLX cells using the TLX antibody (shown in red). D, Immunostaining of coronal brain sections from E14.5 TLX+/− and TLX−/− mice. Scale bar, 200 μm. Enlarged images of the boxed regions are shown below the boxed panels.
To examine more closely the expression pattern of TLX during mammalian neural development, a TLX-specific polyclonal antibody was developed. The specificity of the TLX antibody was revealed by both Western blot and immunofluorescence analyses. The TLX antibody detected a 46-kDa protein specifically in TLX stably transfected 3T3 cells (3T3-TLX), but not in parental 3T3 cells that have no endogenous TLX expression (Fig. 1C). Nuclear staining of TLX was also observed in 3T3-TLX cells specifically, but not in 3T3 cells. The TLX antibody also recognized endogenous TLX in neural stem cells and E14.5 brain lysates as revealed in Western blot analyses (Fig. 1C). Immunostaining of embryonic brain sections using the TLX-specific antibody revealed strong TLX expression in the periventricular zone of E14.5 wild-type and TLX heterozygous brains, whereas no staining was detected in the TLX-null brains (Fig. 1D). These results together indicate that TLX is specifically expressed in the germinal zone of embryonic brains.
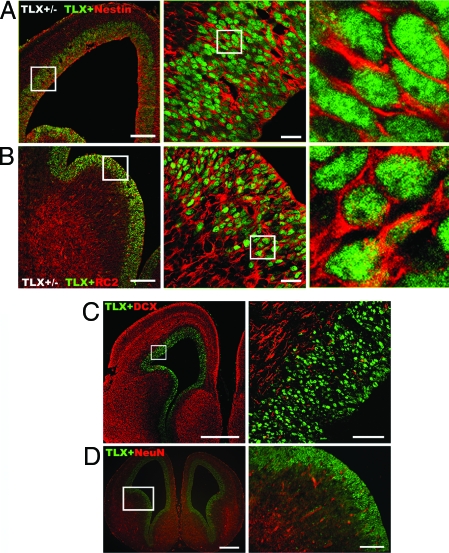
To determine the identity of the TLX-expressing cells, we performed double immunostaining of embryonic brain sections using the TLX-specific antibody and antibodies specific to neural precursors or differentiated neurons. Previous studies suggested that nestin is a common marker of proliferating neural progenitors (15,16). Immunohistological assessment of E14.5 brain sections from TLX+/− mice revealed colocalization of TLX and nestin in the VZ neural progenitor cells, with nuclear TLX staining and cytoplasm/process-specific nestin staining (Fig. 2A). Cortical neural progenitor cells are also marked by expression of RC2, a radial glial marker (17,18,19). Colocalization of TLX with RC2 (Fig. 2B) is evident throughout VZ, further supporting that TLX is expressed in embryonic neural precursor cells. In contrast, costaining of E14.5 brain sections with antibody specific to TLX and antibodies for neuronal markers doublecortin (DCX) or neuronal nuclei (NeuN) revealed distinct staining pattern of TLX and the neuronal markers (Fig. 2, C and D). The TLX-expressing cells are exclusively localized to the VZ, whereas DCX and NeuN-positive cells are specifically distributed to the intermediate zones and cortical plates. These results suggest that the TLX-expressing cells represent VZ-specific neural stem/progenitor cells in embryonic brains.
Figure 2.
TLX-Expressing Cells Are Nestin-Positive Embryonic Neural Precursor Cells
The TLX-expressing cells are nestin (A) and RC2 positive (B), but doublecortin (DCX, panel C) and NeuN (panel D) negative neural stem/progenitor cells in the VZ of mouse embryonic brains. The boxed portions are enlarged and shown in the middle and right panels. Scale bars: A (left), 200 μm; A (middle), 20 μm; scale bars in B are the same as in A; C (left), 500 μm; C (right), 50 μm; D (left), 400 μm; D (right), 100 μm.
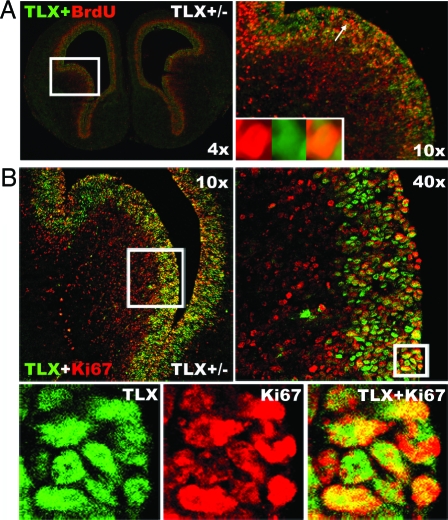
To further examine whether the TLX-expressing cells represent the actively dividing cells in the embryonic brains, we performed double staining of TLX and 5-bromodeoxyuridine (BrdU), a thymidine analog, on brain sections from TLX+/− E14.5 mice pretreated with BrdU. The analysis showed that TLX expression corresponds to both BrdU-positive and BrdU-negative cells in the embryonic brain germinal zone (Fig. 3A). In addition to BrdU labeling, which specifically marks cells at S phase, we also stained E14.5 brain sections with Ki67, a marker that labels all phases of the cell cycle, except the G0/G1 phase (20). Cells that are positive for both TLX and Ki67 staining were detected in the VZ of E14.5 brains (Fig. 3B). Because Ki67 labels a wider range of cells in the cell cycle, more cells were costained with TLX and Ki67 than costained with TLX and BrdU. A fraction of TLX-positive cells that are Ki67 negative was also detected in the VZ. These results suggest that TLX-expressing cells are primarily those actively dividing neural progenitors in cell cycle, but a small proportion could represent quiescent or nondividing cells in the VZ of embryonic brains.
Figure 3.
Costaining of TLX and Proliferative Markers in Embryonic Brains
A, Costaining of TLX and BrdU. Brain sections from mice exposed to a single pulse of BrdU for 2 h before brain harvest were costained with antibodies specific to BrdU (red) and TLX (green). The right panel is the enlargement of the boxed region in the left panel. Insets in the right panel are enlarged images of the arrow-pointed cell shown in BrdU single staining (red), TLX single staining (green), and merged image of double staining. B, Costaining of TLX and Ki67. E14.5 brain sections were costained with antibodies specific for Ki67 (red) and TLX (green). The right panel (×40) is the enlargement of the boxed region in the left panel (×10). The lower panels are the enlargements of the boxed region in the ×40 image panel.
TLX Regulates Cell Cycle Progression in Embryonic Brains
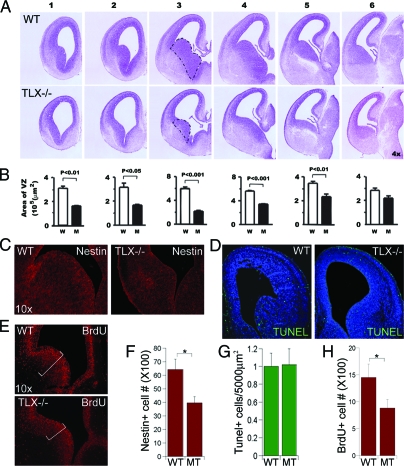
To determine the role of TLX in embryonic brain development, we analyzed developing brains of TLX knockout mice. Serial Nissl staining of embryonic brains of TLX−/− mice at E14.5 revealed reduced areas of VZ and enlarged ventricles in TLX−/− brains, compared with that in wild-type brains (Fig. 4, A and B). The thinning of the VZ is more prominent in rostral sections than that in caudal sections, with the biggest differences in the ganglionic eminence of rostral sections (marked by dotted lines in panel 3).
Figure 4.
Reduced Neural Progenitors and Cell Proliferation in E14.5 TLX−/− Brains
A, Nissl staining of coronal brain sections of E14.5 wild-type (WT) and mutant (MT) mice. The sections were divided into six matched panels from rostral to caudal. B, Quantification of the area of the lateral ganglionic eminence of VZ in each section shown in panel A, P value is indicated for statistical significance. C, Reduced neural progenitor cells in TLX−/− E14.5 brains as revealed by nestin immunostaining (red) of E14.5 wild-type and TLX−/− brains. D, No significant increase of apoptotic cell death in TLX−/− E14.5 brains. TUNEL assay of apoptosis (shown in green) in WT and TLX−/− E14.5 brains. DNA is counterstained with Hoechst 33342 (blue). E, Decreased cell proliferation in TLX−/− E14.5 brains as revealed by BrdU immunostaining (red) of brain sections from E14.5 wild-type and TLX−/− mice. F, Quantification of nestin-positive (nestin+) cells in WT and TLX−/− (MT) E14.5 VZ; *, P < 0.01. G, Quantification of TUNEL-positive cells in WT and TLX−/− (MT) E14.5 brains. H, Quantification of BrdU-positive (BrdU+) cells in WT and TLX−/− (MT) E14.5 VZ; *, P < 0.01.
The reduced areas of the VZ and the enlarged ventricles are probably due to the hypocellularity of the VZ. Therefore we determined whether there are decreased numbers of nestin-positive cells in the VZ of TLX−/− embryonic brains. Brain sections of wild-type and TLX−/− E14.5 embryos were immunostained with nestin-specific antibody. The number of nestin-positive cells was reduced in TLX−/− E14.5 brains (Fig. 4C, quantification in Fig. 4F), in good agreement with the reduced areas of VZ in TLX−/− embryonic brains (Fig. 4, A and B), suggesting that there are decreased numbers of neural precursors in the TLX−/− embryonic brains.
Programmed cell death (apoptosis) occurs during normal brain development (21). Increased cell death may contribute to reduced areas of VZ in TLX−/− embryonic brains. Apoptotic cell death was examined using terminal transferase dUTP nick end labeling (TUNEL) assay in wild-type and TLX−/− brains (Fig. 4D, quantification in Fig. 4G). Counts of total numbers of labeled cells revealed that cell death in transgenic brains was not substantially more than that found in wild-type mice, suggesting that the reduced neural stem/progenitor cells in TLX−/− embryonic brains did not result from increased apoptotic cell death.
Next we examined whether TLX affects cell proliferation in the embryonic brain. We used BrdU to label dividing neural precursor cells by exposing E14.5 embryos to BrdU for 2 h before brain harvest. Immunostaining of embryonic brain sections with BrdU-specific antibody was performed to monitor cell proliferation. Quantification of the BrdU-positive cells in both wild-type and TLX−/− E14.5 VZ revealed a significant decrease of total BrdU-positive cells in TLX−/− brains, compared with wild-type brains (Fig. 4E, quantification in Fig. 4H). These results suggest that TLX plays a role in maintaining at least a subset of proliferative neural precursors in embryonic brains.
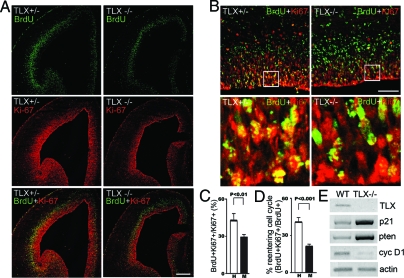
To examine whether the reduced cell proliferation in TLX−/− embryonic brains resulted from decreased mitotic rates, neural progenitor cells were labeled by a 30-min pulse of BrdU in E14.5 brains. The fraction of progenitor cells in S phase, a labeling index, was determined by scoring the percentage of BrdU-labeled neural progenitor cells (22). Progenitor cells were identified by Ki67 immunoreactivity (23,24). Because the length of S phase remains relatively constant whereas the length of G1 phase regulates proliferation in mammalian cells (25), this labeling index provides an estimation of cell cycle length. If the cell cycle is lengthened, the relative fraction of cells labeled by a brief BrdU pulse would decrease. Examination of random fields chosen from eight brains (four wild-type and four mutant brains) revealed that the TLX−/− neural progenitor cells divided slower than did wild-type neural progenitors (Fig. 5A, quantification in Fig. 5C).
Figure 5.
TLX Regulates Cell Cycle Progression
A, Increased cell cycle length in E14.5. TLX−/− brains as revealed by decreased progenitor BrdU labeling index. The percentage of progenitor cells (Ki67, red) labeled with BrdU (green) after a 30-min BrdU pulse (BrdU+Ki67+/Ki67+) is reduced in TLX mutant brains. Scale bar, 200 μm. B, Reduced fraction of cells reentering cell cycles in E14.5 TLX−/− brains. Animals were exposed to a single pulse of BrdU 24 h before being killed; sections were stained with antibodies to BrdU (green) and Ki67 (red). Fraction of cells reentered cell cycle is calculated as BrdU+Ki67+ cells (cell reentered cell cycle)/ BrdU+Ki67+ cells plus BrdU+Ki67− cells (cells left cell cycle). More cells have left cell cycle in the mutant than in wild-type brains. Scale bar, 50 μm. C, Quantification of the percent of BrdU+Ki67+ cells out of Ki67+ cells in E14.5 TLX+/− (H) and TLX−/− (M) brains shown in panel A; P < 0.01 by Student’s t test. D, Quantification of fraction of BrdU+Ki67+ cells out of BrdU+ cells in E14.5 TLX+/− (H) and TLX−/− (M) brains shown in panel B; P < 0.001 by Student’s t test. E, RT-PCR analysis of gene expression in wild type (WT) and TLX−/− E14.5 brains. Cyc D1, Cyclin D1.
To determine whether the decrease in the number of VZ progenitor cells resulted from a reduction in the fraction of progenitor cells that remained undifferentiated, we examined cell cycle exit and reentry by scoring the fraction of cells dividing after pulse labeling with BrdU for 24 h before brain harvest. Cells that had left the cell cycle were identified as BrdU+ and Ki67−, and cells that remained in the cell cycle as BrdU+ and Ki67+. At E15.5, there was about a 2-fold decrease in the proportion of TLX mutant precursors that reentered the cell cycle when compared with wild-type neural precursors (Fig. 5B, quantification in Fig. 5D). These studies suggest that TLX functions in embryonic neural precursors to influence the decision of these cells to reenter the cell cycle instead of exiting cell cycle and becoming differentiated.
What is the molecular basis of TLX-mediated cell cycle regulation? To determine whether cell cycle-related genes are regulated by TLX in embryonic brains, mRNAs were isolated from E14.5 wild-type and from TLX−/− telencephelons, and RT-PCR analyses were performed. As shown in Fig. 5E, the expression of both the cyclin-dependent kinase inhibitor p21 and the tumor suppressor gene pten was up-regulated significantly in E14.5 TLX−/− telencephelons, suggesting that TLX represses both p21 and pten gene expression in embryonic brains. In addition, reduced expression of cyclin D1, a downstream effector of pten (26), was also detected. The up-regulation of a negative cell cycle regulator, p21, and the down-regulation of a positive cell cycle regulator, cyclin D1, provides a molecular mechanism for TLX-regulated cell cycle progression in the germinal zone of embryonic brains.
Transient Knockdown of TLX Led to Outward Migration of VZ Cells in Embryonic Brains
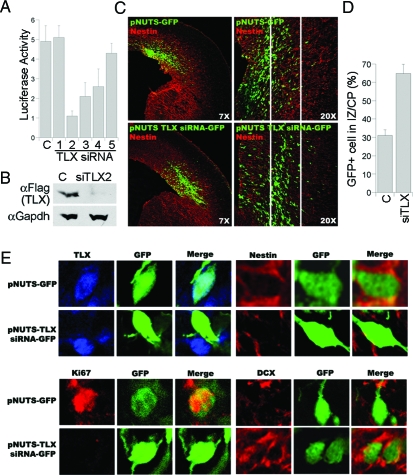
The relatively mild defect in TLX−/− embryonic brains, compared with the much more dramatic defect in TLX−/− adult brains (4), could be due to a systematic compensatory effect in embryonic stage to ensure proper development or redundant molecules that play overlapping roles in embryonic neural precursors. To further explore TLX function in embryonic brains with minimal systemic compensatory effect that could be caused by the systematic TLX knockout, a transient local knockdown approach was used by in utero electroporation (27,28,29), in which plasmid DNAs expressing TLX siRNAs were delivered directly into the VZ of intact embryos. Five TLX sequence-specific siRNAs were screened for the knockdown effect in vitro. Among the siRNAs tested, siRNA 2 had the strongest inhibitory effect on TLX expression (Fig. 6, A and B) and was therefore chosen for the subsequent electroporation-based in vivo studies. TLX siRNA-expressing DNA was introduced into cerebral cortices through in utero electroporation at E13.5, at a time when TLX expression is known to be high (3). Electroporated brains were dissected out and sectioned for analyses at E15.5. Cells that had taken up the siRNAs were labeled green by expression of green fluorescent protein (GFP) that was included in the vector and thus could be readily visualized by fluorescence microscopy. Electroporation of TLX siRNA led to a significant increase in the number of cells that migrate from VZ into intermediate zone and cortical plate 2 d after transfection, compared with that resulting from the transfection with control GFP vector (Fig. 6, C and D). The effectiveness of in utero siRNA knockdown was confirmed by TLX immunohistochemistry on brain sections from in utero electroporated embryos. Expression of TLX siRNA-GFP led to dramatic decrease of TLX expression as revealed by the significant loss of TLX immunostaining, whereas expression of the control GFP vector had no effect on TLX expression (Fig. 6E, upper left panels).
Figure 6.
In Utero Electroporation of TLX siRNA in Embryonic Brains
A, Luciferase assays to determine the repression effect of TLX siRNAs (1,2,3,4,5) using a luciferase reporter gene upstream of TLX coding sequences, compared with control GFP siRNA (C). Results represent the means and sds of three replicates. B, siRNA knockdown of TLX expression in Flag-tagged TLX-transfected human embryonic kidney 293 cells as revealed by Western blot analysis. C, Control GFP siRNA; siTLX2: TLX siRNA 2. Anti-Flag antibody (αFlag) detects the Flag-tagged TLX. Gapdh was included as a loading control. C, pNUTS-GFP or pNUTS-TLX siRNA-GFP was introduced into E13.5 wild-type brains through in utero electroporation. Brains were dissected out at E15.5 for analyses. Cells that had taken up the transfected DNAs were shown green due to expression of a GFP marker. D, Quantification of control vector (C) and TLX siRNA-expressing vector (siTLX)-electroporated cells (GFP+) in intermediate zone (IZ) and cortical plate (CP). E, Immunostaining of cells from pNUTS-GFP or pNUTS-TLX-siRNA-GFP in utero electroporated embryonic brain sections.
Another nuclear receptor, peroxisomal proliferator-activated receptor-γ (PPARγ), has also been shown to regulate embryonic neural stem cells (30). To determine whether PPARγ plays a similar role to that of TLX in neural development, an in vivo knockdown experiment was performed using PPARγ-specific siRNA electroporated into VZ of E13.5 brains. In contrast to TLX siRNA, the PPARγ-specific siRNA (30) did not induce significant migration of VZ cells away from the apical side of the ventricle (data not shown).
The outward migration of the cells affected by TLX siRNA suggested that knockdown of TLX expression in the VZ of embryonic brains could have caused neural stem/progenitor cells to exit cell cycle and differentiate into neurons, which then migrate outward. Alternatively, siRNA knockdown of TLX expression could have induced nondifferentiated neural precursor cells to migrate out. To determine the possible mechanisms of the TLX siRNA-mediated cell migration, immunostaining of sections of siRNA-electroporated brains was done to determine the identity of the transfected cells. The TLX siRNA-transfected cells that migrated out of VZ lost the neural progenitor marker nestin (Fig. 6E, upper right six panels) and the proliferative marker Ki67 (Fig. 6E, lower left six panels). Instead, these cells expressed the neuronal marker, DCX (Fig. 6E, lower right six panels), indicating cell cycle exit and neuronal differentiation. In contrast, the control GFP vector-transfected cells that remained in the VZ were both nestin positive and Ki67 positive and lacked DCX expression (Fig. 6E). These results reinforce that TLX is an important regulator of embryonic neural stem cells by controlling their cell cycle progression and exit.
DISCUSSION
The data presented here indicate that TLX plays an important role in the regulation of cell cycle progression and exit of embryonic neural stem cells. TLX is specifically expressed in neural precursor cells in the germinal zones of embryonic brains. Lack of TLX function during development results in reduced cell proliferation and decreased numbers of nestin-positive neural progenitor cells in the VZ of E14.5 brains. Both lengthened cell cycles and reduced fraction of cells reentering cell cycle could account for the reduced proliferation of progenitor cells in E14.5 TLX−/− brains. The increased expression of a negative cell cycle regulator, p21, and decreased expression of a positive cell cycle regulator, cyclin D1, provide a molecular basis for the lengthened cell cycle progression and reduced cell proliferation in the VZ of TLX−/− embryonic brains.
What are the primary cellular functions exerted by TLX in neural development? TLX could be required in early neural progenitor cells to regulate proliferative divisions and prevent neurogenesis under normal conditions. In support of this hypothesis, our data show that both knockout and transient siRNA knockdown of TLX led to increased cell cycle exit and reduced cell proliferation. TLX could also act by regulating the types of divisions that progenitor cells make. Early progenitor cells expand by symmetric divisions establishing the surface area and number of radial units in the cortex (31). Subsequently, there is a steady increase in the number of differentiative asymmetric divisions, establishing the number of neurons per radial unit and the depth of the cerebral cortex. Therefore the length of the cell cycle and the duration of each phase of division are critical in controlling the size of the cerebral cortex (32,33,34). Our data suggest that progenitor cells in TLX-mutant animals go through fewer cell cycles than that in their wild-type littermates, perhaps by prematurely switching from symmetric divisions to asymmetric divisions. TLX has also been shown to be required to regulate the timing of neurogenesis in the cortex (35) and to control patterning of lateral telencephalic progenitor domains during development (36). The results presented here demonstrated unambiguously that TLX regulates cell cycle progression and exit to maintain embryonic neural stem cells in the undifferentiated and proliferative state through controlling key cell cycle regulator gene expression.
Interestingly, the defect in TLX mutant embryonic brains is modest compared with the dramatic deficiency in TLX-null adult brains. It is a surprise that there are still rigorous cell proliferation and extensive numbers of nestin-positive neural progenitor cells in the VZ of TLX-mutant embryonic brains, in contrast to almost complete loss of cell proliferation in TLX−/− adult brains (4). These results suggest that TLX may not be the sole regulator of neural stem cell maintenance during development. There could be other molecules that play redundant roles in neural stem cell regulation during embryogenesis. Alternatively, the organism could have a fail-safe mechanism to compensate for the systemic TLX loss to ensure proper development. To minimize the potential systemic compensatory effect, we took a transient local knockdown approach. Our observation that a significantly increased number of VZ cells underwent outward migration upon transient local TLX knockdown reinforces an important role of TLX in embryonic neural stem cells and supports the compensatory possibility. In contrast to siRNA knockdown of TLX, siRNA knockdown of another nuclear receptor, PPARγ, which is also expressed in embryonic brains and has been shown to play a role in embryonic neural stem cell regulation (30), did not cause migration of the transfected cells, suggesting that PPARγ plays a distinct role from TLX in embryonic neural stem cells.
The characterization of the TLX-expressing cells in the embryonic brain provides a means to elucidate the molecular and cellular mechanisms underlying embryonic neural stem cell proliferation and differentiation. Although other factors are likely involved, TLX is an important regulator of neural stem cells in the developing brain by regulating the expression of key cell cycle regulators.
MATERIALS AND METHODS
Experimental Animals
All research animals are acquired and used in compliance with federal, state, and local laws and institutional regulations. All animals are maintained in accordance with the NIH Guide for the Care and Use of Laboratory Animals. The studies were approved by the institutional committee on animal care.
Animal Handling and Brain Tissue Preparation
Strain-matched and age-matched mice were used in all experiments. All animal experiments were performed in compliance with institutional and NIH guidelines. TLX heterozygous male and female mice were used for breeding to obtain wild-type, TLX-heterozygous, and null embryos. Timed-pregnant mice were anesthetized with ketamine (120 mg/kg) and xylazine (10 mg/kg). Embryonic brains were dissected and fixed in 4% paraformaldehyde in 0.1 m PBS (pH 7.4) at 4 C overnight, and cryoprotected in 7.5%, 15%, and 30% sucrose in phosphate buffer at 4 C for 2 h, 2 h, and 24 h, respectively. After cryoprotection, brain tissues were embedded in OCT, frozen with dry ice, and stored at −80 C. Coronal sections of forebrains (12 μm) were cut on a cryostat and mounted onto superfrost-plus slides (Fisher Scientific, Pittsburgh, PA).
BrdU Labeling
For BrdU labeling, timed-pregnant mice were injected with 50 mg/kg BrdU (Sigma Chemical Co., St. Louis, MO) in saline ip. Animals were killed in 30 min (E14.5 injection), 2 h (E14.5 injection), or 24 h (E13.5 injection). Embryos were dissected and sectioned as described above. Brain sections were pretreated in 2 n HCl for 30 min at 37 C, followed by neutralization in 0.1 m borate buffer (pH 8.5) for 10 min and blocking with 10% normal donkey serum for 1 h at room temperature, after which immunohistochemistry was performed using a rat anti-BrdU monoclonal antibody (1:300, Accurate) and donkey antirat fluorescein isothiocyanate or cyanine dye 3-conjugated secondary antibody (1:300, Jackson ImmunoResearch Laboratories, Inc., West Grove, PA). BrdU immunostaining was observed using a Nikon Eclipse fluorescent microscope (TE2000-S) and imaged using Spot Camera (version 4.0.8, Diagnostic Instruments, Sterling Heights, MI). The number of BrdU-positive cells was counted in the area of the lateral ventricle zones of E14.5 brains with a minimum of six sections per embryo from five wild-type and five mutant embryos. Cell densities were calculated by dividing cell numbers by the area. Student’s t test was used to determine the P value for statistical significance. All comparisons were made between littermates.
Immunohistochemistry, Nissl Staining, and LacZ Staining
Brain coronal sections (12 μm) from the same litter were used for Nissl staining. The forebrain sections were divided into six matched patterns from rostral to caudal between TLX mutant mice and their littermate wild-type controls. Statistical analysis was performed with Student’s t test. For X-gal staining, embryos were fixed in 4% paraformaldehyde in 0.1 m PBS (pH 7.4) for 3 h, the 40-um frozen sections were fixed with glutaraldehyde and paraformaldehyde and stained in (X-gal; Life Technologies, Inc., Gaithersburg, MD) solution containing 20 mm K-ferricyanide, 20 mm K-ferrocyanide, 0.01% sodium deoxycholate, 0.02% Nonidet P-40, and 2 mm MgCl2. For immunohistochemistry, 12-μm brain sections were incubated with antibodies, including mouse antinestin (1:1,000, PharMingen, San Diego, CA), mouse anti-RC2 (1:30, Hybridoma Bank), rabbit anti-TLX (1:100, Shi laboratory), goat anti-DCX (1:100, Santa Cruz Biotechnology, Inc., Santa Cruz, CA), mouse anti-NeuN (1:1000; Chemicon, Temecula, CA), rabbit anti-Tuj1 (1:4000; Covance Laboratories, Inc., Madison, WI), guinea pig anti-GFAP (1:500, Advanced Immunochemical Inc., Long Beach, CA), rabbit anti-β-gal (1:3500, Cortex Biochem, Inc., San Leandro, CA), rabbit anti-Ki67 (Novocastra Laboratories, Newcastle, UK; 1:100), mouse anti-Ki67 (Novocastra; 1:40), followed by incubation with cyanine dye 3 or fluorescein isothiocyanate-conjugated secondary antibodies (1:400, Jackson ImmunoResearch). Images were visualized by a confocal microscope (Zeiss LSM510 Upright 2 photon; Carl Zeiss, Thornwood, NY). Quantitative studies were based on four or more replicates. Nestin-positive cells were counted within the region of the lateral ganglionic eminence in E14.5 brains of nine wild-type and six TLX−/− embryos. Student’s t test was used to determine the P value for statistical significance. All comparisons were made between littermates.
TLX Polyclonal Antibody Production, Western Blot, and Immunofluorescence
The TLX antigen containing mouse TLX ligand binding domain (amino acid residues 180–385) was produced in bacteria BL21 cells as His-tagged protein and purified using Ni-NTA Agarose (QIAGEN, Hilden, Germany). Rabbit immunizations were performed in the Animal Resource Center in City of Hope. The anti-TLX serum was further purified using SulfoLink Coupling Gel (Pierce Biotechnology, Inc., Rockford, IL). For Western blot, TLX antibody was used at 1:1000 dilution; for immunofluorescence on 3T3 or 3T3-TLX cells, TLX antibody was also used at 1:1000 dilution.
In Utero Electroporation
TLX or PPARγ siRNAs were cloned into pNUTs-GFP, a mouse U6 promoter-based small RNA-expressing vector that contains an ubiquitin promoter-driven enhanced GFP (a gift from David Baltimore, Caltech). pNUTs-GFP, pNUTs-GFP-TLX siRNA, and pNUTs-GFP-PPARγ siRNA vectors were used in in utero electroporation analyses. The plasmids were prepared at 5 μg/μl in H2O with either QIAGEN HighSpeed plasmid maxi kit (QIAGEN) or NucleoBond plasmid purification maxi kit (CLONTECH Laboratories, Inc., Palo Alto, CA). The plasmids have the GFP reporter gene downstream of an Ubiquitin promoter (pNUTS). For in utero electroporation, mice at 12.5-d gestation were anesthetized with an ip injection of ketamine (120 mg/kg) and xylazine (10 mg/kg). The uterine horns were exposed, and 1 μl plasmid DNA (5 μg/μl) was microinjected by pressure using picospritzer III (General valve operation, Fairfield, NJ) through the uterus into the lateral ventricles of embryos by pulled glass capillaries (Drummond Scientific Co., Broomall, PA). Electroporation was accomplished by holding the injected brain through the uterus with forcep-type electrodes CY650-P5 (Protech International, Inc., San Antonio, TX) and delivering square electric pulses (five pulses; duration, 50 msec each; interval, 950 msec) to the embryos using electroporator CUY-21 (Protech International). The mice are allowed to survive 2 d before being euthanized. Brains of embryos were harvested and analyzed.
RT-PCR Analysis
Total RNA was isolated using Trizol reagent (Life Technologies, Inc.). For RT-PCR analysis, reverse transcription was performed using Omniscript RT kit (QIAGEN). PCR primers used were TCT CTA TGT TCC AAA ACC ATT CCA T and TTC CCA AGC ACC TCA TAC TAC CAG C for cyclin D1, ATA TCC AGA CAT TCA GAG CCA CAG G and GGA AAC ACA GAG CTT GGG TTG GGA G for p21, GCT ACC AGA CTC TCA CAG GAG CAA GC and TCA GAC TTT TGT AAT TTG TGA ATG CT for pten, and TCT TCA CCA CCA TGG AGA AGG C and CTG ACA ATC TTG AGT GAG TTG T for actin.
Luciferase Assay
TLX siRNA sequences were cloned into the pCSC vector (4). The reporter vector was constructed by insertion of the TLX coding region into the sicheck 2.2 vector (Promega) downstream of the Renilla luciferase reporter gene. Quantities of 0.64 μg TLX siRNA expressing plasmids, 0.08 μg sicheck reporter construct, and 2 μl Fugene 6 (Roche Clinical Laboratories, Indianapolis, IN) were mixed in 500 μl of cell culture media, incubated at room temperature for 20 min, and added dropwise to three wells of human embryonic kidney 293 cells in 48-well plates. Reporter Renilla luciferase activity and internal control firefly luciferase activity were measured 48 h after transfection using Dual Luciferase Assay kit (Promega). The reporter luciferase activity was normalized with the internal control for transfection efficiency and plotted on the y-axis.
Supplementary Material
Acknowledgments
We thank Drs. Kamil Alzayady, Qiang Lu, Paul Salvaterra, Toshi Tomoda, and John Zaia for critical reading of the manuscript, and Dr. Smitha Reddy Anam for technical help.
Footnotes
This work was supported by Whitehall Foundation, the Margret E. Early Medical Trust, and National Institutes of Health National Institute of Neurological Diseases and Stroke Grants R01NS059546 and R21NS 053350. G.S. is a Herbert Horvitz Postdoctoral Fellow. Y.S. is a Kimmel Scholar.
Disclosure Statement: The authors have nothing to disclose.
First Published Online September 27, 2007
Abbreviations: BrdU, Bromodeoxyuridine; DCX, doublecortin; E8, embryonic d 8; β-gal, β-galactosidase; GFP, green fluorescent protein; NeuN, neuronal nuclei; PPARγ, peroxisomal proliferator-activated receptor-γ; siRNA, short interfering RNA; TUNEL, terminal transferase dUTP nick end labeling; VZ, ventricular zone; X-gal, 5-bromo-4-chloro-3-indolyl-β-galactopyranoside.
References
- Evans RM 2005 The nuclear receptor superfamily: a rosetta stone for physiology. Mol Endocrinol 19:1429–1438 [DOI] [PubMed] [Google Scholar]
- Yu RT, McKeown M, Evans RM, Umesono K 1994 Relationship between Drosophila gap gene tailless and a vertebrate nuclear receptor Tlx. Nature 370:375–379 [DOI] [PubMed] [Google Scholar]
- Monaghan AP, Grau E, Bock D, Schutz G 1995 The mouse homolog of the orphan nuclear receptor tailless is expressed in the developing forebrain. Development 121:839–853 [DOI] [PubMed] [Google Scholar]
- Shi Y, Chichung Lie D, Taupin P, Nakashima K, Ray J, Yu RT, Gage FH, Evans RM 2004 Expression and function of orphan nuclear receptor TLX in adult neural stem cells. Nature 427:78–83 [DOI] [PubMed] [Google Scholar]
- Monaghan AP, Bock D, Gass P, Schwager A, Wolfer DP, Lipp HP, Schutz G 1997 Defective limbic system in mice lacking the tailless gene. Nature 390:515–517 [DOI] [PubMed] [Google Scholar]
- Chiang MY, Evans RM 1997 Reverse genetic analysis of nuclear receptors, RXRg, RARb, and TLX in mice, PhD dissertation, University of California San Diego [Google Scholar]
- Roy K, Thiels E, Monaghan AP 2002 Loss of the tailless gene affects forebrain development and emotional behavior. Physiol Behav 77:595–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young KA, Berry ML, Mahaffey CL, Saionz JR, Hawes NL, Chang B, Zheng QY, Smith RS, Bronson RT, Nelson RJ, Simpson EM 2002 Fierce: a new mouse deletion of Nr2e1; violent behaviour and ocular abnormalities are background-dependent. Behav Brain Res 132:145–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu RT, Chiang MY, Tanabe T, Kobayashi M, Yasuda K, Evans RM, Umesono K 2000 The orphan nuclear receptor Tlx regulates Pax2 and is essential for vision. Proc Natl Acad Sci USA 97:2621–2625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyawaki T, Uemura A, Dezawa M, Yu RT, Ide C, Nishikawa S, Honda Y, Tanabe Y, Tanabe T 2004 Tlx, an orphan nuclear receptor, regulates cell numbers and astrocyte development in the developing retina. J Neurosci 24:8124–8134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura A, Kusuhara S, Wiegand SJ, Yu RT, Nishikawa S 2006 Tlx acts as a proangiogenic switch by regulating extracellular assembly of fibronectin matrices in retinal astrocytes. J Clin Invest 116:369–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang CL, Zou Y, Yu RT, Gage FH, Evans RM 2006 Nuclear receptor TLX prevents retinal dystrophy and recruits the corepressor atrophin1. Genes Dev 20:1308–1320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrahams BS, Mak GM, Berry ML, Palmquist DL, Saionz JR, Tay A, Tan YH, Brenner S, Simpson EM, Venkatesh B 2002 Novel vertebrate genes and putative regulatory elements identified at kidney disease and NR2E1/fierce loci. Genomics 80:45–53 [DOI] [PubMed] [Google Scholar]
- Abrahams BS, Kwok MC, Trinh E, Budaghzadeh S, Hossain SM, Simpson EM 2005 Pathological aggression in “fierce” mice corrected by human nuclear receptor 2E1. J Neurosci 25:6263–6270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lendahl U, Zimmerman LB, McKay RD 1990 CNS stem cells express a new class of intermediate filament protein. Cell 60:585–595 [DOI] [PubMed] [Google Scholar]
- Reynolds BA, Tetzlaff W, Weiss S 1992 A multipotent EGF-responsive striatal embryonic progenitor cell produces neurons and astrocytes. J Neurosci 12:4565–4574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malatesta P, Hartfuss E, Gotz M 2000 Isolation of radial glial cells by fluorescent-activated cell sorting reveals a neuronal lineage. Development 127:5253–5263 [DOI] [PubMed] [Google Scholar]
- Miyata T, Kawaguchi A, Okano H, Ogawa M 2001 Asymmetric inheritance of radial glial fibers by cortical neurons. Neuron 31:727–741 [DOI] [PubMed] [Google Scholar]
- Noctor SC, Flint AC, Weissman TA, Dammerman RS, Kriegstein AR 2001 Neurons derived from radial glial cells establish radial units in neocortex. Nature 409:714–720 [DOI] [PubMed] [Google Scholar]
- Schluter C, Duchrow M, Wohlenberg C, Becker MH, Key G, Flad HD, Gerdes J 1993 The cell proliferation-associated antigen of antibody Ki-67: a very large, ubiquitous nuclear protein with numerous repeated elements, representing a new kind of cell cycle-maintaining proteins. J Cell Biol 123:513–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuan CY, Roth KA, Flavell RA, Rakic P 2000 Mechanisms of programmed cell death in the developing brain. Trends Neurosci 23:291–297 [DOI] [PubMed] [Google Scholar]
- Chenn A, Walsh CA 2002 Regulation of cerebral cortical size by control of cell cycle exit in neural precursors. Science 297:365–369 [DOI] [PubMed] [Google Scholar]
- Scholzen T, Gerdes J 2000 The Ki-67 protein: from the known and the unknown. J Cell Physiol 182:311–322 [DOI] [PubMed] [Google Scholar]
- Kee N, Sivalingam S, Boonstra R, Wojtowicz JM 2002 The utility of Ki-67 and BrdU as proliferative markers of adult neurogenesis. J Neurosci Methods 115:97–105 [DOI] [PubMed] [Google Scholar]
- DiSalvo CV, Zhang D, Jacobberger JW 1995 Regulation of NIH-3T3 cell G1 phase transit by serum during exponential growth. Cell Prolif 28:511–524 [DOI] [PubMed] [Google Scholar]
- Weng LP, Brown JL, Eng C 2001 PTEN coordinates G(1) arrest by down-regulating cyclin D1 via its protein phosphatase activity and up-regulating p27 via its lipid phosphatase activity in a breast cancer model. Hum Mol Genet 10:599–604 [DOI] [PubMed] [Google Scholar]
- Fukuchi-Shimogori T, Grove EA 2001 Neocortex patterning by the secreted signaling molecule FGF8. Science 294:1071–1074 [DOI] [PubMed] [Google Scholar]
- Saito T, Nakatsuji N 2001 Efficient gene transfer into the embryonic mouse brain using in vivo electroporation. Dev Biol 240:237–246 [DOI] [PubMed] [Google Scholar]
- Tabata H, Nakajima K 2001 Efficient in utero gene transfer system to the developing mouse brain using electroporation: visualization of neuronal migration in the developing cortex. Neuroscience 103:865–872 [DOI] [PubMed] [Google Scholar]
- Wada K, Nakajima A, Katayama K, Kudo C, Shibuya A, Kubota N, Terauchi Y, Tachibana M, Miyoshi H, Kamisaki Y, Mayumi T, Kadowaki T, Blumberg RS 2006 Peroxisome proliferator-activated receptor γ-mediated regulation of neural stem cell proliferation and differentiation. J Biol Chem 281:12673–12681 [DOI] [PubMed] [Google Scholar]
- Rakic P 1988 Specification of cerebral cortical areas. Science 241:170–176 [DOI] [PubMed] [Google Scholar]
- Rakic P 1995 A small step for the cell, a giant leap for mankind: a hypothesis of neocortical expansion during evolution. Trends Neurosci 18:383–388 [DOI] [PubMed] [Google Scholar]
- Takahashi T, Nowakowski RS, Caviness Jr VS 1996 The leaving or Q fraction of the murine cerebral proliferative epithelium: a general model of neocortical neuronogenesis. J Neurosci 16:6183–6196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caviness Jr VS, Goto T, Tarui T, Takahashi T, Bhide PG, Nowakowski RS 2003 Cell output, cell cycle duration and neuronal specification: a model of integrated mechanisms of the neocortical proliferative process. Cereb Cortex 13:592–598 [DOI] [PubMed] [Google Scholar]
- Roy K, Kuznicki K, Wu Q, Sun Z, Bock D, Schutz G, Vranich N, Monaghan AP 2004 The Tlx gene regulates the timing of neurogenesis in the cortex. J Neurosci 24:8333–8345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenman JM, Wang B, Campbell K 2003 Tlx controls proliferation and patterning of lateral telencephalic progenitor domains. J Neurosci 23:10568–10576 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.