Abstract
Parturition is a complex mammalian physiological process whose fundamental determinants have remained elusive. The increasing incidence of human preterm birth, a leading cause of infant mortality, highlights the importance of further understanding mechanisms regulating the timing of birth. Parturition is initiated in most nonprimate mammals, including mice, through a decrease in circulating progesterone caused by elevated prostaglandins. In humans, other higher primates, and guinea pigs, no consistent decrease in circulating progesterone occurs before the onset of labor. The divergence in endocrine control of labor initiation between most mammals compared with the great apes and guinea pigs gives rise to the question: how could a mechanism for the initiation of labor not requiring the withdrawal of progesterone evolve? Here, we genetically modulate prostaglandin signaling to determine the role of prostaglandin catabolism in the timing of birth. We find spontaneous preterm labor in the absence of progesterone withdrawal in 15-hydroxyprostaglandin dehydrogenase hypomorphic mice. The onset of labor in these hypomorphic mice is preceded by prematurely increased concentrations of prostaglandin E2 and F2α. Moreover, genetic crosses demonstrate a role for fetal genotype in birth timing. Together, these findings demonstrate a 15-hydroxyprostaglandin dehydrogenase-dependent shift in the physiology of murine parturition to one resembling the physiology of higher primates. Thus, endocrine control of labor has the capacity to plastically adapt to changes in genetically determined prostaglandin signals.
PRETERM LABOR OCCURS in approximately 12% of births in the United States and is the chief cause of neonatal morbidity and mortality (1). Of substantial concern, annual rates of prematurity are increasing. Intervention focused on preventing preterm labor would be an ideal strategy to address this problem, but the mechanisms underlying the timing of normal labor and the pathogenesis of spontaneous idiopathic preterm labor remain unknown (2).
Understanding the mechanism determining the timing of labor onset presents a particularly difficult problem in that it likely involves both redundant signaling mechanisms and integration of input from two distinct genetic components, the mother and the fetus. Moreover, extrapolation of mechanisms from animal model systems to humans has proven problematic. In most mammalian species aside from the great apes, labor is initiated by a decrease in circulating progesterone (progesterone withdrawal) (3,4,5). However, in the great apes, including the human, no such decrease occurs before normal labor (6,7). Despite the difference in regulation of progesterone concentrations, myometrial biochemical changes associated with increased contractility are conserved across all mammalian species. This conservation of the final pathway for myometrial activation has been suggested to reflect that functional progesterone withdrawal occurs in humans to initiate labor (8,9,10). Alternatively, other pathways conserved across mammals, such as prostaglandins (PGs) (11), may supercede the necessity for progesterone withdrawal.
In a range of species, PGE2 and PGF2α facilitate labor as contractile agonists (11). In rodents, PGF2α has an additional role in initiating labor by stimulating luteolysis and progesterone withdrawal (12,13). The enzyme primarily responsible for the breakdown of PGE2 and PGF2α is 15-hydroxyprostaglandin dehydrogenase (15-HPGD) (14,15). The regulation of PG breakdown rather than synthesis as an important determinant of labor onset has been suggested by several observations. In humans, a decrease in 15-HPGD mRNA and activity in the chorion trophoblast cells in laboring women relative to nonlaboring women at term has been measured (16). This difference is further accentuated in women who are laboring preterm (16,17). Analogously, in a mouse model of bacterial-induced preterm labor, induction of labor correlates with a decrease in 15-HPGD mRNA in the fetal membranes and the fetus (18). To test the hypothesis that 15-HPGD-mediated PG breakdown regulates the timing of labor, we generated and analyzed 15-HPGD hypomorphic (HpgdH/H) mice.
RESULTS
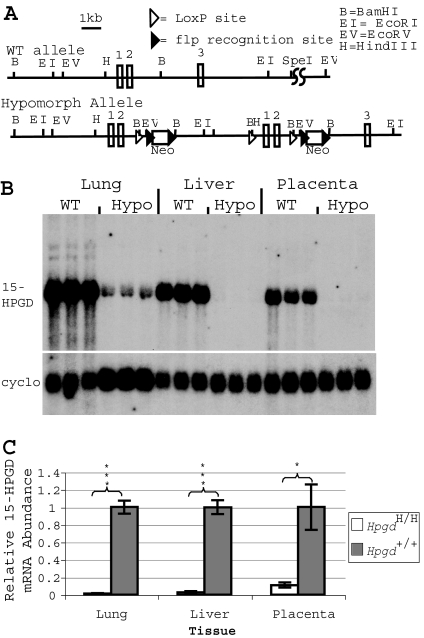
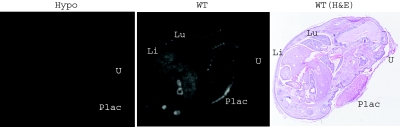
The 15-HPGD hypomorphic allele (HpgdH) arose as a result of an aberrant homologous recombination event resulting in tandem duplication of the 5′ end of the Hpgd gene along with insertion of neomycin resistance cassettes in intronic regions (Fig. 1A). To define the consequences of this integration event on Hgpd gene transcription and activity, we examined 15-HPGD mRNA by Northern blot (Fig. 1, B and C) and in situ hybridization (Fig. 2) and performed activity assays (Fig. 3). 15-HPGD mRNA abundance was measured in the tissues expressing the highest levels of this enzyme: the liver, lung, and placenta (15). In all of these tissues, HpgdH/H mice have a significant decrease in 15-HGPD mRNA relative to control mice (Fig. 1, B and C). In day post coitus (dpc)-17.5 implantation site and fetus, 15-HPGD in situ mRNA hybridization further highlights the developing lung, liver, and placenta in the wild-type mice as tissues of high 15-HPGD expression, with reduced mRNA expression in the HpgdH/H mice (Fig. 2).
Figure 1.
Presence of 15-HPGD Hypomorphic Alleles Results in Decreased 15-HPGD mRNA Gene Expression
A, Wild-type (WT) Hpgd+/+ and hypomorphic HpgdH/H alleles; B, Northern blot analysis of 15-HPGD mRNA in the adult lung, liver, and dpc 15.5 placenta, with cyclophilin mRNA detection provided as loading control; C, quantification of differences in 15-HPGD mRNA abundance in the adult lung, liver, and dpc 15.5 placenta normalized to wild-type values. Values are mean ± sem. ***, P < 0.001; *, P < 0.05; n = 3.
Figure 2.
15-HPGD mRNA Abundance Is Decreased in the dpc 17.5 15-HPGD Hypomorphic Implantation Site and Fetus
In situ hybridization demonstrates abundant 15-HPGD mRNA expression in wild-type (WT) but not hypomorphic (Hypo) fetus and surrounding structures. H&E, Hematoxylin and eosin stain of an adjacent section; Li, liver; Lu, lung; Plac, placenta; U, uterus.
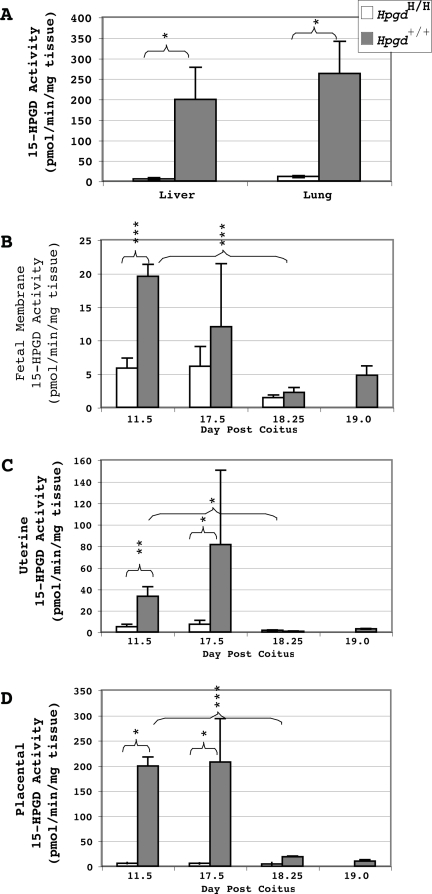
Figure 3.
Presence of 15-HPGD Hypomorphic Alleles Results in Decreased 15-HPGD Activity
The 15-HPGD hypomorphic alleles decrease 15-HPGD activity in adult lung and liver (A), dpc 11.5 fetal membranes (B), dpc 11.5 and 17.5 uterus (C), and dpc 11.5 and 17.5 placenta (D) compared with wild-type mice. 15-HPGD activity significantly decreases after dpc 17.5 in wild-type mice compared with dpc 11.5 in wild-type mice in fetal membranes (B), uterus (C), and placenta (D). Values are mean ± sem. ***, P < 0.001; **, P < 0.01; *, P < 0.05; n = 3–4. Measurements at dpc 18.25 do not differ between genotypes, and only wild-type samples are measured at dpc 19.0.
In both the adult liver and lung, HpgdH/H mice have a significant decrease in PG dehydrogenase activity relative to wild-type mice (Fig. 3A). We also measured PG dehydrogenase activity in the placenta, uterus, and fetal membranes (Fig. 3, B–D) because of their importance in PG production and metabolism during gestation. On dpc 11.5, each of these tissues from HpgdH/H matings demonstrate a significant decrease in 15-HPGD activity relative to control tissues. On dpc 17.5, HpgdH/H placenta and uterus, but not fetal membranes, display a significant decrease in 15-HPGD activity relative to the same wild-type control tissue. In wild-type pregnancy, 15-HPGD activity decreases significantly to activity levels similar to those observed in the hypomorphic gestational tissues at dpc 18.25 and 19.0 (Fig. 3, B–D).
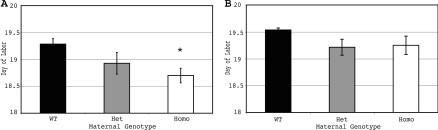
To define the consequences of this alteration in 15-HPGD activity on birth timing, we compared the length of gestation in pregnancies arising after mating hypomorphic mice and wild-type littermates (Fig. 4). When both the female and male are hypomorphic (HpgdH/H), labor occurs more than half a day early relative to wild-type females (Hpgd+/+) mated with the HpgdH/H males (Fig. 4A; P < 0.05) and almost a full day early relative to wild-type pregnancies (Hpgd+/+× Hpgd+/+, Fig. 4B; P < 0.05). However, when HpgdH/H females are mated with wild-type (Hpgd+/+) males, they do not labor significantly early relative to wild-type (Hpgd+/+) females mated with the same males.
Figure 4.
15-HPGD Hypomorphic Pregnancies Labor Early Relative to Wild-Type Pregnancies
A, Gestation length after mating with a 15-HPGD hypomorphic male; B, gestation length after mating with a wild-type (WT) male. Values are mean ± sem. *, P < 0.05 relative to mating between a 15-HPGD female with a wild-type male; n = 7, except n = 14 for wild-type male × female, and n = 15 for 15-HPGD hypomorphic homozygous (homo) male × female. Het, Heterozygous.
Multiple regression was used to examine the relative contributions of maternal genotype and fetal genotype on the timing of birth in these matings. Both maternal and fetal genotypes each have a statistically significant effect on the timing of labor. Maternal genotype when examined alone was found to be statistically significantly predictive for the timing of labor [P = 0.0002; r2 = 0.28; root mean squared error (RMSE) = 0.4252]. Similarly, fetal genotype when examined alone was found to be statistically significantly predictive for the timing of labor (P < 0.0001; r2 = 0.42; RMSE = 0.3886). However, when both maternal and fetal genotypes are included in the regression model, only fetal genotype demonstrated a statistically significant effect on the timing of labor (whole model predicted P < 0.0001; r2 = 0.42; RMSE = 0.3919; maternal genotype leverage predictive P = 0.73; fetal genotype leverage predictive P = 0.01). There was no significant effect of maternal or paternal genotype on pup survival (data not shown).
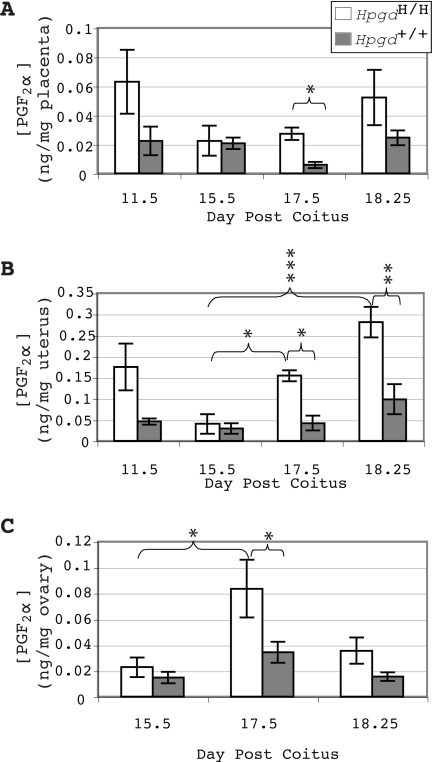
Because PGF2α is primarily inactivated by 15-HPGD (19), and PGF2α has a known prominent role in the induction of labor in mice (13), the concentration of PGF2α was compared in gestational tissues between hypomorphic pregnancies and wild-type pregnancies (Fig. 5, A–C). PGF2α concentrations were significantly elevated in hypomorphic mice relative to the wild-type mice in the uterus and placenta at dpc 17.5 and in the uterus at dpc 18.25. Additionally, PGF2α concentrations were significantly elevated in hypomorphic mice at dpc 17.5 in the uterus and ovaries and in dpc 18.25 uterus relative to hypomorphic mice at dpc 15.5.
Figure 5.
Presence of 15-HPGD Hypomorphic Alleles Leads to Increased Concentrations of PGF2α
15-HPGD hypomorphic pregnancies have increased concentrations of PGF2α in the placenta (A), uterus (B), and ovary (C) on dpc 17.5 and dpc 18.25 (uterus only) relative to control mice. HpgdH/H ovarian PGF2α concentration is significantly increased at dpc 17.5 in comparison with dpc 15.5. Values are mean ± sem. ***, P < 0.001; **, P < 0.01; *, P < 0.05; n = 4–5.
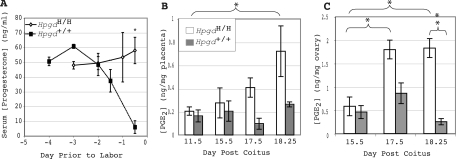
Normally, in mouse pregnancy, signaling of high PGF2α levels through ovarian corpus luteal PGF2α receptors (FP receptors) cause the initiation of labor by inducing luteolysis and a decrease in circulating progesterone (12,13). To determine whether this mechanism underlies the early labor in 15-HPGD hypomorphic mice, serum progesterone was measured during the last several days of pregnancy from hypomorphic (HpgdH/H× HpgdH/H) and wild-type (Hpgd+/+× Hpgd+/+) pregnancies (Fig. 6A). The gravid wild-type females exhibit a progressive fall in serum progesterone as labor approaches similar to what has been observed in inbred C57BL/6 mice previously (20). In contrast, the hypomorphic mice show no decrease in maternal serum progesterone before labor. We also measured serum β-estradiol in hypomorphic and control pregnancies at 17.5 (HpgdH/H 7.5 ± 2.9 pg/ml vs. Hpgd+/+ 9.3 ± 1.0 pg/ml; n = 3) and 18.25 (HpgdH/H 10.3 ± 1.8 pg/ml vs. Hpgd+/+ 5.0 ± 1.7 pg/ml; n = 3) dpc and found no significant differences.
Figure 6.
Measurement of Serum Progesterone and Tissue PGE2 in 15-HPGD Hypomorphic Pregnancies
A, Serum progesterone concentration in hypomorphic and wild-type pregnancies displayed as a function of days before labor onset. Values are mean ± sem. *, P < 0.05; n = 3 for all, except n = 5 for hypomorphic pregnancies one half day before labor. B, PGE2 concentrations in the placenta. *, P < 0.05 compared with all other measures; n = 4. C, PGE2 concentration in ovary. *, P < 0.05; n = 4.
These surprising results prompted us to determine how the corpus luteum is maintained in the context of high PGF2α concentrations. We measured the abundance of the other PG metabolized by 15-HPGD, PGE2, which has been shown to have luteotropic actions and oppose PGF2α luteolytic effects (21,22). PGE2 concentration was significantly elevated in the placenta and ovary of hypomorphic pregnancies at dpc 17.5 and 18.25 (Fig. 6, B and C). Thus, it is likely that the maintenance of the corpus luteum in the context of high PGF2α occurs because of increased trophic effects of PGE2.
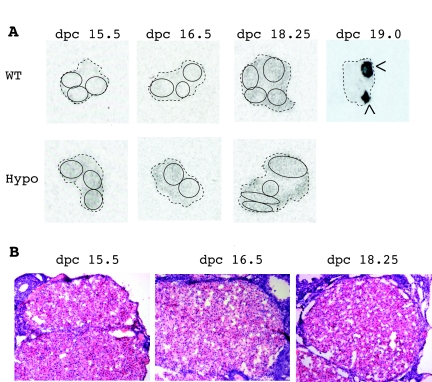
In mice, high PGF2α levels cause the initiation of labor by inducing expression of 20α-hydroxysteroid dehydrogenase (20α-HSD) (23,24) in the corpus luteum leading to luteolysis and a decrease in circulating progesterone concentration. Consistent with the hypothesis that elevated PGE2 maintains ovarian progesterone secretion, we find that despite elevation in ovarian PGF2α concentration in the hypomorphic females at dpc 17.5, no induction of 20α-HSD mRNA occurs (Fig. 7A). This result is in contrast to the robust induction of 20α-HSD mRNA that wild-type females display at dpc 19.0, when progesterone levels are falling just before labor. Consistent with the failure to induce 20α-HSD mRNA, we found no histological evidence for luteolysis in the hypomorphic ovaries at dpc 18.25, with corpora lutea demonstrating prominent vascular spaces, uniform cellular appearance, and organized architecture (Fig. 7B) as opposed to those changes reported in wild-type mice (25).
Figure 7.
No Induction of 20α-HSD mRNA in the Corpus Luteum of 15-HPGD Hypomorphic Ovary at dpc 18.25
A, In situ hybridization for 20α-HSD mRNA detection in dpc 15.5, 16.5, 18.25, and 19.0 ovaries. The corpus luteum is highlighted by circles or arrows (>). B, Hematoxylin- and eosin-stained ovaries from hypomorphic mice show intact corpus luteum histology through d 18.25. Magnification, ×100. Images are representative of n = 2–3 for each genotype and time point.
DISCUSSION
Although several genetically altered murine models exhibit prolonged or delayed labor (12), 15-HPGD hypomorphic mice are the first genetic murine system to exhibit spontaneous preterm labor. This early labor is not entirely unexpected; 15-HPGD is known to have a critical role in metabolizing PGF2α, a PG that causes the initiation of labor through its luteolytic action on the ovaries (13) and directly promotes uterine contractions (11). The decrease in HPGD activity we find in wild-type control placenta, fetal membranes, and uterus late in gestation suggests that down-regulation of HPGD normally occurs to enhance paracrine and more distant actions of PGs. Because HPGD activity is low from at least dpc 11.5, the delay in parturition in the Hpgd+/+ mice to later in pregnancy indicates that the induction of PG synthesis is also an important determinant for parturition timing. In the uterus, induction of cyclooxygenase-1 mRNA and protein has been found to precede the time at which the Hpgd+/+ mice deliver their pups (20,25), consistent with the pattern of tissue PGs that we measure. However, the maintenance of circulating maternal progesterone concentration in association with the early labor was unexpected.
Before this study, another group reported the creation of mice completely lacking 15-HPGD activity (26); however, the labor phenotype in these mice was not described. Unlike our hypomorphic line, these conventional knockout mice die in the perinatal period because of failure to close the ductus arteriosus. We have also generated a complete knockout HPGD line and find similar perinatal mortality (Roizen, J. D., and L. J. Muglia, unpublished data). After indomethacin rescue, mating of HPGD KO males and females results in no pregnancies that last beyond the first week of gestation (Roizen, J. D., and L. J. Muglia, unpublished data), making the current hypomorphic model particularly valuable. Future studies to further mechanistically link augmented uterine PG concentration with the onset of labor in the absence of progesterone withdrawal should be greatly facilitated by the hypomorphic line. Detailed definition of the mechanism of myometrial activation in 15-HPGD hypomorphic mice will offer new insights into how term and preterm labor are initiated in humans and mechanisms of evolution of parturition control in general. Our findings also implicate the utility of evaluating genetic variation in the human HGPD gene as contributing to the risk of human preterm birth.
The observation of the early initiation of labor in the context of sustained circulating progesterone in the 15-HPGD hypomorphic mice is notable in light of the delayed labor observed in the FP receptor knockout mice (13). In the FP receptor knockout mice, labor is delayed due to maintained circulating progesterone. In these mice, there is no evidence of elevated PGE2, however. Although FP receptor activation by its ligand has effects that uniformly accelerate the timing for birth by promoting myometrial contraction and luteolysis, the consequences of PGE2 actions on its receptors (EP receptors) is more complex. In the myometrium, action on the EP2 and EP4 receptors cause myometrial relaxation, whereas action on EP1 and EP3 promote myometrial contraction (27). Both normal gestational changes in relative expression of the FP and EP isoforms in the myometrium, along with changes that may result from the altered PG and progesterone milieu in our hypomorphic line, likely contribute to the timing for preterm birth in the context of high circulating serum progesterone levels. Our results suggest that the increased concentration of PGE2 arising from the placenta may prepare the HpgdH/H uterus for contractions and cause early labor in the setting of maintained circulating progesterone. Alternatively, high concentrations of PGF2α may act via the FP receptor to prepare the uterus for labor even with high progesterone levels, a uterine activity that would not be present in FP receptor knockout mice. This mechanism is also possible because uterine PGF2α is elevated at dpc 17.5 and 18.25 in the gravid HpgdH/H mice.
The profile of PGE2 induction in the HpgdH/H pregnancy further shifts the normal pattern of murine pregnancy to one resembling human pregnancy. Previous studies have shown increased expression of cyclooxygenase isoforms in the placenta as parturition approaches in mice (28), likely accounting for the steady increase in PGE2 that we find in this tissue in the hypomorphic line. The high expression of HPGD in wild-type mice prevents this increase in PGE2 as is demonstrated in unchanged PGE2 in wild-type placenta through dpc 18.25, as we found. The reprogramming of rodent pregnancy from a PGF2α-progesterone-withdrawal system to a PGE2-no-progesterone-withdrawal system suggests that higher primate parturition could have evolved in a one-step process, because an integrated regulatory network already exists to use such a pathway.
In addition to initiating labor in the absence of a decrease in circulating progesterone, the HpgdH/H mice reveal an important role for the fetus in the timing of murine parturition. Hypomorphic females labor significantly early only if their pups are also hypomorphs. Classic studies have previously revealed an important role for the fetal hypothalamic-pituitary-adrenal axis in labor initiation in sheep (29), and recent studies have suggested a novel hormonal role for surfactant apoprotein-A arising from the fetus in contributing to labor initiation in mice (30). The balance of PG signaling during gestation, in contrast to the proposed role of fetal adrenal activity or surfactant apoprotein-A, involves opposing functions of maternal and fetal systems. Previous studies in cycloxygenase-1 (25) or cytosolic phospholipase A2 (31) knockout mice demonstrated that the mother is primarily responsible for the generation of PGs, hastening the onset of labor. We show that the fetus (or fetally derived extraembryonic tissues) has a significant role in breaking down PGs and delaying labor. Such results harmonize well with the current understanding of the relative evolutionary benefits to the mother and the fetus of alterations in the timing of labor (32). Delays in the timing of labor are advantageous to the fetus relative to the mother as a delay increases the chance of further organ and cognitive maturation at the time of delivery, whereas early labor is advantageous to the mother in that it enables her to more easily deliver a smaller fetus, conserve nutrients, and better protect herself from environmental stressors.
MATERIALS AND METHODS
Generation of HpgdH/H Mice
To construct the conditional 15-HPGD targeting vector, loxP sites were inserted into unique restriction sites upstream of exon 1 (HindIII) and downstream of exon 2 (BamHI) in the Hpgd gene. The downstream loxP site was adjacent to a neomycin selection cassette. To obtain embryonic stem (ES) cell clones heterozygous for a targeting event at the Hpgd locus, the linearized construct was electroporated into TC1 ES cells (33). Clones selected for G418 resistance were analyzed by Southern blot for homologous recombination into the endogenous locus. ES clones containing the targeting construct integrated into the endogenous locus were injected into C57BL/6 blastocysts to generate a high percentage of male chimeras. Using this method, two mouse lines have been created; one line is a conditional KO line (data not shown), and the other line through the partial tandem insertion of the construct led to the creation of the HpgdH/H hypomorphic line. All additional mice generated were of a mixed C57BL/6-129/SvJ background, with littermates used as controls. All animal experimentation described was conducted in accord with accepted standards of humane animal care and was approved by the Washington University in St. Louis Animal Studies Committee.
Labor Timing
To examine the effect of a reduction in 15-PGDH activity on the timing of labor, female mice were placed in cages with a male, removed from that cage after mating (determined by detection of a copulation plug), and observed for the timing of birth of the first pup twice daily. Results were analyzed by ANOVA and Tukey’s multiple t tests. Additionally, to examine the relative contributions of maternal and fetal genotype, multiple regression was performed using Jmp (version 5, release 5.1; SAS Institute, Cary, NC) fit model function (standard least squares, effect leverage). Day of labor was set as the dependent variable and maternal and fetal genotypes were set (individually and then together) as independent variables.
Hormone Measurements
Plasma progesterone concentration was determined by RIA kit as described by the manufacturer (Diagnostic Products Corp., Los Angeles, CA) on blood samples obtained by retroorbital phlebotomy. Each measurement was performed on at least three separate pregnancies of each genotype and analyzed for significance by two-way ANOVA, and then each pair was analyzed by Bonferroni post tests. Uterine, placental, fetal membrane, and ovarian PGF2α and PGE2 were measured on tissue harvested and rapidly frozen in liquid nitrogen from three separate pregnancies per genotype at each time point. Tissue was weighed while frozen and then homogenized in 100% ethanol for extraction of PGs. Debris was removed by centrifugation, and each supernatant was assayed in duplicate per the manufacturer’s instructions (Oxford Biomedical Research, Oxford, MI). Measurements were analyzed for statistical significance by ANOVA.
Northern Hybridization Analyses
RNA was prepared from frozen tissue using the RNEasy Midi kit (QIAGEN, Valencia, CA) as described by the manufacturer. Five micrograms of total RNA from each tissue and genotype were subjected to electrophoresis through 1.7% agarose-formaldehyde gels and transferred to nitrocellulose membranes. cRNA probes specific for mouse 15-HPGD mRNA (834-bp fragment from nucleotides 13–846 in pBluescript SK II+) labeled with [α-32P]UTP were generated by transcription with T3 polymerase and hybridized at 65 C in 50% formamide-containing buffer. After being washed, hybridizing probes were quantitated on a Molecular Dynamics (Sunnyvale, CA) PhosphorImager. Each mRNA hybridization signal was corrected for loading and recovery by normalization to cyclophilin A mRNA hybridization on the same filter. Statistical significance was determined by ANOVA.
In Situ Hybridization
In situ hybridization was performed as described previously (25). Fetuses with surrounding membranes and uteri were fixed by immersion in 4% paraformaldehyde in PBS for 24 h at 4 C. Samples were then cryopreserved in 10% sucrose in PBS and embedded in OCT compound (Sakura Finetek, Torrance, CA) for sectioning on a cryostat. Ten-micrometer sections were thaw mounted onto Superfrost plus slides (Fisher Scientific, Pittsburgh, PA) and hybridized to an [α-33P]UTP-labeled 15-HPGD or 20α-HSD antisense riboprobes. After washing, slides were exposed to autoradiographic film and scanned at high resolution. To assess corpus luteum histology, hybridized slides were stained with hematoxylin and eosin and evaluated by light microscopy.
15-HPGD Activity Measurements
15-HPGD activity was assayed as described previously (34) by measuring the transfer of tritium from 15(S)-[15-3H]PGE2 to glutamate by coupling 15-PGDH with glutamate dehydrogenase. Results were analyzed for statistical significance by ANOVA.
Acknowledgments
We thank Drs. Jonathan Gitlin and Peter Nathanielsz for manuscript review and Sherri Vogt for technical assistance.
Footnotes
This work was supported by grants from the March of Dimes (L.J.M.), National Institutes of Health (HL-46296 to H.-H.T.), and Kentucky Lung Cancer Research Program (H.-H.T.).
Disclosure Statement: The authors have nothing to disclose.
First Published Online September 13, 2007
Abbreviations: dpc, Day post coitus; EP receptor, PGE2 receptor; ES, embryonic stem; FP receptor; PGF2α receptor; 15-HPGD, 15-hydroxyprostaglandin; 20α-HSD, 20α-hydroxysteroid dehydrogenase; PG, prostaglandin; RMSE, root mean squared error.
References
- Martin JA, Hamilton BE, Sutton PD, Ventura SJ, Menacker F, Munson ML 2005 Births: final data for 2003. Natl Vital Stat Rep 54:1–116 [PubMed] [Google Scholar]
- Committee on Understanding Premature Birth and Assuring Healthy Outcomes Board on Health Sciences Policy 2006 Preterm birth: causes, consequences, and prevention. Washington, DC: The National Academies Press [Google Scholar]
- Csapo AI, Eskola J, Ruttner Z 1980 The biological meaning of progesterone levels. Prostaglandins 19:203–211 [DOI] [PubMed] [Google Scholar]
- Hall K 1956 Maintenance of pregnancy, parturition and rearing of litters in mice ovariectomized and injected with progesterone, oestradiol and relaxin. J Physiol 134:17P–18P [PubMed] [Google Scholar]
- Thorburn GD, Challis JR 1979 Endocrine control of parturition. Physiol Rev 59:863–917 [DOI] [PubMed] [Google Scholar]
- Challis JR, Matthews SG, Gibb W, Lye SJ 2000 Endocrine and paracrine regulation of birth at term and preterm. Endocr Rev 21:514–550 [DOI] [PubMed] [Google Scholar]
- Smith R, Mesiano S, McGrath S 2002 Hormone trajectories leading to human birth. Regul Pept 108:159–164 [DOI] [PubMed] [Google Scholar]
- Allport VC, Pieber D, Slater DM, Newton R, White JO, Bennett PR 2001 Human labour is associated with nuclear factor-κB activity which mediates cyclo-oxygenase-2 expression and is involved with the ‘functional progesterone withdrawal.’ Mol Hum Reprod 7:581–586 [DOI] [PubMed] [Google Scholar]
- Condon JC, Jeyasuria P, Faust JM, Wilson JW, Mendelson CR 2003 A decline in the levels of progesterone receptor coactivators in the pregnant uterus at term may antagonize progesterone receptor function and contribute to the initiation of parturition. Proc Natl Acad Sci USA 100:9518–9523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesiano S, Chan EC, Fitter JT, Kwek K, Yeo G, Smith R 2002 Progesterone withdrawal and estrogen activation in human parturition are coordinated by progesterone receptor A expression in the myometrium. J Clin Endocrinol Metab 87:2924–2930 [DOI] [PubMed] [Google Scholar]
- Challis JR, Lye SJ, Gibb W 1997 Prostaglandins and parturition. Ann NY Acad Sci 828:254–267 [DOI] [PubMed] [Google Scholar]
- Gross G, Imamura T, Muglia LJ 2000 Gene knockout mice in the study of parturition. J Soc Gynecol Investig 7:88–95 [PubMed] [Google Scholar]
- Sugimoto Y, Yamasaki A, Segi E, Tsuboi K, Aze Y, Nishimura T, Oida H, Yoshida N, Tanaka T, Katsuyama M, Hasumoto K, Murata T, Hirata M, Ushikubi F, Negishi M, Ichikawa A, Narumiya S 1997 Failure of parturition in mice lacking the prostaglandin F receptor. Science 277:681–683 [DOI] [PubMed] [Google Scholar]
- Okita RT, Okita JR 1996 Prostaglandin-metabolizing enzymes during pregnancy: characterization of NAD+-dependent prostaglandin dehydrogenase, carbonyl reductase, and cytochrome P450-dependent prostaglandin ω-hydroxylase. Crit Rev Biochem Mol Biol 31:101–126 [DOI] [PubMed] [Google Scholar]
- Tai HH, Ensor CM, Tong M, Zhou H, Yan F 2002 Prostaglandin catabolizing enzymes. Prostaglandins Other Lipid Mediat 68–69:483–493 [DOI] [PubMed] [Google Scholar]
- Sangha RK, Walton JC, Ensor CM, Tai HH, Challis JR 1994 Immunohistochemical localization, messenger ribonucleic acid abundance, and activity of 15-hydroxyprostaglandin dehydrogenase in placenta and fetal membranes during term and preterm labor. J Clin Endocrinol Metab 78:982–989 [DOI] [PubMed] [Google Scholar]
- Van Meir CA, Ramirez MM, Matthews SG, Calder AA, Keirse MJNC, Challis JRG 1997 Chorionic prostaglandin catabolism is decreased in the lower uterine segment with term labor. Placenta 18:109–114 [DOI] [PubMed] [Google Scholar]
- Wang H, Hirsch E 2003 Bacterially-induced preterm labor and regulation of prostaglandin-metabolizing enzyme expression in mice: the role of toll-like receptor 4. Biol Reprod 69:1957–1963 [DOI] [PubMed] [Google Scholar]
- Jarabak J, Fried J 1979 Comparison of substrate specificities of the human placental NAD- and NADP-linked 15-hydroxyprostaglandin dehydrogenases. Prostaglandins 18:241–246 [DOI] [PubMed] [Google Scholar]
- Winchester SK, Imamura T, Gross G, Muglia LM, Vogt SK, Wright J, Watanabe K, Tai HH, Muglia LJ 2002 Coordinate regulation of prostaglandin metabolism for induction of parturition in mice. Endocrinology 143:2593–2598 [DOI] [PubMed] [Google Scholar]
- Christenson LK, Farley DB, Anderson LH, Ford SP 1994 Luteal maintenance during early pregnancy in the pig: role for prostaglandin E2. Prostaglandins 47:61–75 [DOI] [PubMed] [Google Scholar]
- Henderson KM, Scaramuzzi RJ, Baird DT 1977 Simultaneous infusion of prostaglandin E2 antagonizes the luteolytic action of prostaglandin F2α in vivo. J Endocrinol 72:379–383 [DOI] [PubMed] [Google Scholar]
- Piekorz RP, Gingras S, Hoffmeyer A, Ihle JN, Weinstein Y 2005 Regulation of progesterone levels during pregnancy and parturition by signal transducer and activator of transcription 5 and 20α-hydroxysteroid dehydrogenase. Mol Endocrinol 19:431–440 [DOI] [PubMed] [Google Scholar]
- Stocco CO, Zhong L, Sugimoto Y, Ichikawa A, Lau LF, Gibori G 2000 Prostaglandin F2α-induced expression of 20α-hydroxysteroid dehydrogenase involves the transcription factor NUR77. J Biol Chem 275:37202–37211 [DOI] [PubMed] [Google Scholar]
- Gross GA, Imamura T, Luedke CE, Vogt SK, Olson LM, Nelson DM, Sadovsky Y, Muglia LJ 1998 Opposing actions of prostaglandins and oxytocin determine the onset of murine labor. Proc Natl Acad Sci USA 95:11875–11879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coggins KG, Latour A, Nguyen MS, Audoly L, Coffman TM, Koller BH 2002 Metabolism of PGE2 by prostaglandin dehydrogenase is essential for remodeling the ductus arteriosus. Nat Med 8:91–92 [DOI] [PubMed] [Google Scholar]
- Myatt L, Lye SJ 2004 Expression, localization and function of prostaglandin receptors in myometrium. Prostaglandins Leukot Essent Fatty Acids 70:137–148 [DOI] [PubMed] [Google Scholar]
- Cook JL, Shallow MC, Zaragoza DB, Anderson KI, Olson DM 2003 Mouse placental prostaglandins are associated with uterine activation and the timing of birth. Biol Reprod 68:579–587 [DOI] [PubMed] [Google Scholar]
- McDonald TJ, Nathanielsz PW 1991 Bilateral destruction of the fetal paraventricular nuclei prolongs gestation in sheep. Am J Obstet Gynecol 165:764–770 [DOI] [PubMed] [Google Scholar]
- Condon JC, Jeyasuria P, Faust JM, Mendelson CR 2004 Surfactant protein secreted by the maturing mouse fetal lung acts as a hormone that signals the initiation of parturition. Proc Natl Acad Sci USA 101:4978–4983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonventure JV, Huang Z, Taheri MR, O’Leary E, Li E, Moskowitz MA, Sapirstein A 1997 Reduced fertility and postischaemic brain injury in mice deficient in cytosolic phospholipase A2. Nature 390:622–625 [DOI] [PubMed] [Google Scholar]
- Pike IL 2005 Maternal stress and fetal responses: evolutionary perspectives on preterm delivery. Am J Hum Biol 17:55–65 [DOI] [PubMed] [Google Scholar]
- Deng C, Wynshaw-Boris A, Shen MM, Daugherty C, Ornitz DM, Leder P 1994 Murine FGFR-1 is required for early postimplantation growth and axial organization. Genes Dev 8:3045–3057 [DOI] [PubMed] [Google Scholar]
- Ensor CM, Tai HH 1996 Cysteine 182 is essential for enzymatic activity of human placental NAD+-dependent 15-hydroxyprostaglandin dehydrogenase. Arch Biochem Biophys 333:117–120 [DOI] [PubMed] [Google Scholar]