Abstract
Selective estrogen receptor (ER) modulators (SERMs) are ER ligands whose relative agonist/antagonist activities vary in a cell- and promoter-dependent manner. The molecular basis underlying this selectivity can be attributed to the ability of these ligands to induce distinct alterations in ER structure leading to differential recruitment of coactivators and corepressors. Whether SERM activity is restricted to synthetic ligands or whether molecules exist in vivo that function in an analogous manner remains unresolved. However, the recent observation that oxysterols bind ER and antagonize the actions of 17β-estradiol (E2) on the vascular wall suggests that this class of ligands may possess SERM activity. We demonstrate here that 27-hydroxycholesterol (27HC), the most prevalent oxysterol in circulation, functions as a SERM, the efficacy of which varies when assessed on different endpoints. Importantly, 27HC positively regulates both gene transcription and cell proliferation in cellular models of breast cancer. Using combinatorial peptide phage display, we have determined that 27HC induces a unique conformational change in both ERα and ERβ, distinguishing it from E2 and other SERMs. Thus, as with other ER ligands, it appears that the unique pharmacological activity of 27HC relates to its ability to impact ER structure and modulate cofactor recruitment. Cumulatively, these data indicate that 27HC is an endogenous SERM with partial agonist activity in breast cancer cells and suggest that it may influence the pathology of breast cancer. Moreover, given the product-precursor relationship between 27HC and cholesterol, our findings have implications with respect to breast cancer risk in obese/hypercholesteremic individuals.
THE ESTROGEN RECEPTOR (ER) is a member of the nuclear hormone receptor superfamily of ligand-inducible transcription factors. Upon ligand binding, ER undergoes a conformational change that facilitates receptor dimerization, DNA binding, recruitment of transcriptional coregulators, and modulation of target gene expression. There are two genetically distinct ER isoforms (α and β) that differ in terms of their expression patterns, ligand binding preferences, and biological activities (1,2,3). Although specific biological responses have been attributed to agonist-activated ERα or ERβ, it is also clear that in cells where both receptors are expressed, ERβ functions to dampen ERα transcriptional activity (4). Thus, the pharmacological response of target cells to estrogens and antiestrogens represents the composite activities of both receptors acting as homodimeric or heterodimeric complexes.
Among the endogenous estrogens, 17β-estradiol (E2) is the most potent and functions as a ligand for both ERα and ERβ (5). However, in postmenopausal women, estrone (E1) and estriol (E3) are also likely to be important ER ligands. Besides differences in their pharmacokinetic properties, it is generally considered that the mechanism of action of these endogenous estrogens is similar. This result is in contrast to what has emerged from studies aimed at developing new classes of ER agonists and antagonists. From these efforts have emerged the selective ER modulators (SERMs), compounds whose relative agonist/antagonist activities are manifest in a cell- and promoter-selective manner. The molecular basis of SERM activity is now well established and has been attributed to the ability of these molecules to induce different changes in receptor architecture, an event that engenders the recruitment of functionally distinct cofactors. Until recently, it was not anticipated that there were any endogenous molecules with SERM-like activity. However, the recent observation that the oxysterol 27-hydroxycholesterol (27HC) is a bona fide ER ligand that displays partial agonist activity in the vasculature of ovariectomized mice has raised the possibility that the SERM concept may extend to endogenous ligands (6).
Oxysterols are hydroxylated metabolites of cholesterol that have been previously described as ligands for nuclear receptors, most notably for liver X receptor (LXR) (7). These molecules are produced in many cell types as primary and secondary metabolites of cholesterol. Outside the liver, cholesterol can be hydroxylated via the acidic bile acid synthesis pathway, a process that is initiated by the cytochrome P450 enzyme CYP27A1 (8). This enzyme catalyzes the conversion of cholesterol to 27HC, the principal endogenous oxysterol (Fig. 1), which can then be further metabolized by CYP7B1 into more polar bile acid intermediates (9). Of interest to us was the observation that oxysterol concentrations are particularly high in the vasculature, where the role of ER has been well established (10,11,12,13). In the cardiovascular system, both macrophages and endothelial cells express CYP27A1 and can convert cholesterol to 27HC. This is particularly evident in atherosclerotic lesions where resident macrophages exist. It is not surprising, therefore, given its ability to bind ER, that 27HC has estrogenic activities in the vasculature (6).
Figure 1.
ER Ligand Structures
Estrone (E1), E2, estriol(E3), 27-hydroxycholesterol (27HC), 4-hydroxy-tamoxifen (40HT), and ICI182,780 (ICI).
Of particular interest is the observation that the presence of infiltrating macrophages in breast cancer is associated with decreased disease-free survival (14). This raises the possibility that local production of estrogenic oxysterols by tumor-associated macrophages may have an impact on the biology of ER-positive breast cancer. This prompted us to define the molecular pharmacology of 27HC in established cellular models of estrogen action, a first step in evaluating its role in the pathobiology of ER-positive breast tumors.
RESULTS
27HC Regulates ERα Transcriptional Activity
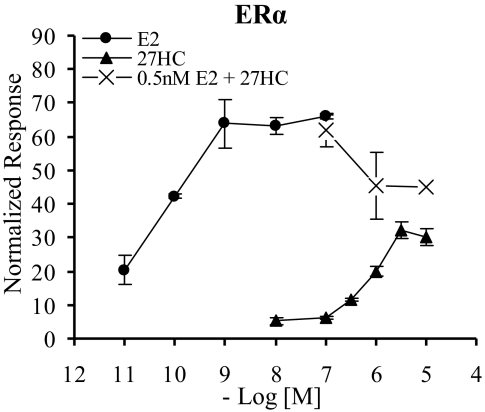
We previously determined that 27HC is an ER ligand that inhibits both the genomic and nongenomic actions of E2 in the cardiovascular system (6). In this study, we focus on defining the molecular mechanisms underlying the distinct pharmacological action of 27HC in a variety of cellular models of estrogen action. Our first objective therefore was to evaluate the ability of 27HC to regulate the transcriptional activity of ERα. For the initial studies, we elected to measure the transcriptional response of this ligand on a classic estrogen response element (ERE) using a well-defined system of transfected receptor and reporter in ER-negative mammalian cell lines. In this experiment, HeLa cells were transiently transfected with ERα and an ERE-luciferase (3XERE-TATA-Luc) reporter. As observed in Fig. 2, 27HC induced transactivation of ERα in a dose-dependent manner. Notably, the maximal transcriptional activity of ERα in the presence of 27HC did not reach that induced by E2. The dose-response curve is shifted to the right, reflecting the difference in affinity of this compound for ERα (27HC Ki = 1.32 μm, E2 Kd = 0.1 nm) (2,6). Similar results were obtained with ERβ, and the findings were reconfirmed in both CV-1 and HepG2 cells, two additional ER-negative mammalian cell lines (data not shown). The concentration range at which 27HC activates ERα transcriptional activity is physiologically relevant, because the circulating concentration of 27HC ranges from 0.15–0.73 μm in a healthy individual, and can reach a local concentration in the millimolar range within atherosclerotic plaques in an individual with severe cardiovascular disease (6,9). In addition, 27HC levels in the mouse aorta were found to be 0.25–0.6 μm in the absence of disease, and notably over 30% of this 27HC was unesterified (6). Interestingly, the Km of 27HC for its catabolic enzyme, CYP7B1, is 24 μm, which is significantly higher than that required to saturate ERα (6). Knowing that 27HC can effectively compete with E2 for binding to ERα at physiological concentrations in an in vitro binding assay (6), we next evaluated its ability to antagonize activation by E2. At a physiologically relevant E2 concentration (0.5 nm), increasing concentrations of 27HC reduced the E2-induced transcriptional activity of ERα (Fig. 2). These data were the first indication that 27HC may in fact be a classic partial agonist or a SERM, with both agonist and antagonist properties.
Figure 2.
Dose-Dependent Induction of ERα Transcriptional Activity by 27HC
Transcriptional activity of ERα was examined in the human ER-negative cell line, HeLa. Cells were transfected overnight with an expression plasmid for ERα and a 3X-ERE-TATA-luc reporter and then treated overnight with vehicle or increasing doses of E2 or 27HC or combinations of both as indicated. After treatment, cells were harvested and assayed for luciferase activity. Luciferase values were normalized to β-galactosidase control. Data are the mean ± sem for one representative experiment performed in triplicate. The combinations of 0.5 nm E2 + 1 μm 27HC and 0.5 nm E2 + 10 μm 27HC are significantly different from 0.5 nm E2 alone (P < 0.05 and P < 0.001 by t test, respectively).
27HC Elicits a Unique Conformational Change in the Structure of ERα
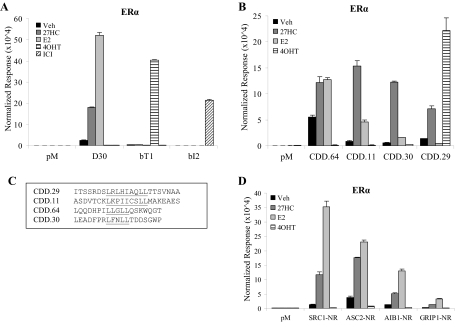
The transcription assays performed above indicated that 27HC functioned as a partial agonist, eliciting a similar response as E2 albeit with lower efficacy. This prompted us to determine whether or not there were mechanistic differences between 27HC and E2 that may help to define its pharmacological identity. One hallmark of ER ligands is that they elicit specific conformational changes within the receptor that dictate their biological response. These ligand-induced conformational changes can be identified using specific peptide conformational probes that bind to differentially exposed protein-protein interaction surfaces (15). Using peptides recognizing either the coactivator or corepressor binding surfaces on the receptor, it is possible to determine the likelihood that a given ligand will function as an agonist or an antagonist. We have previously identified peptides that bind specifically to ERα in the presence of E2, the SERM 4-hydroxy-tamoxifen (4OHT), or the pure antiestrogen ICI 182,780 (ICI) (16,17,18). Therefore, we used these previously identified peptides to see whether the conformation of ERα induced by 27HC shared any similarities with those induced by other ligands. To this end, we analyzed by mammalian two-hybrid assay the interaction of ERα with this set of peptides in the presence of vehicle, 27HC, E2, 4OHT, or ICI. In this assay, ERα was fused to the VP16 transactivation domain, and the peptides were linked to the yeast Gal4 DNA-binding domain (Gal4DBD). Interaction between the receptor and peptide was assessed by measuring the transcriptional readout of a 5XGal4Luc3 luciferase reporter. This analysis revealed that the D30 peptide binds only pure agonist-activated ERα, bT1 binds ERα in the presence of 4OHT, and bI2 binds the receptor in the presence of ICI (16,17). As shown in Fig. 3A, 27HC also leads to recruitment of D30 to ERα, indicating that it induces a more agonist-like receptor conformation. 27HC-bound ERα does not adopt a conformation conducive to binding of bT1 or bI2, confirming our expectations that the conformational change induced by 27HC is similar to E2 and distinct from 4OHT or ICI.
Figure 3.
27HC Induces a Unique Active Conformation of ERα
ER-negative HepG2 cells were transfected overnight with VP16, VP16-ERα, a 5XGal4Luc3 reporter, the β-galactosidase transfection control, and the following peptides: A, pM (vector control), D30, bT1, or bI2; B, pM, CDD.64, CDD.11, CDD.30, or CDD.2; and D, pM, SRC1-NR, ASC2-NR, AIB1-NR, or GRIP1-NR. After transfection, cells were treated overnight with ligands as indicated, including vehicle (Veh), 1 μm 27HC, 1 nm E2, 100 nm 4OHT, or 100 nm ICI. Cells were harvested and assayed for luciferase activity. Data are presented as raw luciferase values normalized to β-galactosidase control values. No significant interaction occurred between VP16 and the peptides or between pM and VP16-ERα. Data are the mean ± sem for one representative experiment performed in triplicate. C, Sequences of select peptides identified in this study.
We next performed combinatorial peptide phage display to identify peptide probes that could be used to evaluate the impact of 27HC on ERα structure and how this differs from E2-activated receptor. For this study, we screened a peptide library containing the LxxLL motif found in nuclear receptor (NR) coactivators (19). Additionally, because 27HC did not exhibit full agonist activity, we also screened a peptide library containing the CoRNR box motif characteristic of corepressor proteins (20,21). Using a modified M13 phage display screen (16), we isolated peptides from both libraries that interacted with 27HC-bound ERα and classified these interacting peptides based on the ligands that elicited an interaction with ERα. The binding profile of representative peptides is shown in Fig. 3B. The majority of the peptides identified recognized the conformations of ERα bound by either 27HC or E2, with varying degrees of selectivity for 27HC over E2. Some peptides interacted equally well with the receptor conformation induced by 27HC or E2 (CDD.64). Importantly, we also identified peptides that preferred the conformation of ERα in the presence of 27HC vs. E2 (CDD.11 and CDD.30) and were intrigued by the finding that this group consisted of peptides containing either an LxxLL or a CoRNR box motif. Interestingly, we also found peptides that bind preferentially to 27HC- and 4OHT-bound ERα but not E2-bound ERα (CDD.29). The sequence of this peptide contains the CoRNR box motif found in corepressors. It is known that 4OHT-bound ERα recruits corepressor proteins, a key element of its ability to manifest antagonist activity. We concluded from these peptide binding studies that 27HC-bound ERα undergoes a conformational change that may allow for the recruitment of both coactivator and corepressor peptides and confirms that 27HC induces a unique active conformation of ERα. This may explain the partial agonist/SERM activity of this oxysterol.
27HC-Bound ERα Recruits Coactivator Peptides
In the presence of E2, the conformational change in ERα allows for the recruitment of peptides containing the NR interaction motifs found within coactivators such as steroid receptor coactivator 1 (SRC1), activating signal cointegrator-2 (ASC2), amplified-in-breast cancer 1 (AIB1), and glucocorticoid receptor interacting protein 1 (GRIP1) (16). We therefore investigated whether the conformational change induced by 27HC also allows for the recruitment of these coactivator peptides. In cells transfected with VP16-ERα, coactivator peptides fused to Gal4DBD, and a 5XGal4Luc3 luciferase reporter, treatment with E2 or 27HC, but not 4OHT, led to recruitment of SRC1-NR, ASC2-NR, AIB1-NR, and GRIP1-NR to ERα (Fig. 3D). These data provided additional support for the idea that 27HC is indeed an estrogen capable of inducing an active activation function-2 (AF-2) conformation and is likely to be a physiologically relevant estrogen in some contexts.
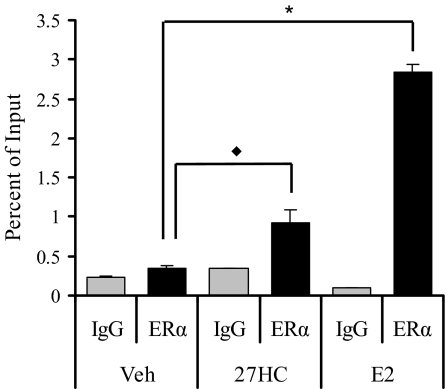
27HC Treatment Increases ERα Occupancy at the pS2 Promoter
Although we have shown that 27HC, like E2, regulates ERα transcriptional activity, we sought to determine whether activation by 27HC allows the recruitment of ERα to DNA elements analogous to that seen with E2. Therefore, we performed chromatin immunoprecipitation in MCF7 cells to analyze ERα recruitment to the well-characterized pS2 promoter. Treatment for 45 min with E2 or 27HC led to a significant recruitment of ERα to the ERE-containing region of the pS2 promoter (Fig. 4) and not to a distal non-estrogen-responsive DNA region as a negative control (data not shown).
Figure 4.
27HC-Bound ERα Is Recruited to the ERE-Containing Region within the pS2 Promoter
Recruitment of ERα to the pS2 promoter was analyzed in MCF7 cells treated with vehicle (Veh), 100 nm E2, or 10 μm 27HC for 45 min. Cells were harvested after cross-linking and subjected to immunoprecipitation with either rabbit IgG control (IgG) or ERα antibody (ERα). After reversal of the cross-linking, DNA was isolated and subjected to qRT-PCR analysis. There was no significant recruitment of ERα to a distal region of the pS2 promoter. There was significant recruitment of ERα to the ERE-containing region within the pS2 promoter in the presence of E2 (*, P < 0.0001, t test) and 27HC (♦, P < 0.05, t test) when compared with vehicle. Data are the mean ± sem for triplicate amplification reactions from one representative experiment.
27HC Regulates Endogenous ERα-Target Gene Expression in MCF7 and T47D Breast Cancer Cells
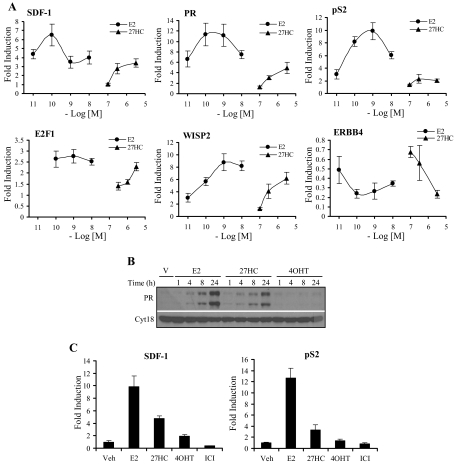
Given that 27HC displayed agonist behavior in the above assays and allows for recruitment of ERα to DNA response elements, we sought to determine whether 27HC acts as an agonist in ERα-positive breast cancer cell lines. Using the ERα-positive MCF7 human breast cancer cell line, we analyzed by quantitative RT-PCR (qRT-PCR) the ability of 27HC to regulate ERα-target gene expression. Increasing concentrations of 27HC led to target gene regulation similar to treatment with increasing concentrations of E2, albeit with lower efficacy in some cases, a reflection of its partial agonist activity (Fig. 5A). Significant regulation of ERα-target gene expression occurred at physiological concentrations of 27HC (0.5–1 μm).
Figure 5.
27HC Has Agonist Activity on Endogenous ERα-Target Genes in Breast Cancer Cells
Expression of ERα-target genes was measured by qRT-PCR in ERα-positive MCF7 (A) and T47D (C) breast cancer cells. MCF7 cells were treated with vehicle or increasing concentrations of E2 or 27HC for either 8 h (SDF-1, PR, pS2, and E2F1) or 24 h (WISP2 and ERBB4). T47D cells were treated with vehicle (Veh), 1 nm E2, 1 μm 27HC, 100 nm 4OHT, or 100 nm ICI 182,780 (ICI) for either 8 h (SDF-1) or 24 h (pS2). After treatment, cells were harvested, total RNA was isolated, and cDNA was prepared for use as a template for gene expression analysis. All values were normalized to the housekeeping gene 36B4. Data are presented as the fold induction over vehicle. Data are the mean ± sem of triplicate amplification reactions from one representative experiment that was repeated with similar results three independent times. B, Expression of PR was analyzed by Western blotting in MCF7 cells. Cells were treated with vehicle (V), 1 nm E2, 10 μm 27HC, or 100 nm 4OHT for 1, 4, 8, or 24 h. Cells were harvested, and 50 μg whole-cell extract was resolved by SDS-PAGE, transferred to nitrocellulose, and subjected to immunoblotting for PR or cytokeratin 18 as a loading control. A representative blot is shown.
ERα is able to regulate gene expression in a direct manner through its interaction with an ERE and by indirect mechanisms through interactions with Fos and Jun at AP1 elements and with Sp1 at GC-rich motifs (22,23). Given that the SERM 4OHT is able to activate transcription of ERα-target genes under control of an AP1 element but not those under control of an ERE (24), we asked whether 27HC exhibited any selectivity with respect to the expression of genes using these two modes of ER-mediated transcriptional regulation. Interestingly, we found that 27HC regulated target gene expression at 1) classical EREs pS2 (trefoil factor 1) and WISP2 (WNT1-inducible signaling pathway protein 2), 2) AP1 elements ERBB4 (v-erb-a erythroblastic leukemia viral oncogene homolog 4) and PR (progesterone receptor), and 3) Sp1 sites PR and E2F1 (E2F transcription factor 1) (Fig. 5A) (25,26,27,28,29,30). Therefore, with respect to this activity of ERα, 27HC most closely resembled E2.
In addition to gene expression, we also analyzed the expression of progesterone receptor (PR) protein, a robust surrogate marker for ER activation. As expected, there was an increase in PR protein beginning at 4 h and increasing through 24 h upon treatment with E2 (Fig. 5B). A similar pattern was observed when the cells were treated with 27HC, albeit with a slight temporal delay. As a control, treatment with 4OHT did not affect PR protein expression.
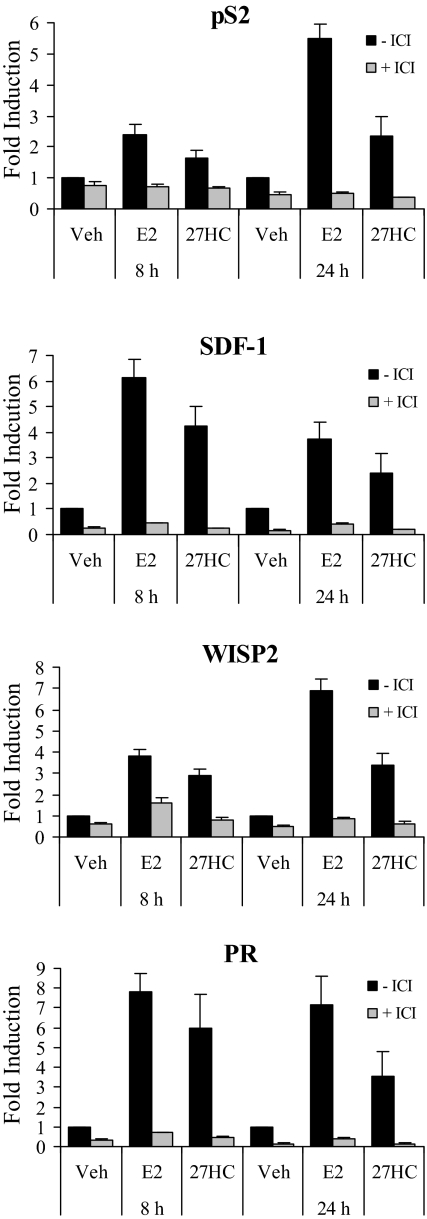
Although the most studied ERα transcriptional targets are up-regulated by treatment with E2, many genes important for growth, differentiation, and signaling are down-regulated upon treatment with E2. These include the growth factor receptor ERBB4, IL1-R1, SMAD3, and Id2 (31). Importantly, these E2 down-regulated genes underwent a similar decrease in transcript level upon treatment with 27HC (Fig. 5A and data not shown), although the kinetics were not identical to that observed in E2-treated cells. It appeared that 27HC may be more active in down-regulating than in up-regulating ERα-target genes, which was not surprising considering the 27HC-ERα complex recruited peptides containing the CoRNR box motif found in corepressors more efficiently than E2-ERα. Similar transcriptional responses were observed in E2- or 27HC-treated T47D breast cancer cells (Fig. 5C). We also demonstrated in both cell lines that target gene induction by E2 and 27HC was abrogated by cotreatment with the pure antagonist ICI (Fig. 6 and data not shown). Cumulatively, these data confirm that 27HC is working through ERα in these breast cancer cell lines and that pharmacologically, it closely resembles E2.
Figure 6.
Activation of ERα by 27HC Is Suppressed by the Pure Antagonist ICI
Expression of the ERα target genes pS2, SDF-1, WISP2, and PR was measured by qRT-PCR in MCF7 cells. Cells were treated with vehicle (Veh), 1 nm E2, or 1 μm 27HC in the presence or absence of 100 nm ICI. After 8 or 24 h, the cells were harvested, total RNA was isolated, and cDNA was prepared for use as a template for gene expression analysis. All values were normalized to the housekeeping gene 36B4. Data are presented as the fold induction over vehicle. Data are the mean ± sem of triplicate amplification reactions from one representative experiment.
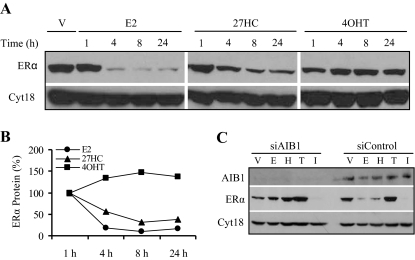
27HC Induces ERα Protein Degradation
One hallmark of ERα agonists is their ability to induce receptor turnover. Treatment with E2 leads to ERα degradation, an event that is linked to transcriptional activity (32,33,34). Therefore, we evaluated the ability of 27HC to regulate ERα turnover. In MCF7 cells, we determined that treatment with 27HC led to a decrease in ERα protein levels over 24 h (Fig. 7A). The percent ERα protein remaining is depicted in Fig. 7B, where it is evident that 27HC is again acting similarly to E2, and mechanistically distinct from 4OHT. 27HC does not induce degradation of ERα as robustly as E2, most likely reflecting the partial agonist activity of 27HC.
Figure 7.
Degradation of 27HC-Bound ERα Required AIB1
A, Ligand-mediated degradation of ERα was examined in MCF7 cells treated for 1, 4, 8, or 24 h with vehicle (V), 1 nm E2, 10 μm 27HC, or 100 nm 4OHT. Cells were harvested, and 50 μg whole-cell extract was resolved by SDS-PAGE, transferred to nitrocellulose, and subjected to immunoblotting for ERα or cytokeratin 18 as a loading control. B, Quantitation of the data in A. Images were scanned and the bands were quantitated using Image J software. Percent ERα protein remaining was not different with vehicle treatment or 1 h ligand treatment. C, The requirement for AIB1 in 27HC-mediated ERα degradation was determined by transiently transfecting MCF7 cells with siRNA to AIB1 (siAIB1) or siRNA control (siControl). After 48 h, cell were treated for 8 h with vehicle (V), 1 nm E2 (E), 10 μm 27HC (H), 100 nm 4OHT (T), or 100 nm ICI (I). Cells were harvested, and whole-cell extract was resolved by SDS-PAGE, transferred to nitrocellulose, and subjected to immunoblotting for AIB1, ERα, or cytokeratin 18. A representative blot is shown.
The coactivator AIB1 has been shown to be required for E2-mediated ERα degradation but not for ICI-mediated degradation (35). Therefore, we investigated the importance of AIB1 in 27HC-induced ERα degradation. Using small interfering RNA (siRNA) technology, we knocked down AIB1 expression and analyzed ERα protein levels after 8 h of ligand treatment (Fig. 7C). As with E2, AIB1 is required for 27HC-mediated ERα degradation, suggesting that the mechanisms of E2- and 27HC-induced ERα turnover are likely very similar.
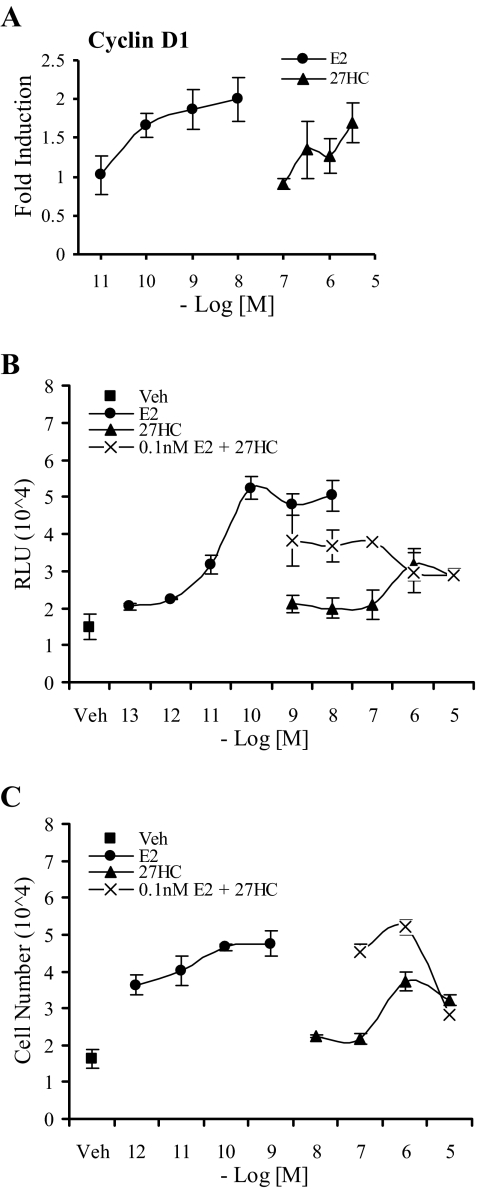
MCF7 Cells Proliferate in Response to 27HC
Treatment of ERα-positive breast cells with E2 leads to the induction of Cyclin D1 expression and a subsequent increase in the number of cells in S-phase (36). Using qRT-PCR, we show that, similar to E2, 24 h of 27HC treatment led to a robust induction in Cyclin D1 expression (Fig. 8A). Not surprisingly, we also demonstrated that, like E2, 27HC increased the number of cells cycling through S-phase (Fig. 8B). Because 27HC acted as an ERα partial agonist, we hypothesized that the same behavior would be observed in a bromodeoxyuridine (BrdU) labeling assay. As shown in Fig. 8B, cotreatment of cells with 27HC and E2 led to a dose-dependent decrease in BrdU incorporation to a level that represented the maximal activity of 27HC. Finally, we determined that treatment over 6 d with either E2 or 27HC led to a dose-dependent increase in cell number compared with vehicle (Fig. 8C). Furthermore, increasing concentrations of 27HC suppressed E2-mediated proliferation, an activity reflecting its partial agonist activity. We conclude therefore that the partial agonist activity of 27HC observed at the level of gene expression is also manifest at the level of cell proliferation.
Figure 8.
27HC Activated Cyclin D1 Expression, Increased Entry into S-Phase, and Induced Proliferation of Human Breast Cancer Cells
A, Expression of Cyclin D1 was measured by qRT-PCR in MCF7 cells. Cells were treated as described in Fig. 5A. Data are the mean ± sem of triplicate amplification reactions from one representative experiment. B, Increase in S-phase entry was quantitated in MCF7 cells treated for 24 h with vehicle (Veh) or increasing concentrations of E2 or 27HC or a combination of both as indicated. Cells were harvested and assayed for BrdU incorporation per manufacturer’s protocol. Data are presented in relative light units (RLU) and represent the mean ± sem for one representative experiment performed in triplicate. C, Increase in cell number was measured in MCF7 cells treated for 6 d with vehicle (Veh) or increasing concentrations of E2 or 27HC or a combination of both as indicated. At the end of 6 d, cells were harvested and assayed for dsDNA content to assess cell number. Data are the mean ± sem for one representative experiment performed in triplicate.
DISCUSSION
We show that 27HC is an endogenous SERM that displays significant partial agonist activity in a variety of cellular models of ER action. As expected for a SERM, the relative estrogenic activity of 27HC varied when assessed on different endpoints. Interestingly, however, the pharmacological activity of 27HC most closely resembles E2 in all systems studied. Both ligands activate the transcriptional activity of ERα, as seen in both exogenous reporter assays and in qRT-PCR analysis of target gene expression. Additionally, both 27HC and E2 induce recruitment of ERα to DNA response elements and trigger ligand-mediated receptor degradation, an event that is dependent on AIB1. Of specific importance was the observation that 27HC, like E2, induces the proliferation of ERα-positive breast cancer cells in vitro. In all these instances, however, 27HC does not exhibit full agonist activity but rather displays classic partial agonist behavior. This is readily apparent in both transcription and proliferation assays where 27HC can antagonize the E2-induced activation of ERα. The fact that 27HC has similar, yet distinct, behavior from E2 is highlighted by the apparent differences in the structure of the ERα-E2 and ERα-27HC complexes as seen in the peptide-binding studies. Specifically, we were able to show using these peptide-binding studies that 27HC-activated ERα adopts a structure that shares features in common with both E2- and 4OHT-activated ERα. Thus we believe that our findings support the idea that 27HC is similar in function to, yet distinct from, E2 in its ability to regulate ER activity in a variety of validated models of estrogen action and that its overall activity is consistent with it being classified as a SERM. We plan to investigate whether oxysterols other than 27HC have similar actions in terms of ER regulation.
We were intrigued by the apparent tissue-specific action of 27HC, given our finding that 27HC has agonist activity in breast cancer cells and the previous observation that it manifests antagonistic activity in the cardiovascular system. Tissue-specific agonist and antagonist actions are classically thought to arise from differential cofactor expression; therefore, it will be interesting to compare the cofactor preferences of 27HC-bound ERα in the breast vs. the vasculature. The fact that we were able to identify peptides that interacted in a highly specific manner with ERα in the presence of 27HC suggests that there are distinct protein-protein interaction surfaces presented on ERα in the presence of this ligand. Defining how this influences the differential recruitment of cofactors is an area of investigation that is currently underway.
27HC as an Endogenous ERα Ligand
In the past, our laboratory and others have generated ER-indicator mice wherein ERE-β-galactosidase or ERE-luciferase reporters are introduced transgenically (37,38,39). In these mice, it was reported that there was a significant level of E2-independent activation of ER-reporter activity that was blocked by treatment with ICI (37,38). This basal activity is currently attributed to ligand-independent activation of ER. However, it is interesting to speculate that the presence of 27HC, or a similar oxysterol, may be responsible for this basal ER-reporter activity, a hypothesis that could be tested by administration of specific CYP27A1 inhibitors (40) or by crossing the CYP27A1 knockout mouse with these ER-indicator mice and examining whether the basal activity is eliminated. These studies are currently planned in our laboratory.
Biological Significance of 27HC Action in Inflammation and Breast Cancer
In the breast, and specifically in breast cancer, E2 is a mitogen through its actions on ERα (41,42,43). We demonstrate that 27HC induces proliferation of ERα-positive breast cancer cells and that this proliferation correlates with increased Cyclin D1 expression and accumulation of cells in the S-phase of the cell cycle. This finding has important implications with respect to the treatment of ERα-positive breast cancers, particularly those treated with aromatase inhibitors. Although these therapies are extremely successful, resistance by an as yet unknown means is a significant clinical issue (44). Given that many of these tumors with acquired resistance continue to rely on ERα, we suggest that the ability of 27HC to regulate proliferation may be a contributing factor. In this way, 27HC may act as an alternate estrogenic ligand that manifests its agonist behavior in a low-estrogen environment.
We examined microarray data from human breast tumor samples and found a trend between CYP7B1 expression and disease-free survival. Among those patients with ERα-positive breast tumors, increased expression of CYP7B1 was associated with an increase in disease-free survival. No such correlation was observed in patients with ERα-negative breast tumors. These findings support our hypothesis that 27HC plays an important role in the development and/or progression of ERα-positive breast cancer. Specifically, CYP7B1 metabolizes 27HC such that increased expression of CYP7B1 would facilitate the conversion of 27HC to downstream products, lowering local concentrations of 27HC. It would be anticipated, therefore, that ERα-positive breast tumors with lower CYP7B1 expression, and therefore higher 27HC levels, would have a growth advantage over those with lower expression, especially in situations of low estrogen.
Interestingly, macrophages exhibit a very high capacity to produce 27HC. This is interesting in light of the observation that an increased number of tumor-infiltrating macrophages is an independent risk factor for reduced survival for breast cancer patients (14). Many hypotheses exist as to how tumor-infiltrating macrophages are co-opted to enter the tumor microenvironment and how they increase tumorigenic behavior (45,46,47). However, a potential explanation stemming from our findings is that increased macrophage presence in breast tumors elevates the local 27HC concentration and subsequently the activation of ER. Although estrogens have an established role in modulating the inflammatory response through repression of cytokine production in macrophages (48), their primary contribution in the etiology of breast cancer is considered to be their ability to function as mitogens. Thus, we hypothesize that the primary action of 27HC in the context of breast cancer is to induce cell proliferation through activation of ER.
There is an increased risk for breast cancer in obese individuals (49,50) that has been linked to increased aromatase activity and resultant estrogen production in adipose tissue (51,52). In a healthy individual, 27HC levels decline alongside estrogen levels during menopause (53). However, circulating levels of 27HC correlate well with cholesterol levels, and obesity often coincides with increased cholesterol levels (54). Thus, this increased level of 27HC in an obese postmenopausal woman may also contribute to the development of breast cancer through the potential mitogenic actions of 27HC in the breast.
The fact that macrophages, epithelial cells, and endothelial cells produce 27HC, which can exit the cell and enter the systemic circulation, raises the possibility that 27HC has a more global function in regulating the activity of ERα and ERβ than just acting as a partial agonist in the breast and the cardiovascular system. ERα and ERβ are expressed in many other tissues, including the liver, bone, lung, and reproductive tract. How 27HC functions to regulate ER activity in these tissues, both in the presence of high estrogen in a premenopausal female and in a low-estrogen environment, remains to be evaluated.
MATERIALS AND METHODS
Biochemicals
PCR reagents were obtained from Bio-Rad (Hercules, CA). E2 and 4-OHT were purchased from Sigma Chemical Co. (St. Louis, MO). 27-HC was purchased from Research Plus, Inc. (Manasquan, NJ). ICI was a kind gift from Dr. A. Wakeling (Zeneca Pharmaceuticals, Macclesfield, UK). Raloxifene was a kind gift from Dr. E. Larson (Pfizer, Inc., Groton, CT). PCR oligos were purchased from Integrated DNA Technologies (Coralville, IA). Cell Proliferation ELISA BrdU kit was obtained from Roche Applied Science (Indianapolis, IN), and the FluoReporter Blue Fluorometric dsDNA Quantitation kit was obtained from Invitrogen (Carlsbad, CA).
Plasmids
pcDNA3.1nv5-ERα is a cytomegalovirus (CMV)-driven expression plasmid containing amino acids 1–595 of human ERα with an N-terminal v5 tag. It was constructed by PCR subcloning full-length ERα from pVP16-ERα (16) and entered into pENTR-TOPO (Invitrogen) to create pENTR-TOPO-ERα, which by LR reaction (recombination between attL and attR sites) into pCDNA3.1nv5-DEST (Invitrogen) yielded pCDNA3.1nv5-ERα. A plasmid expressing ERα as a fusion to the yeast VP16 transactivation domain was used in mammalian two-hybrid assays (pVP16-ERα) and has been previously described (16). The bait peptides are expressed as fusions to the Gal4DBD. GRIP1-NR, SRC1-NR, and D30 have been previously described (16). bI2 and bT1 were described elsewhere (17). AIB1-NR contains amino acids 621–821 of human AIB1. ASC2-NR contains amino acids 746–917 of human ASC2. These fragments were identified in an unrelated screen performed in our laboratory. The 3XERE-TATA-Luc (55) and the 5XGal4Luc3 (16) reporters have both been previously described.
Mammalian Cell Culture and Transient Transfection Assays
All cell lines were obtained from American Type Culture Collection (Manassas, VA). HeLa (human cervical adenocarcinoma) and HepG2 (human hepatocellular carcinoma) cells were maintained in MEM (Invitrogen) supplemented with 8% fetal bovine serum (Hyclone Laboratories, Logan, UT), 1 mm sodium pyruvate, and 0.1 mm nonessential amino acids (Invitrogen). MCF7 (human breast adenocarcinoma) cells were maintained in DMEM/F12 (Invitrogen) supplemented with 8% fetal bovine serum, 1 mm sodium pyruvate, and 0.1 mm nonessential amino acids. All cell lines were grown in a 37 C incubator with 5% CO2.
HeLa cells were used for transactivation assays. For transient transfections, cells were plated in phenol red-free media containing 8% charcoal-stripped serum (Hyclone), 1 mm sodium pyruvate, and 0.1 mm nonessential amino acids in 24-well plates 24 h before transfection. Lipofectin-mediated (Invitrogen) transfection has been described in detail previously (56). Briefly, a DNA-Lipofectin mixture containing a total of 2 μg plasmid for each triplicate sample was added to the cells. Each triplicate sample contained 0.1 μg pCMV-βgal, 1.5 μg 3XERE-TATA-Luc, 5 ng pCDNA3.1nV5-ERα, and 0.395 μg pCDNA3.1nV5-DEST filler vector. Ligands were added to the cells 24 h after transfection, and cells were assayed after overnight treatment. Luminescence and β-galactosidase activity were measured on a Fusion luminometer (PerkinElmer, Waltham, MA). Results are expressed as normalized luciferase activity (normalized to β-galactosidase for transfection efficiency) for one representative experiment performed in triplicate. Error bars indicate the sem for the triplicate wells.
HepG2 cells were used for mammalian two-hybrid assays. Cells were plated in 24-well plates 24 h before transfection in phenol red-free media containing 8% charcoal-stripped serum, 1 mm sodium pyruvate, and 0.1 mm nonessential amino acids. A DNA-Lipofectin mixture containing a total of 3 μg plasmid for each triplicate sample was added to the cells, where each triplicate sample contained 0.1 μg pCMV-βgal, 1.5 μg 5XGal4Luc3, 0.4 μg VP16-ERα, and 1 μg pM-peptide. Ligands were added to the cells 24 h after transfection, and cells were assayed after overnight treatment. Luminescence and β-galactosidase activity were measured as above.
RNA Isolation and qRT-PCR
For RNA analysis, MCF7 or T47D cells were seeded in six-well plates in phenol red-free media containing 8% charcoal-stripped serum, 1 mm sodium pyruvate, and 0.1 mm nonessential amino acids. After 48 h, cells were treated with the appropriate ligand. After the indicated time period, cells were harvested and total RNA was isolated using the Aurum Total RNA Mini Kit (Bio-Rad). One microgram of RNA was reverse transcribed using the iScript cDNA synthesis kit (Bio-Rad). The Bio-Rad iCycler Realtime PCR System was used to amplify and quantitate levels of target gene cDNA. qRT-PCR were performed with 1 μl cDNA, 10 μm specific primers (see Table 1 for sequences), and iQ SYBR Green Supermix (Bio-Rad). Data are normalized to the 36B4 housekeeping gene and presented as fold induction over vehicle. Data are the mean ± sem for triplicate amplification reactions from one representative experiment. Each experiment was repeated at least three independent times with very similar results.
Table 1.
Primer Sequences
| Gene | Primer Sequences |
|---|---|
| SDF-1 | |
| Forward | GTGGTCGTGCTGGTCCTC |
| Reverse | GATGCTTGACGTTGGCTCTG |
| PR | |
| Forward | GCATCGTTGATAAAATCCGCAG |
| Reverse | AATCTCTGGCTTAGGGCTTGGC |
| pS2 | |
| Forward | TCCCCTGGTGCTTCTATCCTAATAC |
| Reverse | GCAGTCAATCTGTGTTGTGAGCC |
| E2F1 | |
| Forward | ACGTGACGTGTCAGGACCT |
| Reverse | GATCGGGCCTTGTTTGCTCT |
| WISP2 | |
| Forward | TGAGAGGCACACCGAAGAC |
| Reverse | ACAGCCATCCAGCACCAG |
| ERBB4 | |
| Forward | GAGAAGATTCTTGGAAACAGAG |
| Reverse | GGATGATCCATACTTGCCAT |
| Cyclin D1 | |
| Forward | CAACTTCCTGTCCTACTACC |
| Reverse | CTCCTCCTCCTCCTCTTC |
| 36B4 | |
| Forward | GGACATGTTGCTGGCCAATAA |
| Reverse | GGGCCCGAGACCAGTGTT |
Cell Proliferation Assays
For both cell proliferation and BrdU-incorporation assays, MCF7 cells were seeded at 5000 cells per well in 96-well plates on d 0 in phenol red-free DMEM/F12 media containing 8% charcoal-stripped serum, 1 mm sodium pyruvate, and 0.1 mm nonessential amino acids. On d 2, the media were replaced with serum- and phenol red-free media for 24 h. For proliferation assays, cells were treated with ligands on d 3 and 5 in media containing 8% charcoal-stripped serum. On d 6, cell proliferation was measured using the FluoReporter Blue Fluorometric dsDNA Quantitation Kit (Invitrogen) according to the manufacturer’s instructions. For BrdU assays, the cells were treated on d 3 with ligand for 22 h, at which time the BrdU labeling reagent was added for 3 h. Cells were then assayed according to the manufacturer’s protocol. For both assays, data are presented as the mean ± sem for triplicate wells in one representative experiment. Each experiment was repeated at least three independent times with very similar results.
Western Blotting
MCF7 cells were seeded in six-well plates in phenol red-free media containing 8% charcoal-stripped serum, 1 mm sodium pyruvate, and 0.1 mm nonessential amino acids. For ERα degradation and protein expression studies, cells were treated after 48 h with ligand for the indicated time. For siRNA experiments, cells were plated in the presence of 40 nm siAIB1 or siRNA control (Stealth siRNA; Invitrogen) using DharmaFECT-1 (Dharmacon, Lafayette, CO) as a transfection reagent. After 48 h of protein knockdown, the cells were treated for 8 h with vehicle, 1 nm E2, 10 μm 27HC, 100 nm 4OHT, or 100 nm ICI. In both experiments, whole-cell extracts were isolated using RIPA buffer [50 mm Tris-HCl (pH 7.5), 150 mm NaCl, 0.1% Nonidet P-40 (NP40), 0.5% sodium-deoxycholate, 0.05% SDS, 1 mm EDTA, and 1× protease inhibitor mixture (EMD Chemicals, Inc., San Diego, CA)]. Whole-cell lysate (50 μg) was resolved by SDS-PAGE and transferred to a nitrocellulose membrane. ERα was detected using monoclonal mouse antibody D12, cytokeratin 18 with the mouse monoclonal antibody DC-10, and AIB1 with the goat polyclonal antibody C-20 (all from Santa Cruz Biotechnology, Santa Cruz, CA). PR was detected using the mouse monoclonal antibody PR1294 (a kind gift from Dr. D. P. Edwards, Baylor College of Medicine, Houston, TX). Secondary antibodies were purchased from Bio-Rad. The scanned images of chemiluminescence were quantitated using Image J software.
M13 Phage Screen
The M13 phage panning protocol has been previously described (16). Modifications to the original protocol are as follows. We used 4 pmol recombinant ERα (Affinity BioReagents, Golden, CO). Approximately 107 plaque-forming units of phage libraries were added to each well for the panning process. Four rounds of panning were performed, with PCR being used to recover peptide inserts from the fourth round, which showed significant enrichment of target binding phage. The PCR products were digested with Xho1 and Xba1 for ligation into the expression vector pM5.1 for mammalian two-hybrid assays.
Chromatin Immunoprecipitation
MCF7 cells were grown to 90% confluence in 15-cm dishes in phenol red-free DMEM/F12 supplemented with 8% charcoal-stripped serum, 1 mm sodium pyruvate, and 0.1 mm nonessential amino acids for 3 d, after which the cells were serum starved for 24 h. After treatment with vehicle, 100 nm E2, or 10 μm 27HC for 45 min, the cells were fixed with 1% formaldehyde for 10 min at room temperature. The reaction was stopped with 250 mm glycine by incubation at room temperature for 5 min. Cells were then washed with ice-cold PBS, harvested in ice-cold PBS, and centrifuged for 5 min. The cell pellet was washed twice with ice-cold PBS before being lysed in 1 ml RIPA buffer [50 mm Tris-HCl (pH 7.5), 150 mm NaCl, 0.1% NP40, 0.5% sodium deoxycholate, 0.05% SDS, 1 mm EDTA, and 1× protease inhibitor mixture) by sonication (13 times for 13 sec each at setting 7; Misonix Microson Ultrasonic Cell Disruptor XL), followed by centrifugation for 15 min at 14000 rpm. Supernatants were collected, diluted in RIPA buffer, and immunocleared in 100 μl Protein A/G-PLUS-Agarose beads [50% slurry in 10 mm Tris-HCl (pH 8.0), 1 mm EDTA, 200 μg/ml sonicated salmon sperm DNA, and 500 μg/ml BSA] for 30 min at 4 C. Immunoprecipitation was performed for 3 h at 4 C with 10 μg ERα-specific antibody (H-184; Santa Cruz Biotechnology) or 10 μg rabbit IgG control. After immunoprecipitation, 100 μl Protein A/G-PLUS-Agarose beads [50% slurry in 10 mm Tris-HCl (pH 8.0), 1 mm EDTA, 200 μg/ml sonicated salmon sperm DNA, and 500 μg/ml BSA] was added and allowed to incubate overnight at 4 C. Precipitates were sequentially washed twice for 5 min each with the following: buffer A [50 mm HEPES (pH 7.8), 140 mm NaCl, 1 mm EDTA, 1% Triton X-100, 0.1% sodium deoxycholate, 0.1% SDS, and 1× protease inhibitor], buffer B [50 mm HEPES (pH 7.8), 500 mm NaCl, 1 mm EDTA, 1% Triton X-100, 0.1% sodium deoxycholate, 0.1% SDS, and 2× protease inhibitor], buffer C [20 mm Tris-HCl (pH 8.0), 1 mm EDTA, 250 mm LiCl, 0.5% NP40, 0.5% sodium deoxycholate, and 1× protease inhibitor], and Tris-EDTA (10 mm Tris-HCl and 1 mm EDTA). Precipitates were eluted twice in 50 mm Tris-HCl (pH 8.0), 1 mm EDTA, and 1% SDS by incubation at 65 C for 10 min, and then the cross-linking was reversed by addition of 230 mm (final concentration) NaCl and incubation at 65 C overnight. DNA was isolated with a QIAquick PCR Purification kit (QIAGEN, Valencia, CA). qRT-PCR were performed with 1 μl immunoprecipitated DNA, 10 μm specific primers, and iQ SYBRGreen Supermix (Bio-Rad). Data are normalized to the input for the immunoprecipitation.
Acknowledgments
We thank the members of the McDonnell and Mangelsdorf laboratories for critical review of the manuscript.
Footnotes
This work was supported by the Howard Hughes Medical Institute (D.J.M.), the Robert A. Welch Foundation (Grant I-1275 to D.J.M.), the Department of Defense Breast Cancer Program Predoctoral Traineeship Award BC050609 (C.D.D.), and National Institutes of Health Grants 5R37DK048807 (D.P.M), R01HL087564 (P.W.S. and D.J.M.), and U19DK62434 (D.J.M.).
Disclosure Summary: The authors have nothing to disclose.
First Published Online September 13, 2007
Abbreviations: AIB1, Amplified-in-breast cancer 1; ASC2, activating signal cointegrator-2; BrdU, bromodeoxyuridine; CMV, cytomegalovirus; E2, 17β-estradiol; ER, estrogen receptor; ERE, estrogen response element; Gal4DBD, Gal4 DNA-binding domain; GRIP1, glucocorticoid receptor interacting protein 1; 27HC, 27-hydroxycholesterol; ICI, ICI 182,780; NP40, Nonidet P-40; NR, nuclear receptor; 4OHT, 4-hydroxy-tamoxifen; PR, progesterone receptor; qRT-PCR, quantitative RT-PCR; siRNA, small interfering RNA; SRC1, steroid receptor coactivator 1.
References
- Green S, Walter P, Kumar V, Krust A, Bornert JM, Argos P, Chambon P 1986 Human oestrogen receptor cDNA: sequence, expression and homology to v-erb-A. Nature 320:134–139 [DOI] [PubMed] [Google Scholar]
- Kuiper GG, Carlsson B, Grandien K, Enmark E, Haggblad J, Nilsson S, Gustafsson JA 1997 Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors α and β. Endocrinology 138:863–870 [DOI] [PubMed] [Google Scholar]
- Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA 1996 Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci USA 93:5925–5930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall JM, McDonnell DP 1999 The estrogen receptor β-isoform (ERβ) of the human estrogen receptor modulates ERα transcriptional activity and is a key regulator of the cellular response to estrogens and antiestrogens. Endocrinology 140:5566–5578 [DOI] [PubMed] [Google Scholar]
- Simpson ER 2003 Sources of estrogen and their importance. J Steroid Biochem Mol Biol 86:225–230 [DOI] [PubMed] [Google Scholar]
- Umetani M, Domoto H, Gormley A, Yuhanna IS, Cummins CL, Javitt NB, Korach KS, Shaul PW, Mangelsdorf DJ 2007 27-Hydroxycholesterol is an endogenous selective estrogen receptor modulator that inhibits the cardiovascular effects of estrogen. Nat Med 13:1185–1192 [DOI] [PubMed] [Google Scholar]
- Janowski BA, Willy PJ, Devi TR, Falck JR, Mangelsdorf DJ 1996 An oxysterol signalling pathway mediated by the nuclear receptor LXRα. Nature 383:728–731 [DOI] [PubMed] [Google Scholar]
- Cali JJ, Russell DW 1991 Characterization of human sterol 27-hydroxylase. A mitochondrial cytochrome P-450 that catalyzes multiple oxidation reaction in bile acid biosynthesis. J Biol Chem 266:7774–7778 [PubMed] [Google Scholar]
- Brown AJ, Jessup W 1999 Oxysterols and atherosclerosis. Atherosclerosis 142:1–28 [DOI] [PubMed] [Google Scholar]
- Rossouw JE 1999 Hormone replacement therapy and cardiovascular disease. Curr Opin Lipidol 10:429–434 [DOI] [PubMed] [Google Scholar]
- Brouchet L, Krust A, Dupont S, Chambon P, Bayard F, Arnal JF 2001 Estradiol accelerates reendothelialization in mouse carotid artery through estrogen receptor-α but not estrogen receptor-β. Circulation 103:423–428 [DOI] [PubMed] [Google Scholar]
- Pare G, Krust A, Karas RH, Dupont S, Aronovitz M, Chambon P, Mendelsohn ME 2002 Estrogen receptor-α mediates the protective effects of estrogen against vascular injury. Circ Res 90:1087–1092 [DOI] [PubMed] [Google Scholar]
- Zhu Y, Bian Z, Lu P, Karas RH, Bao L, Cox D, Hodgin J, Shaul PW, Thoren P, Smithies O, Gustafsson JA, Mendelsohn ME 2002 Abnormal vascular function and hypertension in mice deficient in estrogen receptor β. Science 295:505–508 [DOI] [PubMed] [Google Scholar]
- Steele RJ, Eremin O, Brown M, Hawkins RA 1984 A high macrophage content in human breast cancer is not associated with favourable prognostic factors. Br J Surg 71:456–458 [DOI] [PubMed] [Google Scholar]
- Paige LA, Christensen DJ, Gron H, Norris JD, Gottlin EB, Padilla KM, Chang CY, Ballas LM, Hamilton PT, McDonnell DP, Fowlkes DM 1999 Estrogen receptor (ER) modulators each induce distinct conformational changes in ERα and ERβ. Proc Natl Acad Sci USA 96:3999–4004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Norris JD, Gron H, Paige LA, Hamilton PT, Kenan DJ, Fowlkes D, McDonnell DP 1999 Dissection of the LXXLL nuclear receptor-coactivator interaction motif using combinatorial peptide libraries: discovery of peptide antagonists of estrogen receptors α and β . Mol Cell Biol 19:8226–8239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang HJ, Norris JD, McDonnell DP 2002 Identification of a negative regulatory surface within estrogen receptor α provides evidence in support of a role for corepressors in regulating cellular responses to agonists and antagonists. Mol Endocrinol 16:1778–1792 [DOI] [PubMed] [Google Scholar]
- Norris JD, Paige LA, Christensen DJ, Chang CY, Huacani MR, Fan D, Hamilton PT, Fowlkes DM, McDonnell DP 1999 Peptide antagonists of the human estrogen receptor. Science 285:744–746 [DOI] [PubMed] [Google Scholar]
- Heery DM, Kalkhoven E, Hoare S, Parker MG 1997 A signature motif in transcriptional co-activators mediates binding to nuclear receptors. Nature 387:733–776 [DOI] [PubMed] [Google Scholar]
- Hu X, Lazar MA 1999 The CoRNR motif controls the recruitment of corepressors by nuclear hormone receptors. Nature 402:93–96 [DOI] [PubMed] [Google Scholar]
- Perissi V, Staszewski LM, McInerney EM, Kurokawa R, Krones A, Rose DW, Lambert MH, Milburn MV, Glass CK, Rosenfeld MG 1999 Molecular determinants of nuclear receptor-corepressor interaction. Genes Dev 13:3198–3208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paech K, Webb P, Kuiper GG, Nilsson S, Gustafsson J, Kushner PJ, Scanlan TS 1997 Differential ligand activation of estrogen receptors ERα and ERβ at AP1 sites. Science 277:1508–1510 [DOI] [PubMed] [Google Scholar]
- Porter W, Saville B, Hoivik D, Safe S 1997 Functional synergy between the transcription factor Sp1 and the estrogen receptor. Mol Endocrinol 11:1569–1580 [DOI] [PubMed] [Google Scholar]
- Webb P, Lopez GN, Uht RM, Kushner PJ 1995 Tamoxifen activation of the estrogen receptor/AP-1 pathway: potential origin for the cell-specific estrogen-like effects of antiestrogens. Mol Endocrinol 9:443–456 [DOI] [PubMed] [Google Scholar]
- Fritah A, Redeuilh G, Sabbah M 2006 Molecular cloning and characterization of the human WISP-2/CCN5 gene promoter reveal its upregulation by oestrogens. J Endocrinol 191:613–624 [DOI] [PubMed] [Google Scholar]
- Petz LN, Ziegler YS, Schultz JR, Nardulli AM 2004 Fos and Jun inhibit estrogen-induced transcription of the human progesterone receptor gene through an activator protein-1 site. Mol Endocrinol 18:521–532 [DOI] [PubMed] [Google Scholar]
- Schultz JR, Petz LN, Nardulli AM 2003 Estrogen receptor α and Sp1 regulate progesterone receptor gene expression. Mol Cell Endocrinol 201:165–175 [DOI] [PubMed] [Google Scholar]
- Stack G, Kumar V, Green S, Ponglikitmongkol M, Berry M, Rio MC, Nunez AM, Roberts M, Koehl C, Bellocq P, Gairard B, Renaud JP, Chambon P 1988 Structure and function of the pS2 gene and estrogen receptor in human breast cancer cells. Cancer Treat Res 40:185–206 [DOI] [PubMed] [Google Scholar]
- Wang W, Dong L, Saville B, Safe S 1999 Transcriptional activation of E2F1 gene expression by 17β-estradiol in MCF-7 cells is regulated by NF-Y-Sp1/estrogen receptor interactions. Mol Endocrinol 13:1373–1387 [DOI] [PubMed] [Google Scholar]
- Zhu Y, Sullivan LL, Nair SS, Williams CC, Pandey AK, Marrero L, Vadlamudi RK, Jones FE 2006 Coregulation of estrogen receptor by ERBB4/HER4 establishes a growth-promoting autocrine signal in breast tumor cells. Cancer Res 66:7991–7998 [DOI] [PubMed] [Google Scholar]
- Frasor J, Danes JM, Komm B, Chang KC, Lyttle CR, Katzenellenbogen BS 2003 Profiling of estrogen up- and down-regulated gene expression in human breast cancer cells: insights into gene networks and pathways underlying estrogenic control of proliferation and cell phenotype. Endocrinology 144:4562–4574 [DOI] [PubMed] [Google Scholar]
- Lonard DM, Nawaz Z, Smith CL, O’Malley BW 2000 The 26S proteasome is required for estrogen receptor-α and coactivator turnover and for efficient estrogen receptor-α transactivation. Mol Cell 5:939–948 [DOI] [PubMed] [Google Scholar]
- Nawaz Z, Lonard DM, Dennis AP, Smith CL, O’Malley BW 1999 Proteasome-dependent degradation of the human estrogen receptor. Proc Natl Acad Sci USA 96:1858–1862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijayaratne AL, McDonnell DP 2001 The human estrogen receptor-α is a ubiquitinated protein whose stability is affected differentially by agonists, antagonists, and selective estrogen receptor modulators. J Biol Chem 276:35684–35692 [DOI] [PubMed] [Google Scholar]
- Shao W, Keeton EK, McDonnell DP, Brown M 2004 Coactivator AIB1 links estrogen receptor transcriptional activity and stability. Proc Natl Acad Sci USA 101:11599–11604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland RL, Prall OW, Watts CK, Musgrove EA 1998 Estrogen and progestin regulation of cell cycle progression. J Mammary Gland Biol Neoplasia 3:63–72 [DOI] [PubMed] [Google Scholar]
- Ciana P, Di Luccio G, Belcredito S, Pollio G, Vegeto E, Tatangelo L, Tiveron C, Maggi A 2001 Engineering of a mouse for the in vivo profiling of estrogen receptor activity. Mol Endocrinol 15:1104–1113 [DOI] [PubMed] [Google Scholar]
- Ciana P, Raviscioni M, Mussi P, Vegeto E, Que I, Parker MG, Lowik C, Maggi A 2003 In vivo imaging of transcriptionally active estrogen receptors. Nat Med 9:82–86 [DOI] [PubMed] [Google Scholar]
- Nagel SC, Hagelbarger JL, McDonnell DP 2001 Development of an ER action indicator mouse for the study of estrogens, selective ER modulators (SERMs), and xenobiotics. Endocrinology 142:4721–4728 [DOI] [PubMed] [Google Scholar]
- Brown AJ, Watts GF, Burnett JR, Dean RT, Jessup W 2000 Sterol 27-hydroxylase acts on 7-ketocholesterol in human atherosclerotic lesions and macrophages in culture. J Biol Chem 275:27627–27633 [DOI] [PubMed] [Google Scholar]
- Flototto T, Djahansouzi S, Glaser M, Hanstein B, Niederacher D, Brumm C, Beckmann MW 2001 Hormones and hormone antagonists: mechanisms of action in carcinogenesis of endometrial and breast cancer. Horm Metab Res 33:451–457 [DOI] [PubMed] [Google Scholar]
- Henderson BE, Ross RK, Pike MC, Casagrande JT 1982 Endogenous hormones as a major factor in human cancer. Cancer Res 42:3232–3239 [PubMed] [Google Scholar]
- Hewitt SC, Korach KS 2003 Oestrogen receptor knockout mice: roles for oestrogen receptors α and β in reproductive tissues. Reproduction 125:143–149 [DOI] [PubMed] [Google Scholar]
- Chen S, Masri S, Wang X, Phung S, Yuan YC, Wu X 2006 What do we know about the mechanisms of aromatase inhibitor resistance? J Steroid Biochem Mol Biol 102:232–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JJ, Lin YC, Yao PL, Yuan A, Chen HY, Shun CT, Tsai MF, Chen CH, Yang PC 2005 Tumor-associated macrophages: the double-edged sword in cancer progression. J Clin Oncol 23:953–964 [DOI] [PubMed] [Google Scholar]
- Tang R, Beuvon F, Ojeda M, Mosseri V, Pouillart P, Scholl S 1992 M-CSF (monocyte colony stimulating factor) and M-CSF receptor expression by breast tumour cells: M-CSF mediated recruitment of tumour infiltrating monocytes? J Cell Biochem 50:350–356 [DOI] [PubMed] [Google Scholar]
- Yu JL, Rak JW 2003 Host microenvironment in breast cancer development: inflammatory and immune cells in tumour angiogenesis and arteriogenesis. Breast Cancer Res 5:83–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkonen PL, Vaananen HK 2006 Monocyte-macrophage system as a target for estrogen and selective estrogen receptor modulators. Ann NY Acad Sci 1089:218–227 [DOI] [PubMed] [Google Scholar]
- Furberg AS, Veierod MB, Wilsgaard T, Bernstein L, Thune I 2004 Serum high-density lipoprotein cholesterol, metabolic profile, and breast cancer risk. J Natl Cancer Inst 96:1152–1160 [DOI] [PubMed] [Google Scholar]
- Stoll BA 2002 Upper abdominal obesity, insulin resistance and breast cancer risk. Int J Obes Relat Metab Disord 26:747–753 [DOI] [PubMed] [Google Scholar]
- Simpson ER, Davis SR 2001 Aromatase and the regulation of estrogen biosynthesis: some new perspectives. Endocrinology 142:4589–4594 [DOI] [PubMed] [Google Scholar]
- Zhao Y, Agarwal VR, Mendelson CR, Simpson ER 1997 Transcriptional regulation of CYP19 gene (aromatase) expression in adipose stromal cells in primary culture. J Steroid Biochem Mol Biol 61:203–210 [DOI] [PubMed] [Google Scholar]
- Chen LD, Kushwaha RS, McGill Jr HC, Rice KS, Carey KD 1998 Effect of naturally reduced ovarian function on plasma lipoprotein and 27-hydroxycholesterol levels in baboons (Papio sp.). Atherosclerosis 136:89–98 [DOI] [PubMed] [Google Scholar]
- Burkard I, von Eckardstein A, Waeber G, Vollenweider P, Rentsch KM 2007 Lipoprotein distribution and biological variation of 24S- and 27-hydroxycholesterol in healthy volunteers. Atherosclerosis 194:71–78 [DOI] [PubMed] [Google Scholar]
- Tzukerman MT, Esty A, Santiso-Mere D, Danielian P, Parker MG, Stein RB, Pike JW, McDonnell DP 1994 Human estrogen receptor transactivational capacity is determined by both cellular and promoter context and mediated by two functionally distinct intramolecular regions. Mol Endocrinol 8:21–30 [DOI] [PubMed] [Google Scholar]
- Hall JM, Chang CY, McDonnell DP 2000 Development of peptide antagonists that target estrogen receptor β-coactivator interactions. Mol Endocrinol 14:2010–2023 [DOI] [PubMed] [Google Scholar]