Abstract
A number of amino acids essential for Gs coupling, i.e. hot spots, were identified after in vitro Ala-scanning mutagenesis of the cytosolic extensions of helices 3, 5, and 6 and of intracellular loops 2 and 3 (IL2 and IL3) of the human LH receptor (LHR). Consistent with the results of in vitro experiments involving ligand binding and ligand-mediated signaling in transiently transfected human embryonic kidney 293 cells, computational modeling of the isolated receptor and of the receptor-G protein complexes suggests an important role of the cytosolic extension of helix 3 and the N-terminal portion of the IL2 in Gsα interaction, whereas the contribution of IL3 is marginal. Mapping the hot spots into the computational models of LHR and the LHR-Gs complexes allowed for a distinction between receptor sites required for intramolecular structural changes (i.e. I460, T461, H466, and I549) and receptor sites more likely involved in G protein recognition (i.e. R464, T467, I468, Y470, Y550, and D564). The latter sites include the highly conserved arginine of the (E/D)R(Y/W) motif, which is therefore likely to be a receptor recognition point for Gs rather than a switch of receptor activation. The results of in vitro and in silico experiments carried out in this study represent the first comprehensive delineation of functionality of the individual residues in the intracellular domains of LHR and establish potential switches of receptor activation as well as a map of the primary receptor recognition sites for Gs. A novel way to consider constitutively active mutants was inferred from this study, i.e. receptor states with improved complementarity for the G protein compared to the wild-type receptor.
IT IS ESTIMATED that the human genome contains more than 800 genes encoding G protein-coupled receptors (GPCRs) (1). In response to ligand binding, these heptahelical membrane-associated proteins undergo a conformational change and activate one or more G proteins (2). The patchwork of the most relevant information from in vitro experiments on receptor-G protein recognition suggests that the α4/β6 loop and the C terminus of the G protein α-subunit recognize a solvent accessible cleft on the receptor, formed by amino acids from the extracellular extensions of transmembrane helices 3 and 6 (H3 and H6), from the N terminus of the second intracellular loop (IL2), from the N and C termini of IL3, and from the N terminus of H8 (reviewed in Refs.2,3,4).
GPCRs are allosteric proteins that exist as complex statistical conformation ensembles (5,6,7). They hold regions at high stability (i.e. low flexibility) and regions at low stability (i.e. high flexibility) that communicate with each other, even if distal. The functional properties of a GPCR are related to the distribution of states within the native ensemble, and the distribution is affected differently by ligands and/or interacting proteins and/or amino acid mutations (6,7). Of course, the different oligomeric states of a GPCR may contribute to differentiate the distribution of the receptor states. In this respect, different active state ensembles of a GPCR may show specific G protein coupling. It has also been suggested that GPCRs contain two functional domains: one, an activation domain that is capable of activating multiple G proteins, and the other, a selectivity (or specificity) domain that restricts the coupling to a particular G protein, and thus a specific signaling pathway (8). In many GPCRs, receptor-G protein selectivity is largely restricted to the N- and C-terminal portions of IL3 (9); IL2 may also function in G protein selectivity, in addition to serving as a switch enabling G protein activation (10). In addition, GPCR-G protein coupling is modulated by cellular mechanisms that include the cytoskeleton, the local lipid environment, and proteins of the regulator of G protein signaling family (11,12).
The LH, FSH, and TSH receptors are GPCRs that contain a relatively large ectodomain responsible for high affinity and specific ligand binding (13,14). These three receptors stimulate the cAMP signaling pathway, as well as the inositol phosphate pathway under certain conditions. Various portions of the receptors, including IL2, IL3, the N-terminal portion of H6, and the C-terminal tail, have been implicated in Gs coupling (15,16).
Computational modeling on the isolated wild type (WT) and constitutively active mutants of the human LH receptor (LHR) suggested that ligand-independent activation of LHR involves changes in the interaction pattern of R464(3.50) [the numbering in parenthesis follows the numbering scheme recommended by Ballesteros and Weinstein (17); Fig. 1] of the (E/D)R(Y/W) highly conserved motif and the opening of a solvent-accessible crevice in the neighbors of the conserved arginine (18,19,20). The latter effect is properly marked by the solvent-accessible surface area (SAS) computed over R464(3.50), T467(3.53), I468(3.54), and K563(6.29) at the cytosolic extensions of H3 and H6. This index remains below 50 Å2 in the WT and the nonactive mutants, whereas it increases above that threshold in the structure of almost all the simulated spontaneous and engineered constitutively active mutants (CAMs) (18,19,20). The residues that contribute to the SAS index are, hence, predicted to play a role in Gs recognition. One of them, the (E/D)R(Y/W) arginine, has been postulated to play an important role in GPCR function. Whether the main role of this arginine is to maintain the inactive state of the receptor or to recognize the G protein is not clearly understood and may depend on the receptor system (critically analyzed in Refs. 21 and 22). The role of this amino acid in the LHR function has not yet been unequivocally addressed.
Figure 1.
Rhodopsin and Human LHR Sequence Alignment
Sequence alignment between bovine rhodopsin [PDB code: 1U19 (27)], i.e. template, and the human LHR (target) that was employed for comparative modeling. The amino acid stretches 100–101, 106–107, and 236–242 have been deleted from the 1U19 template. The boxed amino acid in each rhodopsin helix corresponds to the amino acid n.50 according to the Ballesteros and Weinstein nomenclature (17), whereas the boxed amino acid stretch at the end of H7 corresponds to H8. N-TER, N-terminal.
Based on the hypothesis that LHR-Gs coupling involves the cytosolic extensions of H3 and H6, as well as IL2 and IL3, this study was designed to evaluate the roles of these regions of the receptor in Gs coupling. Our approach was to complement computational modeling with in vitro Ala-scanning mutagenesis followed by experimental assessments of receptor function. The combined computational and experimental results emphasize the role of the cytosolic extension of H3, as well as the C- and N-terminal portions of IL2 of the LHR in Gs binding and/or activation. The roles of IL3 and of H5/H6 appear to be much less than that of IL2 and H3, respectively, in Gs coupling.
RESULTS
Prediction of the Likely Receptor-G Protein Interface
In this study, updated computational models of the 323–358 ectodomain sequence of the WT and the D564(6.30)G, D564(6.30)A, and D578(6.44)H CAM forms of human LHR have been achieved by combining comparative modeling with nanosecond molecular dynamics (MD) simulations in an implicit membrane-water model (see Materials and Methods). The comparative structural analysis of each of the four LHR forms was carried out on the structures averaged over the 2000 structures collected during the whole 1-nsec MD trajectory [i.e. average (AVG)1000ps]. The comparisons concerned both intramolecular structural features and interaction modes with heterotrimeric Gs.
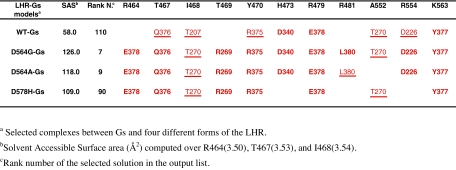
Consistent with previous computational models of LHR (reviewed in Ref.4), the solvent accessibility of selected amino acids at the cytosolic end of H3 turned out to be the main hallmark of functionally different receptor states (i.e. inactive and active). Indeed, the SAS index computed over R464(3.50), T467(3.53), and I468(3.54) was 58 Å2 in WT receptor, whereas it was higher than 100 Å2 in the AVG1000ps structures of the CAMs (Table 1). It is worth noting that this SAS index defined in this study differs from that computed on early LHR models in that K563(6.29) has been excluded.
Figure 7.
Docking Outputs Concerning Different Forms of the LHR and Selected Amino Acids from the Receptor (black) and from the G Protein Involved in Charge-Reinforced H-Bonds (bold) or van der Waals Interactions (underlined)
Rigid body docking simulations have been carried out to investigate whether such an increase in solvent accessibility at the cytosolic end of H3, a feature of the hormone-independent active forms of LHR, could result in improved receptor ability to recognize the cognate G protein and whether the arginine of the (E/D)R(Y/W) motif and its neighbors participate in the receptor-G protein interface. Of the different docking runs launched on the 50 AVGf100ps and AVG1000ps structures of the WT and mutated receptors, only the results concerning one simulation for each form are shown in Table 1, which are closely representative of the majority of the simulation results. These results concern the AVG1000ps structure of each LHR form carrying: 1) two disulfide bridges, i.e. between C336 and C353, and between C439(3.10) and C514; and 2) H482(4.41) and H578(6.44) (the latter only for the D578(6.44)H mutant) in their protonated forms. In Table 1, the SAS computed over R464(3.50), T467(3.53), and I468(3.54) is shown, which properly marks the increase in solvent exposure of the cytosolic end of H3 in the CAM structures compared with the WT.
As described in Materials and Methods, we have employed a distance-based filter to discard most of the false positives in the docking output list. In detail, a distance cutoff of 20 Å between the Cα-atom of R464(3.50) of the receptor and the Cα-atom of L380, the last amino acid of the α-subunit, was employed. In general, more than 90% of the 4000 solutions provided by each docking run did not fulfill the distance-based filter. Moreover, only a minority of the filtered solutions could be considered realistic, i.e. holding an acceptable topology of the N-terminal α-helix of the α-subunit (i.e. αN). Acceptable membrane topologies were considered those characterized by the main axis of αN almost parallel and close enough to the membrane surface to allow the hydrophobic N-acyl and farnesyl modifications of the α- and γ-subunit, respectively, to insert into the membrane.
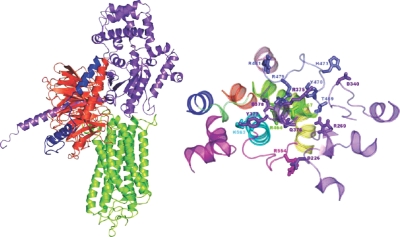
Despite the structural differences between the D564(6.30)G, D564(6.30)A, and D578(6.44)H CAMs, they show common recognition modes to the G protein. For the three mutants, the best scored reliable solutions fall among the best 90 solutions out of 4000 in the ZDOCK output list. In these solutions, 1) the C tail of Gsα docks between H3 and H6 of the receptor; 2) the αN of Gsα docks between H6 and H7 or on H8; and 3) the Gsβ makes contacts with the membrane-facing portions of H5 and H6 and of IL3 (Fig. 2). Most of the LHR-Gsα interactions that are shared by at least three of the selected complexes between Gs and the four different LHR forms involve: 1) R464(3.50), T467(3.53), I469, Y470, R479 (the last three amino acids are from IL2), and K563(6.29) from LHR, and 2) R375, Q376, Y377, and E378 from Gsα. Hence, the major contribution from Gsα comes from the C-terminal stretch. In detail, a recurrent interaction in all the receptor-G protein complexes is the salt bridge between GsαE378 and LHRR464(3.50) of the highly conserved (E/D)R(Y/W) motif. Furthermore, GsαR375, GsαQ376, and GsαY377 are frequently involved in pairwise H bonding or van der Waals interactions with LHRY470, LHRT467(3.53), and LHRK563(6.29), respectively (Table 1). The C-terminal carboxylate of Gsα may be found interacting with LHRR481. Other Gsα domains that are involved in the interface with LHR are the α2/β4, α3/β5, and α4/β6 loops. In this respect, recurrent interactions are those between: 1) GsαD226 and LHRR554; 2) GsαR269 and LHRT469; 3) GsαT270 and LHRI468; and 4) GsαD340 and LHRH473 (Table 1). Finally, the β-subunit of Gs is often involved in interaction with LHRY550.
Figure 2.
Complex between Gs and D564(6.30)G LHR CAM
Left, Side view in a direction parallel to the membrane plane of a selected complex between a AVG1000ps-minimized structure of LHR (green) and Gsαβγ. The Gs α-, β-, and γ-subunits are violet, orange, and blue, respectively. Right, The cytosolic half of the D564(6.30)G LHR CAM and selected domains of Gsα are shown. The complex is seen from the intracellular side in a direction perpendicular to the membrane surface. The amino acid side chains involved in the most recurrent interactions are represented by sticks. Gsα is violet, whereas the receptor domains are colored as follows: H1, H2, H3, H4, H5, H6, and H7 are in blue, orange, green, pink, yellow, cyan, and purple, respectively, whereas IL1, IL2, and IL3 are lime, slate, and magenta, respectively.
Ala-Scanning Mutagenesis of LHR IL2 and the Cytosolic Extensions of H3 and H4
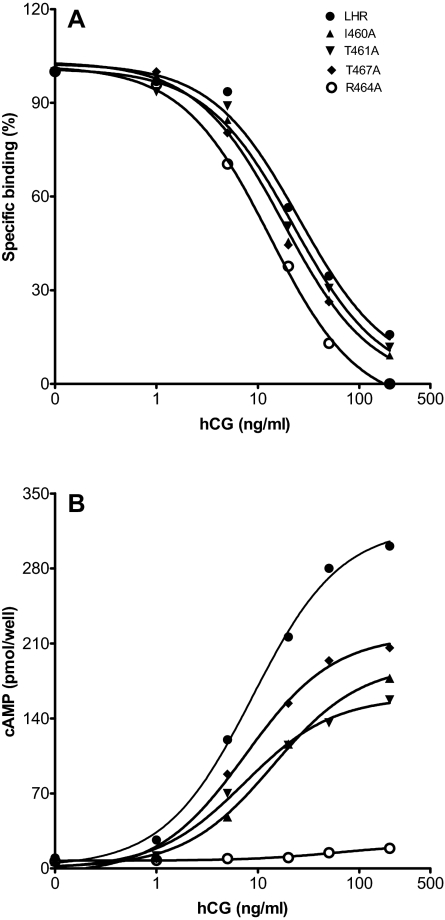
A total of 23 single Ala replacements were made in the cytosolic extensions of H3 and H4, as well as all residues encompassing IL2. Of these, 22 expressed sufficiently well for functional characterization; only E463A failed to express at the cell surface. Figure 3 shows a typical binding isotherm and ligand-mediated cAMP production assay for WT LHR and mutants I460(3.46)A, T461(3.47)A, R464(3.50)A, and T467(3.53)A. A combination of saturation binding, competitive binding, and signaling assays was used to obtain the data presented in Table 2 for Ala replacements of 22 amino acid residues in IL2.
Figure 3.
Representative Competition Binding and cAMP Dose-Response Curves for WT LHR and I460(3.46)A, T461(3.47)A, R464(3.50)A, and T467(3.53)A LHR Mutants
A, Competitive binding of the WT and mutant LHRs. HEK 293 cells were transiently transfected with the various constructs and 48 h later characterized for hCG binding. The binding experiments were performed in Waymouth’s medium/BSA for 6 h at 37 C in the presence of 50 pm [125I]hCG and various concentrations of hCG. B, Concentration-dependent hCG-stimulated cAMP accumulation in HEK 293 cells expressing the WT and mutant LHRs. The transfected cells were stimulated with increasing concentrations of hCG for 30 min at 37 C in the presence of Waymouth’s medium/BSA and 0.8 mm isobutylmethylxanythine.
Table 2.
Summary of the Functional Characteristics of LHR IL2 Mutants after Ala-Scanning Mutagenesis
| LHRa | n | Bmax (%)a | IC50 (nm) | EC50 (nm) | Rmax(%)a | Qmut/Qwta |
|---|---|---|---|---|---|---|
| Wild type | 21 | 100 | 1.10 ± 0.13 | 0.29 ± 0.05 | 100 | 1.00 |
| V459(3.45)A | 3 | 49 ± 13 | 0.47 ± 0.09 | 0.28 ± 0.07 | 63±12 | 1.01 ± 0.06 |
| I460(3.46)A | 4 | 43 ± 10b | 0.59 ± 0.20 | 0.32 ± 0.05b | 47 ± 12b | 0.41 ± 0.003b |
| T461(3.47)A | 3 | 108 ± 26 | 0.64 ± 0.03 | 0.26 ± 0.03b | 51 ± 12b | 0.30 ± 0.02b |
| L462(3.48)A | 3 | 69 ± 20 | 0.42 ± 0.08 | 0.21 ± 0.04 | 89 ± 39 | 1.10 ± 0.03 |
| R464(3.50)A | 8 | 6.4 ± 1.3b | 0.34 ± 0.06b | 2.47 ± 0.43b | 4.6 ± 1.9b | 0.10 ± 0.01b |
| W465(3.51)A | 5 | 10 ± 5b | 0.49 ± 0.06b | 0.42 ± 0.11 | 26 ± 6b | 1.64 ± 0.14b |
| H466(3.52)A | 3 | 58 ± 8 | 0.64 ± 0.03 | 0.48 ± 0.05b | 64 ± 7 | 0.58 ± 0.08b |
| T467(3.53)A | 3 | 181 ± 19b | 1.07 ± 0.05 | 0.26 ± 0.02 | 124 ± 35 | 0.63 ± 0.06b |
| I468(3.54)A | 5 | 19 ± 9b | 0.26 ± 0.05b | 1.00 ± 0.34b | 4.5 ± 1.7b | 0.10 ± 0.01b |
| T469A | 3 | 207 ± 26b | 0.76 ± 0.23 | 0.26 ± 0.04 | 200 ± 8b | 0.91 ± 0.11 |
| Y470A | 3 | 159 ± 22 | 0.64 ± 0.17 | 0.35 ± 0.02b | 136 ± 20 | 0.63 ± 0.04b |
| I472A | 4 | 187 ± 58 | 1.35 ± 0.23 | 0.41 ± 0.09b | 91 ± 27 | 0.34 ± 0.04b |
| H473A | 3 | 183 ± 54 | 0.91 ± 0.20 | 0.24 ± 0.06 | 169 ± 39 | 0.88 ± 0.16 |
| L474A | 3 | 98 ± 24 | 1.10 ± 0.16 | 0.22 ± 0.02 | 78 ± 14 | 0.95 ± 0.03 |
| D475A | 3 | 178 ± 31 | 0.86 ± 0.17 | 0.24 ± 0.05 | 160 ± 2 | 0.94 ± 0.06 |
| Q476A | 3 | 92 ± 14 | 0.86 ± 0.01 | 0.15 ± 0.03 | 90 ± 14 | 0.98 ± 0.17 |
| K477A | 5 | 9.0 ± 2.4b | 0.55 ± 0.07b | 0.51 ± 0.11 | 38 ± 7b | 1.80 ± 0.15b |
| L478A | 3 | 108 ± 18 | 1.40 ± 0.50 | 0.74 ± 0.11b | 54 ± 5b | 0.32 ± 0.01b |
| R479A | 3 | 110 ± 14 | 1.98 ± 0.25 | 0.33 ± 0.03 | 107 ± 17 | 0.94 ± 0.03 |
| L480A | 5 | 44 ± 16 | 0.54 ± 0.13 | 0.44 ± 0.12 | 81 ± 14 | 1.25 ± 0.10 |
| R481A | 4 | 57 ± 13 | 0.69 ± 0.03 | 0.27 ± 0.05 | 64 ± 12 | 0.95 ± 0.05 |
| H482(4.41)A | 3 | 124 ± 46 | 0.74 ± 0.06 | 0.22 ± 0.03 | 90 ± 20 | 0.88 ± 0.02 |
WT LHR and the receptor mutants were expressed in HEK 293 cells. Binding studies with [125I]hCG (saturation and competitive) and hCG-mediated cAMP production assays were conducted to obtain the binding and signaling parameters shown. Basal cAMP values were the same between WT LHR (5.0 ± 0.6 pmol/well, n = 21) and the mutants; Bmax and Rmax values are normalized to those of WT LHR and given as percentages, whereas Q for the mutants is normalized to that of WT LHR and given as a ratio.
Bmax, Rmax, and Q values for WT LHR are 29.0 ± 8.5 fmol/well, 305 ± 35 pmol/well, and 46.7 ± 5.8, respectively.
Significantly different from WT LHR, P < 0.05. The parameters shown for WT LHR represent the mean ± sem for all experiments; statistical analysis, however, was performed on LHR mutant results vs. those for WT LHR in each experiment.
Most of the Ala mutants expressed at levels comparable to that of WT LHR. Four of the mutant receptors, R464(3.50)A, W465(3.51)A, I468(3.54)A, and K477A, exhibited significantly reduced cell surface expression, e.g. 6–20% that of WT LHR. The IC50 values [and dissociation constants (Kds) when measured directly in saturation binding or calculated from competitive binding plots] of the mutants were, in general, equivalent to that of WT LHR; however, the binding affinities of R464(3.50)A, W465(3.51)A, I468(3.54)A, and K477A were 2- to 3-fold higher. None of the mutants harboring a single Ala replacement in IL2 exhibited increased basal cAMP levels, although slight increases may be masked in those mutants that expressed poorly. Several mutants yielded EC50 values slightly different from that of WT LHR, but the EC50 values for R464(3.50)A, I468(3.54)A, and L478A were appreciably increased. Rmax values, i.e. the maximal cAMP level achieved at a saturating concentration of human chorionic gonadotropin (hCG) corrected for basal level, were also comparable to that of WT LHR for many of the mutants, but a number of the single mutants were characterized by lower values, e.g. I460(3.46)A, T461(3.47)A, R464(3.50)A, W465(3.51)A, I468(3.54)A, K477A, and L478A. (One mutant, T469A, exhibited an elevated Rmax relative to WT LHR, but it also overexpressed.) Because four of these Ala mutants are expressed less than WT LHR, a correction for the expression level, i.e. Rmax/Bmax, indicates that T461(3.47)A, R464(3.50)A, I468(3.54)A, and L478A remain less than the ratio for WT LHR, as do I472A and L474A; interestingly, W465(3.51)A, K477A, and L480A exhibit ratios exceeding that of WT LHR.
A more rigorous parameter to compare receptor function is that of the coupling efficiency, Q (23). The coupling efficiency incorporates the major experimentally accessible variables for binding, Bmax and Kd (or IC50 from which the Kd can be obtained), and signaling, Rmax and ED50. In all cases, Q is normalized to 1.0 for WT LHR.
Q = 0.5 [1 + Kd/ED50](Rmax/Bmax)
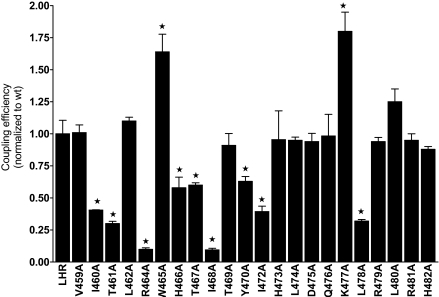
Q values are shown for the 22 mutants in Fig. 4 and Table 2. Of the mutants characterized, nine show reduced Q values compared with WT LHR: I460(3.46)A, T461(3.47)A, R464(3.50)A, H466(3.52)A, T467(3.53)A, I468(3.54)A, Y470A, I472A, and L478A. Of interest, W465(3.51)A and K477A exhibit coupling efficiencies greater than that of WT LHR, suggesting that these two residues have somewhat of an inhibitory role in WT receptor signaling. Computational experiments show that, in the preferential LHR-Gs docking modes, LHRK477 tends to approach GsαR375. We speculate that alanine substitution for LHRK477 would therefore reduce potential electrostatic repulsions with the Gsα arginine, thus favoring a receptor-G protein encounter.
Figure 4.
Coupling Efficiencies of Ala-Substituted Residues in IL2 of LHR
From measurements of expression levels and of binding and signaling parameters, a coupling efficiency, Q, was determined for each Ala-substituted side chain in IL2. *, Significantly different (P ≤ 0.05) from that of WT LHR (Q = 1.00).
Double Mutants of LHR
Three double mutants containing the potent constitutively activating mutation in H6, D578(6.44)H, paired with mutants of reduced coupling efficiencies, T461(3.47)A, R464(3.50)A, and I468(3.54)A, were prepared and characterized. Also, mutants with residues yielding low coupling efficiencies compared with WT LHR, T461(3.47)A, R464(3.50)A, T467(3.53)A, I468(3.54)A, and I472A, were paired in four different double mutants: T461(3.47)A/R464(3.50)A, T461(3.47)A/T467(3.53)A, T461(3.47)A/I468(3.54)A, and I468(3.54)A/I472A. The complete functional characteristics of these double mutants, along with the parameters of the constituent single mutants, are given in Table 3. LHR D578(6.44)H overexpressed relative to WT LHR (5-fold) and exhibited greatly increased basal cAMP, some 34- to 45-fold, over that of WT LHR. The three double mutants containing D578(6.44)H also yielded basal cAMP values ranging from 2- to 42-fold greater than that of WT LHR, the lowest value being observed when pairing D578(6.44)H with R464(3.50)A. Differences in expression levels, however, prevent a quantitative assessment of these results. Because the D578(6.44)H single mutant and D578(6.44)H-containing double mutants respond poorly, if at all, to ligand, the coupling efficiencies are invariably low because Rmax, corrected for basal cAMP levels, is zero or nearly so (Table 3).
Table 3.
Summary of the Functional Characteristics of LHR Double Mutants within IL2 and between IL2 and H6
| LHR | n | Bmax(%)a | IC50 (nm) | bcAMP (pmol/well) | EC50 (nm) | Rmax(%)a | Qmut/Qwta |
|---|---|---|---|---|---|---|---|
| Constituent single mutants | |||||||
| Wild type | 5 | 100 | 0.83 ± 0.11 | 3.0 ± 0.04 | 0.31 ± 0.04 | 100 | 1.00 |
| T461(3.47)A | 3 | 108 ± 26 | 0.64 ± 0.03 | 3.9 ± 0.6 | 0.26 ± 0.03 | 51 ± 12 | 0.30 ± 0.02b |
| R464(3.50)A | 5 | 5.0 ± 0.5b | 0.30 ± 0.06b | 2.5 ± 0.5 | 1.87 ± 0.59b | 3.8 ± 9.7b | 0.22 ± 0.05b |
| T467(3.53)A | 3 | 181 ± 19b | 1.07 ± 0.05 | 4.6 ± 0.8 | 0.26 ± 0.02 | 124 ± 35 | 0.63 ± 0.06b |
| I468(3.54)A | 5 | 19 ± 9 | 0.36 ± 0.07b | 2.6 ± 0.6 | 1.25 ± 0.60b | 4.5 ± 1.7b | 0.10 ± 0.01b |
| I472A | 4 | 187 ± 58 | 1.35 ± 0.23 | 2.3 ± 0.2 | 0.41 ± 0.09b | 91 ± 27 | 0.34 ± 0.04b |
| D578(6.44)H | 3 | 533 ± 95 | 1.62 ± 0.33 | 125 ± 8b | c | c | c |
| Double mutants within IL2 | |||||||
| T461(3.47)A/R464(3.50)A | 4 | 54 ± 20 | 0.71 ± 0.14 | 4.2 ± 0.1 | 5.90 ± 0.50b | 7.3 ± 2.8b | 0.08 ± 0.01b |
| T461(3.47)A/T467(3.53)A | 3 | 194 ± 61 | 1.47 ± 0.09 | 3.9 ± 1.0 | 2.42 ± 0.23b | 38 ± 11b | 0.16 ± 0.01b |
| T461(3.47)A/I468(3.54)A | 3 | 158 ± 37 | 0.80 ± 0.10 | 2.0 ± 0.5 | 3.44 ± 0.79b | 4.8 ± 1.8b | 0.007 ± 0.001b |
| I468(3.54)A/I472A | 4 | 75 ± 27 | 0.69 ± 0.11 | 5.2 ± 0.9 | 34.0 ± 6.6b | 1.1 ± 0.5b | 0.008 ± 0.001b |
| Double mutants between IL2 and H6 | |||||||
| T461(3.47)A/D578(6.44)H | 3 | 1,479 ± 154b | 2.89 ± 0.41b | 118 ± 6b | c | c | c |
| R464(3.50)A/D578(6.44)H | 5 | 20 ± 3b | 0.33 ± 0.08b | 6.5 ± 0.9b | 2.60 ± 0.67b | 6.1 ± 1.7b | 0.05 ± 0.01b |
| I468(3.54)A/D578(6.44)H | 3 | 137 ± 40 | 0.86 ± 0.06 | 91 ± 23b | c | c | c |
WT LHR and mutants (doubles and constituent singles) were expressed in HEK 293 cells, following binding and signaling studies. bcAMP is the basal value, and additional information is provided in the legend to Table 2.
Bmax, Rmax, and Q values for WT LHR are 18.6 ± 3.7 fmol/well, 295 ± 51 pmol/well, and 43.1 ± 11.3, respectively.
Significantly different from WT LHR, P < 0.05 (see footnote b in Table 2).
The D578(6.44)H mutant and two of the D578(6.44)H-containing mutants have high basal cAMP levels and are nonresponsive, or essentially nonresponsive, to hCG. Thus, there are no EC50 values, and the Rmax, as defined, is near zero because the basal and maximal cAMP values are so similar. This feature also renders Q values meaningless.
Like the corresponding single mutants, the double mutants, T461(3.47)A/R464(3.50)A, T461(3.47)A/T467(3.53)A, T461(3.47)A/I468(3.54)A, and I468(3.54)A/I472A, do not exhibit increased basal cAMP levels compared with that of the WT control. The expression levels are good, but hCG-mediated signaling is low, resulting in greatly diminished coupling efficiencies, which compared with WT LHR, are 10–20% for the T461(3.47)A/R464(3.50)A and T461(3.47)A/T467(3.53)A mutants and about 1% for the T461(3.47)A/I468(3.54)A and I468(3.54)A/I472A mutants, much less that that expected from the individual mutants. Three double mutants, pairing R464(3.50)A with I460(3.46)A, T467(3.53)A, and I468(3.54)A, were also prepared and characterized. These mutants expressed very poorly on the cell surface, had basal cAMP levels like that of WT LHR, and were unresponsive to hCG (data not shown).
Ala-Scanning Mutagenesis of LHR IL3 and the Cytosolic Extensions of H5 and H6
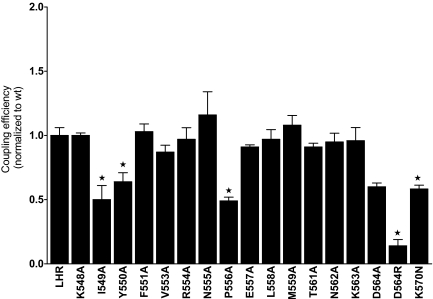
Ala-scanning mutagenesis of a major portion of the cytosolic extensions of H5 and H6 and of IL3 gave the results summarized in Fig. 5 and Table 4. With the exceptions of I549(5.61)A and N562(5.62)A, the expression levels of the single mutants were comparable to that of WT LHR; moreover, the hormone affinities were not affected by Ala replacements. Although a few of the mutants had EC50 values somewhat higher than that of WT LHR, the difference was not large. As monitored by Rmax values, the mutants responded to hCG similarly to WT LHR with the exception of I549(5.61)A, which had an Rmax significantly lower than that of WT LHR. The coupling efficiencies of the mutants were similar to that of WT LHR with the exceptions of I549(5.61)A, Y550(5.62)A, P556A, and D564(6.30)A, each of which yielded Q values of 0.5–0.6 compared with that of WT LHR (normalized to 1.0). A charge reversal replacement was made at position 564 to give D564(6.30)R. Like D564(6.30)A, D564(6.30)R exhibited increased basal cAMP levels, expressed well, and bound hCG with an affinity comparable to WT LHR. Compared with WT LHR, the EC50 of D564(6.30)R was increased, whereas the values of Rmax and Q were decreased. Lastly, to overcome the low expression of K570(6.36)A, an asparagine replacement resulted in good expression, giving IC50 and Rmax values similar to WT LHR; however, EC50 was slightly elevated and Q was diminished. Of the residues examined in IL3, the impact of Ala replacement was relatively modest compared with some of the changes observed in IL2.
Figure 5.
Coupling Efficiencies of LHR Ala Mutants in IL3
From measurements of expression levels and of binding and signaling parameters, Q was determined for each Ala-substituted side chain in IL3. *, Significantly different (P ≤ 0.05) from that of WT LHR (Q = 1.00).
Table 4.
Summary of the Functional Characteristics of LHR IL3 Mutants after Ala-Scanning Mutagenesis
| LHRa | n | Bmax (%)a | IC50 (nm) | EC50 (nm) | Rmax(%)a | Qmut/Qwta |
|---|---|---|---|---|---|---|
| Wild type | 19 | 100 | 1.00 ± 0.12 | 0.35 ± 0.04 | 100 | 1.00 |
| K548(5.60)A | 3 | 117 ± 17 | 0.91 ± 0.15 | 0.47 ± 0.19 | 123 ± 14 | 1.00 ± 0.02 |
| I549(5.61)A | 3 | 13 ± 2b | 0.92 ± 0.13 | 1.55 ± 0.34b | 13.6 ± 2.6b | 0.50 ± 0.11b |
| Y550(5.62)A | 3 | 84 ± 9 | 0.91 ± 0.11 | 0.93 ± 0.33c | 82 ± 8 | 0.64 ± 0.07b |
| F551(5.63)A | 4 | 155 ± 29 | 0.66 ± 0.03 | 0.20 ± 0.05 | 131 ± 15 | 1.03 ± 0.06 |
| V553A | 3 | 130 ± 28 | 0.80 ± 0.12 | 0.22 ± 0.10 | 96 ± 12 | 0.87 ± 0.05 |
| R554A | 3 | 56 ± 4 | 0.87 ± 0.08 | 0.42 ± 0.07 | 66 ± 3 | 0.97 ± 0.09 |
| N555A | 3 | 140 ± 36 | 0.72 ± 0.18 | 0.17 ± 0.07 | 107 ± 27 | 1.16 ± 0.18 |
| P556A | 4 | 157 ± 51 | 0.75 ± 0.12 | 0.42 ±0.09c | 87 ± 12 | 0.49 ± 0.03b |
| E557A | 3 | 175 ± 26 | 0.74 ± 0.01 | 0.19 ± 0.07 | 126 ± 6 | 0.91 ± 0.02 |
| L558A | 3 | 133 ± 10 | 1.64 ± 0.10 | 0.25 ± 0.01 | 94 ± 0.4 | 0.97 ± 0.08 |
| M559A | 3 | 99 ± 25 | 1.92 ± 0.07 | 0.43 ± 0.06 | 117 ± 23 | 1.08 ± 0.08 |
| T561(6.27)A | 3 | 170 ± 25 | 1.60 ± 0.10 | 0.39 ± 0.04 | 108 ± 9 | 0.91 ± 0.03 |
| N562(6.28)A | 3 | 34 ± 9b | 1.34 ± 0.10 | 0.69 ± 0.10 | 66 ± 25 | 0.95 ± 0.07 |
| K563(6.29)A | 3 | 125 ± 36 | 1.00 ± 0.18 | 0.56 ± 0.24 | 95 ± 7 | 0.96 ± 0.10 |
| D564(6.30)A | 3 | 87 ± 8 | 1.62 ± 0.25 | 0.70 ± 0.10b | 84 ± 3 | 0.60 ± 0.03b |
| D564(6.30)R | 5 | 156 ± 36 | 0.81 ± 0.10 | 1.44 ± 0.09b | 27 ± 5b | 0.14 ± 0.05b |
| K570(6.36)N | 3 | 92 ± 36 | 1.87 ± 0.10 | 0.68 ± 0.06c | 77 ± 27 | 0.58 ± 0.05b |
WT LHR and the receptor mutants were expressed in HEK 293 cells and characterized via binding and signaling studies (see additional information in Table 2).
The Bmax, Rmax, and Q values for WT LHR are 34.7 ± 9.5 fmol/well, 394 ± 57 pmol/well, and 44.6 ± 6.1, respectively. Basal cAMP levels for WT LHR were 4.3 ± 0.5 pmol/well. None of the mutants in IL3 exhibited increased basal cAMP values except the D564(6.30)A and D564(6.30)R mutants that had basal cAMP values of 20.6 ± 6.3 (n = 3) and 77.1 ± 10.0 (n = 5) pmol/well, respectively.
Significantly different from WT LHR, P < 0.05 (see footnote b in Table 1).
The EC50 values were shifted somewhat to higher values, but they were not significantly different from WT LHR.
Mapping the Hot Spots onto the Computational Model of WT LHR
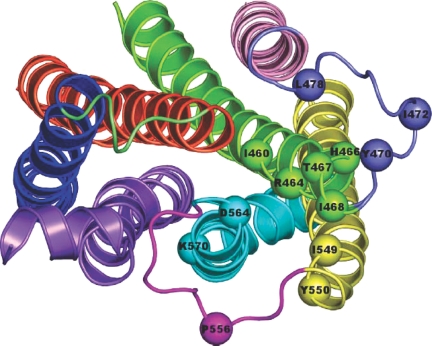
The location of most of the amino acid residues essential for the hormone-induced Gs activation (i.e. hot spots) has been mapped onto one of the selected AVG1000ps structures of WT LHR (Fig. 6). Such mapping clearly shows that the majority of the hot spots lie at the cytosolic end of H3 at the interface with H5 and H6. Some of these amino acids, i.e. I460(3.46), T461(3.47), and H466(3.52), are buried with respect to the cytosol both in the WT and CAM forms. In contrast, R464(3.50), T467(3.53), and I468(3.54) undergo increases in solvent exposure on going from the WT to the CAM forms, i.e. they all contribute to the SAS index that properly differentiates the inactive from the active receptor forms (Table 1). Finally, the IL2 amino acids, Y470 and I472, are almost always solvent exposed, whereas L478 is buried, being directed toward H3. As for the hot spots at the cytosolic end of H5, I459(5.61) is buried in all the simulated LHR forms, being directed toward H3, whereas Y550(5.62) is directed toward H6 and the phospholipids. The only IL3 amino acid that produces a marked decrease in hormone-induced Gs coupling, P556, is solvent exposed in all the simulated LHR forms. As for the hot spots on the cytosolic extension of H6, D564(6.30) is directed toward H3, whereas K570(6.36) is directed toward H7 and the core of the helix bundle in almost all the simulated forms.
Figure 6.
Map of the Hot Spots in LHR
Selected AVG1000ps -minimized structure of WT LHR seen from the intracellular side in a direction perpendicular to the membrane surface. The hot spots resulting from in vitro Ala scanning mutagenesis are highlighted by spheres centered at the Cα atoms. The extracellular domains are not shown. The receptor domains are colored as follows: H1, H2, H3, H4, H5, H6, and H7 are in blue, orange, green, pink, yellow, cyan, and violet, respectively, whereas IL1, IL2, and IL3 are lime, slate, and magenta, respectively.
Collectively, the results of rigid body docking and the mapping of the hot spots highlighted by in vitro mutagenesis suggest that only three amino acids at the cytosolic extension of H3, i.e. R464(3.50), T467(3.53), and I468(3.54), one at the N-terminal end of IL2, i.e. Y470, and two at the cytosolic ends of H5 and H6, i.e. Y550(5.62) and D564(6.30), respectively, could participate in Gs recognition, whereas the other hot spots are likely to play structural/dynamics roles.
DISCUSSION
The results presented herein on LHR establish a critical role for the cytosolic end of H3 and for IL2 in Gs coupling and activation. A combination of computational modeling and experimental data provides a strong framework for a detailed description at the molecular level. The cytosolic extensions of H3 and H4 encompass (approximately) residues 459–468 and 482–483, respectively. In the last 10 amino acid residues of H3, six residues appear to function in some capacity in productive Gs interaction: I460(3.46), T461(3.47), R464(3.50), H466 (3.52), T467(3.53), and I468(3.54). One, E463(3.49), could not be evaluated because LHR E463(3.49)A mutants failed to yield sufficient cell surface expression for characterization. Of the residues assessed in IL2 and IL3, only D564(6.30) appears to be required to maintain the receptor in an inactive conformation in the absence of ligand.
Consistent with the results of in vitro experiments, the results of receptor-G protein docking simulations done in this study emphasize the role of the cytosolic extension of H3 and of the N-terminal end of IL2 as the most important receptor recognition points for the C tail of Gsα. The results of in vitro experiments highlighted I460(3.46), T461(3.47), and I549(5.61) as hot spots for G protein coupling efficiency. These residues are not solvent exposed in the models of the LHR active mutants. This is suggestive of an important structural role rather than an involvement in G protein recognition, at least in the early steps of recognition. The remaining hot spots in the cytosolic end of H3 include R464(3.50), T467(3.53), and I468(3.54), which undergo increases in solvent exposure on going from the WT to the CAM forms. Indeed, these amino acids participate in the SAS index that was found to be a hallmark of the functional receptor state in this and previous studies, increasing over a threshold value in the CAMs but not in the WT and the inactive mutants (4,24). In line with computational analysis of the isolated receptors, the predicted complexes between the different LHR forms and heterotrimeric Gs converge into the involvement of these three hot spots in the receptor-G protein interface (Table 1 and Fig. 2). Each of the R464(3.50)A and I468(3.54)A mutants exhibit IC50 values somewhat lower than that of WT LHR and EC50s significantly higher. The former observation implies that the Ala replacements may lead to conformational changes that are transmitted to the ectodomain via transmembrane reorientations, e.g. interaction of the ectodomain with the extracellular loops, placing the former in a more open conformation for ligand binding, or this may be a result of lower cell surface expression. The increased EC50s of the mutants relative to WT receptor strongly suggest impaired G protein coupling or activation.
The CAMs considered in this study, i.e. D564(6.30)G, D564(6.30)A, and D578(6.44)H, are predicted to share common recognition modes for Gs. In fact, the best complexes are characterized by the docking of the C tail of Gsα in between H3 and H6 of the receptor. A common feature of the receptor-G protein interfaces is the salt bridge between R464(3.50) of the (E/D)R(Y/W) motif and E378 of Gsα (Table 1). These results support a direct involvement of the (E/D)R(Y/W) arginine in G protein recognition. Other recurrent interactions between the C tail of Gsα and the LHR CAMs include those between the following G protein-receptor pairs: GsαR375-LHRY470, GsαQ376-LHRT467(3.53), and GsαY377-LHRK563(6.29) (Table 1).
The most reliable receptor-Gs docking mode shared by the three CAMs is also found in docking simulations involving the WT form of the receptor (Table 1). However, the docking scores concerning the WT receptor are slightly lower than those of the active mutants. This means that, even if the results of computations do not exclude the possibility that LHR and heterotrimeric Gs are constitutively coupled or precoupled, the mutation-induced active forms show better shape and electrostatic complementarities for the G protein than the WT. The hypothesis that Gs and LHR are constitutively coupled or precoupled in the absence of hormone is supported by the significant basal activity of the WT receptor.
Other domains of Gsα like the α2/β4, α3/β5, and α4/β6 loops, may be involved in interaction with the receptor. However, the interaction mode of the latter might be influenced by the presence and conformation of the LHR C tail that is absent in the computational models considered in this study. Therefore, we prefer not to speculate on the interactions made by G protein domains other than the C-terminal amino acids of Gsα, which reach the highest consensus after a large number of simulations.
Collectively, the extensive docking simulations done on different computational models of WT and mutated LHR forms converge in a common recognition mode involving the C tail of Gsα and the cytosolic extensions of H3 and H6, as well as the N-terminal region of IL2 of LHR. This suggests that an effective functional coupling between the two proteins should rely on the establishment of critical interactions between these limited domains. One critical interaction is predicted to be the charge-reinforced H-bond between R464(3.50) of the (E/D)R(Y/W) motif and E378 of Gsα. Such an interaction is predicted to be facilitated by the increase in solvent exposure of the highly conserved arginine, a feature of the mutation-induced active forms, in particular, the D6.30 CAMs.
An earlier study documented the importance of D6.44 of H6 in maintaining an inactive LHR conformation stabilized by its interactions with N7.45 (18). D6.44 is also the locus for the majority of LHR mutations leading to familial and sporadic cases of male-limited precocious puberty (25,26). To extend the comprehensive Ala-scanning mutagenesis approach, a series of double mutants were chosen to pair the potent constitutively activating LHR mutant, D578(6.44)H, with three Ala replacements in the cytosolic extension of H3, T461(3.47)A, T467(3.53)A, R464(3.50)A, and I468(3.54)A, which impair signaling. Differences in expression levels prevent a quantitative assessment of the basal activity of the double mutants involving D578(6.44)H, although the R464(3.50)A/D578(6.44)H mutant exhibited the lowest basal cAMP level. Collectively, the D578(6.44)H single mutant and D578(6.44)H-containing double mutants respond poorly, if at all, to ligand. The expression levels of the four double mutants involving Ala replacements at T461(3.47), R464(3.50), T467(3.53), I468(3.54), and I472, i.e. sites associated with low coupling efficiencies, to give T461(3.47)A/R464(3.50)A, T461(3.47)A/T467(3.53)A, T461(3.47)A/I468(3.54)A, and I468(3.54)A/I472A were comparable to that of WT LHR. The ligand responsiveness was, however, reduced in each case. These findings again reinforce the importance of these side chains in G protein binding and/or activation and strongly support the results from the computational studies.
In conclusion, the results of in vitro and in silico experiments carried out in this study highlight the prominent role of the cytosolic extension of H3 and the N-terminal portion of IL2 in Gsα interaction, whereas the contribution of IL3 is marginal. Furthermore, mapping the hot spots into the computational models of the LHR and of the LHR-Gs complexes allowed for a distinction between receptor sites required for intramolecular structural changes (i.e. I460, T461, H466, and I549) and stretches of receptor sites more likely involved in G protein recognition (i.e. R464, T467, I468, Y470, Y550, and D564). The latter includes the highly conserved (E/D)R(Y/W) arginine that, therefore, is likely to be a receptor recognition point for Gs rather than a switch of receptor activation. The computational experiments carried out in this study provide a novel way to consider LHR CAM structures, i.e. receptor states with improved complementarity for the G protein compared with the WT receptor.
MATERIALS AND METHODS
Materials
Human embryonic kidney (HEK) 293 cells were obtained from American Type Culture Collection (Manassas, VA). DMEM and PBS were from Cellgro Mediatech, Inc. (Herndon, VA). Newborn calf serum, antibiotics, Waymouth’s media, trypsin-EDTA, and lipofectamine were purchased from Life Science Technologies (Gaithersburg, MD), and BSA and isobutylmethylxanythine were from Sigma Chemical Co. (St. Louis, MO). [125I]hCG and [125I]cAMP RIA kits were obtained from DuPont NEN (Boston, MA); [125I]hCG was also radioiodinated by the Iodo-Gen iodination kit from Pierce Biotechnology, Inc. (Rockford, IL) using [Na125I] from Amersham Pharmacia Biotech (Piscataway, NJ).
Mutagenesis of LHR
Mutagenesis of human LHR, cloned in the expression vector pcDNA3, was performed using the QuikChange Site-Directed Mutagenesis kit as recommended by Stratagene (La Jolla, CA). Mutant clones were identified by sequencing (Sequenase Version 2.0 DNA sequencing kit; Amersham), and purified DNA was obtained with the QIAGEN (Chatsworth, CA) plasmid maxi kit.
Cell Culture and Transient Transfection of HEK 293 Cells
HEK 293 cells were grown in monolayer culture in DMEM supplemented with 10% (vol/vol), newborn calf serum, 50 U/ml penicillin, 50 μg/ml streptomycin, 0.125 μg/ml amphotericin B, and 10 mm HEPES (pH 7.4). Cells were maintained at 37 C in humidified air containing 5% CO2. HEK 293 cells were transiently transfected with the WT or mutant cDNA using lipofectamine (Invitrogen, Carlsbad, CA).
Hormone Binding and cAMP Assay
For both hormone binding and cAMP assays, the transfected cells were replated (1 × 105 cells per well) into 12-well tissue culture plates 16–18 h after transfection. After 24 h either binding was performed with addition of [125I]hCG or hCG was added for cAMP determinations. For the binding studies, cells were incubated with [125I]hCG (50 pm and 50–500 pm for the competitive and saturation binding experiments, respectively) for 6 h at 37 C in the presence of Waymouth’s medium or binding buffer (278 mm sucrose, 0.1% glucose, 5 mm HEPES, 5 mm KCl, 1.2 mm MgSO4, 1 mm NaHCO3, 1 mm CaCl2·2 H2O, and 1.2 mm KH2PO4) containing 0.1% BSA, respectively. Increasing concentrations of unlabeled hormone (hCG) were added to each well for competitive binding assays, and nonspecific binding was determined by addition of a 1000-fold excess of unlabeled hormone. For cAMP measurements, the cells were incubated with increasing (or maximal) concentrations of hCG (1–100 ng/ml) for 30 min at 37 C in the presence of 0.8 mm isobutylmethylxanthine. Incubation medium was removed, and the cells were lysed in 100% ethanol at −20 C overnight. The extract was collected, dried under vacuum, and resuspended in the cAMP assay buffer of the [125I]cAMP assays kit. cAMP concentrations were determined by RIA as recommended by DuPont NEN. Both binding and cAMP data were analyzed by the Prism software (GraphPad Software, San Diego, CA), using nonlinear regression analysis. All the results are the average of three to eight experiments each performed in duplicate.
Computational Modeling of the LHR
The details of comparative modeling of the updated model of the human LHR have been already reported elsewhere (27). The human LHR model employed in this study differs slightly from this last reported structure with respect to a slightly different alignment in the N terminus and IL2 as well as the MD setup. The model of the LHR was built by means of the comparative modeling software MODELER (28), by using the latest rhodopsin structure as a template, i.e. Protein Data Base (PDB) code: 1U19 (29). The modeled sequence includes the transmembrane helices, the three intracellular loops (IL1, IL2, and IL3), the three extracellular loops (EL1, EL2, and EL3), as well as the 323–358 ectodomain sequence, which can be reasonably modeled based upon the N terminus of rhodopsin (Fig. 1). A modified rhodopsin template was employed in which the sequences 100–101 and 106–107 were deleted, which correspond to the H2-EL1 junction and the first two amino acids of H3, respectively. The sequence 236–242, corresponding to the C-terminal region of IL3, was deleted as well. During comparative modeling, α-helical restraints were imposed on the LHR sequences 420–423 and 432–439.
Three different alignments were probed, each of which was used to build 200 models by randomizing the Cartesian coordinates of the model through a random number uniformly distributed in an interval from −4 Å to 4 Å (28). The two LHR models finally selected for MD, as characterized by the lowest degrees of restraint violations, were achieved by means of the sequence alignment shown in Fig. 1. These models were subjected to automatic and manual rotation of the side-chain torsion angles when in nonallowed conformations, leading to five models, which were used as input structures for MD. MD simulations were carried out by means of the CHARMM program (30), by using an implicit membrane-water model recently implemented in CHARMM, i.e. the Generalized Born with a Simple Switching module (31). With respect to the physical parameters representing the membrane in the Generalized Born model, the surface tension coefficient (representing the nonpolar solvation energy) was set to 0.03 kcal/(mol·A2). Furthermore, the membrane thickness centered at Z = 0 was set to 30.0 Å with a membrane-smoothing length of 5.0 Å (wm=2.5 Å).
Minimizations were carried out using 1500 steps of steepest descent, followed by Adopted Basis Newton-Raphson minimization until the root mean square gradient was less than 0.001 kcal/mol Å. A disulfide bridge patch was applied to C439(3.10) and C514 (in EL2). MD simulations were also carried out with and without an additional disulfide patching between C336 and C353, both of which are located in the ectodomain.
The all-atom parameter set was used. The lengths of the bonds involving the hydrogen atoms were restrained by the SHAKE algorithm, allowing an integration time step of 0.001 psec. The systems were heated to 300 K with 7.5 K rises every 2500 steps per 100,000 steps by randomly assigning velocities from the Gaussian distribution. After heating, the system was allowed to equilibrate for 100 psec. The secondary structure of the helix bundle was preserved by assigning distance restraints (i.e. minimum and maximum allowed distances of 2.7 Å and 3.0 Å, respectively) between the backbone oxygen atom of residue i and the backbone nitrogen atom of residue i + 4, except for prolines. The scaling factor of such restraints was 10, and the force constant at 300 K was 10 kcal/mol Å. The receptor amino acids, which were found in noncanonical α-helical conformations in the input structure, a condition inherited from the rhodopsin template, were not subjected to any intrabackbone distance restraint. Short (100 psec) equilibrated MD runs were carried out by probing different input structures and different combinations of intrahelical distance restraints. The latter tests included also probing 1) distance restraints on different amino acid stretches in each helix; 2) different parameter sets; 3) different values of membrane thickness (i.e. 30.0 and 35.0 Å); 4) the addition of a disulfide bridge in the ectodomain (i.e. between C336 and C353); and 5) different protonation states of H473 and H482(4.41). Finally, the input structure of the WT receptor form and the computation conditions that, after MD simulation, produced average arrangements characterized by good stereochemical quality as well as structural similarity to rhodopsin were subjected to 1-nsec MD simulations. The selected input structure was used to produce the following constitutively active mutants (CAMs): D564(6.30)G, D564(6.30)A, and D578(6.44)H. As for the latter mutant, H6.44 was simulated in both the prototropic forms and in two different rotameric states. The WT and mutated structures averaged over the first 100 psec (i.e. AVGf100ps) and over the entire 1000-psec trajectories (AVG1000ps) were considered for the docking simulations with heterotrimeric Gs. Thus, for WT LHR and each of the D6.30A and D6.30G mutants, five MD trajectories were employed to produce five AVGf100ps and five AVG1000ps, whereas for the D578(6.44)H mutant eight MD trajectories were employed to produce two sets of eight average minimized structures. Collectively, two sets of 25 average minimized structures of the human LHR, comprising WT and mutant forms, were employed for rigid-body docking simulations with Gsαβ1γ2.
Rigid-Body Docking Simulations
The analysis of the structural complementarity between the cytosolic domains of LHR and Gsαβ1γ2 was done by exhaustively sampling the rototranslational space of one protein (probe) with respect to the other (target) according to a computational protocol already employed for the rhodopsin-transducin system (32,33). The receptor was used as a fixed protein (i.e. target), whereas heterotrimeric Gs was allowed to explore all the possible orientations around the cytosolic domains of the target (i.e. probe). The rigid-body docking algorithm ZDOCK was employed (34). The Gsα model employed in this study was a slightly modified version of the one previously obtained by comparative modeling using the Giα1Gsα chimeric structure as a template (35). The model of the α-subunit is based on the sequence corresponding to the short splice variant of Gα (GenBank accession no. P04896) (35). Differences between the old and new models concerned the backbone conformation of the last 10 amino acids, which in the current model is the same as that of the homologous C-terminal peptide of Gtα determined by nuclear magnetic resonance, i.e. PDB code: 1AQG (36). The novel Gsα model was merged with the βγ-subunits extracted from the structure of the Gα1β1γ2 heterotrimer, i.e. PDB code 1GP2 (37).
To improve sampling efficiency, only the cytosolic domains of LHR, i.e. segments 385–396, 461–484, 545–570, and 625–636, were taken into account in docking simulations. A rotational sampling interval of 6° was employed, and the best 4000 solutions were retained and ranked according to the ZDOCK score. To filter the most reliable solutions from among the 4000 best scored ones, i.e. the Gs orientations fulfilling the membrane topology requirements, an inter-Cα-atom distance cutoff of 20 Å between R464(3.50) of the receptor and L380 of Gsα, was employed.
The solutions fulfilling such distance constraints were subjected to cluster analysis and visual analysis of the cluster centers, i.e. the solution representative of each cluster, following an approach previously described (32). A Cα-root mean square deviation cutoff of 4.0 Å was employed for clustering. The selected receptor-G protein complexes were energy minimized by using the Generalized Born with a Simple Switching-implicit membrane model. The receptor structure in the complex was fully minimized, whereas all the backbone atoms except for those of the first six and last nine amino acids of Gsα and the whole βγ-subunits were kept fixed.
Acknowledgments
We thank Mr. Frank Michel for iodination of hCG, Ms. Judy Gray for expert technical assistance, and Dr. Prema Narayan for many helpful discussions.
Footnotes
This work was supported by grants from the National Institutes of Health (Grant DK33973 to D.P.) and Telethon Italy (Grant S00068TELU to F.F.).
Disclosure Statement: The authors have nothing to disclose.
First Published Online September 13, 2007
Abbreviations: AVG, Average; CAM, constitutively active mutant; CG, chorionic gonadotropin; EL1, EL2, etc., extracellular loops 1 and 2; GPCR, G protein-coupled receptor; H3, H6, etc., transmembrane helices 3 and 6; HEK, human embryonic kidney; IL2, IL3, etc., second and third intracellular loops; LHR, LH receptor; MD, molecular dynamics; PDB, Protein Data Base; SAS, solvent accessible surface area; WT, wild type.
References
- Fredriksson R, Lagerstrom MC, Lundin LG, Schioth HB 2003 The G-protein-coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Mol Pharmacol 6:1256–1272 [DOI] [PubMed] [Google Scholar]
- Bourne HR 1997 How receptors talk to trimeric G proteins. Curr Opin Cell Biol 9:134–142 [DOI] [PubMed] [Google Scholar]
- Hamm HE 2001 How activated receptors couple to G proteins. Proc Natl Acad Sci USA 98:4819–4821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanelli F, De Benedetti PG 2005 Computational modeling approaches to structure-function analysis of G protein-coupled receptors. Chem Rev 105:3297–3351 [DOI] [PubMed] [Google Scholar]
- Freire E 2000 Can allosteric regulation be predicted from structure? Proc Natl Acad Sci USA 97:11680–11682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onaran HO, Scheer A, Cotecchia S, Costa T 2000 A look into receptor efficacy. From the signaling network of the cell to the intramolecular motion of the receptor. In Kenakin T, Angus J, eds. Handbook of experimental pharmacology. Vol 148. Heidelberg: Springer; 217–280 [Google Scholar]
- Kenakin T 2002 Efficacy at G-protein-coupled receptors. Nat Rev Drug Discov 1:103–110 [DOI] [PubMed] [Google Scholar]
- Wong SKF 2003 G protein selectivity is regulated by multiple intracellular regions of GPCRs. Neurosignals 12:1–12 [DOI] [PubMed] [Google Scholar]
- Gether U 2000 Uncovering molecular mechanisms involved in activation of G protein coupled receptors. Endocr Rev 21:90–113 [DOI] [PubMed] [Google Scholar]
- Burstein ES, Spalding TA, Brann MR 1998 The second intracellular loop of the m5 muscarinic receptor is the switch which enables G-protein coupling. J Biol Chem 273:24322–24327 [DOI] [PubMed] [Google Scholar]
- Harder T, Simons K 1997 Caveolae, DIGs, and the dynamics of sphingolipid-cholesterol microdomains. Curr Opin Cell Biol 9:534–542 [DOI] [PubMed] [Google Scholar]
- Alves ID, Salgado GF, Salamon Z, Brown MF, Tollin G, Hruby VJ 2005 Phosphatidylethanolamine enhances rhodopsin photoactivation and transducin binding in a solid supported lipid bilayer as determined using plasmon-waveguide resonance spectroscopy. Biophys J 88:198–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascoli M, Fanelli F, Segaloff, DL 2002 The lutropin/choriogonadotropin receptor, a 2002 perspective. Endocr Rev 23:141–174 [DOI] [PubMed] [Google Scholar]
- Vassart G, Pardo L, Costagliola S 2004 A molecular dissection of the glycoprotein hormone receptors. Trends Biochem Sci 29:119–126 [DOI] [PubMed] [Google Scholar]
- Fernandez LM, Puett D 1997 Evidence for an important functional role of intracellular loop II of the lutropin receptor. Mol Cell Endocrinol 128:161–169 [DOI] [PubMed] [Google Scholar]
- Schulz A, Schoneberg T, Paschke R, Schultz G, Gudermann T 1999 Role of the third intracellular loop for the activation of gonadotropin receptors. Mol Endocrinol 13:181–190 [DOI] [PubMed] [Google Scholar]
- Ballesteros JA, Weinstein H 1995 Integrated methods for the construction of three-dimensional models and computational probing of structure-function relations in G protein-coupled receptors. Methods Neurosci 25:366–428 [Google Scholar]
- Angelova K, Fanelli F, Puett D 2002 A model for constitutive lutropin receptor activation based on molecular simulation and engineered mutations in transmembrane helices 6 and 7. J Biol Chem 277:32202–32213 [DOI] [PubMed] [Google Scholar]
- Fanelli F, Verhoef-Post M, Timmerman M, Zeilemaker A, Martens JW, Themmen AP 2004 Insight into mutation-induced activation of the luteinizing hormone receptor: molecular simulations predict the functional behavior of engineered mutants at M398. Mol Endocrinol 18:1499–1508 [DOI] [PubMed] [Google Scholar]
- Zhang M, Mizrachi D, Fanelli F, Segaloff DL 2005 The formation of a salt bridge between helices 3 and 6 is responsible for the constitutive activity and lack of hormone responsiveness of the naturally occurring L457R mutation of the human lutropin receptor. J Biol Chem 280:26169–26176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capra V, Veltri A, Foglia C, Crimaldi L, Habib A, Parenti M, Rovati GE 2004 Mutational analysis of the highly conserved ERY motif of the thromboxane A2 receptor: alternative role in G protein-coupled receptor signaling. Mol Pharmacol 66:880–889 [DOI] [PubMed] [Google Scholar]
- Flanagan CA 2005 A GPCR that is not “DRY.” Mol Pharmacol 68:1–3 [DOI] [PubMed] [Google Scholar]
- Ballesteros J, Kitanovic S, Guarnieri F, Davies P, Fromme BJ, Konvicka K, Chi L, Millar, RP, Davidson JS, Weinstein H, Sealfon SC 1998 Functional microdomains in G-protein-coupled receptors. The conserved arginine-cage motif in the gonadotropin-releasing hormone receptor. J Biol Chem 273:10445–10453 [DOI] [PubMed] [Google Scholar]
- Fanelli F, De Benedetti PG 2006 Inactive and active states and supramolecular organization of GPCRs: insights from computational modeling. J Comput Aided Mol Des 7–8:449–461 [DOI] [PubMed] [Google Scholar]
- Kosugi S, Van Dop C, Geffner ME, Rabl W, Carel JC, Chaussain JL, Mori T, Merendino Jr JJ, Shenker A 1995 Characterization of heterogeneous mutations causing constitutive activation of the luteinizing hormone receptor in familial male precocious puberty. Hum Mol Genet 4:183–188 [DOI] [PubMed] [Google Scholar]
- Yano K, Hidaka A, Saji M, Polymeropoulos MH, Okuno A, Kohn LD, Cutler Jr GB 1994 A sporadic case of male-limited precocious puberty has the same constitutively activating point mutation in luteinizing hormone/choriogonadotropin receptor gene as familial cases. J Clin Endocrinol Metab 79:1818–1823 [DOI] [PubMed] [Google Scholar]
- Fanelli F 2007 Dimerization of the lutropin receptor: insights from computational modeling. Mol Cell Endocrinol 260–262:59–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sali A, Blundell TL 1993 Comparative protein modeling by satisfaction of spatial restraints. J Mol Biol 234:779–815 [DOI] [PubMed] [Google Scholar]
- Okada T, Sugihara M, Bondar AN, Elstner M, Entel P, Buss V 2004 The retinal conformation and its environment in rhodopsin in light of a new 2.2 A crystal structure. J Mol Biol 34:571–583 [DOI] [PubMed] [Google Scholar]
- MacKerell AD, Bashford D, Bellott M, Dunbrack RL, Evanseck JD, Field MJ, Fischer S, Gao J, Guo H, Ha S, Joseph-McCarthy D, Kushnir L, Kuczera K, Lau FTK, Mattos C, Michnik S, Ngo T, Nguyen DT, Prodhom B, Reiher WE, Roux B, Schlenkrich M, Smith JC, Stote R, Straub J, Watanabe M, Wiorkiewicz-Kuczera J, Yin D, Karplus M 1998 All-atom empirical potential for molecular modeling and dynamics studies of proteins. J Phys Chem B 102:3586–3616 [DOI] [PubMed] [Google Scholar]
- Im W, Feig M, Brooks III CL 2003 An implicit membrane generalized born theory for the study of structure, stability, and interactions of proteins. Biophys J 85:2900–2918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanelli F, Dell’Orco D 2005 Rhodopsin activation follows pre-coupling with transducin: inferences from computational analysis. Biochemistry 44:14695–14700 [DOI] [PubMed] [Google Scholar]
- Dell‘Orco XD, Seeber M, Fanelli F 2007 Monomeric dark rhodopsin holds the molecular determinants for transducin recognition: insights from computational analysis. FEBS Lett 581:944–948 [DOI] [PubMed] [Google Scholar]
- Chen R, Li L, Weng Z 2003 ZDOCK: An initial-stage protein-docking algorithm. Proteins 52:80–87 [DOI] [PubMed] [Google Scholar]
- Fanelli F, Menziani C, Scheer A, Cotecchia S, De Benedetti PG 1999 Theoretical study of the electrostatically driven step of receptor-G protein recognition. Proteins 37:145–156 [PubMed] [Google Scholar]
- Kisselev OG, Kao J, Ponder JW, Fann YC, Gautam N, Marshall GR 1998 Light-activated rhodopsin induces structural binding motif in G protein α subunit. Proc Natl Acad Sci USA 95:4270–4275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall MA, Coleman DE, Lee E, Iniguez-Lluhi JA, Posner BA, Gilman AG, Sprang SR 1995 The structure of the G protein heterotrimer Gi α1 β1 γ2. Cell 83:1047–1058 [DOI] [PubMed] [Google Scholar]