Abstract
Analgesic effects of delta opioid receptor (DOR)-selective agonists are enhanced during persistent inflammation and arthritis. Although the underlying mechanisms are still unknown, membrane density of DOR was shown to be increased 72 h after induction of inflammation, an effect abolished in mu opioid receptor (MOR)-knockout (KO) mice (Morinville et al., 2004b). In this study, we demonstrated a crucial role of MOR in DOR-mediated antihyperalgesia. Intrathecal administration of the DOR selective agonist deltorphin II failed to induce antihyperalgesic effects in MOR-KO mice, whereas it dose-dependently reversed thermal hyperalgesia in wildtype mice. The antihyperalgesic effects of deltorphin II were blocked by naltrindole but not CTOP suggesting that this agonist was mainly acting through DOR. SNC80-induced antihyperalgesic effects in MOR-KO mice were also attenuated as compared to littermate controls. In contrast, kappa opioid receptor knockout did not affect deltorphin II-induced antihyperalgesia. As evaluated using mice lacking endogenous opioid peptides, the regulation of DOR’s effects was also independent of β-endorphin, enkephalins, or dynorphin opioids known to be released during persistent inflammation. We therefore conclude that DOR-mediated antihyperalgesia is dependent on MOR expression but that activation of MOR by endogenous opioids is probably not required.
Introduction
Opioids are the most powerful analgesics for the treatment of moderate to severe pain. Although they bind and activate at least three G protein-coupled receptors referred to as mu (MOR; MOP-R), delta (DOR; DOP-R), and kappa (KOR; KOP-R) opioid receptors, most clinically used opioids act through the MOR subtype to alleviate pain (Bodnar and Klein, 2005). Activation of MOR is, however, associated with multiple undesired side-effects including nausea, respiratory depression, constipation, and sedation (Colpaert, 1996, Kreek, 1996). Most importantly, treatment of chronic pain with repeated or sustained use of opioids induces tolerance and physical dependence (Cowan et al., 1988).
Although delta-selective drugs produce a limited analgesia, their side-effects are weaker and not associated with robust physical dependence (Porreca et al., 1984, May et al., 1989, Szeto et al., 1999, Brandt et al., 2001b, Petrillo et al., 2003, Gallantine and Meert, 2005). Consequently, DOR-selective agonists represent a promising alternative to MOR-selective drugs for the treatment of chronic pain (Mika et al., 2001, Cahill et al., 2003). Over the past few years, it has been suggested that poor analgesic effects associated with DOR could be due to its intracellular localization (Cheng et al., 1995, 1997, Elde et al., 1995, Zhang et al., 1998, Cahill et al., 2001a). Recently, Beaudet and colleagues have shown that plasma membrane expression of DOR in dorsal horn neurons can be increased by repeated activation of MOR by morphine (Cahill et al., 2001b) or other MOR-selective agonists (Morinville et al., 2003). This observation suggests that release of endogenous opioid peptides activating MOR may also increase cell surface expression of DOR. Interestingly, the enhanced density of DOR on neuronal plasma membranes is accompanied by a concomitant increase in the analgesic potency of deltorphin II, a DOR-selective agonist (Cahill et al., 2001b). Thus, it was hypothesized that controlling DOR trafficking could directly alter the analgesic potency of DOR agonists (Zhang et al., 2006, Cahill et al., 2007).
In a model of peripheral inflammation, DOR-selective agonists were shown to have robust antihyperalgesic effects (Desmeules et al., 1993, Stewart and Hammond, 1994, Fraser et al., 2000a, Hurley and Hammond, 2000, Qiu et al., 2000, Brandt et al., 2001a, Cahill et al., 2003, Petrillo et al., 2003). Although the underlying mechanisms controlling this phenomenon are unknown, it has been shown that induction of inflammation increases the density of DOR at the neuronal plasma membrane (Cahill et al., 2003, Morinville et al., 2004b, Gendron et al., 2006). Interestingly, DOR targeting to the cell surface was abolished in MOR-KO mice (Morinville et al., 2004b). These observations suggest that activation of MOR plays a crucial role in the regulation of the trafficking and membrane targeting of DOR in conditions of persistent inflammation. In the present study we tested the hypothesis that inflammation-induced release of endogenous opioids have been responsible for the increased cell surface expression of DOR following inflammation. Because KOR has been shown to interact with DOR (Jordan and Devi, 1999) and in particular because peripheral inflammation induces the release of dynorphin in various brain areas and in the spinal cord (Millan et al., 1986, 1988, Iadarola et al., 1988, Parra et al., 2002), a role for KOR in the regulation of DOR was also considered.
Material and Methods
Animals
Experiments were carried out in adult male C57BL/6 mice (19–26g; Charles River, Wilmington MA, USA). Homozygous MOR (Schuller et al., 1999), KOR (Hough et al., 2000), pENK (Konig et al., 1996), β-END (Rubinstein et al., 1996), pDYN (Sharifi et al., 2001) knockout (KO; −/−) mice were obtained from heterozygous (+/−) breeding pairs arising from >10 backcrosses with C57BL/6 mice. Mice were genotyped using DNA extract from tail samples. Mice were housed in groups of 2–5 and maintained on a 12/12 h light/dark cycle (07:00–19:00). Lab chow and water were available ad libidum. Experiments were either approved by the local animal care committee at University of Washington or at University of Sherbrooke and were in accordance with policies and directives of the Institutional Animal Care and Use Committees (IACUC) and guidelines from the International Association for the study of Pain (IASP).
Induction of inflammation
Unilateral inflammation of the hind limb was induced by a single subcutaneous (s.c.) injection of 50 μl emulsified Complete Freund’s adjuvant (CFA; Calbiochem, San Diego, CA, USA) in the plantar surface of the right hindpaw of mice under brief isoflurane anesthesia (3%; 1L/min). Behavioral testing was carried out 72 h after CFA injection. Control mice received a 50 μl injection of saline (0.9% NaCl) in the plantar surface of the right hindpaw under brief isoflurane anesthesia.
Drugs
Deltorphin II (Lots #074K11261 and #025K12731; Sigma, St Louis, MO, USA), a DOR-selective agonist, was dissolved in sterile saline (0.9% NaCl) at 1 mg/ml and stored at −20°C in aliquots until use. For behavioral testing, deltorphin II was diluted in sterile solution to a final concentration of 1–2.5 μg/5 μl and injected intrathecally (i.t.). SNC80 (Lots #L08806 and #L19847; Alexis Biochemicals, San Diego, CA, USA) was dissolved in sterile, acidic saline solution containing 50% DMSO. Tyr-D-Ala-Gly-N-methyl-Phe-Gly-ol (DAMGO; Tocris Cookson Inc, Ellisville, MS, USA) was diluted in sterile saline at 5 ng/5 μl. Intrathecal injections were done free hand in non-anesthetized mice as described previously (Fairbanks, 2003). Briefly, a 30G ½ needle mounted on a Luer-tip 10 μl-Hamilton syringe was inserted into the L5-L6 intervertebral space (corresponding to the cauda equina) and deltorphin II or SNC80 injected in a volume of 5 μl over 2 s. Saline and acidic saline solution containing 50% DMSO were also used as vehicle controls. Appropriate placement of the needle was confirmed by the observation of a light flick of the tail. Selectivity of deltorphin II for DOR versus MOR was verified by blocking DOR sites with naltrindole (10 mg/kg s.c.; injected 15 min prior to deltorphin II administration; Sigma), and MOR sites with D-Phe-Cys-Tyr-D-Trp-Orn-Thr-Pen-Thr-NH2 (CTOP; 10 ng nmol; Sigma) co-injected i.t. with deltorphin II. These doses of antagonists were chosen based on results presented in a previous study (Guo et al., 2003).
Behavioral testing
Plantar test
Development of hyperalgesia and the antihyperalgesic effects of deltorphin II were assessed using the plantar test (i.e. response to noxious heat stimulus). To test for thermal withdrawal thresholds, mice were acclimatized 15 min to the plantar test environment (IITC Life Science Inc, Woodland Hills, CA, USA), 24 h prior to baseline measurements. The following day, the heat source was positioned under the plantar surface of the hindpaw after a 15 min habituation period, and the latency to paw withdrawal in response to radiant heat was measured twice for each hindpaw in alternation. The intensity of the light beam was adjusted to produce baseline latencies of approximately 10 s for naïve C57BL/6 mice. CFA was then injected in the right hindpaw as described above. Seventy-two hours after injection of CFA, baseline withdrawal latencies (identified as CFA or 0 min) were measured again prior to intrathecal injection of deltorphin II, SNC80, or DAMGO. Thereafter, latencies to paw withdrawal were recorded every 15 min over a period of 60 min. A cutoff time of 20 s was imposed to prevent tissue damage. If an animal reached the cutoff, the light beam was automatically turned off and the animal was assigned the maximum score. The maximum possible antihyperalgesic effect (MPAHE, return to baseline pre-injury withdrawal thresholds) of each agonist in CFA-injected mice was calculated for the inflamed hindpaw (i.e. the paw injected with CFA) according to the following formula: %MPAHE = 100 X [(test latency) – (baseline latency following CFA)]/[(baseline prior to CFA) – (baseline latency following CFA)]. From the latter calculation, a %MPAHE of 0% represents no antihyperalgesic effect of the drug while a %MPAHE of 100% corresponds to a complete relief of the hyperalgesia, i.e. to a response latency to radiant heat identical to baseline prior to CFA injection.
Rotarod
Motor uncoordination/ataxia-like behavior following i.t. injection of deltorphin II was evaluated by measuring motor function on an accelerating rotarod (Columbus Instruments, Columbus, OH, USA). Mice were trained on a 3-day schedule, with three 5-min sessions per day. The first four (4) sessions ramped in a constant acceleration from 4 to 20 rpm over a 5-min period. During the 5 following sessions, the rotarod was set to accelerate from 4 to 30 rpm over a 5-min period. In training sessions 1 through 6, if the mice fell off they were picked up and placed back on the rotarod until 100 s had passed (this was done to accelerate learning). The latency to fall in the last training session was recorded and expressed as the mean performance before deltorphin II injection (in s). On day 3, 15 min after the last training session, mice were injected i.t. with either saline (5 μl) or deltorphin II (1 or 2.5 μg) and tested at 5, 15, and 60 min post-injection. Mouse performance was defined as sec spent on the rotarod.
Calculations and statistical analysis
Calculations were done with Excel 2000, graphs with SigmaPlot 2001 and statistical analysis with Prism Graph Pad 5.0. Data are expressed as the mean ± SEM.
Results
Behavioral observations
Inflammation
Within a few hours after injection of Complete Freund’s adjuvant (CFA; 50 μl) into the plantar surface of the right hindpaw, mice developed edema and swelling that persisted for at least 3 days (72 h). Seventy-two hours after induction of inflammation, mice avoid bearing their body weight on their ipsilateral hind limb, suggesting inflammation-induced spontaneous pain. Such behavior was not observed in control mice injected with saline solution into the plantar surface of the paw.
Motor uncoordination/ataxia-like behavior
In all genotypes of mice tested (i.e. wildtype C57BL/6, MOR-KO, KOR-KO, pENK-KO, β-END-KO, and pDYN-KO and their respective wildtype littermates) in the present study, intrathecal injection of deltorphin II (1–2.5 μg) induced a rapid (3–5 min) but transient (< 30 min) motor uncoordination/ataxia-like behavior restricted to the hind limbs. Following injection of deltorphin II, mice typically adopted an immobile, resting position either in their home cage or in the Plexiglas® enclosure used in the plantar thermal hyperalgesia test. For a short period of time (10–15 min), they barely walked, although they were able to crawl using their front paws. This effect was also accompanied by robust Straub tail symptoms that persisted over a longer period of time (30–40 min). No sedation was observed. Intrathecal injection of saline solution (5 μl) had no effect on motor functions, suggesting that these effects did not result from physical damage to the spinal cord. Comparable ataxia-like responses to deltorphin II were observed in mice injected (in the paw) either with saline or CFA. A similar and transient (~10 min) motor uncoordination/ataxia-like behavior (i.e. hind limbs abduction) was observed following i.t. administration of SNC80, a non peptide DOR-selective agonist. Straub tail symptoms were not observed with SNC80, but mice often had motor convulsions within the first 10 min following SNC80 injection. These effects were absent when the vehicle (acidic saline solution containing 50% DMSO) was delivered i.t. alone.
Antihyperalgesic effect of deltorphin II
As shown in Fig. 1A, injection of deltorphin II (0.1–2.5 μg; i.t.) induced a dose-dependent inhibition of thermal hyperalgesia measured in the ipsilateral hindpaw. For all effective doses of deltorphin II, the effect peaked 15 min after the injection (reaching 5.81±0.73 s, 7.21±0.66 s, and 9.70±0.52 s, respectively for 0.25, 1.0, and 2.5 μg of deltorphin II) and returned to baseline after 30–60 min. At 2.5 μg, deltorphin II-induced antihyperalgesia was more sustained, remaining significantly elevated even 45 min post-injection. Intrathecal saline (5 μl) and 0.1 μg deltorphin II had no effect on the ipsilateral paw withdrawal latency (respectively 4.07±0.76 s and 4.54±0.78 s 15 min after injection). As determined using a graphic representation (Fig. 1B), we calculated an ED50 of 0.72 μg for the antihyperalgesic effect of intrathecal deltorphin II. The percentage maximum possible antihyperalgesic effects (%MPAHE) of deltorphin II were derived from the data presented in Fig. 1A and were calculated at 15 min post-injection. No significant antihyperalgesic effects were observed following saline, 0.1 μg or 0.25 μg deltorphin II (%MPAHESaline = −19.1±13.2%; %MPAHE0.1 = 5.4±13.2%; %MPAHE0.25 = 28.4±13.5%). In contrast, 1.0 and 2.5 μg of intrathecal deltorphin II respectively relieved thermal hyperalgesia by 52.7±11.2% and 80.6±10.5% (F4,28 = 11.51; **, p < 0.01 compared to saline-injected group; one-way ANOVA followed by Dunnett’s multiple comparison test). No analgesic effect of deltorphin II (0–2.5 μg) was observed on the paw withdrawal latency in response to radiant heat applied to the contralateral hindpaw (Fig. 1C).
Figure 1. Antihyperalgesic effect of intrathecal deltorphin II in C57BL/6 mice.
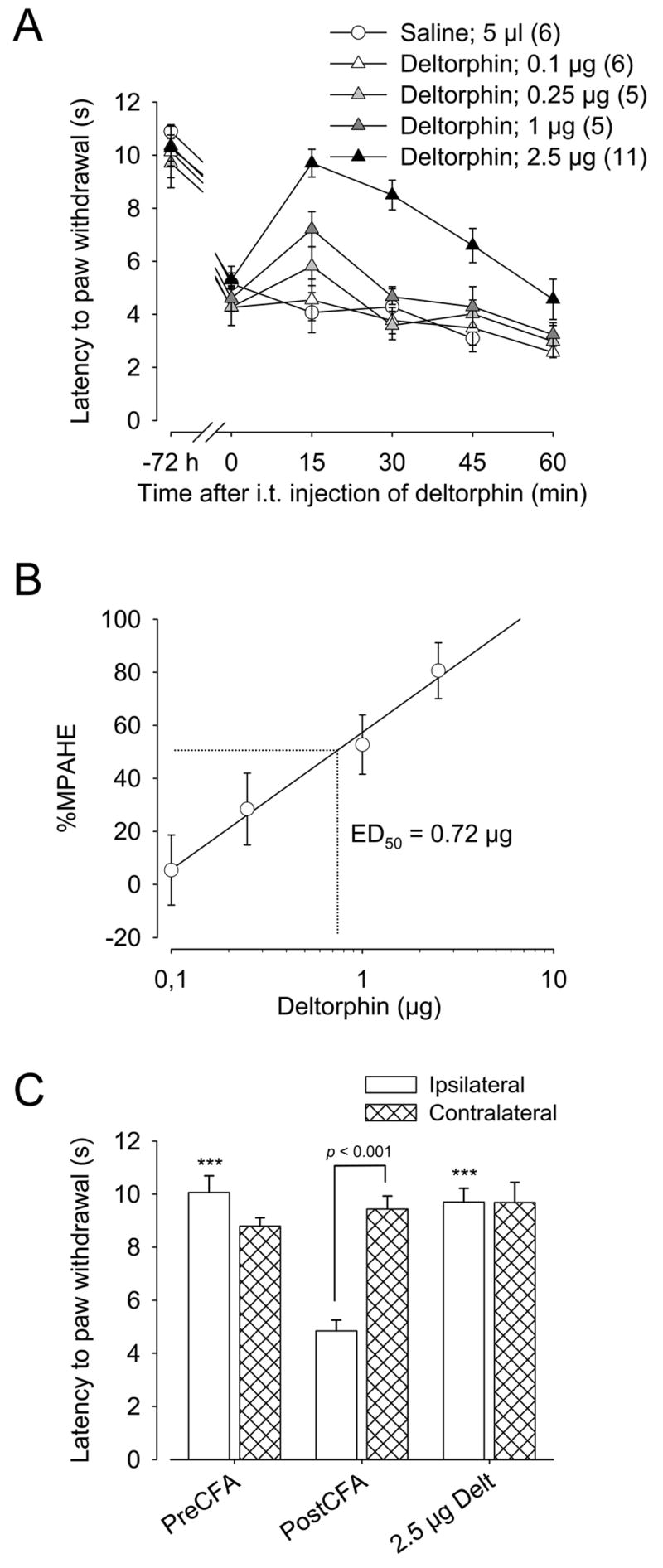
A, C57BL/6 mice were injected with CFA in the plantar surface of the right hindpaw. Seventy-two hours after CFA injection, the latency to paw withdrawal (in s) was tested every 15 min (from 15 to 60 min) after intrathecal injection of saline (5 μl) or deltorphin II (0.1, 0.25, 1, 2.5 μg). Intrathecally-administered deltorphin II induced a dose-dependent relief of thermal hyperalgesia (ipsilateral hindpaw). The antihyperalgesic effect of deltorphin II peaked 15 min after the injection. −72 h indicates the baseline for the latency to paw withdrawal before CFA injection and time 0 the latency to paw withdrawal just before injection of saline or deltorphin II. Number in parenthesis indicates the number of animals in each group. B, Graphic determination of the effective dose of deltorphin II (ED50) inducing a 50% relief of thermal hyperalgesia. C, No effect of deltorphin II was observed on the contralateral hindpaw. ***, p < 0.001 when compared to latency to paw withdrawal of ipsilateral hindpaw 72 h after CFA injection (PostCFA, white bar); one-way ANOVA followed by Bonferroni’s multiple comparison test.
Role of MOR and KOR in deltorphin II-induced antihyperalgesia
The other opioid receptors, MOR and KOR, were previously shown to regulate the trafficking of DOR (Cahill et al., 2001b, Morinville et al., 2003, 2004b, Khotib et al., 2004, Hack et al., 2005, Gendron et al., 2006, Ma et al., 2006). To determine whether MOR and KOR were involved in the regulation of DOR-mediated antihyperalgesic effect in this inflammatory pain model, MOR-KO, KOR-KO, and their respective wildtype littermate mice were injected with CFA into the plantar surface of the right hindpaw. The antihyperalgesic effect of deltorphin II (2.5 μg; intrathecal) was then measured using the plantar test. While no difference in response to deltorphin II was observed between KOR-KO mice and their littermates (Fig. 2A; two-way ANOVA, p = 0.40 for genotypes), deltorphin II-induced antihyperalgesia in MOR-KO mice was significantly reduced (Fig. 2B, black circles; ***, p < 0.001 when compared to MOR+/+ littermates, two-way ANOVA followed by Bonferroni’s multiple comparison test). MOR+/+ littermates (Fig. 2B) and C57BL/6 (Fig. 1A) were similarly sensitive to deltorphin II. As observed in wildtype C57BL/6 mice (cf. Fig. 1C), analgesic effect of deltorphin II (2.5 μg) in MOR+/+, KOR+/+ and KOR−/− mice was restricted to the inflamed-hindpaw (Fig. 2C).
Figure 2. Role for MOR and KOR in the regulation of DOR-mediated antihyperalgesia.
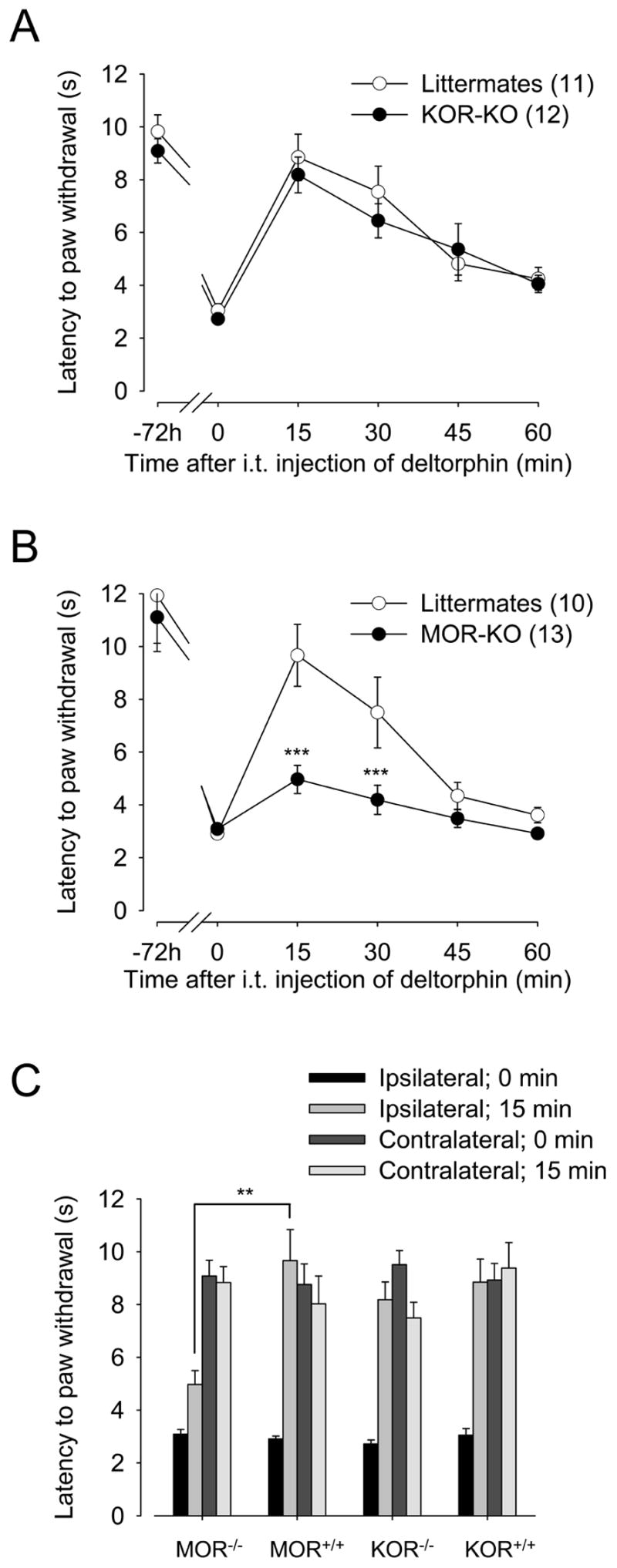
The antihyperalgesic effect of deltorphin II was tested in (A) KOR-KO and (B) MOR-KO mice and in their respective littermate controls 72 h after CFA injection. A and B, Effect of intrathecal injection of deltorphin II (2.5 μg) on the latency to paw withdrawal (for the ipsilateral hindpaw) in response to a noxious heat stimulus. ***, p < 0.001 when compared to MOR+/+ littermates, two-way ANOVA followed by Bonferroni’s multiple comparison test. Number in parenthesis indicates the number of animals in each group. C, Comparison of latencies to paw withdrawal in response to noxious heat stimulus for ipsilateral and contralateral hindpaws regarding genotypes. **, p = 0.0065, two-tailed unpaired t-test.
Pharmacological characterization of deltorphin II-induced antihyperalgesia
Previous reports suggested that deltorphin II and other DOR-selective peptides produce some of their effects via direct interactions with MOR (Guo et al., 2003, Scherrer et al., 2004). To determine whether the antihyperalgesic effects of deltorphin II were DOR-mediated, antagonism studies were performed. As shown in Fig. 3A (left panel), in the presence of naltrindole (10 mg/kg, s.c.), the %MPAHE of deltorphin II was significantly decreased (24.6±10.7% as compared to 80.6±10.5% for deltorphin II alone; F4,33 = 6.759, *, p < 0.05, one-way ANOVA followed by Bonferroni’s multiple comparison test) whereas co-injection of CTOP (10 ng, i.t.) with deltorphin II had no significant effect on the MPAHE (%MPAHE = 63.1±13.6% as compared to 80.6±10.5% for deltorphin alone; F4,33 = 6.759, p > 0.05, one-way ANOVA followed by Bonferroni’s multiple comparison test). When co-injected with DAMGO (5 ng, i.t.) this dose of CTOP (10 ng, i.t.) was sufficient to fully reverse DAMGO-induced antihyperalgesic effect (Fig. 3A, right panel; %MPAHE = −4.2±9.7% as compared to 68.8±23.2% for DAMGO alone; F4,33 = 6.759, *, p < 0.05, one-way ANOVA followed by Bonferroni’s multiple comparison test). In support of these pharmacological observations, we assessed the antihyperalgesic effects of SNC80, a non-peptide DOR-agonist, in MOR-KO mice. Pilot studies revealed that a dose of 45 μg (100 nmol) of i.t. SNC80 was necessary to induce a significant analgesic effect in our murine CFA model of inflammation (not shown). As shown in Fig. 3B, SNC80 induced a significant antihyperalgesic effect in MOR+/+ littermates (%MPAHE = 44.3±12.9% as compared to 3.4±5.8% for vehicle controls; F2,24 = 6.038, *, p < 0.05, one-way ANOVA followed by Bonferroni’s multiple comparison test). Consistent with results using deltorphin II (cf. Fig. 2B), SNC80-induced antihyperalgesia was not evident in MOR-KO mice (MOR−/−; %MPAHE = 5.0 ± 5.7%; F2,24 = 6.038, *, p < 0.05 as compared to MOR+/+ littermates, one-way ANOVA followed by Bonferroni’s multiple comparison test).
Figure 3. Pharmacological characterization of antihyperalgesia induced by deltorphin II.
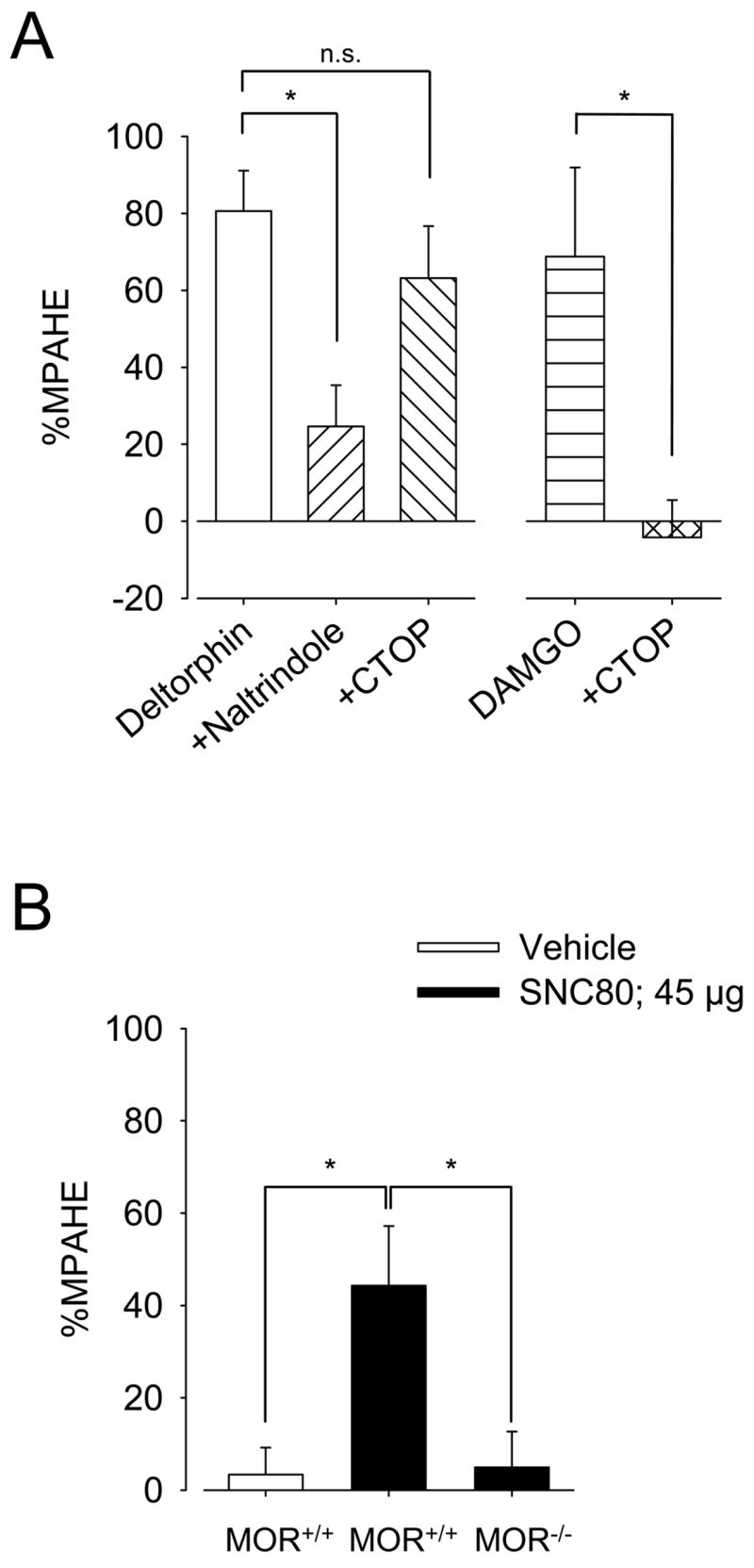
A, The antihyperalgesic effect of deltorphin II in CFA-injected C57BL/6 mice was DOR-mediated since it was completely blocked by naltrindole (n = 9) but not significantly affected by CTOP (n = 6). Naltrindole was administered subcutaneously at a dose of 10 mg/kg, 15 min prior to deltorphin II (2.5 μg) injection. CTOP (10 ng) was co-injected intrathecally with deltorphin II (2.5 μg). This dose of CTOP (10 ng; n = 6) was sufficient to fully reverse DAMGO (5 ng)-induced antihyperalgesia (n = 6), demonstrating the appropriateness of the CTOP dose. *, p < 0.05, one-way ANOVA followed by Bonferroni’s multiple comparison test. B, As for deltorphin II, antihyperalgesic effect of intrathecally administered SNC80 (45 μg), a non-peptide DOR-selective agonist, was significantly weaker in MOR-KO mice (n = 10) than in littermate controls (n = 9). Vehicle control has no effect by itself (n = 8). *, p < 0.05, one-way ANOVA followed by Bonferroni’s multiple comparison test. A and B, the effect of each treatment is expressed as %MPAHE, calculated 15 minutes post-injection.
Contribution of endogenous opioids in deltorphin II-induced antihyperalgesia
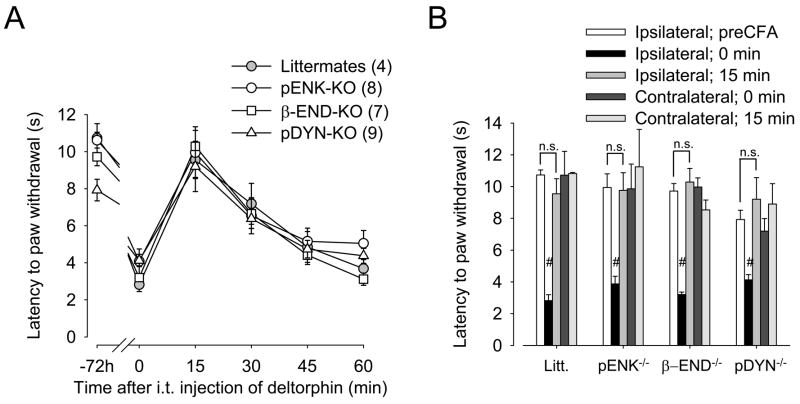
Inflammation is known to induce the release of endogenous opioids (Millan et al., 1986, 1988, Iadarola et al., 1988, Noguchi et al., 1992, Cabot et al., 1997, Hurley and Hammond, 2000, 2001, Parra et al., 2002). To determine whether activation of MOR by an endogenous opioid during inflammation was involved in the regulation of DOR and deltorphin II-mediated analgesia, we tested antihyperalgesic effects of deltorphin II (2.5 μg, intrathecal) in pENK-, β-END-, pDYN-KO mice and littermate controls. As shown in Fig. 4A and B, all strains of mice developed comparable hyperalgesia in their ipsilateral hindpaw 72 h after CFA injection (ipsilateral 0 min as compared with their respective ipsilateral preCFA; #, p < 0.0001, two-tailed unpaired t-test), while no difference was observed in the contralateral hindpaw. Fifteen minutes after intrathecal administration of deltorphin II, the latency to paw withdrawal increased to a level not significantly different from the baseline latency prior to CFA injection (−72 h). No analgesic effect of deltorphin II was observed in the contralateral hindpaw (Fig. 4B, contralateral 15 min compared to their respective contralateral 0 min).
Figure 4. Role of endogenous opioids in the regulation of DOR-mediated antihyperalgesia.
A, Thermal latencies (in s) to noxious heat were recorded every 15 min following intrathecal administration of deltorphin II (2.5 μg) to control littermates (Litt.;
 ), pro-enkephalin-KO (pENK-KO; ○), β-endorphin-KO (β-END-KO; □), and pro-dynorphin-KO (pDYN-KO; △) mice. B, Comparative latencies to paw withdrawal (in s) in control littermates, pENK-, β-END-, and pDYN-KO mice before (0 min) and 15 min after intrathecal injection of deltorphin II (2.5 μg). Number in parenthesis indicates the number of animals in each group. #, p < 0.0001 when ipsilateral 0 min are compared with their respective ipsilateral preCFA; two-tailed unpaired t-test.
), pro-enkephalin-KO (pENK-KO; ○), β-endorphin-KO (β-END-KO; □), and pro-dynorphin-KO (pDYN-KO; △) mice. B, Comparative latencies to paw withdrawal (in s) in control littermates, pENK-, β-END-, and pDYN-KO mice before (0 min) and 15 min after intrathecal injection of deltorphin II (2.5 μg). Number in parenthesis indicates the number of animals in each group. #, p < 0.0001 when ipsilateral 0 min are compared with their respective ipsilateral preCFA; two-tailed unpaired t-test.
Effects of deltorphin II on motor functions
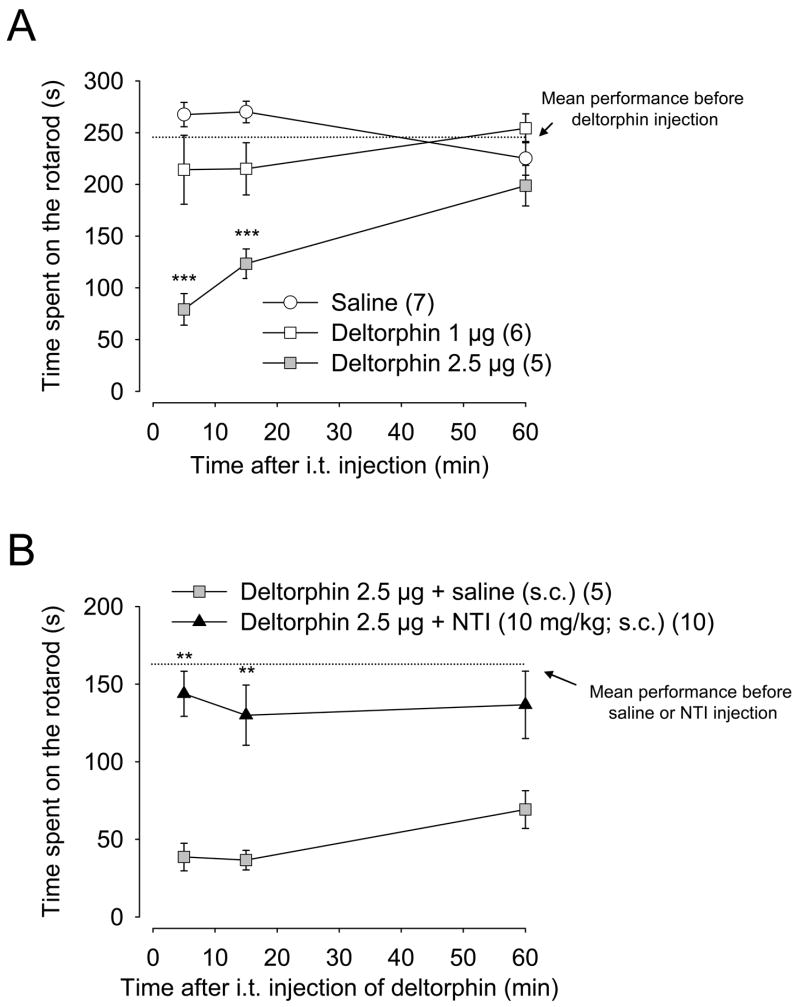
The motor uncoordination/ataxia-like effects observed following deltorphin II injection were measured using the accelerating rotarod. As shown in Fig. 5A, intrathecal deltorphin II administration dose-dependently (1–2.5 μg) decreased wild-type mouse performance on the rotarod (as determined by a significant decrease in the latency to fall as compared with saline-injected mice; ***, p < 0.001; two-way ANOVA followed by Bonferroni’s multiple comparison test). This effect peaked 5 min after injection of deltorphin II and persisted more than 60 min. As shown in Fig. 5B, administration of naltrindole (10 mg/kg; s.c.) 15 min prior to deltorphin II (2.5 μg; intrathecal) prevented the decrease in the latency to fall (**, p < 0.01 as compared to mice pre-treated with saline s.c., two-way ANOVA followed by Bonferroni’s multiple comparison test), demonstrating that this effect was DOR-mediated. While naltrindole blocked the motor uncoordination/ataxia-like effects induced by deltorphin II (Fig. 5B) Straub tail symptoms were still present, but at a lesser extent (not shown).
Figure 5. Effect of deltorphin II on motor functions in C57BL/6 mice.
Rotarod performances were evaluated in C57BL/6 mice following intrathecal injection of deltorphin II. A, Dose-dependent induction of motor uncoordination/ataxia-like behavior induced by deltorphin II (0–2.5 μg). ***, p < 0.001 when compared to saline-injected group (○), two-way ANOVA followed by Bonferroni’s multiple comparison test. B, Blockade of deltorphin II’s effect on motor functions with the DOR-selective antagonist naltrindole (NTI; 10 mg/kg) injected subcutaneously 15 min before administration of deltorphin II (2.5 μg; ▲). Control group (
 ) received an equal volume of saline subcutaneously 15 min prior to deltorphin II. Mean performance before deltorphin (or before saline/NTI) injection represents the mean performance of all mice before treatment. **, p < 0.01 when compared to saline-pretreated group, two-way ANOVA followed by Bonferroni’s multiple comparison test. Number in parenthesis indicates the number of animals in each group.
) received an equal volume of saline subcutaneously 15 min prior to deltorphin II. Mean performance before deltorphin (or before saline/NTI) injection represents the mean performance of all mice before treatment. **, p < 0.01 when compared to saline-pretreated group, two-way ANOVA followed by Bonferroni’s multiple comparison test. Number in parenthesis indicates the number of animals in each group.
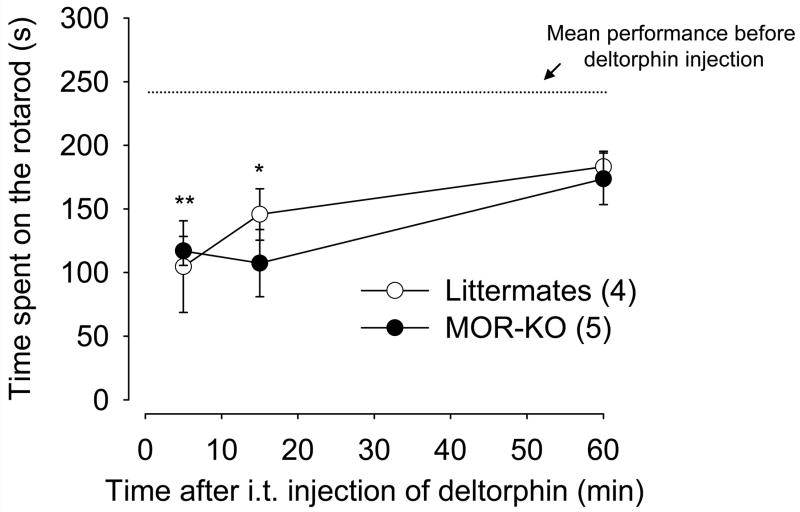
To ensure that deltorphin II-induced antihyperalgesia was not due to motor uncoordination/ataxia-like behavior (i.e. incapability or difficulty to move their paws in response to radiant heat), we measured the ability of deltorphin II (2.5 μg) to affect performance of MOR-KO mice and their wildtype littermate controls on the rotarod. Both in MOR-KO and littermates, deltorphin II administration significantly decreased the time spent on the rotarod as compared with the mean performance before deltorphin II injection (Fig. 6; F6,28 = 5.734, one-way ANOVA followed by Dunnett’s multiple comparison test; *, p < 0.05 and **, p < 0.01), which contrasts with the antihyperalgesic effects of deltorphin II observed in these mice (cf. Fig. 2B). Using this test, no difference was observed between MOR-KO mice and their littermate controls (two-way ANOVA, p = 0.53 for genotypes).
Figure 6. Effect of deltorphin II on motor functions in MOR-KO mice.
Rotarod performances were evaluated following intrathecal injection of deltorphin II (2.5μg) to MOR-KO (●) and their littermate controls (MOR+/+, ○). Mean performance before deltorphin injection corresponds to the mean performance of mice from both groups (MOR-KO and littermates) before treatment. No significant difference was observed between performances before injection of deltorphin II. Intrathecal injection of deltorphin II induced similar motor uncoordination/ataxia-like behavior (*, p < 0.05 and **, p < 0.01 when compared with the mean performance before deltorphin II injection; one-way ANOVA followed by Dunnett’s multiple comparison test) in both groups of mice (p > 0.05 when comparing MOR-KO with littermate controls, two-way ANOVA followed by Bonferroni’s multiple comparison test). Number in parenthesis indicates the number of animals in each group.
Discussion
In the present study, we showed that intrathecal administration of deltorphin II induces a dose-dependent relief of thermal hyperalgesia in a murine model of persistent inflammation. As shown by pharmacological antagonism studies, this effect of deltorphin II was DOR-mediated. Interestingly, DOR-mediated antihyperalgesia was significantly decreased in MOR-KO mice. This DOR function was unaltered in the absence of KOR or in mice lacking endogenous opioids (enkephalin, endorphin, dynorphin). As opposed to antihyperalgesia induced by intrathecal administration of deltorphin II, appearance of motor uncoordination/ataxia-like behavior was not blocked by MOR-gene deletion, but were blocked by naltrindole.
Previous studies have shown that DOR-selective agonists are only slightly analgesic when administrated to naïve animals. However, analgesic potency of DOR agonists can be increased following repeated activation of either MOR (Melchiorri et al., 1992, Cahill et al., 2001b, Morinville et al., 2003, 2004a, 2004b, Hack et al., 2005, Ma et al., 2006, Gendron et al., 2007) or KOR (Khotib et al., 2004), or following induction of peripheral inflammation (Hylden et al., 1991, Desmeules et al., 1993, Stewart and Hammond, 1994, Fraser et al., 2000a, Hurley and Hammond, 2000, Cahill et al., 2003, Petrillo et al., 2003, Morinville et al., 2004b, Gallantine and Meert, 2005, Gendron et al., 2007). The selectivity of given doses of delta agonists in reducing nociception in inflamed but not in normal tissue may potentially have important clinical advantage. In the current study, we first confirmed that peripheral inflammation increased the analgesic potency of deltorphin II, a DOR-selective agonist, in inflamed as compared to normal tissues. However, mechanisms controlling this process are still undefined.
Roles of MOR and KOR in the regulation of DOR functions in persistent inflammation were thus tested in MOR- and KOR-KO mice. While deltorphin II was as potent in KOR-KO mice as in KOR+/+ littermates and wildtype C57BL/6 mice, we demonstrated the necessity of MOR expression for deltorphin II to efficiently relieve thermal hyperalgesia in mice injected with CFA. Indeed, intrathecal administration of deltorphin II in MOR-KO mice had only slight antihyperalgesic effects in comparison to MOR+/+ littermates. This result opposes previous observations by Qiu et al. (2000) who showed that in MOR-KO mice (1:1 hybrids 129/SvEv and C57BL/6J mouse strains), intrathecal deltorphin II produced enhanced analgesia 24 h after CFA injection. The basis for this discrepancy is not understood however the studies were performed in different strains of mice and at different times following CFA injection. Distinct mechanisms of DOR regulation may occur during progression of the inflammation as suggested by our previous report, in which we showed that expression of DOR at the plasma membrane was differentially regulated in dorsal root ganglia (DRG) neurons in rats treated for 48 h than 72 h with CFA (Gendron et al., 2006). It could therefore be hypothesized that the expression of MOR was only be necessary for long term regulation of DOR in this model of inflammatory pain.
As previously reported in DOR-KO mice (Scherrer et al., 2004), one could argue that the antihyperalgesic effect of deltorphin II is mediated via a direct action on MOR, thus accounting for the reduction/annihilation of DOR-selective agonist effects in MOR-KO mice (this study and (Sora et al., 1997a, 1997b, Loh et al., 1998, Matthes et al., 1998, Hosohata et al., 2000, Guo et al., 2003). However, this is unlikely to be the case here because antihyperalgesic effect of deltorphin II was fully reversed by naltrindole (a DOR-selective antagonist) and not significantly affected by co-administration of CTOP (a MOR-selective antagonist). Furthermore, we found that antihyperalgesia induced by the non-peptide DOR agonist SNC80 was also reduced in MOR-KO mice. Therefore, a more plausible hypothesis is that an interaction between MOR and DOR is necessary for complete expression of DOR functions (via regulation of DOR trafficking). Using various techniques, MOR and DOR were previously shown to heterodimerize in vitro (George et al., 2000, Gomes et al., 2000, 2004, Rozenfeld and Devi, 2007), a cell surface process requiring participation of G-proteins (Law et al., 2005). Although this hypothesis was not directly tested in the present study, it is possible that an interaction with MOR is required to maintain DOR at the plasma membrane, at least in a sub-population of cells involved in pain processing. For instance, MOR and DOR were shown to be co-expressed in dorsal root ganglia (DRG) (Fields et al., 1980, Egan and North, 1981, Zieglgansberger et al., 1982) and some dorsal horn spinal cord neurons (Arvidsson et al., 1995, Cheng et al., 1997), suggesting that physical interactions between MOR and DOR are possible in vivo. Despite the fact that DOR was also shown to heterodimerize with KOR (Jordan and Devi, 1999), deltorphin II-induced antihyperalgesia was preserved in KOR-KO mice suggesting that neurons involved in pain transmission do not co-express these two receptors within same cells or, alternatively, that KOR plays only a secondary role and its expression is not sufficient to compensate for the absence of MOR in these neurons.
Persistent peripheral inflammation increases expression and release of endogenous opioids (Millan et al., 1986, 1988, Iadarola et al., 1988, Noguchi et al., 1992, Cabot et al., 1997, Hurley and Hammond, 2000, 2001, Parra et al., 2002). It could therefore be thought that activation of MOR by endogenous opioids may be responsible for the enhanced density of membrane-associated DOR in lumbar spinal cord (Cahill et al., 2003) and DRG neurons (Gendron et al., 2006) in CFA animals and, by way of consequence, for the potentiation of deltorphin II’s analgesic effect reported here.
Using specific knockout models for endogenous opioid precursors (pro-enkephalin; pENK, β-endorphin; β-END, pro-dynorphin; pDYN), we showed that deletion of these naturally occurring agonists did not affect the increase in DOR-mediated analgesia in the CFA model. Thus, the hypothesis that inflammation-induced release of an endogenous opioid caused the MOR-mediated trafficking of DOR was not supported by the present study. However, DOR trafficking may require the concerted action of multiple opioid systems. Because all three opioid peptides (and some metabolites) are known to bind and to activate MOR, it is possible that compensation might be a confounding issue in the knockout experiments, i.e. that an endogenous opioid could compensate the absence of one another. Alternatively, another high affinity opioid for MOR could be involved in the MOR-mediated regulation of DOR-induced antihyperalgesia. Although their respective precursors are still unidentified and their roles not clarified, endomorphin-1 and -2 could be among these candidates (Zadina et al., 1997), especially since endomorphin-immunoreactivity is expressed in substance P-containing primary afferent nociceptors (Sanderson Nydahl et al., 2004). More studies will be needed to show whether or not endogenous opioids and MOR activation are required for the regulation of DOR during persistent inflammation. For instance, the fact that CTOP does not significantly influence the effect of deltorphin II suggests that acute activation of MOR or its occupancy by an antagonist is not involved in the regulation of DOR, which is somehow in contrast with reports from others (He and Lee, 1998, Gomes et al., 2000, Riba et al., 2002).
Recently, Patwardhan et al. have shown that peripheral activation of primary afferent nociceptors with bradykinin (Patwardhan et al., 2005) or trypsin (Patwardhan et al., 2006) enhanced both targeting of DOR to the cell surface and receptor competence. We observed a similar effect on DOR trafficking in small DRG neurons following injection of capsaicin in a rat hindpaw (Gendron et al., 2006). Although whether or not these effects depend on the expression of MOR has not been established, the possibility that under certain circumstances MOR-independent mechanisms might affect the regulation of DOR’s membrane expression should not be excluded. Indeed, protachykinin has recently been shown to regulate plasma-membrane insertion of DORs via stimulus-induced exocytosis of DOR-containing large dense-core vesicles (LDCVs) (Guan et al., 2005).
In rodents, deltorphin analogs and other DOR-selective agonists can interfere with motor functions (Longoni et al., 1991, Miaskowski et al., 1991, Negri et al., 1991, 1996, Spina et al., 1998, Fraser et al., 2000b, Broom et al., 2002, Jutkiewicz et al., 2004). In the present study, we observed that intrathecal administration of deltorphin II induced a rapid, but transient inhibition of motor capacities, an effect mimicked with SNC80 and reversed by the delta-selective antagonist naltrindole. Although we do not know which particular neurons are responsible for these behavioral effects, we hypothesize that motor function impairment is mediated by a direct action of deltorphin II on ventral horn motor neurons of the lumbar spinal cord, as opposed to a dopamine-dependent central effect. Indeed, immunohistochemical and binding studies have shown a wide distribution of DOR in mice lumbar spinal cord, including the ventral horn (Cahill et al., 2001a, Mennicken et al., 2003). Interestingly, as opposed to superficial dorsal horn neurons, internalization of a fluorescent analog of deltorphin in lamina IX motor neurons was not affected by prolonged morphine treatment (Morinville et al., 2004a) nor by CFA-induced inflammation (Gendron et al., 2007), suggesting that this DOR-mediated effect might be regulated by different mechanisms.
Because peak antihyperalgesic effect of deltorphin II was observed after 15 min, it could be hypothesized that the motor uncoordination/ataxia-like behavior may, at least in part, be responsible for the increase in the latency to paw withdrawal. To address this possibility, we measured the effects of intrathecal deltorphin II on motor functions in MOR-KO and MOR+/+ littermates. Interestingly, if DOR-mediated antihyperalgesia requires the expression of MOR, deltorphin II-induced motor uncoordination/ataxia-like behavior does not. The fact that deltorphin II induces motor uncoordination/ataxia-like behavior in MOR-KO mice without increasing the latency to paw withdrawal indicates that these effects are unrelated and, most importantly, implies that deltorphin II’s effect on motor functions does not impair the ability of mice 1) to feel thermal pain and 2) to adequately respond to heat stimuli. These observations are in accordance with a previous report from Miaskowski et al. concluding that DPDPE-induced decrease in rotarod performance does not coincide with its dose-dependent antinociceptive effect (Miaskowski et al., 1991).
Results from the present study suggest that DOR-mediated antihyperalgesia requires the presence of MOR while its motoric effects do not. It is therefore possible that the requirement of MOR for some DOR effects may be region- or neuron-specific. In support to this hypothesis, MOR/DOR interactions were shown to occur in some brain regions but not in peripheral tissues (Franklin and Traynor, 1991, Elliott and Traynor, 1995). Whether or not these interactions may differ within various laminae of the same spinal cord segment and that they play a crucial role in DOR-mediated analgesia in the CFA model of inflammation will need further investigations.
Regardless if DOR needs to directly interact (i.e. heterodimerization) with MOR to relieve hyperalgesia, we found the latter to play an essential role in DOR-mediated antihyperalgesia. Our results also reveal a dichotomy between the antihyperalgesic and the motor uncoordination/ataxia-like behavior effects induced by intrathecally-administered delta-selective agonists. While the former clearly involves MOR, the latter is not dependent on MOR expression. Admitting that the analgesic effects of DOR-selective agonists are directly related to the level of DOR at the membrane (Zhang et al., 2006, Cahill et al., 2007), our results are indeed consistent with previous observations demonstrating that CFA-induced inflammation failed to increase the membrane density of DOR in MOR-KO mice (Morinville et al., 2004b). Overall, this study further supports our previous hypothesis: a better understanding of mechanisms involved in the MOR-mediated regulation of DOR would be helpful to increase the analgesic potency of DOR-selective agonists for the treatment of chronic pain (Cahill et al., 2001b).
Acknowledgments
This work was supported by USPHS grant DA11672 from the National Institute of Health (NIH) to CC and by Fondation de l’Université de Sherbrooke to LG. LG was funded by fellowship MFE-63497 from the Canadian Institutes of Health Research (CIHR). The authors thank Malcolm Low (Oregon Health Science University, OR) for the β-endorphin knockout mice and Ute Hochgeschwender (Oklahoma Medical Research Foundation, OK) for the dynorphin knockout mice. Authors are also grateful to Dan Messinger for his help with breeding and genotyping of knockout mice.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arvidsson U, Riedl M, Chakrabarti S, Lee JH, Nakano AH, Dado RJ, Loh HH, Law PY, Wessendorf MW, Elde R. Distribution and targeting of a mu-opioid receptor (MOR1) in brain and spinal cord. J Neurosci. 1995;15:3328–3341. doi: 10.1523/JNEUROSCI.15-05-03328.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodnar RJ, Klein GE. Endogenous opiates and behavior: 2004. Peptides. 2005;26:2629–2711. doi: 10.1016/j.peptides.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Brandt MR, Furness MS, Mello NK, Rice KC, Negus SS. Antinociceptive effects of delta-opioid agonists in Rhesus monkeys: effects on chemically induced thermal hypersensitivity. J Pharmacol Exp Ther. 2001a;296:939–946. [PubMed] [Google Scholar]
- Brandt MR, Furness MS, Rice KC, Fischer BD, Negus SS. Studies of tolerance and dependence with the delta-opioid agonist SNC80 in rhesus monkeys responding under a schedule of food presentation. J Pharmacol Exp Ther. 2001b;299:629–637. [PubMed] [Google Scholar]
- Broom DC, Jutkiewicz EM, Folk JE, Traynor JR, Rice KC, Woods JH. Nonpeptidic delta-opioid receptor agonists reduce immobility in the forced swim assay in rats. Neuropsychopharmacology. 2002;26:744–755. doi: 10.1016/S0893-133X(01)00413-4. [DOI] [PubMed] [Google Scholar]
- Cabot PJ, Carter L, Gaiddon C, Zhang Q, Schafer M, Loeffler JP, Stein C. Immune cell-derived beta-endorphin. Production, release, and control of inflammatory pain in rats. J Clin Invest. 1997;100:142–148. doi: 10.1172/JCI119506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill CM, Holdridge SV, Morinville A. Trafficking of delta-opioid receptors and other G-protein-coupled receptors: implications for pain and analgesia. Trends Pharmacol Sci. 2007;28:23–31. doi: 10.1016/j.tips.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Cahill CM, McClellan KA, Morinville A, Hoffert C, Hubatsch D, O’Donnell D, Beaudet A. Immunohistochemical distribution of delta opioid receptors in the rat central nervous system: evidence for somatodendritic labeling and antigen-specific cellular compartmentalization. J Comp Neurol. 2001a;440:65–84. doi: 10.1002/cne.1370. [DOI] [PubMed] [Google Scholar]
- Cahill CM, Morinville A, Hoffert C, O’Donnell D, Beaudet A. Up-regulation and trafficking of delta opioid receptor in a model of chronic inflammation: implications for pain control. Pain. 2003;101:199–208. doi: 10.1016/s0304-3959(02)00333-0. [DOI] [PubMed] [Google Scholar]
- Cahill CM, Morinville A, Lee MC, Vincent JP, Collier B, Beaudet A. Prolonged morphine treatment targets delta opioid receptors to neuronal plasma membranes and enhances delta-mediated antinociception. J Neurosci. 2001b;21:7598–7607. doi: 10.1523/JNEUROSCI.21-19-07598.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng PY, Liu-Chen LY, Pickel VM. Dual ultrastructural immunocytochemical labeling of mu and delta opioid receptors in the superficial layers of the rat cervical spinal cord. Brain Res. 1997;778:367–380. doi: 10.1016/s0006-8993(97)00891-3. [DOI] [PubMed] [Google Scholar]
- Cheng PY, Svingos AL, Wang H, Clarke CL, Jenab S, Beczkowska IW, Inturrisi CE, Pickel VM. Ultrastructural immunolabeling shows prominent presynaptic vesicular localization of delta-opioid receptor within both enkephalin- and nonenkephalin-containing axon terminals in the superficial layers of the rat cervical spinal cord. J Neurosci. 1995;15:5976–5988. doi: 10.1523/JNEUROSCI.15-09-05976.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colpaert FC. System theory of pain and of opiate analgesia: no tolerance to opiates. Pharmacol Rev. 1996;48:355–402. [PubMed] [Google Scholar]
- Cowan A, Zhu XZ, Mosberg HI, Omnaas JR, Porreca F. Direct dependence studies in rats with agents selective for different types of opioid receptor. J Pharmacol Exp Ther. 1988;246:950–955. [PubMed] [Google Scholar]
- Desmeules JA, Kayser V, Gacel G, Guilbaud G, Roques BP. The highly selective delta agonist BUBU induces an analgesic effect in normal and arthritic rat and this action is not affected by repeated administration of low doses of morphine. Brain Res. 1993;611:243–248. doi: 10.1016/0006-8993(93)90509-l. [DOI] [PubMed] [Google Scholar]
- Egan TM, North RA. Both mu and delta opiate receptors exist on the same neuron. Science. 1981;214:923–924. doi: 10.1126/science.6272393. [DOI] [PubMed] [Google Scholar]
- Elde R, Arvidsson U, Riedl M, Vulchanova L, Lee JH, Dado R, Nakano A, Chakrabarti S, Zhang X, Loh HH, et al. Distribution of neuropeptide receptors. New views of peptidergic neurotransmission made possible by antibodies to opioid receptors. Ann N Y Acad Sci. 1995;757:390–404. doi: 10.1111/j.1749-6632.1995.tb17497.x. [DOI] [PubMed] [Google Scholar]
- Elliott J, Traynor JR. Evidence for lack of modulation of mu-opioid agonist action by delta-opioid agonists in the mouse vas deferens and guinea-pig ileum. Br J Pharmacol. 1995;114:1064–1068. doi: 10.1111/j.1476-5381.1995.tb13314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks CA. Spinal delivery of analgesics in experimental models of pain and analgesia. Adv Drug Deliv Rev. 2003;55:1007–1041. doi: 10.1016/s0169-409x(03)00101-7. [DOI] [PubMed] [Google Scholar]
- Fields HL, Emson PC, Leigh BK, Gilbert RF, Iversen LL. Multiple opiate receptor sites on primary afferent fibres. Nature. 1980;284:351–353. doi: 10.1038/284351a0. [DOI] [PubMed] [Google Scholar]
- Franklin TG, Traynor JR. Alkylation with beta-funaltrexamine suggests differences between mu-opioid receptor systems in guinea-pig brain and myenteric-plexus. Br J Pharmacol. 1991;102:718–722. doi: 10.1111/j.1476-5381.1991.tb12239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser GL, Gaudreau GA, Clarke PB, Menard DP, Perkins MN. Antihyperalgesic effects of delta opioid agonists in a rat model of chronic inflammation. Br J Pharmacol. 2000a;129:1668–1672. doi: 10.1038/sj.bjp.0703248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser GL, Parenteau H, Tu TM, Ducharme J, Perkins MN, Clarke PB. The effects of delta agonists on locomotor activity in habituated and non-habituated rats. Life Sci. 2000b;67:913–922. doi: 10.1016/s0024-3205(00)00690-1. [DOI] [PubMed] [Google Scholar]
- Gallantine EL, Meert TF. A comparison of the antinociceptive and adverse effects of the mu-opioid agonist morphine and the delta-opioid agonist SNC80. Basic Clin Pharmacol Toxicol. 2005;97:39–51. doi: 10.1111/j.1742-7843.2005.pto_97107.x. [DOI] [PubMed] [Google Scholar]
- Gendron L, Esdaile MJ, Mennicken F, Pan H, O’Donnell D, Vincent JP, Devi LA, Cahill CM, Stroh T, Beaudet A. Morphine priming in rats with chronic inflammation reveals a dichotomy between antihyperalgesic and antinociceptive properties of deltorphin. Neuroscience. 2007;144:263–274. doi: 10.1016/j.neuroscience.2006.08.077. [DOI] [PubMed] [Google Scholar]
- Gendron L, Lucido AL, Mennicken F, O’Donnell D, Vincent JP, Stroh T, Beaudet A. Morphine and pain-related stimuli enhance cell surface availability of somatic delta-opioid receptors in rat dorsal root ganglia. J Neurosci. 2006;26:953–962. doi: 10.1523/JNEUROSCI.3598-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George SR, Fan T, Xie Z, Tse R, Tam V, Varghese G, O’Dowd BF. Oligomerization of mu- and delta-opioid receptors. Generation of novel functional properties. J Biol Chem. 2000;275:26128–26135. doi: 10.1074/jbc.M000345200. [DOI] [PubMed] [Google Scholar]
- Gomes I, Gupta A, Filipovska J, Szeto HH, Pintar JE, Devi LA. A role for heterodimerization of mu and delta opiate receptors in enhancing morphine analgesia. Proc Natl Acad Sci U S A. 2004;101:5135–5139. doi: 10.1073/pnas.0307601101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes I, Jordan BA, Gupta A, Trapaidze N, Nagy V, Devi LA. Heterodimerization of mu and delta opioid receptors: A role in opiate synergy. J Neurosci. 2000;20:RC110. doi: 10.1523/JNEUROSCI.20-22-j0007.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan JS, Xu ZZ, Gao H, He SQ, Ma GQ, Sun T, Wang LH, Zhang ZN, Lena I, Kitchen I, Elde R, Zimmer A, He C, Pei G, Bao L, Zhang X. Interaction with Vesicle Luminal Protachykinin Regulates Surface Expression of delta-Opioid Receptors and Opioid Analgesia. Cell. 2005;122:619–631. doi: 10.1016/j.cell.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Guo XH, Fairbanks CA, Stone LS, Loh HH. DPDPE-UK14,304 synergy is retained in mu opioid receptor knockout mice. Pain. 2003;104:209–217. doi: 10.1016/s0304-3959(03)00007-1. [DOI] [PubMed] [Google Scholar]
- Hack SP, Bagley EE, Chieng BC, Christie MJ. Induction of delta-opioid receptor function in the midbrain after chronic morphine treatment. J Neurosci. 2005;25:3192–3198. doi: 10.1523/JNEUROSCI.4585-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L, Lee NM. Delta opioid receptor enhancement of mu opioid receptor-induced antinociception in spinal cord. J Pharmacol Exp Ther. 1998;285:1181–1186. [PubMed] [Google Scholar]
- Hosohata Y, Vanderah TW, Burkey TH, Ossipov MH, Kovelowski CJ, Sora I, Uhl GR, Zhang X, Rice KC, Roeske WR, Hruby VJ, Yamamura HI, Lai J, Porreca F. delta-Opioid receptor agonists produce antinociception and [35S]GTPgammaS binding in mu receptor knockout mice. Eur J Pharmacol. 2000;388:241–248. doi: 10.1016/s0014-2999(99)00897-3. [DOI] [PubMed] [Google Scholar]
- Hough LB, Nalwalk JW, Chen Y, Schuller A, Zhu Y, Zhang J, Menge WM, Leurs R, Timmerman H, Pintar JE. Improgan, a cimetidine analog, induces morphine-like antinociception in opioid receptor-knockout mice. Brain Res. 2000;880:102–108. doi: 10.1016/s0006-8993(00)02776-1. [DOI] [PubMed] [Google Scholar]
- Hurley RW, Hammond DL. The analgesic effects of supraspinal mu and delta opioid receptor agonists are potentiated during persistent inflammation. J Neurosci. 2000;20:1249–1259. doi: 10.1523/JNEUROSCI.20-03-01249.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley RW, Hammond DL. Contribution of endogenous enkephalins to the enhanced analgesic effects of supraspinal mu opioid receptor agonists after inflammatory injury. J Neurosci. 2001;21:2536–2545. doi: 10.1523/JNEUROSCI.21-07-02536.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hylden JL, Thomas DA, Iadarola MJ, Nahin RL, Dubner R. Spinal opioid analgesic effects are enhanced in a model of unilateral inflammation/hyperalgesia: possible involvement of noradrenergic mechanisms. Eur J Pharmacol. 1991;194:135–143. doi: 10.1016/0014-2999(91)90097-a. [DOI] [PubMed] [Google Scholar]
- Iadarola MJ, Douglass J, Civelli O, Naranjo JR. Differential activation of spinal cord dynorphin and enkephalin neurons during hyperalgesia: evidence using cDNA hybridization. Brain Res. 1988;455:205–212. doi: 10.1016/0006-8993(88)90078-9. [DOI] [PubMed] [Google Scholar]
- Jordan BA, Devi LA. G-protein-coupled receptor heterodimerization modulates receptor function. Nature. 1999;399:697–700. doi: 10.1038/21441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jutkiewicz EM, Eller EB, Folk JE, Rice KC, Traynor JR, Woods JH. Delta-opioid agonists: differential efficacy and potency of SNC80, its 3-OH (SNC86) and 3-desoxy (SNC162) derivatives in Sprague-Dawley rats. J Pharmacol Exp Ther. 2004;309:173–181. doi: 10.1124/jpet.103.061242. [DOI] [PubMed] [Google Scholar]
- Khotib J, Narita M, Suzuki M, Yajima Y, Suzuki T. Functional interaction among opioid receptor types: up-regulation of mu- and delta-opioid receptor functions after repeated stimulation of kappa-opioid receptors. Neuropharmacology. 2004;46:531–540. doi: 10.1016/j.neuropharm.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Konig M, Zimmer AM, Steiner H, Holmes PV, Crawley JN, Brownstein MJ, Zimmer A. Pain responses, anxiety and aggression in mice deficient in pre-proenkephalin. Nature. 1996;383:535–538. doi: 10.1038/383535a0. [DOI] [PubMed] [Google Scholar]
- Kreek MJ. Opioid receptors: some perspectives from early studies of their role in normal physiology, stress responsivity, and in specific addictive diseases. Neurochem Res. 1996;21:1469–1488. doi: 10.1007/BF02532387. [DOI] [PubMed] [Google Scholar]
- Law PY, Erickson-Herbrandson LJ, Zha QQ, Solberg J, Chu J, Sarre A, Loh HH. Heterodimerization of mu- and delta-opioid receptors occurs at the cell surface only and requires receptor-G protein interactions. J Biol Chem. 2005;280:11152–11164. doi: 10.1074/jbc.M500171200. [DOI] [PubMed] [Google Scholar]
- Loh HH, Liu HC, Cavalli A, Yang W, Chen YF, Wei LN. mu Opioid receptor knockout in mice: effects on ligand-induced analgesia and morphine lethality. Brain Res Mol Brain Res. 1998;54:321–326. doi: 10.1016/s0169-328x(97)00353-7. [DOI] [PubMed] [Google Scholar]
- Longoni R, Spina L, Mulas A, Carboni E, Garau L, Melchiorri P, Di Chiara G. (D-Ala2)deltorphin II: D1-dependent stereotypies and stimulation of dopamine release in the nucleus accumbens. J Neurosci. 1991;11:1565–1576. doi: 10.1523/JNEUROSCI.11-06-01565.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Zhang Y, Kalyuzhny AE, Pan ZZ. Emergence of Functional {delta}-Opioid Receptors Induced by Chronic Morphine. Mol Pharmacol. 2006 doi: 10.1124/mol.105.019109. [DOI] [PubMed] [Google Scholar]
- Matthes HW, Smadja C, Valverde O, Vonesch JL, Foutz AS, Boudinot E, Denavit-Saubie M, Severini C, Negri L, Roques BP, Maldonado R, Kieffer BL. Activity of the delta-opioid receptor is partially reduced, whereas activity of the kappa-receptor is maintained in mice lacking the mu-receptor. J Neurosci. 1998;18:7285–7295. doi: 10.1523/JNEUROSCI.18-18-07285.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May CN, Dashwood MR, Whitehead CJ, Mathias CJ. Differential cardiovascular and respiratory responses to central administration of selective opioid agonists in conscious rabbits: correlation with receptor distribution. Br J Pharmacol. 1989;98:903–913. doi: 10.1111/j.1476-5381.1989.tb14620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchiorri P, Maritati M, Negri L, Erspamer V. Long-term sensitization to the activation of cerebral delta-opioid receptors by the deltorphin Tyr-D-Ala-Phe-Glu-Val-Val-Gly-NH2 in rats exposed to morphine. Proc Natl Acad Sci U S A. 1992;89:3696–3700. doi: 10.1073/pnas.89.9.3696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennicken F, Zhang J, Hoffert C, Ahmad S, Beaudet A, O’Donnell D. Phylogenetic changes in the expression of delta opioid receptors in spinal cord and dorsal root ganglia. J Comp Neurol. 2003;465:349–360. doi: 10.1002/cne.10839. [DOI] [PubMed] [Google Scholar]
- Miaskowski C, Sutters KA, Taiwo YO, Levine JD. Comparison of the antinociceptive and motor effects of intrathecal opioid agonists in the rat. Brain Res. 1991;553:105–109. doi: 10.1016/0006-8993(91)90236-o. [DOI] [PubMed] [Google Scholar]
- Mika J, Przewlocki R, Przewlocka B. The role of delta-opioid receptor subtypes in neuropathic pain. Eur J Pharmacol. 2001;415:31–37. doi: 10.1016/s0014-2999(01)00814-7. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Czlonkowski A, Morris B, Stein C, Arendt R, Huber A, Hollt V, Herz A. Inflammation of the hind limb as a model of unilateral, localized pain: influence on multiple opioid systems in the spinal cord of the rat. Pain. 1988;35:299–312. doi: 10.1016/0304-3959(88)90140-6. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Millan MH, Czlonkowski A, Hollt V, Pilcher CW, Herz A, Colpaert FC. A model of chronic pain in the rat: response of multiple opioid systems to adjuvant-induced arthritis. J Neurosci. 1986;6:899–906. doi: 10.1523/JNEUROSCI.06-04-00899.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morinville A, Cahill CM, Aibak H, Rymar VV, Pradhan A, Hoffert C, Mennicken F, Stroh T, Sadikot AF, O’Donnell D, Clarke PB, Collier B, Henry JL, Vincent JP, Beaudet A. Morphine-induced changes in delta opioid receptor trafficking are linked to somatosensory processing in the rat spinal cord. J Neurosci. 2004a;24:5549–5559. doi: 10.1523/JNEUROSCI.2719-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morinville A, Cahill CM, Esdaile MJ, Aibak H, Collier B, Kieffer BL, Beaudet A. Regulation of delta-opioid receptor trafficking via mu-opioid receptor stimulation: evidence from mu-opioid receptor knock-out mice. J Neurosci. 2003;23:4888–4898. doi: 10.1523/JNEUROSCI.23-12-04888.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morinville A, Cahill CM, Kieffer B, Collier B, Beaudet A. Mu-opioid receptor knockout prevents changes in delta-opioid receptor trafficking induced by chronic inflammatory pain. Pain. 2004b;109:266–273. doi: 10.1016/j.pain.2004.01.011. [DOI] [PubMed] [Google Scholar]
- Negri L, Noviello L, Noviello V. Antinociceptive and behavioral effects of synthetic deltorphin analogs. Eur J Pharmacol. 1996;296:9–16. doi: 10.1016/0014-2999(95)00644-3. [DOI] [PubMed] [Google Scholar]
- Negri L, Noviello V, Angelucci F. Behavioural effects of deltorphins in rats. Eur J Pharmacol. 1991;209:163–168. doi: 10.1016/0014-2999(91)90165-m. [DOI] [PubMed] [Google Scholar]
- Noguchi K, Dubner R, Ruda MA. Preproenkephalin mRNA in spinal dorsal horn neurons is induced by peripheral inflammation and is co-localized with Fos and Fos-related proteins. Neuroscience. 1992;46:561–570. doi: 10.1016/0306-4522(92)90144-q. [DOI] [PubMed] [Google Scholar]
- Parra MC, Nguyen TN, Hurley RW, Hammond DL. Persistent inflammatory nociception increases levels of dynorphin 1–17 in the spinal cord, but not in supraspinal nuclei involved in pain modulation. J Pain. 2002;3:330–336. doi: 10.1054/jpai.2002.125185. [DOI] [PubMed] [Google Scholar]
- Patwardhan AM, Berg KA, Akopain AN, Jeske NA, Gamper N, Clarke WP, Hargreaves KM. Bradykinin-induced functional competence and trafficking of the delta-opioid receptor in trigeminal nociceptors. J Neurosci. 2005;25:8825–8832. doi: 10.1523/JNEUROSCI.0160-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patwardhan AM, Diogenes A, Berg KA, Fehrenbacher JC, Clarke WP, Akopian AN, Hargreaves KM. PAR-2 agonists activate trigeminal nociceptors and induce functional competence in the delta opioid receptor. Pain. 2006 doi: 10.1016/j.pain.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Petrillo P, Angelici O, Bingham S, Ficalora G, Garnier M, Zaratin PF, Petrone G, Pozzi O, Sbacchi M, Stean TO, Upton N, Dondio GM, Scheideler MA. Evidence for a selective role of the delta-opioid agonist [8R-(4bS*,8aalpha,8abeta, 12bbeta)]7,10-Dimethyl-1-methoxy-11-(2-methylpropyl)oxycarbonyl 5,6,7,8,12,12b-hexahydro-(9H)-4,8-methanobenzofuro[3,2-e]pyrrolo[2,3-g]iso quinoline hydrochloride (SB-235863) in blocking hyperalgesia associated with inflammatory and neuropathic pain responses. J Pharmacol Exp Ther. 2003;307:1079–1089. doi: 10.1124/jpet.103.055590. [DOI] [PubMed] [Google Scholar]
- Porreca F, Mosberg HI, Hurst R, Hruby VJ, Burks TF. Roles of mu, delta and kappa opioid receptors in spinal and supraspinal mediation of gastrointestinal transit effects and hot-plate analgesia in the mouse. J Pharmacol Exp Ther. 1984;230:341–348. [PubMed] [Google Scholar]
- Qiu C, Sora I, Ren K, Uhl G, Dubner R. Enhanced delta-opioid receptor-mediated antinociception in mu-opioid receptor-deficient mice. Eur J Pharmacol. 2000;387:163–169. doi: 10.1016/s0014-2999(99)00813-4. [DOI] [PubMed] [Google Scholar]
- Riba P, Ben Y, Smith AP, Furst S, Lee NM. Morphine tolerance in spinal cord is due to interaction between mu- and delta-receptors. J Pharmacol Exp Ther. 2002;300:265–272. doi: 10.1124/jpet.300.1.265. [DOI] [PubMed] [Google Scholar]
- Rozenfeld R, Devi LA. Receptor heterodimerization leads to a switch in signaling: {beta}-arrestin2-mediated ERK activation by {micro}-{delta} opioid receptor heterodimers. Faseb J. 2007 doi: 10.1096/fj.06-7793com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein M, Mogil JS, Japon M, Chan EC, Allen RG, Low MJ. Absence of opioid stress-induced analgesia in mice lacking beta-endorphin by site-directed mutagenesis. Proc Natl Acad Sci U S A. 1996;93:3995–4000. doi: 10.1073/pnas.93.9.3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson Nydahl K, Skinner K, Julius D, Basbaum AI. Co-localization of endomorphin-2 and substance P in primary afferent nociceptors and effects of injury: a light and electron microscopic study in the rat. Eur J Neurosci. 2004;19:1789–1799. doi: 10.1111/j.1460-9568.2004.03284.x. [DOI] [PubMed] [Google Scholar]
- Scherrer G, Befort K, Contet C, Becker J, Matifas A, Kieffer BL. The delta agonists DPDPE and deltorphin II recruit predominantly mu receptors to produce thermal analgesia: a parallel study of mu, delta and combinatorial opioid receptor knockout mice. Eur J Neurosci. 2004;19:2239–2248. doi: 10.1111/j.0953-816X.2004.03339.x. [DOI] [PubMed] [Google Scholar]
- Schuller AG, King MA, Zhang J, Bolan E, Pan YX, Morgan DJ, Chang A, Czick ME, Unterwald EM, Pasternak GW, Pintar JE. Retention of heroin and morphine-6 beta-glucuronide analgesia in a new line of mice lacking exon 1 of MOR-1. Nat Neurosci. 1999;2:151–156. doi: 10.1038/5706. [DOI] [PubMed] [Google Scholar]
- Sharifi N, Diehl N, Yaswen L, Brennan MB, Hochgeschwender U. Generation of dynorphin knockout mice. Brain Res Mol Brain Res. 2001;86:70–75. doi: 10.1016/s0169-328x(00)00264-3. [DOI] [PubMed] [Google Scholar]
- Sora I, Funada M, Uhl GR. The mu-opioid receptor is necessary for [D-Pen2,D-Pen5]enkephalin-induced analgesia. Eur J Pharmacol. 1997a;324:R1–2. doi: 10.1016/s0014-2999(97)10016-4. [DOI] [PubMed] [Google Scholar]
- Sora I, Takahashi N, Funada M, Ujike H, Revay RS, Donovan DM, Miner LL, Uhl GR. Opiate receptor knockout mice define mu receptor roles in endogenous nociceptive responses and morphine-induced analgesia. Proc Natl Acad Sci U S A. 1997b;94:1544–1549. doi: 10.1073/pnas.94.4.1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spina L, Longoni R, Mulas A, Chang KJ, Di Chiara G. Dopamine-dependent behavioural stimulation by non-peptide delta opioids BW373U86 and SNC 80: 1. Locomotion, rearing and stereotypies in intact rats. Behav Pharmacol. 1998;9:1–8. [PubMed] [Google Scholar]
- Stewart PE, Hammond DL. Activation of spinal delta-1 or delta-2 opioid receptors reduces carrageenan-induced hyperalgesia in the rat. J Pharmacol Exp Ther. 1994;268:701–708. [PubMed] [Google Scholar]
- Szeto HH, Soong Y, Wu D, Olariu N, Kett A, Kim H, Clapp JF. Respiratory depression after intravenous administration of delta-selective opioid peptide analogs. Peptides. 1999;20:101–105. doi: 10.1016/s0196-9781(98)00141-7. [DOI] [PubMed] [Google Scholar]
- Zadina JE, Hackler L, Ge LJ, Kastin AJ. A potent and selective endogenous agonist for the mu-opiate receptor. Nature. 1997;386:499–502. doi: 10.1038/386499a0. [DOI] [PubMed] [Google Scholar]
- Zhang X, Bao L, Arvidsson U, Elde R, Hokfelt T. Localization and regulation of the delta-opioid receptor in dorsal root ganglia and spinal cord of the rat and monkey: evidence for association with the membrane of large dense-core vesicles. Neuroscience. 1998;82:1225–1242. doi: 10.1016/s0306-4522(97)00341-2. [DOI] [PubMed] [Google Scholar]
- Zhang X, Bao L, Guan JS. Role of delivery and trafficking of delta-opioid peptide receptors in opioid analgesia and tolerance. Trends Pharmacol Sci. 2006;27:324–329. doi: 10.1016/j.tips.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Zieglgansberger W, French ED, Mercuri N, Pelayo F, Williams JT. Multiple opiate receptors on neurons of the mammalian central nervous system. In vivo and in vitro studies. Life Sci. 1982;31:2343–2346. doi: 10.1016/0024-3205(82)90152-7. [DOI] [PubMed] [Google Scholar]