Abstract
Evidence suggests that GABA might mediate the inhibitory influence of centrifugal inputs on taste-evoked responses in the parabrachial nucleus (PBN). Previous studies show that activation of the gustatory cortex (GC), bed nucleus of the stria terminalis (BNST), central nucleus of the amygdala (CeA), and lateral hypothalamus (LH) inhibits PBN taste responses, GABAergic neurons are present in these forebrain regions, and GABA reduces the input resistance of PBN neurons. The present study investigated the expression of glutamic acid decarboxylase immunoreactivity (GAD_67 ir) in GC, BNST, CeA, and LH neurons that project to the PBN in rats. After anesthesia (50 mg/kg Nembutal ip), injections of the retrograde tracer fluorogold (FG) were made in the physiologically defined gustatory PBN. Brain tissue containing the above forebrain structures was processed and examined for FG and GAD_67 ir. Similar to previous studies, each forebrain site contained retrograde labeled neurons. Our results suggest further that the major source of input to the PBN taste region is the CeA (608 total cells) followed by GC (257 cells), LH (106 cells), and BNST (92 cells). This suggests a differential contribution to centrifugal control of PBN taste processing. We further show that despite the presence of GAD_67 neurons in each forebrain area, co-localization was extremely rare, occurring only in 3 out of 1,063 FG labeled cells. If we assume that the influence of centrifugal input is mediated by direct projections to the gustatory region of the PBN, then GABAergic forebrain neurons apparently are not part of this descending pathway.
Keywords: Amygdala, Bed Nucleus, Hypothalamus, Cortex, Taste, GAD
1. Introduction
The major biologic function of the taste system is to determine whether the contents of the oral cavity are ingested or rejected. This gustatory behavioral response, however, is modifiable by learning and immediate physiological state (5; 7). The underlying mechanism appears to involve a change in gustatory hedonic value, i.e. palatability, rather than taste quality. Although palatability is unquestionably a key factor in guiding food intake, little is known about its neural basis. Communication between the forebrain and brainstem, however, is critical because the isolated brainstem is not sufficient to support learned (e.g. conditioned taste aversion) and some forms of unlearned control (e.g. sodium appetite) of taste-guided behavior (12–14).
Recent evidence suggests that the axons necessary for assigning hedonic value to taste stimuli originate in the pontine parabrachial nucleus (PBN), the second central synapse for ascending gustatory information, but do not relay through thalamocortical projections (31). In addition to the thalamocortical pathway, PBN efferents target several ventral forebrain areas directly including the lateral hypothalamus (LH), central nucleus of the amygdala (CeA), and bed nucleus of the stria terminalis (BNST). These same forebrain areas including gustatory insular cortex (GC) also send projections back to the PBN (2; 16; 17; 27; 29; 30; 33; 43).
In normal animals, the induction of behaviors like CTA and sodium appetite, which alter gustatory hedonic value, coincide with changes in taste-evoked responses in the PBN (34; 35). Whether this reflects a causal relationship is unsettled, although communication with forebrain regions is critical because altered taste responses induced by CTA acquisition are abolished following decerebration (42). Furthermore, electrical stimulation of the GC, BNST, CeA, and LH modulates taste-evoked responses in the PBN (20; 22–24; 42). Both excitatory and inhibitory effects were observed, however, inhibition of taste responses predominated suggesting a role for the inhibitory neurotransmitter GABA. GABAergic neurons are present in each of these forebrain regions (1; 3; 9–11; 39), and GABA produces a concentration-dependent reduction in input resistance of neurons in the caudomedial gustatory zone of the PBN (19). However, one study has shown that CeA neurons retrogradely labeled following stereotaxic guidance of WGA-HRP injections into the PBN do not contain GABA (39).
Since the PBN consists of different regions processing gustatory, visceral, and somatosensory signals, the present study tested the hypothesis that LH, CeA, BNST, and GC neurons projecting to the gustatory PBN synthesize GABA. We electrophysiologically isolated the gustatory PBN and iontophoretically injected the retrograde tracer fluorogold (FG). Brain tissue containing the above forebrain structures was subsequently examined for neurons that contain FG and glutamic acid decarboxylase (GAD). Immunohistochemical localization of GAD has been used extensively as a marker for GABAergic neurons (6; 10; 25; 41).
2. Materials and Methods
2.1. Subjects
Five male Sprague-Dawley rats weighing 350–450 g [CrL: CD (SD) BR; Charles River Breeding Laboratories] were used in this study. The animals were maintained in a temperature-controlled colony room on a 12-h light/dark cycle and allowed free access to normal rat chow (Teklad 8604) and distilled water. All procedures conformed to NIH guidelines and were approved by the University of Louisville Institutional Animal Care and Use Committee.
2.2. Surgery
The rats were anesthetized with a 50-mg/kg injection (intraperitoneal, ip) of pentobarbital sodium (Nembutal). Atropine was administered to reduce bronchial secretions. Additional doses of Nembutal (0.1 ml) were administered as necessary to continue a deep level of anesthesia. The animals were placed on a feedback-controlled heating pad and rectal temperature was monitored to maintain body temperature at 37±0.5°C. Animals were secured in a stereotaxic instrument and the skull was exposed with a midline incision then leveled with reference to β and λ. A small hole was drilled through the bone overlying the cerebellum to allow access to the parabrachial nucleus.
2.3. Electrophysiological recording
A 0.1M NaCl solution was applied to the anterior 2/3 of the tongue for 10 sec using a wash bottle and extracellular neural responses were recorded using a glass-insulated tungsten microelectrode oriented 20° off vertical with the tip pointing rostral (1–3MΩ). Only the anterior 2/3 of the tongue was stimulated because numerous prior studies have demonstrated that forebrain activation has a profound influence on brainstem taste cells that receive input via the chorda tympani nerve (8; 21–24; 37; 38). Further, the concentration of NaCl used in the present study has been show to produce a significant neural response in each “best-stimulus” class of PBN neurons (23; 24). Once the gustatory PBN was located, the tungsten electrode was replaced by a micropipette (ID 10 – 20μm) filled with 4% Fluorogold (FG, Biotium Inc) dissolved in saline. The taste responsive area was electrophysiologically relocated and FG was iontophoretically injected (+2μA for 20 min; 2 min on and 1 min off). Cambridge Electronic Design’s Spike2 hardware and software was used to record NaCl-evoked neural responses (Lundy and Norgren, 2001). No attempt was made to isolate single neurons for analysis of response rate; rather we isolated regions in which a response to NaCl applied to the tongue was visually above the baseline discharge (Figure 1A).
Figure 1.
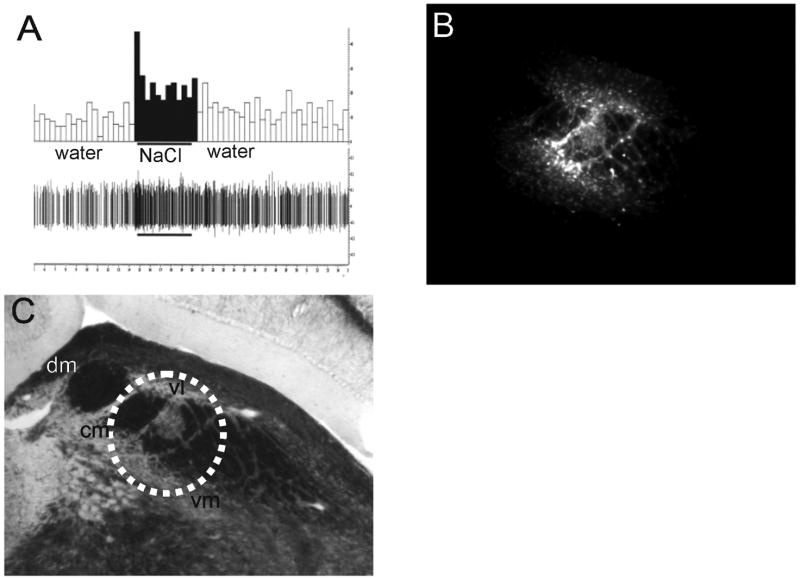
(A) The electrophysiological response to tongue application of 0.1 M NaCl recorded through the FG filled injection pipette. The bottom panel shows the raw neural response and the top panel shows the peristimulus histogram. (B) Fluorescent image of the resultant FG injection (5x magnification). (C) Brightfield image of the same section showing the approximate area of the injection relative to PBN subdivisions (white oval). The PBN section corresponds approximately to Fig. 59 (9.8 mm posterior to bregma) in the Paxinos and Watson Atlas (Paxinos and Watson, 1998). Abbreviations: cm, central medial; dm, dorsal medial; vm, ventral medial; vl, ventral lateral.
2.4. Colchicine treatment
Five days after surgery, the animals were reanesthetized and 3μl of colchicine (20μg/μl dissolved in 0.1 M NaCl, Tokyo Kasei Co. Ltd) was infused into the lateral ventricle ipsilateral to the FG injection site (Coordinates: 0.85 mm anterior to bregma, 1.5 mm lateral to midline and 4.0mm ventral to dura according to Paxinos and Watson, 1998) using a 25-μl Hamilton syringe with a 28 gauge needle mounted in a stereotax microinjection unit (Kopf, model 5000). The animals were perfused 48 h after colchicine administration.
2.5. Perfusion and histology
The animals were administered a lethal dose of Nembutal (100 mg/kg, ip) and perfused through the ascending aorta, initially with 250ml of 0.9% saline containing 5 ml of 100 units/ml heparin followed by 500 ml of 4% paraformaldehyde in 0.1M phosphate buffer (pH 7.4). The brains were removed, blocked just rostral to the PBN, and post fixed overnight at 4°C in the same fixative. Coronal (50 μm) sections were cut using a vibrating microtome (Leica VT 1000S) and every other section was collected for subsequent immunohistochemistry.
2.6. Immunohistochemistry
First, brain sections were incubated in 5% normal goat serum (NGS; Jackson Labs) and 1% bovine serum albumin (BSA; Jackson Labs) mixed in 0.3% triton-x phosphate buffer saline (TPBS) for 1 hr. Sections were then incubated overnight (4°C) in rabbit FG antibody (Chemicon, AB153) diluted 1:2,500 in TPBS. Sections were rinsed several times in PBS followed by 2 h incubation in FITC goat anti-rabbit diluted 1:100 in PBS with 5% NGS. Following several more rinses, sections were incubated in PBS containing 5% normal mouse serum (NMS; Jackson Labs) and 1% BSA for 1 hour, then for 48 hrs at 4°C in mouse monoclonal glutamic acid decarboxylase antibody (Chemicon, catalog number MAB5406) diluted 1:2,000 in PBS (36; 40). The immunogen for this antibody was a recombinant fusion protein containing the unique N terminal regions of GAD67 not shared by GAD65 protein. On day 4 sections were washed several times and incubated for 2 h in Cy-3 goat anti-mouse (Jackson Labs) diluted 1:200 in PBS containing 5% NMS. Following several rinses, the tissue sections were mounted on gelatin-coated slides and cover slipped. For one series of tissue sections the GAD_67 antibody was omitted and, consequently, Cy-3 immunofluorescence.
2.7. Data analysis
Cell bodies positive for FG (FITC; excitation filter: 490 nm; barrier filter: 550 nm) and GAD_67 (Cy-3; excitation filter: 520–554 nm; barrier filter: 580 nm) immunoreactivity in the insular gustatory cortex (GC), central nucleus of the amygdala (CeA), bed nucleus of the stria terminalis (BNST), and lateral hypothalamus (LH) were identified using sequential scanning with an Olympus confocal microscope. All forebrain areas were identified based on the Paxinos and Watson rat brain atlas (32). The GC and LH are the least defined areas and a brief description of the location of retrograde labeled cells follows. GC was identified as the area approximately between the level at which the anterior commissure crosses midline and the disappearance of the genu of the corpus callosum rostrally. The LH was identified as an area bounded laterally by the optic tract, dorsally by the internal capsule, medially by the fornix, and ventrally by the surface of the brain. The number of immunoreactive cells per section (sum of cells divided by the number of sections) was calculated and used for statistical analyses. Only tissue sections that contained retrogradely labeled neurons were analyzed. Comparisons between forebrain sites were performed using One-Way ANOVA and paired-sample T-tests (SPSS 12.0). In some instances, post hoc analyses (Bonferroni) were used to determine the source of statistically significant differences. The results are presented as mean ± S.E. A value of P< 0.05 was considered statistically significant.
3. Results
3.1 Injection sites
In each animal, the taste responsive region of the PBN was relocated with the FG filled injection pipette. An example of the response to 0.1M NaCl applied to the anterior tongue is shown in Fig. 1A. The fluorescent photomicrograph in Fig. 1B shows the resulting FG injection site in the PBN. A brightfield image of the same section is shown in Fig. 1C. Microscopic examination of each injection site revealed that predominately the central medial, ventral medial, and ventral lateral portions of the caudal PBN were targeted with minimal spread into rostrolateral regions. Figure 2 shows a summary of the five FG injections into the gustatory responsive PBN.
Figure 2.
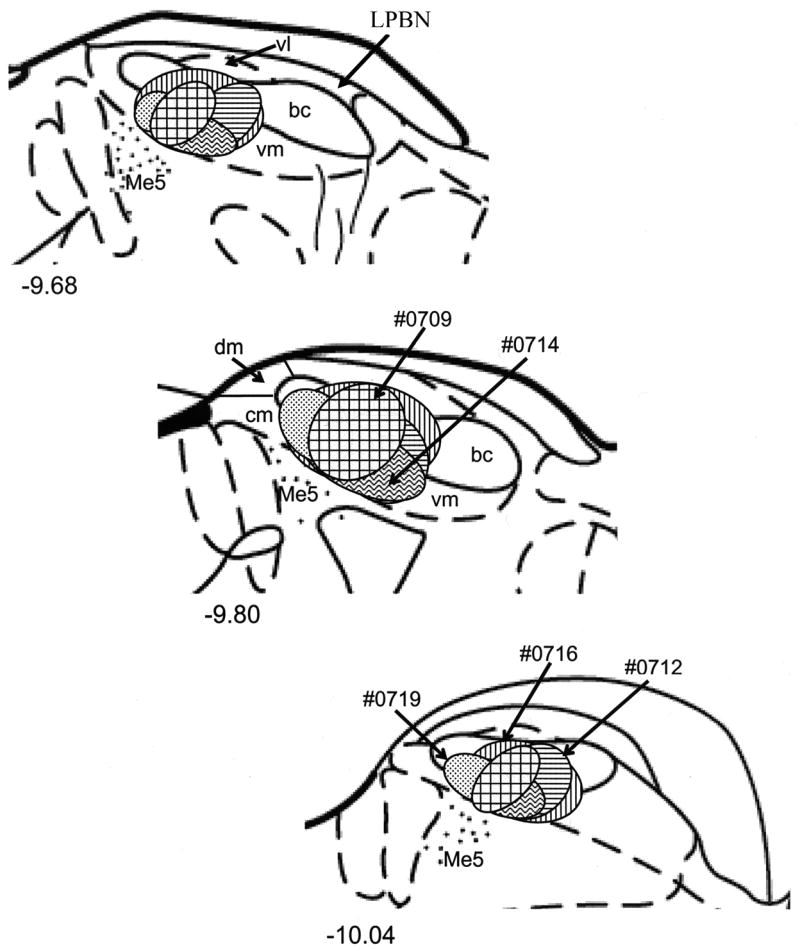
Schematic representation of FG injections in the central medial (cm), dorsal medial (dm), ventral medial (vm), and ventral lateral (vl) subdivisions of the PBN. (top to bottom) Rostral to caudal. The approximate levels relative to bregma are indicated below each figure (Paxinos and Watson, 1998). Me5, mesencephalic trigeminal nucleus; LPBN, corresponds to the caudal portion of the lateral visceral sensitive PBN..
3.2. Retrograde labeling
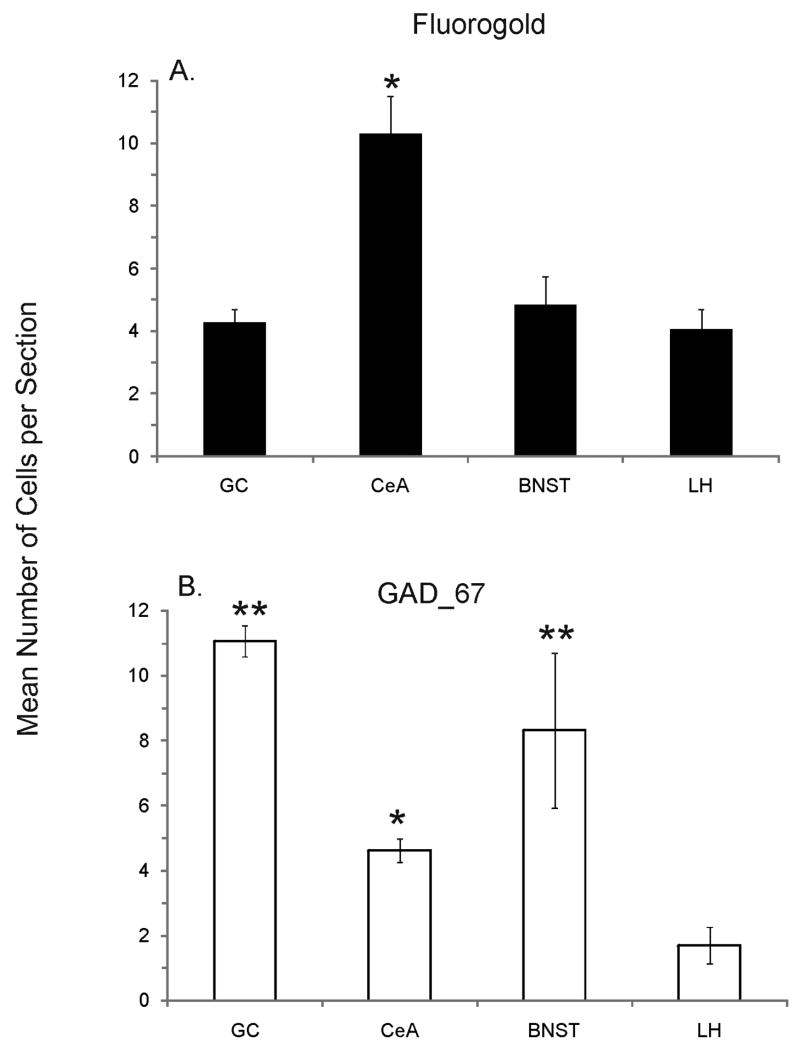
The number of FG-labeled neuronal cell bodies differed between forebrain sites (F3,163 = 11.8, P < 0.01). The CeA contained significantly more retrograde labeled cells compared to the LH, BNST, and GC (P values < 0.01). The LH, BNST, and GC contained similar number of FG-labeled cells per section (Fig. 3A; P’s = 1.0). Nevertheless, the number of GC sections (n=56) with retrogradely filled neuronal cells was far greater compared to the LH (n=26) and BNST (n=19), but not the CeA (n=59). Further analyses using paired-sample T-tests revealed a significantly greater total number of FG labeled neurons in GC (n=257) compared to the LH (n=106; T4 = 4.8, P < 0.01) and BNST (n=92; T4 = 2.5, P = 0.03). The total number of retrograde labeled cells in the CeA (n=608) was significantly greater than each of the other forebrain areas (P’s ≤ 0.05).
Figure 3.
The mean number of cells immunoreactive for FG (A) and GAD_67 (B) in the gustatory cortex (GC), central nucleus of the amygdala (CeA), bed nucleus of the stria terminalis (BNST), and lateral hypothalamus (LH). Panel A (FG label): *, significantly different from GC, BNST, and LH. Panel B (GAD_67 label): *, significantly different from LH; **, significantly different from CeA and LH.
3.3. GAD_67 immunoreactivity
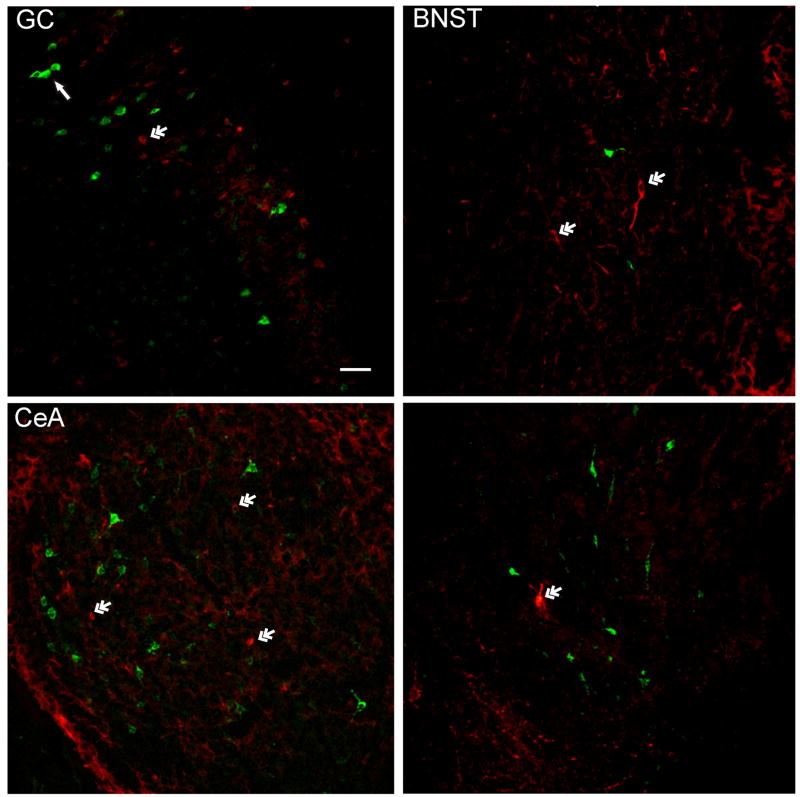
In each forebrain site, immunohistochemical processing for GAD_67 resulted in robust labeling of cells with distinguishable nuclei and short processes (Fig. 4). Quantification of neuronal cells immunoreactive for GAD_67 (GAD_67 ir) in those sections containing FG retrograde labeled cells is shown in Figure 3B. Similar to retrograde labeled cells, the mean number of GAD_67 ir neurons per section differed between the GC, CeA, BNST, and LH (F3,163 = 32.8, P < 0.01). GC and BNST contained significantly more GAD_67 positive neurons compared to the CeA (P’s ≤ 0.01) and LH (P’s < 0.01), but were similar to one another (P = 0.14). Further analyses of total number of GAD ir neurons was not performed, because only GAD positive cells in those sections containing FG labeled neurons were counted. Shown in the photomicrographs of Figure 4, are examples of FG labeled projection neurons and GAD_67 ir neurons in the GC (top left panel), BNST (top right panel), CeA (bottom left panel), and LH (bottom right panel). Despite the presence of GAD positive neurons in each forebrain area, co-localization with FG retrograde labeled cells was extremely rare. Overall, only three neuronal cell bodies were double labeled; one cell each in the GC, BNST, and CeA.
Figure 4.
Photomicrographs of FG labeled projection neurons and GAD positive neurons in the gustatory cortex (GC), central nucleus of the amygdala (CeA), bed nucleus of the stria terminalis (BNST), and lateral hypothalamus (LH). Arrow in GC indicates a group of FG labeled cells (green in each panel). Double arrows indicate GAD_67 ir cells (red in each panel). A scale bar is shown in lower right corner of GC (white, 50 μm).
4. Discussion
This study demonstrated that forebrain neurons projecting to taste responsive sites within the caudal parabrachial nucleus (PBN) do not contain glutamic acid decarboxylase, the enzyme responsible for the conversion of glutamic acid to gamma-aminobutyric acid (GABA). Out of 1,063 retrograde filled forebrain neurons, only three exhibited GAD_67 immunoreactivity. Prior studies show that activation of gustatory insular cortex (GC), bed nucleus of the stria terminalis (BNST), central nucleus of the amygdala (CeA), and lateral hypothalamus (LH) inhibits PBN neural activity suggesting a role for the inhibitory neurotransmitter GABA. The present data do not exclude a role for GABA in mediating descending inhibitory control of taste processing, but do suggest that it is not due to direct input from GABAergic forebrain neurons. The present results also demonstrate that taste responsive sites within the caudal parabrachial nucleus (PBN) were targeted to varying degree by different forebrain areas. The largest source of descending input originated in the CeA followed by GC. The same PBN injections resulted in a considerably smaller number of retrograde labeled neurons in the BNST and LH.
Contribution of forebrain areas to descending input to the PBN
Although each of the forebrain areas examined has been shown to inhibit taste responsive PBN neurons, the magnitude of inhibition and percentage of neurons under inhibitory control appears to vary with forebrain stimulation site. For instance, Lundy and Norgren (2004) showed that more PBN taste neurons were suppressed by activity in the CeA (41/48) and GC (32/45) than by the LH (14/32) in rat. Although the influence of BNST stimulation on PBN taste neurons has not been tested in rat, a recent study in hamster showed that 98% of PBN taste cells was inhibited by BNST activation (20). These data suggest that the BNST, CeA, and GC are the major sources of neurons with axons projecting to the PBN. This is consistent with the present results showing that the CeA and GC contribute significantly more neurons to descending forebrain-gustatory PBN pathways compared to the LH. In the case of the BNST, however, our results from small physiologically defined injections suggest that the BNST contributes fewer neurons with projections back to the PBN compared to the CeA and GC. It might be that the number of BNST neurons projecting to the PBN differs between hamster and rat or the influence of BNST activation on PBN taste cells is, in part, indirect (e.g. a BNST-CeA-PBN pathway).
Modulation of PBN gustatory processing
The predominant inhibition of PBN taste processing by forebrain stimulation suggests a role for the major CNS inhibitory transmitter GABA. Indeed, Kobashi and Bradley (1998) have shown that GABA produces a concentration-dependent reduction in input resistance of neurons in the caudomedial gustatory zone of the PBN. Findings from the present double-label experiments, however, demonstrate that forebrain neurons projecting to central medial, ventral medial, and ventral lateral portions of the caudal gustatory PBN do not express GAD_67. This is consistent with a recent electronmicroscopy study investigating the chemical nature of CeA projections to the PBN (18). Although GABA-positive CeA terminals were found to innervate the lateral visceral portion of the PBN, no such terminals were found in the medial gustatory region of the PBN. Together, these data indicate that forebrain induced inhibition of PBN taste processing is not mediated by direct projections from GABAergic forebrain neurons. One hypothesis is that the visceral sensitive lateral PBN is a necessary way station for the centrifugal projections that modulate taste processing in the medial PBN.
Another hypothesis is that some other neurochemical(s) expressed by neurons in the GC, BNST, CeA, and LH mediate descending control of taste processing. For instance, Moga et al. (1990) provided evidence for at least five distinct neuropeptide-immunoreactive cell populations in the hypothalamus that project to the PBN. PBN projecting neurons originating in the CeA (26) and BNST (28) also are immunoreactive for many of these same neurochemicals including somatostatin (SS), neurotensin (NT), corticotrophin-releasing factor (CRF), enkephalin, substance P, and galanin. Following injections of retrograde tracer centered in the ventrolateral PBN, cells labeled both for the tracer and NT, SS, or CRF were concentrated in the lateral CeA (Moga and Gray 1985). These investigators mentioned in passing that injections centered within the medial or dorsomedial PBN resulted in labeling confined mainly to the medial CeA, but did not present or discuss their neurochemical content. Similarly for the LH, retrograde tracer injections were aimed at the medial and lateral PBN, but the neurochemical content of LH inputs were not presented or discussed in this context (Moga et al. 1990). Thus, the relationship between descending forebrain neurochemical pathways and the various sensory inputs processed within the PBN remains to be established. That is, all these studies used stereotaxic coordinates to place tracer injections, which provided valuable information, but did not discern between gustatory, visceral, and somatosensory regions of the PBN.
Perspectives and Significance
In the present experiments, we confirmed and extended prior data that examined the neurochemical content of forebrain neurons projecting to the gustatory PBN. Considerable evidence suggests that the reciprocal connections between the PBN and ventral forebrain are critical for elaborating and probably for altering the hedonic value of a taste. If we assume that the influence of centrifugal input is mediated by direct projections to the gustatory region of the PBN, then GABAergic forebrain neurons apparently are not part of this descending pathway. In terms of taste response inhibition, one alternative is that intrinsic GABAergic neurons interface between descending axon terminals and PBN gustatory neurons. This synaptic arrangement has been hypothesized to mediate the inhibitory influence of GC on second-order taste neurons in the nucleus of the solitary tract (37). These cells are maintained under tonic GABAergic inhibition and GC-induced inhibition is blocked by local application of the GABAA receptor antagonist bicuculline. Alternatively, centrifugal influences might be mediated by an indirect pathway to PBN taste cells that first synapses in the lateral visceral sensitive region of the PBN. The PBN is a critical substrate for the integration of gustatory and more rostrolaterally processed visceral information, and physiological factors associated with ingestion modulate PBN taste responses (4; 15). GABA-positive CeA terminals innervating the lateral PBN have been reported (18). Future studies using electrophysiological, anatomical, and lesion-behavioral techniques will continue our efforts to determine the neurochemicals and neural circuit(s) that mediate centrifugal control of taste processing in the brainstem.
Acknowledgments
The authors thank Nick Miersma for technical support.
This research was supported by National Institute on Deafness and Other Communication Disorders Grant DC-006698.
Reference List
- 1.Abrahamson EE, Moore RY. The posterior hypothalamic area: chemoarchitecture and afferent connections. Brain Res. 2001;889:1–22. doi: 10.1016/s0006-8993(00)03015-8. [DOI] [PubMed] [Google Scholar]
- 2.Alden M, Besson JM, Bernard JF. Organization of the efferent projections from the pontine parabrachial area to the bed nucleus of the stria terminalis and neighboring regions: a PHA-L study in the rat. J Comp Neurol. 1994;341:289–314. doi: 10.1002/cne.903410302. [DOI] [PubMed] [Google Scholar]
- 3.Araki M, McGeer PL, McGeer EG. Retrograde HRP tracing combined with a pharmacohistochemical method for GABA transaminase for the identification of presumptive GABAergic projections to the habenula. Brain Res. 1984;304:271–277. doi: 10.1016/0006-8993(84)90330-5. [DOI] [PubMed] [Google Scholar]
- 4.Baird JP, Travers SP, Travers JB. Integration of gastric distension and gustatory responses in the parabrachial nucleus. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1581–R1593. doi: 10.1152/ajpregu.2001.281.5.R1581. [DOI] [PubMed] [Google Scholar]
- 5.Berridge KC, Flynn FW, Schulkin J, Grill HJ. Sodium depletion enhances salt palatability in rats. Behav Neurosci. 1984;98:652–660. doi: 10.1037//0735-7044.98.4.652. [DOI] [PubMed] [Google Scholar]
- 6.Blessing WW, Oertel WH, Willoughby JO. Glutamic acid decarboxylase immunoreactivity is present in perikarya of neurons in nucleus tractus solitarius of rat. Brain Res. 1984;322:346–350. doi: 10.1016/0006-8993(84)90131-8. [DOI] [PubMed] [Google Scholar]
- 7.Breslin PA, Spector AC, Grill HJ. A quantitative comparison of taste reactivity behaviors to sucrose before and after lithium chloride pairings: a unidimensional account of palatability. Behav Neurosci. 1992;106:820–836. doi: 10.1037//0735-7044.106.5.820. [DOI] [PubMed] [Google Scholar]
- 8.Cho YK, Li CS, Smith DV. Descending influences from the lateral hypothalamus and amygdala converge onto medullary taste neurons. Chem Senses. 2003;28:155–171. doi: 10.1093/chemse/28.2.155. [DOI] [PubMed] [Google Scholar]
- 9.Day HE, Curran EJ, Watson SJ, Jr, Akil H. Distinct neurochemical populations in the rat central nucleus of the amygdala and bed nucleus of the stria terminalis: evidence for their selective activation by interleukin-1beta. J Comp Neurol. 1999;413:113–128. [PubMed] [Google Scholar]
- 10.Esclapez M, Tillakaratne NJ, Kaufman DL, Tobin AJ, Houser CR. Comparative localization of two forms of glutamic acid decarboxylase and their mRNAs in rat brain supports the concept of functional differences between the forms. J Neurosci. 1994;14:1834–1855. doi: 10.1523/JNEUROSCI.14-03-01834.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fabri M, Manzoni T. Glutamic acid decarboxylase immunoreactivity in callosal projecting neurons of cat and rat somatic sensory areas. Neuroscience. 2004;123:557–566. doi: 10.1016/j.neuroscience.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 12.Grill HJ, Norgren R. The taste reactivity test. I. Mimetic responses to gustatory stimuli in neurologically normal rats. Brain Res. 1978;143:263–279. doi: 10.1016/0006-8993(78)90568-1. [DOI] [PubMed] [Google Scholar]
- 13.Grill HJ, Norgren R. The taste reactivity test. II. Mimetic responses to gustatory stimuli in chronic thalamic and chronic decerebrate rats. Brain Res. 1978;143:281–297. doi: 10.1016/0006-8993(78)90569-3. [DOI] [PubMed] [Google Scholar]
- 14.Grill HJ, Schulkin J, Flynn FW. Sodium homeostasis in chronic decerebrate rats. Behav Neurosci. 1986;100:536–543. doi: 10.1037//0735-7044.100.4.536. [DOI] [PubMed] [Google Scholar]
- 15.Hajnal A, Takenouchi K, Norgren R. Effect of intraduodenal lipid on parabrachial gustatory coding in awake rats. J Neurosci. 1999;19:7182–7190. doi: 10.1523/JNEUROSCI.19-16-07182.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holstege G, Meiners L, Tan K. Projections of the bed nucleus of the stria terminalis to the mesencephalon, pons, and medulla oblongata in the cat. Exp Brain Res. 1985;58:379–391. doi: 10.1007/BF00235319. [DOI] [PubMed] [Google Scholar]
- 17.Hopkins DA, Holstege G. Amygdaloid projections to the mesencephalon, pons and medulla oblongata in the cat. Exp Brain Res. 1978;32:529–547. doi: 10.1007/BF00239551. [DOI] [PubMed] [Google Scholar]
- 18.Jia HG, Zhang GY, Wan Q. A GABAergic projection from the central nucleus of the amygdala to the parabrachial nucleus: an ultrastructural study of anterograde tracing in combination with post-embedding immunocytochemistry in the rat. Neurosci Lett. 2005;382:153–157. doi: 10.1016/j.neulet.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 19.Kobashi M, Bradley RM. Effects of GABA on neurons of the gustatory and visceral zones of the parabrachial nucleus in rats. Brain Res. 1998;799:323–328. doi: 10.1016/s0006-8993(98)00480-6. [DOI] [PubMed] [Google Scholar]
- 20.Li CS, Cho YK. Efferent projection from the bed nucleus of the stria terminalis suppresses activity of taste-responsive neurons in the hamster parabrachial nuclei. Am J Physiol Regul Integr Comp Physiol. 2006;291:R914–R926. doi: 10.1152/ajpregu.00750.2005. [DOI] [PubMed] [Google Scholar]
- 21.Li CS, Cho YK, Smith DV. Taste responses of neurons in the hamster solitary nucleus are modulated by the central nucleus of the amygdala. J Neurophysiol. 2002;88:2979–2992. doi: 10.1152/jn.00239.2002. [DOI] [PubMed] [Google Scholar]
- 22.Li CS, Cho YK, Smith DV. Modulation of parabrachial taste neurons by electrical and chemical stimulation of the lateral hypothalamus and amygdala. J Neurophysiol. 2005;93:1183–1196. doi: 10.1152/jn.00828.2004. [DOI] [PubMed] [Google Scholar]
- 23.Lundy RF, Jr, Norgren R. Pontine gustatory activity is altered by electrical stimulation in the central nucleus of the amygdala. J Neurophysiol. 2001;85:770–783. doi: 10.1152/jn.2001.85.2.770. [DOI] [PubMed] [Google Scholar]
- 24.Lundy RF, Jr, Norgren R. Activity in the hypothalamus, amygdala, and cortex generates bilateral and convergent modulation of pontine gustatory neurons. J Neurophysiol. 2004;91:1143–1157. doi: 10.1152/jn.00840.2003. [DOI] [PubMed] [Google Scholar]
- 25.Mason GF, Martin DL, Martin SB, Manor D, Sibson NR, Patel A, Rothman DL, Behar KL. Decrease in GABA synthesis rate in rat cortex following GABA-transaminase inhibition correlates with the decrease in GAD(67) protein. Brain Res. 2001;914:81–91. doi: 10.1016/s0006-8993(01)02778-0. [DOI] [PubMed] [Google Scholar]
- 26.Moga MM, Gray TS. Evidence for corticotropin-releasing factor, neurotensin, and somatostatin in the neural pathway from the central nucleus of the amygdala to the parabrachial nucleus. J Comp Neurol. 1985;241:275–284. doi: 10.1002/cne.902410304. [DOI] [PubMed] [Google Scholar]
- 27.Moga MM, Herbert H, Hurley KM, Yasui Y, Gray TS, Saper CB. Organization of cortical, basal forebrain, and hypothalamic afferents to the parabrachial nucleus in the rat. J Comp Neurol. 1990;295:624–661. doi: 10.1002/cne.902950408. [DOI] [PubMed] [Google Scholar]
- 28.Moga MM, Saper CB, Gray TS. Bed nucleus of the stria terminalis: cytoarchitecture, immunohistochemistry, and projection to the parabrachial nucleus in the rat. J Comp Neurol. 1989;283:315–332. doi: 10.1002/cne.902830302. [DOI] [PubMed] [Google Scholar]
- 29.Nishijo H, Uwano T, Tamura R, Ono T. Gustatory and multimodal neuronal responses in the amygdala during licking and discrimination of sensory stimuli in awake rats. J Neurophysiol. 1998;79:21–36. doi: 10.1152/jn.1998.79.1.21. [DOI] [PubMed] [Google Scholar]
- 30.Norgren R. Taste pathways to hypothalamus and amygdala. J Comp Neurol. 1976;166:17–30. doi: 10.1002/cne.901660103. [DOI] [PubMed] [Google Scholar]
- 31.Norgren R, Hajnal A, Mungarndee SS. Gustatory reward and the nucleus accumbens. Physiol Behav. 2006;89:531–535. doi: 10.1016/j.physbeh.2006.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Sydney: Academic Press; 1998. [DOI] [PubMed] [Google Scholar]
- 33.Saper CB. Reciprocal parabrachial-cortical connections in the rat. Brain Res. 1982;242:33–40. doi: 10.1016/0006-8993(82)90493-0. [DOI] [PubMed] [Google Scholar]
- 34.Shimura T, Komori M, Yamamoto T. Acute sodium deficiency reduces gustatory responsiveness to NaCl in the parabrachial nucleus of rats. Neurosci Lett. 1997;236:33–36. doi: 10.1016/s0304-3940(97)00745-3. [DOI] [PubMed] [Google Scholar]
- 35.Shimura T, Tanaka H, Yamamoto T. Salient responsiveness of parabrachial neurons to the conditioned stimulus after the acquisition of taste aversion learning in rats. Neuroscience. 1997;81:239–247. doi: 10.1016/s0306-4522(97)00188-7. [DOI] [PubMed] [Google Scholar]
- 36.Singec I, Knoth R, Ditter M, Volk B, Frotscher M. Neurogranin is expressed by principal cells but not interneurons in the rodent and monkey neocortex and hippocampus. J Comp Neurol. 2004;479:30–42. doi: 10.1002/cne.20302. [DOI] [PubMed] [Google Scholar]
- 37.Smith DV, Li CS. GABA-mediated corticofugal inhibition of taste-responsive neurons in the nucleus of the solitary tract. Brain Res. 2000;858:408–415. doi: 10.1016/s0006-8993(99)02484-1. [DOI] [PubMed] [Google Scholar]
- 38.Smith DV, Ye MK, Li CS. Medullary taste responses are modulated by the bed nucleus of the stria terminalis. Chem Senses. 2005;30:421–434. doi: 10.1093/chemse/bji037. [DOI] [PubMed] [Google Scholar]
- 39.Sun N, Yi H, Cassell MD. Evidence for a GABAergic interface between cortical afferents and brainstem projection neurons in the rat central extended amygdala. J Comp Neurol. 1994;340:43–64. doi: 10.1002/cne.903400105. [DOI] [PubMed] [Google Scholar]
- 40.Sweatt AJ, Garcia-Espinosa MA, Wallin R, Hutson SM. Branched-chain amino acids and neurotransmitter metabolism: expression of cytosolic branched-chain aminotransferase (BCATc) in the cerebellum and hippocampus. J Comp Neurol. 2004;477:360–370. doi: 10.1002/cne.20200. [DOI] [PubMed] [Google Scholar]
- 41.Tanaka I, Ezure K, Kondo M. Distribution of glycine transporter 2 mRNA-containing neurons in relation to glutamic acid decarboxylase mRNA-containing neurons in rat medulla. Neurosci Res. 2003;47:139–151. doi: 10.1016/s0168-0102(03)00192-5. [DOI] [PubMed] [Google Scholar]
- 42.Tokita K, Karadi Z, Shimura T, Yamamoto T. Centrifugal inputs modulate taste aversion learning associated parabrachial neuronal activities. J Neurophysiol. 2004;92:265–279. doi: 10.1152/jn.01090.2003. [DOI] [PubMed] [Google Scholar]
- 43.Veening JG, Swanson LW, Sawchenko PE. The organization of projections from the central nucleus of the amygdala to brainstem sites involved in central autonomic regulation: a combined retrograde transport-immunohistochemical study. Brain Res. 1984;303:337–357. doi: 10.1016/0006-8993(84)91220-4. [DOI] [PubMed] [Google Scholar]