Abstract
Objective
We tested whether adding interpretive labels (e.g., “negative test”) to prenatal genetic screening test results changes perceived risk and preferences for amniocentesis.
Study Design
Women (N=1,688) completed a hypothetical pregnancy scenario via the Internet. We randomized participants into two groups: high (12.5/1000) risk of fetal chromosomal problems or low (2/1000) risk. After prenatal screening, estimated risk was identical (5/1000) for all participants, but results were provided either alone or with interpretive labels.
Results
When receiving test results without labels, all participants react similarly. With labels, participants receiving “positive” or “abnormal” results reported higher perceived risk (p<0.001), greater worry (p<0.001), and greater interest in amniocentesis (57% vs. 37%, p<0.001) than those receiving “negative” or “normal” results.
Conclusions
Interpretive labels for test results can induce larger changes to women’s risk perceptions and behavioral intentions than numerical results alone do, creating decision momentum. This finding has broad clinical implications for patient-provider communication.
Keywords: decision making, risk communication, prenatal screening, amniocentesis
INTRODUCTION
Expectant couples face difficult decisions regarding prenatal screening for fetal chromosomal problems. Research into the decision-making processes of parents-to-be regarding such tests is essential to facilitating informed choices. For example, research has assessed both how prenatal screening tests are presented to patients and how they are discussed by health care providers.[1, 2] Research has also considered women’s attitudes regarding testing and evaluated the degree to which women’s decisions whether to have screening tests are informed and consistent with their own preferences.[3–7]. Beyond these considerations, however, it is important to determine whether women are able, once a screening test is performed, to understand the test results and incorporate that information into their decision making about more invasive diagnostic tests such as amniocentesis.
Clinicians face a dilemma when considering how to present the results of prenatal screening tests to patients. On the one hand, clinicians can introduce test results using labels such as “negative” or “positive” to categorize the outcome. “Negative” results emphasize (albeit somewhat counter-intuitively) that the test represents good news (i.e., a final risk estimate that is lower than either the a priori risk or a pre-defined threshold), while a “positive” result implies bad news. In such discussions, the absolute magnitude of the risk may be discussed, but only secondarily. Alternately, clinicians can discuss test results without interpretive labels, instead emphasizing quantitative risk estimates. Doing so stresses the size of the final risk estimate while minimizing whether it is higher or lower than either initial or threshold risk levels.
In this study, we tested whether introducing screening test results with interpretive labels influences perceptions of fetal risk and/or interest in amniocentesis. To do so while controlling for variations in individual family risk factors, we used a randomized controlled trial (RCT) design to systematically vary how test results were described in a short vignette presented to women participating in a larger Internet-administered survey on preferences regarding children and birthing.
MATERIAL AND METHODS
Overview of Study Design
Each participant was asked to imagine being 4 months pregnant and speaking with her physician regarding prenatal screening tests for fetal chromosomal problems. We randomly varied both initial risk status and the format of the hypothetical test results and then assessed participants’ risk perceptions and behavioral intentions. This design received Institutional Review Board exempt status approval as anonymous survey research.
Participants
Study participants were women 18–50 years old who were drawn from a panel of Internet users administered by Survey Sampling International (SSI) and who voluntarily agreed to receive invitations to fill out questionnaires. Email invitations were sent to a stratified random sample of panel members with the goal of approximating the U.S. census on education level, race, and income in the final subject pool. To ensure demographic diversity (but not representativeness) and offset large expected variations in response rates (especially for African-Americans and Hispanic-Americans), we established target response levels for each racial/ethnic group. We also drew three distinct age samples within each race (one-third ages 18–30, one-third ages 30–39, and one-third ages 40–50) to offset lower response rates from younger sample members. The number of email invitations in each demographic sub-sample was dynamically adjusted until all quotas were achieved. Upon completion, participants were entered into a drawing administered by SSI for cash prizes totaling $10,000.
Intervention
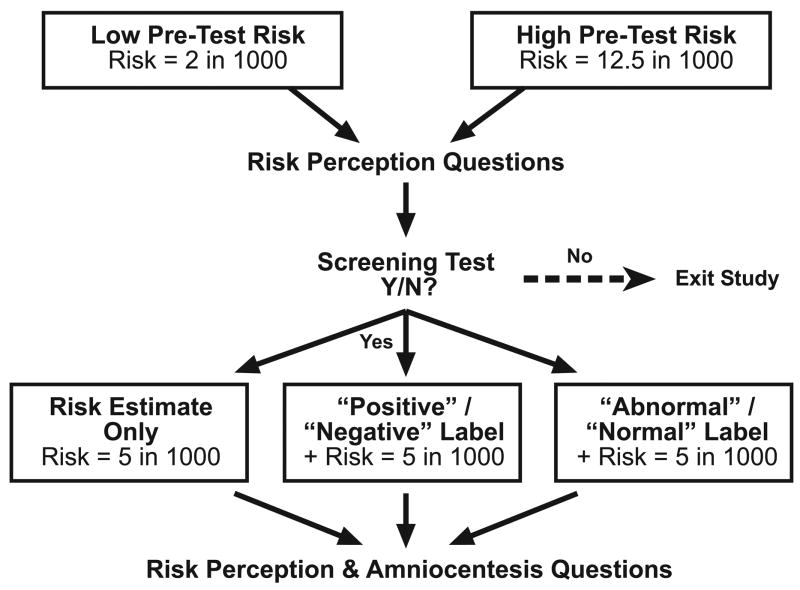
Our scenario was designed so that the estimated risk of fetal chromosomal problems, based on the results of the screening test, was identical for all study participants. However, we used a 2 × 3 factorial RCT design (Figure 1) that varied both the pre-test risk and whether or not the discussion of the test result included labels.
Figure 1.
Experimental design
At the start of the hypothetical scenario, participants were asked to imagine that they and their partner were discussing prenatal screening tests with their health care provider. Each participant was then told that, based on their age and family history, the risk of chromosomal problems with the fetus was either “high” (defined as 12.5 out of 1000) or “low” (defined as 2 out of 1000).
The scenario then described a blood test that could help to clarify the risk of chromosomal problems. Participants rated their pre-test perceptions of the risk of chromosomal problems and their interest in having the blood test. All participants who indicated a desire to take the blood test, regardless of initial risk status, were then informed that the test had been performed and that it indicated an overall risk of 5 in 1000. Thus, all “low risk” women received test results showing increased risk, while all “high risk” women received results showing decreased risk. We used a 5 in 1000 risk because it equals the approximate risk of miscarriage from amniocentesis (as discussed later in our scenario) and because it is often used as the threshold for separating “screen negative” from “screen positive” results. We wanted to see whether other factors, such as the addition of interpretive labels, could bias women’s responses to risk estimates that are right at the tipping point for decision making.
The main experimental manipulation involved varying the format used to present these test results. One-third of our participants were simply informed that the test results indicated a 5 in 1000 risk of fetal chromosomal problems. A second group, however, had their test results preceded by an interpretive label. These participants were first told that the test had come back “positive” (or “negative”) and that this meant that they were at increased (decreased) risk of fetal chromosomal problems. Only on the next page of the scenario were they then provided with the 5 in 1000 post-test risk estimate. To extend our investigation to other types of interpretive labels, a third group received a similarly structured presentation that substituted the labels “abnormal” and “normal” for “positive” and “negative.” All groups then read information about amniocentesis, including the fact that the procedure carries a 5 in 1000 risk of miscarriage. Participants then completed a series of outcome measures and demographic questions. As part of the larger birthing survey, participants also reported whether they had ever given birth or undergone a triple- or quad-screen test before.
We presented all risk estimates as proportions with a denominator of 1000 because research on individual numeracy shows that many people, even those with substantial education, misinterpret risk statistics that utilize varying denominators (e.g., believing a 1 in 384 risk as larger than 1 in 112 risk).[8, 9] Furthermore, using the larger 1000 person denominator minimized the number of fractional numerators that our participants had to interpret.
Pre-Test Measures
We measured pre-test risk perceptions using two questions: “How likely is your fetus to have a chromosome problem?” and “How worried would you be about the risk of fetal chromosome problems?” Participants indicated their responses on 7-point scales, where 0 meant “not at all likely (worried)” and 6 meant “extremely likely (worried).” We then assessed interest in the screening test using a four point scale, with responses defined as “Definitely No,” “Probably No,” “Probably Yes,” and “Definitely Yes.” We dichotomized this variable, with participants answering “No” skipping to the end of the survey and those answering “Yes” continuing on.
Post-Test Measures
After participants read the hypothetical test results, we repeated our measures of risk perceptions and then asked a series of questions about attitudes towards amniocentesis. One question asked the women whether they thought they would want to have amniocentesis done (again using a four-point scale), while a second asked participants to rate “How good of a choice does having amniocentesis seem to you” on another 7-point scale from “a not at all a good choice” to “an extremely good choice.”
Hypotheses
We expected that our manipulation of initial risk status would influence risk perceptions before the screening test. Thus, we predicted that women who were told that they had a “high” risk of 12.5 in 1000 would have greater perceived likelihood of chromosomal problems, greater worry about chromosomal problems, and greater interest in undergoing the blood screening test than women who were told a “low” risk of 2 in 1000.
As noted above, all participants who agreed to undergo the hypothetical blood screening test received identical post-test estimates of the risk of fetal problems: 5 in 1000. We hypothesized that all participants would change their beliefs in response to this new information, with members of the low initial risk group showing increased perceived risk and worry and the high initial risk group showing the opposite pattern. Predicting the magnitude of change, however, is complicated. Rationally, post-test outcome measures for all subjects should converge towards the same point, but “anchoring” effects[10] could reduce participants’ responsiveness to the change in risk estimates.
In our conditions in which post-test risk estimates were introduced by interpretive labels such as “normal” or “positive,” we hypothesized that this small addition would nonetheless be sufficient to amplify any observed changes in women’s beliefs and behavioral intentions. Because inclusion of labels tends to emphasize the good or bad nature of the test outcome, we thought that such presentations could lead some women to make decisions based on whether the test shows an improvement or decrement in risk, rather than on the absolute risk level that is more central to informed decision making. We therefore hypothesized that formerly “low” risk women receiving “positive” or “abnormal” results would, on average, perceive more risk of fetal chromosomal problems, be more worried, and be more interested in amniocentesis than formerly “high” risk women receiving “negative” or “normal” results, even though the true post-test risk was the same for all.
Statistical Analysis
We utilized chi-square tests of proportions to test whether test result format or initial risk status affected willingness to undergo either the blood screening test or amniocentesis. We also used t-tests to compare pre- and post-test risk perceptions, as well as our attitudinal measures, across conditions. We also performed subgroup analyses to assess the influence of demographic and experience variables on the observed effects. All analyses were performed using STATA 8.
RESULTS
A total of 1,785 women reached the survey website and viewed the first content page. Of these, 10 were excluded for reporting ages outside of the requested sample range and 87 failed to complete the relevant sections of the survey. Our analyses focus on the remaining 1,688 participants (94.5%).
Sample mean age was 35 (range 18–50) and, of the 1,627 who reported racial and/or ethnic background information, 84% described themselves as Caucasian, 14% African-American and 13% Hispanic (any race). We observed a wide range of educational achievement, with 29% having completed a Bachelor’s or higher college degree but also 20% with only a High School education or less. Most (64%) reported being parous, and 25% reported prior experience with prenatal triple- or quad-screen tests. As expected given our RCT design, there were no significant variations in sample demographics across the experimental conditions.
Pre-Test Risk Perceptions
Our measures of pre-test risk perception showed that our manipulation of pre-test risk worked as planned: Women who were initially randomized to the high risk condition perceived significantly higher risk of chromosomal problems than those randomized to the low risk condition (M=2.53 vs. 1.29, t=17.37, p<0.001). They also worried more about fetal chromosomal problems (M=3.43 vs. 2.14, t=13.82, p<0.001) and were more interested in the blood screening test (84.7% vs. 80.3%, χ2(1)=5.61, p=0.018).
Post-Test Risk Perceptions
A total of 1,387 women (82.5%) indicated interest in the screening test, continued in the survey, and viewed test results indicating a 5 in 1000 risk of fetal problems. Analyses showed no significant differences among respondents who received the surveys with “positive/negative” labels versus those with “abnormal/normal” labels on any of the post-test outcome measures (all p’s>0.23). We have therefore collapsed these groups, and all subsequent analyses simply compare label versus no-label conditions.
Viewing the test results did change women’s perceived risk from their pre-test levels in all groups. For example, among women who received test results without labels, worry increased from 2.16 to 3.01 (t=5.30, p<0.001) for women in the low initial risk group while decreasing from 3.33 to 3.07 (t=−1.42, p=0.16) among women in the high initial risk group.
More importantly, however, our results show a distinct interaction between our labeling and pre-test risk manipulations. As shown in Table I, when women receive risk estimates without any type of label, their reaction to this information is not significantly influenced by whether the test results raised or lowered their risks. Women initially told that they were in the low risk group had very similar post-test risk perceptions as those previously told that they were in the high risk group.
Table I.
Perceptions and beliefs of women receiving numerical risk estimates without interpretive labels
| Pre-Test Risk | |||
|---|---|---|---|
| Low | High | p-value | |
| Perceived Likelihood of Fetal Chromosomal Problems | 1.98 (1.22) | 2.08 (1.50) | 0.44 |
| Worry about Risk of Fetal Chromosomal Problems | 3.01 (1.81) | 3.07 (1.88) | 0.74 |
| Rating of Amniocentesis As a “Good Choice” | 3.08 (2.01) | 3.07 (2.05) | 0.95 |
Notes: Table reports mean (s.d.) ratings on 7-point (0–6) scales and the significance of associated t-tests. Final risk estimates after testing were identical (5 in 1000) in all conditions.
By contrast, when the test results were accompanied by an interpretive label, women’s reactions were magnified and significantly influenced by whether the test results raised or lowered their risks. As shown in Table II, women in the low pre-test risk group who received test results described as “positive” or “abnormal” perceived a significantly higher likelihood of fetal chromosomal problems and were significantly more worried than women in the high pre-test risk group who received test results labeled as either “negative” or “normal.” In other words, our participants responded differently to the 5 in 1000 risk when that risk information was accompanied by one of the labels. In fact, we observe significant differences among participants in all three age groups and regardless of prior birth or prenatal testing experience.
Table II.
Perceptions and beliefs of women receiving risk estimates introduced by interpretive labels
| Pre-Test Risk | |||
|---|---|---|---|
| Low | High | p-value | |
| Perceived Likelihood of Fetal Chromosomal Problems | 2.38 (1.35) | 1.81 (1.34) | <0.001 |
| Worry about Risk of Fetal Chromosomal Problems | 3.43 (1.68) | 2.80 (1.89) | <0.001 |
| Rating of Amniocentesis As a “Good Choice” | 3.39 (1.93) | 2.66 (2.01) | <0.001 |
Notes: Table reports mean (s.d.) ratings on 7-point (0–6) scales and the significance of associated t-tests. Final risk estimates after testing were identical (5 in 1000) in all conditions. Test results were also described as either “positive” or “abnormal” when pre-test risk was low and “negative” or “normal” when pre-test risk was high.
Impact on Amniocentesis Decisions
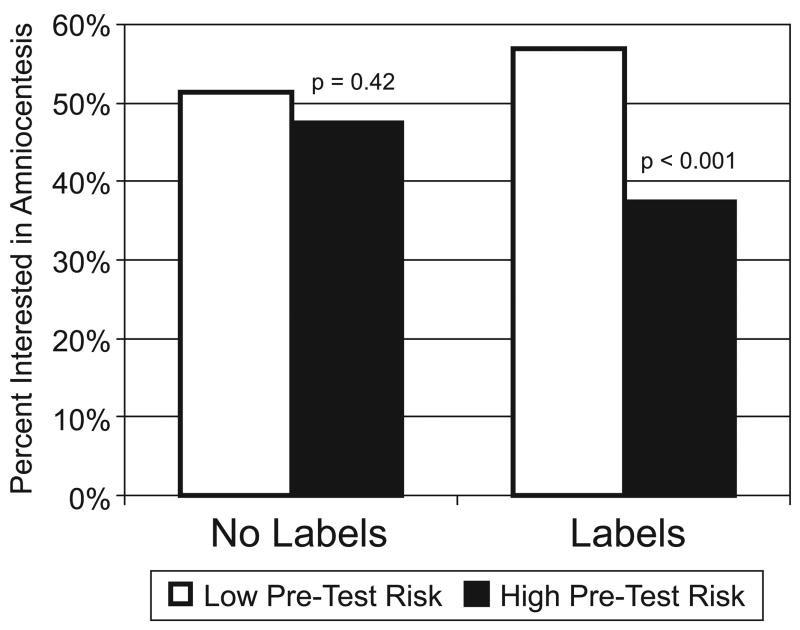
The effect of adding interpretive labels also influenced our participants’ behavioral intentions. In the no-label condition, pre-test risk status had no effect on either intention to have amniocentesis done (Figure 2, left side) or ratings of amniocentesis as a good choice (Table I). Adding interpretive labels, however, led to significant differences in both the percentage of women suggesting that they would pursue amniocentesis (Figure 2, right side) and their assessment of the quality of that option (Table II). Similar significant patterns were observed in subgroup analyses of nulliparous women versus parous women, as well as among those with and without prior prenatal testing experience. Participant age did mediate the effect to some extent, with the strongest effect of labels observed among women 30–39 years old.
Figure 2.
Effect of interpretive labels on interest in amniocentesis
COMMENT
In purely rational terms, decisions regarding invasive tests such as amniocentesis should be based on the estimated risk of fetal problems generated from the screening test and each couple’s personal preferences. While research suggests that women can effectively incorporate such risk information into their decision making,[11] decisions regarding amniocentesis are influenced by the a priori risk level,[12] and do not appear to always correlate clinically with the test results received.[13, 14] Of course, personal beliefs also influence decisions about invasive testing, including confidence that the results would not change behavior, mistaken beliefs that “negative” screening test results imply no risk,[15, 16] and failures of the screening test to reassure.[15]
In addition to these factors, however, the format used to present screening test results to women appears to directly impact decision making. While earlier research suggested that presenting risk estimates in numerical format increased comprehension,[17] our results suggest that the seemingly innocuous practice of labeling a screening test result as “negative/positive” or “normal/abnormal” can influence decision making even when numerical risk information is also available. In our hypothetical scenario, the post-test risk of fetal chromosomal problems was identical for all participants, and the risk perceptions and behavioral intentions of women who received their test results without interpretive labels appropriately reflected that fact. Yet, labeling the same test results as “positive” or “abnormal” led women to perceive more risk and have greater interest in amniocentesis than women receiving results labeled “negative” or “normal.” These study participants displayed a type of decision momentum, making subsequent decisions based more on whether the risk had increased or decreased than on its absolute level.
Our findings are consistent with recent research showing that people’s intuitive perceptions of risk may differ from their objective beliefs about the likelihood of occurrence.[18] For example, people shown the numerical chance that a surgery would be successful were more optimistic when the risk estimate was explained as the result of risk-decreasing reasons (e.g., a simple surgery) versus risk-increasing reasons (e.g., poor blood flow).[19] Like reasons, interpretive labels carry many meaning-laden associations that may bias reactions to quantitative risk information. There is also growing evidence that emotional responses to risk information may mediate cognitive risk perceptions, shape behavior independently, or both.[20–22]
Although our results are based on a hypothetical scenario, they suggest that obstetricians and genetic counselors should be cautious about providing verbal interpretations of prenatal screening test results to patients. Helping couples understand their test results is a challenging task, and it is only natural to want to facilitate comprehension by verbally interpreting the meaning of quantitative data for patients. Labels such as “normal” or “positive” are easy to grasp, and it seems counter-intuitive that using such terms could do anything but help. Yet, informed decision making about prenatal screening requires thoughtful consideration of personal values, possible outcomes, and risk-benefit tradeoffs.[2, 7, 16, 23] Interpretive labels, however, are directive, especially when provided by “expert” medical professionals. They encourage binary categorization of outcomes as either good or bad while inhibiting patients’ ability to integrate the test results within the context of their personal preferences. Over- and under-use of follow-up testing may then result.
Our research has several limitations. First, even though we achieved significant demographic diversity, our survey used an Internet sample which may be non-representative in unidentified ways (e.g, oversampling people who like taking surveys). However, we never intended to achieve a truly representative sample, opting instead to focus on ensuring internal validity by using an RCT design. Furthermore, our previous research using this panel has shown that Internet survey responses closely match those of representative samples.[24] Second, our scenario was entirely hypothetical, and the reactions of actual mothers-to-be may differ. We do not claim that our estimates of perceived risk and intentions to pursue amniocentesis correspond to those of patient populations. In fact, the hypothetical nature of our task may explain why participants’ risk perceptions reacted strongly to the test results even in the absence of interpretive labels, in contrast to prior research showing strong effects of the a priori risk on post-test behavior.[12] Nevertheless, our experimental results suggest that interpretive labels amplify reactions to test results, changing both beliefs and behavioral intentions. In fact, the emotionally-charged nature of discussions of actual screening tests may make real mothers-to-be even more sensitive to interpretive labels than our survey participants were.
While this study focused on prenatal screening, our findings have broad clinical implications for how test results of all types should be discussed with patients. If people automatically translate quantitative test results into simpler “gist” interpretations, then supplementing test reports with interpretive labels may be counterproductive. Adding labels induces people to think in terms of broad categories at times when more detailed consideration of the specific test results may be what is required for informed decision making.
Acknowledgments
The authors wish to acknowledge Jonathan Kulpa for his research assistance and Miriam Kuppermann and two anonymous reviewers for helpful comments on an earlier version of this manuscript.
Financial support for this study was provided by the National Institutes for Health (R01 CA87595 and P50 CA101451). Dr. Zikmund-Fisher is supported by a career development award from the American Cancer Society (MRSG-06-130-01-CPPB) and Dr. Fagerlin is supported by an MREP early career award from the Department of Veterans Affairs. The funding agreements ensured the authors’ independence in designing the study, interpreting the data, and publishing the report.
Footnotes
This work was presented at the annual meeting of the Society for Medical Decision Making, October 16, 2006.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dormandy E, Michie S, Weinman J, Marteau TM. Variation in uptake of serum screening: the role of service delivery. Prenat Diagn. 2002 Jan;22(1):67–9. doi: 10.1002/pd.245. [DOI] [PubMed] [Google Scholar]
- 2.Gekas J, Gondry J, Mazur S, Cesbron P, Thepot F. Informed consent to serum screening for Down syndrome: are women given adequate information? Prenat Diagn. 1999 Jan;19(1):1–7. doi: 10.1002/(sici)1097-0223(199901)19:1<1::aid-pd456>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 3.Dormandy E, Michie S, Hooper R, Marteau TM. Informed choice in antenatal Down syndrome screening: A cluster-randomised trial of combined versus separate visit testing. Patient Educ Couns. 2006;61:56–64. doi: 10.1016/j.pec.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 4.van den Berg M, Timmermans DRM, ten Kate LP, van Vugt JMG, van der Wal G. Informed decision making in the context of prenatal screening. Patient Educ Couns. 2006;63:110–7. doi: 10.1016/j.pec.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 5.Kuppermann M, Learman LA, Gates E, Gregorich SE, Nease RF, Jr, Lewis J, et al. Beyond race or ethnicity and socioeconomic status: Predictors of prenatal testing for Down syndrome. Obstet Gynecol. 2006;107(5):1087–97. doi: 10.1097/01.AOG.0000214953.90248.db. [DOI] [PubMed] [Google Scholar]
- 6.Dormandy E, Michie S, Hooper R, Marteau TM. Low uptake of prenatal screening for Down syndrome in minority ethnic groups and socially deprived groups: a reflection of women’s attitudes or a failure to facilitate informed choices? Int J Epidemiol. 2005;34:346–52. doi: 10.1093/ije/dyi021. [DOI] [PubMed] [Google Scholar]
- 7.Marteau TM, Slack J, Kidd J, Shaw RW. Presenting a routine screening test in antenatal care: practice observed. Public Health. 1992 Mar;106(2):131–41. doi: 10.1016/s0033-3506(05)80390-7. [DOI] [PubMed] [Google Scholar]
- 8.Grimes DA, Snively GR. Patients’ understanding of medical risks: Implications for genetic counseling. Obstet Gynecol. 1999;93:910–4. doi: 10.1016/s0029-7844(98)00567-5. [DOI] [PubMed] [Google Scholar]
- 9.Lipkus IM, Samsa G, Rimer BK. General performance on a numeracy scale among highly educated samples. Med Decis Making. 2001;21(1):37–44. doi: 10.1177/0272989X0102100105. [DOI] [PubMed] [Google Scholar]
- 10.Tversky A, Kahneman D. Judgment under uncertainty: Heuristics and biases. Science. 1974;185:1124–31. doi: 10.1126/science.185.4157.1124. [DOI] [PubMed] [Google Scholar]
- 11.Nicolaides KH, Chervenak FA, McCullough LB, Avgidou K, Papageorghiou A. Evidence-based obstetric ethics and informed decision-making by pregnant women about invasive diagnosis after first-trimester assessment of risk for trisomy 21. Am J Obstet Gynecol. 2005 Aug;193(2):322–6. doi: 10.1016/j.ajog.2005.02.134. [DOI] [PubMed] [Google Scholar]
- 12.Beekhuis JR, De Wolf BT, Mantingh A, Heringa MP. The influence of serum screening on the amniocentesis rate in women of advanced maternal age. Prenat Diagn. 1994 Mar;14(3):199–202. doi: 10.1002/pd.1970140310. [DOI] [PubMed] [Google Scholar]
- 13.Mueller V, Huang T, Summers A, Winsor S. The influence of risk estimates obtained from maternal serum screening on amniocentesis rates. Prenat Diagn. 2005;25:1253–7. doi: 10.1002/pd.1321. [DOI] [PubMed] [Google Scholar]
- 14.Marini T, Sullivan J, Naeem R. Decisions about amniocentesis by advanced maternal age patients following maternal serum screening may not always correlate clinically with screening results: need for improvement in informed consent process. Am J Med Genet. 2002 May 1;109(3):171–5. doi: 10.1002/ajmg.10319. [DOI] [PubMed] [Google Scholar]
- 15.Michie S, Thompson M, Hankins M. To be reassured or to understand? A dilemma in communicating normal cervical screening results. Br J Health Psychol. 2004 Feb;9(Pt 1):113–23. doi: 10.1348/135910704322778768. [DOI] [PubMed] [Google Scholar]
- 16.Smith DK, Shaw RW, Marteau TM. Informed consent to undergo serum screening for Down’s syndrome: the gap between policy and practice. Br Med J. 1994 Sep 24;309(6957):776. doi: 10.1136/bmj.309.6957.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marteau TM, Saidi G, Goodburn S, Lawton J, Michie S, Bobrow M. Numbers or words? A randomized controlled trial of presenting screen negative results to pregnant women. Prenat Diagn. 2000 Sep;20(9):714–8. doi: 10.1002/1097-0223(200009)20:9<714::aid-pd906>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 18.Windschitl PD, Wells GL. Measuring psychological uncertainty: Verbal versus numeric methods. J Exp Psychol Appl. 1996;2(4):343–64. [Google Scholar]
- 19.Flugstad AR, Windschitl PD. The influence of reasons on interpretations of probability forecasts. J Behav Decis Making. 2003;16:107–26. [Google Scholar]
- 20.Loewenstein GF, Weber EU, Hsee CK, Welch N. Risk as feelings. Psychol Bull. 2001;127(2):267–86. doi: 10.1037/0033-2909.127.2.267. [DOI] [PubMed] [Google Scholar]
- 21.Finucane ML, Alhakami A, Slovic P, Johnson SM. The affect of heuristic judgments of risks and benefits. J Behav Decis Making. 2000;13:1–17. [Google Scholar]
- 22.Peters E, Vastfjall D, Slovic P, Mertz CK, Mazzocco K, Dickert S. Numeracy and decision making. Psychol Sci. 2006;17(5):407–13. doi: 10.1111/j.1467-9280.2006.01720.x. [DOI] [PubMed] [Google Scholar]
- 23.Smith DK, Slack J, Shaw RW, Marteau TM. Lack of knowledge in health professionals: a barrier to providing information to patients? Qual Health Care. 1994 Jun;3(2):75–8. doi: 10.1136/qshc.3.2.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lacey HP, Smith DM, Ubel PA. Hope I die before I get old: Mispredicting happiness across the lifespan. J Happiness Stud. 2006;7(2):167–82. [Google Scholar]