Abstract
Production of IL-10, a major immunoregulatory cytokine, by phagocytes during clearance of apoptotic cells is critical to ensuring cellular homeostasis and suppression of autoimmunity. Little is known about the regulatory mechanisms in this fundamental process. We report that IL-10 production stimulated by apoptotic cells is regulated at the level of transcription in a manner dependent on the p38 mitogen-activated protein kinase, partially on the scavenger receptor CD36, and requires cell-cell contact but not phagocytosis. Using a reporter assay, we mapped the Apoptotic Cell Response Element (ACRE) in the human IL-10 promoter, and provide biochemical and physiological evidence that ACRE mediates the transcriptional activation of IL-10 by pre-B-cell leukemia transcription factor-1b and another Hox cofactor Pbx-regulating protein 1 in response to apoptotic cells.
Introduction
In multicelluar organisms, large numbers of apoptotic cells are generated during development, tissue remodeling and inflammation (Aderem and Underhill, 1999). The resolution of inflammation is a dynamically regulated process that involves the suppression of pro-inflammatory gene expression, leukocyte migration and activation followed by clearance of apoptotic cells by phagocytosis. Professional phagocytes such as monocytes, macrophages and neutrophils efficiently phagocytose apoptotic cells to prevent inflammation.
The major difference with respect to phagocytic capacity and efficiency of professional and non-professional phagocytes is the number of different phagocytic receptors present on professional phagocytes (Aderem and Underhill, 1999). Macrophages, in particular, contain a myriad of phagocytic receptors that interact with apoptotic cells. This includes complement receptors, Fc receptors, integins (αvβ3, αvβ5), scavenger receptors (SRA, CD36, CD14, LOX-1) and the presumptive phosphatidylserine receptor (PSR) (Stuart and Ezekowitz, 2005). These receptors interact with their ligands on the surface of the apoptotic cells directly or via bridging proteins. Interestingly, macrophages use the same receptors to recognize both apoptotic cells and pathogens (Stuart and Ezekowitz, 2005). An inflammatory response occurs when macrophages phagocytose pathogens. In contrast, macrophage recognition of apoptotic cells triggers an anti-inflammatory response, which is mediated by the release of IL-10, TGF-β, platelet activating factor (PAF), and prostaglandin E2 (PGE2) with concurrent inhibition of TNFα, IL-12, IL-1β and IL-8 (Voll et al., 1997b). Furthermore, it has been recently demonstrated that the profound suppression of LPS-driven TNF-α release by macrophages requires contact-dependent licensing of phagocytic cell responsiveness to TGF-β by apoptotic cells (Lucas et al., 2006). Both the removal of apoptotic cells and the active suppression of inflammatory cytokines are required to prevent chronic inflammation and autoimmune disorders such as systemic lupus erythematosus (SLE) (Casciola-Rosen et al., 1996; Herrmann et al., 1998; Walport and Lachmann, 1990), retinitis pigmentosa (Camenisch et al., 1999; Gal et al., 2000), cystic fibrosis (Camenisch et al., 1999; Gal et al., 2000).
IL-10 is an important immunoregulatory cytokine originally discovered as a product of Th2 cells to suppress cytokine production by Th1 cells (Fiorentino et al., 1989; Moore et al., 1990). B cells, mast cells, and macrophages also produce IL-10 (de Waal Malefyt et al., 1991a; Hsu et al., 1992). Studies of numerous inflammatory disease models including chronic enterocholitis, cutaneous inflammatory condition, endotoxic shock and Shwartzman reaction, and autoimmune encephalomyelitis in IL-10-deficient mice provided strong indications that IL-10 plays a central role in vivo in restricting inflammatory responses (Berg et al., 1995a; Berg et al., 1995b; Bettelli et al., 1998; Fuss et al., 2002; Kuhn et al., 1993).
IL-10 gene expression in macrophages is usually triggered by the same typical inflammatory stimuli such as lipopolysaccharides (LPS) that induce the release of proinflammatory cytokines. However, the kinetics of its induction differs from those of the proinflammatory mediators (de Waal Malefyt et al., 1991b; de Waal Malefyt et al., 1991c; Yssel et al., 1992). LPS-induced IL-10 production is dependent on the signaling cascade of p38 not p42 (also called extracellular signal regulated kinase 1 or ERK1) mitogen-activated protein kinase (MAPK) (Foey et al., 1998). In contrast, zymosan, a stimulus for TLR2 and dectin-1, induces dendritic cells (DC) to secrete abundant IL-10 but little IL-6 and IL-12 in a mechanism dependent on TLR2- and dectin-1-mediated activation of ERK (Dillon et al., 2006). In macrophages, the activation of ERK following FcγR ligation by immune complexes leads to a remodeling of the chromatin at the il-10 locus, making it more accessible to transcription factors (Lucas et al., 2005). Recent molecular analyses of the murine IL-10 promoter show that IL-10 transcription in macrophage cell types can be regulated by constitutive and ubiquitous transcription factors such as Sp1 and Sp3, suggesting that IL-10 may be produced at low levels constitutively to maintain certain level of control over “baseline” inflammation (Brightbill et al., 2000; Tone et al., 2000). A critical role for Stat3 but not other Stat proteins in LPS-induced IL-10 transcription in a human B cell line was reported by Benkhart and colleagues who demonstrated a direct interaction of Stat3 with the human IL-10 promoter at -120 (Benkhart et al., 2000b). The role of Stat3 as the mediator of autoinduction of IL-10 by IL-10 is also corroborated in human monocyte derived macrophages (Staples et al., 2007).
Voll et al. first observed increased IL-10 and decreased pro-inflammatory cytokines (TNF-α, IL-1β and IL-12) in LPS-activated human peripheral blood mononuclear cells (PBMCs) after exposure to apoptotic peripheral blood lymphocytes (PBL) (Voll et al., 1997a). Byrne et al. observed increased IL-10 and TGF-β production along with decreased TNF-α levels from human monocytes upon simultaneous treatment with LPS and apoptotic human neutrophils (Byrne and Reen, 2002).
Our group previously demonstrated that during phagocytosis of apoptotic T cells, macrophages display profound incapacity to produce IL-12. Furthermore, apoptotic cell-derived signals trigger dephosphorylation and activation of a novel nuclear zinc finger-like protein, named GC-binding protein (GC-BP), which targets a specific site in the IL-12 p35 gene promoter, preventing its transcription (Kim et al., 2004). The objective of the present study was to elucidate the molecular mechanisms involved in the regulation of IL-10 gene expression in macrophages upon interaction with apoptotic cells.
Results
Macrophages produce large amounts of IL-10 in response to apoptotic cells
In this study, we first demonstrated direct induction of IL-10 in murine peritoneal macrophages and human monocyte-derived macrophages (HMDM) following exposure to apoptotic cells. Apoptotsis of Jurkat T cells and autologous T lymphocytes was induced with staurosporine, a protein kinase C (PKC) inhibitor (Tamaoki et al., 1986). Compared to the live cells (Fig 1a, panel 1), staurosporine induced increasing amounts of early apoptosis (Annexin V+, PI-) up to six hours (panels 2 and 3) while extended incubation resulted in “secondary necrosis” marked by the reduction in the Annexin V+/PI- population and increase in Annexin V+/PI+, and Annexin V-/PI+ cells (panel 4). We chose to use the 6h-treated cells for all subsequent experiments.
Figure 1. Macrophages produce IL-10 in response to apoptotic cells.
(a) Apoptosis of Jurkat cells was induced 0, 3, 6, and 12 hrs. The percentage of early and late-apoptotic cells was quantified by flow cytometry analysis using Annexin V and propidium iodide (PI) staining. The numbers indicate percentages of the subpopulations.
(b-d) 0.5 × 105 thioglycollate-elicited peritoneal macrophages (c and e) or HMDM (b), or RAW264.7 cells (d) were stimulated with LPS (0.5μg/ml), apoptotic Jurkat cells, autologous apoptotic CD4+ T cells (ac), live cells (lc), or necrotic cells (nc), (2:1 ratio of ac, lc or nc/macrophages) for indicated times, and analyzed for IL-10 production.
(e) BALB/c mice (3 per group) were injected i.v. with apoptotic or necrotic Jurkat cells at doses of 2×106 and 106 cells/mouse. Blood were collected and sera obtained 4 and 8 hr following the injections. Serum IL-10 levels were measured by ELISA.
JKT ac, apoptotic Jurkat T cells (used in b-e); autologous ac, apoptotic splenic CD4+ T cell (b) or purified human blood CD4+ T cells (c). All data are presented as mean ± SD from a minimum of three individual donors.
We compared the kinetics of apoptotic cell versus LPS-induced IL-10 protein production from HMDM (Fig 1b). The level of LPS-induced IL-10 from HMDM was significantly greater compared to apoptotic T cell- or autologous T cell-induced IL-10. Apoptotic Jurkat T cells were able to induce IL-10 production by 4 hr post stimulation, and this level of cytokine production increased significantly from 8 hr to 24 hr post stimulation. Autologous T cells induced significantly less but similar pattern of IL-10 protein production as apoptotic Jurkat T cells. We also compared the kinetics of apoptotic cell versus LPS-induced IL-10 production from murine peritoneal macrophages (Fig 1c). Unstimulated macrophages produced little IL-10 at all time points. LPS induced IL-10 production gradually from 4 to 48 hr post stimulation. Apoptotic Jurkat T cells were able to induce significant levels of IL-10 production by 4 hr and peaked at 8 hr following stimulation. Autologous apoptotic splenic CD4+ T cells induced less IL-10 production compared with apoptotic Jurkat T cells on a per cell basis but the pattern of induction was very similar.
Since the apoptotic cell population were undergoing early apoptosis and contained ∼25% live cells and some necrotic cells (Fig 1a), they could also have an impact on IL-10 production when macrophages were exposed to a mixture of these cells. Thus, we assessed the effect of live and necrotic cells in RAW264.7 mouse macrophage-like cell line and murine peritoneal macrophages. No IL-10 was produced when peritoneal macrophages were stimulated with live or necrotic cells derived from Jurkat T cells or splenic CD4+ T cells (data not shown). In RAW264.7 cells, live or necrotic Jurkat T cells induced much less IL-10, compared to apoptotic cells (Fig 1d). The low levels of IL-10 induced by live cells could be due to the presence of small amounts of apoptotic cells (∼7%) in the “live” cell population (Fig 1a). Apoptotic or live Jurkat T cells by themselves did not induce any detectable levels of IL-10 (Fig 1d).
To determine the IL-10-inducing effect of apoptotic cells in vivo we injected i.v. apoptotic or necrotic Jurkat cells in two doses into mice, and measured IL-10 levels in the serum 4 or 8 hrs later. As shown in Fig 1e, apoptotic cells induced a potent systemic IL-10 response dose-dependently, much higher than that by necrotic cells. The level at 8 hrs post injection was also more than twice higher than that at 4 hrs similar to the in vitro patterns. This result demonstrates directly the physiological response of apoptotic cells in vivo.
Subsequently, we determined that cell-cell contact between apoptotic cells and macrophages, but not phagocytosis, is required to induce IL-10 production (Supplemental Data, Fig S1), and that apoptotic cells regulate IL-10 expression primarily at the level of transcription (Fig S2, Supplemental Data).
p38 MAPK is crucial for apoptotic cell-induced IL-10 transcription and protein production
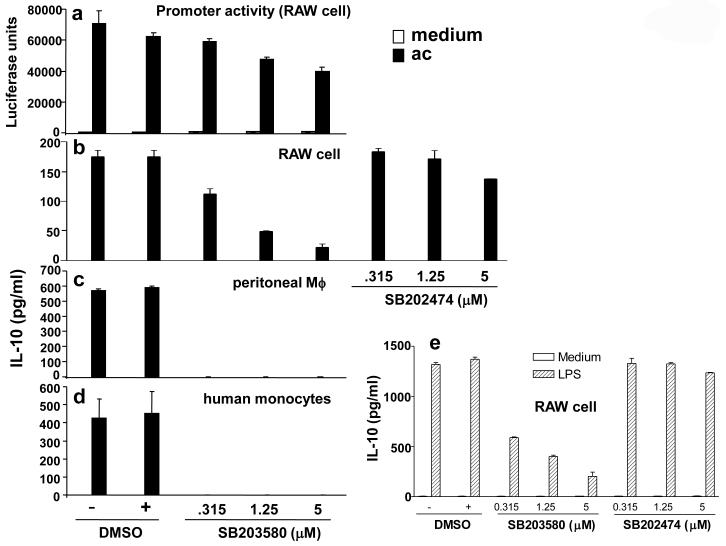
MAPKs are serine/threonine protein kinases, which include the p42/44 ERKs, the p54 and p46 JNK1 and -2/stress-activated protein kinase, and p38 MAPK (Lewis et al., 1998). These three families of MAPK form three parallel signaling cascades activated by distinct and sometimes overlapping set of stimuli (Lewis et al., 1998). It has been shown that LPS-induced p38 MAPK regulates IL-10 synthesis through activation of Sp1 in human monocytes (Ma et al., 2001). We hypothesized that MAPK, and in particular the p38 MAPK, may regulate apoptotic cell-induced IL-10 gene transcription and/or protein production. To address this question, RAW264.7 cells were treated with a pyridinyl imidazole compound, SB203580 (4-(4-fluorophenyl)-2-(4-methylsulfinylphenyl)-5-(4-pyridyl) imidazole), which specifically inhibits p38 MAPK with no significant effect on the activity on the ERK or JNK MAPK subgroups (Cobb and Goldsmith, 1995; Lee and Young, 1996).
RAW264.7 cells transiently transfected with the hIL-10 promoter luciferase reporter construct were treated with SB203580 1 hr prior to apoptotic cell stimulation. Luciferase activity was assayed 8 hr post apoptotic cell stimulation. SB203580, at 5 μM, inhibited apoptotic cell-induced hIL-10 promoter activity by about 50% (Fig 2a). Doses higher than 5μM were not used since these concentrations were cytotoxic (Ma et al., 2001). The role of p38 MAPK on apoptotic cell-induced IL-10 production from RAW264.7 cells was also investigated. Macrophages were treated with the drug 1 hr prior to apoptotic cell stimulation. Cell supernatants were harvested 12 hr post stimulation for analysis of IL-10 production. SB203580 at 1.25 μM inhibited apoptotic cell-induced IL-10 protein production by ∼70% while an inactive analogue of the chemical, SB202474, did not have such an inhibitory effect (Fig 2b). SB203580 at all concentrations totally inhibited apoptotic cell-induced IL-10 production in mouse peritoneal macrophages (Fig 2c), and in primary peripheral blood-derived human monocytes (Fig 2d). Furthermore, SB203580, but not SB202474, similarly inhibited LPS-induced IL-10 production in RAW264.7 cells in a dose-dependent manner (Fig 2e).
Figure 2. p38 MAPK is crucial for apoptotic cell-induced IL-10 promoter and protein production.
(a) Human IL-10 promoter (-129/+30) construct was transfected into RAW264.7 cells as described in Figure 3d. Cells were treated with SB203580 under the indicated concentrations 1 hr prior to apoptotic cell stimulation. Control cells received DMSO instead of the drug.
(b-e) 0.5 × 105 RAW264.7 cells (b, e) or 0.5 × 105 mouse peritoneal macrophages (c) and primary human monocytes (d) were treated with SB203580 or SB202474 (b, e) at the indicated concentrations 1 hr prior to apoptotic cell stimulation (2:1 ratio of apoptotic cells/macrophages) or LPS (e). The supernatants were harvested after 12 hr and analyzed for IL-10 production. All data were presented as mean ± SD from three independent experiments.
Apoptotic cell-induced p38 MAPK activation is partially dependent on CD36
Clearance of apoptotic cells can be mediated by scavenger receptors (SR) including the class SR-A, SR-B1, oxidized low-density lipoprotein receptor-1 and CD36 (Oka et al., 1998; Platt et al., 1996; Savill et al., 1992). CD36, one of the first macrophage receptors to be implicated in the recognition of apoptotic cells (Ren et al., 1995; Savill et al., 1992) was first characterized in the context of lipoprotein metabolism, foam cell formation and atherosclerosis (Savill et al., 1992). CD36 has been identified as a necessary cofactor in PS-mediated recognition of apoptotic cells (Fadok et al., 1998).
To determine the role of CD36 in apoptotic cell-induced IL-10 production, we compared apoptotic cell-induced IL-10 production kinetically between WT and CD36-/- peritoneal macrophages (Fig 3a). LPS-induced IL-10 production in WT and CD36-/- macrophages increased gradually from 4 to 48 hr post stimulation. Compared to the WT, CD36-/- macrophages produced significantly lower levels of LPS-induced IL-10 at 4 and 8 hr (indicated by *), but the level caught up at 24 hr. This implies CD36 signaling is perhaps important during the initial time course of LPS-induced IL-10 production. Apoptotic cells stimulated IL-10 production with a more rapid kinetics compared to LPS. Apoptotic cells stimulated significant levels of IL-10 by 4 hr and peaked at 8 hr post stimulation. At all time points measured, CD36-/- macrophages produced statistically lower levels (∼50% less) apoptotic cell-induced IL-10 when compared to the WT (indicated by #), suggesting that CD36 is significantly involved in apoptotic cell-induced IL-10 production. In addition to IL-10, TNF-α was also measured (Fig 3b). Unstimulated and apoptotic cell-stimulated macrophages produced no TNF-α at all time points. Conversely, LPS induced significant levels of TNF-α from both WT and CD36-/- macrophages by 4 hr and increased gradually over the 48 hr period. There were no statistical differences in LPS-induced TNF-α production between WT and CD36-/- macrophages at all time points. These results imply CD36 is not involved in the LPS signaling pathway for TNF-α production.
Figure 3. CD36 is involved in apoptotic cell-induced IL-10 production.
(a, b) 0.5 × 105 WT and CD36-/- peritoneal macrophages were stimulated with either LPS or apoptotic Jurkat T cells (ac) for indicated times. Supernatants were analyzed for IL-10 (a) and TNF-α production (b).
(c, d) CD36-/- and control C57BL/6 mice (3 per group) were injected i.v. with 107 apoptotic Jurkat cells. Eight hours later, 0.5 ×106 peritoneal macrophages (c) were collected and cultured for 24 hrs without further stimulation, and IL-10 levels were analyzed by ELISA. (d) Peritoneal exudates in these mice were also collected for IL-10 measurement. The values represent the amount of IL-10 in the entire peritoneal exudates of each individual mouse.
(e) 0.5 × 105 RAW264.7cells were stimulated with apoptotic Jurkat T cells (ac) for 12 hr. Oxidized-LDL (ox-LDL) were added simultaneously with apoptotic cells at various concentrations as indicated. Cell-free supernatants were analyzed for IL-10 production.
(f, g) Whole cell lysate were harvested from 2 × 106 WT and CD36-/- peritoneal macrophages under the conditions indicated. Proteins were subject to SDS-PAGE analysis on a 10% polyacrylamide gel and probed with antibodies against total p38, phosphorylated p38 and phosphorylated ERK. SB; SB203580 was used at 25 μM. DS, dimethyl sulfoxide, the medium in which SB203580 was dissolved. *, p<0.05; **, p<0.01; ***, p<0.001 between WT and CD36 KO cells.
To determine the physiological importance of CD36 in apoptotic cell-induced IL-10 production we injected apoptotic Jurkat cells into CD36-/- mice i.v., then measured IL-10 secretion directly from resident macrophages in the peritoneal cavity (Fig 3c) and from the exudates (Fig 3d). Apoptotic cells induced substantial amount of IL-10 from the macrophages in the peritoneum, which was significantly reduced in CD36-/-mice. The degree of reduction was very similar to the in vitro induced IL-10 levels in CD36-/- macrophages (Fig 3a).
Oxidized-LDL (ox-LDL) is one of the physiological ligands for CD36 (Rigotti et al., 1995). However, in our model, ox-LDL alone had no IL-10-inducing activity while it inhibited apoptotic cell-induced IL-10 protein production (Fig 3e). Therefore, ox-LDL appears to function as an antagonist of apoptotic cell-induction of IL-10 production, possibly through interference of CD36’s interaction with apoptotic cells.
The above results demonstrated that both p38 MAPK and CD36 are important for apoptotic cell induced IL-10 gene expression. We then addressed the role of CD36 in apoptotic cell-induced p38 MAPK activation. WT and CD36-/- peritoneal macrophages were stimulated with LPS, necrotic cells, apoptotic cells with or without SB203580 over a period of 6 hr. Whole cell lysates were subject to Western blot analysis with antibodies against phosphorylated p38 and phosphorylated ERK (Fig 3f, g). The level of LPS-induced p38 MAPK activation was similar between WT and CD36-/- macrophages, indicating that LPS-induced p38 MAPK activation does not depend on CD36. Necrotic cells were not able to activate p38 MAPK but activated ERK in WT cells and in CD36-/- cells albeit to a lower degree. Compared with WT macrophages, apoptotic cell-induced p38MAPK activation was substantially reduced in CD36-/- macrophages. These results indicate apoptotic cell-induced p38 MAPK activation is at least partially dependent on CD36. SB203580, at 25 μM, had no significant effect on ERK phosphorylation in both WT and CD36-/- cells while it partially inhibited p38 MAPK activation in both cell types.
Taken together, these results suggest that CD36 is important physiologically but not the only receptor for apoptotic cell induced-IL-10 and p38 MAPK activation is partially dependent on CD36.
An Apoptotic Cell-Response Element, is located at -106/-98 in the IL-10 promoter
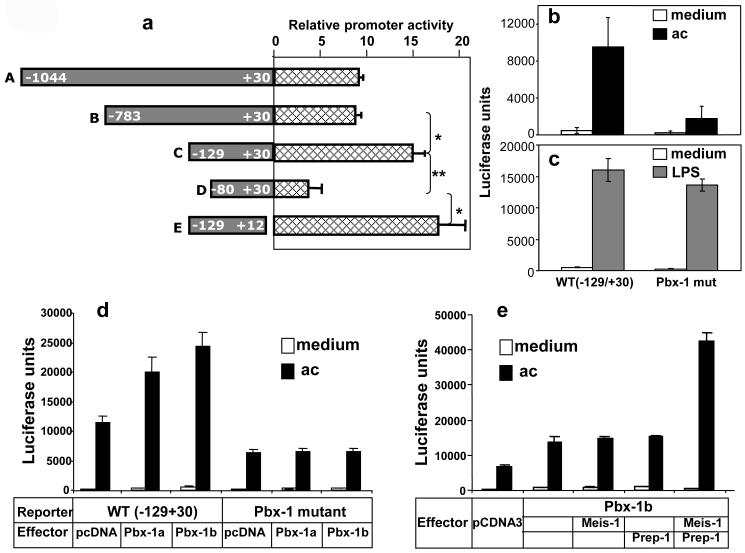
To determine the DNA sequences in the IL-10 promoter that mediate apoptotic cell-induced IL-10 transcription, a series of 5′and 3′ deletion constructs of the -1042/+30 IL-10 promoter-reporter were generated. These constructs were transiently transfected into RAW264.7 cells, stimulated with apoptotic Jurkat cells, and their responses were measured by their luciferase activities (Fig 4a). Notably, there was a dramatic decrease in apoptotic cell-induced luciferase activity when the promoter was deleted from -129 to -80, indicating the presence of a strong positive regulatory element(s) in the 49-bp region of the IL-10 promoter.
Figure 4. The apoptotic cell response element, ACRE, is located from (-106/-98) in the human IL-10 promoter.
(a) The full-length human IL-10 promoter (-1044/+30) and various 5′ and 3′ truncation constructs linked to the firefly luciferase reporter gene were transiently transfected into RAW264.7 cells. Next day, cells were stimulated with apoptotic Jurkat T cells. Luciferase activity was measured 8 hr post stimulation. Data are expressed as fold induction relative to RAW264.7 cells not treated with apoptotic cells, and represent three independent experiments with SD. Student t test was used for statistical analysis of the comparison between two groups (specified by a bracket). *, p<0.05; **, p<0.01.
(b, c) Response of the human IL-10 promoter (-129/+30) construct containing either the WT Pbx-1 binding site or its substitution mutant to apoptotic cells (c) or to LPS (d). The difference in LPS response between the two constructs in (d) was not statistically significant. Base-substitutions were made by transversion.
(d) Response of the human IL-10 promoter (-129/+30) construct (reporter) containing either the WT Pbx-1 binding site or its substitution mutant cotransfected with Pbx-1a and 1b expression vector (effector) or control vector pcDNA3.1 into RAW264.7 cells by electroporation at molar ratios of 1.75:1 or 2:1 (effector:reporter). The next day, cells were stimulated with apoptotic Jurkat T cells. Luciferase activity was measured 8 hr post stimulation.
(e) An identically designed experiment to (d) was carried out with two additional Hox factors, Meis-1 and Prep-1, in a molar ratio of Pbx-1b:Meis-1:Prep-1=1:1:1.
A computer-aided analysis revealed the existence of consensus sequences for two transcription factors: Ets (5′-ggaa-3′ at -113/-110) and the homeodomain protein pre-B-cell leukemia transcription factor-1 (Pbx-1) (5′-ttgattgtg-3′ at -106/-98). A detailed systematic mutagenesis study of the -129/-80 region revealed that the sequence between -95 and -106, which overlaps the putative Pbx-1 motif, was most critical for the apoptotic cell response of the IL-10 promoter (data not shown). Subsequently, we introduced site-specific point mutations into the 9-bp putative Pbx-1 binding site, which reduced the promoter activity by ∼80% in response to apoptotic cells compared to the WT luciferase reporter construct (-129/+30) (Fig 4b) while having little effect on LPS-induced promoter activity (Fig 4c), indicating that this particular sequence constitutes a major apoptotic cell response element (ACRE).
The Pbx homeoproteins can function as cofactors for Hox family of homeodomain-containing transcription factors that pattern the embryonic body axes (Moens and Selleri, 2006). Pbx-1a and Pbx-1b are the two isoforms of Pbx-1. Pbx-1a expression is restricted to neural tissues while Pbx-1b exhibits widespread expression patterns in the mouse embryo (Moens and Selleri, 2006). To further investigate the role and mechanism of Pbx-1 in the regulation of IL-10 transcription during phagocytosis of apoptotic cells we overexpressed Pbx-1a and 1b by transient transfection in RAW264.7 cells, which significantly increased the apoptotic cell-induced luciferase activity in the hIL-10 promoter luciferase reporter construct (-129/+30) (Fig 4d). The augmented effects were totally abrogated with the IL-10 promoter construct containing base substitutions in the putative Pbx-1 binding site, indicating that this sequence is crucial for the transcriptional augmentation by Pbx-1. Both Pbx-1a and 1b had little effect on basal transcription without apoptotic cell stimulation, suggesting that the transcriptional potential of Pbx-1a/b depends on signals from apoptotic cells.
Hox proteins and some orphan homeodomain proteins form complexes with either Pbx or Meis subclasses of homeodomain proteins. This interaction can increase the binding specificity and transcriptional effectiveness of the Hox partner(Moens and Selleri, 2006). To test this possibility on the IL-10 gene we cotransfected Pbx-1b and two Meis proteins: Meis-1 and Pbx-regulating Protein (Prep)-1(also called Meis-4) with the IL-10 reporter (Fig 4e). Expression Meis-1 or Prep-1 individually did not enhance Pbx-1b’s transcriptional stimulation of the IL-10 reporter while the combination of the two factors synergistically augmented Pbx-1b’s transcriptional potency, indicating the functional interaction and cooperation of these three Hox factors in the activation of IL-10 gene transcription. Again, the combination of three Hox factors had little effect on basal transcription without apoptotic cell stimulation.
Pbx-1 is a selective physiological regulator of IL-10 production in response to apoptotic cells
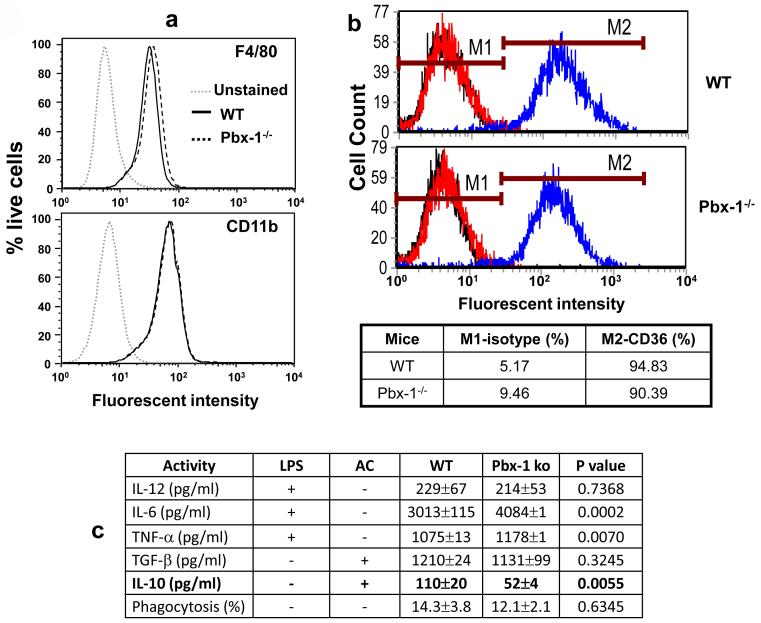
To establish the physiological role of Pbx-1 in the regulation of IL-10 gene expression in response to apoptotic cells we generated macrophages from Pbx-1 knockout embryos. Due to the extraordinary importance of Pbx-1 in mammalian development, thus overwhelming embryonic lethality of the Pbx1-deficiency(Moens and Selleri, 2006), we derived macrophages from E14.5 embryonic liver of heterozygote mating. Cells derived from liver of littermate embryos of the wild type (+/+) and heterozygous (+/-) genotypes were used as control. By flow cytometric analysis, the embryonic liver-derived macrophages were approximately 90% F4/80- and CD11b-positive, irrespective of their Pbx-1 genotypes (Fig 5a). There were equivalent amounts of CD36 surface expression between the wt and ko macrophages (Fig 5b). Functionally, Pbx-1-/- macrophages produced similar amounts of IL-12 and TNF-α in response to LPS stimulation, and TGF-β in response to apoptotic cells whereas LPS-induced IL-6 production was increased and apoptotic cell-induced IL-10 production was significantly reduced (Fig 5c). The phagocytic capacity, as measured by ingestion of E. coli, was also similar between the two types of macrophages. No statistical differences in LPS-stimulated IL-10 production were found between the two groups (result not shown). These results demonstrate that Pbx-1 plays an important and selective physiological role in apoptotic cell-induced IL-10 production, and its transcriptional role is uncoupled from phagocytosis.
Figure 5. Pbx-1 is a selective physiological regulator of IL-10 production.
(a) Flow cytometric analysis of in vitro generated embryonic liver-derived mouse macrophages from littermates of wild type (+/+) and homozygous Pbx-1 knockouts (-/-). The fluorescent intensity (X-axis) of F480 and CD11b staining of live cells (Y-axis) in the cultures are shown.
(b) CD36 expression on macrophages derived from WT and Pbx-1-null embryos. An isotype-matched control antibody was used to set the baseline.
(c) Reduced IL-10 production in Pbx-1-deficient embryonic liver-derived macrophages. After in vitro culture for 7 days, embryonic liver-derived mouse macrophages generated from littermates of wild type (+/+) and homozygous Pbx-1 knockouts (-/-) were stimulated with LPS or apoptotic Jurkat cells. Cell-free supernatant were harvested 12 hr post stimulation and assayed for various cytokine production by ELISA. The numbers of embryos used in this experiment were 3 wild type and 3 Pbx-1-null homozygotes. Phagocytosis of E. coli by embryonic-liver macrophages derived from wild type Pbx-1-/- embryonic liver was measured.
Blocking Pbx-1 expression results in reduction of apoptotic cell-induced IL-10 expression
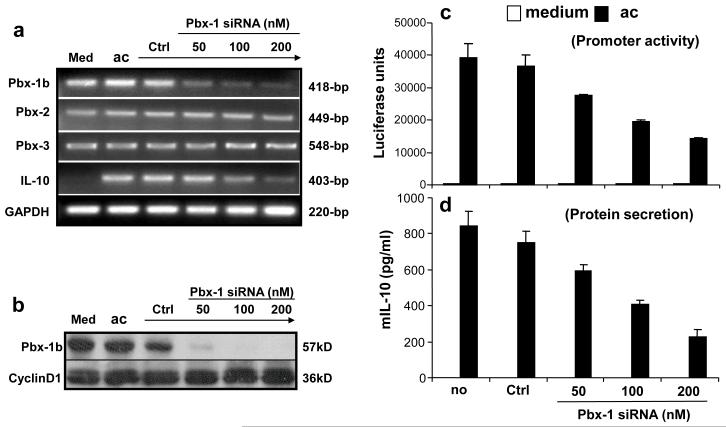
In parallel, we applied the RNAi technology to further corroborate Pbx-1’s role in the regulation of apoptotic cell-induced IL-10 expression, with the prediction that inhibiting the expression of Pbx-1 would result in suppressed IL-10 transcription. For this purpose, a siRNA specific for Pbx-1a/b was transfected into RAW264.7 cells to reduce the level of endogenous Pbx-1 expression. As shown in Fig 6a, Pbx-1b, Pbx-2, and Pbx-3 mRNA were constitutively expressed in RAW264.7 cells while Pbx-1a was not expressed (it would have been amplified as a larger size product by the same primers used to detect Pbx-1b mRNA). The Pbx-1-specific siRNA but not a control siRNA (GFP-specific) selectively inhibited Pbx-1b mRNA expression in a dose-dependent manner, while having little effect on Pbx-2, Pbx-3, and GAPDH mRNA levels. Accompanying the reduction in Pbx-1 mRNA expression, IL-10 mRNA expression was also reduced. At the protein level, the Pbx-1 siRNA strongly inhibited Pbx-1b expression (Fig 6b).
Figure 6. Blocking Pbx-1 expression inhibits apoptotic cell-induced IL-10 expression.
(a, b) Inhibition of Pbx-1 expression by siRNA. RAW264.7 cells were transfected by Oligofectamine with siRNA specific for Pbx-1 at various concentrations as indicated, or GFP (control) at 200 nM for 4 hrs, followed by exposure to apoptotic Jurkat cells. Eight hours later, total RNA was isolated and subject to RT-PCR for the analysis of Pbx-1a, Pbx-1b, Pbx-2, Pbx-3, IL-10, and GAPDH mRNA expression (a). Whole cell lysate were isolated and subject to Western blot analysis for Pbx-1b protein level (b) using a polyclonal antibody for Pbx-1, 2, 3.
(c, d) Inhibition of IL-10 expression by Pbx-1 siRNA. RAW264.7 cells were transfected with the IL-10 promoter construct by electroporation. Twelve hours later, the cells were transfected by Oligofectamine with Pbx-1 siRNA at various concentrations or the control siRNA at 200 nM for 4 hrs followed by exposure to apoptotic Jurkat cells. Luciferase activity (c) was measured 8 hr post exposure to apoptotic cells, and IL-10 protein secretion (d) was analyzed at 24 hr post stimulation.
Consistent with its ability to knock down the endogenous levels of Pbx-1, the Pbx-1 siRNA also dose-dependently inhibited apoptotic cell-induced IL-10 reporter activity by as much as 60% at 200 nM while the control siRNA had little effect at this concentration (Fig 6c). Likewise, the IL-10 protein secretion from the same cells was reduced by approximately the same degrees by the siRNA (Fig 6d).
Taken together, these data demonstrate that Pbx-1b is a physiologically critical mediator of apoptotic cell-induced IL-10 gene transcription.
Characterization of DNA-binding activities of Pbx-1b and its cofactor Prep-1
Based on these observations, we further hypothesized that Pbx-1b may activate IL-10 transcription in response to apoptotic cells by physically interacting with the ACRE. To test this hypothesis, electrophoretic mobility shift assays (EMSAs) were performed using nuclear extracts isolated from RAW264.7 cells, and the ACRE sequence as the probe. As shown in Fig 7a, apoptotic cells strongly induced a DNA-protein complex (lane 3, pointed to by an arrow). This binding activity was partially abolished by polyclonal antibodies against Pbx-1, 2, 3 (lane 6) and Pbx-1 (lane 7) while the isotype-matched control antibody (lane 5) and antibodies against Pbx-2 (lane 8) and Pbx-3 (lane 9) had little effect. Given the fact that Pbx-1a is not expressed in macrophages, this result suggests that Pbx-1b may physically interact with the IL-10 promoter at the ACRE site. The Pbx-1 binding activity was also observed in nuclear extracts isolated from human monocytes, and HMDM stimulated with apoptotic cells, autologous or non-autologous, but not with LPS (Fig S3, Supplemental Data).
Figure 7. Interaction of Pbx-1b and cofactors with ACRE.
(a) EMSA analysis was performed with ACRE oligonucleotides and nuclear extracts from RAW264.7 cells with or without stimulation by apoptotic cells in the presence or absence of antibodies against members of the Pbx family, or control IgG or PBS.
(b) ChIP analysis of in vivo binding to the IL-10-ACRE by Pbx-1 in RAW264.7 cells under unstimulated (med) or apoptotic cell (ac)-stimulated conditions. Control antibody (rat IgG) was used and a 230 bp region 1.3 kb upstream of the ACRE was probed as a negative control.
(c) Whole cell extracts were prepared from WT and CD36-/- peritoneal macrophages following stimulation with LPS or apoptotic cells (AC). Immunoprecipitation (IP) was performed using anti-phosphotyrosine (α-pY), anti-phosphoserine (α-pS), and anti-phosphothreonine (α-pT) mAbs, followed by Western blot with anti-Pbx-1 or anti-Prep-1 Ab. The relative ratios of tyrosine phosphorylation of Prep-1 are indicated below the image.
(d) ChIP analysis of in vivo binding to the IL-10-ACRE by Pbx-1, Meis-1, and Prep-1 in WT and CD36-/- peritoneal macrophages under unstimulated (med) or apoptotic cell (ac)-stimulated conditions. Control antibodies (mouse and rat IgG) were used as negative controls.
To confirm direct binding of Pbx-1b to ACRE in vivo chromatin immunoprecipitation (ChIP) assays were performed in RAW264.7 cells. Fig 7b shows Pbx-1-specific binding to the vicinity of the ACRE as a 214-bp amplified band, which was slightly present in resting cells (lane 5) and increased significantly following apoptotic cell stimulation (lane 6, top). The binding was site-specific because it was not observed at an irrelevant site 1.3 kb upstream of ACRE, amplified as a 230-bp fragment (bottom).
To investigate the relationship between Pbx-1b and CD36 engagement by apoptotic cells we analyzed the expression and phosphorylation of Pbx-1b in CD36-/- peritoneal macrophages stimulated with LPS or apoptotic cells. Fig 7c shows that surprisingly, Pbx-1b expression was not altered before and after stimulation with LPS or apoptotic cells, nor by the CD36-deficiency. Moreover, phosphorylation of Pbx-1b at tyrosine (Y), serine (S), and threonine (T) residues was not altered under these conditions in wild type or CD36-/- cells. These results indicate that CD36-mediated signaling does not directly regulate Pbx-1 expression or phosphorylation.
Because of the functional importance of Pbx-1 dimerizing partners Meis-1 and Prep-1 (Fig 4e), we next examined their phosphorylation. While tyrosine, serine, and threonine phosphorylation of Meis-1 was not detectable (data not shown) threonine phosphorylation of Prep-1 was constitutive and strong but not altered by the stimuli or CD36 deficiency (Fig 7c). In contrast, tyrosine phosphorylation of Prep-1 was further stimulated by apoptotic cells but not by LPS, and moreover it was strongly impaired in CD36-/- cells. These results suggest that Prep-1 is an apoptotic cell- and CD36-regulated molecule through tyrosine phosphorylation.
To further investigate the direct functional role of cofactors Meis-1 and Prep-1 in Pbx-1b-mediated IL-10 transcription we performed ChIP analysis of these Hox factors in CD36-/- macrophages. As shown in Fig 7d, binding by all three molecules were induced to various degrees in WT peritoneal macrophages encountering apoptotic cells whereas their binding activities were abolished in the absence of CD36 (lanes 4-6), demonstrating that these Hox factors interact specifically with the ACRE in an apoptotic cell- and CD36-dependent manner.
Discussion
Many phagocytic receptors are implicated in the recognition, tethering and ingestion of apoptotic cells (Somersan and Bhardwaj, 2001) as well as in mediating apoptotic cell-induce IL-10 production. In humans, the ternary complex consisting of CD36, TSP-1 and the vitronectin receptor (αvβ3) has been shown to mediate the uptake of apoptotic cells (Lucas et al., 2006; Savill et al., 1990; Savill et al., 1992). CD36 is involved in the differentiation of monocytes and foam cell formation in macrophages as a result of exposure to oxidized low density lipid (oxLDL) (Huh et al., 1996; Nicholson et al., 1995; Nozaki et al., 1995). A domain (155-183) on CD36 was identified as important in the phagocytosis of apoptotic neutrophils (Navazo et al., 1996). Incubation of LPS-activated human PBMCs and monocytes with FA6-152, an agonistic monoclonal antibody against the TSP-1-binding site of CD36 resulted in the production of IL-10 and the inhibition of inflammatory cytokines TNF-α, IL-1β and IL-12 (Voll et al., 1997a), suggesting the anti-inflammatory effect is achieved via CD36 interaction with TSP-1 expressed on the surface of apoptotic cells. While previous studies used LPS-activated human macrophages to investigate the role of CD36 in apoptotic cell-induced IL-10 production, our study took a more direct approach examining apoptotic cell-induced IL-10 with respect to the role of CD36 using CD36-deficient macrophages. Our data demonstrate that CD36 is responsible for ∼50% of apoptotic cell-induced IL-10 production.
The evidence presented here strongly suggests that Pbx-1b is a major physiological and selective transcriptional mediator of apoptotic cell-induced IL-10 gene expression through the ACRE. Pbx proteins are composed of Pbx-1, 2, 3, 4 that can function as Hox cofactors in vertebrates as they do in Drosophila and pattern the body axes of animal embryos (Moens and Selleri, 2006). Pbx1-/- mouse embryos exhibit profound anemia and embryonic lethality at embryonic day 15 (E15) or E16 (DiMartino et al., 2001). Hematopoietic stem cells from Pbx1-/- embryos have reduced colony-forming activity and are unable to establish multilineage hematopoiesis in competitive reconstitution experiments. Common myeloid progenitors (CMPs) are markedly depleted in Pbx1-/- embryos at E14 and display clonogenic defects in erythroid colony formation (DiMartino et al., 2001). Thus, Pbx1 is essential for the function of hematopoietic progenitors and its loss creates a proliferative block at the level of the CMP. Hox and Pbx genes have also been implicated as proto-oncogenes in human leukemia. Pbx1 was originally isolated as a proto-oncogene at the site of the t(1:19) rearrangement in human pre-B acute lymphocytic leukemia and in a small number of acute T-lymphoid and myeloid leukemias, whereby its homeodomain is fused to the transcriptional activation domains of the immunologulin enhancer-binding protein E2a (Kamps et al., 1990). The derived chimeric oncoprotein retains the ability to bind DNA as a complex with Hox proteins and causes aberrant proliferation of hematopoietic progenitors and leukemic transformation (Dedera et al., 1993; Kamps and Baltimore, 1993).
The IL-10 deficiency in Pbx-1-null macrophages encountering apoptotic cells is not due to global developmental impairment because Pbx-1-deficient embryonic hematopoietic stem cells can differentiate normally into macrophages indistinguishable with respect to F4/80 and CD11b expression (Fig 5a). They exhibited no defect in their ability to ingest apoptotic cells (Fig 5b), and to produce several cytokines (Fig 5c). However, it should be pointed out that Pbx-1 is not the only transcription factor driving the apoptotic cell-induced IL-10 expression since deletion of Pbx-1 resulted in ∼50% loss in IL-10 production (Fig 5c). Moreover, Pbx-1b is not regulated at the level of expression or phosphorylation by apoptotic cells nor altered in CD36-deficient cells (Fig 6a, b, Fig 7e), while its binding to IL-10 promoter was highly inducible (Fig 7a-d). This suggests that additional, apoptotic cell-induced factors are involved that facilitate Pbx-1b binding to DNA and these factors, not Pbx-1b itself, may be lacking or inactive in CD36-deficient macrophages. In other words, CD36 does not directly regulate Pbx-1b and that Pbx-1b plays the role of a constitutive but essential transcription factor for IL-10 in the apoptotic cell pathway.
Hox proteins and some orphan homeodomain proteins form complexes with either Pbx or Meis subclasses of homeodomain proteins. This interaction can increase the binding specificity and transcriptional effectiveness of the Hox partner (Swift et al., 1998). However, Pbx has also been shown to interact with non-homeodomain proteins such as myogenic bHLH proteins myoD and myf5 (Berkes et al., 2004). Indeed, our data indicated that both Meis-1 and Prep-1 can enhance Pbx-1b-mediated transcriptional activation of IL-10 gene expression in response to apoptotic cells (Fig 4e). Furthermore, apoptotic cells stimulate Prep-1 tyrosine phosphorylation in a CD36-dependent manner (Fig 7e), and the DNA-binding activities by all three Hox factors are dependent on both apoptotic cell-induced signaling and the presence of CD36 (Fig 7f), although Pbx-1b itself is not regulated by these signals. These results suggest that Prep-1 may be the bridging molecule linking the two pathways, one inducible through the CD36 contact with apoptotic cells, the other constitutive through Pbx-1b, for IL-10 transcription. The involvement of tyrosine phosphorylation of Prep-1 is consistent with the observation that p38 MAPK may not be the only kinase important for Pbx-1b binding since SB203580 could only inhibit partially Pbx-1 binding (Fig 7c).
In summary, we demonstrate that CD36, Pbx-1b, and Prep-1 are key regulators of IL-10 gene transcription induced by apoptotic cells. This finding establishes a novel link between two seemingly unrelated physiological pathways, one that regulates development and one that controls autoimmune responses. This study carries the prospect to open up a completely new area for future exploration at the intersection between cellular homeostasis and regulation of immune responses to exogenous pathogens as well as to endogenous danger signals.
Experimental Procedures
Animals and Cell lines
C57BL/6 mice (6-8 wk old) were purchased from the Jackson Laboratory (Bar Harbor, ME). CD36-/- mice were maintained at the Weill Medical College of Cornell University (New York, NY). Jurkat, a leukemic human T-cell line, was kindly provided by Dr. Gary Koretzky of the University of Pennsylvania (Philadelphia, PA).
Reagents and Antibodies
Staurosporine was from Cayman Chemical (Ann Arbor, MI); Cytochalasin D was from Sigma-Aldrich; (Saint Louis, MO); LPS from Escherichia coli, Pyridinyl imidazole, SB203580 and SB20247 were from Calbiochem (San Diego, CA); Antibodies against total p38, phosphorylated p38 and phosphorylated ERK were from Cell Signaling Technology (Danver, MA); Antibodies against CD8 (TIB 210), MHC II (212.A1), Mac-1 (CD11b), NK1.1 (PK 136), and CD19 (ID3) were generously provided by Dr. Derek Sant’Angelo of the Memorial Sloan-Kettering Cancer Center (New York, NY). PE-anti-mouse-CD36 and PE-rat IgG2a isotype control were from eBioscience, and all anti-Pbx, anti-Meis-1, anti-Prep-1 antibodies were from Santa Cruz Biotechnologies (Santa Cruz, CA) unless mentioned otherwise. Oxidized LDL was from INTRACELL, Frederick, MD, (cat. #RP-047).
Murine Splenic CD4+ T cell Isolation
Enrichment of CD4+CD8- T cells from mouse spleens was performed using Dynabeads M-450 (Dynal, Oslo, Norway). After enrichment, >75% of the T cells were CD4+.
Human Monocytes, HMDMs and Human T cell Isolation
Leukocytes from healthy human donors were purchased from the New York Blood Center. The desired sub-cell populations were prepared as described (Kim et al., 2004) .
Induction of Apoptosis and Necrosis
The induction of apoptosis and necrosis was as described previously (Kim et al., 2004).
Construction of Luciferase Reporter Gene Vectors
The human IL-10 promoter-luciferase construct pIL-10 (-1044/+30)-luc was generously provided by Dr. L. Ziegler-Heitbrock of the University of Leicester (Leicester, U.K.) (Benkhart et al., 2000a). All sub-constructs containing deletions or base-substitutions were generated by PCR and verified by sequencing.
Transient Transfection of RAW264.7 cells
Transfection using electroporation was performed as described previously (Cao et al., 2002).
Quantitative Real-time PCR
Reverse transcription was performed using 2μg total RNA and 0.2μg of random hexamer primers (Fermentas) following the manufacturer’s protocol for first synthesis of first strand cDNA. Real-time PCR was performed using SyBr Green PCR Master Mix (Applied Biosystem, Piscataway, NJ) containg 5ng of cDNA mixed with pairs of primers (10μM) specific for IL-10 or GAPDH in a 10μl volume. Data were processed using GeneAmp software (Applied Biosystems, Foster City, CA). All results were expressed as fold changes in mRNA expression relative to unstimulated cells and normalized to mGAPDH expression. Data represented the mean of triplicates.
EMSA
EMSAs were performed as described (Cao et al., 2002). The oligonocleotide sequences for ACRE wt: 5′-d(AAA CCT TGA TTG TGG CTT T)-3′; ACRE mutant: 5′-d(AAA CCG ATC AGA ATG CTT T)-3′, where the critical nucleotides are in bold face.
Immunoprecipitation
400 μg of whole cell extract isolated from LPS- or apoptotic treated macrophages was used with various phospho-specific antibodies (10 μl of anti-phospho-Tyrosine, 15 μl of anti-phospho-Serine and 30 μl of anti-phospho-Threonine antibodies). After overnight incubation at 4°c with rotation, 100 μl of proetein A agarose beads were added into the solution and incubated for 2 h at 4°c. The protein-bound beads were collected, washed by PBS and resuspended in 60 μl of 2x loading buffer and resolved by SDS-PAGE, blotted, and detected by the anti-Pbx-1 or anti-Prep-1 antibody.
Phagocytosis Assay
Apoptotic cells
as described previously (Kim et al., 2004)
E. coli
The phagocytosis assay was performed according to the manufacture’s protocol (Molecular probes, Invitrogen). Briefly, 0.1×106 of embryonic liver derived macrophages was inoculated in each well of 96 well assay black plate (BD Biosciences) in 100 ml of complete DMEM medium. After 2 hrs incubation at 37°C to allow the cells to adhere to the microplate surface, the culture medium was removed by aspiration and 100 ml of prepared fluorescent-labeled E. coli was added to all wells. After 2 hrs incubation, BioParticle loading suspension was removed and 100 ml of trypan blue was added to all of the wells. The trypan blue suspension was aspirated after 1 min incubation at room temperature followed by measuring the fluorescent immediately using ∼480 nm excitation, ∼520 nm emission. The relative fluorescence was calculated by subtract the average fluorescence intensity of control wells that without adding the fluorescent-labeled E. coli from that of E. coli loaded group, and verse the negative-control wells that without cells. Each group included 4 wells.
SiRNA
The siRNA for Pbx-1 was purchased from Dharmacon (Lafayette, CO). Sense sequence: 5′GGCGGAAGAGACGGAAUUUdTdT-3′, Anti-sense sequence: 5′-AAAUUCCGUCUCUUCCGCCdTdT-3′. It does not discriminate the closely related Pbx-1a and Pbx-1b isoforms. The control siRNA was specific for GFP. Transfection of siRNA was carried out by using Oligofectamine (Invitrogen) following the Supplier’s instructions. RAW264.7 cells, plated at 2 × 106 cell/well in 6-well culture plates (Falcon), were transfected with siRNA 4 hr prior to apoptotic cell incubation. Total whole cell lysates, RNA and supernatants were harvested.
Generation of embryonic liver derived macrophages
Embryonic liver derived macrophages were generated from E 14.5 embryos derived from Pbx-1 +/- × Pbx-1 +/- mating. Genotypes of the embryos were determined by PCR. Embryonic livers were gently homogenized into single-cell suspension, plated at 1×106 cells/well in a 48-well plate and cultured for 7 days in the presence of DMEM supplemented with 20% L-cell medium. Culture medium was replaced every 3 days during the period of macrophage differentiation. Pbx-1 knockout cells lack both Pbx-1a and Pbx-1b (Moens and Selleri, 2006). These macrophages were ∼90% F480+ and CD11b+, as revealed by flow cytometric analysis.
Chromatin Immunoprecipitation Assay
The chromatin immunoprecipitation (ChIP) procedure was performed using a kit (Upstate Biotechnology, Lake Placid, NY) following the manufacturer’s instructions, with 2.5 × 107 RAW264.7 cells or peritoneal macrophages/condition. The input DNA was diluted 200 times before PCR. The input and immunoprecipitated DNA were PCR amplified with the primers encompassing the ARCE in the mouse IL-10 promoter (upper primer: TTTTCTACCAGCAGCAAGCA and lower primer: ATGCTAGCTGGGTCTTGAGC. The control primers are: upper: TTC CCT AGG ATC AGG GAG GT, and lower: AAT GGG CCT CTC TTT CCA GT. This pair of primers amplifies a 230-bp fragment 1.3 kb upstream of the ACRE. The samples were amplified for 35 cycles (RAW264.7) or 37 cycles (primary peritoneal macrophages) by PCR and were analyzed by electrophoresis on a 1.2% agarose gel.
Statistical Analysis
All transfection and cytokine studies were performed in duplicates. The results were expressed as mean ± S.D. from at least three independent experiments. All statistical analyses were performed with two-tailed Student’s t test. Data were considered significant if the p <0.05.
Supplementary Material
Acknowledgement
This work was supported by a grant from the NIH (AI45899) to X.M. and a grant from the Mary Kirkland Foundation for Lupus Research to X.M.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aderem A, Underhill DM. Mechanisms of phagocytosis in macrophages. Annu Rev Immunol. 1999;17:593–623. doi: 10.1146/annurev.immunol.17.1.593. [DOI] [PubMed] [Google Scholar]
- Benkhart EM, Siedlar M, Wedel A, Werner T, Ziegler-Heitbrock HW. Role of Stat3 in lipopolysaccharide-induced IL-10 gene expression. J Immunol. 2000a;165:1612–1617. doi: 10.4049/jimmunol.165.3.1612. [DOI] [PubMed] [Google Scholar]
- Benkhart EM, Siedlar M, Wedel A, Werner T, Ziegler-Heitbrock HW. Role of Stat3 in lipopolysaccharide-induced IL-10 gene expression. J Immunol. 2000b;165:1612–1617. doi: 10.4049/jimmunol.165.3.1612. [DOI] [PubMed] [Google Scholar]
- Berg DJ, Kuhn R, Rajewsky K, Muller W, Menon S, Davidson N, Grunig G, Rennick D. Interleukin-10 is a central regulator of the response to LPS in murine models of endotoxic shock and the Shwartzman reaction but not endotoxin tolerance. J Clin Invest. 1995a;96:2339–2347. doi: 10.1172/JCI118290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg DJ, Leach MW, Kuhn R, Rajewsky K, Muller W, Davidson NJ, Rennick D. Interleukin 10 but not interleukin 4 is a natural suppressant of cutaneous inflammatory responses. J Exp Med. 1995b;182:99–108. doi: 10.1084/jem.182.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkes CA, Bergstrom DA, Penn BH, Seaver KJ, Knoepfler PS, Tapscott SJ. Pbx marks genes for activation by MyoD indicating a role for a homeodomain protein in establishing myogenic potential. Mol Cell. 2004;14:465–477. doi: 10.1016/s1097-2765(04)00260-6. [DOI] [PubMed] [Google Scholar]
- Bettelli E, Das MP, Howard ED, Weiner HL, Sobel RA, Kuchroo VK. IL-10 is critical in the regulation of autoimmune encephalomyelitis as demonstrated by studies of IL-10- and IL-4-deficient and transgenic mice. J Immunol. 1998;161:3299–3306. [PubMed] [Google Scholar]
- Brightbill HD, Plevy SE, Modlin RL, Smale ST. A prominent role for Sp1 during lipopolysaccharide-mediated induction of the IL-10 promoter in macrophages. J Immunol. 2000;164:1940–1951. doi: 10.4049/jimmunol.164.4.1940. [DOI] [PubMed] [Google Scholar]
- Byrne A, Reen DJ. Lipopolysaccharide induces rapid production of IL-10 by monocytes in the presence of apoptotic neutrophils. J Immunol. 2002;168:1968–1977. doi: 10.4049/jimmunol.168.4.1968. [DOI] [PubMed] [Google Scholar]
- Camenisch TD, Koller BH, Earp HS, Matsushima GK. A novel receptor tyrosine kinase, Mer, inhibits TNF-alpha production and lipopolysaccharide-induced endotoxic shock. J Immunol. 1999;162:3498–3503. [PubMed] [Google Scholar]
- Cao S, Liu J, Chesi M, Bergsagel PL, Ho IC, Donnelly RP, Ma X. Differential regulation of IL-12 and IL-10 gene expression in macrophages by the basic leucine zipper transcription factor c-Maf fibrosarcoma. J Immunol. 2002;169:5715–5725. doi: 10.4049/jimmunol.169.10.5715. [DOI] [PubMed] [Google Scholar]
- Casciola-Rosen L, Rosen A, Petri M, Schlissel M. Surface blebs on apoptotic cells are sites of enhanced procoagulant activity: implications for coagulation events and antigenic spread in systemic lupus erythematosus. Proc Natl Acad Sci U S A. 1996;93:1624–1629. doi: 10.1073/pnas.93.4.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb MH, Goldsmith EJ. How MAP kinases are regulated. J Biol Chem. 1995;270:14843–14846. doi: 10.1074/jbc.270.25.14843. [DOI] [PubMed] [Google Scholar]
- de Waal Malefyt R, Abrams J, Bennett B, Figdor CG, de Vries JE. Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991a;174:1209–1220. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Waal Malefyt R, Abrams J, Bennett B, Figdor CG, de Vries JE. Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991b;174:1209–1220. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Waal Malefyt R, Haanen J, Spits H, Roncarolo MG, te Velde A, Figdor C, Johnson K, Kastelein R, Yssel H, de Vries JE. Interleukin 10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II major histocompatibility complex expression. J Exp Med. 1991c;174:915–924. doi: 10.1084/jem.174.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedera DA, Waller EK, LeBrun DP, Sen-Majumdar A, Stevens ME, Barsh GS, Cleary ML. Chimeric homeobox gene E2A-PBX1 induces proliferation, apoptosis, and malignant lymphomas in transgenic mice. Cell. 1993;74:833–843. doi: 10.1016/0092-8674(93)90463-z. [DOI] [PubMed] [Google Scholar]
- Dillon S, Agrawal S, Banerjee K, Letterio J, Denning TL, Oswald-Richter K, Kasprowicz DJ, Kellar K, Pare J, van Dyke T, et al. Yeast zymosan, a stimulus for TLR2 and dectin-1, induces regulatory antigen-presenting cells and immunological tolerance. J Clin Invest. 2006;116:916–928. doi: 10.1172/JCI27203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMartino JF, Selleri L, Traver D, Firpo MT, Rhee J, Warnke R, O’Gorman S, Weissman IL, Cleary ML. The Hox cofactor and proto-oncogene Pbx1 is required for maintenance of definitive hematopoiesis in the fetal liver. Blood. 2001;98:618–626. doi: 10.1182/blood.v98.3.618. [DOI] [PubMed] [Google Scholar]
- Fadok VA, Warner ML, Bratton DL, Henson PM. CD36 is required for phagocytosis of apoptotic cells by human macrophages that use either a phosphatidylserine receptor or the vitronectin receptor (alpha v beta 3) J Immunol. 1998;161:6250–6257. [PubMed] [Google Scholar]
- Fiorentino DF, Bond MW, Mosmann TR. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med. 1989;170:2081–2095. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foey AD, Parry SL, Williams LM, Feldmann M, Foxwell BM, Brennan FM. Regulation of monocyte IL-10 synthesis by endogenous IL-1 and TNF-alpha: role of the p38 and p42/44 mitogen-activated protein kinases. J Immunol. 1998;160:920–928. [PubMed] [Google Scholar]
- Fuss IJ, Boirivant M, Lacy B, Strober W. The interrelated roles of TGF-beta and IL-10 in the regulation of experimental colitis. J Immunol. 2002;168:900–908. doi: 10.4049/jimmunol.168.2.900. [DOI] [PubMed] [Google Scholar]
- Gal A, Li Y, Thompson DA, Weir J, Orth U, Jacobson SG, Apfelstedt-Sylla E, Vollrath D. Mutations in MERTK, the human orthologue of the RCS rat retinal dystrophy gene, cause retinitis pigmentosa. Nat Genet. 2000;26:270–271. doi: 10.1038/81555. [DOI] [PubMed] [Google Scholar]
- Herrmann M, Voll RE, Zoller OM, Hagenhofer M, Ponner BB, Kalden JR. Impaired phagocytosis of apoptotic cell material by monocyte-derived macrophages from patients with systemic lupus erythematosus. Arthritis Rheum. 1998;41:1241–1250. doi: 10.1002/1529-0131(199807)41:7<1241::AID-ART15>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Hsu DH, Moore KW, Spits H. Differential effects of IL-4 and IL-10 on IL-2-induced IFN-gamma synthesis and lymphokine-activated killer activity. Int Immunol. 1992;4:563–569. doi: 10.1093/intimm/4.5.563. [DOI] [PubMed] [Google Scholar]
- Huh HY, Pearce SF, Yesner LM, Schindler JL, Silverstein RL. Regulated expression of CD36 during monocyte-to-macrophage differentiation: potential role of CD36 in foam cell formation. Blood. 1996;87:2020–2028. [PubMed] [Google Scholar]
- Kamps MP, Baltimore D. E2A-Pbx1, the t(1;19) translocation protein of human pre-B-cell acute lymphocytic leukemia, causes acute myeloid leukemia in mice. Mol Cell Biol. 1993;13:351–357. doi: 10.1128/mcb.13.1.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamps MP, Murre C, Sun XH, Baltimore D. A new homeobox gene contributes the DNA binding domain of the t(1;19) translocation protein in pre-B ALL. Cell. 1990;60:547–555. doi: 10.1016/0092-8674(90)90658-2. [DOI] [PubMed] [Google Scholar]
- Kim S, Elkon KB, Ma X. Transcriptional suppression of interleukin-12 gene expression following phagocytosis of apoptotic cells. Immunity. 2004;21:643–653. doi: 10.1016/j.immuni.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- Lee JC, Young PR. Role of CSB/p38/RK stress response kinase in LPS and cytokine signaling mechanisms. J Leukoc Biol. 1996;59:152–157. doi: 10.1002/jlb.59.2.152. [DOI] [PubMed] [Google Scholar]
- Lewis TS, Shapiro PS, Ahn NG. Signal transduction through MAP kinase cascades. Adv Cancer Res. 1998;74:49–139. doi: 10.1016/s0065-230x(08)60765-4. [DOI] [PubMed] [Google Scholar]
- Lucas M, Stuart LM, Zhang A, Hodivala-Dilke K, Febbraio M, Silverstein R, Savill J, Lacy-Hulbert A. Requirements for Apoptotic Cell Contact in Regulation of Macrophage Responses. J Immunol. 2006;177:4047–4054. doi: 10.4049/jimmunol.177.6.4047. [DOI] [PubMed] [Google Scholar]
- Lucas M, Zhang X, Prasanna V, Mosser DM. ERK activation following macrophage FcgammaR ligation leads to chromatin modifications at the IL-10 locus. J Immunol. 2005;175:469–477. doi: 10.4049/jimmunol.175.1.469. [DOI] [PubMed] [Google Scholar]
- Ma W, Lim W, Gee K, Aucoin S, Nandan D, Kozlowski M, Diaz-Mitoma F, Kumar A. The p38 mitogen-activated kinase pathway regulates the human interleukin-10 promoter via the activation of Sp1 transcription factor in lipopolysaccharide-stimulated human macrophages. J Biol Chem. 2001;276:13664–13674. doi: 10.1074/jbc.M011157200. [DOI] [PubMed] [Google Scholar]
- Moens CB, Selleri L. Hox cofactors in vertebrate development. Dev Biol. 2006;291:193–206. doi: 10.1016/j.ydbio.2005.10.032. [DOI] [PubMed] [Google Scholar]
- Moore KW, Vieira P, Fiorentino DF, Trounstine ML, Khan TA, Mosmann TR. Homology of cytokine synthesis inhibitory factor (IL-10) to the Epstein-Barr virus gene BCRFI. Science. 1990;248:1230–1234. doi: 10.1126/science.2161559. [DOI] [PubMed] [Google Scholar]
- Navazo MD, Daviet L, Savill J, Ren Y, Leung LL, McGregor JL. Identification of a domain (155-183) on CD36 implicated in the phagocytosis of apoptotic neutrophils. J Biol Chem. 1996;271:15381–15385. doi: 10.1074/jbc.271.26.15381. [DOI] [PubMed] [Google Scholar]
- Nicholson AC, Frieda S, Pearce A, Silverstein RL. Oxidized LDL binds to CD36 on human monocyte-derived macrophages and transfected cell lines. Evidence implicating the lipid moiety of the lipoprotein as the binding site. Arterioscler Thromb Vasc Biol. 1995;15:269–275. doi: 10.1161/01.atv.15.2.269. [DOI] [PubMed] [Google Scholar]
- Nozaki S, Kashiwagi H, Yamashita S, Nakagawa T, Kostner B, Tomiyama Y, Nakata A, Ishigami M, Miyagawa J, Kameda-Takemura K, et al. Reduced uptake of oxidized low density lipoproteins in monocyte-derived macrophages from CD36-deficient subjects. J Clin Invest. 1995;96:1859–1865. doi: 10.1172/JCI118231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka K, Sawamura T, Kikuta K, Itokawa S, Kume N, Kita T, Masaki T. Lectin-like oxidized low-density lipoprotein receptor 1 mediates phagocytosis of aged/apoptotic cells in endothelial cells. Proc Natl Acad Sci U S A. 1998;95:9535–9540. doi: 10.1073/pnas.95.16.9535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt N, Suzuki H, Kurihara Y, Kodama T, Gordon S. Role for the class A macrophage scavenger receptor in the phagocytosis of apoptotic thymocytes in vitro. Proc Natl Acad Sci U S A. 1996;93:12456–12460. doi: 10.1073/pnas.93.22.12456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Y, Silverstein RL, Allen J, Savill J. CD36 gene transfer confers capacity for phagocytosis of cells undergoing apoptosis. J Exp Med. 1995;181:1857–1862. doi: 10.1084/jem.181.5.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigotti A, Acton SL, Krieger M. The class B scavenger receptors SR-BI and CD36 are receptors for anionic phospholipids. J Biol Chem. 1995;270:16221–16224. doi: 10.1074/jbc.270.27.16221. [DOI] [PubMed] [Google Scholar]
- Savill J, Dransfield I, Hogg N, Haslett C. Vitronectin receptor-mediated phagocytosis of cells undergoing apoptosis. Nature. 1990;343:170–173. doi: 10.1038/343170a0. [DOI] [PubMed] [Google Scholar]
- Savill J, Hogg N, Ren Y, Haslett C. Thrombospondin cooperates with CD36 and the vitronectin receptor in macrophage recognition of neutrophils undergoing apoptosis. J Clin Invest. 1992;90:1513–1522. doi: 10.1172/JCI116019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somersan S, Bhardwaj N. Tethering and tickling: a new role for the phosphatidylserine receptor. J Cell Biol. 2001;155:501–504. doi: 10.1083/jcb.200110066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staples KJ, Smallie T, Williams LM, Foey A, Burke B, Foxwell BM, Ziegler-Heitbrock L. IL-10 induces IL-10 in primary human monocyte-derived macrophages via the transcription factor Stat3. J Immunol. 2007;178:4779–4785. doi: 10.4049/jimmunol.178.8.4779. [DOI] [PubMed] [Google Scholar]
- Stuart LM, Ezekowitz RA. Phagocytosis: elegant complexity. Immunity. 2005;22:539–550. doi: 10.1016/j.immuni.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Swift GH, Liu Y, Rose SD, Bischof LJ, Steelman S, Buchberg AM, Wright CV, MacDonald RJ. An endocrine-exocrine switch in the activity of the pancreatic homeodomain protein PDX1 through formation of a trimeric complex with PBX1b and MRG1 (MEIS2) Mol Cell Biol. 1998;18:5109–5120. doi: 10.1128/mcb.18.9.5109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaoki T, Nomoto H, Takahashi I, Kato Y, Morimoto M, Tomita F. Staurosporine, a potent inhibitor of phospholipid/Ca++dependent protein kinase. Biochem Biophys Res Commun. 1986;135:397–402. doi: 10.1016/0006-291x(86)90008-2. [DOI] [PubMed] [Google Scholar]
- Tone M, Powell MJ, Tone Y, Thompson SA, Waldmann H. IL-10 gene expression is controlled by the transcription factors Sp1 and Sp3. J Immunol. 2000;165:286–291. doi: 10.4049/jimmunol.165.1.286. [DOI] [PubMed] [Google Scholar]
- Voll RE, Herrmann M, Roth EA, Stach C, Kalden JR, Girkontaite I. Immunosuppressive effects of apoptotic cells. Nature. 1997a;390:350–351. doi: 10.1038/37022. [DOI] [PubMed] [Google Scholar]
- Voll RE, Roth EA, Girkontaite I, Fehr H, Herrmann M, Lorenz HM, Kalden JR. Histone-specific Th0 and Th1 clones derived from systemic lupus erythematosus patients induce double-stranded DNA antibody production. Arthritis Rheum. 1997b;40:2162–2171. doi: 10.1002/art.1780401210. [DOI] [PubMed] [Google Scholar]
- Walport MJ, Lachmann PJ. Complement deficiencies and abnormalities of the complement system in systemic lupus erythematosus and related disorders. Curr Opin Rheumatol. 1990;2:661–663. doi: 10.1097/00002281-199002040-00018. [DOI] [PubMed] [Google Scholar]
- Yssel H, De Waal Malefyt R, Roncarolo MG, Abrams JS, Lahesmaa R, Spits H, de Vries JE. IL-10 is produced by subsets of human CD4+ T cell clones and peripheral blood T cells. J Immunol. 1992;149:2378–2384. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.