Abstract
Hypoxia-ischemia is relatively common in human infants. Hypoxia-ischemia can occur as a result of complications associated with prematurity or birth, frequently leading to altered brain development and cognitive and behavioral deficits that persist throughout life. Despite the relative frequency of neonatal hypoxic ischemic encephalopathy, the immature brain sustains relatively less damage than an adult who experiences a similar crisis of oxygen and nutrient deprivation. Therefore, factors may be present that protect the developing brain. During late gestation, the infant brain encounters high levels of the steroid hormone 17β-estradiol. This observation, combined with evidence supporting 17β-estradiol as a neuroprotective agent, led us to hypothesize that increasing the basal level of 17β-estradiol would reduce the amount of hypoxia-ischemia induced injury to the neonatal brain. To test that hypothesis we administered 17β-estradiol using either a repeated dosing paradigm or a single dose paradigm to immature male and female rats. Here we show that the repeated dosing paradigm (three doses of 17β-estradiol) provided approximately 70% protection of the hippocampus, basal ganglia, and amygdala. By contrast, a single administration of 17β-estradiol 24 hours prior to hypoxia-ischemia conferred little protection. The only exception was the pyramidal layer of the female hippocampus, which was modestly protected (16% reduction in damage). The protection afforded by the multiple administrations of 17β-estradiol was similar for females and males, with the only exception being the male amygdala, which displayed less damage than the female amgydala. We conclude that 17β-estradiol acts as a potent neuroprotective agent against hypoxia-ischemia induced damage to the developing brain, and that pretreating infants at risk for hypoxic ischemic injury may be advisable.
Introduction
Hypoxia, or a lack of oxygen, and the accompanying reduced blood flow, called ischemia, can impair brain function over the entire lifespan (Andine et al., 1990; Nyakas et al., 1996). Pioneering work by Vanucci and others has demonstrated that similar to human infants, the young (postnatal day 6) rat is susceptible to hypoxic-ischemic brain injury (Rice et al., 1981; Vanucci et al., 1993; Vanucci, 1993). And although a topic of debate, the developmental stage of the postnatal day 6–14 rat is considered to be equivalent to that of the newborn human infant (Dobbing and Sands, 1979; Romijn et al., 1991; Clancy et al., 2007). Using the technique of carotid artery ligation (modified from Levine, 1960), combined with a period of hypoxia, Vanucci and others have demonstrated profound pathological impairments in the hippocampus, basal ganglia and cerebral cortex in the ipsilateral hemisphere (Rice et al., 1981; Andine et al., 1990; Vanucci et al., 1993). The extent of damage is proportional to the duration of hypoxia (Nyakas et al., 1996). Much data has shown that hypoxia-ischemia induced in young rats produces anatomical and behavioral deficits similar to those observed in asphyxiated newborn humans (Vanucci, 1990; Simon, 1999). Intriguingly, while the immature mammal is susceptible to/readily encounters hypoxia-ischemia, the repercussions of injury are less severe than in adulthood. This leads us to speculate if some factor or group of factors in the developing brain buffer the extent of damage.
As mentioned above, many infants experience one or more hypoxic ischemic insult, yet have only moderate brain damage or behavioral problems. To produce significant brain damage in neonates, immature mice are typically exposed to a combination of hypoxia and ischemia for 70 minutes (Brazel et al., 2004). In contrast, to produce a similar extent of injury in the adult brain, adult mice are exposed to less than 25 minutes of hypoxia and ischemia (Basu et al., 2002). The fact that a longer insult is required to induce damage to the immature rodent indicates that the immature brain is relatively less vulnerable than the adult brain to hypoxia-ischemia. Of potential interest is that during late gestation, the infant brain encounters high levels of steroid hormones including 17β-estradiol, testosterone and progesterone.
Around the time of birth in both rat and human infants, circulating steroid hormone levels (i.e. testosterone, estradiol, and progesterone) are elevated, comparable to levels in adulthood (Warne et al., 1977; Forest, 1979; Weisz and Ward, 1980; Corbier et al., 1992; Knickmeyer and Baron-Cohen, 2006). Levels of testosterone and estradiol are elevated from embryonic day 17 though embryonic day 21 in rats, with another surge in hormone levels occurring immediately after birth. Elevated levels persist through the first postnatal week of life in rats (Dohler and Wuttke, 1974; Forest, 1979; Weisz and Ward, 1980; Corbier et al., 1992). In humans, testosterone biosynthesis begins around gestational week 9, with elevated levels of both testosterone and estradiol (due to aromatization from testosterone) persisting through gestational week 24 (Warne et al., 1977; Knickmeyer and Baron-Cohen, 2006). Levels increase again around the time of birth, with levels slowly dropping over the first three postnatal months (Forest, 1979). Progesterone levels in rats and humans are elevated prior to birth, dropping precipitously at birth (Warne et al., 1977; Forest, 1979). Testosterone and 17β-estradiol levels are relatively equivalent between immature rats and humans (Forest, 1979; Corbier et al., 1992), however sex differences exist in testosterone production (males>females) (Warne et al., 1977; Forest, 1979; Corbier et al., 1992; Knickmeyer and Baron-Cohen, 2006). The developmental production of steroid hormones (i.e. testosterone, progesterone and estradiol), comes from four distinct sources: 1) originating from the fetus, produced by the gonads or adrenal glands (Gerall et al., 1991), 2) originating from the mother, produced by her gonads and the placenta (Weisz and Ward, 1980), 3) locally converted from a biochemical precursor via the activity of an enzyme such as neuronal aromatase within rat pups (George and Ojeda, 1982), and 4) de novo synthesis in rat pups (Amateau et al., 2004).
The steroid hormones 17β-estradiol and progesterone are potent neuroprotective agents. 17β-estradiol attenuates injury due to stroke (Toung et al., 1998; Green et al., 2001), seizures (Veliskova et al., 2000), oxygen-glucose deprivation (Harms et al., 2001) and oxidative stress (Mize et al., 2003) in the adult central nervous system (CNS). Protection occurs via receptor-dependent and receptor-independent mechanisms including increasing levels of Bcl-2 (Singer et al., 1998; Dubal et al., 1999; Stoltzner et al., 2001; Zhao et al., 2004), decreasing the production of reactive oxygen species and enhanced uptake of free radicals (Prokai et al., 2003), attenuation of calcium currents (Huang et al., 2004; Hilton et al., 2006), and activation of survival-promoting signal transduction cascades (Linford et al., 2000; Jover et al., 2002). In contrast, little is known regarding the neuroprotective action of 17β-estradiol in the developing CNS. A recent study by McCarthy and colleagues documented that 17β-estradiol attenuated glutamate-mediated damage via decreasing mGluR1 protein levels, with a concomitant decrease in intracellular calcium (Hilton et al., 2006). While our knowledge of the developmental actions of progesterone is incomplete (Golub et al., 2006), there is a growing body of literature on the neuroprotective potential of progesterone (for a review, see Stein et al., 2007). In the adult, progesterone protects against traumatic brain injury, stroke and oxygen-glucose deprivation (Ardeshiri et al., 2006; Cutler et al., 2006). One potential mechanism of action is via activation of the GABAA receptor by the progesterone metabolite allopregnanolone (Ardeshiri et al., 2006).
Given that the immature brain is less vulnerable to hypoxia-ischemia and also experiences high levels of the steroid hormone estradiol, we hypothesized that elevating the levels of 17β-estradiol to slightly higher than physiologic circulating concentrations would render the immature brain even less vulnerable to hypoxia-ischemia induced injury. The experiments that we report herein were designed to test that hypothesis.
Materials and Methods
Perinatal Hypoxia Ischemia Model
Timed Pregnant Wistar rats were purchased from Charles River Laboratories (Charles River, Wilmington, DE). Animals were housed in individual cages and fed standard lab chow. After normal delivery, the litter size was adjusted to 12 pups per litter. Cerebral hypoxia-ischemia (H/I) was produced in male and female 6-day-old rats (day of birth being postnatal day 0). Briefly, pups were anesthetized with isoflurane (4% induction, 2% maintenance). Once anesthetized, a midline neck incision was made and the right common carotid artery (CCA) was identified. The CCA was separated from the vagus nerve and then cauterized. Animals were returned to the dam for 1.5 hours. The pups were then pre-warmed in jars for 20 minutes in a 37° C water bath and then exposed to systemic 8% 02 balance N2 gas (humidified) at 37° C for 90 minutes. After hypoxia the pups were returned to their dam for a recovery period of 7 days, at which time they were deeply anesthetized with pentobarbital and sacrificed by intracardiac perfusion. This protocol has been optimized such that only two animals (both females) died following hypoxia-ischemia. All experimental animal procedures were approved by the UMDNJ IACUC Committee. The animal experimentation in this paper was in accordance with the Society for Neuroscience’s policy on the appropriate use of animals for neuroscience research. The authors also certify that all experiments on animals were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication 80–23, rev. 1996). The authors further attest that all efforts were made to minimize the number of animals used and their suffering.
Hormone Manipulation
One group of animals received 17β-estradiol (50 μg/0.05 ml dissolved in sesame oil) administered IP on postnatal days (PN) 1, 3 and 5, with H/I performed on day 6 (repeated estradiol). Initial body weights were not statistically significant between the groups, therefore a fixed concentration was administered. Another group of pups received the same dose of 17β-estradiol on PN 5 only (single dose estradiol). Administration of the 50μg dose of 17β-estradiol on postnatal day one and two has previously been shown to elevate hippocampal tissue levels of estradiol (when collected on postnatal day two) to 53.5% greater than vehicle treated animals at the same time point. The estradiol concentration on postnatal day two is only 32% higher than the peak hippocampal tissue levels of estradiol observed on postnatal day one (Amateau et al., 2004). Vehicle treated animals received a 0.05ml injection of sesame oil (n=5 total for all groups).
Histology
Animals were perfusion fixed with 4% paraformaldehyde in 0.1M phosphate buffer. Brains were removed and immersion fixed in the same fixative overnight at 4° C. The brains were then blocked and prepared for paraffin embedding using standard methods. Coronal sections were cut through the entire brain at 6 μm and mounted onto coated slides prior to staining for cresyl violet.
Stereology
The anterior to posterior extent of four brain regions: 1) hippocampus, 2) dentate gyrus, 3) basal ganglia and 4) amygdala were measured for each animal. The anterior-most tissue section containing the hippocampus, dentate gyrus, basal ganglia or amygdala was noted as section 0H, section 0D, section 0B or section 0A, respectively. The first section that underwent volumetric analysis using the Cavalieri estimator was a randomly chosen tissue section that was within 30μm (five tissue sections) of the anterior-most tissue section (section 0H, 0D, 0B or 0A) containing the particular brain region of interest. Each subsequent tissue section that underwent volumetric analysis was exactly 120μm (twenty tissue sections) from the previously analyzed tissue section. The last tissue section selected for volumetric analysis was the last plane in which the particular region of interest was present, with a total of 8 to 10 tissue sections analyzed / animal. Cavalieri estimation was performed using the 4X objective and 75μm grid spacing for the hippocampus, dentate gyrus and basal ganglia, and the 2X objective and 150μm grid spacing for the amygdala. The Stereo Investigator program package (MicrobrightField version 6.01, Colchester, VT) was used to convert the volume of each tissue plane (tissue thickness multiplied by the surface area, as estimated using the Cavalieri method), to a total volume of a particular brain region by summing the areas across all tissue planes investigated (8–10 total), and through the entire depth of the tissue planes sampled (between 1500 and 1920μm).
Statistical Analysis
Two way analysis of variance (sex, treatment) was performed using the Systat program package (version 11.0, Richmond, CA) on hippocampal, dentate gyrus, basal ganglia and amygdala volume, followed by the post-hoc Tukey’s test (p<0.05 to obtain significance).
Results
Ammon’s Horn of the Hippocampus
There was a significant main effect of treatment (F2,24=156.67, p<0.0001), and a treatment by sex interaction (F2,24=4.027, p<0.035) on the volume of Ammon’s Horn (Figure 1A). Perinatal hypoxia-ischemia (H/I) led to an 85–89% reduction in hippocampal volume in young animals, with no difference between the sexes in the magnitude of injury. In both males and females, repeated treatment (three administrations) with physiologic levels of 17β-estradiol resulted in significant attenuation of damage – a 72.93% reduction in males, and a 63.32% reduction in females as compared to their same-sex vehicle treated counterparts (Tukey’s, p<0.001 for each measure) (Figure 2A–D). While a single administration of 17β-estradiol had no effect on H/I-induced damage to male pyramidal neurons, there was a significant measure of protection (15.85%) of female pyramidal neurons afforded by a single administration of 17β-estradiol (Tukey’s, p<0.05).
Figure 1.
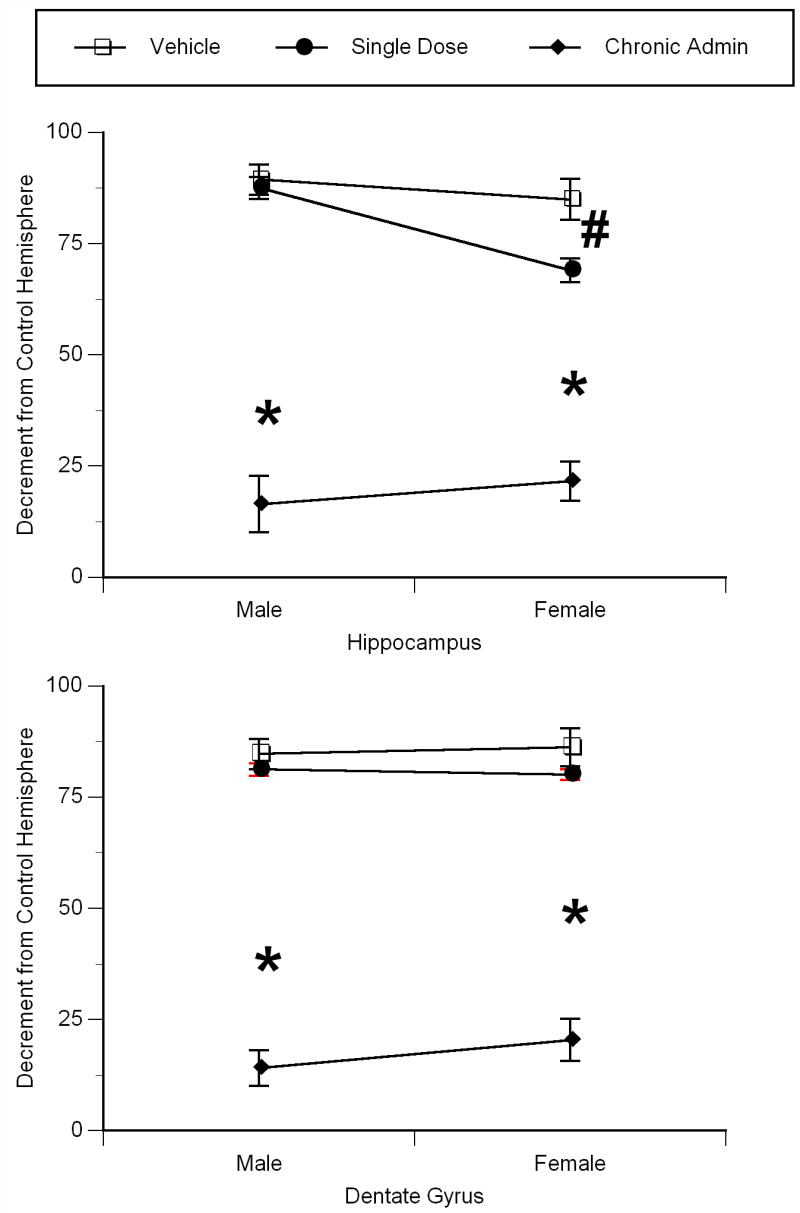
Chronic pretreatment with physiologic levels of 17β-estradiol protects the neonatal hippocampus from the damaging effects of perinatal hypoxia/ischemia. A) Ammons horn and, B) dentate gyri of P13 male and female rats. 17β-estradiol (50μg) was administered on postnatal days 1, 3 and 5, with H/I occurring on postnatal day 6 (postnatal day 0 is the day of birth). Data represent the mean decrement in volume as compared to the unaffected hemisphere ± SEM values, obtained from five animals in each group. * indicates significant difference from vehicle treated and single dose 17β-estradiol treated animals of the same sex (Tukey’s, p<0.01). # indicates significant difference from vehicle treated animals of the same sex (Tukey’s, p<0.05).
Figure 2.
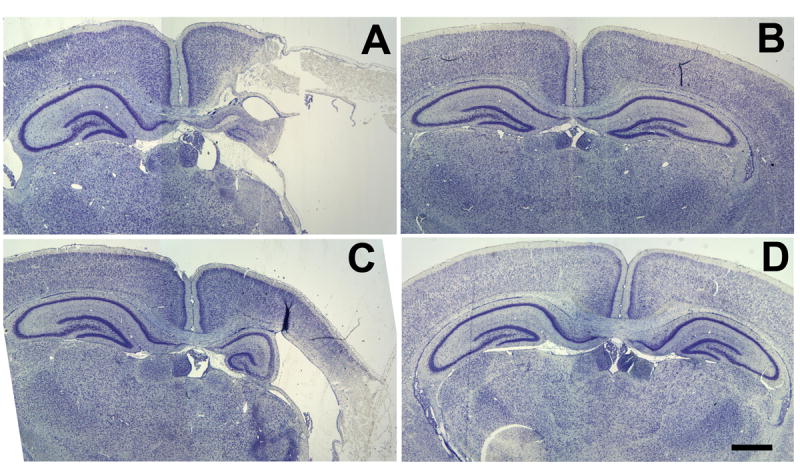
Repeated 17β-estradiol protects the forebrain of both sexes from H/I. animals. Photomicrographs of cresyl violet stained brains illustrate effects of perinatal H/I on the neocortex, Ammon’s horn and dentate gyrus of the hippocampus. A) vehicle treated males, B) chronic 17β-estradiol treated males, C) vehicle treated females and D) chronic 17β-estradiol treated females. In all photomicrographs, the H/I hemisphere is the right hemisphere. Scale bar = 500μm.
Dentate Gyrus
There was a significant main effect of treatment (F2,24=240.079, p<0.0001) on dentate granule cell layer volume (Figure 1B). Perinatal H/I reduced dentate granule cell volume by 84–86%, with no sex difference in the magnitude of injury. As in the ammon’s horn, both males and females who received the repeated 17β-estradiol treatment regiment showed attenuated damage – a 70.56% reduction in males, and a 65.75% reduction in females when compared to their same-sex vehicle treated counterparts (Tukey’s, p<0.001 for each measure) (Figure 2A–D). A one-time pretreatment with 17β-estradiol had no effect on H/I-induced damage to the male and female dentate gyrus.
Basal Ganglia
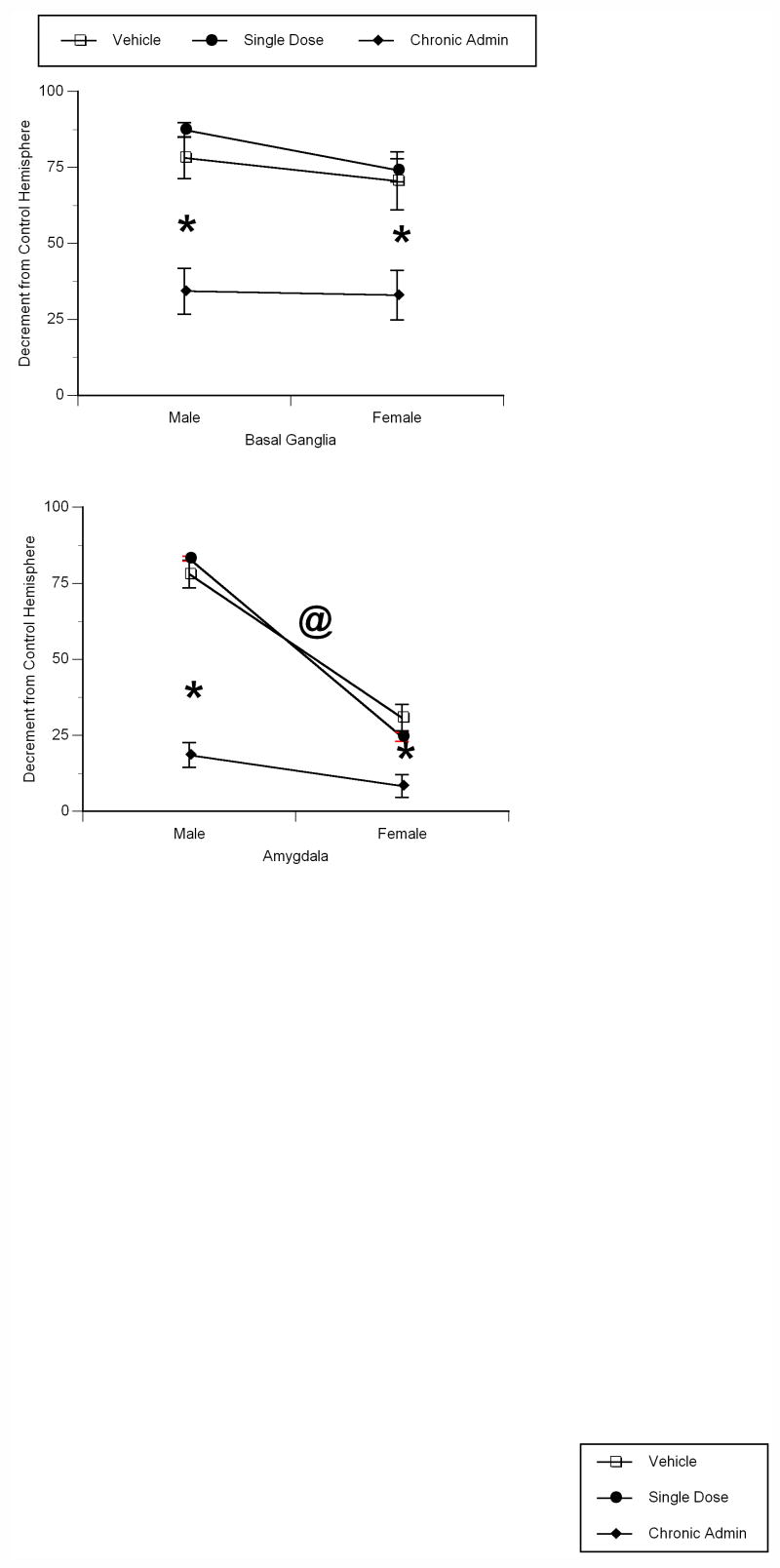
There was a significant main effect of treatment (F2,24=27.577, p<0.0001) on the volume of the basal ganglia (Figure 3A). There was a 71–78% reduction in basal ganglia volume following H/I in young animals, with no difference between the sexes in the magnitude of injury. Males and females who were repeatedly administered 17β-estradiol prior to H/I displayed significantly attenuated damage – a 43.89% reduction (as compared to vehicle treated animals) in males, and a 37.60% reduction (as compared to vehicle treated animals) in females (Tukey’s, p<0.001 for each measure). The single dose administration of 17β-estradiol had no effect on H/I-induced damage to the male and female basal ganglia.
Figure 3.
Chronic pretreatment with physiologic levels of 17β-estradiol protects the basal ganglia and amygdala from the damaging effects of perinatal hypoxia/ischemia in the A) basal ganglia and B) amygdala of young male and female rats. Data represent the mean decrement in volume as compared to the unaffected hemisphere ± SEM values, obtained from five animals in each group. * indicates significant difference from vehicle treated and single dose 17β-estradiol treated animals of the same sex (Tukey’s, p<0.01). @ indicates significant difference from vehicle treated animals of the opposite sex (Tukey’s, p<0.01).
Amygdala
There was a significant main effect of treatment (F2,24=90.556, p<0.0001), sex (F1,24=183.956, p<0.0001), and a treatment by sex interaction (F2,24=22.267, p<0.0001) on volume of the amygdala (Figure 3B). H/I differentially affected the male and female amygdala, resulting in a 77.70% reduction in male amygdala volume, as compared to a 30.76% reduction in female amygdala volume (Tukey’s, p<0.01) (Figure 4A–C). In both males and females, repeated treatment with 17β-estradiol significantly attenuated amygdala damage – a 56.17% reduction in males, and a 22.44% reduction in females (Tukey’s, p<0.001 for each measure). Consistent with the basal ganglia and dentate gyrus, a single pretreatment with 17β-estradiol had no effect on H/I-induced damage to the amygdala in both males and females.
Figure 4.
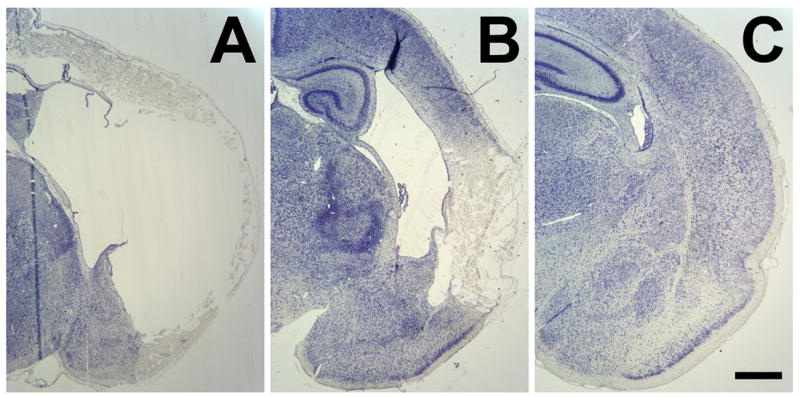
Severe extent of amygdalar damage in vehicle treated animals of both sexes, along with the attenuation of deficits in the chronic 17β-estradiol pretreated animals. Photomicrographs of cresyl violet staining illustrate the effects of perinatal H/I on the neocortex and amygdaloid region of A) vehicle treated males, B) vehicle treated females and C) chronic 17β-estradiol treated females. Scale bar = 500μm.
Discussion
Hypoxia-ischemia is relatively common in full term human infants, occurring in approximately 4 every 1000 births. The incidence of hypoxia-ischemia is increased dramatically to 50% in prematurely born human infants (Billards et al., 2006; Jensen, 2006). Given the pervasiveness of hypoxia-ischemia in human infants, and the persistence of deficits caused by hypoxia-ischemia throughout the lifespan, it is surprising that relatively few pharmacologic methods of neuroprotection are available for infants. Here we investigated the effectiveness of 17β-estradiol, an endogenously produced steroid hormone whose levels are naturally elevated during late gestation, on hypoxia-ischemia (H/I) induced injury to the developing male and female rat brain. The neuroprotective action of 17β-estradiol has been documented in the adult and aged animal against stroke and H/I-induced injury. Data have illustrated that at physiologic levels, 17β-estradiol protects the mature nervous system (Wise et al., 2001). However, 17β-estradiol should not be considered a panacea – there is much controversy regarding the effects of differing doses and treatment schedules of 17β-estradiol, with these controversies leading to the need for further investigation (Chen et al., 2006; De Butte-Smith et al., 2007).
The results of the present study support the conclusion that an extended period of high physiological levels of 17β-estradiol protects against H/I-induced damage to the hippocampal formation (including Ammons horn and the dentate gyrus), basal ganglia and amygdala in neonatal male and female rats. By the end of the first postnatal week in rats (when H/I was administered), estradiol levels are naturally waning (Dohler and Wuttke, 1974; Weisz and Ward, 1980). Similarly, following birth, estradiol levels in humans are dropping (Forest, 1979; Corbier et al., 1992). Therefore, the treatment regiment used in the present study may be applicable not only to other animal models of postnatal brain injury, but may also be appropriate for human infants at risk for traumatic events such as H/I. The paradigm of 50μg 17β-estradiol treatment every other day ensures maintained elevated estradiol levels that are in the high physiologic range (Nuñez and McCarthy, 2003; Amateau et al., 2004). However promising, much more research is needed prior to taking this lab bench research to the bedside.
17β-estradiol has been demonstrated to confer neuroprotection via genomic and non-genomic mechanisms (Culmsee et al., 1999; Dubal et al., 1999; Linford et al., 2000; Jover et al, 2002; Zhao et al., 2005). The non-genomic mechanisms include reduction in calcium influx and decreases in the production of reactive oxygen species and other free radicals (Chen et al., 1998; Culmsee et al., 1999). These events are unaffected by treatment with the estrogen receptor antagonists tamoxifen or ICI 182,780. An alternative manner of neuroprotection involving the non-genomic actions of estradiol, yet dependent upon the binding of estrogen to the estrogen receptor (occuring within the cytosol – prior to translocation into the nucleus), involves an increase in ERK1/2 phosphorylation, activation of p42/44 MAP kinase, tyrosine kinase, protein kinase A and PI-3 kinase cascades, along with attenuation of caspase-3 activation (Bi et al., 2000; Linford et al., 2000). All of the receptor-independent, non-genomic neuroprotective effects of estradiol are rapid – occurring within minutes to hours of estradiol exposure (Fugger et al., 2001; Marin et al., 2005; Balthazart et al., 2006). While in vitro models of injury allow for the use of selective estrogen receptor antagonists (Heyer et al., 2005), the majority of pharmacologic mechanisms for distinguishing between genomic and non-genomic mechanisms of neuroprotection in vivo are fraught with complications as a consequence of the lack of selective estrogen receptor antagonists (Musa et al., 2007; Zhang et al., 2007). A relatively short (less than 24 hour pretreatment), administration paradigm is thought to activate the rapid, non-genomic actions of 17β-estradiol, while multiple administrations of 17β-estradiol over a series of days initiate the long term, genomic actions of 17β-estradiol. We saw a dramatic difference in outcome between the acute (single administration) and longer term (repeated) 17β-estradiol treatment paradigms. Also, with the exception of the female hippocampus, the single administration of 17β-estradiol was without effect on H/I-induced damage.
The repeated administration of 17β-estradiol reduced damage by approximately 70% in all brain regions examined and in across both sexes. While the potential exists that the differential effects of single versus repeated administration are the result of a threshold effect (a certain concentration of 17β-estradiol is required for action, which is only attained by the long term administration paradigm), we hypothesize that the genomic actions of 17β-estradiol are responsible for neuroprotection against H/I-induced injury. The genomic effects of 17β-estradiol are important in increasing Bcl-2, BDNF and p75 neurotrophin receptor expression, along with decreasing ischemia and stroke-induced cellular damage in adulthood (Toung et al., 1998; Dubal et al., 1999; Jover et al, 2002). These events are less rapid, occurring within days (Abraham and Herbison, 2005). Thus, the protective effects of 17β-estradiol are multi-faceted: in adult models of brain injury, 17β-estradiol can confer a fast acting, non-genomic, receptor-independent means of neuroprotection by activating survival-promoting cascades and buffering the changes in intracellular pH. 17β-estradiol can also activate a longer term, genomic, receptor-dependent mechanisms which inhibit apoptosis. Given the implication that 17β-estradiol is protective against neonatal H/I via genomic mechanisms, future work using selective estrogen receptor antagonists, estrogens that have little binding affinity for the estrogen receptor, and selective estrogen receptor α and β agonists need to be employed.
Sex differences exist in basic anatomical parameters and physiologic properties that may differentially predispose one sex versus the other to brain injury (Arnold and Gorski, 1984). As mentioned previously, developing male and female brains are exposed to elevated levels of steroid hormones, including estradiol and testosterone (Weisz and Ward, 1980; Gerall et al., 1991). Males encounter higher circulating levels of both testosterone and estradiol than females (Weisz and Ward, 1980). Also, estrogen receptor (predominantly the α isoform) immunoreactive neurons and protein are present in the developing hippocampus and basal ganglia by postnatal day 3–4, and the amygdala by postnatal day one (Yokosuka et al., 1997; Solum and Handa, 2001; Perez et al., 2003). However, there has been little systematic research investigating sex differences in estrogen receptor presence or binding in these regions of the developing brain. It is hypothesized that the sex differences in physiology and neuroendocrine milieu may play a role in the response to traumatic events (Nuñez and McCarthy, 2003; Nuñez et al., 2005). There is a growing body of literature showing that males are more sensitive to deleterious events that may affect the developing brain than females (Hall et al., 1991; Lauterbach et al., 2001; Yager et al., 2005). In the current investigation, we documented sex differences on the response to H/I-induced injury. We observed no sex differences in the magnitude of H/I-induced damage in both the hippocampal formation and basal ganglia. However, we did observe a significant effect of sex on H/I-induced damage to the amygdala, with the amygdala of males sustaining a greater magnitude of damage than the amygdala of females.
Differences exist between brain regions in response to the deleterious effects of H/I. These differences extend to the subregional level, with findings of differential effects among the subfields of the hippocampus in response to H/I and excitotoxicity (Mattson et al., 1989; Xu et al., 2001). In males, we documented equal sensitivity amongst all the brain regions investigated to H/I-induced damage. While damage was greatest in the pyramidal cells of the hippocampus and dentate gyrus (90% loss of volume), it was less in the basal ganglia and amygdala (80% loss of volume). In females, the amygdala was markedly less sensitive to H/I-induced damage than all other regions – we documented a reduction in volume of 85% in the hippocampus and dentate gyrus, 70% in the basal ganglia, and strikingly, only 35% in the amygdala. Further research is needed to understand the natural resistance of the female amygdala to H/I-induced injury.
While repeated 17β-estradiol administration protected the hippocampus, amygdala and basal ganglia from structural damage following neonatal H/I, further investigation is required to document the extent of functional protection. Also, the protected cells may be permanently altered such that their electrophysiological responses may be different from those in normal animals. Future work from this lab will address these two issues, particularly as they related to hippocampal function.
This study highlights the neuroprotective potential of 17β-estradiol in a model of H/I-induced brain injury. Both males and females displayed significantly attenuated damage in the hippocampus, basal ganglia and amygdala following pretreatment with 17β-estradiol. Protection from the damaging effects of H/I appears to require repeated, long term administration. In contrast, there was little to no protection afforded by a single administration of 17β-estradiol 24 hours prior to H/I. These data indicate that administering 17β-estradiol to premature infants who are at risk for H/I is advisable. However, caution is warranted as work from our lab has also shown that 17β-estradiol may exacerbate developmental injuries with underlying GABAA receptor involvement. These include fetal alcohol syndrome and seizures (Dzhala et al., 2005; Galindo et al., 2005; Khalilov et al., 2005; Young et al., 2005). While 17β-estradiol exerts substantial neuroprotection from hypoxia/ischemia, the benefit it confers may be dependent upon the type of injury and the age of the infant.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrahám IM, Herbison AE. Major sex differences in non-genomic estrogen actions on intracellular signaling in mouse brain in vivo. Neuroscience. 2005;131:945–51. doi: 10.1016/j.neuroscience.2004.10.046. [DOI] [PubMed] [Google Scholar]
- Amateau SK, Alt JJ, Stamps CL, McCarthy MM. Brain estradiol content in newborn rats: sex differences, regional heterogeneity, and possible de novo synthesis by the female telencephalon. Endocrinology. 2004;145:2906–17. doi: 10.1210/en.2003-1363. [DOI] [PubMed] [Google Scholar]
- Andine P, Thordstein M, Kjellmer I, Nordborg C, Thiringer K, Wennberg E, Hagberg H. Evaluation of brain damage in a rat model of neonatal hypoxic-ischemia. J Neurosci Methods. 1990;35:253–60. doi: 10.1016/0165-0270(90)90131-x. [DOI] [PubMed] [Google Scholar]
- Ardeshiri A, Kelley MH, Korner IP, Hurn PD, Herson PS. Mechanisms of progesterone neuroprotection of rat cerebellar Purkinje cells following oxygen-glucose deprivation. Eur J Neurosci. 2006;24:2567–2574. doi: 10.1111/j.1460-9568.2006.05142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold AP, Gorski RA. Gonadal steroid induction of structural sex differences in the central nervous system. Annu Rev Neurosci. 1984;7:413–42. doi: 10.1146/annurev.ne.07.030184.002213. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Cornil CA, Taziaux M, Charlier TD, Baillien M, Ball GF. Rapid changes in production and behavioral action of estrogens. Neuroscience. 2006;138:783–91. doi: 10.1016/j.neuroscience.2005.06.016. [DOI] [PubMed] [Google Scholar]
- Basu A, Krady JK, O’Malley M, Stryen SD, DeKosky ST, Levison SW. The type 1 interleukin-1 receptor is essential for the efficient activation of microglia in response to brain injury and the expression of specific pre-inflammatory mediators. J Neurosci. 2002;22:6071–6082. doi: 10.1523/JNEUROSCI.22-14-06071.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi R, Broutman G, Foy MR, Thompson RF, Baudry M. The tyrosine kinase and mitogen-activated protein kinase pathways mediate multiple effects of estrogen in hippocampus. Proc Natl Acad Sci USA. 2000;97:3602–7. doi: 10.1073/pnas.060034497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billiards SS, Pierson CR, Haynes RL, Folkerth RD, Kinney HC. Is the late preterm infant more vulnerable to gray matter injury than the term infant? Clin Perinatol. 2006;33:915–33. doi: 10.1016/j.clp.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Brazel CY, Rosti RT, Boyce S, Rothstein RP, Levison SW. Perinatal hypoxia-ischemia damages and depletes progenitors from the mourse subventricular zone. Develop Neurosci. 2004;26:266–274. doi: 10.1159/000082143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Adachi N, Liu K, Arai T. The effects of 17beta-estradiol on ischemia-induced neuronal damage in the gerbil hippocampus. Neuroscience. 1998;87:817–22. doi: 10.1016/s0306-4522(98)00198-5. [DOI] [PubMed] [Google Scholar]
- Chen S, Nilsen J, Brinton RD. Dose and temporal pattern of estrogen exposure determines neuroprotective outcome in hippocampal neurons: therapeutic implications. Endocrinology. 2006;147:5303–5313. doi: 10.1210/en.2006-0495. [DOI] [PubMed] [Google Scholar]
- Clancy B, Finlay BL, Darlington RB, Anand KJ. Extrapolating brain development from experimental species to humans. Neurotoxicology. 2007 doi: 10.1016/j.neuro.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbier P, Edwards DA, Roffi J. The neonatal testosterone surge: a comparative study. Arch Int Phys Biochem Biophys. 1992;100:127–131. doi: 10.3109/13813459209035274. [DOI] [PubMed] [Google Scholar]
- Culmsee C, Vedder H, Ravati A, Junker V, Otto D, Ahlemeyer B, Krieg JC, Krieglstein J. Neuroprotection by estrogens in a mouse model of focal cerebral ischemia and in cultured neurons: evidence for a receptor-independent antioxidative mechanism. J Cereb Blood Flow Metab. 1999;19:1263–9. doi: 10.1097/00004647-199911000-00011. [DOI] [PubMed] [Google Scholar]
- Cutler SM, VanLandingham JW, Murphy AZ, Stein DG. Slow-release and injected progesterone treatments enhance acute recovery after traumatic brain injury. Pharmacol Biochem Behav. 2006;84:420–428. doi: 10.1016/j.pbb.2006.05.029. [DOI] [PubMed] [Google Scholar]
- De Butte-Smith M, Nguyen AP, Zukin RS, Etgen AM, Colbourne F. Failure of estradiol to ameliorate global ischemia-induced CA1 sector injury in middle-aged female gerbils. Brain Res. 2007;1153:214–220. doi: 10.1016/j.brainres.2007.03.082. [DOI] [PubMed] [Google Scholar]
- Dobbing J, Sands J. Comparative aspects of the brain growth spurt. Early Hum Dev. 1979;3:79–83. doi: 10.1016/0378-3782(79)90022-7. [DOI] [PubMed] [Google Scholar]
- Dohler KD, Wuttke W. Changes with age in levels of serum gonadotropins, prolactin and gonadal steroids in prepubertal male and female rats. Endocrinology. 1975;97:898–907. doi: 10.1210/endo-97-4-898. [DOI] [PubMed] [Google Scholar]
- Dubal DB, Shughrue PJ, Wilson ME, Merchenthaler I, Wise PM. Estradiol modulates bcl-2 in cerebral ischemia: a potential role for estrogen receptors. J Neurosci. 1999;19:6385–93. doi: 10.1523/JNEUROSCI.19-15-06385.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzhala VI, Talos DM, Sdrulla DA, Brumback AC, Mathews GC, Benke TA, Delpire E, Jensen FE, Staley KJ. NKCC1 transporter facilitates seizures in the developing brain. Nat Med. 2005;11:1205–13. doi: 10.1038/nm1301. [DOI] [PubMed] [Google Scholar]
- Forest MG. Plasma androgens (testosterone and 4-androstenedione) and 17-hydroxyprogesterone in the neonatal and peripubertal periods in the human and rat: differences between species. J Steroid Biochem. 1979;11:543–548. doi: 10.1016/0022-4731(79)90080-3. [DOI] [PubMed] [Google Scholar]
- Fugger HN, Kumar A, Lubahn DB, Korach KS, Foster TC. Examination of estradiol effects on the rapid estradiol mediated increase in hippocampal synaptic transmission in estrogen receptor alpha knockout mice. Neurosci Lett. 2001;309:207–9. doi: 10.1016/s0304-3940(01)02083-3. [DOI] [PubMed] [Google Scholar]
- Galindo R, Zamudio PA, Valenzuela CF. Alcohol is a potent stimulant of immature neuronal networks: implications for fetal alcohol spectrum disorder. J Neurochem. 2005;94:1500–11. doi: 10.1111/j.1471-4159.2005.03294.x. [DOI] [PubMed] [Google Scholar]
- George FW, Ojeda SR. Changes in aromatase activity in the rat brain during embryonic, neonatal, and infantile development. Endocrinology. 1982;111:522–9. doi: 10.1210/endo-111-2-522. [DOI] [PubMed] [Google Scholar]
- George FW, Wilson JD. Sex determination and differentiation. In: Nobil, Neill, editors. The Physiology of Reproduction. New York: Raven Press; 1994. [Google Scholar]
- Gerall AA, Moltz H, Ward IL. Handbook of Behavioral Neurobiology: Sexual Differentiation. In: Gerall, Moltz, Ward, editors. Handbook of Behavioral Neurobiology. Vol. 11. NewYork: Plenum Press; 1991. [Google Scholar]
- Golub MS, Kaufman FL, Campbell MA, Li LH, Donald JM. “Natural” progesterone: information on fetal effects. Birth Defects Res. 2006;77:455–470. doi: 10.1002/bdrb.20089. [DOI] [PubMed] [Google Scholar]
- Green PS, Yang SH, Nilsson KR, Kumar AS, Covey DF, Simpkins JW. The nonfeminizing enantiomer of 17beta-estradiol exerts protective effects in neuronal cultures and a rat model of cerebral ischemia. Endocrinology. 2001;142:400–406. doi: 10.1210/endo.142.1.7888. [DOI] [PubMed] [Google Scholar]
- Hall ED, Pazara KE, Linseman KL. Sex differences in postischemic neuronal necrosis in gerbils. J Cereb Blood Flow Metab. 1991;11:292–8. doi: 10.1038/jcbfm.1991.61. [DOI] [PubMed] [Google Scholar]
- Harms C, Lautenschlager M, Bergk A, Katchanov J, Freyer D, Kapinya K, Herwig U, Megow D, Dirnagl U, Weber JR, Hortnagl H. Differential mechanisms of neuroprotection by 17 beta-estradiol in apoptotic versus necrotic neurodegeneration. J Neurosci. 2001;21:2600–2609. doi: 10.1523/JNEUROSCI.21-08-02600.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyer A, Hasselblatt M, von Ahsen N, Häfner H, Sirén AL, Ehrenreich H. In vitro gender differences in neuronal survival on hypoxia and in 17beta-estradiol-mediated neuroprotection. J Cereb Blood Flow Metab. 2005;25:427–30. doi: 10.1038/sj.jcbfm.9600056. [DOI] [PubMed] [Google Scholar]
- Hilton GD, Nuñez JL, Bambrick LL, Thompson SM, McCarthy MM. Glutamate-mediated excitotoxicity in neonatal hippocampal neurons is mediated by mGluR-induced release of calcium from intracellular stores and is prevented by estradiol. Eur J Neurosci. 2006;24:3008–3016. doi: 10.1111/j.1460-9568.2006.05189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Huang YL, Zhang S, Zhu YC, Yao T. Estradiol acutely attenuates glutamate-induced calcium overload in primarily cultured rat hippocampal neurons through a membrane receptor-dependent mechanism. Brain Res. 2004;1026:254–260. doi: 10.1016/j.brainres.2004.08.038. [DOI] [PubMed] [Google Scholar]
- Jensen FE. Developmental factors regulating susceptibility to perinatal brain injury and seizures. Curr Opin Pediatr. 2006;18:628–33. doi: 10.1097/MOP.0b013e328010c536. [DOI] [PubMed] [Google Scholar]
- Jover T, Tanaka H, Calderone A, Oguro K, Bennett MV, Etgen AM, Zukin RS. Estrogen protects against global ischemia-induced neuronal death and prevents activation of apoptotic signaling cascades in the hippocampal CA1. J Neurosci. 2002;22:2115–24. doi: 10.1523/JNEUROSCI.22-06-02115.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalilov I, Le Van Quyen M, Gozlan H, Ben-Ari Y. Epileptogenic actions of GABA and fast oscillations in the developing hippocampus. Neuron. 2005;48:787–96. doi: 10.1016/j.neuron.2005.09.026. [DOI] [PubMed] [Google Scholar]
- Knickermeyer RC, Baron-Cohen S. Fetal testosterone and sex differences. Early Human Develop. 2006;82:755–760. doi: 10.1016/j.earlhumdev.2006.09.014. [DOI] [PubMed] [Google Scholar]
- Lauterbach MD, Raz S, Sander CJ. Neonatal hypoxic risk in preterm birth infants: the influence of sex and severity of respiratory distress on cognitive recovery. Neuropsychology. 2001;15:411–20. [PubMed] [Google Scholar]
- Linford N, Wade C, Dorsa D. The rapid effects of estrogen are implicated in estrogen-mediated neuroprotection. J Neurocytol. 2000;29:367–74. doi: 10.1023/a:1007113323582. [DOI] [PubMed] [Google Scholar]
- Marin R, Guerra B, Alonso R, Ramírez CM, Díaz M. Estrogen activates classical and alternative mechanisms to orchestrate neuroprotection. Curr Neurovasc Res. 2005;2:287–301. doi: 10.2174/156720205774322629. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Guthrie PB, Kater SB. A role for Na+-dependent Ca2+ extrusion in protection against neuronal excitotoxicity. FASEB J. 1989;3:2519–26. doi: 10.1096/fasebj.3.13.2572500. [DOI] [PubMed] [Google Scholar]
- Mize AL, Shapiro RA, Dorsa DM. Estrogen receptor-mediated neuroprotection from oxidative stress requires activation of the mitogen-activated protein kinase pathway. Endocrinology. 2003;144:306–312. doi: 10.1210/en.2002-220698. [DOI] [PubMed] [Google Scholar]
- Musa MA, Khan MO, Cooperwood JS. Medicinal chemistry and emerging strategies applied to the development of selective estrogen receptor modulators (SERMs) Curr Med Chem. 2007;14:1249–61. doi: 10.2174/092986707780598023. [DOI] [PubMed] [Google Scholar]
- Nuñez JL, Bambrick LL, Krueger BK, McCarthy MM. Prolongation and enhancement of gamma-aminobutyric acid receptor mediated excitation by chronic treatment with estradiol in developing rat hippocampal neurons. Eur J Neurosci. 2005;21:3251–61. doi: 10.1111/j.1460-9568.2005.04175.x. [DOI] [PubMed] [Google Scholar]
- Nuñez JL, McCarthy MM. Estradiol exacerbates hippocampal damage in a model of preterm infant brain injury. Endocrinology. 2003;144:2350–9. doi: 10.1210/en.2002-220840. [DOI] [PubMed] [Google Scholar]
- Nyakas C, Buwalda B, Luiten PG. Hypoxia and brain development. Prog Neurobiol. 1996;49:1–51. doi: 10.1016/0301-0082(96)00007-x. [DOI] [PubMed] [Google Scholar]
- Perez SE, Chen EY, Mufson EJ. Distribution of estrogen receptor alpha and beta immunoreative profiles in the postnatal rat brain. Develop Brain Res. 2003;145:117–139. doi: 10.1016/s0165-3806(03)00223-2. [DOI] [PubMed] [Google Scholar]
- Prokai L, Prokai-Tatrai K, Perjesi P, Zharikova AD, Perez EJ, Liu R, Simpkins JW. Quinol-based cyclic antioxidant mechanism in estrogen neuroprotection. Proc Natl Acad Sci U S A. 2003;100:11741–11746. doi: 10.1073/pnas.2032621100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice JE, Vannucci RC, Brierley JB. The influence of immaturity on hypoxic-ischemic brain damage in the rat. Ann Neurol. 1981;9:131–41. doi: 10.1002/ana.410090206. [DOI] [PubMed] [Google Scholar]
- Romijn HJ, Hofman MA, Gramsbergen A. At what age is the developing cerebral cortex of the rat comparable to that of the full-term newborn human baby? Early Hum Dev. 1991;26:61–7. doi: 10.1016/0378-3782(91)90044-4. [DOI] [PubMed] [Google Scholar]
- Simon NP. Long-term neurodevelopmental outcome of asphyxiated newborns. Clin Perinatol. 1999;26:767–78. [PubMed] [Google Scholar]
- Singer CA, Rogers KL, Dorsa DM. Modulation of Bcl-2 expression: a potential component of estrogen protection in NT2 neurons. Neuroreport. 1998;9:2565–2568. doi: 10.1097/00001756-199808030-00025. [DOI] [PubMed] [Google Scholar]
- Solum DT, Handa RJ. Localization of estrogen receptor alpha in pyramidal neurons of the developing rat hippocampus. Develop Brain Res. 2001;128:165–175. doi: 10.1016/s0165-3806(01)00171-7. [DOI] [PubMed] [Google Scholar]
- Stein DG, Wright DW, Kellermann AL. Does progesterone have neuroprotective properties. Ann Emerg Med. 2007 doi: 10.1016/j.annemergmed.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Stoltzner SE, Berchtold NC, Cotman CW, Pike CJ. Estrogen regulates bcl-x expression in rat hippocampus. Neuroreport. 2001;12:2797–800. doi: 10.1097/00001756-200109170-00009. [DOI] [PubMed] [Google Scholar]
- Toung TJ, Traystman RJ, Hurn PD. Estrogen-mediated neuroprotection after experimental stroke in male rats. Stroke. 1998;29:1666–70. doi: 10.1161/01.str.29.8.1666. [DOI] [PubMed] [Google Scholar]
- Vannucci RC. Experimental models of perinatal hypoxic-ischemic brain damage. APMIS Suppl. 1993;40:89–95. [PubMed] [Google Scholar]
- Vannucci RC. Experimental biology of cerebral hypoxia-ischemia: relation to perinatal brain damage. Pediatr Res. 1990;27:317–26. doi: 10.1203/00006450-199004000-00001. [DOI] [PubMed] [Google Scholar]
- Vannucci RC, Christensen MA, Yager JY. Nature, time-course, and extent of cerebral edema in perinatal hypoxic-ischemic brain damage. Pediatr Neurol. 1993;9:29–34. doi: 10.1016/0887-8994(93)90006-x. [DOI] [PubMed] [Google Scholar]
- Warne GL, Faiman C, Reyes FI, Winter JS. Studies on human sexual development. V. Concentrations of testosterone, 17-hydroxyprogesterone and progesterone in human amniotic fluid through gestation. J Clin Endocrinol Metab. 1977;44:934–939. doi: 10.1210/jcem-44-5-934. [DOI] [PubMed] [Google Scholar]
- Weisz J, Ward IL. Plasma testosterone and progesterone titers of pregnant rats, their male and female fetuses, and neonatal offspring. Endocrinology. 1980;106:306–16. doi: 10.1210/endo-106-1-306. [DOI] [PubMed] [Google Scholar]
- Wise PM, Dubal DB, Wilson ME, Rau SW, Böttner M, Rosewell KL. Estradiol is a protective factor in the adult and aging brain: understanding of mechanisms derived from in vivo and in vitro studies. Brain Res Brain Res Rev. 2001;37:313–9. doi: 10.1016/s0165-0173(01)00136-9. [DOI] [PubMed] [Google Scholar]
- Xu L, Sapolsky RM, Giffard RG. Differential sensitivity of murine astrocytes and neurons from different brain regions to injury. Exp Neurol. 2001;169:416–24. doi: 10.1006/exnr.2001.7678. [DOI] [PubMed] [Google Scholar]
- Yager JY, Wright S, Armstrong EA, Jahraus CM, Saucier DM. A new model for determining the influence of age and sex on functional recovery following hypoxic-ischemic brain damage. Dev Neurosci. 2005;27:112–20. doi: 10.1159/000085982. [DOI] [PubMed] [Google Scholar]
- Yokosuka M, Okamura H, Hayashi S. Postnatal development and sex difference in neurons containing estrogen receptor alpha immunoreactivity in the preoptic brain, the diencephalon, and the amygdale in the rat. J Comp Neurol. 1997;389:81–93. doi: 10.1002/(sici)1096-9861(19971208)389:1<81::aid-cne6>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Young C, Jevtovic-Todorovic V, Qin YQ, Tenkova T, Wang H, Labruyere J, Olney JW. Potential of ketamine and midazolam, individually or in combination, to induce apoptotic neurodegeneration in the infant mouse brain. Br J Pharmacol. 2005;146:189–97. doi: 10.1038/sj.bjp.0706301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Milatovic D, Aschner M, Feustel PJ, Kimelberg HK. Neuroprotection by tamoxifen in focal cerebral ischemia is not mediated by an agonist action at estrogen receptors but is associated with antioxidant activity. Exp Neurol. 2007;204:819–27. doi: 10.1016/j.expneurol.2007.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Chen S, Ming Wang J, Brinton RD. 17beta-estradiol induces Ca2+ influx, dendritic and nuclear Ca2+ rise and subsequent cyclic AMP response element-binding protein activation in hippocampal neurons: a potential initiation mechanism for estrogen neurotrophism. Neuroscience. 2005;132:299–311. doi: 10.1016/j.neuroscience.2004.11.054. [DOI] [PubMed] [Google Scholar]
- Zhao L, Wu TW, Brinton RD. Estrogen receptor subtypes alpha and beta contribute to neuroprotection and increased Bcl-2 expression in primary hippocampal neurons. Brain Res. 2004;1010:22–34. doi: 10.1016/j.brainres.2004.02.066. [DOI] [PubMed] [Google Scholar]