Abstract
Associative recognition memory often is thought to rely primarily on recollection processes, but opinions differ regarding the possible contribution of familiarity. The current experiments capitalized on hypothesized event-related potential (ERP) measures of familiarity and recollection to assess the contribution of each process to associative recognition. In two ERP experiments participants studied pairs of fractals and were later tested on their ability to recognize the studied pairs. Early (100 – 175 ms) visual ERP components were sensitive to the novelty of individual fractals, but later components hypothesized to be indicative of familiarity and recollection were sensitive to the novelty of the association between fractals. These relationships suggest that accurate memory for visual associations may be dependent on both familiarity and recollection processes.
Keywords: Human Memory, Event-Related Potentials (ERPs), Associative Recognition, Familiarity
1. Introduction
The ability to rapidly form and later recognize associations between previously unrelated visual objects is central to our everyday lives. For example, recognizing an intersection based on the surrounding buildings is often critical to arriving at an appointment on time. This ability to remember associations between objects is distinct from the ability to remember the objects themselves: barring any neuropsychological disorders we can easily recognize a familiar building regardless of where it appears (e.g., in a picture vs. in its natural context). The dissociability of associative and item recognition is evidenced by both behavioral and neuroimaging studies. Associative recognition is more impaired than item recognition in healthy older adults (Bastin & Van der Linden, 2006), and in patients with neuropsychological disorders (e.g., schizophrenia; Achim & Lepage, 2003) or neurological damage (e.g., amnesia; Holdstock, Mayes, Gong, Roberts, & Kapur, 2005; Mayes, et al., 2004). Different brain regions are activated during associative and item recognition, (Achim & Lepage, 2005), and item information is more susceptible to forgetting than associative information (Hockley, 1992).
Some dual-process theories of recognition memory have suggested that these differences arise because associative and item recognition are supported by different processes (for a review, see Yonelinas, 2002). Familiarity is thought to rely on a continuous, content-free assessment of memory strength. On the other hand, recollection involves the ability to retrieve specific details associated with the original experience. This characterization of recollection as a process of bringing to mind details about the original study experience has led dual-process theorists to suggest that differences between associative and item recognition arise because associative recognition is supported solely by recollection processes (Yonelinas, 2002). In line with this hypothesis, associative recognition generates higher proportions of remember judgments (thought to reflect recollection; Tulving, 1985) than item recognition (Hockley & Consoli, 1999). Also, receiver operating curves (ROCs) for associative, but not item, recognition can be linear, suggesting that whereas recollection contributes to both item and associative recognition, familiarity contributes to only item recognition (Yonelinas, 1997; but see Parks & Yonelinas, 2007; Wixted, 2007).
However, recent dual-process conceptualizations of the role of familiarity and recollection in associative recognition suggest that there are certain conditions in which familiarity can contribute to associative recognition. These theories suggest that when individual items can be easily unitized into a single representation, such as studying the word pair “TRAFFIC-JAM”, both familiarity and recollection may contribute to associative recognition. Amnesic patients who typically have impaired recollection are able to recognize associations such as “SEA-HORSE” (Quamme, Yonelinas, & Norman, 2007). In addition, ROCs for recognition of associations between internal facial features (eyes, nose, etc.) and external facial features (hair, clothes) are curvilinear (Yonelinas, Kroll, Dobbins, & Soltani, 1999), suggesting that both familiarity and recollection contribute to associative recognition in unitized stimuli, such as faces.
In contrast to dual-process theories proposing that the use of familiarity in associative recognition tasks requires unitization, global matching models do not set any constraints on the ability for familiarity to contribute to associative recognition. These models suggest that a single strength value reflecting the degree of match between study and test can be based on both item familiarity and study context (Hintzman, 1988; Humphreys, Bain, & Pike, 1989; Shiffrin & Steyvers, 1997). In this way, when a pair of items is presented at test, the familiarity of the individual items in each pair, as well as the familiarity of their co-occurrence, combine to produce a single strength value in memory. An exact match between a study pair and a test pair would lead to a high familiarity signal at test (due to high item familiarity and high associative familiarity), but a test pair consisting of studied items not presented in the same pair during study (a rearranged pair) would lead to a lower familiarity signal at test (due to high item familiarity but low associative familiarity). Similar views have been expressed by dual-process theories that propose a role for familiarity in associative recognition even in the absence of unitization processes (e.g., Diana, Reder, Arndt, & Park, 2006; Norman & O’Reilly, 2003).
Recent developments in the ERP literature have identified two components that appear to index familiarity and recollection processes, and provide a useful method for distinguishing the roles of familiarity and recollection in recognition memory (for reviews, see Curran, Tepe, & Piatt, 2006; Mecklinger, 2006; Rugg & Curran, 2007; for a different view of FN400 effects see Paller, Voss, & Boehm, 2007). Familiarity has been related to a frontal component (the FN400), that is more negative for “new” items than for “old” items approximately 300–500 ms after stimulus onset, whereas recollection has been related to a posterior parietal component that is more positive for “old” items than for “new” items approximately 400–800 ms after stimulus onset. For example, in one study participants distinguished between studied items (e.g., “CAT”), plurality-reversed lures (e.g., “CAT”), and new items (e.g., “APPLE”). The FN400 component discriminated between familiar (studied items and lures) and unfamiliar (new items) stimuli, but the parietal component discriminated between recollected (studied items) and non-recollected (lures and new items) stimuli (Curran, 2000). These two ERP components have also been dissociated using pharmacological methods. Midazolam, an amnesia-inducing drug that has a larger effect on recollection than on familiarity processes (Hirshman, Fisher, Henthorn, Arndt, & Passannante, 2002), severely reduces the parietal old/new effect, but leaves the FN400 effect intact (Curran, DeBuse, Woroch, & Hirshman, 2006).
If recollection but not familiarity contributes to associative recognition, then these two components should show different effects during associative recognition tasks. A handful of studies have found initial evidence that the FN400 and parietal old/new components are differentially sensitive to associations between words, objects, and/or pictures (Jäger, Mecklinger, & Kipp, 2006; Rhodes & Donaldson, 2007; Tsivillis, Otten, & Rugg, 2001). However, these studies have either not tested traditional associative recognition (Jäger et al., 2006; Tsivillis et al., 2001), or have not compared the FN400 component on rearranged and old pairs (Rhodes & Donaldson, 2007), although Donaldson & Rugg (1998) compared responses to rearranged and old pairs on the parietal component. The typical comparison when looking at the FN400 and parietal old/new components in item recognition tasks is between old and completely new items. Associative recognition, on the other hand, is defined as the ability to distinguish old from rearranged pairs (Hockley, 1992), rather than old from new pairs or old from new items. In order to test the sensitivity of the FN400 and parietal old/new effects to associative recognition, it is necessary to determine whether the response to rearranged pairs is different from the response to old pairs, and whether the response to new pairs is different from the response to rearranged pairs. The one previous study that has compared old and new pairs found that the FN400 is sensitive to prior associations (Tsivillis, Otten, & Rugg, 2001), although this study tested item recognition with associative context being incidental to subjects’ task. The primary aim of the current studies was to use electrophysiological measures of familiarity and recollection to determine the way in which familiarity contributes to associative recognition. If familiarity processes contribute to associative recognition, then the FN400 component should show a larger negative response to rearranged pairs than to studied pairs.
A secondary aim of the current study was to test the hypothesis that an earlier component would be sensitive to item but not associative familiarity. Recent associative recognition studies using visual stimuli have observed an early positive component between 100 and 200 ms after test stimulus onset that appears to be sensitive to item rather than associative familiarity. This component may be related to perceptual priming processes, which act at the level of individual visual objects rather than pairs of objects (Curran & Dien, 2003; Duarte, Ranganath, Winward, Hayward, & Knight, 2004; Duzel, Habib, Guderian, & Heinze, 2004; Ecker, Zimmer, Groh-Bordin, Mecklinger, 2007; Jäger, et al., 2006; Tsivillis, et al., 2001). For example, when studying objects in the context of scenes (e.g., Ecker, et al., 2007; Tsivillis, et al., 2001), an early component observed between 100 and 300 ms after test stimulus onset differentiated new objects in new scenes from several conditions, including conditions containing at least one old object and/or scene, regardless of study-list pairing. This early component may track the familiarity of individual items rather than contextual/associative familiarity. Given the early onset of these effects, this effect might be due to priming or novelty-detection mechanisms that are important in visual recognition (Ecker, et al., 2007; Tsivillis, et al., 2001).
Participants in the current experiments studied pairs of fractals. EEG data were then collected while participants were tested on their memory for pairs of fractals presented during study. To determine whether the strength of the association influences familiarity and recollection processes indexed by the FN400 and parietal old/new effects, the frequency with which fractals were paired together during study varied (following Bunge, Burrows, & Wagner, 2004). Each individual fractal was viewed five times during study to equate item familiarity. On each presentation a given fractal was either a) always paired with the same fractal (leading to strong familiarity for the association), b) paired with the same fractal 2 times and with different fractals 3 times (leading to weak familiarity for the association), or c) always paired with a different fractal (leading to familiarity for the individual fractals but not for the association between fractals). In the first experiment the position of fractals within pairs was constant on each presentation (e.g., AB and AB), and in the second experiment the position of the fractals within a pair varied randomly on each presentation (e.g., AB and BA). This manipulation of position was included to decrease the likelihood that participants would view the fractal pairs as a unit (i.e., decrease the likelihood that unitization would occur). Critically, each studied fractal in both experiments was seen an equal number of times and all were equal in terms of their item familiarity. At test, participants distinguished old pairs (strong and weak) from non-studied pairs (rearranged pairs of studied fractals, and pairs of new fractals in which neither fractal had appeared during study) while brain activity was recorded (see Figure 1). These different test conditions were compared across the FN400 and parietal components, to determine the degree to which familiarity and recollection contribute to associative recognition. In addition, the test conditions were examined in the early P1 component to determine whether this early component might be related to item familiarity. By comparing ERP components to test stimuli across the different study conditions, we were able examine the processes that contribute to the recognition of novel, visual associations, and determine the nature of the role familiarity plays in associative recognition.
Figure 1.
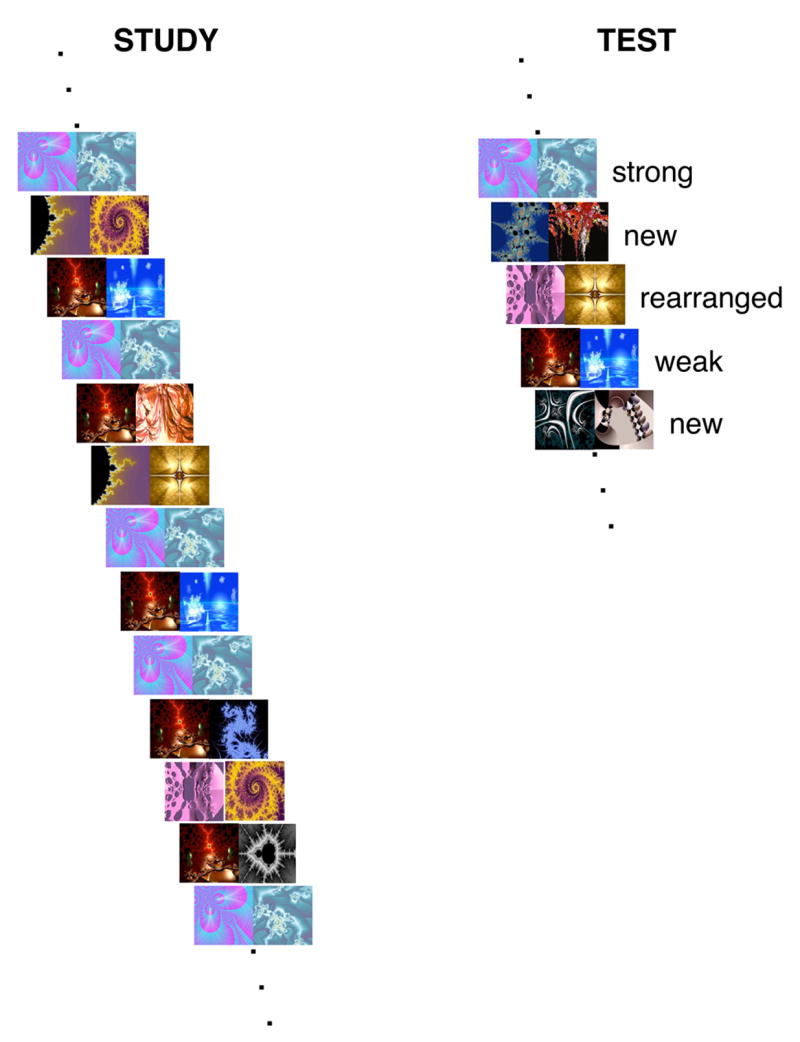
Examples of fractals and conditions used in the current study. In the variable location group, the position of each fractal within a pair varied across presentations.
2. Results
Behavioral
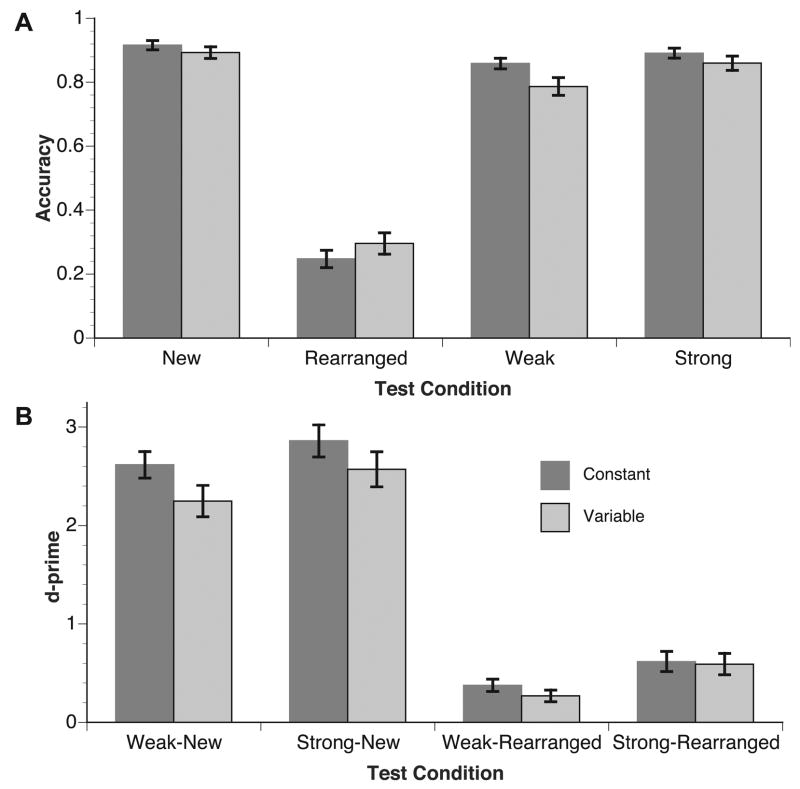
A 2 (group: constant and variable) × 4 (condition: strong, weak, rearranged, and new) analysis of variance (ANOVA) compared accurate performance on each condition within each group. For this ANOVA, as well as the ERP ANOVAs below, all p values associated with more than 1 degree of freedom have been corrected according to the conservative Geisser-Greenhouse procedure for sphericity violations (Winer, 1971). Figure 2a shows that accuracy on new pairs was greater than strong, weak, and rearranged pairs. This relationship was confirmed by a main effect of condition, F(3, 138) = 305.17, p < .001. Follow-up tests determined that new and strong pair performance did not differ, t(47) = 1.82, p = .08, and both were significantly more accurate than weak or rearranged pairs, smallest t(47) = 4.19, p < .001. The main effect of group and the interaction of group with condition were not statistically significant, F(1, 46) = 2.30, p = .14, and F(3, 138) = 2.12, p = .10. D-prime analyses comparing hits to strong and weak pairs with false alarms to new and rearranged pairs showed no differences across groups, largest t(46) = 1.77, p = .08 (see Figure 2b). Although d’ values computed using false alarms to new pairs were larger than d’ values computed using false alarms to rearranged pairs, all d’ values for each group were statistically different from zero, smallest t(23) = 4.55, p < .001.
Figure 2.
Behavioral performance. A) Accuracy was highest for new pairs and lowest for rearranged pairs. B) Discrimination was highest when comparing old pairs with new pairs, and lowest when comparing old pairs with rearranged pairs.
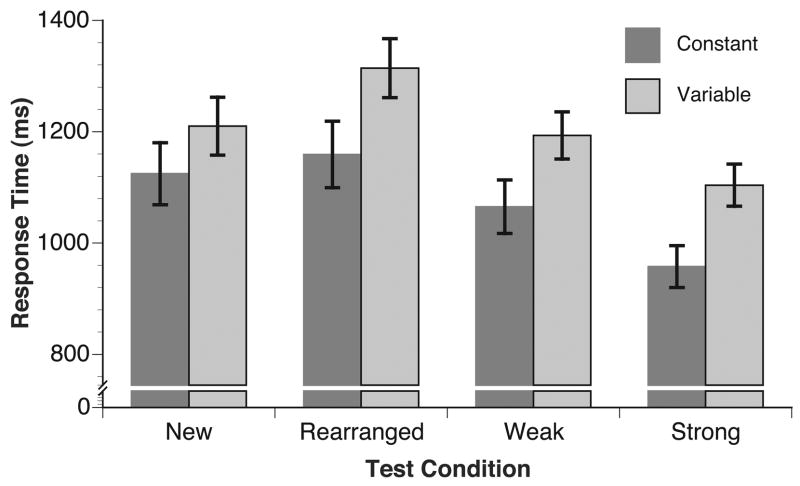
A second ANOVA compared mean response times for each condition within each group. For strong, weak, and new pairs, mean response times were computed for accurate responses; for rearranged pairs, mean response times were computed for inaccurate responses (as in the ERP analyses reported below). Figure 3 shows that strong pairs produced the fastest response times, and rearranged pairs produced the slowest responses, and response times in the variable position group were slower overall than in the constant position group. The differences across groups and conditions were confirmed by the ANOVA, F(1, 46) = 4.13, p = .05, and F(3, 138) = 63.74, p < .001. Follow-up tests demonstrated that response times to strong pairs were faster than weak pairs, which did not differ from new pairs, t(47) = 1.51, p = .14. Response times to rearranged pairs were slower than to all other pairs, smallest t(47) = 2.36, p = .02. The interaction of group and condition was not statistically significant, F(3, 138) = .94, p = 42.
Figure 3.
Response times. Response times were fastest for strong pairs, and slowest for rearranged pairs.
ERP
To obtain a sufficient number of trials within each condition, given the obtained accuracy rates, the ERP analyses only considered correct trials in the strong, weak, and new conditions; and only incorrect trials in the rearranged condition. There were not enough participants with sufficient numbers of correct trials in the rearranged condition to analyze correctly rejected rearranged pairs. The number of trials/subject/condition used to calculate ERPs to test pairs were as follows: new (M = 55.69, Range = 39–67), rearranged (M = 44.31, Range = 18–61), weak (M = 50.10, Range = 29–63), and strong (M = 53.42, Range = 32–67). Figure 4 shows the channel groups over which ERPs were averaged, as plotted in Figures 5 (constant) and 6 (variable). A series of ANOVAs analyzed the three spatiotemporal regions of interest (P1, FN400, and parietal old/new). Each ANOVA tested a priori predictions using a 2 (group) × 4 (condition) × 2 (hemisphere) within-subjects ANOVA for each region of interest. Follow-up tests for statistically significant main effects and interactions were conducted using additional ANOVAs, collapsing across factors that were not involved in the main effects or interactions.
Figure 4.
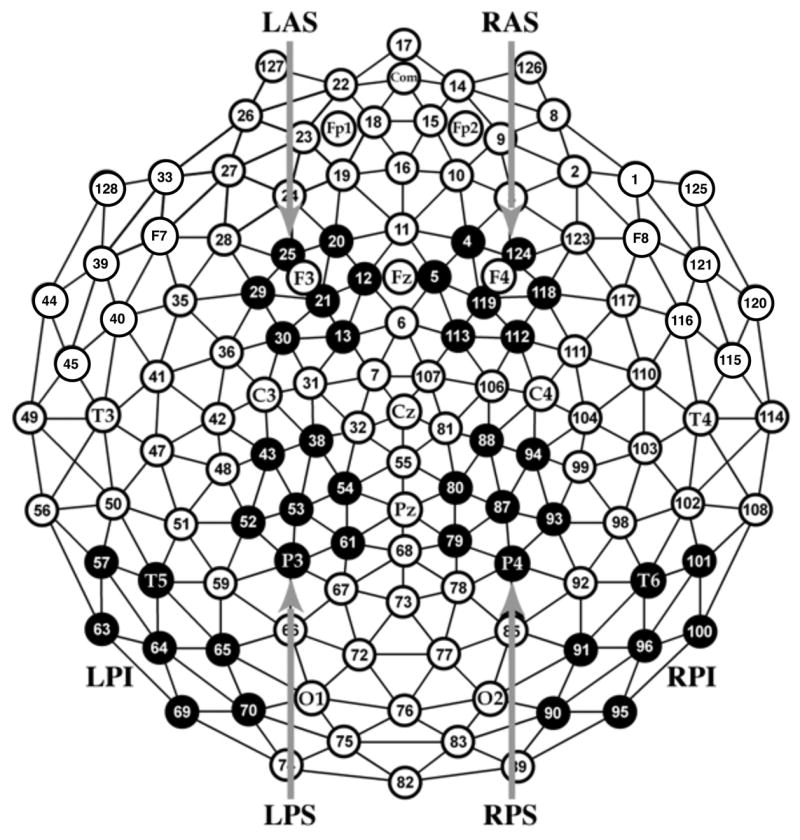
Channel groups used to generate average ERPs. R = right, L = left; A = anterior, P = posterior; I = Inferior, S = Superior.
Figure 5.
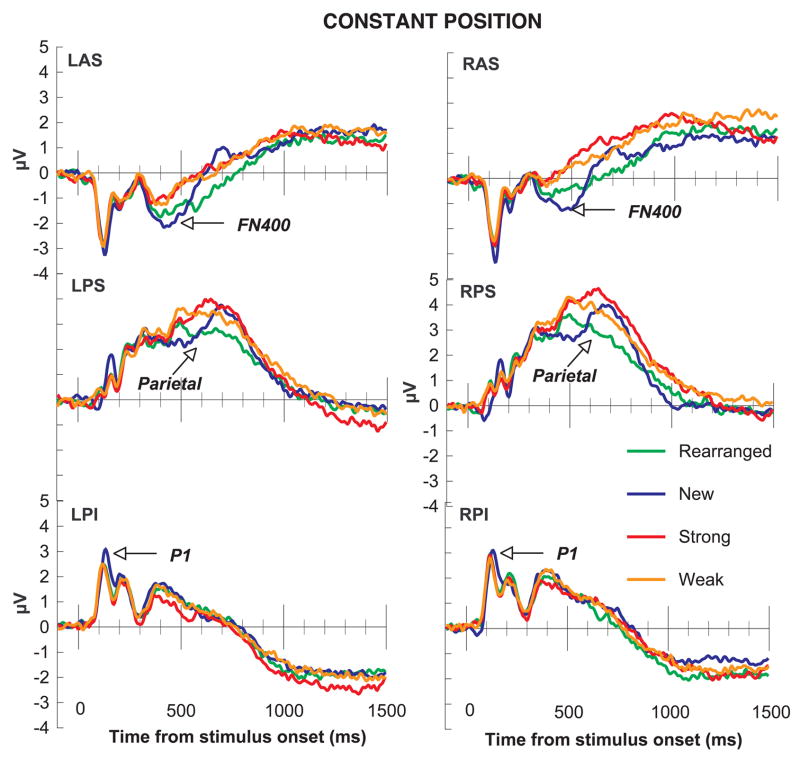
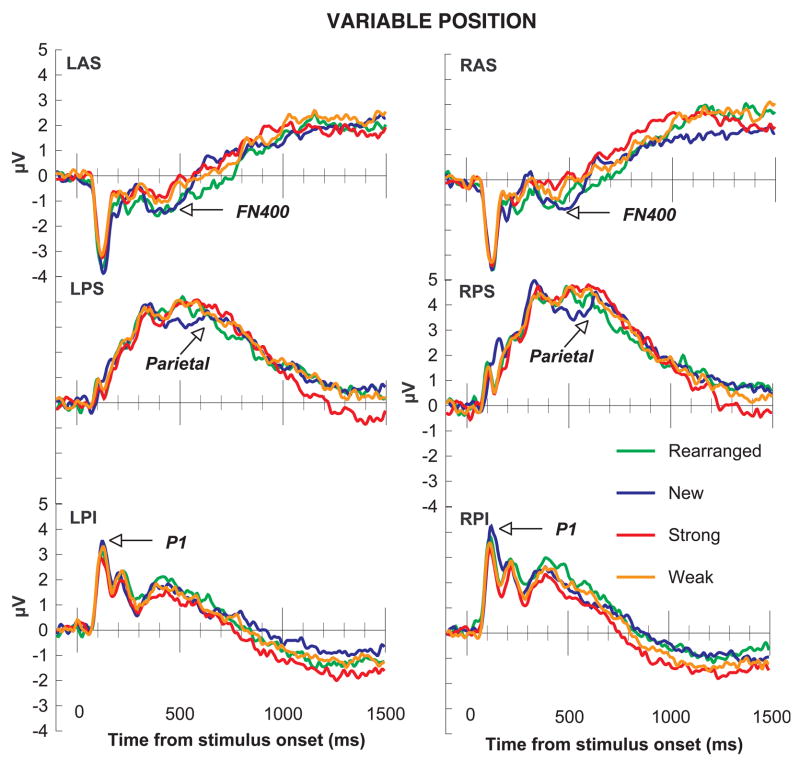
ERPs for the constant position group. In the constant position group, early posterior effects were sensitive to the novelty of the items (P1). New pairs were associated with a larger amplitude response than rearranged, weak, or strong pairs. The FN400 and Parietal old/new effects both showed sensitivity to the novelty of the associations. Strong and weak pairs produced larger amplitude responses than rearranged or new pairs.
P1 old/new effects
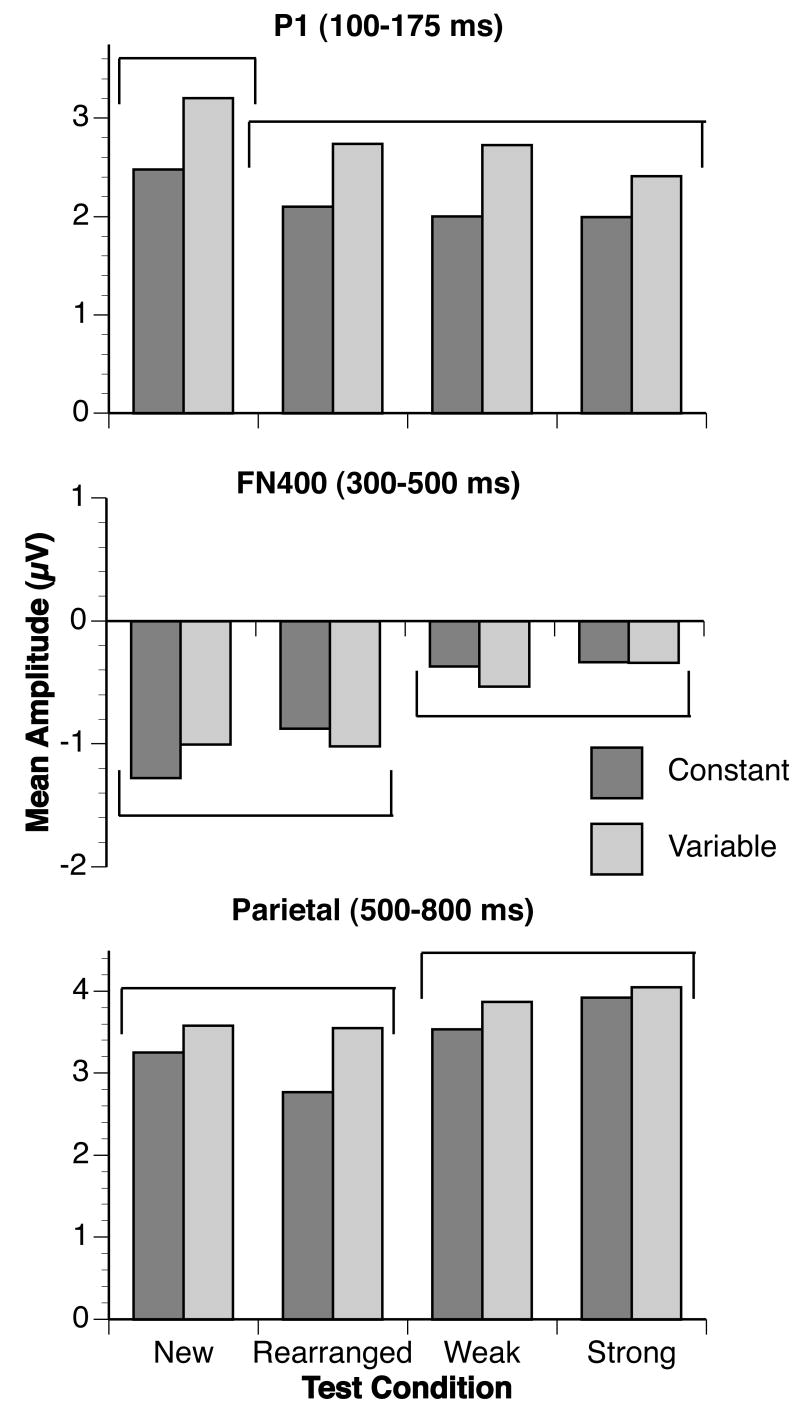
The P1 region was defined as the mean amplitude across posterior inferior channels where the P1 was maximal (LPI and RPI in Figure 4) between 100 and 175 ms. Figures 5–9 show that the P1 response was largest for new pairs. The main effect of condition was statistically significant, F(3, 138) = 9.98, p < .001, with a greater P1 response to new items, smallest F(1, 46) = 10.68, p = .002, than to all other items, which did not differ significantly from each other, largest F(1, 46) = 3.01, p = .09. No other main effects or interactions were statistically significant, largest F = 2.42, p = .08.
Figure 9.
Mean amplitudes for each ERP effect. Mean amplitudes for the P1 effect (channels LPI and RPI, 100–175 ms after test stimulus onset) were sensitive to item familiarity: Mean amplitudes for new fractal pairs were larger than mean amplitudes for rearranged, weak, and strong fractal pairs. Mean amplitudes for the FN400 (LAS and RAS, 300–500 ms after test stimulus onset) and parietal (LPS and RPS, 500–800 ms after test stimulus onset) effects were sensitive to prior associations: strong and weak pairs produced more positive amplitudes than familiar or rearranged pairs. Conditions with amplitudes that differed from each other are grouped into separate brackets; conditions that did not differ in amplitude are grouped into the same bracket.
FN400 old/new effects
The FN400 region was defined as the mean amplitude across anterior superior channels between 300 and 500 ms (LAS and RAS in Figure 4; following Curran, 2000; Curran & Cleary, 2003; Curran, DeBuse, Woroch, & Hirshman, 2006). Figures 5–9 show that the FN400 response was largest for the new and rearranged pairs, and this relationship was confirmed by a statistically significant effect of condition, F(3,138) = 11.41, p < .001. Follow-up tests confirmed that the mean amplitude to new and rearranged pairs, which did not differ, was larger than the mean amplitude to weak and strong pairs, which did not differ, largest nonsignificant F(1,46) = 1.16, p = .29, smallest significant F(1,46) = 10.49, p = .002. No other main effects or interactions were statistically significant, largest F = 2.20, p = .14.
Parietal old/new effects
The parietal old/new region was defined as the mean amplitude across posterior superior channels between 500 and 800 ms (LPS and RPS in Figure 4; following Curran, 2000; Curran & Cleary, 2003; Curran, DeBuse, Woroch, & Hirshman, 2006). Figures 5–9 show that the parietal response was largest for strong and weak pairs. This pattern was confirmed by a statistically significant effect of condition, F(3, 138) = 9.16, p < .001. The responses to new and rearranged pairs did not differ, F(1, 46) = 2.15, p = .15, but responses to rearranged pairs were smaller than the responses to both weak and strong pairs, smallest F(1, 46) = 16.32, p < .001. Although responses to strong pairs were statistically larger than responses to new pairs, F(1, 46) = 10.86, p = .002, there was only a trend for larger responses to weak pairs than to new pairs, F(1, 46) = 3.70, p = .06. There was also a trend for larger responses to strong than weak pairs, F(1, 46) = 2.96, p = .09. No other main effects or interactions were statistically significant, largest F = 1.42, p = .24.
Topographic comparison of FN400 and parietal effects
Prior studies using single item recognition have demonstrated that the FN400 and parietal components are distinct, with the FN400 maximal over anterior, superior locations, and the parietal effect maximal over posterior, superior locations. A final ANOVA tested the hypothesis that this distinction extends to associative recognition. For this analysis, old/new differences were computed in each of the three main test conditions (strong-new, weak-new, rearranged-new). These differences were rescaled around the range of the data within each test condition to compare the topography of these effects in a manner that is not compromised by overall amplitude differences (McCarthy & Wood, 1985). These range-normalized differences were entered into a 2 (group) × 2 (time; 300–500 ms and 500–800 ms) × 3 (difference; strong-new, weak-new, and rearranged-new) × 2 (hemisphere) × 2 (anterior and superior) ANOVA, using the four regions of interest shown in Figure 4
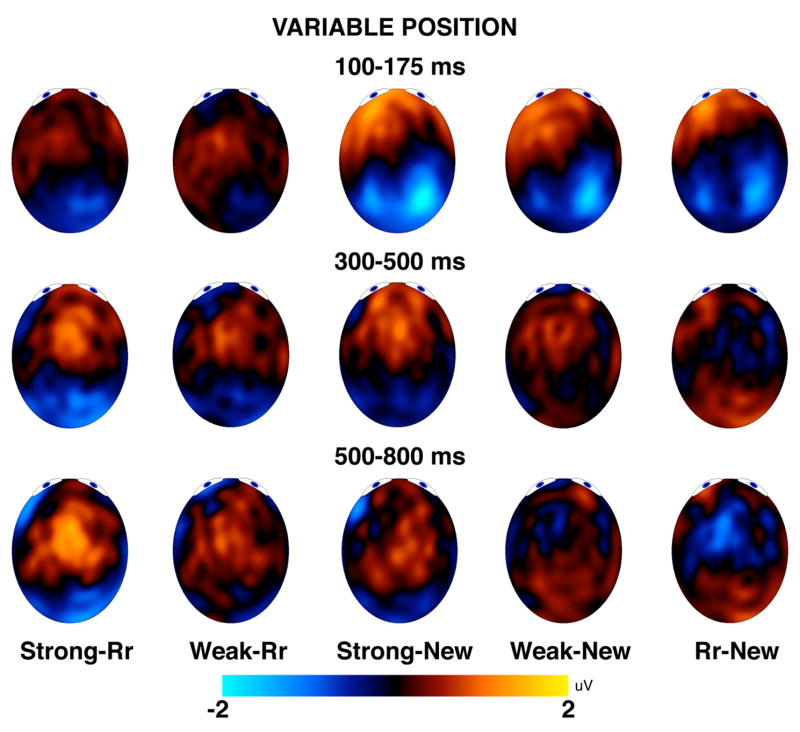
Figures 7 and 8 suggest that differences between new test pairs and strong, weak, or rearranged pairs during the 300–500 ms window were larger for anterior channel groups than for posterior channel groups, and differences during the 500–800 ms window were larger for posterior channel groups than for anterior channel groups. This dissociation was confirmed by a statistically significant interaction between time and anterior/posterior in the analysis of range-normalized old/new differences, F(1, 46) = 10.96, p = .002. This effect was qualified by an interaction of time, anterior/posterior, and hemisphere, F(1, 46) = 5.18, p = .03, which indicated that the interaction between time and anterior/posterior was especially pronounced over the left hemisphere, whereas right hemisphere differences tended to be more even between anterior and posterior sites from 500–800 ms. None of the remaining main effects or interactions involving condition or time reached statistical significance, largest F = 1.90, p = .16.
Figure 7.
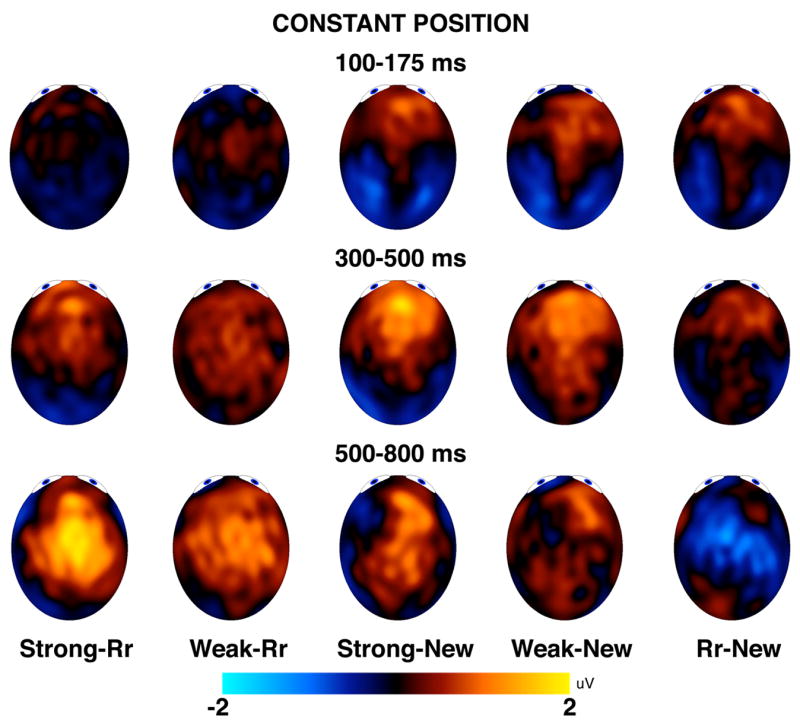
Topographical maps for ERPs in the constant position group. The topographical maps show average amplitude differences over the three temporal windows for the constant position group. Rr = rearranged.
Figure 8.
Topographical maps for ERPs in the variable position group. The topographical maps show average amplitude differences over the three temporal windows for the variable position group. Rr = rearranged.
3. Discussion
The results of this study suggest that both familiarity processes, indexed by FN400 old/new effects, and recollection processes, indexed by parietal old/new effects, play a role in recognizing perceptual associations. Participants showed larger amplitude FN400 and parietal old/new responses to studied pairs of fractals than to pairs of new fractals or pairs of rearranged fractals. These differences persisted under conditions designed to minimize unitization processes that may have facilitated familiarity-based recognition: Varying the position of the fractals within each pair from one trial to the next had no effect on the FN400 and parietal old/new effects. In both experiments, weak and strong fractal pairs generated more positive ERPs than rearranged or new pairs.
The involvement of familiarity in associative recognition is inconsistent with some dual-process theories which assume that familiarity cannot contribute to associative recognition unless the to-be-associated items are unitized into a single representation (Jäger, Mecklinger, & Kipp, 2006; Quamme, Yonelinas, & Norman, 2007; Rhodes & Donaldson, 2007; Yonelinas, 1997; Yonelinas, Kroll, Dobbins, & Soltani, 1999). The current results could be explained in terms of those dual-process theories if the assumption is made that participants were unitizing the fractal pairs. This explanation seems unlikely given the highly abstract visual stimuli and the lack of an effect of the constant/variable position manipulation. Although weak fractal pairs were repeated twice, in the variable position condition, the position of the fractals within each pair changed on each presentation (e.g., AB, BA rather than AB, AB). However, there were no effects of this position manipulation on any of the three ERP components. Although it is possible that two presentations of the weak fractal pairs were sufficient to drive unitization of the fractals despite position changes, it seems unlikely given that the position manipulation had no effect on the ERP (and behavioral) data. We cannot rule out the possibility that this lack of group differences may have been due to low power in the between-subjects group variable. However, it is clear from the data and analyses that both groups show associative recognition effects in the FN400 and parietal old/new components (e.g., strong/weak test pairs > rearranged/new test pairs). If group differences did arise, they would arise from differences in the magnitude of these effects. For instance, there might be a larger associative recognition effect in the constant group than in the variable group, if the constant group had been able to unitize the fractal pairs. However, because both groups already show associative recognition effects in the FN400 and parietal old/new components, that effect would not disappear by increasing the ability to detect group differences. Whether or not there are group differences in the magnitude of these associative recognition effects, the FN400 and parietal old/new effects in both groups were sensitive to associative rather than item recognition.
Global matching theories and dual-process theories which do not set constraints on the ability for familiarity to contribute to associative recognition (Diana, et al., 2006; Hintzman, 1988; Humphreys, et al., 1989; Norman & O’Reilly, 2003; Shiffrin & Steyvers, 1997) offer a more parsimonious explanation of the current data by suggesting that both item and contextual (i.e., associative) familiarity contribute to associative recognition memory. According to these theories, item and contextual familiarity contribute to recognition judgments. Thus in tasks that require associative recognition judgments, the familiarity of the individual items, as well as the familiarity of their co-occurrence combine to produce a single strength value, which then leads to an “old” or “new” judgment for a given pair of items. The fact that the FN400 discriminated between old and rearranged pairs in the current experiments suggests that models that do not presume associated stimuli are unitized may best characterize the familiarity process. These results suggest that familiarity may more generally contribute to associative recognition memory, and theories of familiarity that take into account contextual and item familiarity may provide an accurate account of this more general role of familiarity in the ability to recognize associations between previously unrelated items.
Both the FN400 and the parietal old/new effects were sensitive to the novelty of the association (e.g., showed larger responses to old than to rearranged pairs). Despite their similar modulation by the present manipulations, topographic analyses indicated that these components were spatially distinct by showing that FN400 effects were larger over anterior than posterior locations, whereas the parietal effects were larger over posterior locations, as is typically observed (see Figures 7 and 8). This finding that the FN400 and parietal old/new effects were both sensitive to the novelty of the association contrasts with the results of two other recent studies demonstrating that only the parietal old/new effect is sensitive to the recognition of arbitrary associations between items (Jäger, et al., 2006; Rhodes & Donaldson, 2007). These studies have shown an effect of associative recognition on the FN400 only when the individual items in a pair of stimuli can be unitized, and not when there is an arbitrary relationship between the items in the studied pair. However, each of these studies is limited in some respects (Jäger, et al., 2006; Rhodes & Donaldson, 2007).
In one experiment, participants studied pairs of semantically related words (e.g., “CEREAL-BREAD”), associated words (e.g., “TRAFFIC-JAM”), or semantically related and associated words (e.g., “LEMON-ORANGE”; Rhodes & Donaldson, 2007). At test participants were given old word pairs, rearranged word pairs, or word pairs consisting of entirely new items, but only the ERPs for old and new pairs were analyzed. The FN400 component showed a more negative response for new pairs than for old pairs only for word pairs that contained associated words, and not for word pairs that contained semantically related words. However, because this study did not analyze ERPs to the rearranged pairs, it is not clear whether the FN400 component was showing an effect of associative recognition for any of the conditions. A true associative recognition response would be reflected in a more positive ERP to old pairs than to rearranged pairs, rather than a more positive response to old pairs than to new pairs. Without that comparison, the observed old/new effect for associated word pairs may have been due to differences among the items in each pair.
In a second experiment, participants studied pairs of faces that were either morphed versions of the same person (intra-item) or two different people (inter-item; Jäger et al., 2006). Memory tests included an initial single-face recognition judgment followed a 2-alternative forced-choice test of the initial face’s pair mate. FN400 old/new effects to the single test faces were observed only when followed by correct intra-item association judgments, whereas parietal old/new effects were observed only when followed by correct inter-item associations. This double dissociation is consistent with familiarity having greater sensitivity to intra-item associations, but recollection having greater sensitivity to inter-item associations (Aggleton & Brown, 1999; Norman & O’Reilly, 2003). However, in the intra-item conditions, old faces were always morphs but new faces were always non-morphs, so these stimulus differences could have contributed to the FN400 effects rather than reflecting a truly associative influence. Although we agree with the conclusions of these two previous studies, we believe the results of the experiments presented here provide the clearest ERP evidence to date regarding the possible contribution of familiarity-related processes, indexed by the FN400, to associative recognition.
It is somewhat unexpected that there were no differences in ERP amplitude between the weak and strong pairs. Although item familiarity was the same in both conditions, associative familiarity should have been greater with five rather than two presentations of identical pairs. This enhanced associative familiarity should have been associated with larger FN400 old/new effects for strong than weak pairs. In addition, recollection should have been greater with increased presentation of identical pairs, leading to a larger parietal old/new effect for strong than weak pairs. However, the behavioral strong/weak effects were small, indicating that the lack of ERP effects may reflect low statistical power rather than a true absence of strong/weak effects. Indeed, there was a trend for a larger parietal old/new effect for strong compared to weak pairs.
The behavioral data suggest that although the FN400 and parietal old/new components were sensitive to associations, participants were relying heavily on the identities of the individual fractals to decide whether two fractals were studied together during encoding. Participants were more able to accurately discriminate studied pairs from pairs of novel fractals than to accurately discriminate studied pairs from rearranged pairs. Neither the FN400 nor the parietal old/new effect demonstrated this pattern. In both ERP components, the response to rearranged pairs of fractals was statistically identical to the response to completely novel pairs of fractals. However, the early P1 component that has been observed in recent associative recognition studies using visual rather than verbal stimuli showed a larger response to rearranged, weak, and strong pairs of fractals than to novel fractals at test. Following previous studies (Duzel, et al., 2004; Ecker, et al., 2007; Jäger, et al., 2006; Tsivillis, et al., 2001), this early component responded to the familiarity of individual items rather than to contextual/associative familiarity. Given the early onset of these effects, others have suggested that this effect might be due to priming mechanisms or novelty-detection mechanisms that may contribute to recognition judgments (Ecker, et al., 2007; Tsivillis et al., 2001). The results of the current study add to this evidence to suggest that this early component, regardless of whether it is related to perceptual priming, may be important for recognition memory. The high false alarm rate in the rearranged pair condition suggests that participants may have relied heavily on this type of perceptual mechanism to decide whether a pair of fractals was old or new. This interpretation would be consistent with the perspective that multiple processes may contribute to familiarity-based recognition, including processes indexed by both P1 and FN400 old/new effects (Rugg & Curran, 2007).
The separation of the behavioral data from the FN400 and parietal old/new components suggests that these later components are not reflecting the decision process, but instead may indicate the actual status of the studied pairs in memory. Data from functional magnetic resonance imaging studies (fMRI studies) have implicated parietal regions in perceived rather than veridical oldness (Wagner, Shannon, Kahn, & Buckner, 2005). That is, activation in parietal regions is greater for hits and false alarms than for misses and correct rejections, suggesting that parietal regions respond to the old/new decision, regardless of the accuracy of that decision. In the current study, the parietal component was greater for hits than for false alarms to rearranged pairs and correct rejections of new pairs, which did not differ. That is, the parietal component was sensitive to the old/new response status of the studied pairs. This pattern of results suggests that fMRI and ERP studies may be measuring two different processes in parietal cortex, with fMRI activation indexing the perceived oldness of studied information, and ERP components measuring the veridical oldness of studied information.
The fact that the FN400 showed a larger negative response to new than to old pairs of objects argues against a recent hypothesis that the FN400 effects are due to conceptual priming rather than familiarity processes (Paller et al., 2007). The current studies used conceptually impoverished visual images (fractals), but the old/new FN400 effect persisted. If the FN400 effect were due to conceptual priming, then the old/new differences in the FN400 component should have been severely reduced or eliminated in the current studies. The old/new FN400 differences persisted across both experiments, suggesting that the familiarity hypothesis, rather than the conceptual priming hypothesis, provides a more appropriate description of the cognitive processes underlying the FN400 component.
To the degree that the FN400 and parietal old/new components reflect familiarity and recollection processes, respectively, it appears that both processes are involved in the recognition of perceptual associations between arbitrary items. Across two experiments, both the FN400 and parietal old/new ERP components were sensitive to prior associations between studied items. This pattern of results contrasted with that of an earlier, posterior component that was sensitive to the novelty of the items, rather than the novelty of the association between items. Taken together, these results suggest that accurate memory for perceptual associations is dependent on a combination of perceptual priming, familiarity, and recollection processes.
4. Experimental Procedure
Participants
Forty-eight right-handed students at the University of Colorado participated in these experiments for course credit or a cash stipend (Experiment 1: n = 24, ages 18–26, 8 women; Experiment 2: n = 24, ages 18–27, 8 women). Data from an additional 29 participants were discarded due to insufficient trial counts (< 18) in each condition arising from high blink rates (n= 12), excessive EEG noise (n=8), failure to return for the second session (n= 3), low accuracy (n=4), and equipment failure (n=2). Participants did not have a history of neurological problems. Informed consent was obtained in accordance with the guidelines set by the Human Research Committee at the University of Colorado at Boulder.
Materials
Stimuli consisted of a set of 792 fractals, divided into two lists of 396 fractals (the two lists were counterbalanced across sessions). Pairs of fractals during study and test were presented on a Dell computer running E-prime (Psychology Software Tools, Pittsburgh, PA), and always appeared in the center of a 15 in flat panel computer monitor with a visual angle of approximately one degree (individual fractals measured 1.90 × 1.90 cm). A serial response box (Electrical Geodesics, Inc., Eugene, OR) was used to record participant responses.
Design and Procedure
Participants studied pairs of fractals, and were asked to distinguish studied from non-studied pairs at test. Each participant completed nine study-test blocks in each of two, two-hour sessions. The first study-test block on the first day was considered practice, and these data were not included in any analyses. During study, each individual fractal was viewed five times. In the strong condition, each fractal always appeared in the same pair during study. In the weak condition, each fractal appeared in the same pair twice during study, and was paired with three different fractals (one from each of the other three weak pairs) during three additional study presentations. In the rearranged condition, each fractal was presented with a different fractal five times during study (always with a fractal from one of the other rearranged pairs). An important feature of this design is that individually the fractals in each condition were seen five times during study and the fractals were therefore all equally familiar at the item level for the recognition test.
Study lists consisted of 74 presentations of fractal pairs: two pairs each of recency and primacy buffers, 20 presentations of the four strong study pairs, 8 presentations of the four weak study pairs, 12 presentations of fractals in the weak study pairs presented in novel arrangements (to control for item familiarity in the weak study condition), and 30 presentations of the six rearranged fractal pairs. In the first experiment (the constant position group), fractals always appeared in the same position on the screen at study and test, regardless of which fractal they were paired with during study. In the second experiment (the variable position group), the position of the fractals on the screen during study and test was counterbalanced across presentations. For example, in the strong condition, A-B would appear three times during the study list, and B-A would appear twice during the study list and once during the test list). Each fractal pair remained on the screen for 1900 ms, and was followed by a 100 ms inter-trial interval. Participants were instructed to remember the pairs for a later memory test.
Test lists consisted of 16 fractal pairs: the four strong and four weak pairs from the study list, four rearranged fractal pairs, and four new fractal pairs consisting of entirely novel fractals. Strong pairs were studied together five times. Weak pairs were studied together two times, but studied with other fractals in their other three presentations. Rearranged pairs were never studied together, but were studied with five different fractals (from other rearranged pairs) during each of their study-list presentations. New pairs included two fractals that never appeared on the study list. Each test trial began with a fixation cross whose duration varied randomly between 1250 and 1750 ms. The test pairs remained on the screen until participants indicated whether the pair was old (i.e., the fractals had appeared together during the study list), or new (i.e., the fractals had not appeared together during the study list, even if they had both appeared during the study list). Participants responded with the index finger of both hands, with the assignment of left and right hands to old and new responses counterbalanced across participants. Participants received accuracy and response time feedback at the end of each block, and were asked to keep their average accuracy above 90%, and their average response time under 2000 ms.
EEG/ERP Recording & Analysis
During the test list, scalp voltages were collected with a 128-channel Geodesic Sensor Net™ (Tucker, 1993) connected to an AC-coupled, 128-channel, high-input impedance amplifier (200 MΩ, Net Amps™, Electrical Geodesics Inc., Eugene, OR). Amplified analog voltages (0.1–100 Hz bandpass) were digitized at 250 Hz. Individual sensors were adjusted until impedances were less than 50 kΩ. The EEG was digitally low-pass filtered at 40 Hz. Trials in the strong, weak, and new conditions were discarded from analyses if they contained incorrect responses, but trials in the rearranged condition were discarded if they contained correct responses (performance on these trials was too low to generate a sufficient number of accurate trials for analysis). Trials were also discarded if they contained eye movements (EOG over 70 μV), or more than 20% of channels were bad (average amplitude over 100 μV or transit amplitude over 50 μV). Individual bad channels were replaced on a trial-by-trial basis with a spherical spline algorithm (Srinivasan, Nunez, Tucker, Silberstein, & Cadusch, 1996). EEG was measured with respect to a vertex reference (Cz), but an average-reference transformation was used to minimize the effects of reference-site activity and accurately estimate the scalp topography of the measured electrical fields (Dien, 1998; Picton, Lins, & Sherg, 1995). The average reference was corrected for the polar average reference effect (Junghöfer, Elbert, Tucker, & Braun, 1999). Event-related potentials (ERP) were obtained by stimulus-locked averaging of the EEG recorded in each condition. ERPs were baseline-corrected with respect to a 200-ms prestimulus recording interval.
Figure 6.
ERPs for the variable position group. In the variable position group, early posterior effects were sensitive to the novelty of the items (P1). New pairs were associated with a larger amplitude response than rearranged, weak, or strong pairs. The FN400 and Parietal old/new effects both showed sensitivity to the novelty of the associations. Strong and weak pairs produced larger amplitude responses than rearranged or new pairs.
Acknowledgments
This research was supported by a NIH F32 grant to NKS (#MH074215), a NIH R01 grant to TC (#MH64812), a grant from the James S. McDonnell Foundation supporting the Perceptual Expertise Network, and the Temporal Dynamics of Learning Center (NSF Science of Learning Center SBE-0542013). Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the TDLC or the National Science Foundation. We thank Kristin Becker, Alexis Billings, Chris Bird, Julia Cox, Casey DeBuse, Jeanne Doyle, Tatsuko GoHollo, Tessa Hill, Sarah Jirkovsky, Megan Lipsett, Jessica Pachter, Brion Woroch, and Brent Young for help with participant testing.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Achim AM, Lepage M. Is associative recognition more impaired than item recognition memory in Schizophrenia? A meta-analysis Brain Cognition. 2003;53:121–124. doi: 10.1016/s0278-2626(03)00092-7. [DOI] [PubMed] [Google Scholar]
- Achim AM, Lepage M. Neural correlates of memory for items and for associations: an event-related functional magnetic resonance imaging study. J Cognitive Neurosci. 2005;17:652–667. doi: 10.1162/0898929053467578. [DOI] [PubMed] [Google Scholar]
- Aggleton JP, Brown MW. Interleaving brain systems for episodic and recognition memory. Trends Cogn Sci. 1999;10:455–463. doi: 10.1016/j.tics.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Bastin C, Van der Linden M. The effects of aging on the recognition of different types of associations. Exp Aging Res. 2006;32:61–77. doi: 10.1080/03610730500326291. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Burrows B, Wagner AD. Prefrontal and hippocampal contributions to visual associative recognition: interactions between cognitive control and episodic retrieval. Brain Cognition. 2004;56:141–152. doi: 10.1016/j.bandc.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Curran T. Brain potentials of recollection and familiarity. Mem Cognition. 2000;28:923–938. doi: 10.3758/bf03209340. [DOI] [PubMed] [Google Scholar]
- Curran T, Cleary AM. Using ERPs to dissociate recollection from familiarity in picture recognition. Cognitive Brain Res. 2003;15:191–205. doi: 10.1016/s0926-6410(02)00192-1. [DOI] [PubMed] [Google Scholar]
- Curran T, DeBuse C, Woroch B, Hirshman E. Combined pharmacological and electrophysiological dissociation of familiarity and recollection. J Neurosci. 2006;26:1979–1985. doi: 10.1523/JNEUROSCI.5370-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran T, Dien J. Differentiating amodal familiarity from modality-specific memory processes: An ERP study. Psychophysiology. 2003;40:979–988. doi: 10.1111/1469-8986.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran T, Tepe KL, Piatt C. ERP explorations of dual processes in recognition memory. In: Zimmer HD, Mecklinger A, Lindenberger U, editors. Binding in human memory: A neurocognitive approach. Oxford University Press; Oxford: 2006. pp. 467–492. [Google Scholar]
- Diana RA, Reder LM, Arndt J, Park H. Models of recognition: A review of arguments in favor of a dual-process account. Psychon B Rev. 2006;13:1–21. doi: 10.3758/bf03193807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dien J. Issues in the application of the average reference: Review, critiques, and recommendations. Behav Res Meth Ins C. 1998;30:34–43. [Google Scholar]
- Donaldson DI, Rugg MD. Recognition memory for new associations: electrophysiological evidence for the role of recollection. Neuropsychologia. 1998;36:377–395. doi: 10.1016/s0028-3932(97)00143-7. [DOI] [PubMed] [Google Scholar]
- Duarte A, Ranganath C, Winward L, Hayward D, Knight RT. Dissociable neural correlates for familiarity and recollection during the encoding and retrieval of pictures. Cognitive Brain Res. 2004;18:255–272. doi: 10.1016/j.cogbrainres.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Duzel E, Habib R, Guderian S, Heinze HJ. Four types of novelty-familiarity responses in associative recognition memory of humans. Eur J Neurosci. 2004;19:1408–1416. doi: 10.1111/j.1460-9568.2004.03253.x. [DOI] [PubMed] [Google Scholar]
- Ecker UKH, Zimmer HD, Groh-Bordin C, Mecklinger A. Context effects on familiarity are familiarity effects of context - An electrophysiological study. Int J Psychophysiol. 2007;64:146–156. doi: 10.1016/j.ijpsycho.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Hintzman DL. Judgments of frequency and recognition memory in a multiple-trace memory model. Psychol Rev. 1988;95:528–551. [Google Scholar]
- Hirshman E, Fisher J, Henthorn T, Arndt J, Passannante A. Midazolam amnesia and dual-process models of the word frequency mirror effect. J Mem Lang. 2002;47:499–516. [Google Scholar]
- Hockley WE. Item versus associative information: Further comparisons of forgetting rates. J Exp Psychol Learn. 1992;18:1321–1330. [Google Scholar]
- Hockley WE, Consoli A. Familiarity and recollection in item and associative recognition. Mem Cognition. 1999;27:657–664. doi: 10.3758/bf03211559. [DOI] [PubMed] [Google Scholar]
- Holdstock JS, Mayes AR, Gong QY, Roberts N, Kapur N. Item recognition is less impaired than recall and associative recognition in a patient with selective hippocampal damage. Hippocampus. 2005;15:203–215. doi: 10.1002/hipo.20046. [DOI] [PubMed] [Google Scholar]
- Humphreys MS, Bain JD, Pike R. Different ways to cue a coherent memory system: A theory for episodic, semantic, and procedural tasks. Psychol Rev. 1989;96:208–233. [Google Scholar]
- Jäger T, Mecklinger A, Kipp KH. Intra- and inter-item associations doubly dissociate the electrophysiological correlates of familiarity and recollection. Neuron. 2006;52:535–545. doi: 10.1016/j.neuron.2006.09.013. [DOI] [PubMed] [Google Scholar]
- Junghöfer M, Elbert T, Tucker DM, Braun C. The polar average reference effect: a bias in estimating the head surface integral in EEG recording. Clin Neurophysiol. 1999;110:1149–1155. doi: 10.1016/s1388-2457(99)00044-9. [DOI] [PubMed] [Google Scholar]
- Mayes AR, Holdstock JS, Isaac CL, Montaldi D, Grigor J, et al. Associative recognition in a patient with selective hippocampal lesions and relatively normal item recognition. Hippocampus. 2004;14:763–784. doi: 10.1002/hipo.10211. [DOI] [PubMed] [Google Scholar]
- McCarthy G, Wood CC. Scalp distributions of event-related potentials: An ambiguity associated with analysis of variance models. Electroen Clin Neuro. 1985;62:203–208. doi: 10.1016/0168-5597(85)90015-2. [DOI] [PubMed] [Google Scholar]
- Mecklinger A. Electrophysiological measures of familiarity memory. Clin EEG Neurosci. 2006;37:292–299. doi: 10.1177/155005940603700406. [DOI] [PubMed] [Google Scholar]
- Norman KA, O’Reilly RC. Modeling hippocampal and neocortical contributions to recognition memory: A complementary learning systems approach. Psychol Rev. 2003;110:611–646. doi: 10.1037/0033-295X.110.4.611. [DOI] [PubMed] [Google Scholar]
- Paller KA, Voss JL, Boehm SG. Validating neural correlates of familiarity. Trends Cogn Sci. 2007 doi: 10.1016/j.tics.2007.04.002. in press. [DOI] [PubMed] [Google Scholar]
- Parks CM, Yonelinas AP. Moving beyond pure signal-detection models: Comment on Wixted (2007) Psychol Rev. 2007;114:188–202. doi: 10.1037/0033-295X.114.1.188. [DOI] [PubMed] [Google Scholar]
- Picton TW, Lins OG, Sherg M. The recording and analysis of event-related potentials. In: Boller F, Grafman J, editors. Handbook of Neuropsychology. Vol. 10. Elsevier; Amsterdam: 1995. pp. 3–73. [Google Scholar]
- Quamme JR, Yonelinas AP, Norman KA. The effect of unitization on associative recognition in amnesia. Hippocampus. 2007;17:192–200. doi: 10.1002/hipo.20257. [DOI] [PubMed] [Google Scholar]
- Rhodes SM, Donaldson DI. Electrophysiological evidence for the influence of unitization on the processes engaged during episodic retrieval: Enhancing familiarity based remembering. Neuropsychologia. 2007;45:412–424. doi: 10.1016/j.neuropsychologia.2006.06.022. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Curran T. Trends Cogn Sci. 2007. Event-related potentials and recognition memory. in press. [DOI] [PubMed] [Google Scholar]
- Shiffrin RM, Steyvers M. A model for recognition memory: REM--retrieving effectively from memory. Psychon B Rev. 1997;4:145–166. doi: 10.3758/BF03209391. [DOI] [PubMed] [Google Scholar]
- Srinivasan R, Nunez PL, Tucker DM, Silberstein RB, Cadusch PJ. Spatial sampling and filtering of EEG with spline laplacians to estimate cortical potentials. Brain Topogr. 1996;8:355–366. doi: 10.1007/BF01186911. [DOI] [PubMed] [Google Scholar]
- Tsivillis D, Otten LJ, Rugg MD. Context effects on the neural correlates of recognition memory: An electrophysiological study. Neuron. 2001;31:497–505. doi: 10.1016/s0896-6273(01)00376-2. [DOI] [PubMed] [Google Scholar]
- Tucker DM. Spatial sampling of head electrical fields: the geodesic sensor net. Electroen Cli Neuro. 1993;87:154–163. doi: 10.1016/0013-4694(93)90121-b. [DOI] [PubMed] [Google Scholar]
- Tulving E. Memory and consciousness. Can Psycho. 1985;26:1–12. [Google Scholar]
- Wagner AD, Shannon BJ, Kahn I, Buckner RL. Parietal lobe contributions to episodic memory retrieval. Trends Cogn Sci. 2005;9:445–453. doi: 10.1016/j.tics.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Winer BJ. Statistical principles in experimental design. 2. New York: McGraw-Hill; 1971. [Google Scholar]
- Wixted JT. Dual process theory and signal detection theory of recognition memory. Psychol Rev. 2007;114:152–176. doi: 10.1037/0033-295X.114.1.152. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP. Recognition memory ROCs for item and associative information: the contribution of recollection and familiarity. Mem Cognition. 1997;25:747–763. doi: 10.3758/bf03211318. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP, Kroll NE, Dobbins IG, Soltani M. Recognition memory for faces: when familiarity supports associative recognition judgments. Psychon B Rev. 1999;6:654–661. doi: 10.3758/bf03212975. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP. The nature of recollection and familiarity: A review of 30 years of research. J Mem Lang. 2002;46:441–517. [Google Scholar]