Abstract
Inbred strains of mice are useful model systems for studying the interactions of genetic and environmental contributions during neurodevelopmental stages. We recently reported an inbred strain, BTBR T + tf/J (BTBR), which, as compared to the commonly used C57BL/6J (B6) strain, displays lower social interactions as juveniles, lower social approach in adult ages, and higher levels of repetitive self-grooming throughout developmental stages. The present study investigated whether the early postnatal maternal environment contributes substantially to the unusually low expression of social behaviors and high self-grooming in BTBR as compared to B6. Within 24 hours of birth, entire litters of pups were cross-fostered to either a dam of the same strain or a dam of the opposite strain. Control litters were left with their own mothers. Offspring were tested for juvenile play at postnatal day 21±1, for sociability at 8 weeks of age in an automated 3-chambered social approach test, and for self-grooming at 9-11 weeks of age. Results indicate that deficits in play behaviors in juvenile BTBR pups were not rescued by a B6 maternal environment. Similarly, a BTBR maternal environment did not induce play deficits in B6 pups. Cross-fostering had no effect on sociability scores in adults. The high self-grooming in BTBR and low self-grooming in B6 were not affected by maternal environment. These findings favor a genetic interpretation of the unusual social behaviors and self-grooming traits of BTBR, and support the use of the BTBR inbred strain as a mouse model to study genetic mechanism of autism.
Keywords: inbred strains of mice, behavioral development, maternal factor, postnatal environment, social interaction, juvenile play, autism
1. Introduction
Accumulating evidence indicates that early postnatal environment can substantially influence a broad range of behavioral traits in the offspring (Meaney, 2001; Kaffman and Meaney, 2007). Human studies showed that childhood trauma and neglect are associated with increased risk of developing various psychiatric disorders later in life (Dube et al., 2001; Kendler et al., 2002; Chapman et al., 2004; Kendler et al., 2004; Holmes et al., 2005; Millstein and Holmes, 2007). In rodents, adult rats born and raised by high licking-grooming and arched-back nursing (LG-ABN) mothers exhibited lower levels of novelty-induced fear-like behaviors and milder HPA responses to acute stress, as compared to offspring of low LG-ABN mothers (Caldji et al., 1998). Periodic maternal deprivation during the early postnatal life was associated with increased anxiety-like behaviors (Ogawa et al., 1994; Wigger and Neumann, 1999; Macri et al., 2004) and enhanced hypothalamo-pituitary-adrenal (HPA) axis responses to stress (Plotsky and Meaney, 1993; Suchecki et al., 1995; Ladd et al., 2004). Mice raised in a communal nesting environment, where multiple females share care-giving for their litters, displayed higher levels of social interactions than mice raised in standard laboratory homecages (D’Andrea et al., 2007).
Cross-fostering is a experimental procedure widely used to dissociate relative influences of genetic versus early environmental factors on various neurobiological phenotypes in rodents (Caldji et al., 2004; Priebe et al., 2005; Prakash et al., 2006; Champagne et al., 2007) and primates (Maestripieri, 2003). In an elegant study, (Francis et al., 1999) showed that rats born to low LG-ABN mothers but raised by high LG-ABN mothers were more likely to explore a novel open field than rats raised by low LG-ABN mothers, including biological offspring of high LG-ABN mothers, indicating the importance of non-genomic factors on anxiety/fear-like behaviors in the rat. (Bartolomucci et al., 2004) found that cross-fostered male Swiss CD1 male mice exhibited lower levels of anxiety-like behaviors in the free-exploratory paradigm as compared to non-fostered males, suggesting that the cross-fostering procedure itself could have significant impact on behavioral development.
Inbred strains of mice offer valuable translational tools to study the interplay of genetic and environmental factors in neurodevelopmental disorders. Recent studies (McFarlane et al., 2007; Moy et al., 2007) demonstrated that the BTBR T + tf/J (BTBR) inbred strain displays behavioral phenotypes with analogies to all three diagnostic symptoms of autism, which are aberrant reciprocal social interactions, qualitative impairments in communication, and restricted repetitive and stereotyped patterns of behavior, interests and activities (DSM-IV 2000; Lord et al., 2001, 2006; Volkmar et al., 2004), and thus might be a useful model for the disorder. Compared to the commonly used C57BL/6J (B6) strain, BTBR showed lower levels of play soliciting behaviors at postnatal day 21 (first diagnostic symptom), lower levels of adult social approach (first diagnostic symptom), reduced social transmission of food preference as adults (first and second diagnostic symptoms), and higher levels of repetitive self-grooming throughout developmental stages (third diagnostic symptom). Moreover, comprehensive physical examination and behavioral tests confirmed that BTBR were normal on measures of general health, sensory abilities, motor functions, learning and memory, and anxiety-related tasks These data indicate that the BTBR inbred strain exhibits traits with reasonable face validity to all three of the diagnostic criteria for autism. Following the initial discovery, subsequent research in our laboratory has begun to investigate underlying mechanisms of the autism-like phenotype in BTBR mice.
Cross-fostering studies in rodents have mostly focused on stress and/or anxiety related processes. Data on effects of cross-fostering on mouse social behaviors are scarce. As such, the present study was carried out to investigate the influences of early postnatal maternal environment on subsequent expression of social behaviors in BTBR and B6 mice. Social and grooming behaviors were compared in BTBR and B6 raised by either a dam of the same strain (intra-strain cross-fostering) or a dam of the opposite strain (inter-strain cross-fostering). Control BTBR and B6 mice were raised by their biological mothers. The most critical comparison is between BTBR offspring fostered onto B6 and B6 offspring raised by BTBR mothers. Three behavioral tests were conducted. The juvenile play test was carried out on postnatal day 21±1. Social approach and self-grooming were tested between 8 to 11 weeks of age. This experimental design tests the hypothesis that the unusually low social behaviors and high self-grooming trait in BTBR are shaped primarily by maternal characteristics, observational learning, and/or homecage environment. Alternatively, if inter-strain cross-fostering does not significantly alter social and/or self-grooming behaviors, then it is probable that these traits are attributable to genomic variations between the BTBR and the B6 strain. Given the strong evidence for a genetic component in autism (Wassink and Piven, 2000; Muhle et al., 2004; Bacchelli and Maestrini, 2006; DiCicco-Bloom et al., 2006; Schanen, 2006), a genetic rather than early maternal environment determinant of autism-like traits in BTBR would support the use of this inbred strain in searching for new candidate genes for autism.
2. Materials and methods
2.1. Animals
Breeding, housing, and behavioral testing were conducted in strict compliance with the NIH guidelines for the Care and Use of Laboratory Animals and approved by the National Institute of Mental Health Animal Care and Use Committee. Mice were weaned at postnatal day 21-22, then group housed by sex in standard mouse cages containing 2-4 mice. Animals were bred and housed in colony rooms with controlled temperature (20°C) and humidity (~55%). Standard rodent chow and tap water were available ad libitum. In addition to standard bedding, a Nestlet square and a cardboard tube were provided in each cage. The colony room was on a reverse 12:12 light/dark cycle, with lights on at 9:00 PM. All experiments were conducted between 10:00 AM and 4:00 PM, during the dark phase of the circadian cycle, in a procedure room illuminated by a single 25-watt red light. Recent experiments from our laboratory indicated similar behavioral phenotypes of BTBR and B6 tested during the dark and light phases of their circadian cycles (Yang et al., submitted).
2.2. Cross-fostering
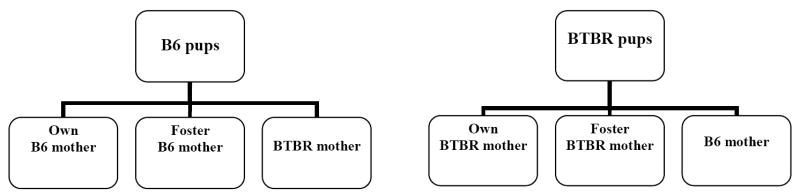
Cross-fostering was conducted within 24-h of parturition. All pups from the litter were removed from the biological mother and placed in a clean cage containing a small amount of bedding taken from the cage of the foster mother. After 5 min, pups were placed in the cage of the foster mother. With this method, pups born to B6 mothers were cross-fostered to either other B6 mothers (intra-strain cross-fostering) or BTBR mothers (inter-strain cross-fostering). Similarly, pups born to BTBR mothers were cross-fostered to either other BTBR mothers (intra-strain cross-fostering) or B6 mothers (inter-strain cross-fostering). Control B6 and BTBR subjects were raised by their own mothers. Figure 1 illustrates the six treatment groups. Each experimental group consisted of 8-12 subjects, drawn from 3-5 litters. Litter sizes ranged from 5-11 pups per cage. Litters were not culled because litter size could be an important aspect of the early social milieu (Ricceri et al., 2007). Moreover, the whole litter cross-fostering method has been described recently (Caldji et al., 2004).
Figure 1.
Schematic illustration of the six treatment groups in the present study. BTBR and B6 pups were either transferred to another female of the same strain (inter-strain cross-fostering) or to a female of the opposite strain (intra-strain cross-fostering). Control animals were raised by their own mothers.
2.3. Behavioral assays
2.3.1. Juvenile Play
Juvenile play was conducted in the Noldus PhenoTyper arena (Noldus, Leesburg VA, USA) as previously described (McFarlane et al., 2007), at postnatal day 21±1. One day before the play test, and 1-h after being singly housed in cages with clean bedding, each subject was allowed to acclimate to the entire empty arena for 10 min. The arena was cleaned with 70% ethanol and water between subjects. On the day of the play test, subjects were again individually housed for 1-h prior to the play test. Two sibling males were then placed in the testing arena and their interactions were recorded for 30 min. Behaviors were subsequently scored from digital videotapes using the Noldus Observer 5.0 system, by a highly trained observer unaware of the fostering group assignment. Behaviors analyzed included nose-to-nose sniffing (sniffing of the head and snout region of the partner), front approach (walking straight towards the partner, facing head-to-head), push and crawl (push = squeezing between the wall and the partner; crawl = crawling over or under the partner’s body), and exploring (walking around the arena, sniffing or digging the bedding). All behaviors were analyzed for frequency of occurrence.
2.3.2 Sociability test
Social approach behaviors were tested in an automated 3-chambered apparatus using methods similar to those previously described (Moy et al., 2004; Nadler et al., 2004; Crawley et al., 2007; McFarlane et al., 2007; Moy et al., 2007). Briefly, the apparatus was a rectangular, three-chambered box made from clear polycarbonate. Retractable doorways built in the two dividing walls allowed access to the side chambers. Quantification of entries and duration in the chambers was automatically measured by photocells embedded in the doorways. The apparatus was cleaned with 70% ethanol and water between subjects.
Animals used as “strangers” were male 129Sv/ImJ and AJ mice, aged 8-14 weeks old, bred in the NIMH vivarium from breeding pairs originally obtained from The Jackson Laboratory (Bar Harbor, ME). Strangers were habituated to the apparatus and to the wire cup enclosure before the start of experiments, for 10 min per day for three consecutive days. The subject mouse was allowed to acclimate to the apparatus for 20 min before the sociability test, 10 min in the central chamber with the doors closed and another 10 min in the entire empty arena with the doors open. The subject was then briefly confined to the center chamber while a novel object (inverted wire cup, Galaxy Cup, Kitchen Plus, http://www.kitchen-plus.com) was introduced into one of the side chambers. A stranger mouse enclosed in an identical wire cup was placed in the other side chamber. An upright plastic drinking cup, held in place by a lead weight in the cup, was placed on the top of each inverted wire cup to prevent the subject from climbing onto the top of the wire cup. The location for the novel object and the stranger mouse alternated between the left and right chambers across subjects. After both stimuli were positioned, the doors were simultaneously re-opened and the subject was allowed access to all three chambers for 10 min. Measures taken include time spent in each chamber, time spent sniffing each cup, and number of entries. An observer uninformed of the genotypes scored time spent sniffing with a stopwatch.
2.3.3. Self-grooming
The test was performed as previously described (McFarlane et al., 2007). Each subject was placed individually in a clean standard mouse cage and allowed to acclimate for 10 min. Following this habituation period, subjects were observed for another 10 min, during which time cumulative time spent in self-grooming was scored by an experimenter sitting approximately 2 meters from the test cage. A silenced stopwatch was used for scoring cumulative time spent grooming during the 10 min test session.
2.4. Statistical analysis
For the automated social approach task, Repeated Measures Analysis of Variance (ANOVA) was used to compare time spent in the chamber. Since times spent in each of the three chambers were not independent, the test condition factor compared time spent only in the right versus left chambers. Time spent in the center chamber is shown in the graphs for illustrative purposes. Time spent sniffing the novel object versus the stranger mouse was similarly analyzed. Number of entries to the side chamber in the social approach test, the juvenile play test, and grooming parameters were analyzed using a 2 × 3 ANOVA, with subject strain (B6 vs. BTBR) and maternal environment (own, inter-strain cross-fostering, intra-strain cross-fostering) as independent variables. Newman-Keuls test was used for post-hoc pairwise comparisons following a significant overall F value. Litter sizes were compared for strain differences by Student’s t-test. P < .05 was used to define statistically significant differences.
3. Results
3.1. Litter sizes
Student’s t-test showed that B6 mothers had smaller litters than BTBR mothers, t(22)=2.71, p<.01 (number of pups: B6=7.50±0.4; BTBR=9.58±0.6;). Male : female ratio (B6=1.54±0.3; BTBR=1.34±0.4) did not differ significantly between the two strains, t(22)=0.42, p=0.68.
3.2. Juvenile Play
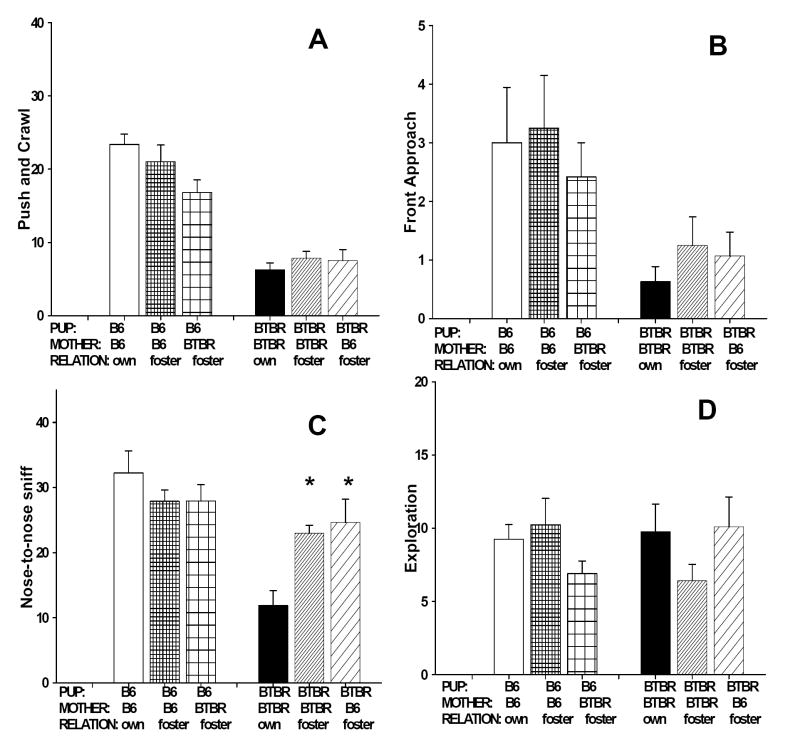
Results of the juvenile play test are illustrated in Figure 2. A) Push/Crawl. The main effect of maternal environment was not significant for the number of push/crawl (F2, 56=1.82, NS). The overall strain difference was significant (F1, 56=104.36, p<.0001), with BTBR pups showing fewer push/crawl compared to B6 pups. The strain x maternal environment interaction was not significant (F2, 56=3.10, NS). B) Front approach. The main effect of maternal environment was not significant for the number of front approach (F2, 56=0.42, NS). The overall strain difference was significant (F1, 56=14.41, p<.001), with BTBR pups showing fewer front approach compared to B6 pups. The strain x maternal environment interaction was not significant (F2, 56=0.38, NS). C) Nose-to-nose sniff. The main effect of maternal environment was not significant for the number of nose sniff (F2, 56=1.14, NS). The overall strain difference was significant (F1, 56=16.52, p<.001), with BTBR pups showing fewer nose sniffs compared to B6 pups. The strain x maternal environment interaction was significant (F2, 56=5.00, p<.01). Post-hoc Newman-Keuls test indicated that BTBR pups raised by foster BTBR mothers or B6 mothers showed more nose sniffs than BTBR raised by their own mothers. D) For the number of arena exploration, no significant difference was found for maternal environment (F2, 56=0.27, NS), the overall strain difference (F1, 56=0.002, NS), or the strain x maternal environment interaction (F2,56=2.63, NS).
Figure 2.
Juvenile play test. No significant maternal strain or significant strain x maternal environment interaction was detected for push/crawl, front approach, and exploration. Significant pup strain differences were found for push/crawl, front approach, nose sniff, but not exploration. (A) B6 pups showed higher levels of push/crawl than BTBR pups (B) B6 pups showed higher levels of front approach than BTBR pups. (C) B6 pups showed higher levels of nose sniff than B6 pups raised in any maternal environment. A significant strain x maternal environment interaction was detected for nose sniff. BTBR pups raised by foster BTBR mother or B6 mothers showed more nose sniff as compared to BTBR raised by their own mothers. (D) All groups showed similar levels of exploration, indicating no strain difference or maternal effect on locomotor activity. *, p<.05 as compared to BTBR pups raised by their own mothers. Data are shown as mean + standard error of the mean in Figures 2-4.
3.3. Social approach test
Figure 3 illustrates social approach behaviors in B6 and BTBR mice. B6 mice raised by their own mother spent more time in the chamber containing the stranger mouse than the chamber containing the novel object (Fig. 3A, F1, 10=28.17, p<.001) and spent more time sniffing the stranger than sniffing the novel object (Fig. 3B, F1, 10=74.65, p<.001). B6 mice raised by foster B6 mothers spent more time in the chamber containing the stranger mouse than the chamber containing the novel object (Fig. 3A, F1,7=40.53, p<.001) and spent more time sniffing the stranger mouse than the novel object (Fig. 3B, F1,7=54.02, p<.001). B6 mice raised by BTBR mothers spent more time in the chamber containing the stranger mouse than the chamber containing the novel object (Fig. 3A, F1, 8=16.15, p<.01) and spent more time sniffing the stranger mouse than the novel object (Fig. 3B, F1, 8=23.59, p<.001). The same genotype determination was found in BTBR subjects. BTBR mice raised by their own mothers failed to spend more time in the chamber containing the stranger mouse than the chamber containing the novel object (Fig. 3A, F1,11=0.37, NS) and failed to spend more time sniffing the stranger mouse than the novel object (Fig. 3B, F1,11=4.44, p=.059). Similarly, BTBR mice raised by foster BTBR mothers failed to spend more time in the chamber containing the stranger mouse than the chamber containing the novel object (Fig. 3A, F1,8=0.75, NS) and failed to spend more time sniffing the stranger mouse than the novel object (Fig. 3B, F1,8=0.18, NS). Further, BTBR mice reared by B6 mothers failed to spend more time in the chamber containing the stranger mouse than the chamber containing the novel object (Fig. 3A, F1,11=0.73, NS) and failed to spend more time sniffing the stranger mouse than the novel object (Fig. 3B, F1,11=0.22, NS). Numbers of entries to the side chambers were analyzed using subject strain x maternal environment ANOVA. As Fig. 3C shows, a significant strain difference was found for entries (F1, 55=8.94, p<.01), with B6 making more entries than BTBR. The maternal environment (F2, 55=1.57, NS) and the strain x maternal environment interaction (F2, 55=0.83, NS) were not significant.
Figure 3.

(A) Adult B6 mice raised by their own mothers, foster B6 mothers, or BTBR mothers all showed normal sociability, spending more time in the chamber containing the stranger mouse than in the chamber containing the novel object. Adult BTBR mice raised by their own mothers, foster BTBR mothers, or B6 mothers all showed deficits in sociability, failing to spend more time in the chamber containing the stranger mouse than in the chamber containing a novel object. (B) Similarly, all three groups of B6 mice spent more time sniffing the stranger mouse than the novel object, whereas BTBR mice raised under any maternal environment failed to spend more time sniffing the stranger mouse than the empty wire cup. (C) B6 made more entries than BTBR.
3.4. Self-grooming
Figure 4 shows the amount of time spent in self-grooming in a clean empty cage. Subject strain x maternal environment ANOVA revealed no significant maternal environment effect (F2, 56=2.80, NS). The overall strain difference was significant (F1, 56=153.46, p<.0001), with BTBR subjects showing substantially higher levels of grooming than B6 subjects. The strain x maternal environment interaction was not significant (F2, 56=2.84, NS).
Figure 4.
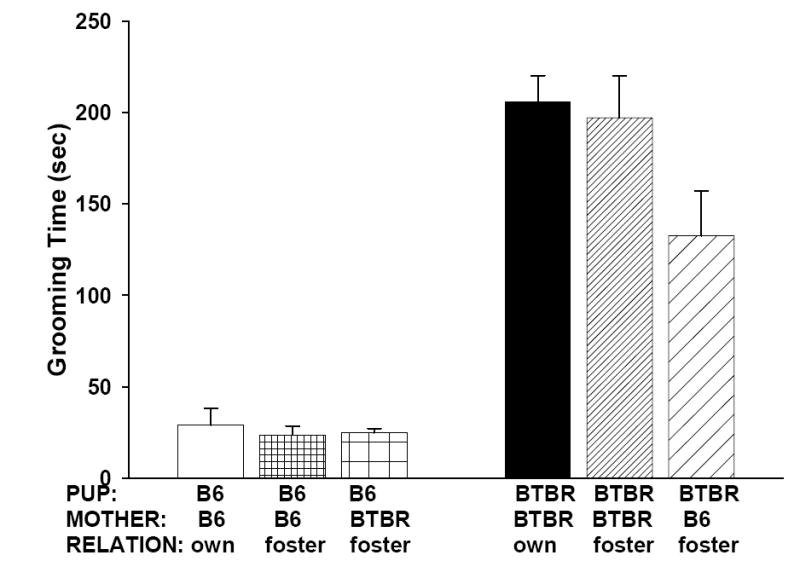
Adult BTBR mice raised by their own mothers, foster BTBR mother, or B6 mothers all showed substantially higher levels of repetitive self-grooming in comparison to B6 raised under any maternal environment. The low self-grooming in B6 was not raised by BTBR maternal environment.
4. Discussion
Early environmental influences on adult rodent behaviors have been extensively described (Meaney, 2001). Prior studies in rats and mice have focused primarily on anxiety-like and stress-related responses in the adults (Caldji et al., 2004; Priebe et al., 2005; Prakash et al., 2006). In the present experiments, we used B6 and BTBR inbred strains, which are characterized by high versus low social approach tendencies, and tested the hypothesis that early maternal environment is responsible for the unusually low social traits and unusually high self-grooming in BTBR mice. In contrast to previous reports which demonstrated that maternal strain has substantial influences on subsequent stress-related and/or anxiety-like traits in rodent offspring, our findings indicate that maternal environment did not have significant impacts on expression of social and self-grooming traits in the offspring.
The juvenile play test demonstrated that the primary social parameters were generally unrelated to the strain of the mother. Push and crawl, i.e. the most commonly used social play behaviors, were not altered by either inter-strain or intra-strain cross-fostering. All three groups of BTBR pups exhibited substantially lower levels of push/crawl in comparison to all three groups of B6 pups. This result replicated a recent finding reported by (McFarlane et al., 2007) and further indicated that the level of push/crawl reflects a largely genetically determined social tendency in juvenile mice. Front approach, a social approach behavior, is lower in BTBR groups as compared to B6 groups and was not affected by maternal environment. Nose-to-nose sniff, a social investigative behavior that could be considered analogous to human eye contact (McFarlane et al., 2007), was lower in BTBR pups than B6 pups. Maternal environment did not have significant effect on nose-to-nose sniff. The present front approach and nose sniff data are fully in agreement with the finding that BTBR pups exhibit lower levels of social investigation in comparison to B6 pups (McFarlane et al., 2007). All groups showed similar levels of exploration of the test arena, indicating that strain differences in social play behavior were not due to variations in general exploratory locomotion.
Adult social approach testing revealed a complete lack maternal effect on sociability in BTBR and B6 male mice. BTBR mice raised by their own mother, by another BTBR mother, or by a B6 mother, all exhibited profound deficits in sociability, failing to spend more time in the chamber containing the stranger mouse as compared to time in the chamber containing the novel object. Similarly, all three BTBR groups failed to spend more time sniffing the stranger mouse than the novel object. In contrast, B6 mice raised by their own mother, by another B6 mother, or by a BTBR mother, all exhibited high levels of sociability, spending significantly more time in the chamber containing the stranger mouse than in the chamber containing the novel object. Similarly, all three groups of B6 spent significantly more time sniffing the stranger mouse than the novel object. These data provided strong evidence that the high sociability in B6 mice and the low sociability in BTBR are not affected by the strain of the mother that provided the maternal care. Instead, the contrasting sociability profiles in BTBR and B6 mice appear to be attributable to genetic differences between these two inbred strains. Future discovery of specific gene differences would shed light on genetic mechanisms of sociability.
It should be noted that B6 made more entries to the side chambers than BTBR. This result is similar to a recent study which showed that B6 tend to make more entries than BTBR in the social approach test ((Moy et al., 2007) Interestingly, BTBR mice exhibited similar levels of locomotor activity as compared to B6 mice in the non-social open field environment (McFarlane et al., 2007). Based on the current result and previous reports, we suggest that BTBR mice do not have abnormalities in motor functions but tend to be less active in a social environment.
Similarly, adult self-grooming result favors a genetic explanation for high repetitive self-grooming in BTBR. All three BTBR groups exhibited substantially higher levels of grooming than B6 mice raised under either maternal condition, demonstrating that excessive repetitive self-grooming in BTBR was not alleviated by a B6 maternal environment. Conversely, B6 mice raised by BTBR mother did not show increased levels of self-grooming compared to intra-strain fostered or non-fostered B6 mice, demonstrating that a BTBR maternal environment was not sufficient to induce the BTBR-typical high self-grooming in the low self-grooming B6 strain.
Results from the social behavior tests, i.e. juvenile play and social approach, are in accord with several previous studies that found no effect of cross-fostering on other categories of social behaviors. It was reported that inter-strain cross-fostered B6 and C3H females exhibited maternal aggression akin to their biological mothers, suggesting that maternal defensive response towards a stranger is largely shaped by genetic factors (Haug and Pallaud, 1981). Similarly, it has been shown that female rhesus macaques exhibited affiliative and aggressive behaviors that are more similar to their biological mothers than to foster mothers (Maestripieri, 2003). Present data are not in agreement with the finding that cross-fostered white-foot mice and California mice exhibited aggressive behaviors similar to their foster parents (Bester-Meredith and Marler, 2001). The discrepancy might be related to the fact that the Bester-Meredith and Marler study used wild-dwelling mice instead of genetically homogeneous laboratory inbred mice. The present findings of low self-grooming of B6 in either maternal condition are consistent with previous findings that B6 mice display low anxiety-like traits that are resistant to changes in maternal environment. Several groups have reported that B6 mice raised by either B6 or BALB/c mothers exhibited similarly low levels of fear/anxiety-like behaviors. In contrast, BALB/c mice cross-fostered to B6 mothers showed decreased levels of fear/anxiety-like behaviors compared to their peers raised by BALB/c mothers (Anisman et al., 1998; Caldji et al., 2004; Priebe et al., 2005).
It is recognized that maternal behaviors of BTBR and B6 dams were not systematically analyzed in the present experiment. Initial unpublished observations did not reveal major differences in maternal care given by BTBR and B6 dams. Nevertheless, it remains to be investigated of whether maternal behaviors differ significantly between BTBR and B6 dams and whether they treat fostered pups and their own pups similarly. Another point emerging from the present experimental design is the similarity of traits in mice raised by their own mother and by another mother of the same strain. Cross-fostering per se did not appear to influence subsequent social interaction traits.
In conclusion, we demonstrated that several unusual behavioral traits of BTBR mice, including deficits in juvenile social interactions, low levels of sociability, and high levels of repetitive self-grooming, were not altered in subjects raised by the more social B6 strain. These data support an interpretation that these phenotypes in BTBR are stable, genetically based traits that are not malleable to different maternal environments during early development. Consistent social deficits and high self-grooming in BTBR raised by mothers of divergent genotypes support the use of the BTBR strain as a mouse model to seek background genes responsible for impaired sociability and repetitive self-grooming, toward generating new hypotheses about genes underlying the diagnostic symptoms of autism.
Acknowledgments
Supported by the National Institute of Mental Health Intramural Research Program.
Footnotes
Sept. 21st, 2007 revision for International Journal of Developmental Neuroscience
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anisman H, Zaharia MD, Meaney MJ, Merali Z. Do early-life events permanently alter behavioral and hormonal responses to stressors? Int J Dev Neurosci. 1998;16:149–164. doi: 10.1016/s0736-5748(98)00025-2. [DOI] [PubMed] [Google Scholar]
- Bacchelli E, Maestrini E. Autism spectrum disorders: molecular genetic advances. American journal of medical genetics. 2006;142:13–23. doi: 10.1002/ajmg.c.30078. [DOI] [PubMed] [Google Scholar]
- Bartolomucci A, Gioiosa L, Chirieleison A, Ceresini G, Parmigiani S, Palanza P. Cross fostering in mice: behavioral and physiological carry-over effects in adulthood. Genes, brain, and behavior. 2004;3:115–122. doi: 10.1111/j.1601-183x.2003.00059.x. [DOI] [PubMed] [Google Scholar]
- Bester-Meredith JK, Marler CA. Vasopressin and aggression in cross-fostered California mice (Peromyscus californicus) and white-footed mice (Peromyscus leucopus) Hormones and behavior. 2001;40:51–64. doi: 10.1006/hbeh.2001.1666. [DOI] [PubMed] [Google Scholar]
- Caldji C, Tannenbaum B, Sharma S, Francis D, Plotsky PM, Meaney MJ. Maternal care during infancy regulates the development of neural systems mediating the expression of fearfulness in the rat. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:5335–5340. doi: 10.1073/pnas.95.9.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldji C, Diorio J, Anisman H, Meaney MJ. Maternal behavior regulates benzodiazepine/GABAA receptor subunit expression in brain regions associated with fear in BALB/c and C57BL/6 mice. Neuropsychopharmacology. 2004;29:1344–1352. doi: 10.1038/sj.npp.1300436. [DOI] [PubMed] [Google Scholar]
- Champagne FA, Curley JP, Keverne EB, Bateson PP. Natural variations in postpartum maternal care in inbred and outbred mice. Physiology & behavior. 2007;91:325–334. doi: 10.1016/j.physbeh.2007.03.014. [DOI] [PubMed] [Google Scholar]
- Chapman DP, Whitfield CL, Felitti VJ, Dube SR, Edwards VJ, Anda RF. Adverse childhood experiences and the risk of depressive disorders in adulthood. Journal of affective disorders. 2004;82:217–225. doi: 10.1016/j.jad.2003.12.013. [DOI] [PubMed] [Google Scholar]
- Crawley JN, Chen T, Puri A, Washburn R, Sullivan TL, Hill JM, Young NB, Nadler JJ, Moy SS, Young LJ, Caldwell HK, Young WS. Social approach behaviors in oxytocin knockout mice: comparison of two independent lines tested in different laboratory environments. Neuropeptides. 2007;41:145–163. doi: 10.1016/j.npep.2007.02.002. [DOI] [PubMed] [Google Scholar]
- D’Andrea I, Alleva E, Branchi I. Communal nesting, an early social enrichment, affects social competences but not learning and memory abilities at adulthood. Behavioural brain research. 2007 doi: 10.1016/j.bbr.2007.05.029. [DOI] [PubMed] [Google Scholar]
- Diagnostic and Statistical Manual of Mental Disorders. Fourth Edition. American Psychiatric Publishing; Arlington, VA: 2000. [Google Scholar]
- DiCicco-Bloom E, Lord C, Zwaigenbaum L, Courchesne E, Dager SR, Schmitz C, Schultz RT, Crawley J, Young LJ. The developmental neurobiology of autism spectrum disorder. J Neurosci. 2006;26:6897–6906. doi: 10.1523/JNEUROSCI.1712-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube SR, Anda RF, Felitti VJ, Chapman DP, Williamson DF, Giles WH. Childhood abuse, household dysfunction, and the risk of attempted suicide throughout the life span: findings from the Adverse Childhood Experiences Study. Jama. 2001;286:3089–3096. doi: 10.1001/jama.286.24.3089. [DOI] [PubMed] [Google Scholar]
- Francis D, Diorio J, Liu D, Meaney MJ. Science. Vol. 286. New York, N.Y: 1999. Nongenomic transmission across generations of maternal behavior and stress responses in the rat; pp. 1155–1158. [DOI] [PubMed] [Google Scholar]
- Haug M, Pallaud B. Effect of reciprocal cross-fostering on aggression of female mice toward lactating strangers. Developmental psychobiology. 1981;14:177–180. doi: 10.1002/dev.420140305. [DOI] [PubMed] [Google Scholar]
- Holmes A, le Guisquet AM, Vogel E, Millstein RA, Leman S, Belzung C. Early life genetic, epigenetic and environmental factors shaping emotionality in rodents. Neuroscience and biobehavioral reviews. 2005;29:1335–1346. doi: 10.1016/j.neubiorev.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Kaffman A, Meaney MJ. Neurodevelopmental sequelae of postnatal maternal care in rodents: clinical and research implications of molecular insights. Journal of child psychology and psychiatry, and allied disciplines. 2007;48:224–244. doi: 10.1111/j.1469-7610.2007.01730.x. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Sheth K, Gardner CO, Prescott CA. Childhood parental loss and risk for first-onset of major depression and alcohol dependence: the time-decay of risk and sex differences. Psychological medicine. 2002;32:1187–1194. doi: 10.1017/s0033291702006219. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Kuhn JW, Prescott CA. Childhood sexual abuse, stressful life events and risk for major depression in women. Psychological medicine. 2004;34:1475–1482. doi: 10.1017/s003329170400265x. [DOI] [PubMed] [Google Scholar]
- Ladd CO, Huot RL, Thrivikraman KV, Nemeroff CB, Plotsky PM. Long-term adaptations in glucocorticoid receptor and mineralocorticoid receptor mRNA and negative feedback on the hypothalamo-pituitary-adrenal axis following neonatal maternal separation. Biological psychiatry. 2004;55:367–375. doi: 10.1016/j.biopsych.2003.10.007. [DOI] [PubMed] [Google Scholar]
- Lord C, Leventhal BL, Cook EH., Jr Quantifying the phenotype in autism spectrum disorders. American journal of medical genetics. 2001;105:36–38. [PubMed] [Google Scholar]
- Lord C, Risi S, DiLavore PS, Shulman C, Thurm A, Pickles A. Autism from 2 to 9 years of age. Archives of general psychiatry. 2006;63:694–701. doi: 10.1001/archpsyc.63.6.694. [DOI] [PubMed] [Google Scholar]
- Macri S, Mason GJ, Wurbel H. Dissociation in the effects of neonatal maternal separations on maternal care and the offspring’s HPA and fear responses in rats. The European journal of neuroscience. 2004;20:1017–1024. doi: 10.1111/j.1460-9568.2004.03541.x. [DOI] [PubMed] [Google Scholar]
- Maestripieri D. Similarities in affiliation and aggression between cross-fostered rhesus macaque females and their biological mothers. Developmental psychobiology. 2003;43:321–327. doi: 10.1002/dev.10143. [DOI] [PubMed] [Google Scholar]
- McFarlane HG, Kusek GK, Yang M, Phoenix JL, Bolivar VJ, Crawley JN. Autism-like behavioral phenotypes in BTBR T+tf/J mice. Genes, brain, and behavior. 2007 doi: 10.1111/j.1601-183X.2007.00330.x. [DOI] [PubMed] [Google Scholar]
- Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annual review of neuroscience. 2001;24:1161–1192. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- Millstein RA, Holmes A. Effects of repeated maternal separation on anxiety- and depression-related phenotypes in different mouse strains. Neuroscience and biobehavioral reviews. 2007;31:3–17. doi: 10.1016/j.neubiorev.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Perez A, Barbaro RP, Johns JM, Magnuson TR, Piven J, Crawley JN. Sociability and preference for social novelty in five inbred strains: an approach to assess autistic-like behavior in mice. Genes, brain, and behavior. 2004;3:287–302. doi: 10.1111/j.1601-1848.2004.00076.x. [DOI] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Young NB, Perez A, Holloway LP, Barbaro RP, Barbaro JR, Wilson LM, Threadgill DW, Lauder JM, Magnuson TR, Crawley JN. Mouse behavioral tasks relevant to autism: phenotypes of 10 inbred strains. Behavioural brain research. 2007;176:4–20. doi: 10.1016/j.bbr.2006.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhle R, Trentacoste SV, Rapin I. The genetics of autism. Pediatrics. 2004;113:e472–486. doi: 10.1542/peds.113.5.e472. [DOI] [PubMed] [Google Scholar]
- Nadler JJ, Moy SS, Dold G, Trang D, Simmons N, Perez A, Young NB, Barbaro RP, Piven J, Magnuson TR, Crawley JN. Automated apparatus for quantitation of social approach behaviors in mice. Genes, brain, and behavior. 2004;3:303–314. doi: 10.1111/j.1601-183X.2004.00071.x. [DOI] [PubMed] [Google Scholar]
- Ogawa T, Mikuni M, Kuroda Y, Muneoka K, Mori KJ, Takahashi K. Periodic maternal deprivation alters stress response in adult offspring: potentiates the negative feedback regulation of restraint stress-induced adrenocortical response and reduces the frequencies of open field-induced behaviors. Pharmacology, biochemistry, and behavior. 1994;49:961–967. doi: 10.1016/0091-3057(94)90250-x. [DOI] [PubMed] [Google Scholar]
- Plotsky PM, Meaney MJ. Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Brain Res Mol Brain Res. 1993;18:195–200. doi: 10.1016/0169-328x(93)90189-v. [DOI] [PubMed] [Google Scholar]
- Prakash P, Merali Z, Kolajova M, Tannenbaum BM, Anisman H. Maternal factors and monoamine changes in stress-resilient and susceptible mice: cross-fostering effects. Brain research. 2006;1111:122–133. doi: 10.1016/j.brainres.2006.06.089. [DOI] [PubMed] [Google Scholar]
- Priebe K, Romeo RD, Francis DD, Sisti HM, Mueller A, McEwen BS, Brake WG. Maternal influences on adult stress and anxiety-like behavior in C57BL/6J and BALB/cJ mice: a cross-fostering study. Developmental psychobiology. 2005;47:398–407. doi: 10.1002/dev.20098. [DOI] [PubMed] [Google Scholar]
- Ricceri L, Moles A, Crawley J. Behavioral phenotyping of mouse models of neurodevelopmental disorders: relevant social behavior patterns across the life span. Behavioural brain research. 2007;176:40–52. doi: 10.1016/j.bbr.2006.08.024. [DOI] [PubMed] [Google Scholar]
- Schanen NC. Epigenetics of autism spectrum disorders. Human molecular genetics. 2006;15(Spec No 2):R138–150. doi: 10.1093/hmg/ddl213. [DOI] [PubMed] [Google Scholar]
- Suchecki D, Nelson DY, Van Oers H, Levine S. Activation and inhibition of the hypothalamic-pituitary-adrenal axis of the neonatal rat: effects of maternal deprivation. Psychoneuroendocrinology. 1995;20:169–182. doi: 10.1016/0306-4530(94)00051-b. [DOI] [PubMed] [Google Scholar]
- Volkmar FR, Lord C, Bailey A, Schultz RT, Klin AJ. Autism and pervasive developmental disorders. Child Psychol Psychiatry. 2004;45:135–170. doi: 10.1046/j.0021-9630.2003.00317.x. [DOI] [PubMed] [Google Scholar]
- Wassink TH, Piven J. The molecular genetics of autism. Current psychiatry reports. 2000;2:170–175. doi: 10.1007/s11920-000-0063-x. [DOI] [PubMed] [Google Scholar]
- Wigger A, Neumann ID. Periodic maternal deprivation induces gender-dependent alterations in behavioral and neuroendocrine responses to emotional stress in adult rats. Physiology & behavior. 1999;66:293–302. doi: 10.1016/s0031-9384(98)00300-x. [DOI] [PubMed] [Google Scholar]