Abstract
Delayed removal of amelogenins, which are initially hydrolyzed by matrix metalloproteinase MMP-20, is a characteristic of enamel fluorosis. In this study, we investigated the regulation of MMP-20 and possible effects of fluoride on MMP-20 expression in human ameloblast lineage cells. Protein expression and signaling pathways of human ameloblast lineage cells, exposed to 10 μM fluoride, were compared to control cells without fluoride exposure. The role of activator protein-1 in MMP-20 regulation was analyzed by DNA-protein affinity precipitation and luciferase reporter gene assays. MMP-20 protein levels in human ameloblast lineage cells decreased in the presence of fluoride, while amelogenin and TIMP-2 were not altered. Fluoride also decreased the transcription of a luciferase reporter gene driven by the MMP-20 promoter. Down-regulation of MMP-20 by fluoride was related to suppression of JNK/c-Jun phosphorylation. In contrast, the JNK activator elevated the expression of MMP-20. Three c-Jun binding sites on the MMP-20 promoter were identified for the first time, and were occupied by c-Jun as MMP-20 was induced. Deletion of any one of AP-1 binding sites on the MMP-20 promoter significantly reduced the transcription of downstream luciferase reporter. These in vitro findings suggest that c-Jun is a key regulatory element for MMP-20 expression, and human ameloblast lineage cells can respond to fluoride by down-regulating MMP-20 transcription through the JNK/c-Jun signaling pathway.
Keywords: matrix metalloproteinase, amelogenin, tissue inhibitor of matrix metalloproteinase, mitogen activated protein kinase, Jun N-terminal kinase, activator protein-1
INTRODUCTION
Fluorosed enamel has been shown to have more amelogenin proteins at the late-secretory/early maturation stage of enamel formation (Aoba and Fejerskov, 2002; DenBesten et al., 1985). This retention of amelogenins could delay mineralization of the enamel matrix, resulting in a more porous enamel structure. Amelogenins, which constitute 90% of the enamel organic matrix, are hydrolyzed by enamel matrix proteinases (Robinson et al., 1998). Alteration of proteinase expression and/or activity by fluoride would result in a delay in the removal of amelogenins, with a subsequent retention of these matrix proteins and delayed enamel matrix mineralization.
Both amelogenins and the enamel matrix proteinases are synthesized by epithelially derived ameloblasts. Enamel matrix proteases include matrix metalloproteinase 20 (MMP-20, enamelysin) and kallikrein 4 (KLK-4, enamel matrix serine protease 1, EMSP1) (Hu et al., 2002). MMP-20 is primarily responsible for the initial hydrolysis of amelogenins and the regulation of matrix formation at the secretory and early maturation stages of amelogenesis (Llano et al., 1997). Enamel formation in the MMP-20 knockout mouse model is severely compromised (Caterina et al., 2002), with enamel mineral content reduced by 50% and hardness decreased by 37% (Bartlett et al., 2004). Therefore any alteration in normal MMP-20 expression and/or activity by fluoride is likely to affect enamel formation.
Expression of MMPs are primarily regulated at the transcriptional level (Chakraborti et al., 2003). Mitogen-activated protein kinase (MAPK) signaling pathways have been reported to regulate numerous MMPs in response to various extracellular stimuli, such as growth factors, cytokines, various chemical agents (e.g. phorbol esters) and stress (Chakraborti et al., 2003). MAPKs are a group of protein serine/threonine kinases that serve as information relays, by connecting cell-surface receptors to specific transcription factors such as c-fos and c-jun in the nucleus, to regulate almost all cellular process (Sternlicht and Werb, 2001). How the tooth-specific MMP-20 gene (Turk et al., 2006) is regulated at the transcriptional level is not clearly known.
At the post-transcriptional level, MMP activity is precisely regulated by the natural inhibitors.i.e, the tissue inhibitors of metalloproteinases (TIMPs). TIMPs consist of four members TIMPs-1, -2, -3 and -4 (Gomez et al., 1997), which inactivate MMPs by forming non-covalent stoichiometric complexes with the zinc-binding site of the active form of MMPs (Gomis-Ruth et al., 1997). MMP-20 activity can be completely inhibited by TIMP-2 (Llano et al., 1997).
An alteration in the balance between MMP-20 and its substrate amelogenins could be due to either a matrix effect as fluoride reduces MMP-20 activity or cellular effects, reducing the synthesis of MMP-20 in the presence of increased exogenous fluoride. In this study we explored the possible effects of fluoride on amelogenin and MMP-20 expression of cultured ameloblast lineage cells. Since fluoride is a highly electronegative ion with a wide range of effects at the cellular level, an important consideration for these studies was to use physiologically relevant fluoride levels.
Studies in rodents show that relatively constant plasma fluoride concentrations of approximately 5 μM are sufficient to consistently produce enamel hypomineralization (Angmar-Mansson and Whitford, 1982; Angmar-Mansson and Whitford, 1984). The plasma fluoride concentrations of pigs that developed dental fluorosis range from 5 μM to 12 μM at the time of slaughter (Richards et al., 1985). We assume that humans require similar micromolar plasma fluoride levels to develop fluorosis, whereas higher millimolar fluoride levels may have additional cytotoxic effects not relevant to the formation of fluorosis in vivo. Therefore, we believe that the micromolar level of fluoride (10 μM) applied in this paper is physiologically relevant for the study of fluorosis.
There is little evidence of the effect of fluoride on the biological activity of ameloblasts, in particular protease expression. This has been in part due to the lack of suitable ameloblast culture systems. Our laboratory has successfully isolated, cultured and characterized primary ameloblast lineage cells (DenBesten et al., 2005; Yan et al., 2006). In this current study, we investigated the effects of micromolar fluoride on MMP-20 expression and the molecular pathway transmitting a fluoride-induced alteration of MMP-20 in ameloblast lineage cells.
RESULTS
Micromolar fluoride reduced MMP-20 protein levels, but had no effect on amelogenin and tissue inhibitor of matrix metalloprotease 2 (TIMP-2) expression by ameloblast lineage cells
Western blot analysis of expression of amelogenin, MMP-20 and TIMP-2 in ameloblast lineage cells exposed to 10 μM fluoride for 24 h, showed a significant decrease (approximately 21%) in MMP-20 protein, as compared to the cells that were not exposed to fluoride (Fig. 1A). There was no significant difference in the expression of amelogenin and TIMP-2 in fluoride-treated samples as compared to controls (Fig. 1B).
FIG. 1. Effect of 10 μM fluoride on amelogenin, MMP-20 and TIMP-2 levels in human fetal ameloblast lineage cells.
(A) Western blot analyses for the amounts of amelogenin, MMP-20 and TIMP-2 after 24 h of fluoride exposure. Actin served as a loading control. (B) The graph shows the relative intensity of each immunoreactive band measured with NIH Image software. Fluoride significantly decreased MMP-20 levels by 21% compared to the untreated sample as determined by t-test, *, P < 0.05 (n=3). Amounts of amelogenin and TIMP-2 in ameloblast lineage cells were not significantly affected by fluoride.
Fluoride suppressed transcriptional activity of the MMP-20 promoter
To further investigate whether fluoride regulates the expression of MMP-20 at the transcriptional level, plasmids carrying a luciferase reporter gene driven either by SV40 or MMP-20 promoter (designated as pGL3-control or pGL3-MMP20) were separately transfected into NIH 293 cells. A promoterless luciferase reporter construct (designated as pGL3-basic) was used as a negative control. The transcriptional activity of the luciferase reporter gene, assessed 24 h post-transfections, showed luciferase activity was detected in the cells transfected with pGL3-MMP20 and pGL3-control plasmid, but not with the pGL3-basic vector. As expected, cells transfected with pGL3-control plasmid displayed a stronger luciferase activity than that of the cells transfected with pGL3-MMP20 (Fig. 2B). When NIH 293 cells transfected with either pGL3-control or pGL3-MMP20 plasmids, were exposed to either 0 or 10 μM of fluoride, luciferase activity in the cells transfected with pGL3-MMP-20 exposed to fluoride (average relative light units, ARLU: 115.30), was 35% lower compared to the non-treated cells (ARLU:176.63) (Fig. 2B). The same amount of fluoride did not affect the luciferase activity of cells transfected with pGL3-control.
FIG. 2. Effect of 10 μM fluoride on the transcriptional activity of a luciferase reporter gene driven by the MMP-20 promoter.
(A) Schematic diagrams of the plasmids that were used to transfect NIH 293 cells. Plasmids pGL3-basic and pGL3-control served as a promoterless negative and positive control for the luciferase reporter gene assay. A DNA fragment containing 1855 bp 5′ flanking sequence upstream of the initiating ATG of human MMP-20 gene was cloned into pGL3-basic vector in the sense orientation, and designated as pGL3-MMP20. (B) Twenty-four hours after transfection, cells transfected with pGL3-control and pGL3-MMP20 displayed large amounts of luciferase activity compared to cells transfected with a promoterless pGL3-basic plasmid. Addition of fluoride to the pGL3-MMP20 transfected cells decreased their luciferase activity by 35%. One way ANOVA shows p < 0.05, Tukey’s multiple comparison post-test shows * is significantly different from the rest of samples (n=3). Fluoride had no effect on the cells transfected with pGL3-control.
MMP-20 expression of ameloblast lineage cells was down-regulated by a JNK kinase inhibitor
To determine the signaling pathway that regulates MMP-20 expression, kinase inhibitors specific to subgroups of the MAPK family were used to identify factors responsible for the expression of MMP-20. Ameloblast lineage cells were incubated for 24 h with inhibitors specific for p38 kinase (2.5 μM SB202190, 5 μM SB203580), ERK1/2 kinase (10 μM U0126), JNK kinase (40 μM SP600125) or a negative control SB202474 (2.5 μM). Western blot analysis showed that SP600125, a JNK kinase inhibitor down-regulated MMP-20 expression (Fig. 3A). Expression levels of MMP-20 were significantly decreased by 24%, as compared to that of negative control (Fig. 3B).
FIG. 3. Effect of MAPK inhibitors on the expression of MMP-20 in ameloblast lineage cells.

(A) Western blot was performed to assess the expression of MMP-20 after 24 h of incubation with MAPK inhibitors specific to p38, MEK1/2 or JNK subgroup. Expression of actin served as a loading control. (B) Densitometry analyses of the immunoreactive bands show only SP600125, the JNK specific inhibitor had an effect. It lowered MMP-20 protein levels by 24% compared to control. The difference is significant based on one-way ANOVA followed by Tukey’s multiple comparison post-test (n=3).
Down-regulation of MMP-20 by 10 μM of fluoride was associated with the down-regulation of phosphorylated JNK and c-Jun in ameloblast lineage cells
After exposure to 10 μM of fluoride, ameloblast lineage cells were harvested at various time courses (0 min, 15 min, 30 min and 60 min) and 20 μg of the purified total protein was loaded on a 10% SDS-PAGE for Western blot analysis (Fig. 4A). The expression of pan JNK and c-Jun remained consistent. However, the levels of phosphorylated JNK and c-Jun were significantly suppressed by fluoride after a 30-min exposure, as compared to the level at 0 min control (Fig. 4B).
FIG. 4. Effect of 10 μM fluoride on the phosphorylation of JNK and c-Jun.
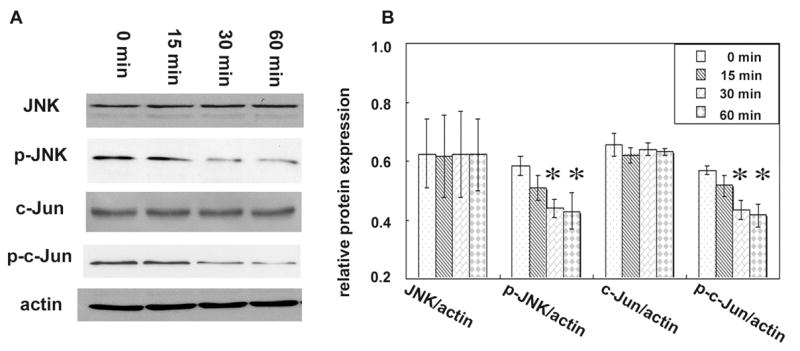
(A) Total proteins of ameloblast lineage cells exposed to 10 μM of fluoride for 0, 15 min, 30 min or 60 min time course were analyzed by Western blot assay. Actin served as a loading control. (B) Densitometry analyses of the immunoreactive bands show that fluoride did not have a significant effect on pan JNK and c-Jun protein levels. However, phosphorylation levels of JNK and c-Jun in the ameloblast lineage cells were significantly decreased after 30 min and 60 min duration. One way ANOVA shows p < 0.05, Tukey’s multiple comparison post-test shows * is significantly different from the 0 min control (n=3).
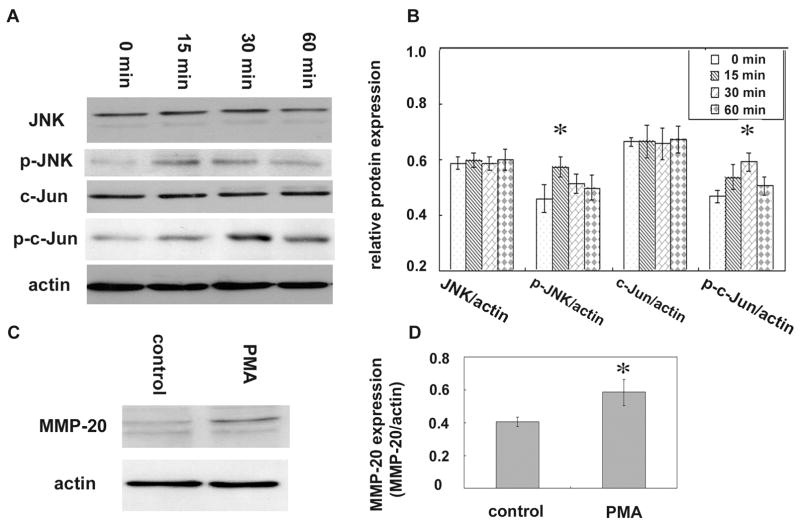
Up-regulation of MMP-20 was induced by PMA, and was associated with elevated phosphorylated JNK and Jun
Ameloblast lineage cells were subjected to exposure with 400 nM of the protein kinase C activator PMA. This activator has been shown to indirectly activate the JNK cascade (Lopez-Bergami et al., 2005). Cells incubated with PMA were collected at various time courses at 0 min, 15 min, 30 min and 60 min. The same amount of total protein was loaded on a 10% SDS-PAGE and probed by JNK and c-Jun antibody for Western blot assay (Fig. 5A). Data analysis showed that PMA induced the phosphorylation of JNK and c-Jun. The level of phosphorylated JNK after 15 min exposure was increased by 25%, and phosphorylated c-Jun at 30 min was increased by 26% compared to that of the controls at 0 min (Fig. 5B). Expression of MMP-20 was significantly increased in the ameloblast lineage cells after 24 h exposure to PMA (Fig. 5C, D).
FIG. 5. Up-regulation of MMP-20 induced by PMA is associated with elevated phosphorylated JNK and Jun.
(A) Total proteins of the ameloblast lineage cells exposed to 400 nM of PMA for either 0, 15 min, 30 min or 60 min were resolved on a 10% SDS-PAGE gel, then transferred to nitrocellulose membrane for Western blot assay. Actin served as a loading control. (B) Densitometry analyses of the immunoreactive bands shows PMA increased the phosphorylation of JNK or c-Jun at 15 min or 30 min duration. One way ANOVA shows p < 0.05, Tukey’s multiple comparison post-test shows * is significantly different from the 0 min control (n=3). (C) Total proteins from ameloblast lineage cells incubated with 400 nM of PMA for 24 h, were subjected to Western blot analysis to asses the expression of MMP-20. Actin served as loading control. (D) Densitometry analyses of the immunoreactive bands show PMA up-regulated the expression of MMP-20 in those cells. * versus control, p < 0.05, determined by t-test (n=3).
The c-Jun transcription factor in ameloblast lineage cells was precipitated by biotinylated double-stranded oligonucleotides containing consensus AP-1 binding sites found within the MMP-20 promoter
There are four potential AP-1 binding motifs (-1456AP-1 tgactca, -711AP-1 ttaatca, -112AP-1 tgaatca and -64AP-1 gagctca) within upstream 1855 bp proximal to the start codon of human MMP-20 gene (Fig. 6A). Three of these are predicted by the software www.gene-regulation.com, and an additional potential -64AP-1 binding site was predicted by Bartlett and co-workers (Turk et al., 2006). Biotinylated oligonucleotides containing the AP-1 binding motifs described above and the corresponding flanking sequences in the MMP-20 gene were incubated with total cell lysates of ameloblast lineage cells. The precipitated proteins were eluted, resolved on 10% SDS-PAGE, and Western blot was applied to probe the presence of c-Jun protein. Oligonucleotides representing the -1456 bp, -711 bp and -112 bp AP-1 binding motifs of MMP-20 were capable of precipitating c-Jun proteins, but not oligonucleotides containing the -64 bp AP-1 binding motif (Fig. 6B).
FIG. 6. . Identification of AP-1 binding motifs within promoter of human gene.
(A) A schematic diagram shows the sequences and location of four potential AP-1 binding motifs within the human MMP-20 promoter, predicted by computer analysis and published report. The numbers indicate the distance from the transcription initiation site to the most distal sequence of the AP-1 binding motif within the promoter of the human MMP-20 gene. (B) Immobilized biotinylated double-stranded oligonucleotides containing the sequences of each of AP-binding motif and flanking region were used to precipitate proteins from the total cell lysate of ameloblast lineage cells. Western blot analysis shows that oligonucleotides representing the -112AP-1, -711AP-1 or -1456 AP-1 binding motif precipitated c-Jun protein from total cell lysate. (C) Immobilized rabbit anti-human pan c-Jun antibody or rabbit non-immune IgG was incubated with cross-linked and fragmented DNA/protein complex isolated from ameloblast lineage cells treated by PMA. The precipitated DNA/protein complex was reverse cross-linked and the DNA was extracted. PCR was performed to amplify sequences flanking individual AP-1 binding site within the MMP-20 promoter by using extracted DNA as template and oligonucleotides complimentary with the end of the flanking sequence of -112AP-1, -711AP-1 or -1456 AP-1 binding motif as primers (product was designated as c-Jun). DNA/protein complex precipitated by immobilized c-Jun antibody served as a negative control (−), and DNA extracted from reverse cross-linked of fragmented total DNA/protein complex served as a positive control (+). PCR products at length 176 bp, 230 bp or 162 bp containing sequences flanking -112AP-1, -711AP-1 or -1456 AP-1 binding motif were visualized on a 1.2% agarose gel. The results suggest that c-Jun binds to the AP-1 sites on the promoter of the MMP-20 gene as MMP-20 was induced.
Chromatin immunoprecipitation confirmed that the regulatory protein c-Jun occupied the promoter region of the MMP-20 gene as MMP-20 was induced
DNA of the ameloblast lineage cells stimulated with PMA was cross-linked to regulatory proteins by adding formaldehyde into the culture media. The purified nuclei extract was sonicated into resultant fragmented DNAs at approximately 300~1000 bp (data not shown). Immobilized rabbit anti-c-Jun antibody or non-immue rabbit IgG was incubated with the sonicated cell lysate to precipitate c-Jun and bound DNA. After elution and reverse cross-linking the associated proteins, sequences flanking AP-1 sites within the MMP-20 promoter were amplified by PCR. DNA/protein complex precipitated by immobilized c-Jun antibody, without reverse cross-linked, served as a negative control, and DNA extracted from reverse cross-linked fragmented total DNA/protein complex served as a positive control. PCR products with sequences flanking -112AP-1, -711AP-1 or -1456AP-1 binding site were detectable on agarose gel in both the positive control and the c-Jun antibody precipitated sample at size 176 bp, 230 bp or 162 bp (Fig. 6C).
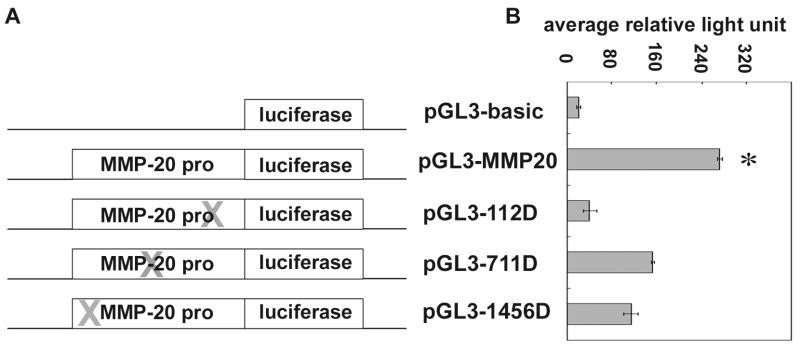
Deletion of AP-1 binding motifs within the MMP-20 promoter significantly repressed the transcriptional activity of downstream luciferase reporter gene
In order to characterize the role of AP-1 binding motifs within the MMP-20 promoter region on transcriptional regulation, -112 AP-1, -711 AP-1 and -1456 AP-1 binding motifs in pGL3-MMP20 construct were deleted individually. The structure of mutated plasmids is diagramed in Fig. 7A. Plasmids were transfected into NIH 293 cells and luciferase activity was assessed 24 h post-transfection. Deletion of either -112 AP-1, -711 AP-1 or -1456 AP-1 binding sites decreased the transcriptional activity of the luciferase reporter gene by 85%, 44% and 58% respectively, compared to the signal of the pGL3-MMP20 transfected cells (Fig. 7B).
FIG. 7. . Characterization of AP-1 binding motifs within the promoter of the human MMP-20 gene.
(A) Schematic diagrams show the structure of the plasmid carrying luciferase reporter gene driven by either a wild type or mutated MMP-20 promoter. QuikChange II site–directed mutagenesis kit was used to delete the various AP-1 binding sites at a time on the pGL3-MMP20 construct. Plasmids were used to transfect NIH 293 cells and its luciferase reporter activity was assessed. (B) Luciferase activity measured 24 h after transfection, dramatically decreased by 85%, 44% or 58% in NIH 293 cells transfected with mutated plasmid with a deletion of either -112AP-1, -711AP-1 or -1456AP-1 binding site respectively compared to cells transfected with the pGL3-MMP20 construct. One way ANOVA shows p < 0.05, Tukey’s multiple comparison post-test shows * is significantly different from the rest of the samples (n=3).
DISCUSSION
In these studies, we found that micromolar levels of fluoride can down-regulate MMP-20 expression in human ameloblast lineage cells. This is consistent with previous studies using real-time PCR to demonstrate the down-regulation of MMP-20 mRNA in vitro (Zhang et al., 2006). We also demonstrated that the same amount of fluoride decreased the transcription of a luciferase reporter gene driven by the MMP-20 promoter in NIH 293 cells. Therefore, we conclude that the reduction of MMP-20 by micromolar levels of fluoride can occur at the transcription level. Our results from in vitro studies are similar to that from in vivo studies by Jing and co-workers, who found MMP-20 but not TIMP-2 was down-regulated in the enamel epithelial cells of Wistar rats exposed to fluoride in their drinking water (Jing et al., 2006).
Fluoride exerts diverse cellular effects in a dose- and cell-type dependent manner. In bone, fluoride at micromolar levels is considered an effective anabolic agent because it promotes bone cell proliferation and alkaline phosphatase actvity both in vivo and in vitro (Farley et al., 1983; Kleerekoper and Mendlovic, 1993). The molecular mechanisms of the osteogenic action of fluoride have been proposed to involve the MAPK signal pathway (Lau and Baylink, 1998). Proliferation of bone cells is inhibited by millimolar levels of fluoride (Farley et al., 1983). Fluoride induces apoptosis of epithelial lung cells through activating P38 and possibly the JNK pathway (Thrane et al., 2001). These studies emphasize the importance of using a relevant cell system and physiological fluoride concentrations to determine the cellular effect of fluoride.
Since fluoride has been shown to affect MAPK signaling pathways in other cell types, we hypothesized that MAPK signaling is responsible for fluoride-induced MMP-20 reduction in ameloblast lineage cells. MAPKs consist of three well-characterized subgroups: extra-cellular signal regulated kinases (ERKs), JNKs and P38. ERKs phosphorylate TCF/ELK-1 and therefore induce c-Fos synthesis. JNKs phosphorylate c-Jun and ATF2 (Karin, 1995). P38 phosphorylates HSP-27 and ATF1/2. In our study, only SP600125, a JNK inhibitor significantly suppressed MMP-20 expression. SP600125 selectively inhibits JNK phosphorylation by competitively responding to ATP (Bennett et al., 2001). In addition, fluoride-induced reduction of MMP-20 was concomitant with a decrease in JNK and c-Jun phosphorylation. Conversely, MMP-20 expression increased in response to PMA, a JNK activator. These results suggest that the JNK/c-Jun signaling pathway is involved in fluoride-induced MMP-20 down-regulation.
Activated JNKs can translocate to the nucleus where they regulates transcription through their effectors: c-Jun, activating transcription factor (ATF-2) and other transcription factors. Homodimers and heterodimers of Jun, Fos or ATF-2 make up of the activator protein (AP-1) transcription factors. Jun-Jun and Jun-Fos dimers preferentially bind to the palindromic TRE sequence TGA(C/G)TCA. Jun-ATF dimmers or ATF homodimers prefer to bind to the cAMP-responsive element, which has the base sequence TGACGTCA (Karin et al., 1997). AP-1 transcription factors are regulated by abundance, phosphorylation and stability. In the case of c-Jun, the phosphorylation of serines 63 and 73 by JNK reduces the ubiquitination of this protein and hence its degradation (Musti et al., 1997), increasing its stability.
We tested whether the JNK effector- c-Jun was able to bind to the MMP-20 promoter region and regulate its transcription. NIH 293 cells were used for the luciferase reporter gene assay because these cells are relatively easy to transfect, and have been used to express a recombinant wild-type human MMP-20 constructs (Ozdemir et al., 2005). This assay showed that our cloned MMP-20 promoter was capable of initiating the transcription of the downstream luciferase reporter gene. Computer analyses and published reports predict four potential c-Jun binding motifs within this region, but no ATF binding sites. For the first time, we determined that three functional AP-1 binding sites on the human MMP-20 promoter, regulate MMP-20 expression. When the 5′-TGAATCA- 3′ sequence at -112 was deleted, transcriptional activity of luciferase gene driving by mutated MMP-20 promoter was reduced by 85%. This transcription site is also found in MMP-3 and MMP-10 promoters (Chakraborti et al., 2003). The 5′-TGACTCA-3′ sequence at -1456 is present on most MMPs’ promoters. The 5′-TTAATCA-3′ at -717 is somewhat divergent, occurring within MMP-1, MMP-3, MMP-7 and MMP-11 promoters. Deletion any of the three AP-1 sites within the MMP-20 promoter significantly reduced the transcription of the downstream luciferase reporter gene.
These motifs were occupied by c-Jun as MMP-20 was induced. Therefore, we conclude that c-Jun regulates the MMP-20 gene. BenBow and coworkers proposed that the presence of more than one AP-1 binding site in the MMP promoter may have a role in the tissue-specific expression pattern of MMPs (Benbow and Brinckerhoff, 1997). Our results indicate the importance of AP-1 on the regulation of MMP-20 expression and imply that any factors (such as fluoride or phorbol ester) that affect AP-1 activity could affect MMP-20 transcription. Although AP-1 is essential to switch on the human MMP-20 gene, transactivation of the MMP-20 promoter in the tooth organ in vivo, may be dependent on a complex network with other transcriptional factors binding to additional regulatory elements in the 5′-flanking regions of the MMP-20 gene, such as NF-E1 (Marchenko et al., 2002).
MMP-20 expression plays a pivotal role in guiding enamel formation. Understanding the regulation of MMP-20 enhances our overall knowledge of amelogenesis, as well as dental fluorosis. Further studies to determine how AP-1 integrates with other transcriptional factors to activate MMP-20 transcription in tooth organ will allow us to determine the tissue specific regulation of MMP-20 in tooth formation.
EXPERIMENTAL PROCEDURES
Antibodies and reagents
Polyclonal rabbit anti-recombinant amelogenin antibody was purified from anti-serum by using protein A affinity chromatography. Mouse anti-TIMP-2 antibody was purchased from Chemicon (Temecula, CA). Rabbit anti-human MMP-20 antibody, rabbit IgG were purchased from Sigma (St. Louis, MO). Rabbit anti-human actin antibody was purchased from Santa Cruz Biotechnology, INC (Santa Cruz, CA). Rabbit anti-human pan JNK antibody, rabbit anti-human phosphorylated JNK antibody, rabbit anti-human Pan c-Jun, rabbit anti-human phosphorylated c-Jun (Ser 73) were purchased from Cell Signaling (Beverly, MA). MAPK inhibitors SB202190, SB203580, U0126, SP600125, negative control SB202474 and phorbol-12-myristate-13-acetate (PMA) were purchased from Calbiochem (La Jolla, CA). Immobilized NeutrAvidin gel was purchased from Pierce (Rockford, IL). Sheared salmon sperm DNA solution was purchased from Invitrogen (Carlsbad, CA). POLY(dI-dC).POLY(dI-dC) was purchased from Amersham Biosciences (Piscataway, NJ). Cloned Pfu DNA polymerase, QuikChange II site–directed mutagenesis kit were purchased from Stratagene (La Jolla, CA). Human genomic DNA was purchased from BD Biosciences (Palo Alto, CA). Plasmid pGL3 basic, pGL3 control vectors, Glo lysis buffer and Bright-Glo™ Luciferase Assay System were purchased from Promega (Madison, WI). Lipofectamine™ 2000 Transfection Reagent was purchased from Invitrogen (Carlsbad, CA).
Cell culture
Tooth organs were dissected from approximately 21-week old human fetal cadaver tissues. All tissues were collected under the approval of the UCSF committee on human research. Ameloblast lineage cells were isolated as previously described (Yan et al., 2006) and grown in supplemented keratinocyte growth media (KGM-2) (Cambrex, MD) with 0.05 mM calcium, 1 % penicillin and streptomycin. This cell population was further enhanced by removing fibroblast-like cells with STV solution impregnated cloning discs (Scienceware, GA). Selected ameloblast lineage cells were characterized by immunochemical staining with cytokeratin 14, amelogenin and MMP-20 antibodies (Tabata et al., 1996; Yan et al., 2006). For fluoride treated samples, 10 μM NaF was added into the growth medium as cells reached 80% confluence, following overnight synchronization in growth factor-free media (KBM).
Western blot analysis
Cultured cells were washed twice with cold PBS and lysed in TNE buffer (150 mM NaCl, 50 mM Tris, pH 7.4, 5 mM EDTA, 0.1% SDS, 1% Nonidet P-40, 1% sodium deoxycholate) containing protease inhibitor mixtures I and II (Sigma, MO) for 15 min at 4°C. The lysates were pelleted by 15 min of centrifugation at 13,000 × g at 4°C, and the protein concentration in supernatant was determined with a bicinchoninic acid assay kit from Piece (Rockford, IL). After 5 min of boiling, equal amounts of protein (20 ug of total protein) from each sample were resolved by SDS-PAGE and then transferred to nitrocellulose membrane (Amersham Biosciences, NJ, USA). After 1 h of blocking with 5 % not-fat milk, the membranes were probed with primary antibodies overnight at 4°C. A control blot incubated with anti-human actin antibody was used to normalize the amount of protein loaded in each sample. The membrane was washed and then probed with a species-specific horseradish peroxidase-conjugated secondary antibody for 1 h at room temperature, followed by ECL detection (Amersham Biosciences, NJ). The intensities of the immunoreactive bands were scanned and measured with NIH Image, version 1.30.
Plasmid construction
Commercial human genomic DNA was used as a template to amplify an 1855bp DNA fragment upstream of the human MMP-20 gene start codon ATG (The GenBank accession number of the human MMP-20 gene is AY673603). Primers containing Nhe I and Xho I restriction enzyme cutting sites (5′ ctagctagcttttagcagctgttgaagataagg 3′ and 5′ccgctcgagcccctcacagtagcttggtaatta 3′) were synthesized by Elim Biopharmaceutical, Inc (Hayward, CA). The gel purified PCR product was digested with Nhe I and Xho I, and subsequently subcloned into the NheI/XhoI site of a promoterless luciferase reporter pGL3-basic vector. The construct was confirmed by sequencing and designated as pGL3-MMP20. A QuikChange II site–directed mutagenesis kit was used to separately delete -112AP-1 site (tgaatca), -711AP-1 site (ttaatca) and -1456AP-1 site (tgactca) within the construct by following the manufacturer’s protocol. The primer sequences are listed in TABLE 1. The mutated plasmids were transformed into XL1-blue competent cells and their sequences were confirmed by sequencing. The structures of promoterless pGL-3 basic vector, SV40 promoter driven luciferase reporter vector pGL3-control and MMP-20 driven luciferase reporter vector pGL3-MMP20 are diagramed in Fig. 2A.
TABLE 1.
Oligonucleotide primers used for the precipitation assays and deletion constructs
| Name | Sequence | Orientation | Experiment |
|---|---|---|---|
| B-64AP-1 | 5′-taaaaggagctcataaaaggagctcaaggtcgaagt -3′ | sense | biotinylated DNA IP |
| B-64AP-1 | 5′-bio-acttcgaccttgagctccttttatgagctcctttta -3′ | antisense | biotinylated DNA IP |
| B-112AP-1 | 5′-gagacatgaatcagagacatgaatcatccttgctcg -3′ | sense | biotinylated DNA IP |
| B-112AP-1 | 5′-bio-cgagcaaggatgattcatgtctctgattcatgtctc -3′ | antisense | biotinylated DNA IP |
| B-717AP-1 | 5′-tccatcttaatcatccatcttaatcagacccaggaa -3′ | sense | biotinylated DNA IP |
| B-717AP-1 | 5′-bio-ttcctgggtctgattaagatggatgattaagatgga -3′ | antisense | biotinylated DNA IP |
| B-1456AP-1 | 5′-cttagttgactcacttagttgactcacagttttaca -3′ | sense | biotinylated DNA IP |
| B-1456AP-1 | 5′-bio-tgtaaaactgtgagtcaactaagtgagtcaactaag -3′ | antisense | biotinylated DNA IP |
| CHIP-112AP-1 | 5′-gactctttttctccttttgcc -3′ | sense | CHIP |
| CHIP-112AP-1 | 5′-ttttatagagtcaaacaggtc -3′ | antisense | CHIP |
| CHIP-711AP-1 | 5′-cccaacatcacacagctagga -3′ | sense | CHIP |
| CHIP-711AP-1 | 5′-aattaaggcaagagttacgtc -3′ | antisense | CHIP |
| CHIP-1456AP-1 | 5′-tgtgtgtattagtccattctc -3′ | sense | CHIP |
| CHIP-1456AP-1 | 5′-caagaaggtgcctgcttcccc -3′ | antisense | CHIP |
| PGL3-112D | 5′-gtataattactgtttgagacatccttgctcggagggtccag -3′ | sense | site mutagenesis |
| PGL3-112D | 5′-ctggaccctccgagcaaggatgtctcaaacagtaattatac -3′ | antisense | site mutagenesis |
| PGL3-711D | 5′-catctctggatccaaatccatcgacccaggaaaaacaatatg -3′ | sense | site mutagenesis |
| pGL3-711D | 5′-catattgtttttcctgggtcgatggatttggatccagagatg -3′ | antisense | site mutagenesis |
| pGL3-1456D | 5′-taaaggaaagaggcttagtcagttttacatggctggggag -3′ | sense | site mutagenesis |
| pGL3-1456D | 5′-ctccccagccatgtaaaactgactaagcctctttccttta -3′ | antisense | site mutagenesis |
Transient transfection and luciferase reporter assay
Plasmids were transformed into DH 5α competent cells for propagation. DNA was purified using QIAGEN Endofree Plasmid Maxi Kit (Valencia, CA). NIH 293 cells from American Type Culture Collection (ATCC) were maintained in minimum essential medium with 2 mM L-glutamine, 10% FBS and 1% penicillin and streptomycin according to the manufacturer’s protocol. NIH 293 cells were seeded at a density of 5×105 cells per well of a six-well plate in antibiotic free medium. The following day, cells were transfected with 5 μg of DNA and 10 μl of Lipofectamine™ 2000 according to the manufacturer’s instructions. Twenty-four hours after transfection, the cells were washed with PBS, lysed with 500 μl Glo lysis buffer for 5 min. Then 100 μl of cell lysate was transferred into a clear bottom/black 96-well plate (Becton Dickinson Labware, NJ). The same volume of Bright-GloTM assay reagent was added to each well, the plates were incubated for 5 min at room temperature, and then luminescence was measured by using a Geminixs luminometer (Molecular Devices, Sunnyvale, CA).
Biotinylated DNA affinity precipitation
Confluent ameloblast lineage cells were rinsed twice by PBS, and lysed by lysis buffer (10 mM Hepes, pH 7.9, 100 mM KCl, 5 mM MgCl2, 10% Glycerol, 0.1% NP-40). Cells were sonicated three times with 10 seconds/each stroke, then rotated at 4 °C for 20 min. The cell lysate was then spun at 14,000× g at 4 °C for 20 min. The supernatant was collected and the protein concentration was determined by bicinchoninic acid assay (BCA). Biotinylated oligos containing the potential -64AP-1, -112AP-1, -711AP-1 or -1456AP-1 binding sites and the corresponding flank sequences within the human MMP-20 gene promoter region were synthesized by Elim Biopharmaceutical, Inc. (TABLE 1). The synthesized single strand oligos were dissolved with buffer (20 mM Tris, pH 7.4, 150 mM NaCl), and the same molar ratios of sense and antisense oligos were mixed and heated to 94°C for 5 min, then allowed to gradually cool to form double-stranded oligos. Neutravidin agarose and 20 nmol of annealed double-stranded DNA were incubated at 4°C for 2 h with rotation. Unbound oligos were removed by washing with PBS three times. The agarose/oligo complex was incubated with 1 mg of ameloblast cell lysate and 5 ug of poly(dI-dc) and were rotated at 4 °C overnight. After overnight incubation, the agarose was washed three times with PBS. Then the agarose was resuspended with 30 ul of 2×sample buffer and boiled for 10 min. Thirty microlitres of suspension were loaded on a 10% SDS-PAGE for Western blot analysis.
Chromatin immunoprecipitation (CHIP) assays
Ameloblast lineage cells at 80% confluence were incubated in 10-cm diameter dishes overnight with KGM-2 medium supplemented with 400 nM phorbol-12-myristate-13-acetate (PMA). Then DNA and proteins were cross-linked by treatment with formaldehyde (final concentration, 1%) for 10 min at 37°C. After thoroughly rinsing with phosphate-buffered saline, cells were collected and resuspended in SDS lysis buffer (1% SDS, 10 mM EDTA, 50 mM Tris-HCl, pH 8.0) with protease inhibitor cocktail. The lysate was subjected to sonication to break down the genomic DNA into 300 to 1000 bp long fragments, then diluted with dilution buffer (0.01%SDS, 1.1% TritonX-100, 1.2 mM EDTA, 16.7 mM Tris-HCl, pH 8.1, 167 mM NaCl), and precleared by incubation with salmon sperm DNA/protein A agarose for 60 min at 4°C. The precleared supernatant was then incubated with 4 μg of anti-human-c-Jun antibody or non-immune rabbit IgG overnight at 4°C. Immunocomplexes were sequentially incubated with a salmon sperm DNA/protein A agarose for 4 h at 4°C, eluted after stringent washings, and reversed cross-linked by heating the complex at 65°C for 4 h, followed by treatment with 2 μl of 10 mg/ml proteinase K at 45°C for 60 min. DNA was extracted by phenol-chloroform extraction and ethanol precipitation and used as a template for PCR to amplify the DNA fragments flanking AP-1 sites within the MMP-20 promoter. The sequences of primer pairs used in PCR are listed in TABLE 1. The PCR products were separated and visualized on a 1.2 % agarose gel.
Statistics analysis
All data were collected in triplicate experiments and analyzed by either t-test or one-way ANOVA following Tukey’s multiple comparison post-test by using Prism software (GraphPad Software Inc, San Diego).
Acknowledgments
This study was supported by NIDCR grants R01-DE013508 to P.D.B. and R01-DE015821 to W.L. We are grateful to Dr. Chen Liu for helpful discussion and Dr. Francoise Chanut for editorial assistance.
The abbreviations used are
- MMP
matrix metalloproteinase
- TIMP
tissue inhibitor of metalloproteinase
- KGM
keratinocyte growth media
- MAPK
mitogen activated protein kinase
- ERK
extra-cellular signal regulated kinase
- JNK
Jun N-terminal linase
- AP-1
activator protein-1
- PMA
phorbol-12-myristate-13-acetate
- FBS
fetal bovine serum
- PBS
phosphate-buffered saline
- PCR
polymerase chain reaction
- BCA
bicinchoninic acid assay
- ANOVA
analysis of variance
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Angmar-Mansson B, Whitford GM. Plasma fluoride levels and enamel fluorosis in the rat. Caries Res. 1982;16:334–339. doi: 10.1159/000260617. [DOI] [PubMed] [Google Scholar]
- Angmar-Mansson B, Whitford GM. Enamel fluorosis related to plasma F levels in the rat. Caries Res. 1984;18:25–32. doi: 10.1159/000260743. [DOI] [PubMed] [Google Scholar]
- Aoba T, Fejerskov O. Dental fluorosis: chemistry and biology. Crit Rev Oral Biol Med. 2002;13:155–170. doi: 10.1177/154411130201300206. [DOI] [PubMed] [Google Scholar]
- Bartlett JD, Beniash E, Lee DH, Smith CE. Decreased mineral content in MMP-20 null mouse enamel is prominent during the maturation stage. J Dent Res. 2004;83:909–913. doi: 10.1177/154405910408301204. [DOI] [PubMed] [Google Scholar]
- Benbow U, Brinckerhoff CE. The AP-1 site and MMP gene regulation: what is all the fuss about? Matrix Biol. 1997;15:519–526. doi: 10.1016/s0945-053x(97)90026-3. [DOI] [PubMed] [Google Scholar]
- Bennett BL, Sasaki DT, Murray BW, O’Leary EC, Sakata ST, Xu W, Leisten JC, Motiwala A, Pierce S, Satoh Y, et al. SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proc Natl Acad Sci U S A. 2001;98:13681–13686. doi: 10.1073/pnas.251194298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caterina JJ, Skobe Z, Shi J, Ding Y, Simmer JP, Birkedal-Hansen H, Bartlett JD. Enamelysin (matrix metalloproteinase 20)-deficient mice display an amelogenesis imperfecta phenotype. J Biol Chem. 2002;277:49598–49604. doi: 10.1074/jbc.M209100200. [DOI] [PubMed] [Google Scholar]
- Chakraborti S, Mandal M, Das S, Mandal A, Chakraborti T. Regulation of matrix metalloproteinases: an overview. Mol Cell Biochem. 2003;253:269–285. doi: 10.1023/a:1026028303196. [DOI] [PubMed] [Google Scholar]
- DenBesten PK, Crenshaw MA, Wilson MH. Changes in the fluoride-induced modulation of maturation stage ameloblasts of rats. J Dent Res. 1985;64:1365–1370. doi: 10.1177/00220345850640120701. [DOI] [PubMed] [Google Scholar]
- DenBesten PK, Machule D, Zhang Y, Yan Q, Li W. Characterization of human primary enamel organ epithelial cells in vitro. Arch Oral Biol. 2005;50:689–694. doi: 10.1016/j.archoralbio.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Farley JR, Wergedal JE, Baylink DJ. Fluoride directly stimulates proliferation and alkaline phosphatase activity of bone-forming cells. Science. 1983;222:330–332. doi: 10.1126/science.6623079. [DOI] [PubMed] [Google Scholar]
- Gomez DE, Alonso DF, Yoshiji H, Thorgeirsson UP. Tissue inhibitors of metalloproteinases: structure, regulation and biological functions. Eur J Cell Biol. 1997;74:111–122. [PubMed] [Google Scholar]
- Gomis-Ruth FX, Maskos K, Betz M, Bergner A, Huber R, Suzuki K, Yoshida N, Nagase H, Brew K, Bourenkov GP, et al. Mechanism of inhibition of the human matrix metalloproteinase stromelysin-1 by TIMP-1. Nature. 1997;389:77–81. doi: 10.1038/37995. [DOI] [PubMed] [Google Scholar]
- Hu JC, Sun X, Zhang C, Liu S, Bartlett JD, Simmer JP. Enamelysin and kallikrein-4 mRNA expression in developing mouse molars. Eur J Oral Sci. 2002;110:307–315. doi: 10.1034/j.1600-0722.2002.21301.x. [DOI] [PubMed] [Google Scholar]
- Jing FQ, Wang Q, Liu TL, Guo LY, Liu H. [Effects of overdosed fluoride on rat’s incisor expression of matrixmetalloproteinase-20 and tissue inhibitors of metalloproteinase-2] Hua Xi Kou Qiang Yi Xue Za Zhi. 2006;24:199–201. [PubMed] [Google Scholar]
- Karin M. The regulation of AP-1 activity by mitogen-activated protein kinases. J Biol Chem. 1995;270:16483–16486. doi: 10.1074/jbc.270.28.16483. [DOI] [PubMed] [Google Scholar]
- Karin M, Liu Z, Zandi E. AP-1 function and regulation. Curr Opin Cell Biol. 1997;9:240–246. doi: 10.1016/s0955-0674(97)80068-3. [DOI] [PubMed] [Google Scholar]
- Kleerekoper M, Mendlovic DB. Sodium fluoride therapy of postmenopausal osteoporosis. Endocr Rev. 1993;14:312–323. doi: 10.1210/edrv-14-3-312. [DOI] [PubMed] [Google Scholar]
- Lau KH, Baylink DJ. Molecular mechanism of action of fluoride on bone cells. J Bone Miner Res. 1998;13:1660–1667. doi: 10.1359/jbmr.1998.13.11.1660. [DOI] [PubMed] [Google Scholar]
- Llano E, Pendas AM, Knauper V, Sorsa T, Salo T, Salido E, Murphy G, Simmer JP, Bartlett JD, Lopez-Otin C. Identification and structural and functional characterization of human enamelysin (MMP-20) Biochemistry. 1997;36:15101–15108. doi: 10.1021/bi972120y. [DOI] [PubMed] [Google Scholar]
- Lopez-Bergami P, Habelhah H, Bhoumik A, Zhang W, Wang LH, Ronai Z. RACK1 mediates activation of JNK by protein kinase C [corrected] Mol Cell. 2005;19:309–320. doi: 10.1016/j.molcel.2005.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchenko GN, Marchenko ND, Leng J, Strongin AY. Promoter characterization of the novel human matrix metalloproteinase-26 gene: regulation by the T-cell factor-4 implies specific expression of the gene in cancer cells of epithelial origin. Biochem J. 2002;363:253–262. doi: 10.1042/0264-6021:3630253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musti AM, Treier M, Bohmann D. Reduced ubiquitin-dependent degradation of c-Jun after phosphorylation by MAP kinases. Science. 1997;275:400–402. doi: 10.1126/science.275.5298.400. [DOI] [PubMed] [Google Scholar]
- Ozdemir D, Hart PS, Ryu OH, Choi SJ, Ozdemir-Karatas M, Firatli E, Piesco N, Hart TC. MMP20 active-site mutation in hypomaturation amelogenesis imperfecta. J Dent Res. 2005;84:1031–1035. doi: 10.1177/154405910508401112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards A, Kragstrup J, Nielsen-Kudsk F. Pharmacokinetics of chronic fluoride ingestion in growing pigs. J Dent Res. 1985;64:425–430. doi: 10.1177/00220345850640030601. [DOI] [PubMed] [Google Scholar]
- Robinson C, Brookes SJ, Shore RC, Kirkham J. The developing enamel matrix: nature and function. Eur J Oral Sci. 1998;106(Suppl 1):282–291. doi: 10.1111/j.1600-0722.1998.tb02188.x. [DOI] [PubMed] [Google Scholar]
- Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabata MJ, Matsumura T, Liu JG, Wakisaka S, Kurisu K. Expression of cytokeratin 14 in ameloblast-lineage cells of the developing tooth of rat, both in vivo and in vitro. Arch Oral Biol. 1996;41:1019–1027. doi: 10.1016/s0003-9969(96)00087-8. [DOI] [PubMed] [Google Scholar]
- Thrane EV, Refsnes M, Thoresen GH, Lag M, Schwarze PE. Fluoride-induced apoptosis in epithelial lung cells involves activation of MAP kinases p38 and possibly JNK. Toxicol Sci. 2001;61:83–91. doi: 10.1093/toxsci/61.1.83. [DOI] [PubMed] [Google Scholar]
- Turk BE, Lee DH, Yamakoshi Y, Klingenhoff A, Reichenberger E, Wright JT, Simmer JP, Komisarof JA, Cantley LC, Bartlett JD. MMP-20 is predominately a tooth-specific enzyme with a deep catalytic pocket that hydrolyzes type V collagen. Biochemistry. 2006;45:3863–3874. doi: 10.1021/bi052252o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Q, Zhang Y, Li W, DenBesten PK. Differentiation of human ameloblast-lineage cells in vitro. Eur J Oral Sci. 2006;114(Suppl 1):154–158. doi: 10.1111/j.1600-0722.2006.00304.x. discussion 164-155, 380–151. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Yan Q, Li W, DenBesten PK. Fluoride down-regulates the expression of matrix metalloproteinase-20 in human fetal tooth ameloblast-lineage cells in vitro. Eur J Oral Sci. 2006;114(Suppl 1):105–110. doi: 10.1111/j.1600-0722.2006.00303.x. discussion 127-109, 380. [DOI] [PubMed] [Google Scholar]