Abstract
The retinal ganglion cell (RGC)-like RGC-5 line can be differentiated with staurosporine to stop dividing, extend neurites, and increase levels of several ganglion cell markers. This allows study of regulation of neurite development on a single cell basis. However, it is unclear whether the neurites induced by differentiation have features characteristic of dendrites or axons. To address this question, RGC-5 cells were differentiated with staurosporine and then immunoblotted for microtubule-associated protein 2 (MAP2) and actin, or stained immunocytochemically for different MAP2 isoforms, tau, growth-associated protein 43 (GAP-43), or the neuronal marker β-III-tubulin. We found that staurosporine-induced differentiation led to an upregulation of MAP2c, a MAP2 isoform expressed in developing neurons. Some neurites expressed MAP2c but not the dendritic markers MAP2a and b, consistent with an axonal phenotype. Some neurites expressed the axonal marker tau in a characteristic proximal-to-distal gradient, and had GAP-43 labeling characteristic of axonal growth cones. The presence of MAP2c in differentiated RGC-5 cells is indicative of RGC-like neurite development, and the pattern of staining for the different MAP2 isoforms, as well as positivity for tau and GAP-43, indicates that differentiation induces axon-like and dendrite-like neurites.
Introduction
Retinal ganglion cells (RGCs) are central neurons that carry visual information from the retina to the lateral geniculate nucleus and superior colliculus via the optic nerve. The development and function of RGCs are of particular interest for both normal and pathological states, and much work has been done to characterize the factors guiding normal axonal pathfinding in RGCs to appropriate sites in the brain (Oster et al., 2004). Studies have also characterized factors controlling the formation of the dendritic arbor and its stratification in the retina during development, including cell density (Troilo et al., 1996), neurotrophin levels (Lom et al., 2002), and other factors (Wingate, 1996). However, none of these studies can monitor neurite development over time in a single ganglion cell. Purified RGCs have been cultured and their neurite outgrowth has been studied extensively by others (Goldberg et al., 2002a; Goldberg et al., 2002b; Inatani et al., 2001; Steinbach et al., 2001), but these cells have undergone injury to their existing neurites in the process of purification and culture, and may not accurately represent the behavior of normal developing RGCs.
Microtubule-associated protein 2 (MAP2) is one of a family of proteins responsible for microtubule stabilization and organization, and is particularly involved in cytoskeletal changes associated with neuronal differentiation (Caceres et al., 1986). MAP2 exists in several isoforms, the most prevalent of which are MAP2a, b and c. MAP2a and b are present in dendrites of mature neurons (Bernhardt and Matus, 1984; Matus et al., 1981). MAP2c is a smaller splice variant and is present in developing neurites (Meichsner et al., 1993), including axons of developing RGCs (Tucker and Matus, 1988), but is absent in mature neurons (Riederer and Matus, 1985). Previous studies have shown that expression of MAP2 is necessary for retinoic acid-induced differentiation of embryonal carcinoma cells (Dinsmore and Solomon, 1991), and that specific expression of MAP2c is sufficient to induce microtubule reorganization in a neuronal cell line and initiate neurite outgrowth (Dehmelt et al., 2003). Due to the challenges of studying neurite formation in RGCs, relatively few studies have looked at MAP proteins in developing RGCs (Bates et al., 1993; Okabe et al., 1989; Tucker and Matus, 1988).
Another microtubule-associated protein expressed in neuronally differentiated cells is tau. Tau is expressed exclusively in the axon in vivo (Binder et al., 1985), but is also found in the soma of cultured cells (Brandt et al., 1995). Expression of tau in cultured differentiating neurons characteristically presents as a gradient, with greater amounts of tau binding microtubules at the distal axon (Kempf et al., 1996). The presence of tau has been demonstrated in the axons of developing RGCs, and its localization to the axon plays a role in proper axon development and, ultimately, RGC survival (Wang et al., 2000).
Growth-associated protein 43 (GAP-43) is a neuronal growth protein with expression localized to neurite growth cones, specifically those of axons (Goslin et al., 1988). Initially, the protein is equally distributed among all growth cones in the neuron, but reactivity is decreased in all other neurites when neuronal polarity is established and one outgrowth becomes classifiable as an axon (Goslin et al., 1990). It has been shown that developing RGCs express GAP-43 (Avwenagha et al., 2003; Ekstrom and Johansson, 2003), and expression of the gene plays an important role in axonal growth and guidance in forming appropriate tectal connections in the brain (Strittmatter et al., 1995; Zhu and Julien, 1999).
The RGC-5 cell line is a transformed proliferating cell line that expresses RGC-specific markers similar to RGCs, but with a non-neuronal morphology (Krishnamoorthy et al., 2001). Previous work in our laboratory demonstrated that sub-lethal treatment with staurosporine (SS) could induce differentiation of RGC-5 cells to a non-mitotic phenotype with neurite outgrowth and expression of at least one neuronal ion channels (Frassetto et al., 2006), making them a better model for the study of RGCs in culture than non-differentiated RGC-5 cells. Differentiation of the RGC-5 cells also allows for the study of developing neurites in uninjured RGC-like cells in vitro. However, the correlation between known events in RGC differentiation and the changes induced by SS in RGC-5 cells has not been determined.
We set out to study the nature of neurite formation in RGC-5 cells after SS treatment, as well as to characterize the neurite outgrowth as axonal or dendritic. We found that differentiation induces a strong upregulation in expression of MAP2c compared to untreated cells, suggesting that some of the outgrowth may be axonal in nature. Immunocytochemical staining confirmed that some neurites had predominantly MAP2c staining, compared to other MAP2 isoforms. The axonal nature of some neurites was further confirmed by positive staining for tau and GAP-43.
Materials and Methods
Materials
Dimethyl sulfoxide (DMSO) were from Sigma-Aldrich (St. Louis, MO); and SS (from Streptomyces staurosporeus; ≥98% purity; catalog number 380-014) from Alexis Biochemicals (San Diego, CA). Cell culture reagents, unless noted, were from BioWhittaker (Rockland, ME). Fluorescent secondary antibodies and fluorescent dyes were from Molecular Probes (Eugene, OR). Peroxidase-conjugated secondary antibodies were from Jackson ImmunoResearch Laboratories (West Grove, PA).
Cell Culture
RGC-5 cells were cultured in Dulbecco’s modified Eagle’s medium (Mediatech, Inc., Herndon, CA) containing 1 g/L glucose with L-glutamine, supplemented with 10% fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin. Cells were incubated at 37°C in humidified 5% CO2.
Immunoblot Analysis
Rat RGC-5 cells were grown to approximately 70% confluence on 100-mm tissue culture plates (BD Biosciences, Bedford, MA) and either treated with SS to a final concentration of 316 nM for 3 days or harvested without treatment at 70% confluence. Cells were lysed in PBS containing 1% Igepal CA-630, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate (Fisher Scientific), and a protease inhibitor (Complete Mini Protease Inhibitor Cocktail Tablet; 5 mg/mL; Roche Diagnostics, Mannheim, Germany) for 15 minutes at 4°C, scraped off the plate, collected, and incubated on ice for 60 minutes. After centrifugation at 10,000g for 10 minutes at 4v, the pellets were sonicated on ice for two 15-second bursts and centrifuged again at 10,000g for 10 minutes. Protein from differentiated and undifferentiated cells was heated to 80 °C for 5 minutes in the presence of 4x lithium dodecyl sulfate (LDS) sample buffer and 10x sample reducing agent (Invitrogen, Carlsbad, CA), resolved on a Bis-Tris 4% to 12% polyacrylamide gel (NuPAGE; Invitrogen), and transferred overnight at 50 mA to nitrocellulose membrane in a transfer apparatus (Mini Protean II; Bio-Rad Laboratories, Hercules, CA).
After transfer, the membrane was blocked with 5% nonfat milk in TBS (pH 8.0) for 30 to 60 minutes and then probed with primary antibodies to MAP2 in blocking buffer. Antibodies used included affinity-purified rabbit polyclonal anti-microtubule-associated protein 2 (1:1000; Chemicon, Temecula, CA) and polyclonal rabbit anti-actin (1:1000; Sigma-Aldrich). Blots were rinsed three times with TBS containing 0.05% Tween-20 (Fisher Scientific), then washed 5 times for 10 minutes each at room temperature on an orbital shaker. Secondary antibodies used were affinity-purified horse radish peroxidase (HRP)-conjugated goat anti-rabbit IgG and purified HRP-conjugated goat anti-mouse IgG (1:5000; Jackson ImmunoResearch Laboratories, West Grove, PA) and were incubated for 1 hour at room temperature, followed by 3 rinses and five 10-minute washes with TBS containing Tween-20 at room temperature on an orbital shaker. Blots were treated with freshly prepared ECL solution containing 100 mM Tris-HCl [pH 8.5], 1.25 mM luminol, 225 μM p-coumaric acid (Sigma-Aldrich), and 1 mM H2O2 for 1 minute, and excess solution was allowed to drip off. The blots were then exposed to film (Blue Lite Autorad, ISC BioExpress, Kaysville, UT) and developed. Blots probed for MAP2 levels were rinsed with TBS, then placed in a solution containing 0.2 M glycine and 0.05% Tween-20 at 55 °C for 30 minutes to remove the bound antibodies. The blot was blocked again, and probed for actin in an identical manner to control for differences in protein loading. The films were scanned at 1600 dpi and band density was determined by comparing total intensity in an area containing the band of interest to the intensity of an equal size area of background using NIH ImageJ software. Band density readings are presented with respect to the density of the band from the control, untreated cell condition.
Immunocytochemistry
RGC-5 cells plated on coverslips were fixed with 4% paraformaldehyde in PBS (pH 7.2) for 20 minutes at room temperature, rapidly rinsed with Tris-buffered saline (TBS; 100 mM Tris [pH 7.6], 0.9% NaCl) three times for a few seconds each and two times for 5 minutes each, and blocked with 5% normal goat serum (BioWhittaker) in TBS for 30 minutes at room temperature. MAP2 (all isoforms) was probed using affinity-purified rabbit polyclonal anti-MAP2 antibody (Chemicon; Temecula, CA) at 1:250 dilution overnight at 4°C, followed by Alexa Fluor 488 goat anti-rabbit IgG at 10 μg/mL at room temperature for 60 minutes. Alexa Fluor 488 fluorescence was detected with a FITC filter set (excitation 470 ± 20 nm, dichroic 505 nm long-pass, emission 540 ± 20 nm). MAP2 (a and b isoforms only) were detected by incubating with affinity-purified mouse monoclonal anti-MAP2 antibody (Chemicon; clone AP20) at 10 μg/mL overnight at room temperature, followed by Alexa Fluor 594 (Molecular Probes, Eugene, OR) goat anti-mouse IgG at 10 μg/mL in blocking buffer at room temperature for 90 minutes. Alexa Fluor 594 fluorescence was detected with a Texas red filter set (excitation 560 ± 20 nm, dichroic 595 nm long-pass, emission 630 ± 30 nm). Tau was detected by incubating with affinity-purified mouse monoclonal anti-tau antibody (Chemicon) at 7 μg/mL overnight at room temperature, followed by Alexa Fluor 594 (Molecular Probes, Eugene, OR) goat anti-mouse IgG at 10 μg/mL in blocking buffer at room temperature for 90 minutes. Alexa Fluor 594 fluorescence was detected with a Texas red filter set (excitation 560 ± 20 nm, dichroic 595 nm long-pass, emission 630 ± 30 nm). GAP-43 was detected by incubating with affinity-purified mouse monoclonal anti-GAP-43 antibody (Chemicon) at 0.75 μg/mL overnight at room temperature, followed by Alexa Fluor 594 (Molecular Probes, Eugene, OR) goat anti-mouse IgG at 10 μg/mL in blocking buffer at room temperature for 90 minutes. Alexa Fluor 594 fluorescence was detected with a Texas red filter set (excitation 560 ± 20 nm, dichroic 595 nm long-pass, emission 630 ± 30 nm). β-III-tubulin was visualized after fixing and blocking by incubating with mouse anti-β-III-tubulin monoclonal IgG (Sigma-Aldrich, St. Louis, MO) at 1:750 dilution (from ascites) overnight at 4°C, followed by Alexa Fluor 594 (Molecular Probes, Eugene, OR) goat anti-mouse IgG at 10 μg/mL in blocking buffer at room temperature for 90 minutes. Alexa Fluor 594 fluorescence was detected with a Texas red filter set (excitation 560 ± 20 nm, dichroic 595 nm long-pass, emission 630 ± 30 nm). Coverslips were then transferred and mounted on microscope slides (Gel/Mount; Biomeda Corp., Foster City, CA). Slides were viewed with an upright microscope (Axiophot; Carl Zeiss Meditec, Dublin CA) with DIC optics and epifluorescence, and images acquired (Axiovision software; Carl Zeiss Meditec) at 1300 × 1030 resolution.
Statistics
Pictures acquired for statistical analysis were taken by an observer masked to the expected staining pattern, in order to eliminate bias in field and cell selection. Twenty fields were acquired for each condition from slides prepared from 3 independent experiments. Cells were subsequently assessed for dendrite- and axon-specific features in neurites, such as differential staining for MAP2 isoforms, a tau gradient increasing from soma to growth cone, or strong staining at the growth cone of a single neurite with GAP-43. Results were calculated as the number of cells with positive features divided by the total number of cells summed across all twenty images. More than 100 cells were assessed per experiment. Results were compared by Fisher’s exact test.
Results
RGC-5 cells express MAP2
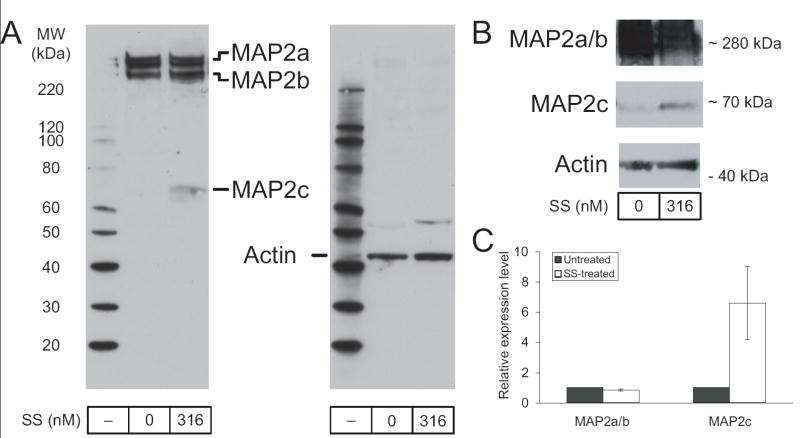
We previously found that MAP2 is present in both differentiated and undifferentiated RGC-5 cells in its two high-molecular weight isomers. This result was examined again by immunoblotting, demonstrating the presence of MAP2a and MAP2b (Figure 1A). In addition, longer exposure revealed MAP2c expression at low levels in undifferentiated RGC-5 cells (Figure 1B).
Figure 1. Staurosporine-induced differentiation of RGC-5 cells increases MAP2c expression.
(A) RGC-5 cells differentiated in the presence of 316 nM staurosporine (SS) for 3 days showed roughly equal levels of MAP2a and MAP2b compared to undifferentiated (0 nM SS) RGC-5 cells. However, levels of the low molecular weight isoform, MAP2c, showed increased expression. (B) MAP2c expression in undifferentiated cells is more evident at longer exposure times, although MAP2a and MAP2b tend to be overexposed at these times (bands shown are from a separate experiment than in A). (C) Quantitation of band strength demonstrated that the increase was 2- to 16-fold after differentiation (6.5 ± 2.4).
Staurosporine-induced differentiation of RGC-5 cells increases MAP2c expression
As MAP2c has been shown by others to have a role in initializing neurite outgrowth (Dehmelt et al., 2003), we were intrigued by its presence in undifferentiated RGC-5 cells and were curious how expression in those cells compared to levels after differentiation with SS. RGC-5 cells were treated with SS and allowed to differentiate for 3 days before protein preparation. Proteins from differentiated and undifferentiated RGC-5 cells were prepared in parallel and again examined by immunoblotting for MAP2. While levels of the MAP2a and MAP2b isomers were not consistently altered by differentiation, MAP2c underwent a significant increase in expression levels (Figure 1A,B). Quantitation of this upregulation showed a 2- to 16-fold increase (6.5 ± 2.4; n=5) in MAP2c after differentiation, normalized to actin (Figure 1C).
Staurosporine-induced differentiation leads to the development of dendrite-like neurites
Having confirmed the presence of MAP2 in three major isoforms in differentiated RGC-5 cells, we used the presence of MAP2 isoforms and other neuronal markers to determine the nature of the neurites formed by SS-induced differentiation. Staining with antibodies against β-III-tubulin, present in both dendritic and axonal processes, showed labeling of all neurite outgrowth induced by differentiation (Figure 2A,B: DIC and fluorescence). Use of an antibody specific to MAP2a and MAP2b (AP20 clone), which are not present in mature axons, showed staining in the cell body as well as the vast majority of neurites (Figure 2C,D: DIC and fluorescence). Interestingly, staining with both MAP2 antibodies (all isoform reactive/AP20 clone) revealed similar staining levels in the soma, while some of the neurites from cells were stained preferentially or exclusively with the antibody to all isoforms (Figure 2E-H). This exclusive staining of some neurites suggests that they may be axonal in nature, unlike those that are immunoreactive for MAP2a and b isoforms only. However, this staining pattern was not found in any cells imaged at 3 or 7 days after differentiation.
Figure 2. Staurosporine-induced differentiation leads to the development of dendrite-like neurites.
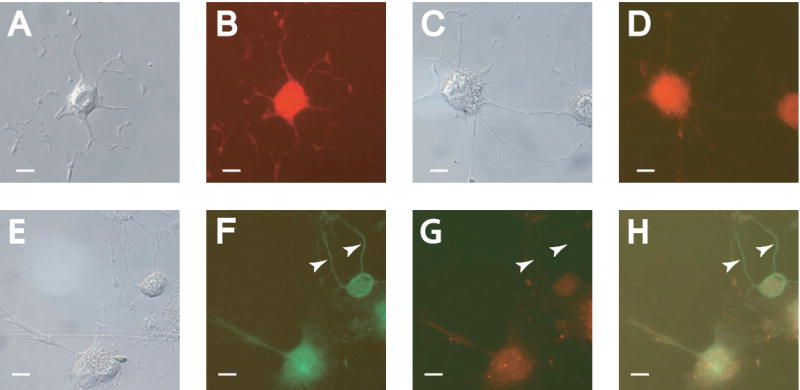
RGC-5 cells were differentiated for 48 hours and photographed with DIC optics (A, C, E), or under filters appropriate for immunocytochemical staining (B, D, F-H). (A-B) Differentiated cells showed staining for the neuronal marker β-III-tubulin strongly in all neurites. (C-D) Staining for the dendritic markers MAP2a and MAP2b revealed staining in the majority of neurites. (F). Labeling for all isoforms of MAP2 showed staining in all neurites. (G) Concurrent staining for the MAP2a and MAP2b isoforms revealed some unlabeled neurites. (H) Overlay of (F) and (G). Arrows indicate neurites showing exclusive labeling for MAP2c, suggesting an axonal phenotype. Scale bar in all panels indicates 10 μm.
Staurosporine-induced differentiation leads to the development of axon-like neurites
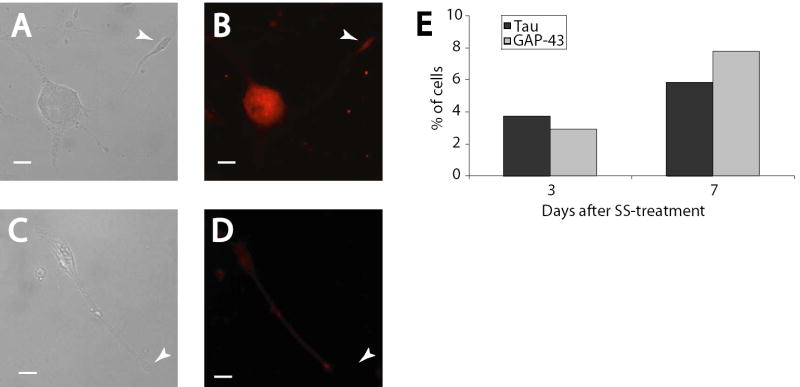
While differential staining for MAP2c suggests the neurites may be axonal in nature, the presence of axon-specific markers in differentiated cells is a more definitive measure of axon-like neurite growth. Staining for tau, a microtubule-associated protein localized to the soma and axon of cultured neurons, confirmed the axonal nature of some neurites (Figure 3A,B: DIC and fluorescence). A single, longer projection from the pictured cell showed a gradient of tau distribution reaching its maximum at the base of the growth cone, while other shorter projections had minimal staining. We found this pattern of staining exhibited in 3.7% of cells 3 days after differentiation, and 5.8% of cells after 7 days (Figure 3E). Additional confirmation of axonal outgrowth was found by staining for GAP-43, a specific marker of axonal growth cones, which stained intensely at the growth cone of a single neurite in the pictured cell (Figure 3C,D: DIC and fluorescence). As with tau staining, this pattern was observed in only 2.9% of cells after 3 days of differentiation with SS, with the proportion increasing to 7.9% after 7 days (Figure 3E). No undifferentiated cells showed these characteristic staining patterns, instead demonstrating only faint somal staining and a lack of neurite outgrowth (data not shown). Together with the MAP2 results, these patterns of gene expression indicate primarily dendrite formation, with evidence of the establishment of neuronal polarity in a small proportion of the cells.
Figure 3. Staurosporine-induced differentiation leads to the development of axon-like neurites.
RGC-5 cells were differentiated for 7 days and photographed with DIC optics (A, C) or for immunocytochemical staining of tau (B) or GAP-43 (D). In these cells, tau staining was strong in a single neurite and was distributed in a gradient characteristic of axonal outgrowth (B), while GAP-43 showed strong staining in a single growth cone (D). (E) Analysis of a large number of cells indicated that these characteristic staining patterns were evident in only a portion of the cells. The number of cells expressing the staining is similar between tau and GAP-43, and the number of cells is not significantly higher at 7 days than at 3 days. Scale bar in all panels indicates 10 μm.
Discussion
We used immunoblotting to examine levels of MAP2 expression and immunocytochemical staining to localize tau, GAP-43, and MAP2 in differentiating RGC-5 cells. We found levels of mature MAP2 to be relatively stable after differentiation, but the juvenile form, MAP2c, was expressed at higher levels. By concurrently labeling for adult and juvenile forms of MAP2, we were able to characterize the nature of the neurites after SS differentiation as primarily dendritic, but with some displaying an axonal phenotype, demonstrated by a lack of MAP2a and MAP2b, as well as a typical axonal morphology. Tau and GAP-43 staining confirmed the axonal nature of some neurite outgrowth in a small percentage of differentiated cells at 7 days.
The use of SS-differentiated RGC-5 cells as a model for neurite formation in RGCs has potential shortcomings. While RGC-5 cells take on a distinct neuronal phenotype after treatment, it is unclear whether this differentiation program is similar to what occurs during differentiation of primary RGCs. Others have shown that MAP2c is expressed in the axons of embryonic RGCs (Tucker and Matus, 1988), consistent with what has been observed during differentiation of other immature neurons and neuronal cell lines. We therefore compared several aspects of RGC-5 differentiation with known changes involved in RGC differentiation. The results of this study confirmed that SS-induced RGC-5 differentiation occurs by a similar manner, with upregulation of MAP2c from 2-to 16-fold. While other characteristics of mature RGCs in RGC-5 cells differentiated with staurosporine are not yet defined, the similarities in gene expression and morphology seen so far suggest that these cells have useful features for modeling RGC differentiation.
We used an antibody for immunocytochemistry that recognizes all three of the major isoforms of MAP2. After determining by immunoblotting that MAP2c had substantial and likely non-compartmentalized expression, we then examined expression of only the adult (a and b) forms. As MAP2c specific antibodies cannot be generated, examination of differential staining patterns of MAP2 isoforms, and the exclusion of MAP2a and b, is the only immunochemical way to determine the distribution of MAP2c within the cell, which in turn identified neurites more likely to be developing axons. The majority of neurite outgrowth showed positive staining for MAP2a and MAP2b, indicating that they are dendrites. Nearly all cells did not show evidence of axonal outgrowth by this method. However, as others have shown that the large molecular weight isoforms (a and b) of MAP2 can sometimes be found in developing axons (Kempf et al., 1996), the GAP-43 staining of growth cones and presence of a tau gradient we observed in some more mature differentiated cells confirms the development of axons and the establishment of neuronal polarity in these cells.
Acknowledgments
Grant Support: NIH R0112492 and P30EY016665, Retina Research Foundation, and an unrestricted departmental grant from Research to Prevent Blindness, Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Avwenagha O, Campbell G, Bird MM. Distribution of GAP-43, beta-III tubulin and F-actin in developing and regenerating axons and their growth cones in vitro, following neurotrophin treatment. J Neurocytol. 2003;32:1077–1089. doi: 10.1023/B:NEUR.0000021903.24849.6c. [DOI] [PubMed] [Google Scholar]
- Bates CA, Trinh N, Meyer RL. Distribution of microtubule-associated proteins (MAPs) in adult and embryonic mouse retinal explants: presence of the embryonic map, MAP5/1B, in regenerating adult retinal axons. Dev Biol. 1993;155:533–544. doi: 10.1006/dbio.1993.1050. [DOI] [PubMed] [Google Scholar]
- Bernhardt R, Matus A. Light and electron microscopic studies of the distribution of microtubule-associated protein 2 in rat brain: a difference between dendritic and axonal cytoskeletons. J Comp Neurol. 1984;226:203–221. doi: 10.1002/cne.902260205. [DOI] [PubMed] [Google Scholar]
- Binder LI, Frankfurter A, Rebhun LI. The distribution of tau in the mammalian central nervous system. J Cell Biol. 1985;101:1371–1378. doi: 10.1083/jcb.101.4.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt R, Leger J, Lee G. Interaction of tau with the neural plasma membrane mediated by tau’s amino-terminal projection domain. J Cell Biol. 1995;131:1327–1340. doi: 10.1083/jcb.131.5.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caceres A, Banker GA, Binder L. Immunocytochemical localization of tubulin and microtubule-associated protein 2 during the development of hippocampal neurons in culture. J Neurosci. 1986;6:714–722. doi: 10.1523/JNEUROSCI.06-03-00714.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehmelt L, Smart FM, Ozer RS, Halpain S. The role of microtubule-associated protein 2c in the reorganization of microtubules and lamellipodia during neurite initiation. J Neurosci. 2003;23:9479–9490. doi: 10.1523/JNEUROSCI.23-29-09479.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinsmore JH, Solomon F. Inhibition of MAP2 expression affects both morphological and cell division phenotypes of neuronal differentiation. Cell. 1991;64:817–826. doi: 10.1016/0092-8674(91)90510-6. [DOI] [PubMed] [Google Scholar]
- Ekstrom P, Johansson K. Differentiation of ganglion cells and amacrine cells in the rat retina: correlation with expression of HuC/D and GAP-43 proteins. Brain Res Dev Brain Res. 2003;145:1–8. doi: 10.1016/s0165-3806(03)00170-6. [DOI] [PubMed] [Google Scholar]
- Frassetto LJ, Schlieve CR, Lieven CJ, Utter AA, Jones MV, Agarwal N, Levin LA. Kinase-dependent differentiation of a retinal ganglion cell precursor. Invest Ophthalmol Vis Sci. 2006;47:427–438. doi: 10.1167/iovs.05-0340. [DOI] [PubMed] [Google Scholar]
- Goldberg JL, Espinosa JS, Xu Y, Davidson N, Kovacs GT, Barres BA. Retinal ganglion cells do not extend axons by default: promotion by neurotrophic signaling and electrical activity. Neuron. 2002a;33:689–702. doi: 10.1016/s0896-6273(02)00602-5. [DOI] [PubMed] [Google Scholar]
- Goldberg JL, Klassen MP, Hua Y, Barres BA. Amacrine-signaled loss of intrinsic axon growth ability by retinal ganglion cells. Science. 2002b;296:1860–1864. doi: 10.1126/science.1068428. [DOI] [PubMed] [Google Scholar]
- Goslin K, Schreyer DJ, Skene JH, Banker G. Development of neuronal polarity: GAP-43 distinguishes axonal from dendritic growth cones. Nature. 1988;336:672–674. doi: 10.1038/336672a0. [DOI] [PubMed] [Google Scholar]
- Goslin K, Schreyer DJ, Skene JH, Banker G. Changes in the distribution of GAP-43 during the development of neuronal polarity. J Neurosci. 1990;10:588–602. doi: 10.1523/JNEUROSCI.10-02-00588.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inatani M, Honjo M, Otori Y, Oohira A, Kido N, Tano Y, Honda Y, Tanihara H. Inhibitory effects of neurocan and phosphacan on neurite outgrowth from retinal ganglion cells in culture. Invest Ophthalmol Vis Sci. 2001;42:1930–1938. [PubMed] [Google Scholar]
- Kempf M, Clement A, Faissner A, Lee G, Brandt R. Tau binds to the distal axon early in development of polarity in a microtubule- and microfilament-dependent manner. J Neurosci. 1996;16:5583–5592. doi: 10.1523/JNEUROSCI.16-18-05583.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamoorthy RR, Agarwal P, Prasanna G, Vopat K, Lambert W, Sheedlo HJ, Pang IH, Shade D, Wordinger RJ, Yorio T, Clark AF, Agarwal N. Characterization of a transformed rat retinal ganglion cell line. Brain Res Mol Brain Res. 2001;86:1–12. doi: 10.1016/s0169-328x(00)00224-2. [DOI] [PubMed] [Google Scholar]
- Lom B, Cogen J, Sanchez AL, Vu T, Cohen-Cory S. Local and target-derived brain-derived neurotrophic factor exert opposing effects on the dendritic arborization of retinal ganglion cells in vivo. J Neurosci. 2002;22:7639–7649. doi: 10.1523/JNEUROSCI.22-17-07639.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matus A, Bernhardt R, Hugh-Jones T. High molecular weight microtubule-associated proteins are preferentially associated with dendritic microtubules in brain. Proc Natl Acad Sci U S A. 1981;78:3010–3014. doi: 10.1073/pnas.78.5.3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meichsner M, Doll T, Reddy D, Weisshaar B, Matus A. The low molecular weight form of microtubule-associated protein 2 is transported into both axons and dendrites. Neuroscience. 1993;54:873–880. doi: 10.1016/0306-4522(93)90581-y. [DOI] [PubMed] [Google Scholar]
- Okabe S, Shiomura Y, Hirokawa N. Immunocytochemical localization of microtubule-associated proteins 1A and 2 in the rat retina. Brain Res. 1989;483:335–346. doi: 10.1016/0006-8993(89)90178-9. [DOI] [PubMed] [Google Scholar]
- Oster SF, Deiner M, Birgbauer E, Sretavan DW. Ganglion cell axon pathfinding in the retina and optic nerve. Semin Cell Dev Biol. 2004;15:125–136. doi: 10.1016/j.semcdb.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Riederer B, Matus A. Differential expression of distinct microtubule-associated proteins during brain development. Proc Natl Acad Sci U S A. 1985;82:6006–6009. doi: 10.1073/pnas.82.17.6006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbach K, Bauch H, Stier H, Schlosshauer B. Tissue-specific neuro-glia interactions determine neurite differentiation in ganglion cells. Eur J Cell Biol. 2001;80:245–255. doi: 10.1078/0171-9335-00151. [DOI] [PubMed] [Google Scholar]
- Strittmatter SM, Fankhauser C, Huang PL, Mashimo H, Fishman MC. Neuronal pathfinding is abnormal in mice lacking the neuronal growth cone protein GAP-43. Cell. 1995;80:445–452. doi: 10.1016/0092-8674(95)90495-6. [DOI] [PubMed] [Google Scholar]
- Troilo D, Xiong M, Crowley JC, Finlay BL. Factors controlling the dendritic arborization of retinal ganglion cells. Vis Neurosci. 1996;13:721–733. doi: 10.1017/s0952523800008609. [DOI] [PubMed] [Google Scholar]
- Tucker RP, Matus AI. Microtubule-associated proteins characteristic of embryonic brain are found in the adult mammalian retina. Dev Biol. 1988;130:423–434. doi: 10.1016/0012-1606(88)90338-7. [DOI] [PubMed] [Google Scholar]
- Wang SW, Gan L, Martin SE, Klein WH. Abnormal polarization and axon outgrowth in retinal ganglion cells lacking the POU-domain transcription factor Brn-3b. Mol Cell Neurosci. 2000;16:141–156. doi: 10.1006/mcne.2000.0860. [DOI] [PubMed] [Google Scholar]
- Wingate RJ. Retinal ganglion cell dendritic development and its control. Filling the gaps. Mol Neurobiol. 1996;12:133–144. doi: 10.1007/BF02740650. [DOI] [PubMed] [Google Scholar]
- Zhu Q, Julien JP. A key role for GAP-43 in the retinotectal topographic organization. Exp Neurol. 1999;155:228–242. doi: 10.1006/exnr.1998.6989. [DOI] [PubMed] [Google Scholar]