Abstract
The tomato Cf-4 and Cf-9 genes are the founder members of a large gene family of homologues of Cladosporium fulvum resistance gene Cf-9 (Hcr9 genes), several of which confer resistance against C. fulvum through recognition of different pathogen-encoded avirulence determinants. Three loci of tandemly repeated Hcr9 genes—Southern Cross (SC), Milky Way (MW), and Northern Lights (NL)—are located on the short arm of tomato chromosome 1. Comparisons between 2 SC-Hcr9s, 11 from MW, and 5 from NL implicated sequence exchange between gene family members in their evolution. The extent to which novel variants can be generated by recombination depends on the degree of sequence polymorphism available within the gene family. Here we show that physical separation of Hcr9 genes can be associated with elevated sequence divergence. Two diverged subclasses of Hcr9s could be defined. These are physically separated from each other, with members of one class exclusively residing at Northern Lights. One exceptional Hcr9 at Northern Lights carried sequence features specific for Hcr9s at other loci, suggesting a recent transfer of this gene by an interlocus recombination event. As members of diverged subclasses are brought into physical vicinity within a tandem repeat, a larger spectrum of sequence variants can potentially be generated by subsequent interhomologue sequence exchange.
Biological surveillance systems for the detection of nonself molecules are essential for the defense of higher eukaryotes against pathogens. As pathogens evolve to evade these recognition systems, the generation of novel recognition specificities appears crucial for fitness and long-term survival of the host species. In plants, recognition of pathogens is mediated by resistance genes (R genes). The cloning of several different classes of R genes over the last few years enables investigation of their evolution. Certain R genes are members of multigene families (1–3), with distinct specificities encoded by individual family members. For example, the highly homologous L6 and M genes of flax confer resistance toward races of flax rust carrying different avirulence genes (4). The tomato Cf-4 and Cf-9 genes for resistance to the leaf mould fungus Cladosporium fulvum belong to a large family of homologues of C. fulvum resistance gene Cf-9 (Hcr9s) (5–7). This gene family is ideally suited for the analysis of evolutionary mechanisms, because at least five functional R genes have been identified encoding at least four distinct recognition specificities (5, 8–10). Furthermore, genetical and physical mapping as well as sequence data of multiple Hcr9s are available (5–7, 9, 11). Different recognition specificities within a gene family are conceptually based on amino acid sequence polymorphism. In R genes which consist of leucine-rich repeats (LRRs; refs. 11 and 12), the highest degree of amino acid variability is found in predicted solvent exposed residues of the LRR parallel β-sheet structure, which implicated this domain in the determination of recognition specificity (5, 13). In a previous study we observed that Hcr9 genes located at the complex Cf-4/9 locus consist of patchwork or mosaic genes, which suggested that novel sequence variants are generated by sequence exchange between family members (5). Conceptually, such a mechanism allows for the generation of novel specificities; however, excessive exchange would result in sequence homogenization within the gene family and the concomitant loss of specificities. Therefore, mechanisms must exist that allow for the conservation and coexistence of homologous R genes in tandem arrays. We suggested earlier that the unique sequence block composition of the Hcr9 intergenic regions (IRs) at the Cf-4/9 locus reduces the frequency of interhomologue sequence exchange, thereby limiting homogenization (5). Here, we analyze the ORFs and IRs of seven Hcr9s originating from additional gene clusters proximal and distal to the Cf-4/9 locus. We show that physical separation of Hcr9s can be associated with increased sequence divergence. We describe the molecular traces of an interlocus recombination event which apparently transferred an entire Hcr9 including flanking sequences into the center of another Hcr9 cluster. This finding provides a mechanism by which sequence polymorphism that has built up during physical separation can be exploited. By occasional recruitment of diverged sequences, chimeras comprised of sequences derived from diverged family members can be generated.
MATERIALS AND METHODS
DNA sequences were established as described previously (5). Sequencing reads were assembled by using xbap and gap4 of the Staden package (14). Sequences were aligned and analyzed by using Compare, Bestfit, Gap, and Pileup of GCG (Wisconsin Package, Versions 8 and 9, Genetics Computer Group, Madison, WI). Alignments were optimized using the sequence editor of gcg9, and informative polymorphic sites (IPSs) were displayed with Sequence Output (B. G. Spratt, University of Sussex, Brighton, U.K.).
RESULTS
High Sequence Similarity Between Southern Cross (SC) and Milky Way (MW).
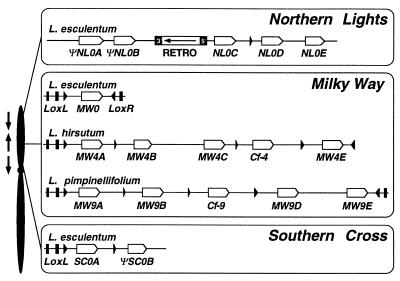
Hcr9s are organized in three loci comprising clusters of tandemly repeated genes (7), called SC, MW, and Northern Lights (NL) (Fig. 1). Comparisons between SC and MW revealed a stretch of 8.8 kb of near sequence identity between the Lycopersicon esculentum haplotype of SC and the Lycopersicon hirsutum haplotype of MW. This homologous region comprises the SC0A gene as well as its flanking regions. It is delimited at the 5′ end by the extent of the sequenced region and at the 3′ end by the ΨSC0B gene. All the sequenced haplotypes at MW carry a lipoxygenase sequence at their 5′ end (5) and a highly homologous sequence is present at SC (Fig. 1). Strikingly, the SC0A gene itself is more closely related to MW4A and MW9A—the most 5′ located Hcr9 genes in the Cf-4 and Cf-9 clusters at MW—than to any other Hcr9 gene. The nucleotide sequence of the IR between SC0A and ΨSC0B is homologous to the IR between the MW4A and MW4B genes (Fig. 2) with which it also shares the same relative position within the cluster (Fig. 1). This sequence synteny between SC and MW is less clear for the ΨSC0B gene for which no nearest evolutionary neighbor could be defined.ΨSC0B carries two frameshift mutations and is, therefore, predicted to be a nonfunctional gene (7). However, comparisons of its nucleotide sequence with Hcr9s at MW showed extensive stretches of shared IPSs (Fig. 3), clearly indicating a close evolutionary relationship. The high degree of homology between SC and MW suggests these to be the products of a large duplication event encompassing tandemly arranged Hcr9s and flanking regions. This cluster duplication was either a very recent event, which is unlikely given the occurrence of both clusters in different Lycopersicon species, or sequences did not diverge because homogenizing sequence exchange still occurred after the physical separation of MW and SC. Because ectopic recombination involving reciprocal sequence exchange would lead to chromosomal rearrangements, these recombination events, if they occur, are more likely to involve gene conversions.
Figure 1.
Map position and physical structure of the NL, MW, and SC clusters of Hcr9 genes. On the left is a schematic genetical map of tomato chromosome 1 showing the position of three Hcr9 loci relative to each other. Arrows indicate the transcriptional polarity of Hcr9s at the different loci—e.g., MW Hcr9s are transcribed toward the telomere which is the opposite direction to Hcr9s at SC and NL. On the right, the physical organization of Hcr9 clusters is shown. All of the displayed haplotypes have been entirely sequenced (5, 7). Open arrows indicate the position and transcriptional polarity of Hcr9s. The haplotypes at MW are all flanked by convergently orientated lipoxygenase genes (LoxL and LoxR). The exons of Lox are indicated by black boxes. The 3′ most exons are shown by black triangles, indicating the transcriptional polarity. Two types of fragments originating from the 3′ end of LoxL are interspersed between Hcr9 genes at MW, SC, and NL, and are also represented by black triangles. Hcr9 pseudogenes are labeled with a Ψ prefix. RETRO denotes a retrotransposon insertion in the NL0 haplotype in which the terminal repeats are shown by black boxes. The transcriptional direction of the polyprotein gene is shown by an arrow.
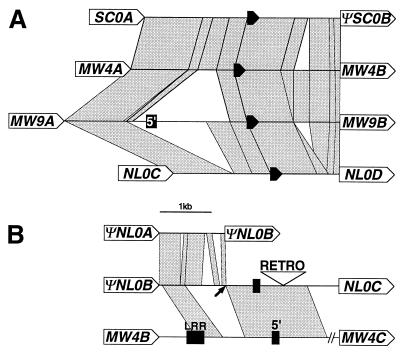
Figure 2.
Sequence affiliations between IRs. Stretches of high homology (>90%) are indicated by gray segments. The positions of Hcr9 ORFs (or homologous regions in case of ΨNL0A,ΨNL0B, and ΨSC0B) are shown by arrows (not to scale) labeled with the gene name. The positions of a truncated fragment of the LoxL gene are shown by filled arrows. 5′: Hcr9 fragment with homology to a region flanking the transcriptional start site. LRR: sequence with homology to a region encoding LRRs 20–22 of Cf-9. (A) Stretches of near sequence identity between the IRs SC0A/ΨSC0B, MW4A/MW4B, MW9A/MW9B and NL0C/NL0D. (B) The IR ΨNL0B/NL0C schematically aligned to most closely related IRs ΨNL0A/ΨNL0B and MW4B/MW4C. Only the relevant region of the latter sequence is shown. The arrow indicates the position at which homology in the ΨNL0B/NL0D IR switches between NL and MW. This point, therefore, marks a potential recombination breakpoint involved in the insertion of the NL0C gene into the NL cluster. The open triangle (RETRO) marks a retrotransposon insertion (not to scale). Homologies between IRs in A and B as well as some short insertions or deletions and imperfect repeats are not shown.
Figure 3.
Sequence relationships between Hcr9 genes. Display of all 517 IPS (a polymorphic nucleotide which is shared between at least two Hcr9 sequences within the alignment of 18 Hcr9 genes). Dots indicate identity with the consensus; dashes indicate deletions. The position of each site relative to the first nucleotide of the ATG start codon within the consensus sequence is given by vertical numbers above each site. Highlighted are sequence patches of at least three consecutive IPS that deviate from the consensus sequence and are shared between at least two Hcr9s. Patches shared with ΨNL0A, ΨNL0B, NL0D, or NL0E are shown by white letters in black boxes. Patches shared with NL0C, but not with the other NL Hcr9s, are highlighted by gray boxes. Patches involving the SC Hcr9s SC0A and ΨSC0B are surrounded by black lines. Sequence affiliations between Hcr9s at MW have been published elsewhere (5) and are only shown here when shared with genes in other clusters. The close relationship between Hcr9s at SC and MW is indicated by almost continous sequence affiliation between SC0A, MW4A, and MW9A and the shared patches between ΨSC0B and Cf-4 and Cf-9. In contrast, sequence affiliations of the NL genes ΨNL0A, ΨNL0B, NL0D, or NL0E are mostly confined to these four genes. ΨNL0A carries an imperfect direct repeat of the sequence encoding LRRs 17–21 of which the first repeat unit was aligned.
Specific Features of Hcr9s at NL.
The overall structure of Hcr9 genes at NL is similar to Cf-9, although variations do exist. NL0E carries a deletion of exactly 4 LRRs relative to Cf-9 starting after amino acid 14 of LRR 7. NL0E is the only Hcr9 that does not encode the potential endoplasmic reticulum retrieval signal KKRY at the C terminus as the two lysines are replaced by glutamate residues (EERY).
ΨNL0A carries a 2-bp deletion at position 501 and an in-frame stop codon at position 2269, whereas in ΨNL0B the ORF is disrupted by insertion of 1 bp at position 452 and a deletion of 17 bp at position 1087. Therefore, we consider them to be pseudogenes. The 3′ half of ΨNL0A is unique in that sequences homologous to the Cf-9 region encoding LRRs 17–21 are imperfectly duplicated. The repetitive DNA structure of the region encoding LRRs might promote unequal crossing-over. Recombination between DNA repeats encoding different LRRs has been proposed to be responsible for the variability in LRR number and position in the Hcr2 family (15) and for intragenic duplications in the RPP5 (16) and L6 (1) genes. The deletion of precisely four LRRs in NL0E, two LRRs in Cf-4 (6), and the duplication of sequences encoding four LRRs in ΨNL0A suggests that intragenic unequal recombination is also involved in the diversification of Hcr9s. However, it occurs to a lesser extent than in the evolution of Hcr2s, as most Hcr9s contain 27 LRRs. Intragenic unequal crossing over between Hcr2s is probably facilitated by the repetitive central part that encodes two types of highly conserved LRRs, whereas in Cf-9 all the individual LRRs are distinct from each other (8, 9, 15, 17).
A Diverged Subclass of Hcr9s Resides at NL.
The ΨNL0A, ΨNL0B, NL0D, and NL0E genes constitute a diverged subclass within the Hcr9 gene family, the members of which all reside at NL. A quantitative analysis revealed that 16.7–26.8% of IPS are specific for NL; i.e., they are not found in Hcr9s at the MW or SC loci (Table 1). Likewise, 7.8–12% of the IPS in Hcr9s at MW are specific for MW and SC. In contrast, not a single IPS was found to be specific for SC Hcr9s, reflecting the homogeneity with Hcr9s from MW (Table 1 and Fig. 3). The members of the NL subclass are more related to each other than to the remainder of the gene family. They share extensive patches of IPS sequence, and the most closely related genes vary with the position in the alignment (Fig. 3). For example, ΨNL0A and NL0D share IPS sequence from position 1054 to 1291, but ΨNL0A is most closely related toΨNL0B from position 2483 to 2683. This patchwork pattern indicates the occurrence of sequence exchange between ΨNL0A, ΨNL0B, NL0D, and NL0E, possibly as a result of unequal crossing-over or gene conversion.
Table 1.
Polymorphic nucleotides in Hcr9s
| Hcr9 | Length, bp | Unique PS | IPS | Shared IPS*MW/NL/SC | Specific IPS†
|
|
|---|---|---|---|---|---|---|
| NL | MW/SC | |||||
| ΨNLOA | 2956 | 67 | 507 | 371 | 136 | 0 |
| ΨNLOB | 2555 | 63 | 487 | 370 | 117 | 0 |
| NLOD | 2559 | 82 | 485 | 365 | 120 | 0 |
| NLOE | 2304 | 75 | 450 | 375 | 75 | 0 |
| NLOC | 2565 | 22 | 505 | 394 | 6† | 105† |
| MW4A | 2595 | 3 | 517 | 457 | 0 | 60 |
| MW9A | 2595 | 5 | 516 | 454 | 0 | 62 |
| MW4B | 2160 | 16 | 414 | 372 | 0 | 42 |
| MW9B | 2595 | 11 | 515 | 467 | 0 | 48 |
| MW4C | 2586 | 8 | 510 | 455 | 0 | 55 |
| Cf-9 | 2589 | 11 | 512 | 464 | 0 | 48 |
| Cf-4 | 2418 | 19 | 465 | 412 | 0 | 53 |
| MW9D | 2598 | 17 | 516 | 476 | 0 | 40 |
| MW4E | 2565 | 18 | 496 | 442 | 0 | 54 |
| MW9E | 2586 | 4 | 511 | 457 | 0 | 54 |
| MWO | 2535 | 26 | 503 | 460 | 0 | 43 |
| SCOA | 2595 | 7 | 516 | 456 | 0 | 60 |
| ΨSCOB | 2289 | 27 | 454 | 420 | 0 | 34 |
Unique PS, number of polymorphic sites (PS) with nucleotides not found at the corresponding position in any other Hcr9. IPS, number of informative polymorphic sites (IPS) per sequence. This number differs between Hcr9s because of specific deletions and the coincidence of unique nucleotides at otherwise informative sites. Shared IPS, number of IPS carrying nucleotides found in sequences from other clusters.
Specific IPS, number of IPS carrying nucleotides found only in sequences from NL or MW. No sites with IPS specific for SC exist.
For NLOC the number of IPS shared with both NL and MW is given.
For NLOC the number of sites shared only with NL (specific IPS NL) or only with MW (specific IPS MW) is given.
Genetic evidence for unequal crossing-over near or within complex R gene loci has been obtained for the rp1 complex in maize (18) and the M locus in flax (4). Accumulating evidence suggests unequal recombination is a major mechanism diversifying R gene sequences (3–6, 15, 19, 20). The finding of intergenic recombination within the NL Hcr9 subclass is in full agreement with these results.
In contrast, only limited sequence information appears to be exchanged between the NL subclass and Hcr9s at other loci. Only three stretches longer than 5 IPSs were shared between the NL subclass and Hcr9s from SC or MW, all located 5′ of position 1109 of the consensus alignment. A single patch of three IPSs is shared between ΨNL0A and ΨSC0B from position 2312 to 2315 (Fig. 3). The divergence of the NL subclass is probably a consequence of its genetic isolation. An ancient duplication in combination with a low frequency of interlocus recombination could have provided the environment for such an independent evolution.
Under the assumption of stable mutation rate and absence of recombination, the number of acquired point mutations leading to polymorphisms could be taken as a measure for the relative age of a gene family. All Hcr9 sequences at MW carry a low number of unique polymorphic nucleotides, suggesting a relatively young age. However, the finding of more diverged Hcr9s at NL shows that the gene family is older than suggested by the inspection of genes at MW alone. The homogeneity of sequences at MW contrasts with the age of the family and suggests that acquired point mutations were rapidly distributed at this locus. This independently supports the concept of sequence exchange between Hcr9s, which we concluded earlier from the patchwork pattern of sequence identities (5).
In the absence of recombination, the vast majority of polymorphic nucleotides are expected to be unique for a given sequence. Recombination between Hcr9s will lead to sequence exchange that influences the distribution of acquired point mutations in the gene family. The higher the frequency of sequence exchange, the higher the ratio of shared versus unique polymorphic nucleotides in each sequence. To obtain an independent measure for the degree of homogenization, we determined the ratio of unique polymorphic sites versus shared IPS in each Hcr9. The vast majority (>94%) of polymorphic nucleotides in an Hcr9 sequence from MW or SC are shared with at least one other Hcr9, suggesting a high rate of sequence exchange relative to the mutation rate. In contrast, in ΨNL0A, ΨNL0B, NL0D, and NL0E a higher percentage of the polymorphic sites are unique, suggesting that homogenization at NL does not occur as rapidly as at MW (Table 1).
The analysis of recombination events within the MW locus has implicated Hcr9 IRs in determining the position and frequency of unequal crossing-over events (5). IRs within the NL0 cluster exhibit a high degree of polymorphism. Their length varies between 1253 bp (betweenΨNL0A and ΨNL0B) and 9542 bp (between ΨNL0B and NL0C, including a retrotransposon). Sequence homologies between IRs at NL0 are generally restricted to interrupted segments shorter than 600 bp and with the overall level of homology usually as low as 80% (data not shown). Only two longer stretches of homology were detected. The first 1243 bp of the sequence immediately 3′ of ΨNL0A and the sequence immediately 3′ of ΨNL0B (Fig. 2) are the most closely related IRs within the NL cluster, with an overall level of identity of 96%. A 1.1-kb stretch of only 81% identity interrupted by multiple insertions or deletions is shared between the sequences immediately flanking the 3′ ends of NL0C and NL0D.
NL0C: An Hcr9 of the MW Subclass at NL.
Although physically positioned in the center of the NL cluster, the NL0C gene is clearly different from the other genes at NL. Strikingly, NL0C exhibits the sequence signature of Hcr9s at MW. A strong affiliation with Hcr9s at MW and SC is indicated by an extensive sharing of polymorphic nucleotides (Table 1). NL0C shares only a single patch of IPSs with NL0D but nine patches with Hcr9s at MW or SC (Fig. 3). The inconsistent sequence signature of Hcr9 genes at NL suggests NL0C was translocated from another locus. This idea is also supported from analysis of flanking IRs that comprise extensive stretches of homology to IRs at MW and SC (Fig. 2), suggesting they could have arrived at NL together with NL0C. The sequence immediately 5′ of the ATG of NL0C has homology to the putative promoter regions of Hcr9s from different loci and is, therefore, not informative. Further 5′, a 1.5-kb region exhibits near sequence identity to the IR between the MW4B and MW4C genes (Fig. 2). This stretch of MW homology is flanked by a sequence with near identity outside multiple insertions or deletions to the IR between ΨNL0A and ΨNL0B (Fig. 2). The transition from MW to NL homology within the IR is possibly the result of an insertion of MW sequences into the NL cluster. The point of transition possibly defines a recombination breakpoint 5′ of the NL0C gene implicated in the insertion event (marked by an arrow in Fig. 2). The IR 3′ of the NL0C gene exhibits near sequence identity over almost the entire length with the IR between MW9A and MW9B (Fig. 2), encompassing a fragment with homology to the lipoxygenase sequence that flanks the MW and SC clusters at their 5′ ends (Fig. 1). This further supports the idea of translocation, because lipoxygenase sequences are otherwise not detectable at NL (Fig. 1 and 2). No NL specific sequences could be identified in this IR, but the NL0D gene itself exhibits features of the NL subclass. We hypothesize that the ≈2-kb putative promoter region that shares homology with Hcr9s from all three clusters might have served as a recombination template which promoted the integration of the DNA segment carrying the NL0C gene.
DISCUSSION
Ectopic Recombination Between Diverged R Gene Clusters.
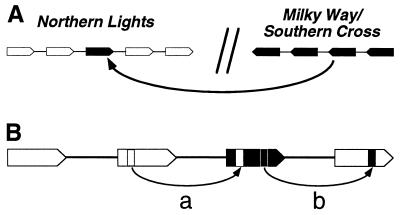
The characterization of three clusters of Hcr9 genes at the genetic, physical and sequence level allows us to correlate sequence relationships between Hcr9s with their physical location. The analysis of IPS led to the definition of two subclasses of Hcr9s. The members of both subclasses of Hcr9s tend to be separated from each other in individual clusters. The NL subclass is physically confined to NL. Restriction-fragment-length polymorphism analysis of NL haplotypes allowed the identification of polymorphism between different Lycopersicon species or L. esculentum cultivars (7). Among the cultivars analyzed, the L. esculentum Verticillium wilt, Fusarium wilt, root knot nematode, tobacco mosaic virus (VFNT) Cherry haplotype of NL resembled most closely the L. esculentum Moneymaker (Cf0) haplotype analyzed in the present study. Restriction-fragment-length polymorphism and partial sequence analysis suggested the presence of four Hcr9s at NLV that are orthologous to ΨNL0A, ΨNL0B, NL0D, and NL0E. However, a gene orthologous to NL0C was not detectable at NL in VFNT Cherry (7). Only the NL class of Hcr9s are found within the VFNT Cherry haplotype of NL. Likewise at MW, only the MW subclass is present in haplotypes originating from three species. Given this ordered occurrence, the presence of an Hcr9 of the MW subclass in the NL0 cluster is inconsistent and most likely the result of an ectopic recombination event (Fig. 4). In this scenario, the VFNT Cherry haplotype resembles a hypothetical progenitor locus of Cf0 prior to the insertion of the NL0C gene.
Figure 4.
Interactions between Hcr9 genes and clusters. (A) Genetic isolation between Hcr9 clusters allows sequence polymorphism to accumulate (members of the two diverged subclasses of Hcr9s are represented by black and white pentagons). The arrow symbolizes a hypothetical intercluster recombination event transferring a member of the SC/MW subclass of Hcr9 genes to the NL locus. Because NL otherwise only comprises Hcr9s of the NL subclass, such recombination events occurred at a frequency too low to homogenize the sequences between distant Hcr9 clusters. (B) By recombination between diverged clusters the receiving cluster (white Hcr9s) acquires sequence novelty (black Hcr9). By subsequent intracluster shuffling of sequence stretches (a and b) more diverse Hcr9 variants can be generated than without the input of diverged family members.
Due to the alternating orientation of the three loci each cluster is inverted with respect to its nearest cluster (Fig. 1). This arrangement results in deletions or duplications of intervening chromosomal segments upon recombination between loci, whereas a direct orientation would create dicentric bridges and acentric fragments. There is also the possibility of intrachromosomal exchange which would lead to inversion of the intervening chromosomal segment from recombination between inverted loci but a deletion in the direct orientation. The inverted orientation of the loci might be conducive to pairing, which allows rare interlocus gene conversion or unequal exchange while minimizing the risk of gross chromosomal rearrangement.
Ectopic recombination events have been implicated in the evolution of R gene homologues in cereal genomes. In barley, rice, and foxtail millet, homologues of the nucleotide binding site LRR type of R genes (21) are mostly organized in genetically linked clusters comprising diverged members (22). The composition and size of these clusters exhibited strong intra- and interspecific variation, suggesting rapid reorganization (22). Similar ectopic events might have been involved in the generation of the Dm3 complex of lettuce, in which diverged R gene homologues are dispersed over several Mb (13, 23). The L and M loci in flax are composed of members of the same gene family which suggests a common progenitor; however, the loci reside on different chromosomes (4). Comparative analyses of sequences of members at the M and L loci might reveal whether sequence exchange between the loci still occurred after the duplication event that separated the loci physically.
R Gene Evolution: A Balance Between Diversification and Conservation.
Conceptually, two opposing forces determine the evolutionary dynamics in an R gene family. The need to generate sequence novelty is imposed by the evolution of the pathogen. Positive selection for diversification of the putative recognition domain of R genes has been revealed by the analysis of synonymous and nonsynonymous substitution rates (5, 13, 19, 20, 24).
The chances of generating a gain of function allele by random mutation alone are extremely low. A strategy that also involves sequence exchange allows successful DNA fragments that have been selected for in one homologue to be tested in different R gene backgrounds. The validity of this concept has recently been demonstrated in vitro. By sequence shuffling between cephalosporinase genes of different bacterial species, alleles 270–540 times more active than any of the progenitor genes have been obtained (25). The higher the existing sequence diversity between functional genes, the larger the sequence repertoire that can be generated by DNA shuffling.
On the other hand, functional genes have to be conserved and protected against the eroding force of homogenization that is invariably associated with sequence exchange. Sequence homogenization of gene families is a well-recognized phenomenon termed “concerted evolution” (26). Selection by a specific pathogen is only imposed on a plant population at intervals and many generations may set seed without experiencing a specific avirulence determinant. Therefore, successful alleles have to be maintained in the absence of the pathogen during phases in which selection is absent. The generation and maintenance of sequence diversity, therefore, requires a reduction of the frequency at which homogenizing recombination occurs. Our analysis of Hcr9 clusters indicates that their genetic map position, the degree of polymorphism within a cluster and the physical separation of clusters all affect the degree of homogenization.
It is well documented that recombination frequencies can vary greatly along plant chromosomes. Within the NL0 cluster, we observe a significantly higher degree of Hcr9 sequence divergence than at MW and SC. Although IRs at both MW and NL are polymorphic, their structure suggests IR sequence homogenization occurs to a lesser degree at NL. IRs at MW are composed of segments of high homology between multiple IRs and polymorphism is mainly due to the distinct segment composition of each IR (5). In contrast, IRs within NL0 exhibit a generally low level of homology. Therefore, it is possible that NL is located in a less recombinogenic region of tomato chromosome 1 than MW and SC.
Likewise, the frequency at which mispairing followed by unequal crossing-over occurs, is affected by the degree of sequence homology between the substrates. It is possible that polymorphic IRs reduce the rate of sequence exchange at NL both between IRs and the adjacent Hcr9 coding regions.
The generally localized occurrence of the two subclasses of Hcr9s at NL and MW/SC also shows that physical separation of clusters can provide genetic niches within which sequences can diverge from the remainder of the gene family. The degree of divergence between the two subclasses of Hcr9 genes is probably not sufficient to abolish sequence exchange between the classes, as suggested by few patches of shared IPS sequence (Fig. 3 and 4).
Genetic isolation of parts of a gene family might ultimately result in a degree of divergence that will split the family into nonrecombining classes. Members of the Xa21 R gene family of rice belong to two classes that are physically linked in a cluster of tandemly repeated genes. Within each class more than 95% of DNA sequence identity was observed on average, but the members of the two classes were typically only 63.5% identical (3). Recombination between both diverged classes only occurred in a short stretch of high homology near the 5′ end (3). The similar overall structure and the homology in the C-terminal portion of the Hcr2 and Hcr9 proteins together with the similar exon–intron structure of their genes suggests the Hcr2 and Hcr9 genes originated from a common ancestor. The Hcr genes as a whole are an extreme example of sequence diversification that illustrates how genetic isolation can ultimately lead to the generation of independent gene families.
The balance between diversification and conservation within the Hcr9 gene family is accommodated by the physical distribution of clustered sequences and exploitation of the standard recombination machinery. Sequence exchange occurs in tandem arrays of Hcr9 genes and the frequency appears to be adjusted by the polymorphism of the IRs and the position on the chromosome. The spatial organization in three clusters provides the environment for sequence polymorphism to build up and coexist. The discovery of intercluster gene transfer suggests that the machinery generating variability is fuelled by slow acquisition of this polymorphism (Fig. 4).
Acknowledgments
We thank Brande B. H. Wulff, David A. Jones, and Colwyn M. Thomas for help and useful suggestions, Erik van der Biezen for critical reading of the manuscript, and Brian G. Spratt for providing the Sequence Output software. M.P. was supported by fellowships of the European Molecular Biology Organization (EMBO) and the European Community. Research at the Sainsbury Laboratory is funded by the Gatsby Charitable Foundation.
ABBREVIATIONS
- LRR
leucine-rich-repeat
- IR
intergenic region
- IPS
informative polymorphic site
Footnotes
References
- 1.Anderson P A, Lawrence G J, Morrish B C, Ayliffe M A, Finnegan E J, Ellis J G. Plant Cell. 1997;9:641–651. doi: 10.1105/tpc.9.4.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Salmeron J M, Oldroyd G, Rommens C, Scofield S R, Kim H S, Lavelle D T, Dahlbeck D, Staskawicz B J. Cell. 1996;86:123–133. doi: 10.1016/s0092-8674(00)80083-5. [DOI] [PubMed] [Google Scholar]
- 3.Song W Y, Pi L Y, Wang G L, Gardner J, Holsten T, Ronald P C. Plant Cell. 1997;9:1279–1287. doi: 10.1105/tpc.9.8.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ellis J, Lawrence G, Ayliffe M, Anderson P, Collins N, Finnegan J, Frost D, Luck J, Pryor T. Annu Rev Phytopathol. 1997;35:271–291. doi: 10.1146/annurev.phyto.35.1.271. [DOI] [PubMed] [Google Scholar]
- 5.Parniske M, Hammond-Kosack K E, Golstein C, Thomas C M, Jones D A, Harrison K, Wulff B B H, Jones J D G. Cell. 1997;91:821–832. doi: 10.1016/s0092-8674(00)80470-5. [DOI] [PubMed] [Google Scholar]
- 6.Thomas C M, Jones D A, Parniske M, Harrison K, Balint-Kurti P J, Hatzixanthis K, Jones J D G. Plant Cell. 1997;9:2209–2224. doi: 10.1105/tpc.9.12.2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parniske M, Wulff B B H, Bonnema G, Thomas C M, Jones D A, Jones J D G. Mol Plant Microbe Interact. 1999;12:93–102. doi: 10.1094/MPMI.1999.12.2.93. [DOI] [PubMed] [Google Scholar]
- 8.Jones D A, Thomas C M, Hammond-Kosack K E, Balint-Kurti P J, Jones J D G. Science. 1994;266:789–793. doi: 10.1126/science.7973631. [DOI] [PubMed] [Google Scholar]
- 9.Thomas C M, Dixon M S, Parniske M, Golstein C, Jones J D G. Phil Trans R Soc London B. 1998;353:1413–1424. doi: 10.1098/rstb.1998.0296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takken F L W, Schipper D, Nijkamp H J J, Hille J. Plant J. 1998;14:401–411. doi: 10.1046/j.1365-313x.1998.00135.x. [DOI] [PubMed] [Google Scholar]
- 11.Jones D A, Jones J D G. Advances In Botanical Research Incorporating Advances In Plant Pathology. 1997;24:89–167. [Google Scholar]
- 12.Kobe B, Deisenhofer J. Trends Biochem Sci. 1994;19:415–421. doi: 10.1016/0968-0004(94)90090-6. [DOI] [PubMed] [Google Scholar]
- 13.Meyers B C, Shen K A, Rohani P, Gaut B S, Michelmore R W. Plant Cell. 1998;10:1833–1846. doi: 10.1105/tpc.10.11.1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Staden R. Mol Biotech. 1996;5:233–241. doi: 10.1007/BF02900361. [DOI] [PubMed] [Google Scholar]
- 15.Dixon M, Hatzixanthis K, Jones D A, Harrison K, Jones J D G. Plant Cell. 1998;10:1915–1926. doi: 10.1105/tpc.10.11.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parker J E, Coleman M J, Szabo V, Frost L N, Schmidt R, van der Biezen E A, Moores T, Dean C, Daniels M J, Jones J D G. Plant Cell. 1997;9:879–894. doi: 10.1105/tpc.9.6.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dixon M S, Jones D A, Keddie J S, Thomas C M, Harrison K, Jones J D G. Cell. 1996;84:451–459. doi: 10.1016/s0092-8674(00)81290-8. [DOI] [PubMed] [Google Scholar]
- 18.Hulbert S H. Annu Rev Phytopathol. 1997;35:293–310. doi: 10.1146/annurev.phyto.35.1.293. [DOI] [PubMed] [Google Scholar]
- 19.Botella M A, Parker J E, Frost L N, Bittner-Eddy P D, Beynon J L, Daniels M J, Holub E B, Jones J D. Plant Cell. 1998;10:1847–1860. doi: 10.1105/tpc.10.11.1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McDowell J M, Dhandaydham M, Long T A, Aarts M G, Goff S, Holub E B, Dangl J L. Plant Cell. 1998;10:1861–1874. doi: 10.1105/tpc.10.11.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van der Biezen E A, Jones J D G. Curr Biol. 1998;8:R226–R227. doi: 10.1016/s0960-9822(98)70145-9. [DOI] [PubMed] [Google Scholar]
- 22.Leister D, Kurth J, Laurie D A, Yano M, Sasaki T, Devos K, Graner A, Schulze-Lefert P. Proc Natl Acad Sci USA. 1998;95:370–375. doi: 10.1073/pnas.95.1.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meyers B C, Chin D B, Shen K A, Sivaramakrishnan S, Lavelle D O, Zhang Z, Michelmore R W. Plant Cell. 1998;10:1817–1832. doi: 10.1105/tpc.10.11.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang G L, Ruan D L, Song W Y, Sideris S, Chen L L, Pi L Y, Zhang S P, Zhang Z, Fauquet C, Gaut B S, et al. Plant Cell. 1998;10:765–779. doi: 10.1105/tpc.10.5.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crameri A, Raillard S-A, Bermudez E, Stemmer W P C. Nature (London) 1998;391:288–291. doi: 10.1038/34663. [DOI] [PubMed] [Google Scholar]
- 26.Dover G A, Linares A R, Bowen T, Hancock J M. Methods Enzymol. 1993;224:525–541. doi: 10.1016/0076-6879(93)24039-w. [DOI] [PubMed] [Google Scholar]