Abstract
Fluorescent labeling of a short sequence of double-stranded DNA (dsDNA) was achieved by ligating a labeled dsDNA fragment to a stem–loop triplex forming oligonucleotide (TFO). After the TFO has wound around the target sequence by ligand-induced triple helix formation, its extremities hybridize to each other, leaving a dangling single-stranded sequence, which is then ligated to a fluorescent dsDNA fragment using T4 DNA ligase. A non-repeated 15 bp sequence present on lambda DNA was labeled and visualized by fluorescence microscopy after DNA combing. The label was found to be attached at a specific position located at 4.2 ± 0.5 kb from one end of the molecule, in agreement with the location of the target sequence for triple helix formation (4.4 kb from one end). In addition, an alternative combing process was noticed in which a DNA molecule becomes attached to the combing slide from the label rather than from one of its ends. The method described herein provides a new tool for the detection of very short sequences of dsDNA and offers various perspectives in the micromanipulation of single DNA molecules.
INTRODUCTION
Recent advances in ultrasensitive instrumentation have contributed to the development of single molecule studies, which have provided important information that complements conventional biochemical approaches regarding the kinetic and mechanical aspects of biomolecular interactions. In the field of DNA structure and protein interactions, these studies have shown the existence of new structural motifs (1) and shed new light into the mechanism of DNA processing enzymes. The effects on DNA of mechanical constraints (2) or protein interactions (3–8) can be observed by single molecule manipulation techniques combined with force measurements, but DNA molecules can also be visualized directly using electron, atomic force or optical microscopy (8–11). Different methods have been used for the observation of single DNA molecules by optical microscopy. For example, DNA elongated by a laminar flow or an electric field and stained with the dye YOYO has been used for the study of chromatin assembly (12) or exonuclease activity (13), respectively. One particularly interesting technique for stretching DNA in a homogenous and reproducible manner is DNA combing (14,15). This method has been extensively used for the physical mapping of genomes by in situ hybridization (16) and it has been extended recently to the study of DNA–protein interactions. It has been possible for example to observe an enzymatic process such as RNA polymerization on combed DNA molecules (17).
Various methods have been described for the sequence-specific recognition of dsDNA. Oligopurine-oligopyrimidine sequences can be targeted by Peptide Nucleic Acids (PNA) (18) or Triplex Forming Oligonucleotides (TFO) (19). Recognition involves strand displacement followed by triplex formation on one DNA strand in the former case or direct recognition of the DNA major groove in the latter. Polyamides composed of N-methyl-3-hydroxypyrrole, N-methylpyrrole and N-methylimidazole can specifically target any short sequence of dsDNA by binding in the minor groove (20). A sequence-specific label can also be introduced by circularizing an oligonucleotide around a single-stranded target after local opening of the DNA double helix using so-called PNA-openers (21), or after heat denaturation of AT-rich sequences within supercoiled DNA (22). We have also recently reported a method by which TFOs can be circularized around specific double-stranded DNA sequences (23,24). Most of these strategies can be used for the observation of sequence-specific labeled dsDNA by electron and atomic force microscopy (21,22,24–27).
Very few methods have been described for the detection of double-stranded DNA sequences by optical microscopy. Fluorescently labeled sequence-specific DNA binding proteins have been visualized on stretched lambda DNA (28,29). Fluorescently labeled PNAs (30), TFOs (31), as well as minor groove binding agents (32) have been used for in situ labeling without denaturation, but in all cases the targeted sequences were present in multiple copies, as it occurs in centromeric and telomeric repeats. Here, we describe a novel method for linking a highly fluorescent probe to double-stranded DNA in a sequence-specific way. This probe has been used for detecting a short target sequence on combed lambda DNA by optical microscopy.
MATERIALS AND METHODS
Oligonucleotides and chemicals
The sequence of the 59mer stem–loop TFO and 39mer linear TFO were 5′GAGCCCTAGTACTCGACGCTAAAAGTG TTGTGGTGGTGTAAAAGTGGAGCTGTACTAGG3′ and 5′GAGCCCTAGTACTCGACGCTAAAAGTGTTGTGGT GGTGT3′, respectively. An 8mer adapter 5′GTACTAGG3′ was used with the 39mer linear TFO. The sequences of the primers used for PCR were 5′CGCTTGGTCTCTGCTCGG TATCAGCTCACTCAAAG3′ (fw), and 5′GGCGATAAGT CGTGTCTTAC3′ (rv). The fw primer was biotinylated at the 5′ end during synthesis. All these oligonucleotides were obtained from Eurogentec (Seraing, Belgium); their concentration was calculated using a nearest-neighbor model for absorption coefficients.
The TFO was 5′-phosphorylated for the ligation reactions using the following protocol: 300 pmol of oligonucleotide were incubated in 50 µl of T4 DNA ligase buffer [50 mM Tris–HCl, 10 mM MgCl2, 10 mM DTT, 1 mM ATP and 25 µg/ml BSA, pH 7.8 at 25°C, New England Biolabs (NEB)] with 10 U T4 PNK (NEB), for 1 h 30 min at 37°C.
Synthesis of the triplex stabilizing agent BQQ (6- [3-(dimethylamino)propyl]amino-11-methoxy-benzo[f]quino- [3,4-b]quinoxaline) has been described previously (33).
Preparation of lambda DNA
Lambda DNA was purchased from Amersham. In order to avoid concatemerization during the ligation reactions, the single-stranded ends were partially filled; 25 µg of lambda DNA were incubated in 100 µl of Eco buffer (NEB) supplemented with 50 µM of dATP, 50 µM of dGTP and 5 U of Klenow fragment (NEB) for 3 h at 37°C. The polymerase was then inactivated by heating for 20 min at 75°C and the DNA was ethanol precipitated. This resulted in lambda molecules with 9 base overhangs that cannot be ligated to each other.
Preparation of DNA fragments
PCR primers were designed in order to produce a fragment of approximately 500 bp starting from the pBluescript SK+ plasmid (Stratagene). The sequence of the fw primer was chosen in order to introduce a cleavage site for the BsaI restriction enzyme. The PCR reaction was carried out by mixing each primer (1.6 µM) in Taq buffer (Promega) with 2 mM MgCl2, 50 µM of each of dATP, dCTP and dGTP, 33 µM of dTTP, 17 µM of dUTP-Alexa Fluor 546 (Molecular Probes), 10 pg/µl of pBluescript and 0.1 U/µl of Taq polymerase (Promega). After 30 cycles of 30 s at 94°C, 30 s at 61°C and 1 min at 72°C (this last time increasing by 10 s per cycle), the concluding extension was carried out for 10 min at 72°C. Usually, 900 µl of PCR mix was prepared and aliquotted into eighteen 50 µl tubes. Primers and unincorporated dNTP were removed using Qiagen PCR purification kits, using the standard protocol. Then the PCR products were digested overnight at 50°C with 100 U BsaI (NEB) in a total volume of 200 µl. The biotinylated extremities and the non-digested biotinylated PCR products were removed using streptavidin-coated magnetic beads (Dynabeads). The labeled fragments were then ethanol precipitated and quantified by absorption spectroscopy. Absorbance maxima were measured near 260 nm and near 553 nm. The extinction factor and the correction factor (absorbance ratio A260/Amax for the free Alexa Fluor 546 dye) indicated by the provider allowed us to estimate the concentration of the fragments and the amount of dye per fragment. Unlabeled fragments were prepared in the same way except that the PCR was carried out using 200 µM of each unmodified dNTP and the elongation step was maintained at 1 min at 72°C for all the cycles.
For some experiments, the DNA fragments had to be ligated to the linear or stem–loop TFOs before any contact with lambda DNA. For this purpose, 100 nM of the fragment was mixed with 200 nM of the stem–loop TFO or with 200 nM of the linear TFO and 400 nM of the 8mer adapter in 50 µl of T4 DNA ligase buffer (NEB), heated to 65°C and slowly cooled to room temperature. 400 U of T4 DNA ligase (NEB) were added and the sample was incubated overnight at 20°C. The fragments were purified from the excess oligonucleotides using a Qiagen PCR purification kit.
Attachment of DNA fragments to the target site
Lambda DNA (1 µg; 3 nM) was incubated in 10 µl of T4 DNA ligase buffer with 20 µM of the triplex stabilizing ligand BQQ, 100 nM of TFO and 200 nM of labeled fragment (for fluorescence microscopy analysis) or unlabeled fragment (for gel retardation assay). The sample was heated to 80°C and slowly cooled to room temperature. 400 U of T4 DNA ligase were then added and the sample was incubated overnight at 20°C.
When removal of the fragment was necessary, the previously described spermidine precipitation procedure was used (34). Briefly, 10 µl of the ligation reaction were diluted to a final volume of 100 µl in a precipitation buffer containing 20 mM Tris pH 8.0, 40 mM NaCl, 8 mM MgCl2 and 12.5 mM of ice-cold spermidine. The sample was incubated for 30 min at room temperature and centrifuged for 15 min at 14 000 r.p.m. The pellet was rinsed with 100 µl of a 1:1 v/v mix of isopropanol and a solution containing 600 mM NaCl, 20 mM MgCl2 and 50 mM EDTA. It was resuspended in 100 µl of T4 DNA ligase buffer supplemented with 4 µM BQQ and 150 nM YOYO-1 (Molecular Probes).
Then 50 ng of YOYO stained DNA were loaded on a 1% agarose gel in 0.5× TBE buffer at 14°C. The migration conditions were 6 V/cm, 120°, 1 to 6 s pulses, for 20 h in a Chef DRIII pulsed-field gel electrophoresis system (Bio-Rad). The gel was then stained with ethidium bromide.
Attachment site analysis
The specificity of the labeling reaction was checked by digesting lambda DNA with the ApaI restriction enzyme. After the ligation reaction, the sample was heated for 20 min at 65°C to inactivate the ligase. 200 ng of lambda DNA (2 µl of the ligation reaction) were incubated with 5 U of ApaI (Promega) in 20 µl of the recommended buffer (10 mM Bis Tris Propane–HCl, 10 mM MgCl2, 1 mM dithiothreitol pH 7.0 at 25°C) for 3 h at 37°C. The digested sample was analyzed by pulsed-field gel electrophoresis as described above except that the migration time was 12 h.
Molecular combing
Glass surfaces were rendered hydrophobic by polystyrene spin-coating: a drop of polystyrene (5% w/v in toluene) was placed on a clean coverslip and spread by spin-coating at 3000 r.p.m. for 20 s. Then 50 ng of DNA stained with YOYO-1 (at a ratio of 1 dye for 100 bp) was added in a reservoir containing 3 ml of MES 50 mM, pH 5.5 at 4°C. The coverslip was dipped into the reservoir for 2 min and then slowly and regularly pulled up with a Combing Apparatus (Institut Pasteur).
The proposed mechanism of molecular combing (35) suggests that, at an appropriate pH, DNA in the reservoir binds to the coverslip’s surface mainly by one of its extremities. When the coverslip is pulled up, the DNA molecules are aligned and uniformly overstretched by the receding meniscus like ‘algae by the receding tide’ (35).
Images
The coverslips were scanned using an Olympus inverted microscope equipped with a 100×, 1.4 numerical aperture oil immersion objective lens, in combination with an Hg lamp and filter sets chosen for the detection of YOYO-1 and AlexaFluor 546 (OmegaFilters). Pictures were recorded with a CCD camera (CoolSnap, Roper Scientific) (exposure time 100 ms). The length of the DNA molecules and the position of the labels were analyzed with MetaVue (Roper Scientific) and ImageJ software.
RESULTS
Strategy for labeling double-stranded DNA
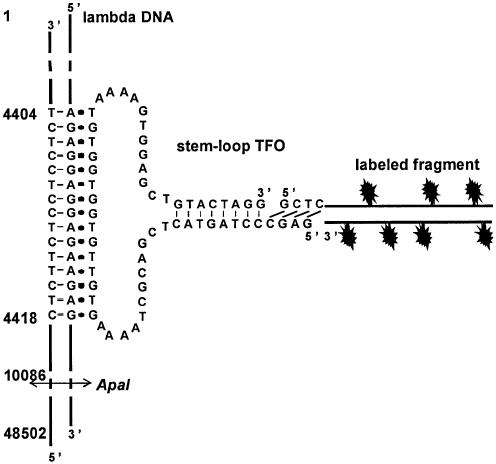
Triple helix forming oligonucleotides bind to dsDNA in a very sequence-specific way. In order to achieve fluorescent labeling of dsDNA, several fluorophores should be attached to a TFO that is stably bound to its target sequence. We have recently described a method for attaching peptides to dsDNA in which a stem–loop TFO is ligated to a peptide-conjugated hairpin oligonucleotide (36). In a similar approach, the short hairpin oligonucleotide may be replaced by a longer double-stranded DNA fragment in which several fluorescent compounds could be incorporated (Fig. 1).
Figure 1.
Description of the labeling strategy used in this study. The sequence of the 59mer stem–loop TFO is shown. The 59mer oligonucleotide can form a triple helix by binding to a 15 bp oligopurineoligopyrimidine target sequence, located between 4404 and 4418 bp from the extremity of the phage. A fluorescently labeled fragment made by PCR is digested by a class IIS restriction enzyme in order to produce a sticky end that can be ligated to the 5′-end of the TFO. The restriction site for ApaI is indicated.
A lambda DNA molecule was chosen as the dsDNA to be labeled. A 15 bp oligopurineoligopyrimidine target sequence, located 4404 bp from the closest end of the molecule was selected, and a 59mer stem–loop oligonucleotide was designed with a central part capable of forming a triple helix by binding to this sequence. This triple helix is made of T.AxT and C.GxG base-triplets and is very stable in the presence of the triplex stabilizing agent BQQ (34). Complementary sequences were introduced in the 3′- and 5′-regions of the TFO so that they can hybridize to each other, forming a 8 bp double-stranded stem with a 4 nucleotide dangling 5′ end (Fig. 1).
The labeled fragment of approximately 500 bp was produced by PCR using pBluescript as a template. A site for the class IIS restriction enzyme BsaI, which enables it to produce any 4 base overhang after digestion, was introduced close to one end of the fragment in order to obtain a sticky end complementary to the 5′ end of the oligonucleotide. A non-self-complementary sequence was chosen in order to avoid self ligation of the oligonucleotide or the fragment.
A fluorophore-coupled dUTP was incorporated during the PCR reaction. Several conditions and several fluorophore-modified dUTP were tried for preparation of the labeled fragment, and for each of them the quantity of final product as well as the number of fluorophores that have been incorporated have been measured by absorption spectroscopy. dUTP carrying AlexaFluor 546 was found to be incorporated efficiently. The conditions we used (see Materials and Methods) resulted in the reproducible incorporation of at least 20 fluorophores in the 500 bp fragment with an acceptable decrease of the PCR reaction yield. The quantity of PCR product per tube was indeed 30% of that obtained under standard conditions with unmodified dNTPs.
Attachment of the DNA fragment to the specific target
We checked first whether the DNA fragment could be linked to its target site. To prevent auto-ligation of the complementary ends of the lambda molecules during the ligation reaction, the 12 nucleotide single-stranded ends of the lambda molecules were partially filled using a bacterial DNA polymerase (Klenow fragment of Escherichia coli DNA polymerase). The TFO, the DNA fragment (unlabeled) and lambda DNA were mixed in the presence of the triplex-specific ligand BQQ in T4 DNA ligase buffer. The samples were heated to 80°C to permit the opening of the TFO stem–loop structure, and then slowly cooled down to allow first triple helix formation followed by hybridization of the ends of the TFO to each other. T4 DNA ligase was then added and the mixture was incubated overnight at 20°C in order to ligate the TFO to the fragment’s sticky end.
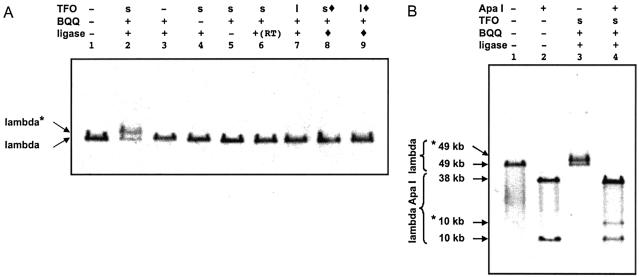
The formation of the labeling complex was assessed by a gel shift assay as the labeled lambda molecules migrate more slowly than the unlabeled molecules when analyzed by pulsed-field gel electrophoresis (Fig. 2A, lane 2). The observed shift was probably larger than what would be expected upon addition of 500 bp to a lambda molecule. This may be due to the branched structure of the labeled lambda molecule. This assay allowed us to measure a yield for the labeling reaction between 50 and 60%. No shift was observed in the absence of TFO (lane 3), BQQ (lane 4), or ligase (lane 5), or when the samples were not heated before addition of ligase (lane 6). This suggests that attachment of the fragment necessitates ligand-induced triple helix formation and opening of the stem–loop structure, although the heating step may in fact be required only for the purpose of annealing.
Figure 2.
Attachment of the labeled fragment to its specific target site as revealed by pulsed-field gel electrophoresis. (A) Lambda DNA (previously treated in order to prevent concatemerization) was incubated with the stem–loop (s) or the linear (l) TFO and the DNA fragment in the presence of BQQ and the enzymatic ligation reaction was performed. The TFO was omitted in lanes 1 and 3. BQQ was omitted in lanes 1 and 4. The ligase was omitted in lanes 1 and 5. Sample shown in lane 6 was not heated before ligation. In lanes 8 and 9, the stem–loop (s, filled lozenge) or linear (l, filled lozenge) TFO had been ligated (filled lozenge) to the labeled fragment before performing the incubation with lambda DNA. (B) Lambda DNA loaded on lanes 1 and 3 was identical to that of lanes 1 and 2 in (A), respectively. These unlabeled and labeled phage molecules were digested with ApaI (lanes 2 and 4), which generates a 38 kb and a 10 kb fragment. Bands *49 kb and *10 kb correspond to the shifted (labeled) 49 kb lambda DNA and 10 kb fragment, respectively.
We wondered whether the TFO was wound around the target sequence. First, we ligated the DNA fragment to the stem–loop TFO before the formation of a complex with lambda DNA. No band shift was observed (lane 8). The ligation prevents the opening of the TFO stem–loop structure, and it is unlikely that the TFO would thread along lambda DNA to reach its target sequence. Then, a 39mer linear TFO was designed based on the 59mer stem–loop TFO, which lacks the 3′-side of the triplex-forming sequence. This oligonucleotide can be ligated to the DNA fragment upon addition of a short adapter oligonucleotide whose sequence corresponds to the eight nucleotides on the 3′-end of the 59mer. No shift was observed when this linear TFO was used, whether the ligation was performed before (lane 9) or after (lane 7) triple helix formation. These results show that the only complex that can be detected in the gel shift assay is the one where the TFO can wind around its target sequence.
To check whether the DNA fragment was attached on the right target sequence, we digested the labeled lambda DNA with an enzyme that yields a 10 kb DNA molecule containing the target site and a 38 kb DNA molecule. Analysis of the samples by pulsed-field gel electrophoresis showed that only the lower band, i.e. the 10 kb molecule, was shifted (Fig. 2B, lane 4), thus indicating that the DNA fragment was attached to the 10 kb segment, which contains the target sequence and not to the longer 38 kb segment. No shift was observed in control experiments when the labeling complex had not been formed (Fig. 2B, lane 2). All these results suggest that the DNA fragment can be attached in a sequence-specific way to a target site on lambda DNA.
Visualization of the fluorescent label on combed DNA
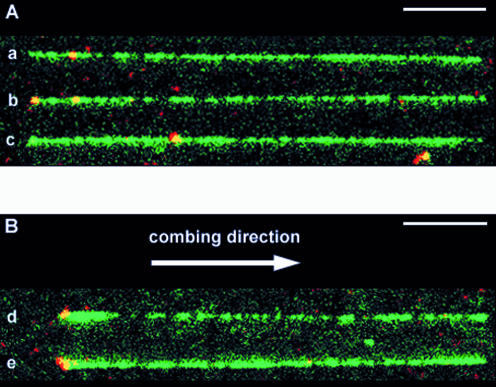
Fluorescence microscopy was then used to observe the labeled fragment attached to combed lambda DNA molecules. Since labeled fragments may bind to the combing surface, the large excess of fragments that are not ligated to phage molecules had to be removed. This was achieved by spermidine selective precipitation. More than 95% of the labeled fragments were removed while more than 95% of the lambda DNA were preserved, as assessed by agarose gel electrophoresis (not shown). The purified lambda DNA was then stained with YOYO-1 and combed on a polystyrene coated slide. Images were acquired separately for each fluorophore (YOYO-1 and Alexa Fluor 546) and then superimposed. The green images showed the elongated YOYO-1 stained lambda DNA molecules, as usually observed after the combing process, and bright monodisperse fluorescent dots spread over the slide. The red images also revealed bright monodisperse fluorescent dots which colocalize with the green ones, giving yellow dots on the superimposed images (Fig. 4). The red signal showed low bleaching and a signal/noise ratio of 4. The size of the spots (300 nm) is diffraction limited. Therefore, each spot may correspond to any combed DNA molecule with a length below 700 bp. These observations suggest that these dots represent single DNA label molecules that have incorporated several Alexa Fluor 546 and are also stained with YOYO-1. In each field of view (100 µm × 100 µm), an average of 12 lambda DNA molecules and 80 fluorescent dots were usually observed. Thus the probability to observe a label on a lambda molecule only by chance was low and the probability to observe it at the expected position was very low.
Figure 4.
Visualisation of labels on combed lambda molecules. DNA was stained with YOYO-1 (green). The labeled DNA fragments contained Alexa Fluor 546 (red); they therefore appear as yellow spots. The bars represent 5 µm. A montage of different patterns of combing and labeling is produced: (A) aligned longer (26–27.5 µm) molecules. The combed molecules were aligned so that the red internal labels were located on the left side, but they were equally distributed on both sides with respect to the combing direction (see text). (B) Aligned shorter molecules (23.5–25.5 µm). In this case, combed molecules were oriented with respect to the combing direction, i.e. the left side is the side where the DNA molecule sticks first to the glass surface, before being stretched in the direction indicated by the arrow.
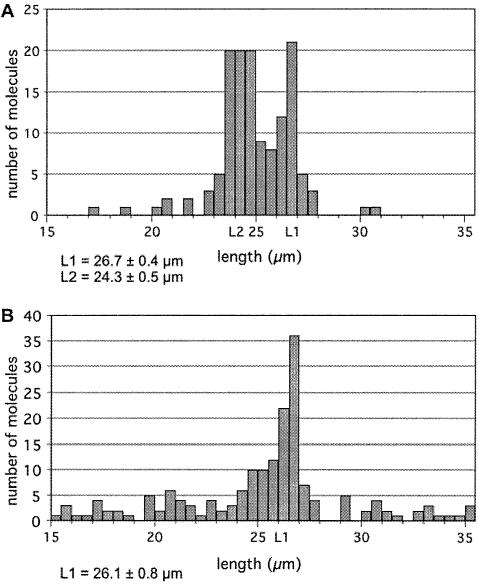
We first measured the size of the combed molecules in order to control their integrity. Few short molecules were observed, which probably resulted from phage molecules that have been broken during the combing process; the very few molecules with a length below 15 µm were not analyzed. Very long molecules were not observed as the sample had been previously treated to prevent lambda concatemerization. Surprisingly two sets of molecules could be distinguished among the 15–30 µm analyzed molecules: about one-third of the molecules were 26.7 ± 0.4 µm long, while the other two-thirds were 24.3 ± 0.5 µm long (Fig. 3A). In a control experiment where lambda molecules were combed without any pretreatment, the same analysis showed only one set of full-length molecules (26.1 ± 0.8 µm) (Fig. 3B); the length distribution for the labeled molecules is significantly different from that observed for the non-labeled molecules (P < 0.001).
Figure 3.
Analysis of the length of combed lambda DNA molecules. Lambda DNA molecules either labeled (A) or untreated (B) were combed on polystyrene coated slides. Images were captured and analysed. 135 (A) and 159 (B) molecules were measured.
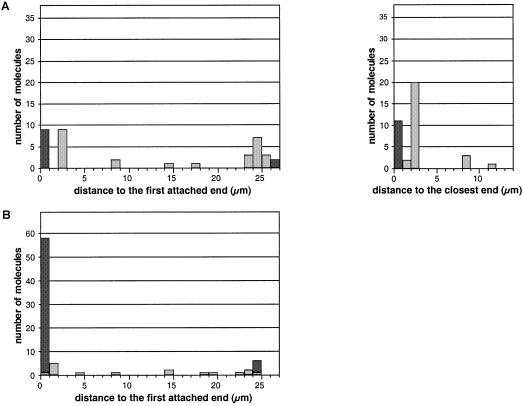
The two sets of molecules were further analyzed separately for the position of red labels with respect to entire lambda molecules. The first set consisted of 38 molecules with a length between 26.0 and 27.5 µm. Within this set, 22 molecules (58%) exhibited a red label at 2.3 ± 0.3 µm from the nearest extremity (Figs 4A, a, b and 5A). This position corresponds to the location of the target sequence, at 4404 bp (2.4 µm) from the extremity of the 48 502 bp fully extended (26.7 µm) lambda molecule. The labeling yield (58%) appeared to be in good agreement with the results of gel-shift experiments (labeling yield between 50 and 60%). No distinctive orientation of the lambda molecules could be noted: the label was equally distributed on both sides of the combed molecule with respect to the combing direction (Fig. 5A, left). Four molecules (11%) were observed with a label at a different position from the expected one (Fig. 4A, c). It is not obvious whether these labeled fragments were attached to the lambda molecules at a non-specific site or whether they were not removed during the purification process and became attached to the slide close to a lambda molecule. Eleven molecules (29%) were observed with a label at their extremity (Fig. 4A, b), nine of which were on the same side relative to the combing direction, i.e. on the side that sticks first to the glass surface. This suggests that there could be some non-specific interactions during the combing process between this extremity and the labeled fragment, or that labeled fragments could be ligated at the end of lambda molecules and then favor attachment to the slide during the combing process.
Figure 5.
Histograms presenting the position of labels on lambda DNA molecules. Distances were measured between the label and the end of the molecule that becomes attached first to the slide during the combing process. Data are shown for the first set of molecules (26.0–27.5 µm) (A, left) and for the second set (23.5–25.5 µm) (B). The labels located exactly at the extremity of the molecules are scored in black, whereas those located at an internal position are scored in grey. For the first set of molecules, the distance between the label and the closest end of the molecule is also presented (A, right).
The second set consisted of 69 molecules with a length between 23.5 and 25.5 µm. Within this set, 57 molecules (84%) exhibited a label at one of their extremities (Figs 4B, d, e and 5B). It is interesting to note that all those molecules were oriented relative to the combing direction: the label was always located on the DNA extremity that becomes attached first to the slide during the combing process. The difference between the length of both sets of molecules (26.7–24.3 µm) was the same as the distance between the label and the extremity of the lambda molecule (2.3 µm). Moreover, some of these shorter combed molecules had a larger width on the side of attachment, over a short length that does not exceed 2.5 µm (Fig. 4B d). These observations strongly support the hypothesis that the molecules of this shorter set have first bound to the slide surface at the labeling site, then the DNA on both sides of this site have completed the combing process. Folding of the molecules at the attachment point would explain the larger width of some molecules close to this site. These shorter length molecules are therefore labeled at the correct target site and the presence of the labeled fragment interferes with the combing process by providing a new internal attachment site. The results of DNA combing experiments are consistent with those obtained from gel shift experiments, showing that our strategy results in the introduction of a stable sequence-specific label on double-stranded DNA. They prove that labeled combed molecules can be observed by optical microscopy and therefore confirm the validity of our labeling strategy.
DISCUSSION
The aim of this study was to develop a new strategy to visualize a short double-stranded DNA sequence by optical microscopy. For this purpose, we linked a fluorescent DNA fragment to a TFO. This labeled fragment was efficiently catenated to lambda DNA in a sequence-specific way. Formation of a catenated complex resulted from the ligation of the sticky end of the fragment to the end of a stem–loop triplex forming oligonucleotide that is wound around its target sequence. In our previous work where the same type of stem–loop oligonucleotide was ligated to a short hairpin oligonucleotide, the complex formed by the circularized oligonucleotides and the plasmid containing the target sequence for triplex formation resisted the denaturing conditions, which proved the formation of a catenated structure (36). It is unlikely that replacing the hairpin oligonucleotide by a longer dsDNA fragment would prevent the stem–loop TFO winding around its dsDNA target. In the present work, the target is located on a linear DNA. Therefore, the structure formed by the lambda DNA and the DNA-ligated TFO should be a pseudorotaxane and the topological link formed between the two components of this structure would be disrupted upon complete denaturation. Our previous studies support the idea that TFOs that are wound around their target form triple helices that are much more stable than those made by linear TFOs. Indeed their stability approaches that of triple helices where the TFO has been linked covalently to its target. Based on this concept, control experiments were designed. The only complex that we detected by gel shift experiments is the one where the TFO can wind around its target, which suggests that this complex has a pseudorotaxane structure. A more simple labeling strategy has been designed in which a linear TFO is ligated to a labeled DNA fragment, before or after the TFO has bound to its target sequence. However, this approach did not result in a complex that was stable enough to be observed by gel shift experiment. Thus the pseudorotaxane structure is required for formation of a very stable complex that does not dissociate during migration in a gel and during the combing process.
The labeling yield was comparable those obtained in our previous reports (60%) (24,36). It did not improve when using higher concentrations of TFOs or labeled fragments and, as previously reported, oligonucleotide quality is probably the major factor which limits the yield of these labeling procedures.
At least 20 molecules of Alexa Fluor 546 were incorporated in the 500 bp DNA fragment made by PCR, which proved to be sufficient for visualization. Statistical analysis of the position of the label on the lambda molecule showed that it was attached only to the target site. The triplex stabilizing agent (BQQ) has not been removed, which results in a very stable complex in which the TFO is not able to slide from its target sequence, as previously reported in one of our previous studies (34). Some non-specific interactions between the labels and the ends of the lambda molecule were noticed, which may be due to the presence of short single stranded sequences at the extremity of phage DNA molecules. It may also be due to the ligation of the PCR label at the end of the lambda molecule, although there is no evident base-pairing scheme that would explain such a result. Very few labels were seen at a non-specific position within the lambda DNA molecules, and no secondary binding site was observed. These non-specific signals are probably explained by an incomplete elimination of the non-bound labeled fragments.
To our knowledge, this is the first time that a non-repeated sequence as short as 15 bp has been visualized by optical microscopy. Another original feature of this approach is that the DNA sequence is recognized in its double-stranded form. Although formation of DNA triple helices is associated with certain sequence restrictions, progress has been made that extends the repertory of sequences that can be bound by TFOs (37). We have been able to use this labeling strategy on oligopurineoligopyrimidine target sequences ranging from 12 to 20 bp (not shown). There are 15 such sequences in the lambda genome, and the overrepresentation of oligopurineoligopyrimidine sequences has been demonstrated for eukaryotic genomes (38). Moreover, our approach may be applied with other DNA recognition strategies (22,39).
Some DNA molecules were found to be anchored to the polystyrene coated surface at the labeling site. Attachment probably occurs through the single-stranded DNA surrounding the target site, since the combing process is thought to involve attachment of DNA to the slide via its single-stranded ends. If anchoring via the labeled site may be a problem if one wants to observe the precise position of a label after combing long DNA molecules, it may also be exploited to specifically capture the tagged molecules from a large pool of DNA molecules, or to attach long DNA molecules to a surface or to a bead by an internal site and not only by its extremities, which presently remains an experimental challenge (2,3,6,7,40). The labeling strategy described here may therefore constitute a new tool for the manipulation of single DNA molecules.
Single molecule studies of DNA by optical microscopy have been carried out using YOYO-stained molecules (12,13). However, staining with a fluorescent dye may inhibit several interactions between DNA and proteins (41). The method we describe here may be used for introducing one or two labels for the localization or measurement of single DNA molecules.
Since no amplification reaction such as enzymatic DNA polymerization or successive additions of antibody layers is necessary, and the exposure time is short (100 ms), the labeled DNA molecules may be observed in real time in order to allow the visualization of dynamic behavior. It has been shown that the TFO can slide along the double helix if the triplex stabilizing agent (BQQ) is removed (34). Thus the labeled fragment described here may be displaced by DNA and RNA polymerases and proteins that translocate along double-stranded DNA (42). Therefore, the labeled fragment could be an interesting tool for the study of protein movement and may be used as a probe for observing their one-dimensional motion.
In conclusion, the labeling strategy described in this paper represents an original approach for the visualization of short sequences of double-stranded DNA by optical microscopy. This strategy may also find applications as a tool for single molecule studies of DNA and its interaction with proteins.
Acknowledgments
ACKNOWLEDGEMENTS
We acknowledge insightful discussion with Ron Lebofsky. We thank Aurélien Crut for technical advice and assistance, and Jeff Sperinde for careful reading of the manuscript. B.G.-L. was supported by a grant from Ministère de la Recherche. T.R. was supported by the AFM (Association Française contre les Myopathies).
REFERENCES
- 1.Tiner W.J. Sr, Potaman,V.N., Sinden,R.R. and Lyubchenko,Y.L. (2001) The structure of intramolecular triplex DNA: atomic force microscopy study. J. Mol. Biol., 314, 353–357. [DOI] [PubMed] [Google Scholar]
- 2.Strick T., Allemand,J., Croquette,V. and Bensimon,D. (2000) Twisting and stretching single DNA molecules. Prog. Biophys. Mol. Biol., 74, 115–140. [DOI] [PubMed] [Google Scholar]
- 3.Wang M.D., Schnitzer,M.J., Yin,H., Landick,R., Gelles,J. and Block,S.M. (1998) Force and velocity measured for single molecules of RNA polymerase. Science, 282, 902–907. [DOI] [PubMed] [Google Scholar]
- 4.Harada Y., Funatsu,T., Murakami,K., Nonoyama,Y., Ishihama,A. and Yanagida,T. (1999) Single-molecule imaging of RNA polymerase–DNA interactions in real time. Biophys. J., 76, 709–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Strick T.R., Croquette,V. and Bensimon,D. (2000) Single-molecule analysis of DNA uncoiling by a type II topoisomerase. Nature, 404, 901–904. [DOI] [PubMed] [Google Scholar]
- 6.Davenport R.J., Wuite,G.J., Landick,R. and Bustamante,C. (2000) Single-molecule study of transcriptional pausing and arrest by E. coli RNA polymerase. Science, 287, 2497–2500. [DOI] [PubMed] [Google Scholar]
- 7.Maier B., Bensimon,D. and Croquette,V. (2000) Replication by a single DNA polymerase of a stretched single-stranded DNA. Proc. Natl Acad. Sci. USA, 97, 12002–12007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ha T., Rasnik,I., Cheng,W., Babcock,H.P., Gauss,G.H., Lohman,T.M. and Chu,S. (2002) Initiation and re-initiation of DNA unwinding by the Escherichia coli Rep helicase. Nature, 419, 638–641. [DOI] [PubMed] [Google Scholar]
- 9.Kabata H., Kurosawa,O., Arai,I., Washizu,M., Margarson,S.A., Glass,R.E. and Shimamoto,N. (1993) Visualization of single molecules of RNA polymerase sliding along DNA. Science, 262, 1561–1563. [DOI] [PubMed] [Google Scholar]
- 10.Washizu M., Kurosawa,O., Arai,I., Suzuki,S. and Shimamoto,N. (1995) Applications of electrostatic stretch-and-positioning of DNA. IEEE Trans. Ind. Appl., 31, 447–456. [Google Scholar]
- 11.Harada Y., Ohara,O., Takatsuki,A., Itoh,H., Shimamoto,N. and Kinosita,K.,Jr (2001) Direct observation of DNA rotation during transcription by Escherichia coli RNA polymerase. Nature, 409, 113–115. [DOI] [PubMed] [Google Scholar]
- 12.Ladoux B., Quivy,J.P., Doyle,P., du Roure,O., Almouzni,G. and Viovy,J.L. (2000) Fast kinetics of chromatin assembly revealed by single-molecule videomicroscopy and scanning force microscopy. Proc. Natl Acad. Sci. USA, 97, 14251–14256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matsuura S., Komatsu,J., Hirano,K., Yasuda,H., Takashima,K., Katsura,S. and Mizuno,A. (2001) Real-time observation of a single DNA digestion by lambda exonuclease under a fluorescence microscope field. Nucleic Acids Res., 29, e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bensimon A., Simon,A., Chiffaudel,A., Croquette,V., Heslot,F. and Bensimon,D. (1994) Alignment and sensitive detection of DNA by a moving interface. Science, 265, 2096–2098. [DOI] [PubMed] [Google Scholar]
- 15.Michalet X., Ekong,R., Fougerousse,F., Rousseaux,S., Schurra,C., Hornigold,N., van Slegtenhorst,M., Wolfe,J., Povey,S., Beckmann,J.S. and Bensimon,A. (1997) Dynamic molecular combing: stretching the whole human genome for high-resolution studies. Science, 277, 1518–1523. [DOI] [PubMed] [Google Scholar]
- 16.Aston C., Mishra,B. and Schwartz,D.C. (1999) Optical mapping and its potential for large-scale sequencing projects. Trends Biotechnol., 17, 297–302. [DOI] [PubMed] [Google Scholar]
- 17.Gueroui Z., Place,C., Freyssingeas,E. and Berge,B. (2002) Observation by fluorescence microscopy of transcription on single combed DNA. Proc. Natl Acad. Sci. USA, 99, 6005–6010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nielsen P.E. (1999) Peptide nucleic acid: a versatile tool in genetic diagnostics and molecular biology. Curr. Opin. Biotechnol., 12, 16–20. [DOI] [PubMed] [Google Scholar]
- 19.Thuong T.N. and Hélène,C. (1993) Sequence-specific recognition and modification of double-helical DNA by oligonucleotides. Angew. Chem. Int. Ed. Engl., 32, 666–690. [Google Scholar]
- 20.Dervan P.B. and Bürli,R.W. (1999) Sequence-specific DNA recognition by polyamides. Curr. Opin. Chem. Biol., 3, 688–693. [DOI] [PubMed] [Google Scholar]
- 21.Kuhn H., Demidov,V.V. and Frank-Kamenetskii,M.D. (1999) Topological links between duplex DNA and a circular DNA single strand. Angew. Chem. Int. Ed. Engl., 38, 1446–1449. [DOI] [PubMed] [Google Scholar]
- 22.Potaman V.N., Lushnikov,A.Y., Sinden,R.R. and Lyubchenko,Y.L. (2002) Site-specific labeling of supercoiled DNA at the A+T rich sequences. Biochemistry, 41, 13198–13206. [DOI] [PubMed] [Google Scholar]
- 23.Escudé C., Garestier,T. and Hélène,C. (1999) Padlock oligonucleotides for duplex DNA based on sequence-specific triple helix formation. Proc. Natl Acad. Sci. USA, 96, 10603–10607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roulon T., Coulaud,D., Delain,E., Lecam,E., Hélène,C. and Escudé,C. (2002) Padlock oligonucleotides as a tool for labeling superhelical DNA. Nucleic Acids Res., 30, e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cherny D.I., Malkov,V.A., Volodin,A.A. and Frank-Kamenetskii,M.D. (1993) Electron microscopy visualization of oligonucleotide binding to duplex DNA via triplex formation. J. Mol. Biol., 230, 379–383. [DOI] [PubMed] [Google Scholar]
- 26.Svinarchuk F., Cherny,D., Debin,A., Delain,E. and Malvy,C. (1996) A new approach to overcome potassium-mediated inhibition of triplex formation. Nucleic Acids Res., 24, 3858–3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pfannschmidt C., Schaper,A., Heim,G., Jovin,T.M. and Langowski,J. (1996) Sequence-specific labeling of superhelical DNA by triple helix formation and psoralen crosslinking. Nucleic Acids Res., 24, 1702–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oana H., Ueda,M. and Washizu,M. (1999) Visualization of a specific sequence on a single large DNA molecule using fluorescence microscopy based on a new DNA-stretching method. Biochem. Biophys. Res. Commun., 265, 140–143. [DOI] [PubMed] [Google Scholar]
- 29.Taylor J.R., Fang,M.M. and Nie,S. (2000) Probing specific sequences on single DNA molecules with bioconjugated fluorescent nanoparticles. Anal. Chem., 72, 1979–1986. [DOI] [PubMed] [Google Scholar]
- 30.Zijlmans J.M., Martens,U.M., Poon,S.S., Raap,A.K., Tanke,H.J., Ward,R.K. and Lansdorp,P.M. (1997) Telomeres in the mouse have large inter-chromosomal variations in the number of T2AG3 repeats. Proc. Natl Acad. Sci. USA, 94, 7423–7428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnson M.D. III, and Fresco,J.R. (1999) Third-strand in situ hybridization (TISH) to non-denatured metaphase spreads and interphase nuclei. Chromosoma, 108, 181–189. [DOI] [PubMed] [Google Scholar]
- 32.Maeshima K., Janssen,S. and Laemmli,U.K. (2001) Specific targeting of insect and vertebrate telomeres with pyrrole and imidazole polyamides. EMBO J., 20, 3218–3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zain R., Marchand,C., Sun,J.-S., Nguyen,C.H., Bisagni,E., Garestier,T. and Hélène,C. (1999) Design of a triple-helix-specific cleaving reagent. Chem. Biol., 6, 771–777. [DOI] [PubMed] [Google Scholar]
- 34.Roulon T., Hélène,C. and Escudé,C. (2001) A ligand-modulated padlock oligonucleotide for supercoiled plasmids. Angew. Chem. Int. Ed. Engl., 40, 1523–1526. [DOI] [PubMed] [Google Scholar]
- 35.Allemand J.F., Bensimon,D., Jullien,L., Bensimon,A. and Croquette,V. (1997) pH-dependent specific binding and combing of DNA. Biophys. J., 73, 2064–2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roulon T., Hélène,C. and Escudé,C. (2002) Coupling targeting peptides to supercoiled plasmids using a new type of padlock oligonucleotide. Bioconjug. Chem., 13, 1134–1139. [DOI] [PubMed] [Google Scholar]
- 37.Sun J.S. (1999) New targets for triple helix forming oligonucleotides. In Malvy,C., Harrel-Bellan,A. and Pritchard,L.L. (eds), Triple Helix Forming Oligonucleotides. Kluwer Academic Publishing, Dordrecht, The Netherlands, pp. 273–284. [Google Scholar]
- 38.Behe M.J. (1995) An overabundance of long oligopurine tracts occurs in the genome of simple and complex eukaryotes. Nucleic Acids Res., 23, 689–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Demidov V.V., Kuhn,H., Lavrentieva-Smolina,I.V. and Frank-Kamenetskii,M.D. (2001) Peptide nucleic acid-assisted topological labeling of duplex DNA. Methods, 23, 123–131. [DOI] [PubMed] [Google Scholar]
- 40.Bockelmann U., Thomen,P., Essevaz-Roulet,B., Viasnoff,V. and Heslot,F. (2002) Unzipping DNA with optical tweezers: high sequence sensitivity and force flips. Biophys. J., 82, 1537–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meng X., Cai,W. and Schwartz,D.C. (1996) Inhibition of restriction endonuclease activity by DNA binding fluorochromes. J. Biomol. Struct. Dyn., 13, 945–951. [DOI] [PubMed] [Google Scholar]
- 42.Firman K. and Szczelkun,M.D. (2000) Measuring motion on DNA by the type I restriction endonuclease EcoR124I using triplex displacement. EMBO J., 19, 2094–2102. [DOI] [PMC free article] [PubMed] [Google Scholar]