Abstract
The role of unsaturated fatty acids in membrane lipids in the tolerance of the photosynthetic machinery to salt stress was studied by comparing the desA−/desD− mutant of Synechocystis sp. PCC 6803, which contained monounsaturated fatty acids, with the wild-type strain, which contained a full complement of polyunsaturated fatty acids. In darkness, the loss of oxygen-evolving photosystem II activity in the presence of 0.5 M NaCl or 0.5 M LiCl was much more rapid in desA−/desD− cells than in wild-type cells. Oxygen-evolving activity that had been lost during incubation with 0.5 M NaCl in darkness returned when cells were transferred to conditions that allowed photosynthesis or respiration. Recovery was much greater in wild-type than in desA−/desD− cells, and it was prevented by lincomycin. Thus, the unsaturation of fatty acids is important in the tolerance of the photosynthetic machinery to salt stress. It appears also that the activity and synthesis of the Na+/H+ antiporter system might be suppressed under high-salt conditions and that this effect can be reversed, in part, by the unsaturation of fatty acids in membrane lipids.
Keywords: salt tolerance, photosystem II, Na+, H+ antiport, desA−, desD−
The regulation of the unsaturation of fatty acids in membrane lipids is important in the acclimation of poikilothermic organisms to changing environmental conditions, in particular, temperature (1–4). The effects of temperature on the desaturation of fatty acids have been extensively studied in cyanobacteria (for reviews, see refs. 2 and 5). When the ambient temperature is shifted from high to low, cyanobacterial cells synthesize fatty acid desaturases that introduce double bonds into the fatty acids of membrane lipids.
In a previous study we isolated a desA−/desD− mutant strain of the cyanobacterium Synechocystis sp. PCC 6803 in which the desA and desD genes for the Δ12 and Δ6 desaturases, respectively, had been inactivated by targeted mutagenesis (6). The desA−/desD− cells contain monounsaturated but not polyunsaturated fatty acids, whereas wild-type cells contain polyunsaturated fatty acids such as di-, tri-, and tetraunsaturated fatty acids (7). By comparing these two strains we found that polyunsaturated fatty acids are essential for growth in Synechocystis sp. PCC 6803 at low temperatures (6).
In the present study, we investigated the contribution of the unsaturation of fatty acids in membrane lipids to tolerance to salt stress by comparing desA−/desD− cells to wild-type cells of Synechocystis sp. PCC 6803. We demonstrated that the unsaturation of fatty acids is associated with the ability of the photosynthetic machinery to tolerate salt stress.
MATERIALS AND METHODS
Cells, Culture Conditions, and Preparation of Thylakoid Membranes.
Wild-type and desA−/desD− (6) cells of Synechocystis sp. PCC 6803 were grown photoautotrophically in BG-11 medium (pH 7.5) (23), which contained 20 mM Na+ ions at 35°C under constant illumination from incandescent lamps (70–80 μE⋅m−2⋅s−1, in which E indicates an einstein, 1 mol of photons) with aeration by sterile air that contained 1% CO2 (8). After 4 days, cells were harvested by centrifugation at 9,000 × g for 10 min at 35°C and resuspended in fresh BG-11 medium. They were then incubated at 35°C with mild stirring every 5 min in BG-11 medium that contained various concentrations of NaCl, LiCl, and sorbitol at a chlorophyll (Chl) concentration of 10 μg/ml in glass tubes (20 ml) in darkness or in light (50 μE⋅m−2⋅s−1). Thylakoid membranes were isolated from wild-type and desA−/desD− cells as described (9) with minor modifications.
Quantitation of Glucosylglycerol (GG).
GG was extracted from cells as described by Mikkat et al. (10). The extracted samples were dissolved in 2 ml of water and subjected to HPLC (11) on a combination of an octadecyl silica column (ODS; pore size, 5 μm; 250 mm × 4 mm i.d.; Merck) and an Aminex carbohydrate column (HPX-87C; 250 mm × 4 mm i.d.; Bio-Rad) with a refractive index detector (RI-930; Shimadzu). GG (95% purity; provided by M. Hagemann) was used for calibration.
Measurement of Photosynthetic Activities.
Activities of photosystem (PS) II in intact cells were measured with a Clark-type oxygen electrode (Hansatech Instruments, Kings Lynn, U.K.) in the presence of 1.0 mM 1,4-benzoquinone (BQ) or 1.0 mM 2,6-dichlorobenzoquinone (DCBQ) as described previously (6). The light-induced reduction of dichloroindophenol (DCIP) by isolated thylakoid membranes was measured with a dual-wavelength spectrophotometer (UV-300; Shimadzu) as described previously (9). The incubation and reaction mixture contained 50 mM Hepes-NaOH (pH 7.5), 800 mM sorbitol, 5 mM CaCl2, and 1.0 mM 6-amino-n-caproic acid. Concentrations of Chl were determined as described by Arnon (12).
Measurement of Na+/H+ Antiport Activity.
The Na+/H+ antiport activity of intact cells was measured by monitoring the fluorescence of acridine orange (13). To generate outwardly directed Na+ gradients, a suspension of cells (20 μl) was diluted 100-fold in a solution that contained 5 μM acridine orange, 35 mM N-methylglucamine-gluconate (pH 7.8), and 0.6 M mannitol. After fluorescence had attained a steady state in 3 min, 90 μl of a 2.0 M solution of NaCl was added to give a final NaCl concentration of 100 mM. After the fluorescence had once again attained a steady state in 4 min, 85 μl of a 1% solution of Triton X-100 was added to give a final concentration of 0.04% and a final steady-state level of fluorescence was recorded. Fluorescence was monitored with a spectrofluorimeter (RF-500; Shimadzu) at excitation and emission wavelengths of 495 nm and 540 nm, respectively, at room temperature. The activity of the Na+/H+ antiport was calculated from the initial rate of recovery of fluorescence quenching upon addition of NaCl, divided by the difference between the level of fluorescence before the addition of NaCl and the steady-state level of fluorescence 2 min after the addition of Triton X-100.
RESULTS
Salt-Induced Inactivation of the Oxygen-Evolving Machinery in Vivo.
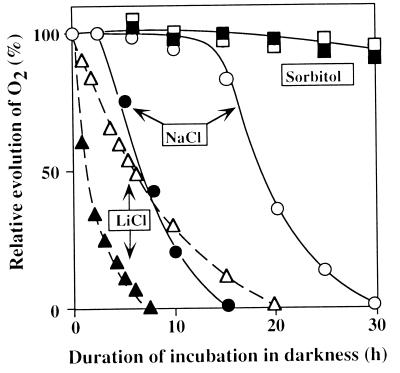
Fig. 1 shows changes in the oxygen-evolving activity of wild-type and desA−/desD− cells during incubation in darkness in the presence of NaCl, LiCl, or sorbitol. The evolution of oxygen was markedly depressed in both types of cell in the presence of 0.5 M LiCl. However, desA−/desD− cells were much more sensitive to LiCl than were wild-type cells. The time required for 50% inactivation was 1 h in the case of desA−/desD− cells, whereas it was 6 h in the case of wild-type cells.
Figure 1.
Changes in the photosynthetic oxygen-evolving activity of wild-type and desA−/desD− cells during incubation in darkness in the presence of 0.5 M NaCl, 0.5 M LiCl, or 1.0 M sorbitol. At designated times, a portion of the cell suspension was withdrawn and oxygen-evolving activity was examined at 35°C after addition of 1.0 mM BQ. The activities of wild-type and desA−/desD− cells that corresponded to 100% were 610 ± 24 and 556 ± 18 μmol of O2 per mg of Chl per h, respectively. Open symbols, wild-type cells; filled symbols, desA−/desD− cells. Each point represents the average of results from four independent experiments.
The inactivation of the oxygen-evolving machinery in the presence of 0.5 M NaCl was slower than in the presence of 0.5 M LiCl. However, desA−/desD− cells appeared, once again, to be more sensitive to NaCl than were wild-type cells. The time required for 50% inactivation was 7 h and 18 h in desA−/desD− and wild-type cells, respectively. The oxygen-evolving activity remained at the original level initially and then started to decline. When we examined the effect of NaCl on the oxygen-evolving activity with 2,6-dichlorobenzoquinone as an artificial electron acceptor, we obtained essentially the same result as with BQ with respect to the difference between wild-type and desA−/desD− cells in their responses to salt stress (data not shown).
Sorbitol (1.0 M), which does not penetrate through the plasma membrane of Synechocystis sp. PCC 6803 (14), had almost no effect on the oxygen-evolving activity in either wild-type or desA−/desD− cells (Fig. 1). Thus, the effects of LiCl and NaCl were due to the cationic effect of Li+ and Na+ and not to an osmotic effect.
Effects of Light on NaCl-Induced Inactivation.
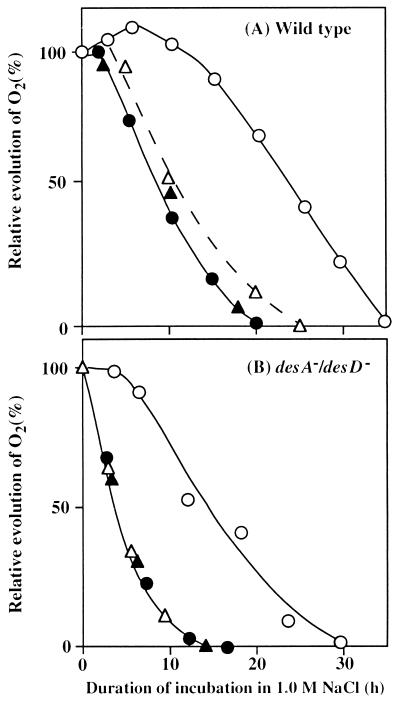
Fig. 2 shows the effects of light and lincomycin on the inactivation by 1.0 M NaCl of the oxygen-evolving machinery in wild-type and desA−/desD− cells. Light at 50 μE⋅m−2⋅s−1 protected the oxygen-evolving machinery against NaCl-induced inactivation in both types of cell. The time required for 50% inactivation increased from 9 h to 25 h in wild-type cells and from 3.5 h to 14 h in desA−/desD− cells.
Figure 2.
Effects of light and lincomycin on the NaCl-induced inactivation of the oxygen-evolving machinery. Wild-type (A) and desA−/desD− (B) cells were incubated with 1.0 M NaCl in darkness or in light at 50 μE⋅m−2⋅s−1 in the absence or presence of 25 μg/ml lincomycin. At designated times, a portion of the cell suspension was withdrawn and the oxygen-evolving activity was measured at 35°C after addition of 1.0 mM BQ. The activities of wild-type and desA−/desD− cells that corresponded to 100% were 590 ± 22 and 532 ± 16 μmol of O2 per mg of Chl per h, respectively. ○, Light in the absence of lincomycin; ▵, light in the presence of lincomycin; ●, darkness in the absence of lincomycin; and ▴, darkness in the presence of lincomycin. Each point represents the average of results from four independent experiments.
To clarify the involvement of protein synthesis in the tolerance to salt stress, we exposed cells to lincomycin (25 μg/ml), an inhibitor of protein synthesis, in the presence of 1.0 M NaCl. Fig. 2 shows that, in darkness, lincomycin had no effect on the NaCl-induced inactivation of the oxygen-evolving machinery in either wild-type or desA−/desD− cells. In a parallel experiment in light (50 μE⋅m−2⋅s−1), lincomycin eliminated the protective effect of light on the NaCl-induced inactivation. Essentially the same results were obtained with 0.5 M NaCl, although the inactivation was delayed. These observations suggest that synthesis of proteins was involved in the light-induced protection against salt-induced inactivation.
To examine the involvement of the Na+/H+ antiport system (see Discussion) in the tolerance to salt stress, we investigated the effect of N,N-dicyclohexylcarbodiimide (DCCD), an inhibitor of H+-ATPase (15), on the NaCl-induced inactivation of the oxygen-evolving machinery. This inhibitor at 50 μM accelerated the inactivation in light and darkness in both wild-type and desA−/desD− cells (data not shown).
Effects of Light on Recovery of the Oxygen-Evolving Machinery.
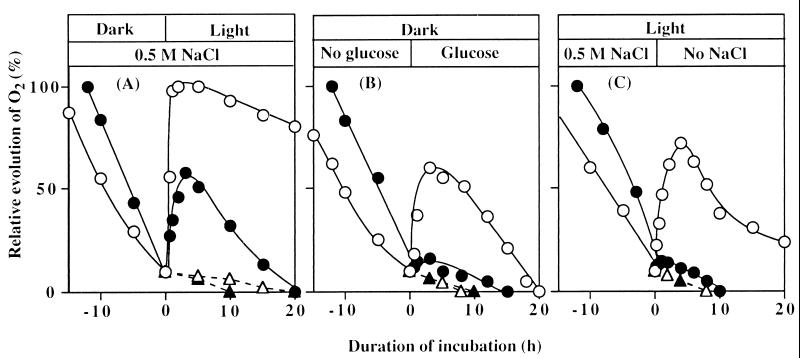
Fig. 3A shows that oxygen-evolving activity was restored by light after it had fallen to a certain level in the presence of NaCl. After incubation of cells with 0.5 M NaCl in darkness to reduce the oxygen-evolving activity to approximately 10% of the original level, they were exposed to light at 50 μE⋅m−2⋅s−1. Under these conditions, the oxygen-evolving activity returned to the original level within 2 h in wild-type cells, whereas in desA−/desD− cells only 50% of the original activity was restored. The oxygen-evolving activity then began to decrease, and it had completely disappeared within 20 h in desA−/desD− cells and within 35 h in wild-type cells. Lincomycin completely prevented restoration of oxygen-evolving activity in both types of cell.
Figure 3.
Effects of light, glucose, and removal of NaCl on restoration of oxygen-evolving activity in wild-type and desA−/desD− cells after NaCl-induced inactivation. (A) Wild-type and desA−/desD− cells were incubated for 25 h and 12 h, respectively, in darkness in the presence of 0.5 M NaCl. Then cells were exposed to light of 50 μE⋅m−2⋅s−1 in the presence of 25 μg/ml lincomycin (broken lines) or its absence (solid lines). (B) Wild-type and desA−/desD− cells were incubated in darkness in the presence of 0.5 M NaCl as in A. Then glucose was added to a final concentration of 5 mM. (C) Wild-type and desA−/desD− cells were incubated with 0.5 M NaCl in light at 50 μE⋅m−2⋅s−1 for 45 h and 25 h, respectively. Then cells were collected by centrifugation, resuspended in fresh BG-11 medium with no added NaCl, and incubated in light. ○, Wild-type cells; ●, desA−/desD− cells in the absence of lincomycin; ▵, wild-type cells; and ▴, desA−/desD− cells in the presence of lincomycin. Each point represents the average of results from four independent experiments.
Effects of Glucose on Recovery of the Oxygen-Evolving Machinery in Darkness.
Fig. 3B shows the effects of glucose on the recovery of the oxygen-evolving machinery after NaCl-induced inactivation in wild-type and desA−/desD− cells. After incubation of cells in the presence of 0.5 M NaCl in darkness to reduce the oxygen-evolving activity to about 10% of the original level, 5 mM glucose was added to induce respiration. Under these conditions, the oxygen-evolving activity in wild-type cells returned to 60% of the original level within 3 h. However, the addition of glucose did not restore oxygen-evolving activity in desA−/desD− cells. Lincomycin completely eliminated the glucose-induced restoration of oxygen-evolving activity in wild-type cells.
Effects of Removal of NaCl on the Restoration of Oxygen-Evolving Activity.
Fig. 3C shows that oxygen-evolving activity was restored on removal of NaCl from the culture medium after the activity had been reduced by incubation with NaCl. After wild-type and desA−/desD− cells were incubated with 0.5 M NaCl in light at 50 μE⋅m−2⋅s−1, which caused 90% inactivation, they were washed with BG-11 medium by centrifugation and resuspension and illuminated. Wild-type cells recovered 75% of their original activity within 3 h, whereas in desA−/desD− cells the restoration of oxygen-evolving activity was very limited. Once again, lincomycin completely eliminated the restoration of oxygen-evolving activity in wild-type cells.
NaCl-Induced Inactivation of the Oxygen-Evolving Machinery in Vitro.
We compared the effects of NaCl on the oxygen-evolving activity of thylakoid membranes isolated from wild-type and desA−/desD− cells. During incubation in the presence of 0.5 M NaCl in darkness, the transport of electrons from H2O to DCIP in thylakoid membranes was inhibited much more rapidly than in intact cells of both types. Moreover, the inactivation of thylakoid membranes from desA−/desD− cells occurred much more rapidly than that of membranes from wild-type cells. The time required for 50% inactivation was 1.5 h and 4 h for thylakoid membranes from desA−/desD− and wild-type cells, respectively. By contrast, the inactivation in thylakoid membranes from both types of cell was very slow in the absence of NaCl. The transport of electrons from 1,5-diphenylcarbazide (DPC) to DCPIP, which bypasses the oxygen-evolving site (16), was scarcely inhibited during incubation with 0.5 M NaCl. Another set of experiments indicated that light (50 μE⋅m−2⋅s−1) had no effect on the NaCl-induced inactivation of the oxygen-evolving machinery in isolated thylakoid membranes. These observations demonstrate that incubation with NaCl resulted primarily in damage to the oxygen-evolving site in the PS II complex and that the oxygen-evolving site in the PS II complex from wild-type cells was more tolerant to NaCl than was that from desA−/desD− cells.
Accumulation of GG During Salt Stress.
One of the main responses of Synechocystis sp. PCC 6803 cells to salt stress is the accumulation of GG (10, 11). To examine whether the difference in sensitivity to salt stress was related to accumulation of GG, we quantitated GG during incubation of cells in the presence of 0.5 M or 1.0 M NaCl. At each concentration the level of GG increased at about the same rate in both types of cell and reached 200 ± 40 μg/mg of Chl in wild-type cells and 180 ± 30 μg/mg of Chl in desA−/desD− cells after incubation in light for 30 h or 20 h in the presence of 0.5 M or 1.0 M NaCl, respectively. However, we found no GG prior to salt stress and 5 μg of GG per mg of Chl after incubation in darkness for 30 h in either type of cell. The requirement of light for the synthesis of GG was previously demonstrated in another strain of the cyanobacterium (17).
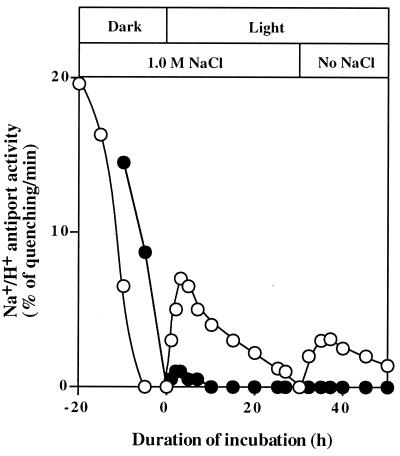
NaCl-Induced Decrease in Na+/H+ Antiport Activity.
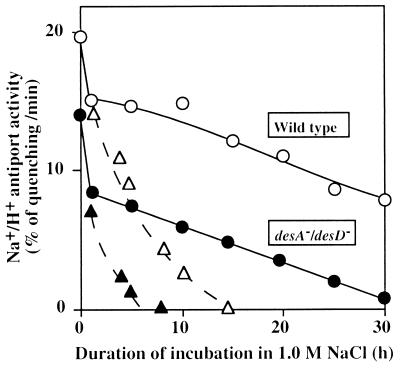
Changes in the Na+/H+ antiport activity during incubation of wild-type and desA−/desD− cells were examined in the presence of 1.0 M NaCl (Fig. 4). In darkness, the decline in the activity in desA−/desD− cells was much more rapid than that in wild-type cells; the activity was totally lost within 6 h in desA−/desD− cells, whereas it was lost within 15 h in wild-type cells. In light (50 μE⋅m−2⋅s−1), the activity fell rapidly for 1 h and then declined slowly in both types of cell. However, after incubation in 1.0 M NaCl for 30 h, desA−/desD− cells had lost almost all antiport activity, whereas wild-type cells retained about half of the original activity. Moreover, the absolute activity before incubation in 1.0 M NaCl was higher in wild-type cells than in desA−/desD− cells. These observations suggest that the Na+/H+ antiport system in desA−/desD− cells was less active under nonstressed conditions and was also more sensitive to salt stress than was that in wild-type cells.
Figure 4.
Changes in the Na+/H+ activity of wild-type and desA−/desD− cells during incubation in the presence of 1.0 M NaCl in darkness and in light at 50 μE⋅m−2⋅s−1. At designated times, 20 μl of the suspension was withdrawn and diluted 100-fold with Na+-free medium that contained 5 μM acridine orange. The Na+/H+ antiport activity was determined as described in the text. ○, Wild-type cells in light; ▵, wild-type cells in darkness; ●, desA−/desD− cells in light; and ▴, desA−/desD− cells in darkness. Each point represents the average of results from four independent experiments.
Similar experiments were conducted in light in the presence of 25 μg/ml lincomycin. The loss of antiport activity occurred much more rapidly than in the absence of this drug. These findings suggest that maintenance of antiport activity in the light depended on the synthesis of proteins and, very probably, on the synthesis of the Na+/H+ antiport system.
Effects of Light and Removal of NaCl on the Restoration of Na+/H+ Antiport Activity.
Fig. 5 shows that Na+/H+ antiport activity was restored by light after it had fallen to a certain level in the presence of NaCl. Incubation of wild-type and desA−/desD− cells with 1.0 M NaCl in darkness resulted in total inactivation of the Na+/H+ antiport system. When cells were then exposed to light at 50 μE⋅m−2⋅s−1, the Na+/H+ antiport activity in wild-type cells returned to 30% of the original level within 2 h. By contrast, in desA−/desD− cells, only 5% of the original activity was restored. The Na+/H+ antiport activity then began to decrease. Lincomycin completely prevented restoration of Na+/H+ antiport activity in both wild-type and desA−/desD− cells (data not shown).
Figure 5.
Effects of light and removal of NaCl on restoration of the Na+/H+ antiport activity in wild-type and desA−/desD− cells after NaCl-induced inactivation. Wild-type and desA−/desD− cells were incubated for 20 h and 12 h, respectively, in darkness in the presence of 1.0 M NaCl. Then cells were exposed to light at 50 μE⋅m−2⋅s−1. After incubation in light for 30 h, cells were collected by centrifugation, resuspended in fresh BG-11 medium with no added NaCl, and incubated in light. Experimental conditions were the same as those described in the legend to Fig. 4. ○, Wild-type cells; and ●, desA−/desD− cells. Each point represents the average of results from three independent experiments.
Fig. 5 also shows that the Na+/H+ antiport activity was restored on removal of NaCl from the culture medium after the activity had been lost during incubation in the presence of 1.0 M NaCl. Wild-type cells recovered 15% of their original activity within 3 h, whereas in desA−/desD− cells the restoration of antiport activity was very limited. Again, lincomycin completely eliminated the restoration of Na+/H+ antiport activity in both types of cell (data not shown).
The time-dependent profile of restoration of the Na+/H+ antiport activity was very similar to that of the oxygen-evolving activity when both types of cell had been incubated in the presence of 1.0 M NaCl. These findings suggest that the NaCl-dependent inactivation of the oxygen-evolving machinery and its recovery might be closely related to the inactivation and recovery of the Na+/H+ antiport system.
DISCUSSION
A Possible Site of NaCl-Induced Inactivation in the PS II Complex.
In the present study, we examined the NaCl-induced inactivation of the oxygen-evolving machinery in two types of cell in which the extent of unsaturation of fatty acids in membrane lipids differed. We demonstrated clearly that the unsaturation of fatty acids was important for the protection against salt-induced inactivation of the oxygen-evolving machinery of the PS II complex (Fig. 1). Because NaCl and LiCl, but not sorbitol, interfered with the photosynthetic evolution of oxygen (Fig. 1), it appeared that the salt-induced inactivation was due to the effects of cations and not of osmotic pressure.
The site of inactivation was examined in an experiment in vitro. NaCl interfered with the transport of electrons from H2O to DCIP but not from diphenylcarbazide (DPC) to DCIP. Because DPC donates electrons to P680 (16), it is probable that the oxygen-evolving machinery was inactivated during incubation of cells in the presence of NaCl. In previous studies in vitro (18, 19), we demonstrated that high concentrations of NaCl specifically dissociated the extrinsic proteins of the oxygen-evolving machinery of the PS II complex. It is likely that the extrinsic proteins of the oxygen-evolving machinery were dissociated under salt stress both in thylakoid membranes within cyanobacterial cells and in thylakoid membranes isolated from these cells. This dissociation is expected to result in inactivation of the oxygen-evolving machinery.
Protection of the Oxygen-Evolving Machinery Against NaCl-Induced Inactivation by Protein Synthesis.
Light at 50 μE⋅m−2⋅s−1 reversed the NaCl-induced inactivation of the oxygen-evolving machinery (Figs. 2 and 3). This effect of light was eliminated by lincomycin, an inhibitor of protein synthesis. Oxygen-evolving activity that had been lost during incubation of cells with NaCl in darkness was restored in light, but this restoration was prevented by lincomycin (Fig. 3A). Restoration of activity was also achieved in darkness by the addition of glucose to cultures, which accelerated respiration. Again, lincomycin prevented such restoration of activity (Fig. 3B). These results suggest that light and respiration allowed production of high-energy compounds, such as ATP, which allowed the synthesis de novo of the proteins necessary for reactivation of the oxygen-evolving machinery. Removal of NaCl from the medium in light resulted in reactivation of the oxygen-evolving machinery, and this effect was eliminated by lincomycin (Fig. 3C). These results suggest that the synthesis of proteins was inhibited in the presence of NaCl and that the energy produced by photosynthesis and respiration overcame the NaCl-induced inhibition of protein synthesis.
The previous study by Hagemann et al. (20) on the synthesis of proteins in Synechocystis sp. PCC 6803 under salt conditions indicated that a high concentration of NaCl inhibited the synthesis of most proteins but specifically induced the synthesis of several proteins that might be related to salt stress. Our results in the present study are consistent with these observations.
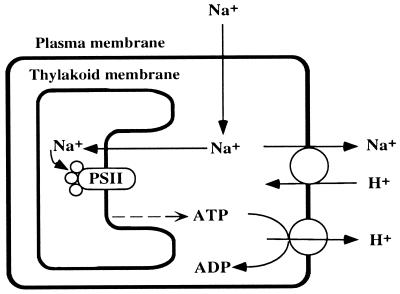
A Schematic Explanation of the Effects of NaCl.
Our results can be explained by the Na+/H+ antiport system in Fig. 6, which includes cooperation of H+-ATPase and Na+/H+ antiporter. The involvement of the H+-ATPase is suggested by the finding that the NaCl-induced inactivation of the oxygen-evolving machinery was accelerated by N,N-dicyclohexylcarbodiimide, an inhibitor of H+-ATPase. The higher effect of LiCl than NaCl on the inactivation of the oxygen-evolving machinery (Fig. 1) suggests that the Na+/H+ antiporter contributes to the tolerance to salt, because this antiporter does not pump out Li+ as efficiently as Na+ (15). When the extracellular concentration of Na+ increases, Na+ ions leak through the plasma membrane into the cytosol. The Na+/H+ antiport system pumps out Na+, with consumption of ATP, to maintain a certain low cytosolic concentration of Na+. During incubation with NaCl in darkness, the supply of ATP becomes insufficient and, as a result, the Na+/H+ antiport system becomes inoperative, with a resultant increase in the cytosolic concentration of Na+. Then Na+ ions leak through the thylakoid membranes to increase the concentration of Na+ in the intrathylakoid space (lumen) to inactivate the oxygen-evolving machinery. When ATP (or energy) is produced by photosynthesis or by respiration, the Na+/H+ antiport system is reactivated and decreases cytosolic and intrathylakoid concentrations of Na+.
Figure 6.
A schematic explanation of the effects of Na+ ions and the unsaturation of fatty acids in membrane lipids on the oxygen-evolving activity of the PS II complex in the cyanobacterial cells.
As noted above, the presence of NaCl inhibited protein synthesis in darkness and the energy produced by photosynthesis or respiration enhanced protein synthesis. It is likely that the Na+/H+ antiport system is the relevant protein(s) that is synthesized with the energy from photosynthesis or respiration and that this system is responsible for the maintenance of oxygen-evolving activity. Our scheme explains why light or glucose each so dramatically restored oxygen-evolving activity. Synechocystis sp. PCC 6803 has five genes for putative Na+/H+ antiporters (21). The genes responsible for maintenance of the Na+/H+ antiport system and for the protective effects of photosynthesis and respiration on the oxygen-evolving machinery remain to be identified.
Possible Sites Affected by the Unsaturation of Fatty Acids in Membrane Lipids.
The desA−/desD− cells were more sensitive to NaCl and less able to recover from its effects than were wild-type cells (Figs. 1–3). This observation can be explained as follows. (i) The oxygen-evolving machinery in thylakoid membranes isolated from the desA−/desD− cells was more sensitive to NaCl than that from wild-type cells, suggesting that the unsaturation of fatty acids in membrane lipids might act directly to protect the oxygen-evolving machinery against salt-induced inactivation. (ii) The activity of the Na+/H+ antiport system was higher in the wild-type cells than in the desA−/desD− cells (Fig. 4), suggesting that the unsaturation of fatty acids in membrane lipids might activate the Na+/H+ antiport system by means of enhanced fluidity of the membrane. This hypothesis is supported by results, obtained in studies of other membrane systems, indicating that the activities of various membrane-bound enzymes can change with changes in membrane fluidity (22). (iii) The Na+/H+ antiport activity was more sensitive to NaCl in desA−/desD− cells than in wild-type cells (Fig. 4), and the recovery of Na+/H+ antiport activity in wild-type cells was much more efficient than in desA−/desD− cells (Fig. 5). These findings suggest that the unsaturation of fatty acids might also stimulate synthesis of the protein(s) in the Na+/H+ antiport system.
Acknowledgments
We are grateful to Dr. M. Hagemann (Rostock University, Germany) for his kind gift of GG and his critical reading of the manuscript. We also thank Dr. G. Papageorgiou (National Research Center for Scientific Research, Demokritos, Greece) for his helpful discussion of the manuscript. This work was supported, in part, by a Grant-in-Aid for Specially Promoted Research (no. 08102011) from the Ministry of Education, Science and Culture, Japan, and by the National Institute for Basic Biology Cooperative Research Program on the Stress Tolerance of Plants.
ABBREVIATIONS
- E
einstein
- Chl
chlorophyll
- GG
glucosylglycerol
- PS
photosystem
- BQ
1,4-benzoquinone
- DCIP
dichloroindophenol
References
- 1.Nishida I, Murata N. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:541–568. doi: 10.1146/annurev.arplant.47.1.541. [DOI] [PubMed] [Google Scholar]
- 2.Sato N, Murata N. Biochim Biophys Acta. 1980;619:353–366. doi: 10.1016/0005-2760(80)90083-1. [DOI] [PubMed] [Google Scholar]
- 3.Berry J, Bjorkman O. Annu Rev Plant Physiol. 1980;31:491–543. [Google Scholar]
- 4.Quinn P J, Williams W P. Biochim Biophys Acta. 1983;737:223–266. [Google Scholar]
- 5.Murata N, Wada H. Biochem J. 1995;308:1–8. doi: 10.1042/bj3080001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tasaka Y, Gombos Z, Nishiyama Y, Mohanty P, Ohda T, Ohki K, Murata N. EMBO J. 1996;15:6416–6425. [PMC free article] [PubMed] [Google Scholar]
- 7.Wada H, Murata N. Plant Cell Physiol. 1989;30:971–978. [Google Scholar]
- 8.Ono T, Murata N. Plant Physiol. 1981;67:176–181. doi: 10.1104/pp.67.1.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mamedov M, Hayashi H, Wada H, Mohanty P, Papageorgiou G, Murata N. FEBS Lett. 1991;294:271–274. doi: 10.1016/0014-5793(91)81446-f. [DOI] [PubMed] [Google Scholar]
- 10.Mikkat S, Effmert U, Hagemann M. Arch. Microbiol. 1997;167:112–118. [PubMed] [Google Scholar]
- 11.Schoor A, Erdmann N, Effmert U, Mikkat S. J Chromatography A. 1995;704:89–97. [Google Scholar]
- 12.Arnon D I. Plant Physiol. 1949;24:1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blumwald E, Wolosin T M, Packer K. Biochem Biophys Res Commun. 1984;122:452–459. doi: 10.1016/0006-291x(84)90497-2. [DOI] [PubMed] [Google Scholar]
- 14.Papageorgiou G C, Alygizaki-Zorba A. Biochim Biophys Acta. 1997;1335:1–4. doi: 10.1016/s0304-4165(96)00156-0. [DOI] [PubMed] [Google Scholar]
- 15.Padan E, Schuldiner S. Biochim Biophys Acta. 1994;1185:129–151. doi: 10.1016/0005-2728(94)90204-6. [DOI] [PubMed] [Google Scholar]
- 16.Yamashita T, Butler W L. Plant Physiol. 1969;44:435–438. doi: 10.1104/pp.44.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Erdmann N, Berg C, Hagemann M. Arch Hydrobiol. 1989;144:521–530. [Google Scholar]
- 18.Miyao M, Murata N. Biochim Biophys Acta. 1983;725:87–93. [Google Scholar]
- 19.Murata N, Miyao M. Trends Biochem Sci. 1985;10:122–124. [Google Scholar]
- 20.Hagemann M, Techel D, Rensing J. Arch Microbiol. 1991;155:587–592. [Google Scholar]
- 21.Kaneko T, Sato S, Kotani H, Tanaka A, Asamizu E, Nakamura Y, Miyajima N, Hirosawa M, Sugiura M, Sasamoto S, et al. DNA Res. 1996;3:109–136. doi: 10.1093/dnares/3.3.109. [DOI] [PubMed] [Google Scholar]
- 22.Kamada Y, Jung U S, Piotrowski J, Levin D E. Genes Dev. 1995;9:1559–1571. doi: 10.1101/gad.9.13.1559. [DOI] [PubMed] [Google Scholar]
- 23.Stanier R Y, Kunisawa R, Mandel M, Cohen-Bazire G. Bacteriol Rev. 1971;35:171–205. doi: 10.1128/br.35.2.171-205.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]