Abstract
Herein we characterized various genetic markers and the biological behavior of a natural recombinant strain of Toxoplasma gondii (P-Br). From nine genetic markers analyzed, three (B1, ROP1, and SAG1) and three (cS10-A6, GRA6, and SAG3) markers belong to parasites from the type I and type III lineages, respectively. The SAG2 and L363 loci were shown to be type I-III chimera alleles. The cB2l-4 microsatellite marker showed a unique haplotype. The P-Br strain presented low virulence in the acute phase of infection and was cystogenic during the chronic infection. The interleukin 12/gamma interferon axis and inducible nitric oxide synthase were main determinants of resistance during the acute infection with the P-Br strain. As opposed to infection with the type II strain of T. gondii (ME-49), peroral infection with the P-Br strain led only to a light inflammatory infiltrate and no major lesions in the intestine of the C57BL/6 mice. In addition, the BALB/c (resistant to ME-49) and C57BL/6 (susceptible to ME-49) mice were shown, respectively, to be more susceptible and more resistant to cyst formation and toxoplasmic encephalitis when infected with the P-Br strain. Further, the C57BL/KsJ and DBA2/J congenic strains containing major histocompatibility complex (MHC) haplotype “d” were more resistant than the parental strains (C57BL/6 and DBA1/J), when infected with the ME-49 but not with the P-Br strain. Together, our results indicate that resistance to cyst formation and toxoplasmic encephalitis induced during infection with P-Br is not primarily controlled by the MHC haplotype d, as previously reported for type II strains of T. gondii.
Toxoplasma gondii is a protozoan parasite, distributed worldwide, that has been known to infect more than 30 species of birds and 300 species of mammals, including humans (9). It is believed that one-third of the world population is chronically infected with this parasite (12). Toxoplasmosis is common and establishes itself as a lifelong chronic infection after consumption of undercooked meat harboring tissue cysts or from accidental ingestion of oocysts shed in cat feces. In most individuals the infection is asymptomatic, whereas severe pathology and lethality due to toxoplasmosis are a common finding in congenitally infected or immunodeficient individuals (8). In addition, toxoplasmosis is one of the most common causes of infectious uveitis in both imunocompetent and imunocompromised persons (24). In fact, ocular uveitis is found in 2 to 20% of the T. gondii-infected human population, depending on the geographic area (18, 38). Variation in the clinical presentation and severity of disease in susceptible persons has been attributed to several factors, including the genetic heterogeneity of the host and the genotype of the infective parasite (24, 27, 42).
Some aspects of host resistance to pathogenesis and lethality observed during infection with T. gondii have been elucidated, whereas the influence of parasite strains on outcome of disease during toxoplasmosis is largely unknown. Nevertheless, recent studies show that the structure of the T. gondii population is clonal, since most strains fall into one of the three categories of lineage denominated type I, type II and type III (27, 42). The type I lineage was shown to exclusively contain those strains that are highly virulent, whereas type II and type III strains display lower virulence in mice. A small percentage of strains are recombinant between two of three parasite lineages and vary in terms of their virulence phenotype in mice (20). Molecular clinical epidemiology studies have shown an association of certain parasite lineages and disease outcome in humans. For instance, most cases of reactivation of T. gondii infection in AIDS patients is associated with type II strains (27). In contrast, type I or recombinant type I-III strains are more often found in patients with ocular toxoplasmosis (22).
Different studies performed with mice show the important role of cytokines, such as IL-12, TNF-α, and IFN-γ, and generation of RNI as mediators of host resistance to early T. gondii infection (1, 7). Thus, animals deficient in IL-12, IFN-γ, and iNOS or those treated with neutralizing antibodies, anticytokines, or specific inhibitors of iNOS are highly susceptible to infection with T. gondii (14, 15, 23, 41, 44, 46). Acquired immunity to T. gondii is associated with a Th1-type response (14, 17). During chronic infection, neutralization of either IFN-γ or TNF-α results in the reactivation of disease and the development of TE (13, 14, 16, 45, 47). Further, other host genetic factors, including MHC alleles, are important determinants of host resistance and susceptibility to early infection, as well as controlling cyst numbers and encephalitis at later stages of infection with T. gondii in mice (2, 3, 4, 29, 30, 37). Consistently, both CD4+ T as well as CD8+ T lymphocytes are important components in host resistance to this parasite (4, 14, 17).
In the present study, we characterize a particular recombinant (type I-III) strain of T. gondii, named P-Br, which presents low virulence and is cystogenic in mice. We also determined some immunological components involved in host resistance to infection with this parasite strain. As previously shown for other strains of T. gondii, we found that the IL-12/IFN-γ axis and iNOS are important components of early resistance to the P-Br strain. More importantly, we demonstrate here that resistance to cyst formation and TE induced during infection with P-Br is not primarily controlled by the MHC haplotype “d,” as previously reported for type II strains of T. gondii (2, 4). Thus, our results show that the involvement of the MHC haplotype in host resistance to cyst formation and TE may also vary according to the lineage of the infective strain of T. gondii.
MATERIALS AND METHODS
Abbreviations.
CNS, central nervous system; IL-12, interleukin 12; INF-γ, gamma interferon; iNOS, inducible nitric oxide synthase; KO, knockout; MHC, major histocompatibility complex; RFLP, restriction fragment length polymorphism; RNI, reactive nitrogen intermediates; ROP, rhoptry protein; SAG, surface antigen; TE, toxoplasmic encephalitis; and TNF-α, tumor necrosis factor alpha.
Animals.
BALB/c, C3H/He, C57BL/6, DBA1/J, IL-12 KO, IFN-γ KO, and iNOs KO mice and the congenic mice (C57BL/KsJ and DBA2/J) were bred as homozygotes and were kept in the animal house of the Biological Sciences Institute, Federal University of Minas Gerais. The KO mice were all in the C57BL/6 background. Mice were maintained in microisolators to minimize infection with environmental pathogens. All animals used for the experiments were females or males aged 8 weeks, as indicated in the figure legends and tables, when appropriated.
Parasite strains.
The following representative strain types were used for standardization of PCR assays: strains RH (type I) (40), ME-49 (36), and PTG (32) (type II), as well as VEG (11) and CTG (39) (type III). ME-49 and P-Br (28) strains were maintained in Swiss female mice.
DNA isolation.
For DNA extraction, 106 tachyzoites were incubated in lysis buffer (10 mM Tris-HCl [pH 8.0], 0.1 M EDTA, and 0.5% sodium dodecyl sulfate) at 37°C. After 1 h of incubation, 100 μg of proteinase K (Promega Biosciences, Inc., San Luis Obispo, Calif.)/ml was added to tachyzoites solubilized in lysis buffer, following overnight incubation at 50°C. After inactivation of the proteinase K, we added phenol:chloroform for 15 min, following centrifugation for 300 × g for 15 min, and the supernatant precipitated with sodium acetate (3 M) and ethanol. The suspensions were incubated during 1 h at −20°C and was centrifuged for 15 min at 4°C. The DNA samples were resuspended in ethanol (70%) and were centrifuged for 15 min at 4°C. The DNA precipitates were dissolved in milli-Q-treated water and were stored at −20°C.
Genotype analysis.
The lineage type was determined by restriction fragment of amplified SAG1 (27), SAG2 (25), SAG3 (22), B1 (21), ROP1 (26), cB2l-4, cS10-A6, GRA6, and L363. The mix PCRs consisted of 60 ng of DNA, 0.2 mM deoxynucleoside triphosphate, 3 μl of 10× PCR buffer, 50 mM MgCl2, 1.5 U of Taq DNA polymerase (Cenbiot, Porto Alegre, Brazil), and 5 pmol of each primer in a final volume of 30 μl. The PCRs were performed in a thermal cycler. The first step of amplification was 3 min of denaturation at 94°C. This step was followed by 30 cycles at 94°C for 60 s, and the annealing step temperature for each pair of primers consisted of 67°C for SAG1, 62°C for SAG2, 65°C for B1, 66°C for ROP1, and 55°C for CB21-4, cS10-A6, GRA6, L363, and SAG3, followed by incubation at 72°C during 60 s. The final cycle was followed by an extension step of 10 min at 72°C. Amplification and generation of polymorphic restriction fragments from each cDNA were performed by using the following primers: 5′CAATGTGCACCTGTAGGAAGC 3′ and 5′CAACGGTAATCACTCACGCG 3′ for SAG1, producing a fragment 1,183 bp long; 5′GAAATGTTTCAGGTTGCTGC3′ and 5′AACGTTTCACGAAGGCACAC3′ for SAG2, producing a fragment 1,310 bp long; 5′ACCCATCTGCGAAGAAAACG3′ and 5′ATTTCGACCAGCGGGAGCAC3′ for SAG2, producing a 546-bp fragment; 5′CAACTCTCACCATTCCACCC3′ and 5′GCGCGTTGTTAGACAAGACA3′ for SAG3, producing a 311-bp fragment; 5′TGTCTGTCCTATCGCAACG3′ and 5′ACGGATGCAGTTCCTTTCTG3′, for B1, producing a 577-bp fragment; 5′CGTGACATATTACTGCACTGACG3′ and 5′ACCATCTGGAAACTCGATCAC3′ for ROP1, producing a 1,346-bp fragment; 5′CCAGGTGTTTCGATATTGAT3′ and 5′GCCTGTGTGGTGTTCGAATC3′ for CB21-4, producing a 503-bp fragment; 5′CTGGTTACATTTTCGCCTATCA3′ and 5′CCTAGTCCAAACTAGGGCTTGA3′, for cS10-A6, producing a 341-bp fragment; 5′ATTTGTGTTTCCGAGCAGGT3′ and 5′TCGCCGAAGAGTTGACATAG3′, for GRA6, producing a 351-bp fragment; and 5′GGCTATTCGGCAAACAACAC3′ and 5′GCAATCCAGTGAGTCACCAA3′ for L363, producing a 505-bp fragment. The PCR products and the restriction fragments were fractionated in either 6% acrylamide or a 2.5% agarose gel.
DNA sequencing and analysis.
The codifying region of the SAG1, SAG2 and SAG3 genes of P-Br were amplified. The amplification products were cloned into PCR4-Topo vector (Invitrogen, Carlsbad, Calif.) and were sequenced by using the DYEnamic Et Dye Terminator Cycle Sequencing kit for MegaBace (Amershan Pharmacia Biotech, Little Chalfont, Buckinghamshire, United Kingdom). We performed a homology search by using the National Center for Biotechnology Information gene bank database.
Experimental infections.
The P-Br strain of T. gondii, isolated from a dog in Brazil (28), and the ME-49 strain of T. gondii, isolated from a sheep (36), were used to infect mice in the experiments described in this study. Cysts were harvested from Swiss mice that had been inoculated 2 months beforehand with approximately 10 cysts, by the oral route. The mice were sacrificed by cervical dislocation; the brains were removed and homogenized in 1 ml of phosphate-buffered saline, pH 7.2. For experimental infections, mice received 4, 20, or 100 cysts in a volume of 0.2 ml orally.
Splenocyte cultures.
Mice infected with either the T. gondii ME-49 or P-Br strain were sacrificed on the 7th and 15th days postinfection and had their spleens removed. For controls we used spleens from uninfected mice (day 0). Suspensions of splenocytes were washed in RPMI medium and were treated for 2 min with lysing buffer (9 volumes of 0.16 M NH4Cl and 1 volumes of 0.17 M Tris-HCl, pH 7.5). The erythrocyte-free cells were then washed three times and were adjusted to 5 × 106 cells/ml in RPMI supplemented with 10% heat-inactivated fetal calf serum. The cell suspension was distributed (100 μl/well) in a 24-well tissue culture plate and was cultured with RPMI medium alone, for 48 h and 72 h at 37°C in 5% CO2. The supernatants were subsequently collected for cytokine and nitrite measurements.
Nitrite concentrations.
The nitrite concentration in the cell culture supernatants were measured by using the Griess assay (19). The levels of nitrite in the culture supernatants after 48 h was assayed in a 96-well microplate by mixing 0.1 ml of culture supernatant with 0.1 ml of Griess reagent. The plates were read for absorbance at 490 nm after 10-min incubations, and the nitrite concentrations were determined in reference to a standard curve of 1 to 500 μM NaNO2.
Cytokine measurement.
IL-10 and TNF-α were quantified in 48-h supernatants of the spleen cells by using the Duo Set kit (R&D Systems, Minneapolis, Minn.). IL-12 (p70) and IFN-γ were quantified in 72-h supernatants of the spleen cells by using the enzyme-linked immunosorbent assay DuoSet kit (Genzyme, Cambridge, Mass.). The development was made with streptavidin-peroxidase conjugate. The plates were read at 405 nm and concentration calculated in reference to a standard curve employing respective recombinant cytokines.
Histopathology and immunocytochemistry.
A histological examination was carried out in the brain and intestine of mice. The brain was evaluated 45 days after infection. The intestine was evaluated 7 days postinfection. Following processing, the samples were set in paraffin and were sectioned in slices of 4-μm width. The material was stained with hematoxylin-eosin, subjected to immunocytochemical analysis, and examined under a light microscope. For immunocytochemistry, deparaffinized sections were incubated for 30 min at 37°C in 2% unlabeled sheep serum to reduce nonspecific binding and were then incubated in polyclonal rabbit antibody against whole parasites of strain P-Br of T. gondii at 4°C overnight. Secondary biotinylated antibodies were sheep anti-rabbit antibodies. The sensitivity was improved with the avidin-biotin technique (ABC kit, PK-4000; Vector Laboratories, Inc., Burlingame, Calif.). The reaction was visualized by incubating the section with 3,3-diaminobenzidine tetrahydrochloride (Amresco, Solon, Ohio) for 5 min. Control slides were incubated in the unrelated rabbit serum. The slides were studied with an Olympus microscope and were photographed with Kodak film (100 ASA).
Statistical analysis.
The statistical significance of differences between survival curves in 15, 30, and 45 days postinfection, as well as cyst numbers, was determined by the Kruskal-Wallis nonparametric test. The intensity of inflammatory reaction in the brain or small intestine, as well as cytokine and nitrite levels in the supernatants from cultures of splenocyte obtained from different groups of mice, was determined by analysis of variance. For all statistical tests mentioned above, the difference was considered statistically significant when P was < 0.05.
RESULTS
P-Br is a recombinant (type I-III) strain of T. gondii.
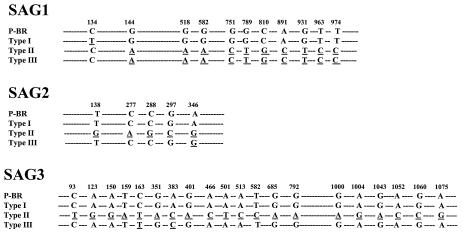
To identify the alleles carried by different genes or regions present in the genome of P-Br strain of T. gondii, different restriction endonucleases defined in Table 1 were used to digest B1, cS10-A6, GRA6, L363, ROP1, SAG1, SAG2, and SAG3 amplicons. Strains RH and P-Br exhibited identical restriction patterns for B1, CB21-4, ROP1, and SAG1, and this was different from ME-49/PTG (type II) and VEG/CTG (type III) strains. These findings indicate that P-Br possesses a type I haplotype for several loci. In contrast, for cS10-A6, GRA6, and SAG3, strain P-Br displayed a genotype identical to that of the CTG strain and differed from RH and PTG strains, indicating that P-Br has haplotype III at these loci. Interestingly, for L363 and SAG2 loci, the P-Br strain has a mixture of haplotypes seen in type I and III strains. In the case of SAG2, we used four different restriction enzymes, since HhaI, HinfI, and TaqI yielded fragments identical to type 1, whereas digestion with Sau3AI generated a type III allele. For the cB21-4 microsatellite marker, the RFLP generated a unique haplotype. The sequencing of the coding region from the SAG1, SAG2, and SAG3 genes showed that nucleotide sequences from P-Br strain presented higher to lower homology to genes from RH (type I), VEG (type III), and ME-49 (type II) (Fig. 1).
TABLE 1.
Genotyping of SAG1, SAG2, SAG3, B1, ROP1, cB21-4, cS10-A6, GRA6, and L363 loci from T. gondii strains
| Locus | Endonuclease(s) | Allele type
|
||||
|---|---|---|---|---|---|---|
| RH (I) | ME49 or PTG (II) | VEG or CTG (III) | P-Br | P-Br haplotype | ||
| SAG1 | HaeII | 1 | 2 | 2 | 1 | I |
| DdeI | 1 | 2 | 2 | 1 | ||
| SAG2 | HhaI | 1 | 2 | 3 | 1 | Unique (combination of I-III) |
| Sau3aI | 1 | 2 | 3 | 3 | ||
| TaqI | 1 | 2 | 1 | 1 | ||
| HinfI | 1 | 1 | 2 | 1 | ||
| SAG3 | NciI | 1 | 2 | 3 | 3 | III |
| B1 | XhoI | 1 | 2 | 2 | 1 | I |
| PmlI | 1 | 2 | 2 | 1 | ||
| ROP1 | DdeI | 1 | 2 | 2 | 1 | I |
| HhaI | 1 | 2 | 2 | 1 | ||
| cB21-4 (microsatellite and RFLP marker) | HaeIII | 1 | 2 | 3 | 4 | Unique |
| cS10-A6 | RsaI | 1 | 1 | 2 | 2 | III |
| HpyCH4IV | 1 | 2 | 1 | 1 | ||
| GRA6 | MseI | 1 | 2 | 3 | 3 | III |
| L363 | HpaII | 1 | 2 | 2 | 1 | Unique (combination of I-III) |
| HpyCH4IV | 1 | 1 | 2 | 2 | ||
FIG. 1.
The coding region of the SAG1 (GenBank accession no. AY187278) gene from strain P-Br presented 99.9, 97, and 97% homology with SAG1 from RH, ME-49, and VEG, respectively. The coding region of the SAG2 gene (GenBank accession no. AY187279) from P-Br presented 100, 98, and 99% homology with SAG2 from RH, ME-49, and VEG, respectively. The coding region of the SAG3 gene (GenBank accession no. AY187280) from P-Br presented 100, 98, and 99% homology with SAG1 from RH, ME-49, and VEG, respectively. Underlined letters indicate natural nucleotide mutations of type I, type II, and type III standard strains in relation to the sequence of the same DNA from the P-Br strain.
Distinct biological behavior of strain P-Br of T. gondii in isogenic mouse strains.
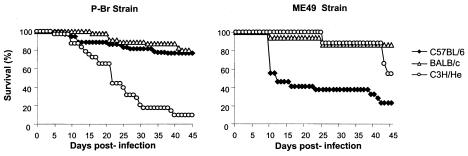
Peroral infection of BALB/c, C3H/He, and C57BL/6 mice with four cysts from the P-Br strain resulted, at 45 days postinfection, in 81, 9, and 76% survival, respectively. In contrast, infection with ME-49, a well-established type II strain, in BALB/c, C3H/He, and C57BL/6 mice resulted in 87, 56, and 23% survival, respectively (Table 2; Fig. 2). Thus, strain P-Br exhibited high virulence in C3H/He and low virulence in the BALB/c and C57BL/6 mice, whereas strain ME-49 exhibited high virulence in C57BL/6, moderate virulence in C3H/He, and low virulence in BALB/c mice.
TABLE 2.
Histopathological analysis of the CNS and small intestine of female BALB/c, C3H/HeJ, and C57BL/6 mice after peroral infection with four cysts of strain P-Br or ME-49 of T. gondiia
| Mouse type | Oral infection results for each strain used
|
|||||||
|---|---|---|---|---|---|---|---|---|
| P-Br
|
ME-49
|
|||||||
| No. of animalsb,f evaluated | No. of cystsc | TEd | SIe | No. of animals evaluated | No. of cysts | TE | SI | |
| BALB/c H-2d | 22/27 (aA) | 727 ± 358 (aA) | +++ | + | 28/32 (aA) | 202 ± 71 (aB) | + | + |
| C3H/He H-2k | 3/35 (bB) | 13,925 ± 2,015 (cA) | ++++ | + | 9/16 (aA) | 28,141 ± 15,285 (bA) | ++++ | + |
| C57BL/6 H-2b | 41/54 (aA) | 151 ± 40.5 (bA) | + | + | 5/22 (bB) | 4,175 ± 3,103 (bB) | +++ | +++ |
Mice were perorally infected with four cysts of the ME-49 or P-Br strain of T. gondii. These data are pooled from three to five experiments for each mouse-parasite combination, which yielded similar results.
Indicates the number of survivors out of the total number of animals used in the experiment. All the survivors were infected, as confirmed by the presence of cysts in the brain.
The number of brain cysts and intensity of TE were evaluated at 45 days postinfection.
Two sagittal sections from each mouse were examined and scored for pathological changes, by hematoxylin-and-eosin staining. +, mild; ++, moderate; +++, severe; ++++, very severe.
The small intestine (SI) was evaluated after 7 days postinfection and was scored for pathological changes by hematoxylin-and-eosin staining.
Different lowercase letters indicate statistically significant differences between mouse lineages infected with the same strain, while different capital letters indicate statistically significant differences between forms of behavior of parasite strains within the same mouse lineage, as indicated by the Kruskal-Wallis test (P < 0.05).
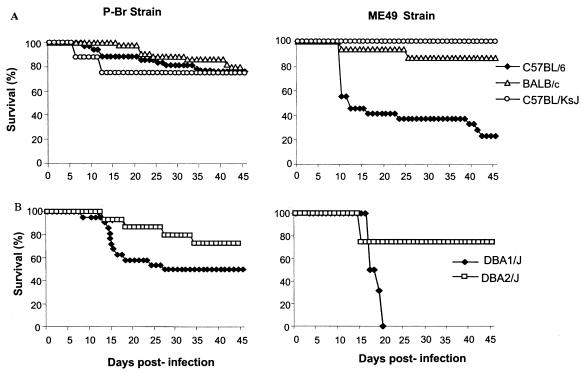
FIG. 2.
Survival of female BALB/c, C3H/He, and C57BL/6 mice after peroral infection with four cysts of either strain P-Br or ME-49 of T. gondii. Infection with P-Br resulted in low (3 of 35 mice), high (22 of 27 mice), and high (41 in 54 mice) survival in C3H/HeJ, BALB/c and C57BL/6 mice, respectively. Infection with ME-49 resulted in low (5 of 22), moderate (9 of 16), and high (28 of 32) survival in C57BL/6, C3H/He, and BALB/c mice, respectively. The experiment was repeated three to five times and provided similar results. The presented results are pooled from the experiments for each parasite and mouse lineage combination. All the survivors were infected, as confirmed by the presence of cysts in the brain. The significance of survival rate differences between lineages infected with the same strain and of differences between strains in terms of behavior in the same mouse lineage was determined by the Kruskal-Wallis test (P < 0.05) 15, 30, and 45 days postinfection. This analysis indicates that the survival curve of C3H/He mice was statistically different from those of C57BL/6 and BALB/c mice infected with P-Br, that the survival curve of C57BL/6 mice was statistically different from those of C3H/He and BALB/c mice infected with the ME-49 strain, and that the survival curves of C3H/He and C57BL/6 mice were statistically significant when mice infected with P-Br and ME-49 were compared.
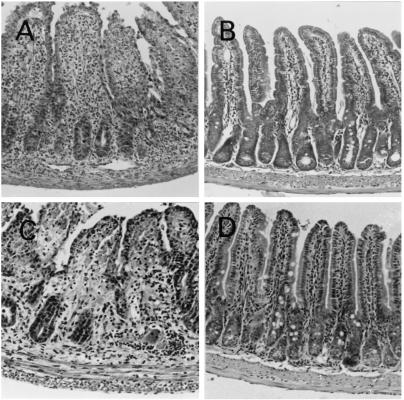
In terms of cyst numbers and TE, the C3H/He mice showed high susceptibility to infection with either the P-Br or ME-49 strain of T. gondii. As expected, BALB/c and C57BL/6 mice were resistant and highly susceptible to cyst formation and TE, when infected with the ME-49 strain. Surprisingly, the BALB/c mice were more susceptible to cyst formation and TE than C57BL/6 mice, when infected with the P-Br strain. It is also known (35) that one of the main pathologies observed in C57BL/6 mice acutely infected with ME-49 is necrosis in the intestinal mucosa. Here, we compared the ability of the P-Br and ME-49 strains to cause lesions in the intestinal tissues. The results shown in Fig. 3 and Table 2 reveal that strain ME-49 but not P-Br causes intense tissue pathology in the intestinal mucosa from C57BL/6 mice. Neither the ME-49 or P-Br strain causes large lesions in the intestine of BALB/c or C3H/HeJ mice.
FIG. 3.
Histological changes in the ilea of C57BL/6 mice infected perorally with T. gondii. Animals were infected with 4 (A and B) or 20 (C and D) cysts of either the ME-49 (left) or P-Br (right), and histological analyses of small intestine were performed at 7 days postinfection. The villi clearly visible in panels B and D are almost indiscernible in panels A and C due to inflammatory infiltration and initial stages of necrosis in mice that were infected with ME-49. Six sections of the entire length of small intestine from each mouse were examined. Five mice were used for each group. Slides were stained with hematoxylin and eosin. Magnification, ×60.
To further confirm the reverse phenotype of the P-Br strain in the BALB/c and C57BL/6 mice, we performed a second set of experiments. BALB/c and C57BL/6 mice were infected with 4, 20, or 100 cysts of each parasite strain. Infection of BALB/c mice with 4, 20, and 100 cysts of P-Br resulted in 100, 100, and 66% survival, respectively. Similar results were obtained in terms of mortality in the C57BL/6 mice, except that only 10% of the mice died when infected with 100 cysts of the P-Br strain. Infection with 4, 20, and 100 cysts of the ME-49 strain resulted in 100, 80, and 70% survival in the BALB/c mice, respectively. In contrast, we observed 23, 0, or 0% survival in C57BL/6 infected with 4, 20, or 100 cysts of the ME-49 strain (Table 3). The severity of pathology (inflammation and necrosis) in the small intestine did correlate with the intensity of intestinal tissue parasitism in BALB/c and C57BL/6 after peroral infection with 4, 20, or 100 cysts of either P-Br or ME-49. Indeed, the intestinal mucosa of C57BL/6 presented from 5- to 15-fold more parasites when infected with the ME-49 than when infected with the same dose of P-Br. Importantly, giving higher doses (i.e., 20 or 100 cysts) of cysts from P-Br to BALB/c mice resulted in a dramatic increase of cysts in the brain, at 45 days postinfection. In contrast, increasing the number of infective cysts of ME-49 and P-Br to BALB/c and C57BL6 mice, respectively, resulted in a rather small increment of cysts in the CNS at 45 days postinfection.
TABLE 3.
Analysis of CNS and small intestine of female BALB/c and C57BL/6 mice after peroral infection with 4, 20, or 100 cysts of strain P-Br or ME-49 of T. gondiia
| Mouse type | No. of cysts (used to infect mice) | Results for strain:
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| P-Br
|
ME-49
|
||||||||
| Rate of survivalb,d (%) | SIc (immuno- cytochemistry) | SIc (H&E) | No. of cysts (CNS) | Rate of survival (%) | SI (immuno- cytochemistry) | SI (H&E) | No. of cysts (CNS) | ||
| BALB/c H-2d | 4 | 100 (aA) | 3.3 ± 5.8 (aA) | + | 420 ± 334 (aA) | 100 (aA) | 3.7 ± 1.2 (aA) | + | 131.2 ± 75 (aB) |
| 20 | 100 (aA) | 3 ± 4.2 (aA) | + | 1,077 ± 794 (a, bA) | 80 (aA) | 23.3 ± 14.6 (bB) | ++ | 266.6 ± 28.8 (aB) | |
| 100 | 66 (aA) | 18 ± 11.3 (aA) | + | 8,140 ± 6,264 (cA) | 70 (aA) | 42.5 ± 12 (bB) | ++ | 707 ± 287 (bB) | |
| C57BL/6 H-2b | 4 | 100 (aA) | 2.7 ± 3.8 (aA) | + | 120 ± 44 (dA) | 23 (bB) | 17.5 ± 7.8 (bB) | +++ | 4,325 ± 2,015 (cB) |
| 20 | 100 (aA) | 4 ± 1.4 (aA) | + | 195 ± 92 (d) | 0 (cB) | 38.3 ± 6.4 (bB) | ++++ | *e | |
| 100 | 90 (aA) | 10 ± 3.5 (aA) | + | 361 ± 134 (a,d) | 0 (cB) | 147.3 ± 3.8 (cB) | +++++ | * | |
Mice were perorally infected with 4, 20, or 100 cysts of the ME-49 or P-Br strain of T. gondii. Five mice from each strain were examined per group.
Percentage of survival and the number of brain cysts in BALB/c (9 animals per group) and C57BL/6 (10 animals per group) mice infected with 4, 20, or 100 cysts of strain P-Br or ME-49 strain of T. gondii 45 days postinfection.
Small intestine (SI) was cut and rolled on itself in order to make a “Swiss roll.” Tissue parasitism was visualized by immunocytochemistry. The score of tissue parasitism was obtained by counting the number of microscopic fields (1 × 400) per histopathological section from the entire small intestine infected by T. gondii. A total of six sections was examined for each animal. SI was evaluated 7 days postinfection. H&E, hematoxylin and eosin.
Different lowercase letters indicate statistically significant differences between doses of infective cysts in mouse lineages infected with the same parasite strain, while different capital letters indicate statistically significant differences between forms of behavior of parasite strains within the same mouse lineage, as indicated by analysis of variance (P < 0.05).
*, all animal succumbed to infection by day 45 postinfection. These data are from one representative experiment.
Role of IL-12/IFN-γ axis and iNOS in early resistance to the P-Br strain of T. gondii.
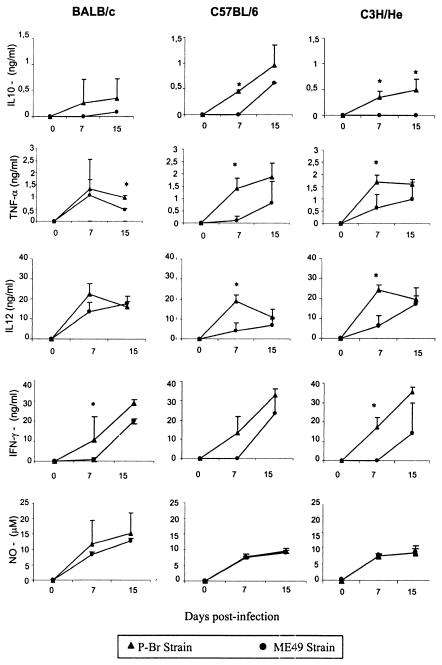
IL-12 has been postulated to play a key role in induction of Th1 responses, whereas IFN-γ and TNF-α are important cytokines in triggering effector mechanisms by macrophages and nonprofessional phagocytic cells. Production of IL-10 has also been shown to be stimulated during infection with T. gondii and to be involved in regulating the production of IL-12, IFN-γ, and TNF-α. To study the ability of P-Br to stimulate cytokine synthesis in vivo, we measured the production of cytokines 7 and 15 days postinfection by spleen cells from mice infected with T. gondii cultured in vitro in the absence of antigen (Fig. 4). Our experiments demonstrated high levels of these cytokines and RNI in the supernatants of splenocytes of BALB/c, C57BL/6, and C3H/He mice infected with either the P-Br or ME-49 strain. Interestingly, peroral infection with P-Br was shown to elicit earlier (7 days postinfection) IFN-γ, IL-12, IL-10, and TNF-α production than did infection with ME-49, which in most cases induced noticeable cytokine production only by 15 days postinfection. The kinetics of RNI produced by splenocytes was similar in mice infected with either the P-Br or ME-49 strain. The levels of RNI and cytokines produced by spleen cells from uninfected mice were below the limits of sensitivity of the assays employed in our study.
FIG. 4.
Production of IFN-γ, IL-10, IL-12, TNF-α, and RNI by spleen cells from BALB/c, C3H/He, and C57BL/6 mice after peroral infection with four cysts of either the P-Br or ME-49 strain of T. gondii. Cytokine and nitrite levels were evaluated 7 and 15 days after inoculation of the parasite. The values shown are the mean of three animals per data points. The experiment was repeated twice and provided similar results.
IL-12 KO, IFN-γ KO, and iNOS mice were infected with four cysts of P-Br, and their survival was monitored over a 45-day period. Mortality experiments for each mouse strain were repeated two times, and the data presented are representative of one experiment. Infection in IL-12 and IFN-γ KO mice resulted in 100% mortality around 15 to 20 days postinfection (data not shown). Infection of iNOS KO mice resulted in 20% survival. The survivor iNOS KO was sacrificed at 45 days postinfection and showed 4,100 cysts/brain. All C57BL/6-infected mice survived and showed an average of 90 cysts/brain.
MHC haplotype d is not a main determinant of mouse resistance to the P-Br strain of T. gondii.
The influence of MHC on survival and brain cyst numbers was examined in congenic mouse strains carrying the d haplotype. Experiments were performed to compare survival rates and number of brain cysts after peroral infection with the P-Br and ME-49 strains of T. gondii. The congenic strains named C57BL/KsJ and DBA2/J, containing MHC haplotype d, were more resistant (as measured by survival rates and cyst numbers) to infection by ME-49 than were the parental lineages (i.e., C57BL/6 and DBA1/J). In contrast, the presence of MHC haplotype d in congenic strains compared to its presence in parental strains did not affect resistance and/or susceptibility to cyst formation or rates of survival of acute infection in animals infected with strain P-Br. In female BALB/c, C57BL/6, and C57BL/KsJ mice infected with P-Br, we observed, respectively, 81, 76, and 75% survival at 45 days postinfection. In contrast, after infection with ME-49, we observed in female BALB/c, C57BL/6, and C57BL/KsJ mice 87, 23, and 100% survival, respectively (Table 4; Fig. 5). Female DBA1/J (haplotype q) mice presented high and moderate susceptibility to infection with the ME-49 and P-Br strains, respectively, displaying 100 and 50% mortality before 30 days postinfection (Fig. 5). Female DBA2/J mice displayed high survival (80%) when infected with ME-49 or P-Br. The difference between survival curves was statistically significant only when DBA1/J and DBA2/J mice infected with ME-49 were compared. To measure the number of cysts in the DBA congenic strains, we used the male, which in general is more resistant to infection with T. gondii (30). Consistent with the results obtained from females, the male DBA1/J mouse presented significantly higher number of cysts when infected with the ME-49 strain (2,442 ± 741) than did the DBA2/J mouse, which presented on average 500 ± 223 cysts per animal at 45 days postinfection. In DBA1/J and DBA2/J mice infected with the P-Br strain, we observed 1,035 ± 275 and 783 ± 377 cysts in the brain, respectively.
TABLE 4.
Number of cysts in the brain of BALB/c, C57BL/6, C57BL/BKsJ, DBA1/J, and DBA2/J mice after peroral infection with four cysts of strains P-Br and ME-49 of T. gondii
| Expt no. and mouse type | Results for:
|
|||
|---|---|---|---|---|
| Strain P-Bra
|
Strain ME-49a
|
|||
| No. of animals evaluatedb,d | No. of cystsc | No. of animals evaluatedb | No. of cystsc | |
| Expt 1 | ||||
| BALB/c H-2d (female) | 22/27 (aA) | 727.6 ± 358 (aA) | 28/32 (aA) | 202 ± 71 (aB) |
| C57BL/6 H-2b (female) | 41/54 (aA) | 151 ± 40 (bA) | 5/22 (bB) | 4,175 ± 3,103 (bB) |
| C57BL/KsJ H-2d (female) | 6/8 (aA) | 243 ± 220 (bA) | 7/7 (aA) | 62.5 ± 17.7 (aA) |
| Expt 2 | ||||
| DBA1/J H-2q (female) | 11/22 (aA) | 1,525 ± 740 (aA) | 0/15 (bB) | *e |
| DBA2/J H-2d (female) | 11/15 (aA) | 725 ± 204 (aA) | 9/11 (aA) | 203 ± 5 (aB) |
Mice were perorally infected with four cysts of the ME-49 or P-Br strain of T. gondii. These data are pooled from three to five experiments for each mouse, parasite combination that yielded similar results.
Indicates the number of survivors out of total number of animals used in all experiments. All the survivors were infected, as confirmed by presence of cysts in the brain.
The number of brain cysts was evaluated at 45 days postinfection.
Different lowercase letters indicate statistically significant differences between mouse lineages infected with the same strain, while different capital letters indicate statistically significant differences between forms of behavior of parasite strains within the same mouse lineage, as indicated by the Kruskal-Wallis test (P < 0.05).
*, all animal succumbed by day 45 postinfection.
FIG. 5.
Survival of female BALB/c, C57BL/6, and C57BL/KsJ (A) and DBA1/J and DBA2/J (B) mice after peroral infection with four cysts of either strain P-Br or ME-49 of T. gondii. Infection with P-Br resulted in high survival of BALB/c (22 of 27), C57BL/6 (41 of 54), and C57BL/KsJ (6 of 8) mice, respectively. Infection with P-Br resulted in moderate survival of DBA1/J (11 of 22) and high survival of DBA2/J (11 of 15) mice. Infection with ME-49 resulted in low and high survival of C57BL/6 (5 of 22) and C57BL/KsJ (7 of 7) mice, as well as high survival of BALB/c (28 of 32) mice, respectively. Infection with ME-49 resulted in high and low survival of DBA2/J (9 in 11) and DBA1/J (0 in 15) mice, respectively. All the survivors were infected, as confirmed by the presence of cysts in the brain. The experiment was repeated three times and provided similar results. The results presented are the pooled data of three experiments. The significance of survival differences between lineages infected with the same strain and of differences between strains in terms of parasite behavior in the same mouse lineage was determined by the Kruskal-Wallis test (P < 0.05) in 15, 30, and 45 days postinfection. This analysis indicate that the survival curve of C57BL/6 mice was statistically different from those of C57BL/KsJ and BALB/c mice infected with the ME-49 strain, that the survival curve of DBA1/J mice was statistically different from that of DBA2/J mice infected with ME-49 strain, and that the survival curves of C57BL/6 mice were statistically significant when mice infected with P-Br and ME-49 were compared.
DISCUSSION
T. gondii strains can be divided in three main lineages based on various genetic markers (42). Studies with mice have shown that infection with each of the three lineages of T. gondii results in different outcomes: type I strains are highly virulent, whereas type II and III strains are relatively avirulent (42). Type I differs genetically by 1% or less from type II and type III (27). However, the main determinants that dramatically affect the virulence of different T. gondii strains in the host and pathogenesis of toxoplasmosis are poorly understood.
Type II strains of T. gondii appear to be dominant in U.S. territory and are frequently isolated from AIDS patients with TE (27). Interestingly, different studies suggest the involvement of type I and type I-III strains in the development of ocular disease. Thus, different studies show a higher prevalence of type I and type III strains in Brazil (10, 42), where the occurrence of acquired ocular toxoplasmosis is more frequent (43). Consistently, type I was identified as responsible for a toxoplasmosis outbreak associated with a high rate of development of acquired ocular disease (5). In addition, a recent study indicates a high frequency of type I as well as type I-III recombinant isolates from ocular lesions in patients from the United States (22). In the present report, we characterized a natural recombinant type I-III strain of T. gondii and established the involvement of cytokines as well as MHC haplotype in mouse resistance and susceptibility to infection with this particular parasite strain.
Different cytokines, such as IL-12, IFN-γ, and TNF-α, as well as RNI, have been shown to play an important role in resistance to early as well as late stages of infection with T. gondii parasites. Thus, IFN-γ inhibits replication of T. gondii within macrophages and somatic cells (7) and can confer protection against lethal Toxoplasma challenge (46). The action of IFN-γ appears to be only partially dependent on the production of RNI, and other mechanisms are apparently involved (6). In addition, the counterregulatory cytokine IL-10 has been shown to be essential in preventing an overwhelming and lethal immune response during acute infection with T. gondii (16).
Here we compared the ability of the ME-49 and P-Br strains to elicit cytokine response at the initial stage of infection, in different mouse lineages (BALB/c, C57BL/6, and C3H/He). Our results show that both parasite strains elicited type I cytokines (i.e., TNF-α, IL-12, and IFN-γ) as well as the counterregulatory cytokine IL-10. In addition, both parasite strains induced the production of high levels of RNI by spleen cells. However, we did not observe any major difference in the ability of ME-49 and P-Br strains to elicit cytokine production that could explain the different behavior of the studied T. gondii strains in the various inbred mouse lineages. Importantly, cytokine production by spleen cells elicited during infection with the P-Br strain was in most cases high by 7 days postinfection, whereas production of cytokine by ME-49 was mainly noticeable by day 15 postinfection. In addition, the cytokine production elicited by P-Br was higher than that elicited by ME-49 in all mouse lineages. To confirm the importance of these cytokines in host resistance to the P-Br strains, we used the IFN-γ KO, IL-12 KO, and iNOS KO mice. As previously shown for the ME-49 strain (6, 41, 48), all the KO mice employed here were highly susceptible to infection with the P-Br strain.
A well-known mechanism of pathogenesis and death during acute infection of C57BL/6 mice with the ME-49 strain of T. gondii is the inflammatory process and necrosis observed in the small intestine (35). These pathological effects are thought to be mediated at least in part by CD4+ T lymphocytes, TNF-α, IFN-γ, and RNI (33, 34, 35). Despite the similar (or even higher) cytokine and RNI levels produced in spleen cells from mice infected with P-Br, we observed no (or very little) pathology in the small intestine of C57BL/6 mice infected with P-Br strain compared to that found in the intestine of mice infected with ME-49. A possible explanation for these discrepant findings would be the intensity of intestinal tissue parasitism yielded during infection with each parasite strain. Indeed, we found that infection with the ME-49 always led to a much higher load of parasite in the small intestine. Thus, as previously postulated (34, 35), we favor the hypothesis that, even though the immune response is actively involved in this pathological process (33, 34, 35), the intensity of tissue parasitism is also an important component for eliciting inflammation and causing necrosis in the small intestine of C57BL/6 mice acutely infected with T. gondii.
Inbred strains of mice markedly differ in their susceptibility to peroral infection with T. gondii. The pattern of resistant, intermediately resistant, and susceptible strains of mice indicates that genetic regulation of resistance to peroral T. gondii infection is polygenic (2, 37). The relative pathogenicity of T. gondii can be influenced by route of inoculation age, gender, genotype of the host, and life cycle stage of the parasite as well as parasite strain (2, 30, 31). Brown and McLeod (4) reported the importance of MHC class I genes at H-2L in the regulation of formation of T. gondii cysts in brain of mice. The number of cysts that form after peroral infection with T. gondii is associated with the level of parasitemia in initial infection (37). Further, resistance to cyst formation and development of TE has been linked in H-2d mice to the MHC class I L gene (4, 45). Mortality is substantially greater in mice that have H-2b than in mice possessing an H-2d background (2, 3). In addition, mortality at the acute phase of infection appears to be controlled by MHC class II genes (30).
However, most in vivo studies described above have employed parasite strains of type II lineage. A recent study (29) shows that infection with the type II, but not type I, strain of T. gondii elicits a CD8+-lymphocyte-mediated Ld-restricted killing of parasite-infected targets. Herein, we evaluated the biological behavior in mice of a natural recombinant (type I-III) strain of T. gondii displaying relatively low virulence in mice and performed the experiments side by side with ME-49, which is a standard type II strain. Interestingly, we found that, while the C57BL/6 (H-2b) mice were highly resistant, BALB/c (H-2d) mice were more susceptible to cyst formation and TE induced during infection with P-Br. Furthermore, C57BL/6 mice infected with the P-Br strain were resistant to the typical intestinal lesions induced by infection with the ME-49 strain.
The involvement of MHC molecules (H-2b and H-2d) in host resistance and/or susceptibility to P-Br compared to results for the ME-49 strain of T. gondii was further investigated by using congenic mouse strains. These studies confirmed the importance and the lack of importance of the H-2d haplotype in mouse resistance to cyst formation and TE elicited during infection with the ME-49 and P-Br strains, respectively. Thus, C57BL/KsJ and DBA2/J mice containing the H-2d haplotype were highly resistant, compared to their progenitor strains C57BL/6 (H-2b) and DBA1/J (H-2q) infected with ME-49. In contrast, the resistance of C57BL/KsJ and DBA2/J was not affected when compared to C57BL/6 and DBA1/J mouse strains infected with the P-Br strain.
In conclusion, we showed in inbred mouse lineages that the balance of host resistance and/or susceptibility to cyst formation and TE infection with T. gondii also depends on the parasite strain. We demonstrated the importance of the IL-12/IFN-γ axis and iNOS in host resistance to a low-virulence recombinant strain type I-III of T. gondii. However, the difference in the ability to elicit cytokines appears not to justify the different behavior of the type II and type I-III T. gondii strains in inbred mice. Interestingly, our results indicate that MHC haplotype d is not primarily involved in host resistance to cyst formation and TE elicited during infection with T. gondii strain type I-III and therefore differs from resistance to type II strains (2, 4). Therefore, we believe that this study provides new information for understanding the importance of host and parasite genetic variability in pathogenesis and host resistance to infection with T. gondii.
Acknowledgments
We thank Catherine Ropert for helpful discussion, Carolina Damas Rocha as well as Júlio César Menezes Vieira for technical assistance, and Andrea Macedo for providing the DBA2/J and C57BL/KsJ mice. We are also grateful to Ivan Sampaio from the Department of Zootechnology (Veterinary School, Federal University of Minas Gerais) for advice and support on statistical analyses.
This work was supported by the Fundação de Amparo a Pesquisa do Estado de Minas Gerais (FAPEMIG—EDT 24000) and a grant from the National Institutes of Health (AI36629 to D.S.). R.T.G. and R.W.A.V. are Research Fellows from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). B.F. has a postdoctoral fellowship from the FIOCRUZ/CNPq program.
Editor: J. M. Mansfield
REFERENCES
- 1.Alexander, J., T. M. Scharton-Kersten, G. Yao, C. W. Roberts, F. Y. Liew, and A. Sher. 1997. Mechanisms of innate resistance to Toxoplasma gondii infection. Philos. Trans. R. Soc. Lond. B 352:1355-1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blackwell, J. M., C. W. Roberts, and J. Alexander. 1993. Influence of genes within the MHC on mortality and brain cyst development in mice infected with Toxoplasma gondii: kinetics of immune regulation in BALB H-2 congenic mice. Parasite Immunol. 15:317-324. [DOI] [PubMed] [Google Scholar]
- 3.Brown, C. R., C. S. David, S. J. Khare, and R. McLeod. 1994. Effects of human class I transgene on Toxoplasma gondii cyst formation. J. Immunol. 152:4537-4541. [PubMed] [Google Scholar]
- 4.Brown, C. R., and R. McLeod. 1990. Class I MHC genes and CD8+ T cells determine cyst number in Toxoplasma gondii infection. J. Immunol. 145:3438-3441. [PubMed] [Google Scholar]
- 5.Burnett, A. J., S. G. Short, J. Isaac-Renton, A. King, D. Werker, and W. R. Bowie. 1998. Multiple cases of acquired toxoplasmosis retinitis presenting in an outbreak. Ophthalmology 105:1032-1037. [DOI] [PubMed] [Google Scholar]
- 6.Collazo, C. M., G. S. Yap, S. Hieny, P. Caspar, C. G. Feng, G. A. Taylor, and A. Sher. 2002. The function of gamma interferon-inducible GTP-binding protein IGTP in host resistance to Toxoplasma gondii is Stat1 dependent and requires expression in both hematopoietic and nonhematopoietic cellular compartments. Infect. Immun. 70:6933-6939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Denkers, E. Y., and R. T. Gazzinelli. 1998. Regulation and function of T-cell-mediated immunity during Toxoplasma gondii infection. Clin. Microbiol. Rev. 11:569-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Desmonts, G., and J. Couvreur. 1974. Toxoplasmosis in pregnancy and its transmission to the fetus. Bull. N.Y. Acad. Med. 50:146-159. [PMC free article] [PubMed] [Google Scholar]
- 9.Dubey, J. P., and C. P. Beattie. 1988. Toxoplasmosis of animals and man. CRC Press, Boca Raton, Fla.
- 10.Dubey, J. P., D. H. Graham, C. R. Blackston, T. Lehmann, S. M. Gennari, A. M. A. Ragozo, S. M. Nishi, S. K. Shen, O. C. H. Kwok, D. E. Hill, and P. Thulliez. 2002. Biological and genetic characterization of Toxoplasma gondii isolates from chickens (Gallus domesticus) from São Paulo, Brazil: unexpected findings. Int. J. Parasitol. 32:99-105. [DOI] [PubMed] [Google Scholar]
- 11.Dubey, J. P. 1996. Infectivity and pathogenicity of Toxoplasma gondii oocysts for cats. J. Parasitol. 82:957-961. [PubMed] [Google Scholar]
- 12.Dubey, J. P. 1998. Advances in the life cycle of Toxoplasma gondii. Int. J. Parasitol. 28:1019-1024. [DOI] [PubMed] [Google Scholar]
- 13.Gazzinelli, R. T., I. Eltoum, T. A. Wynn, and A. Sher. 1993. Acute cerebral toxoplasmosis is induced by in vivo neutralization of TNF-alpha and correlates with the down-regulated expression of inducible nitric oxide synthase and other markers of macrophage activation. J. Immunol. 7:3672-3681. [PubMed] [Google Scholar]
- 14.Gazzinelli, R. T., F. T. Hakim, S. Hieny, G. M. Shearer, and A. Sher. 1991. Synergic role of CD4+ and CD8+ T lymphocytes in IFN-γ production and protective immunity induced by an attenuated Toxoplasma gondii vaccine. J. Immunol. 146:286-292. [PubMed] [Google Scholar]
- 15.Gazzinelli, R. T., M. Wysocka, S. Hayashi, E. Y. Denkers, S. Hieny, G. Caspar, G. Trinchieri, and A. Sher. 1994. Parasite-induced IL-12 stimulates early IFN-γ synthesis and resistance during acute infection with Toxoplasma gondii. J. Immunol. 153:2533-2543. [PubMed] [Google Scholar]
- 16.Gazzinelli, R. T., M. Wysocka, S. Hieny, T. Scharton-Kersten, A. Cheever, R. Kuhn, W. Muller, G. Trinchieri, and A. Sher. 1996. In the absence of endogenous IL-10, mice acutely infected with Toxoplasma gondii succumb to a lethal immune response dependent on CD4+ T cells and accompanied by overproduction of IL-12, IFN-gamma and TNF-alpha. J. Immunol. 157:798-805. [PubMed] [Google Scholar]
- 17.Gazzinelli, R. T., Y. Xu, A. Cheever, and A. Sher. 1992. Simultaneous depletion of CD4+ and CD8+ T lymphocytes is required to reactivate chronic infection with Toxoplasma gondii. J. Immunol. 149:175-179. [PubMed] [Google Scholar]
- 18.Glasner, P. D., C. Silveira, D. Kruszon-Moran, M. C. Martins, M. Burnier, Jr., S. Silveira, M. E. Camargo, R. B. Nussenblatt, R. A. Kaslow, and R. Belfort, Jr. 1992. An unusually high prevalence of ocular toxoplasmosis in southern Brazil. Am. J. Ophthalmol. 14:136-144. [DOI] [PubMed] [Google Scholar]
- 19.Green, L. C., S. R. Tannenbaum, and P. Goldman. 1981. Nitrate biosynthesis in the germfree and conventional rat. Science 212:56-58. [DOI] [PubMed] [Google Scholar]
- 20.Grigg, M. E., S. Bonnefoy, A. B. Hehl, Y. Suzuki, and J. C. Boothroyd. 2001. Success and virulence in Toxoplasma as the results of sexual recombination between two distinct ancestries. Science 294:161-165. [DOI] [PubMed] [Google Scholar]
- 21.Grigg, M. E., and J. C. Boothroyd. 2001. Rapid identification of virulent type 1 strains of the protozoan pathogen Toxoplasma gondii by PCR-restriction fragment length polymorphism analysis at the B1 gene. J. Clin. Microbiol. 39:398-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grigg, M. E., J. Ganatra, J. C. Boothroyd, and T. P. Margolis. 2001. Unusual abundance of atypical strains associated with human ocular toxoplasmosis. J. Infect. Dis. 184:633-639. [DOI] [PubMed] [Google Scholar]
- 23.Hayashi, S., C. C. Chan, R. Gazzinelli, and F. G. Roberge. 1996. Contribution of nitric oxide to the host parasite equilibrium in toxoplasmosis. J. Immunol. 4:1476-1481. [PubMed] [Google Scholar]
- 24.Holland, G. N. 1999. Reconsidering the pathogenesis of ocular toxoplasmosis. Am. J. Ophthalmol. 128:502-505. [DOI] [PubMed] [Google Scholar]
- 25.Howe, D. K., S. Honore, F. Derouin, and L. D. Sibley. 1997. Determination of genotypes of Toxoplasma gondii strains isolated from patients with toxoplasmosis. J. Clin. Microbiol. 6:1411-1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Howe, D. K., and L. D. Sibley. 1994. Toxoplasma gondii: analysis of different laboratory stocks of the RH strain reveals genetic heterogeneity. Exp. Parasitol. 2:242-245. [DOI] [PubMed] [Google Scholar]
- 27.Howe, D. K., and L. D. Sibley. 1995. Toxoplasma gondii comprises three clonal lineages: correlation of parasite genotype with human disease. J. Infect. Dis. 172:1561-1566. [DOI] [PubMed] [Google Scholar]
- 28.Jamra, L. M. F., and M. P. L. Vieira. 1991. Isolamento do Toxoplasma gondii de exsudato peritoneal e órgãos de camundongos com infecção experimental. Rev. Inst. Med. Trop. São Paulo 33:435-441. [PubMed] [Google Scholar]
- 29.Johnson, J. J., C. W. Roberts, C. Pope, F. Roberts, M. J. Kirisits, R. Estes, E. Mui, T. Krieger, C. R. Brown, J. Forman, and R. McLeod. 2002. In vitro correlates of Ld-restricted resistance to toxoplasmic encephalitis and their critical dependence on parasite strain. J. Immunol. 169:966-973. [DOI] [PubMed] [Google Scholar]
- 30.Johnson, J., Y. Suzuki, D. Mack, E. Mui, R. Estes, D. Chella, E. Skamene, J. Forman, and R. McLeod. 2002. Genetic analysis of influences on survival following Toxoplasma gondii infection. Int. J. Parasitol. 32:179-185. [DOI] [PubMed] [Google Scholar]
- 31.Johnson, L. L. 1994. Resistance to Toxoplasma gondii in mice infected as neonates or exposed in utero. Infect. Immun. 8:3075-3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kasper, L. H., and P. L. Ware. 1985. Recognition and characterization of stage-specific oocyst/sporozoite antigens of Toxoplasma gondii by human antisera. J. Clin. Investig. 75:1570-1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khan, I. A., J. D. Schwazman, T. Matsuura, and L. H. Kasper. 1997. A dichotomous role for nitric oxide during acute Toxoplasma gondii infection in mice. Proc. Natl. Acad. Sci. USA 94:13955-13960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liesenfeld, O., H. Kang, D. Park, T. A. Nguyen, C. V. Parkhe, H. Watanabe, T. Abo, A. Sher, J. S. Remington, and Y. Suzuki. 1999. TNF-α, nitric oxide and IFN-γ are all critical for development of necrosis in the small intestine and early mortality in genetically susceptible mice infected perorally with Toxoplasma gondii. Parasite Immunol. 21:365-376. [DOI] [PubMed] [Google Scholar]
- 35.Liesenfeld, O., J. Kosek, J. S. Remington, and Y. Suzuki. 1996. Association of CD4+ T cell-dependent, interferon-gamma-mediated necrosis of the small intestine with genetic susceptibility of mice to peroral infection with Toxoplasma gondii. J. Exp. Med. 184:597-607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lunde, M. N., and L. Jacobs. 1983. Antigenic differences between endozoites and cystozoites of Toxoplasma gondii. J. Parasitol. 69:806-808. [PubMed] [Google Scholar]
- 37.McLeod, R., P. Eisenhauer, D. Mack, C. Brown, G. Felice, and G. Spitalny. 1989. Immune responses associated with early survival after peroral infection with Toxoplasma gondii. J. Immunol. 142:3247-3249. [PubMed] [Google Scholar]
- 38.Perkins, E. S. 1973. Ocular toxoplasmosis. Br. J. Ophthalmol. 57:1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pfefferkorn, E. R., and L. H. Kasper. 1983. Toxoplasma gondii genetic crosses reveal phenotypic suppression of hydroxyurea resistance by fluorodeoxyuridine resistance. Exp. Parasitol. 55:207-218. [DOI] [PubMed] [Google Scholar]
- 40.Sabin, A. B. 1941. Toxoplasmic encephalitis in children. JAMA 116:801-807. [Google Scholar]
- 41.Scharton-Kersten, T. M., T. A. Wynn, E. Y. Denkers, S. Bala, E. Grunvald, S. Hieny, R. T. Gazzinelli, and A. Sher. 1996. In the absence of endogenous IFN-gamma, mice develop unimpaired IL-12 responses to Toxoplasma gondii while failing to control acute infection. J. Immunol. 157:4045-4054. [PubMed] [Google Scholar]
- 42.Sibley, L. D., and J. C. Boothroyd. 1992. Virulent strains of Toxoplasma gondii comprise a single clonal lineage. Nature 359:82-85. [DOI] [PubMed] [Google Scholar]
- 43.Silveira, C., R. Belfort, Jr., C. Muccioli, M. T. Abreu, M. C. Martins, C. Victora, R. B. Nussenblatt, and G. N. Holland. 2001. A follow-up study of Toxoplasma gondii infection in southern Brazil. Am. J. Ophthalmol. 131:351-354. [DOI] [PubMed] [Google Scholar]
- 44.Suzuki, Y., F. K. Conley, and J. H. Remington. 1989. Importance of endogenous IFN-gamma for prevention of toxoplasmic encephalitis in mice. J. Immunol. 143:2045-2050. [PubMed] [Google Scholar]
- 45.Suzuki, Y., K. Joh, M. A. Orellana, F. K. Conley, and J. S. Remington. 1991. A gene(s) within the H-2D region determines the development of toxoplasmic encephalitis in mice. Immunology 74:732-739. [PMC free article] [PubMed] [Google Scholar]
- 46.Suzuki, Y., M. A. Orellana, R. D. Schreiber, and J. S. Remington. 1988. Interferon-gamma: the major mediator of resistance against Toxoplasma gondii. Science 240:516-518. [DOI] [PubMed] [Google Scholar]
- 47.Suzuki, Y., M. A. Orellana, S. Y. Wong, F. K. Conley, and J. S. Remington. 1993. Susceptibility to chronic infection with Toxoplasma gondii does not correlate with susceptibility to acute infection in mice. Infect. Immun. 61:2284-2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yap, G., M. Pesin, and A. Sher. 2000. Cutting edge: IL-12 is required for the maintenance of IFN-gamma production in T cells mediating chronic resistance to the intracellular pathogen, Toxoplasma gondii. J. Immunol. 165:628-631. [DOI] [PubMed] [Google Scholar]