Abstract
Antibodies that inhibit Plasmodium falciparum adhesion to the placental receptor chondroitin sulfate A are associated with a reduced risk of placental malaria, but whether these antibodies lead to improved pregnancy outcomes is unknown. We measured antiadhesion antibody levels in parturient women in western Kenya, where malaria transmission is intense. Secundigravid women with antiadhesion activity in their plasma delivered babies that were on average 398 g heavier (P = 0.019) and 2 weeks more mature (P = 0.002) than babies delivered to secundigravidas without antiadhesion activity. Our findings support the development of antiadhesion vaccines to prevent poor fetal outcomes due to pregnancy malaria.
Children born to mothers with pregnancy malaria commonly suffer low birth weight (LBW), and Plasmodium falciparum-induced LBW kills 62,000 to 363,000 neonates in sub-Saharan Africa each year (4, 5). Women develop increasing resistance to pregnancy malaria over successive pregnancies (12). This pattern of parity-specific resistance has been related to the acquisition of antibodies that inhibit adhesion of P. falciparum-infected erythrocytes (IEs) to the placental receptor, chondroitin sulfate A (CSA) (7).
These antiadhesion antibodies are related to a reduced risk of placental parasitemia (7). The antigens targeted by antiadhesion antibodies have conserved features (7, 13), suggesting that a pregnancy malaria vaccine comprising a limited number of antigens may be globally effective.
No previous studies have related a specific antimalarial immune response to an improvement in pregnancy outcomes. We collected plasma samples from parturients in an area of high malaria transmission in western Kenya and assayed them for their ability to inhibit placental parasite adhesion to CSA. We then examined relationships between plasma antiadhesion activity and newborn birth weight, gestational age, or maternal hemoglobin levels.
All parturients at the New Nyanza General Provincial Hospital in Kisumu, Kenya, 18 years of age and older were asked to participate in the study and gave signed informed consent after receiving a study explanation form and oral explanation from a nurse in their native language. This study was approved by the ethical committees of the Walter Reed Army Institute of Research and Kenya Medical Research Institute. Infants were weighed immediately after delivery, and gestational age was estimated according to the modified Dubowitz examination. Placental blood samples were obtained by compressing fresh tissue in a tissue grinder. Hemoglobin levels were measured by Coulter model T-890. Placental parasite densities (percent IEs) were determined by microscopic examination of Giemsa-stained blood smears.
Representative plasma samples were randomly selected from among the samples donated by women of different parities and from infected and uninfected women. Plasma samples were tested for their ability to inhibit adhesion of a median of two placental parasite isolates (mean, 2.8; range, 1 to 9 isolates). Plasma samples collected early in the series and those with common ABO type (plasma samples were assayed against ABO-matched IEs) were assayed more frequently than plasma collected later in the series or those with uncommon ABO type. Individual plasma samples give highly similar results against different parasite isolates (7).
The antiadhesion antibody assay was described previously (7). Thirty-microliter volumes of CSA (Sigma, St. Louis, Mo.) at 10 μg/ml in phosphate-buffered saline (PBS) were adsorbed onto petri dishes (8). Wells were blocked with bovine serum albumin (Sigma) at 2 mg/ml in PBS. The parasites used in the binding assay were freshly collected isolates from infected placentas in Kisumu, Kenya. Placental IE suspensions (5 to 20% parasitemia, 5% hematocrit) in RPMI medium (GIBCO, Grand Island, N.Y.) were preincubated for 1 h at 37°C with placental plasma (diluted 1:5) and then allowed to bind to the immobilized CSA for 30 min. After three gentle washes with PBS, bound cells were fixed in 0.5% glutaraldehyde (Sigma) in PBS and stained with 1% modified Giemsa (Sigma) in tap water.
Adhesion was quantified as the number of parasites bound per 20 high-power fields. Antiadhesion activity was measured as the percentage of binding of the control, where control binding is binding that occurred in pooled AB plasma from the United States. Plasma that reduced parasite binding to a level below 35% of control binding was considered to have antiadhesion activity. The level of 35% is one standard deviation (14%) above the mean level of binding (21%) in the presence of plasma from multigravid women (n = 53) in western Kenya and has been used previously to define protective levels of antiadhesion activity (7).
Differences between groups were analyzed by nonparametric methods (Mann-Whitney or Kruskal-Wallis tests). Correlations were examined by Spearman rank test. Analysis of variance (ANOVA) was used to study the effects of antibody levels and other variables on pregnancy outcomes. The significance limit was chosen at a P of 0.05, and tied P values are given. Statistical analyses were performed with Statview version 5.0.1 (SAS Institute, Cary, N.C.) on a Macintosh computer.
Among 1,485 mothers of singleton, live, vaginally delivered infants who participated, maternal age, maternal hemoglobin, infant birth weight, and gestational age were fully documented in 1,329 mothers, and these constitute the whole cohort for our analysis. Demographic characteristics were similar among the whole cohort compared to the subset of women whose plasma was selected for assays.
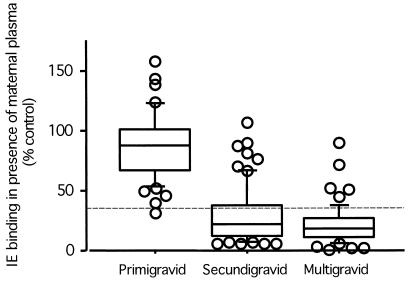
Within gravid groups, women without antiadhesion activity did not differ significantly in age from women with antiadhesion activity (P > 0.2 for all comparisons, Mann-Whitney test). Only 1 of 47 plasma samples from primigravid women reduced binding below 35% of the control level, compared to 47 of 68 samples from secundigravid women and 48 of 55 samples from multigravid women (Fig. 1). Increasing levels of antiadhesion activity correlated significantly with decreasing placental parasite density in both primigravid (ρ = 0.317; P = 0.03) and secundigravid (ρ = 0.469; P = 0.0001) women. Among multigravid women, no relationship was observed between levels of antiadhesion activity and parasite density in the placenta—probably because antiadhesion activity was fairly uniform among multigravid women. Parasite density of infected placentas was significantly lower in multigravid women than in primigravid women (mean percentages of IEs, 0.5% versus 6.1%, respectively; P = 0.03).
FIG. 1.
Antiadhesion antibodies in placental plasma samples from parturients of different gravidities. Antibodies were quantified as the number of IEs binding to CSA while in the presence of maternal plasma and expressed as percentage of binding in the presence of control, nonimmune plasma. Decreasing numbers of bound IEs indicate increasing levels of antiadhesion antibodies. Antiadhesion activity was defined as present or protective when plasma inhibited binding to a level below 35% of control plasma (indicated by the dashed line), as in previous studies (3). Box plots indicate medians (center lines), 75th percentiles (boxes), and 90th percentiles (bars). The remaining values are individually plotted as circles.
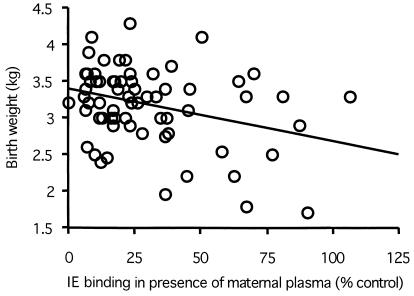
Among secundigravid women, antiadhesion activity in the plasma correlated with increasing birth weight in the newborn (Fig. 2; ρ = −0.239, P = 0.050), and women with antiadhesion antibodies had significantly heavier babies than did women without these antibodies (P = 0.019, Mann-Whitney test). By ANOVA, antiadhesion antibody levels in secundigravidas retained a significant influence on birth weight (P = 0.05) when placental parasitemia was included as a covariate. Women without antiadhesion activity delivered babies that were, on average, 398 g smaller than infants of women with antiadhesion activity in their plasma: birth weight (mean ± standard error) 2.907 ± 0.145 versus 3.305 ± 0.059 kg.
FIG. 2.
Scattergram of infant birth weight and maternal plasma antiadhesion activity measured in secundigravid women. Plasma samples that reduced IE binding to CSA were associated with increased birth weight. The regression line is indicated.
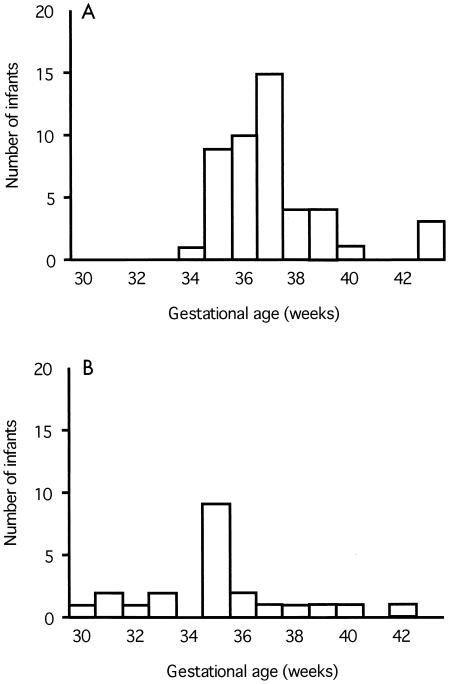
Secundigravid women with antiadhesion activity (Fig. 3A) delivered infants with significantly higher gestational ages (P = 0.002, Mann-Whitney test) compared to women without activity (Fig. 3B). By ANOVA, antiadhesion activity in secundigravid plasma retained a significant influence on gestational age (P = 0.04) when placental parasite density was included as a covariate. Secundigravid women without antiadhesion activity delivered babies that were, on average, 2 weeks more premature than did women with antibodies: gestational age (mean ± standard error), 35.14 ± 0.65 versus 37.09 ± 0.31 weeks.
FIG. 3.
Relationship between antiadhesion activity and gestational age of infants born to secundigravid women. Shown is a frequency distribution of gestational ages of infants born to women with (A) or without (B) plasma antiadhesion activity. Plasma that reduced parasite binding to a level below 35% of control binding was considered to have antiadhesion activity.
Antiadhesion activity and maternal hemoglobin levels were not related within the gravid groups. In the subset of women studied for plasma antiadhesion activity, differences in hemoglobin level in women with or without placental malaria did not achieve significance (P = 0.29).
Our principal finding is that antiadhesion antibodies in maternal plasma are associated with significantly increased birth weights and gestational ages in neonates from western Kenya, where malaria transmission is intense. LBW is the strongest risk factor for mortality during infancy (10). Reducing the incidence of LBW deliveries should reduce infant mortality in sub-Saharan Africa, which has ranged between 133 and 176 deaths per 1,000 live births during recent studies in areas of high transmission (3, 11, 14).
Secundigravid women with antiadhesion antibodies delivered infants who were on average 398 g heavier and 2 weeks older than infants born to women without antibodies. In a recent study from an area of Kenya where transmission is seasonal, neonates born to chronically infected mothers were 395 to 636 g larger when antibodies (VSAPAM immunoglobulin G [IgG]) specific for variant surface antigens of CSA-binding parasites were present (T. Staalsoe et al., personal communication). These results from disparate areas with distinct malaria transmission intensities suggest that the protective effect of antibodies targeting placental parasites may be a general phenomenon.
Antiadhesion antibodies were not associated with increased hemoglobin levels in mothers participating in our study. In a coastal area of Kenya, VSAPAM IgG antibodies have been associated with increased hemoglobin levels among mothers with chronic malaria (Staalsoe et al., personal communication). Because placental malaria is associated with severe maternal anemia (15) and because antiadhesion antibodies are associated with a reduced risk for placental malaria (7), we had hypothesized that antiadhesion antibodies would also be related to improved hemoglobin levels. However, several factors could contribute to anemia in our cohort, including micronutrient deficiency, and this may explain why women of different parities did not differ in their hemoglobin levels (Table 1). The sample size in this study may have been insufficient to detect an effect of protective antibodies on an outcome with complex etiology. Future studies should examine the relationship between antiadhesion antibodies and maternal hemoglobin levels by using larger cohorts. The value of a pregnancy malaria vaccine would be reduced if it did not prevent malaria-induced maternal anemia.
TABLE 1.
Characteristics of the cohort used in this studya
| Group (n) | Age (yr) | Hemoglobin level (g/dl) | Birth wt (kg) | Gestational age (wk) |
|---|---|---|---|---|
| All mothers | ||||
| Primigravid (571) | 19.8 ± 2.3 | 9.8 ± 2.5 | 3.08 ± 0.52 | 36.4 ± 3.7 |
| Secundigravid (305) | 21.8 ± 2.8 | 9.9 ± 2.8 | 3.23 ± 0.50 | 36.8 ± 3.2 |
| Multigravid (453) | 28.0 ± 5.2 | 9.7 ± 2.5 | 3.36 ± 0.50 | 36.7 ± 3.4 |
| Mothers included in antibody studies | ||||
| Primigravid (47) | 20.4 ± 2.7 | 9.6 ± 2.8 | 3.19 ± 0.49 | 36.4 ± 2.7 |
| Secundigravid (68) | 21.2 ± 2.7 | 9.7 ± 3.1 | 3.18 ± 0.53 | 36.5 ± 2.6 |
| Multigravid (53) | 26.4 ± 5.7 | 9.2 ± 2.2 | 3.41 ± 0.44 | 37.4 ± 2.3 |
The whole cohort of women who entered the study is compared to the subset of mothers randomly selected for studies of antiadhesion antibodies. Values are means ± standard errors. Women whose plasma samples were studied for antiadhesion activity did not differ significantly from the primary cohort on the basis of age, hemoglobin level, birth weight, or gestational age of their neonates. P > 0.05 for all comparisons within gravid groups (Mann-Whitney test).
CSA is the principal receptor for adhesion of parasites in the placenta (1, 8, 9). Hyaluronic acid (2) and nonimmune antibody adsorbed on the surface of parasitized erythrocytes (6) have been implicated as additional receptors in studies of placental parasites elsewhere. Whether antibodies that block adhesion mediated by hemagglutinin or IgG are required for protection remains unknown.
In this study, we found that antibodies to placental parasites are associated with reduced levels of placental parasitemia and increased birth weights and gestational ages of newborns. In work from eastern Kenya, where malaria is seasonal, maternal antibodies are associated with maternal hemoglobin levels as well as birth weight in the subset of women with chronic placental malaria (Staalsoe et al., personal communication). The findings from these studies support efforts to develop a vaccine against pregnancy malaria and suggest that birth weight may be used as an end point for defining the efficacy of such a vaccine during clinical trials.
Acknowledgments
We acknowledge the women delivering at the New Nyanza General Provincial Hospital who generously donated samples for this study. Richard Muga and Ambrose Misore were instrumental in establishing the facilities for sample collection at the hospital, and Ramadhan Mtalib, Livingstone Wanyama, and James Gitonga provided excellent laboratory support for these studies.
This work was supported by funds from NIAID/NIH (R01 AI 43680 and R01 AI52059 to P.E.D.) and the Bill & Melinda Gates Foundation.
The views expressed in this paper do not necessarily reflect those of the U.S. Department of Defense.
Editor: J. M. Mansfield
REFERENCES
- 1.Beeson, J. G., G. V. Brown, M. E. Molyneux, C. Mhango, F. Dzinjalamala, and S. J. Rogerson. 1999. Plasmodium falciparum isolates from infected pregnant women and children are associated with distinct adhesive and antigenic properties. J. Infect. Dis. 180:464-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beeson, J. G., S. J. Rogerson, B. M. Cooke, J. C. Reeder, W. Chai, A. M. Lawson, M. E. Molyneux, and G. V. Brown. 2000. Adhesion of Plasmodium falciparum-infected erythrocytes to hyaluronic acid in placental malaria. Nat. Med. 6:86-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bloland, P., L. Slutsker, R. W. Steketee, J. J. Wirima, D. L. Heymann, and J. G. Breman. 1996. Rates and risk factors for mortality during the first two years of life in rural Malawi. Am. J. Trop. Med. Hyg. 55:82-86. [DOI] [PubMed] [Google Scholar]
- 4.Breman, J. G. 2001. The ears of the hippopotamus: manifestations, determinants, and estimates of the malaria burden. Am. J. Trop. Med. Hyg. 64:1-11. [DOI] [PubMed] [Google Scholar]
- 5.Breman, J. G., A. Egan, and G. T. Keusch. 2001. The intolerable burden of malaria: a new look at the numbers. Am. J. Trop. Med. Hyg. 64:iv-vii. [DOI] [PubMed] [Google Scholar]
- 6.Flick, K., C. Scholander, Q. Chen, V. Fernandez, B. Pouvelle, J. Gysin, and M. Wahlgren. 2001. Role of nonimmune IgG bound to PfEMP1 in placental malaria. Science 293:2098-2100. [DOI] [PubMed] [Google Scholar]
- 7.Fried, M., F. Nosten, A. Brockman, B. J. Brabin, and P. E. Duffy. 1998. Maternal antibodies block malaria. Nature 395:851-852. [DOI] [PubMed] [Google Scholar]
- 8.Fried, M., and P. E. Duffy. 1996. Adherence of Plasmodium falciparum to chondroitin sulfate A in the human placenta. Science 272:1502-1504. [DOI] [PubMed] [Google Scholar]
- 9.Fried, M., R. M. Lauder, and P. E Duffy. 2000. Plasmodium falciparum: adhesion of placental isolates modulated by sulfation characteristics of the glycosaminoglycan receptor. Exp. Parasitol. 95:75-78. [DOI] [PubMed] [Google Scholar]
- 10.McCormick, M. C. 1985. The contribution of low birth weight to infant mortality and childhood morbidity. N. Engl. J. Med. 312:82-90. [DOI] [PubMed] [Google Scholar]
- 11.McElroy, P. D., F. O. ter Kuile, A. W. Hightower, W. A. Hawley, P. A. Phillips-Howard, A. J. Oloo, A. A. Lal, and B. L. Nahlen. 2001. All-cause mortality among young children in western Kenya. VI. The Asembo Bay Cohort Project. Am. J. Trop. Med. Hyg. 64:18-27. [DOI] [PubMed] [Google Scholar]
- 12.McGregor, I. A., M. E. Wilson, and W. Z. Billewicz. 1983. Malaria infection of the placenta in The Gambia, West Africa; its incidence and relationship to stillbirth, birth weight and placental weight. Trans. R. Soc. Trop. Med. Hyg. 77:232-244. [DOI] [PubMed] [Google Scholar]
- 13.Ricke, C. H., T. Staalsoe, K. Koram, B. D. Akanmori, E. M. Riley, T. G. Theander, and L. Hviid. 2000. Plasma antibodies from malaria-exposed pregnant women recognize variant surface antigens on Plasmodium falciparum-infected erythrocytes in a parity-dependent manner and block parasite adhesion to chondroitin sulfate A. J. Immunol. 165:3309-3316. [DOI] [PubMed] [Google Scholar]
- 14.Salum, F. M., T. J. Wilkes, K. Kivumbi, and C. F. Curtis. 1994. Mortality of under-fives in a rural area of holoendemic malaria transmission. Acta Trop. 58:29-34. [DOI] [PubMed] [Google Scholar]
- 15.Verhoeff, F. H., B. J. Brabin, L. Chimsuku, P. Kazembe, and R. L. Broadhead. 1999. An analysis of the determinants of anaemia in pregnant women in rural Malawi—a basis for action. Ann. Trop. Med. Parasitol. 93:119-133. [DOI] [PubMed] [Google Scholar]