Abstract
Plasmodium falciparum rifin proteins, belonging to the largest known family of variable infected-erythrocyte surface-expressed proteins encoded by rif genes, were recently shown to be capable of inducing a strong immune response in P. falciparum-infected adults living in an area in Gabon where malaria is endemic. In the present study, the levels of antirifin antibodies were analyzed in serum obtained from 60 children from the same area who were admitted to hospital and diagnosed with severe malaria. High antirifin antibody concentrations in these individuals correlated significantly with their capacity to rapidly clear their parasites from the circulation after the start of chemotherapy. A doubling of antirifin antibody concentrations reduced the clearance time by 5 h (95% confidence interval, 4.1 to 6.9 h). In the same group of children, who were followed up for 2 years, antirifin antibody levels did not correlate with a reduced rate of reinfection or with a delay in the time to the first reinfection. However, the initial antirifin antibody levels were sustained over the study period. The likelihood that these antibodies could confer a certain degree of protection against malaria is supported by our findings of statistically higher levels of antirifin antibodies to all four rifin proteins in a group of 42 asymptomatic parasitemic children.
In areas of holoendemic transmission of malaria, where the most severe form of human malaria is caused by Plasmodium falciparum, protective immunity is acquired during the first 5 to 10 years of life, so that most of the severe disease is found in young children (20). Manifestations of clinical symptoms occur during the blood stage of infection, when parasites grow and replicate within red blood cells. The damage caused by the disease is linked to parasite-induced changes of the infected erythrocyte (IE) surface, which are responsible for mediating adhesion to a variety of host receptors on microvascular endothelium and on uninfected erythrocytes. This leads to sequestration of IEs in the microvasculature and rosetting of uninfected red blood cells, with resulting obstruction of microvascular blood flow. Parasite-induced changes include changes in the rigidity of the cell, alterations in metabolite transport, and insertion of parasite proteins into knobbed structures on the surface of the IE membrane (6).
The best-characterized variant antigen, designated P. falciparum erythrocyte membrane protein 1 (PfEMP-1) and encoded by the var gene family, is anchored in knobs and shown to mediate cytoadhesion to host endothelial receptors (2, 16, 17). In close association with this var gene family found in clusters at the telomeres of the parasite chromosome are found the rif (for “repetitive interspersed family”) (5) and stevor (for “subtelomeric variable open reading frame) (9) genes. Recently, the P. falciparum genome project reported the presence of 59 var, 149 rif, and 28 stevor genes in the 3D7 strain, some of which also appear to be truncated pseudogenes (10).
Evidence has now accumulated that surface-expressed PfEMP-1 proteins are strong inducers of protective immunity (4). Clonally variant rifins, products of the rif genes, have also been reported to be expressed on the surface of IEs (8). stevor proteins are less polymorphic but have a certain degree of sequence similarity to rifins. The functions of these two gene products are not yet known.
Unlike var genes, rif genes are expressed only at late ring or early trophozoite stage, although they appear on the surface of the IE at the same time (12). This colocalization has prompted speculation that their expression and trafficking to the surface are linked. A large cross-sectional survey of individuals exposed to natural P. falciparum infections was carried out recently to evaluate the presence in their sera of specific antirifin antibodies capable of recognizing recombinant rifin proteins. The most significant finding was the high frequency of recognition by malaria-exposed immune adults whereas semi-immune children were less reactive (1). In this study, serum samples available from a longitudinal study were analyzed for specific antirifin antibodies to find whether rifin antibodies play a role in natural immunity to malaria.
MATERIALS AND METHODS
Study site.
Human plasma samples were collected from children attending the Albert Schweitzer Hospital in Lambaréné, Gabon. The prevalence of P. falciparum infection in this rain forest area is high, with an entomological inoculation rate of about 50 infectious bites per person per year, and transmission is intense and perennial (18).
Study cohort.
Ethical clearance was obtained from the Ethics Committee of the International Foundation of the Albert Schweitzer Hospital for the study. All study participants or their parents or guardians gave informed consent. Some of the samples for this work originated from the 1/95C study, a longitudinal study during which 100 children with severe malaria were recruited. Relevant information such as treatment, clinical and follow-up surveillance, and hematological and biochemical measurements of the participants has been previously described (13).
For the 100 children of the 1/95C study who had severe malaria, only 60 serum samples were still available for this study. The median age of these individuals was 39 months, with a minimum of 14 months and a maximum of 118 months. The patients had severe anemia (hemoglobin, <5 g/dl) and/or P. falciparum hyperparasitemia (>250,000 parasites/μl). From the day of admission, patients were followed up twice daily by carrying out Giemsa-stained calibrated thick blood smear examinations (15) of their peripheral blood until the parasitemia was cleared. Thereafter, microscopic examination of peripheral blood continued every 2 weeks to detect new P. falciparum infections. During the 2-year follow-up period, all children in whom symptomatic reinfections were detected (P. falciparum parasitemia and rectal temperature of ≥38.3°C) were given standard antimalarial treatment with sulfadoxine-pyrimethamine. Through strict intervention, these individuals did not develop any further severe disease; nevertheless, their rates of reinfection (estimated as the number of reinfections detected during the follow-up period) were still high, in some patients up to 11 reinfections. The time to first reinfection was defined as the time from the malarial attack at admission until the next P. falciparum-positive thick smear.
The same longitudinal study included a group of age and sex-matched children who suffered from mild malaria, which in its uncomplicated form is considered of less clinical importance than severe disease. For this reason, the present study addressed only the group of children with severe disease.
The asymptomatic group of children (n = 42; median age of 33 months, with a minimum of 6 months and a maximum of 121 months) was defined as children who had no clinical symptoms of malaria but had a positive blood smear. These children, of both sexes, were randomly selected from our study area and systematically examined once daily for 7 days for the presence of parasites by performing thick blood smears. Thereafter, they were followed up once every two days until they showed clinical symptoms of malaria (defined as a rectal temperature of >38.3°C). In these cases, the children were given standard antimalarial treatment. To be able to perform the analysis with a reasonable sample size, children who were asymptomatic for at least 5 days were included in our analysis.
Plasma samples.
Blood samples were collected in sterile tubes containing EDTA. Plasma was separated from whole blood by centrifugation and stored in aliquots at −20°C until use. Plasma samples were collected at four different times. The first sample (month 0) was taken on admission (pretreatment). The second sample (month 1) was taken 1 month posttreatment for the severe cases, during the convalescent stage of the disease. The third and the fourth samples from the patients with severe cases (for simplification referred to as month 6 and month 24, respectively) were collected when the individual was free of malaria attacks. During this so-called healthy phase, samples were collected only when the child was free of any clinically obvious intercurrent infection and tested P. falciparum negative by thick blood smear on at least three consecutive occasions before sample collection. As such, the occasions during which the third and fourth samples were collected did not coincide precisely with month 6 or month 24. The number of samples in the group of patients with severe infections which were available for the study was 60 for month 0, 15 for month 1, 26 for month 6, and 24 for month 24.
Antigen preparation and antibody assay.
The recombinant rifins used in this study were purified on affinity columns to 95% homogeneity, and plasma immunoglobulin G antibody responses to the purified His6-Rif proteins were measured by a standard enzyme-linked immunosorbent assay by using previously established procedures and conditions (1). Blank values were determined in parallel with each individual test serum with no antigen in the well.
Statistical analysis.
Statistical analysis was performed using JMP version 5 (SAS Institute Inc., Cary, N.C.). The background corrected optical density values (measured antibody levels from which blank values were subtracted) were correlated with age and parasite clearance by using Pearson's correlation coefficient. Antibody levels were compared among the study groups (severe and asymptomatic) using two-sample t tests. Changes in antibody response were analyzed longitudinally using Student's t test for paired data. Two-sided P values of <0.05 were considered to indicate statistical significance.
RESULTS
Comparison between antirifin antibody levels and age.
Plasma samples collected from 60 children with severe malaria were analyzed for antirifin antibody levels during the acute phase of the disease. Antirifin antibody levels measured during disease did not significantly correlate with the ages of the individuals for all four rifins (data not shown).
Correlation between antirifin antibody levels and parasite clearance.
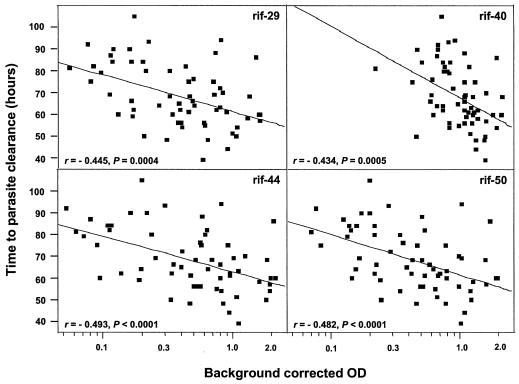
For the 60 individuals in this study who were admitted to the hospital, blood smears were performed every 12 h to determine parasitemia. The microscopic analysis continued until parasites reached undetectable levels, and the number of hours it took for the patients to clear parasites from the circulation was recorded. An analysis of the parasite clearance time as a function of the antibody response to recombinant rifin proteins showed that the higher the antirifin antibody levels measured at the start of the clinical attack, the faster the parasites were eliminated (Fig. 1). An association between rapid clearance of P. falciparum parasites with the levels of antibody to all four recombinant Rif proteins was observed. A doubling of antirifin antibody concentrations reduced the clearance time by 5 h (95% confidence interval, 4.1 to 6.9 h). There was no significant difference in the reduction time among the four rifins.
FIG. 1.
Correlation between the parasite clearance time in hours and the difference between the measured antirifin antibody levels and the corresponding blank values (log scale) (n = 60). Correlation coefficients are given for each protein. Fitting a linear model to all rifins simultaneously, it is noted that doubling any of the rifin antibody concentration reduces the clearance time by 5 h (95% confidence interval, 4.1 to 6.9 h), with no significant difference in the reduction time for all four rifins. OD, optical density.
Relationship between antirifin antibody levels and reinfection.
To further investigate whether high antirifin antibody titers in children correlated with other measures of natural immunity to malaria, additional data were analyzed. During the 2-year period between sample collections, our records show that many children became frequently reinfected. However, antibody levels were not found to correlate with the annual rate of reinfection. In addition, there was no correlation between antibody levels and the time between the first two infections. On the basis of these findings, we conclude that antirifin antibody responses did not appear to protect children against reinfections or against the occurrence of clinical malaria during follow-up.
Longevity of antirifin antibody responses in patients during follow-up.
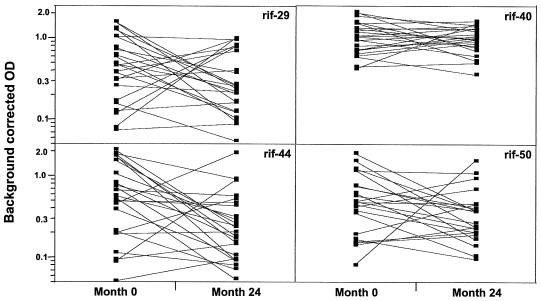
Samples collected in a longitudinal study were used to shed more light on the possible role of antirifin antibodies in malaria immunity. Antibody levels in plasma samples taken at different phases of infections, namely, the acute, convalescent, and healthy phases, were measured. In total, there were only six matched samples available for all time points at months 0, 1, 6, and 24. In these individuals, there was a common pattern where the antibody levels during the entire follow-up period were not highly variable but peaked consistently at month 1 (data not shown). That the antirifin antibody levels measured at least 24 months after hospital admission were sustained at the same high level is an interesting observation, especially since at blood-sampling times during the healthy phases of months 6 and 24, none of the children was sick from malaria. Matched samples taken from 24 individuals at the start and end of the study were available for further analysis (Fig. 2). For all recombinant proteins, the recognition pattern was consistent in that there was an increase in the antibody levels in about half the samples, whereas the response either remained the same or was only transient and declined in the other half. However, when considered together, the overall reactivities of all 24 samples measured at both time points were not statistically different from each other. Our observations therefore indicate that rifin proteins can induce an adequate antibody response that is maintained over time under natural conditions.
FIG. 2.
Antibody responses to recombinant rifin proteins. Antibody levels (log scale) at month 0 and month 24 were available for 24 patients. According to paired t tests, no significant differences were detectable. OD, optical density.
Detection of high antirifin antibody levels in asymptomatic children.
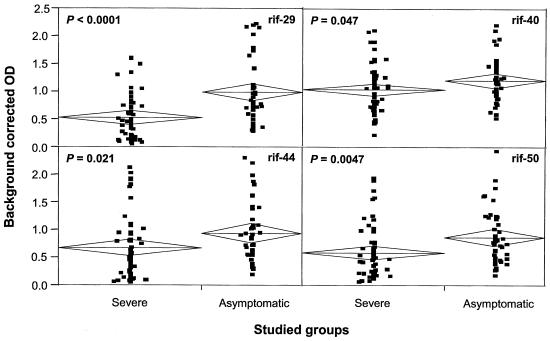
To investigate whether these responses are also induced in other groups of individuals, we analyzed plasma samples from 42 children who had P. falciparum parasites in their blood but were asymptomatic for malaria. By analyzing samples from children who appeared to tolerate malaria infections, we were able to ask whether the presence of antirifin antibodies in these children could be related to the capacity of the children to suppress malaria. An analysis of the antirifin responses in the symptomatic group with severe disease at admission and in asymptomatic children showed that both groups of children had high antibody levels (Fig. 3). Notably, antirifin antibody concentrations were significantly higher in the asymptomatic group as determined for all rifin proteins. In a recent study, anti-glycosylphosphatidylinositol antibodies were also higher in children with asymptomatic infections than in those with high parasite levels in their circulation and suffering clinical symptoms of malaria, even though this difference was not of statistical significance (7). Our results suggest that the presence of antirifin antibodies in some individuals may explain why they do not manifest disease symptoms.
FIG. 3.
Detection of antibodies to rifins in severely symptomatic and asymptomatic children. Plasma samples from 42 asymptomatic children were compared with those from 60 children with severe malaria. Even though the antibody concentrations were high in both groups, asymptomatic individuals showed a higher response to all rifin proteins. The center line in each diamond shows the group mean, and the vertical spans of the diamond show the 95% confidence interval.
DISCUSSION
In a previous serological study using a subset of rifin proteins cloned and produced in Escherichia coli, a naturally acquired immune response to these proteins was demonstrated in adults living in an area of high malaria endemicity. In this study, we sought to identify a role of these antibodies by analyzing serum samples from patients with severe malaria from part of a longitudinal study. By combining our serological findings with well-defined clinical and parasitological data available for each study participant, we obtained insight into the functional relevance of the antirifin antibody responses. The most striking observation was the finding that the intensity of immune responses to purified recombinant rifins measured at enrollment was highly correlated with the time of parasite clearance after the start of treatment. Thus, malaria patients who had high antibody levels were able to clear their parasites more efficiently than were those with low antibody responses. This observation indicates that these antibodies may have a protective effect against disease progression. However, the biological significance of this association is not clear, and until the mechanism by which antibodies mediate this effect is elucidated, the possibility remains that the measured antibody responses are surrogate markers of other biological activities.
From the longitudinal study, we observed that this novel family of rifin antigens can induce a positive response in some study participants and that the response can persist for 2 years. The induction and maintenance of such a long-lasting response could be considered important for protection against P. falciparum malaria, even though nothing is known about the natural production of antirifin antibodies. In fact, 4 of the 24 participants in our longitudinal study were not reinfected during the 2 years, and the antibody levels in 2 of these individuals were either elevated or sustained at the initial levels. These are thought to be long-lived antibodies rather than being induced as a result of continued exposure to malaria infections. The case is confounded in the other individuals who became reinfected. Here, it is not possible to distinguish between the existence of long-lasting antibodies and those resulting from repeated boosting due to previously eliminated infections or to subpatent infections, since the children were from an area with high-intensity transmission and were suffering frequent clinical attacks. Since each parasite genome has 149 rifin genes and several rifin products are expressed on the erythrocyte surface at any one time, antibody responses to rifins may already have developed after only a few malaria episodes.
Levels of antirifin antibodies in the study participants did not correlate with age when using the recombinant rifin proteins. Our earlier analysis using sera from children in a cross-sectional study also showed no association between antirifin antibody levels and age (range, 1.3 to 6.5 years). This result was attributed both to the small number of samples tested and to the likelihood that antibody responses have not yet been built up in children younger than 6 years. The age range of our cohort is 14 to 118 months, and for the older children in particular, it is an unexpected finding that the levels of antibodies to the tested recombinant rifins did not correlate with age.
A significant number of asymptomatic individuals who appeared to control their parasitemia and who did not experience symptoms of malaria had elevated levels of antibodies to rifins. Clearly, this is one of many studies to investigate antibodies to P. falciparum antigens in symptomatic and asymptomatic individuals. Until a true protective effect of these antibodies can be demonstrated, we will not be able to rule out the possibility that infections may have been present in an asymptomatic child longer than in a child experiencing severe attacks. In this scenario, antigenic stimulation may last long enough for antibody boosting and the measured antibody levels may thus be a consequence of the current infection.
Since high antibody levels were not associated with time to reinfection in the longitudinal study, it is very likely that rifin antibodies alone do not help explain protection against malaria. It can be speculated that antibodies to rifins could also, in a fashion similar to that described for anti-glycosylphosphatidylinositol antibodies, control the disease by inhibiting certain processes such as those that lead to cytoadhesion (3, 14). Some children in whom antirifin antibodies persist could therefore eliminate or tolerate parasitemia by regulating parasite density and/or suppressing clinical symptoms of malaria. Tebo et al. (19) showed residual antibody-mediated opsonization of IEs after protease treatment, suggesting that protease-resistant rifins may play a role in phagocytosis.
Several studies have been carried out to extensively analyze the antibody responses to parasite-derived erythrocyte surface-exposed proteins, now generally termed P. falciparum parasite-induced erythrocyte surface antigens (PIESAs) (11). In dissecting anti-PIESA responses in malaria-exposed individuals, the PfEMP-1 family of variant proteins is by far the best characterized as a major target antigen of these responses. Our data presented here demonstrate that the immune response to PIESAs that develops in most children includes antibody responses to an even larger multicopy family of variant antigens, the rifin proteins. While the surface location of this family of proteins has been previously demonstrated by other groups (8, 12), our failure to confirm these results in our previous work (1) appears to be related to the fact that the recombinant rifin proteins used to affinity purify specific antibodies from human sera did not represent conformationally intact molecules. In conclusion, this study represents the first longitudinal study showing the acquisition of specific antirifin antibodies in children after clinical episodes of malaria and a potential relevance of these antibodies in protection against P. falciparum malaria.
Acknowledgments
We thank Adrian Luty and Steffen Borrmann for helpful discussions.
This work received financial support from the Fortune-Programme of the Medical Faculty of the University of Tübingen (863-0-1) and the European Commission (QLK2-CT-1999-01293 and QLK2-CT-2002-01197).
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Abdel-Latif, M. S., A. Khattab, C. Lindenthal, P. G. Kremsner, and M. Q. Klinkert. 2002. Recognition of variant rifin antigens by human antibodies induced during natural Plasmodium falciparum infections. Infect. Immun. 70:7013-7021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baruch, D. I., B. L. Pasloske, H. B. Singh, X. Bi, X. C. Ma, M. Feldman, T. F. Taraschi, and R. J. Howard. 1995. Cloning the P. falciparum gene encoding PfEMP1, a malarial variant antigen and adherence receptor on the surface of parasitized human erythrocytes. Cell 82:77-87. [DOI] [PubMed] [Google Scholar]
- 3.Boutlis, C. S., D. C. Gowda, R. S. Naik, G. P. Maguire, C. S. Mgone, M. J. Bockarie, M. Lagog, E. Ibam, K. Lorry, and N. M. Anstey. 2002. Antibodies to Plasmodium falciparum glycosylphosphatidylinositols: inverse association with tolerance of parasitemia in Papua New Guinean children and adults. Infect. Immun. 70:5052-5057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bull, P. C., B. S. Lowe, M. Kortok, C. S. Molyneux, C. I. Newbold, and K. Marsh. 1998. Parasite antigens on the infected red cell surface are targets for naturally acquired immunity to malaria. Nat. Med. 4:358-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng, Q., N. Cloonan, K. Fischer, J. Thompson, G. Waine, M. Lanzer, and A. Saul. 1998. stevor and rif are Plasmodium falciparum multicopy gene families which potentially encode variant antigens. Mol. Biochem. Parasitol. 97:161-176. [DOI] [PubMed] [Google Scholar]
- 6.Deitsch, K. W., and T. E. Wellems. 1996. Membrane modifications in erythrocytes parasitized by Plasmodium falciparum. Mol. Biochem. Parasitol. 76:1-10. [DOI] [PubMed] [Google Scholar]
- 7.de Souza, J. B., J. Todd, G. Gowdahalli, D. C. Gowda, D. Kwiatkowski, and E. M. Riley. 2002. Prevalence and boosting of antibodies to Plasmodium falciparum glycosylphosphatidylinositols and evaluation of their association with protection from mild and severe clinical malaria. Infect. Immun. 70:5045-5051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fernandez, V., M. Hommel, Q. Chen, P. Hagblom, and M. Wahlgren. 1999. Small, clonally variant antigens expressed on the surface of the Plasmodium falciparum-infected erythrocyte are encoded by the rif gene family and are the target of human immune responses. J. Exp. Med. 190:1393-1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gardner, M. J., H. Tettelin, D. J. Carucci, et al. 1998. Chromosome 2 sequence of human malaria parasite Plasmodium falciparum. Science 282:1126-1132. [DOI] [PubMed]
- 10.Gardner, M. J., N. Hall, E. Fung, et al. 2002. Genome sequence of the human malaria parasite Plasmodium falciparum. Nature 419:498-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kinyanjiu, S. M., P. Bull, C. I. Newbold, and K. Marsh. 2003. Kinetics of antibody responses to Plasmodium falciparum-infected erythrocyte variant surface antigens. J. Infect. Dis. 187:667-674. [DOI] [PubMed] [Google Scholar]
- 12.Kyes, S., R. Pinches, and C. Newbold. 2000. A simple RNA analysis method shows var and rif multigene family expression patterns in Plasmodium falciparum. Mol. Biochem. Parasitol. 105:311-315. [DOI] [PubMed] [Google Scholar]
- 13.Luty, A. J. F., S. Ulbert, B. Lell, L. Lehman, R. Schmidt-Ott, D. Luckner, B. Greve, P. Matousek, D. Schmid, K. Herbich, B. Dubois, P. Deloron, and P. G. Kremsner. 2000. Antibody responses to Plasmodium falciparum: evolution according to the severity of a prior clinical episode and association with subsequent reinfection. Am. J. Trop. Med. Hyg. 62:566-572. [DOI] [PubMed] [Google Scholar]
- 14.Marsh, K., and R. W. Snow. 1997. Host-parasite interaction and morbidity in malaria endemic areas. Philos. Trans. R. Soc. Lond. Ser. B 352:1385-1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Planche, T., S. Krishna, M. Kombila, K. Engel, J. F. Faucher, E. Ngou-Milama, and P. G. Kremsner. 2001. Comparison of methods for the rapid laboratory assessment of children with malaria. Am. J. Trop. Med. Hyg. 65:599-602. [DOI] [PubMed] [Google Scholar]
- 16.Smith, J. D., C. E. Chitnis, A. G. Craig, D. J. Roberts, D. E. Hudson-Taylor, D. S. Peterson, R. Pinches, C. I. Newbold, and L. H. Miller. 1995. Switches in expression of Plasmodium falciparum var genes correlate with changes in antigenic and cytoadherent phenotypes of infected erythrocytes. Cell 82:101-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Su, X. Z., V. M. Heatwole, S. P. Wertheimer, F. Guinet, J. A. Herrfeldt, D. S. Peterson, J. A. Rayetch, and T. E. Wellems. 1995. The large diverse gene family var encodes proteins involved in cytoadherence and antigenic variation of Plasmodium falciparum-infected erythrocytes. Cell 82:89-100. [DOI] [PubMed] [Google Scholar]
- 18.Sylla, E. H. K., J. F. J. Kun, and P. G. Kremsner. 2000. Mosquito distribution and entomological inoculation rates in three malaria-endemic areas in Gabon. Trans. R. Soc. Trop. Med. Hyg. 94:652-656. [DOI] [PubMed] [Google Scholar]
- 19.Tebo, A. E., P. G. Kremsner, and A. J. F. Luty. 2002. Fcγ receptor-mediated phagocytosis of Plasmodium falciparum-infected erythrocytes in vitro. Clin. Exp. Immunol. 130:300-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Health Organization. 1997. World malaria situation in 1994. I. Population at risk. Wkly. Epidemiol. Rec. 72:269-274. [PubMed] [Google Scholar]