Abstract
Trypanosoma cruzi metacyclic trypomastigotes invade and replicate in the gastric mucosal epithelium after oral infection. In this study we analyzed the process of infection by T. cruzi isolates deficient in the expression of gp82, the metacyclic stage-specific surface glycoprotein implicated in target cell entry in vitro and in promoting mucosal infection in mice after oral challenge. Mice infected by the oral route with metacyclic forms of gp82-deficient isolate 569 or 588 developed patent parasitemia but at greatly reduced levels compared to those infected with the gp82-expressing isolate CL. Metacyclic forms of both isolates expressed gp30, a surface glycoprotein detectable by monoclonal antibody (MAb) 3F6 directed to gp82. Otherwise, the gp82-deficient isolates displayed a surface profile similar to that of the CL isolate and also entered epithelial HeLa cells in a manner inhibitable by MAb 3F6 and dependent on the parasite signal transduction that involved the activation of protein tyrosine kinase and Ca2+ mobilization from thapsigargin-sensitive stores. Like gp82, gp30 triggered the host cell Ca2+ response required for parasite internalization. Purified gp30 and the recombinant gp82 inhibited HeLa cell invasion of metacyclic forms of isolates 569 and 588 by ∼90 and ∼70%, respectively. A cell invasion assay performed in the presence of gastric mucin, mimicking the in vivo infection, showed an inhibition of 70 to 75% in the internalization of gp82-deficient isolates but not of the CL isolate. The recombinant gp82 exhibited an adhesive capacity toward gastric mucin much higher than that of gp30. Taken together, our findings indicate that target cell entry of metacyclic trypomastigotes can be mediated either by gp82 or gp30 but that efficient mucosal infection depends on the expression of gp82.
Studies performed with Trypanosoma cruzi metacyclic trypomastigotes, the developmental forms that initiate infection in the mammalian host, have indicated that the stage-specific surface glycoprotein gp82 mediates mucosal invasion, leading to systemic T. cruzi infection after oral challenge (17), a route to which is attributed the microepidemics responsible for more than half of the acute cases of Chagas' disease recorded between 1968 and 2000 in Brazilian Amazon (5). Orally delivered insect-derived metacyclic forms consistently infect 100% of BALB/c mice, and evidence has been found that the parasites invade and replicate in the gastric mucosal epithelium, in contrast to the bloodstream trypomastigotes, which rarely initiate mucosal infection (10, 11).
gp82 appears to be the main metacyclic trypomastigote surface molecule specialized for adhesion to gastric mucin and for subsequent penetration into underlying epithelial cells. It binds in vitro to gastric mucin in a dose-dependent manner, whereas the binding of other surface molecules such as gp90 and gp35/50 is negligible, and oral administration to BALB/c mice of metacyclic forms preincubated with monoclonal antibody (MAb) 3F6, which reacts with gp82 and inhibits target cell invasion in vitro, greatly reduces parasitemia (17). gp82-mediated binding of metacyclic forms to the host cell induces in both cells the activation of signal transduction pathways, leading to intracellular Ca2+ mobilization (20, 30), which is a requirement for T. cruzi internalization (7, 14, 23, 28). We have recently shown that noninfective epimastigotes, which do not express detectable levels of gp82 and are unable to induce a Ca2+ response, when stably transfected with a T. cruzi expression vector carrying the metacyclic stage gp82 cDNA, produced a functional gp82, which bound to and triggered Ca2+ responses in HeLa cells (13).
Expression of gp82 has been detected by MAb 3F6 in metacyclic forms of 10 different T. cruzi isolates examined to date (20) in a survey that did not include isolates from chronically infected Chagas' disease patients. Recently we found for the first time two T. cruzi samples deficient in expression of gp82, which were isolated from individuals at the chronic phase with severe clinical manifestations. In the present study we performed a series of in vivo and in vitro experiments to investigate the mechanisms of infection of these gp82-deficient isolates.
MATERIALS AND METHODS
Parasites, mammalian cells, and cell invasion assay.
The following T. cruzi samples were used in this study: 569 (MHOM/BR97/GOCH569), isolated in 1997 by xenodiagnosis from a 59-year-old chronically infected woman with megaesophagus and megacolon; 588 (MHOM/BR99/GOCH588), isolated in 1999 by xenodiagnosis from a 40-years-old chronically infected man with the cardiac form of the disease; and CL, from Triatoma infestans (3). T. cruzi samples 569 and 588 were isolated by one of us (A. Luquetti) and were not characterized before the present study. The parasites were maintained alternately in mice and in liver infusion tryptose medium. Metacyclic forms, harvested from cultures at the stationary growth phase, were purified by passage through a DEAE-cellulose column, as described previously (25). HeLa cells, a human carcinoma-derived epithelial cell line, were grown at 37°C in Dulbecco's minimal essential medium supplemented with 10% fetal calf serum, streptomycin (100 μg/ml), and penicillin (100 U/ml) in a humidified 5% CO2 atmosphere. Experiments on mammalian cell invasion by T. cruzi were performed essentially as previously described (29).
Oral infection.
Purified T. cruzi metacyclic forms were introduced by the intrapharyngeal route into 4- to 5-week-old female BALB/c mice through a plastic tube adapted to a 1-ml plastic syringe. Each mouse received 4 × 105 parasites suspended in phosphate-buffered saline (PBS). Starting on day 13 postinoculation, parasitemia was monitored by twice-weekly phase-contrast microscopy examination of 5-μl peripheral blood samples collected from the tail.
Purification of native and recombinant T. cruzi molecules.
To purify the surface molecule gp30, parasite extracts were prepared by solubilizing metacyclic trypomastigotes in 1% Nonidet P-40 in the presence of protease inhibitors. After centrifugation at 12,000 × g for 5 min, the supernatant was collected and applied to the antibody affinity column prepared by coupling MAb 3F6 to CNBr-activated Sepharose 4B. After 2 h at room temperature with constant shaking, the resin was packed in a 10-ml plastic syringe and washed several times with PBS. The bound antigen was eluted with 0.1 M glycine (pH 2.8), neutralized with Tris-HCl, dialyzed against double-distilled water, vacuum dried, and stored at −20°C until use. Recombinant protein J18, comprising the full-length gp82 sequence in frame with glutathione S-transferase, was generated in Escherichia coli and purified as described previously (21), by excising from the sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) gel the band of high intensity and of the expected size for the recombinant protein. After electroelution as described previously (27), using a buffer containing 25 mM Tris-HCl, 192 mM glycine-HCl, and 20% methanol, the sample was dialyzed against 10 mM ammonium bicarbonate for 24 h and thereafter against distilled water for another 24 h. The purity of the isolated protein was assessed by staining on SDS-PAGE gel with Coomassie blue, and its specificity was ascertained by immunoblotting using MAb 3F6.
Antibodies to T. cruzi molecules.
Polyclonal monospecific antibodies to gp30 or to the recombinant protein J18 were generated by immunizing BALB/c mice intraperitoneally with four doses of the purified molecule (5 μg/mouse) plus A1(OH)3 as adjuvant (0.5 mg/mouse) at 10-day intervals. MAb 3F6, recognizing gp82 (25), was purified as described previously (19). MAb 1C3, directed to Leishmania amazonensis gp63 (2), was kindly provided by C. L. Barbieri, Universidade Federal de São Paulo.
Binding assay of gp30 and gp82 to gastric mucin.
The binding assay was performed using mucin from porcine stomach (type III mucin; Sigma), as follows: 96-well microtiter plates coated with gastric mucin in PBS (10 μg/well) were blocked for 1 h at room temperature with PBS containing 10% fetal calf serum (PBS-FCS) and then incubated sequentially, at 37°C for 1 h, with gp30 or with recombinant gp82, polyclonal monospecific antibody to gp30 or recombinant gp82, and peroxidase-conjugated anti-mouse IgG, in PBS-FCS. The final reaction was revealed by using o-phenylenediamine as described previously (19).
Immunoblotting and flow cytometry.
To analyze the expression of metacyclic trypomastigote surface molecules by immunoblotting, we used a procedure described elsewhere (29). The final reaction was revealed both by chemiluminescence, using the ELC Western blotting detection reagent and Hyperfilm-MP from Amersham, and by using diaminobenzidine plus H2O2. For analysis by flow cytometry, live metacyclic trypomastigotes (3 × 107) were incubated for 1 h on ice with anti-T. cruzi MAb or with unrelated MAb 1C3. After three washings in PBS, the parasites were fixed with 2% paraformaldehyde in PBS for 30 min. The fixative was washed out, and the parasites were incubated with fluorescein-labeled goat anti-mouse immunoglobulin G for 1 h at room temperature. Following two more washes, the number of fluorescent parasites was estimated with a Becton-Dickinson cytometer.
Enzymatic treatment of parasites.
Treatment with endoglycosidase H was carried out as follows: parasites were lysed with 1% Nonidet P-40 and centrifuged at 12,000 × g for 5 min, and the supernatant was incubated overnight at 37°C with 1,000 U of endoglycosidase H in 10× denaturing buffer (5% SDS, 10% β-mercaptoethanol) and 10× G5 buffer (0.5 M sodium citrate [pH 5.5]) in a total volume of 50 μl. The enzyme-treated samples and the untreated controls were analyzed by immunoblotting.
Detection of tyrosine-phosphorylated T. cruzi proteins.
Parasites (5 × 107) were incubated for 20 min at 37°C in the absence or presence of HeLa cell extract, equivalent to 160 μg of protein per ml, in a total volume of 100 μl. After being washed with PBS, the parasites were disrupted at 4°C in a lysis solution (50 mM Tris-HCl [pH 7.4] 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, 1 mM NaVO4, 1 mM NaF). Lysates were dissolved in loading buffer and subjected to electrophoresis in an SDS-10% polyacrylamide gel under reducing conditions, and the proteins were analyzed by immunoblotting using antiphosphotyrosine antibodies (Sigma) and chemiluminescence reagent as described previously (8).
Preparation of HeLa cell extract.
The cell extracts used in phosphorylation experiments were prepared as follows. After being washed in PBS, HeLa cells were detached by scraping, suspended in PBS containing protease inhibitors (1 mM phenylmethylsulfonyl fluoride, 1 mM iodoacetamide, 25 μg of leupeptin per ml, 25 μg of antipain per ml), and then sonicated on ice in an XL ultrasonic processor (two pulses of 30 s each). After disruption of the cells was confirmed by phase-contrast microscopy, the sonicated preparation was centrifuged at 12,000 × g for 5 min and the supernantant was collected and immediately used for experiments or stored at −80°C until use.
RESULTS
Profile of surface molecules of T. cruzi isolates 569 and 588.
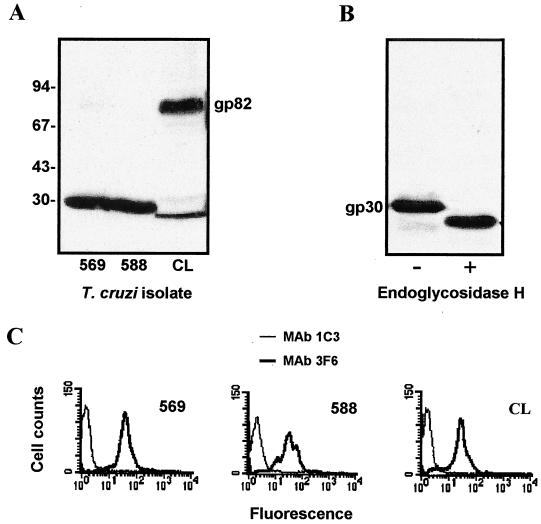
First, metacyclic trypomastigotes of isolates 569 and 588 were analyzed by Western blotting. A component of ∼30 kDa was revealed by MAb 3F6 in both isolates, whereas gp82 was the predominant molecule recognized by this antibody in isolate CL (Fig. 1A). gp82 was undetectable in isolate 588 and barely detectable in isolate 569. To determine whether the MAb 3F6-reactive 30-kDa molecule was glycosylated, extracts of metacyclic forms of isolate 569 were treated with endoglycosidase H, an enzyme that specifically cleaves the high-mannose-type oligosaccharide side chain linked to N-asparagine (24), and were then analyzed by immunoblotting using MAb 3F6. The endoglycosidase H treatment fully converted the 30-kDa molecule to a component of ∼25 kDa (Fig. 1B). Next, we determined the levels of gp30 on the parasite surface. Live metacyclic forms were incubated with MAb 3F6 or with isotype-matched unrelated MAb 1C3 and processed for analysis by flow cytometry. Expression of gp30 in isolates 569 and 588 was comparable (Fig. 1C). Neither isolate reacted with MAb 1G7, directed to the metacyclic stage-specific surface molecule gp90 (25), but they were recognized by MAb 5E7 directed to a variant gp90 (data not shown). Both reacted with MAb 2B10 but not with MAb 10D8, directed to different epitopes of the mucin-like glycoprotein gp35/50 (15, 29), thus displaying a surface profile very similar to that of the gp82-expressing isolate CL metacyclic forms (20).
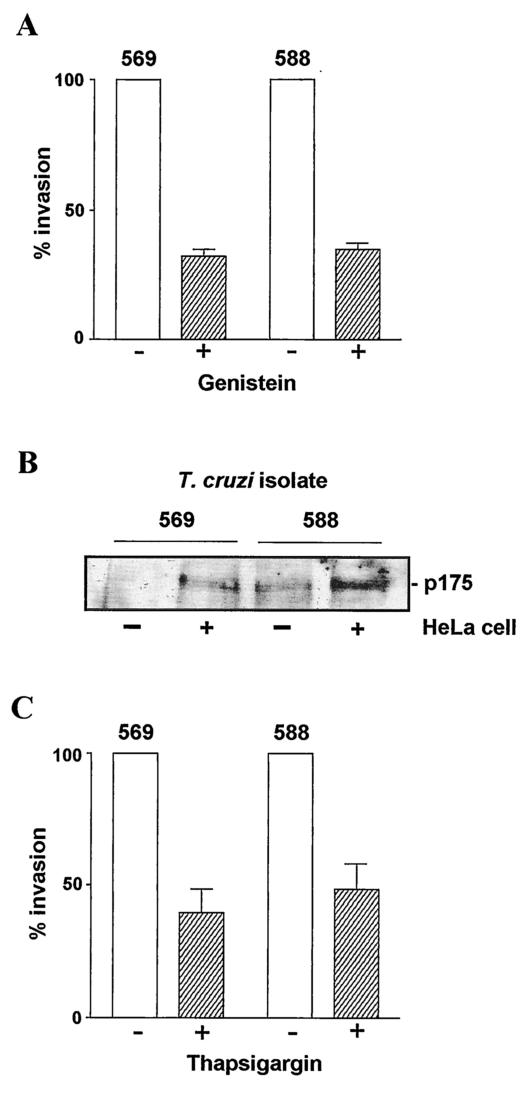
FIG. 1.
Expression of surface glycoprotein gp30 in T. cruzi isolates 569 and 588. (A) Metacyclic trypomastigotes were analyzed by immunoblotting using MAb 3F6. Note the component of ∼30 kDa recognized by MAb 3F6 in both isolates and the absence of gp82, which is predominant in isolate CL. Positions of molecular size markers are shown on the left in kilodaltons. (B) Detergent extracts of metacyclic forms of isolate 569 were subjected to treatment with endoglycosidase H, as described in Materials and Methods. The immunoblot with MAb 3F6 shows the conversion of the 30-kDa molecule into a smaller component, indicating its glycoprotein nature. (C) Live parasites were incubated for 1 h on ice with MAb 3F6 or with unrelated control, MAb 1C3, and processed for analysis by flow cytometry. Note that gp30 is similarly expressed on the surface of isolates 569 and 588.
Mucosal infection by gp82-expressing and gp82-deficient T. cruzi isolates.
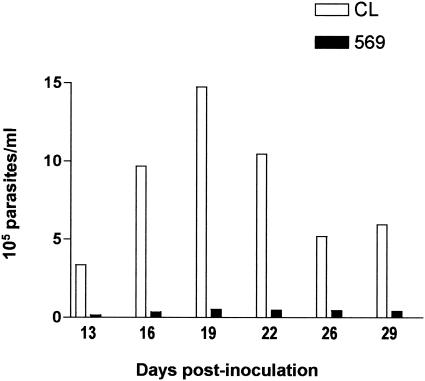
BALB/c mice received 4 × 105 metacyclic forms of isolate 569 or CL by the intrapharingeal route. Starting on day 13 postinoculation, 5-μl blood samples from individual animals were examined twice a week for the presence of parasites. In mice infected with isolate 569, the parasitemia levels were very low, in contrast to the animals inoculated with isolate CL, which developed high parasitemias (Fig. 2). Repeated experiments gave essentially the same results. Oral infection of mice with metacyclic forms of isolate 588 also resulted in reduced parasitemias, similar to those produced by isolate 569 (data not shown). When inoculated by the intraperitoneal route into BALB/c mice, metacyclic forms of isolates CL, 569, and 588 produced comparable parasitemia levels (data not shown).
FIG. 2.
Oral infection of mice with gp82-expressing or gp82-deficient T. cruzi isolates. Each mouse received 4 × 105 metacyclic trypomastigotes of gp82-expressing isolate CL or gp82-deficient isolate 569. Each datum point corresponds to the mean parasitemia of six animals in each group of mice.
Host cell invasion by gp82-deficient T. cruzi isolates expressing gp30.
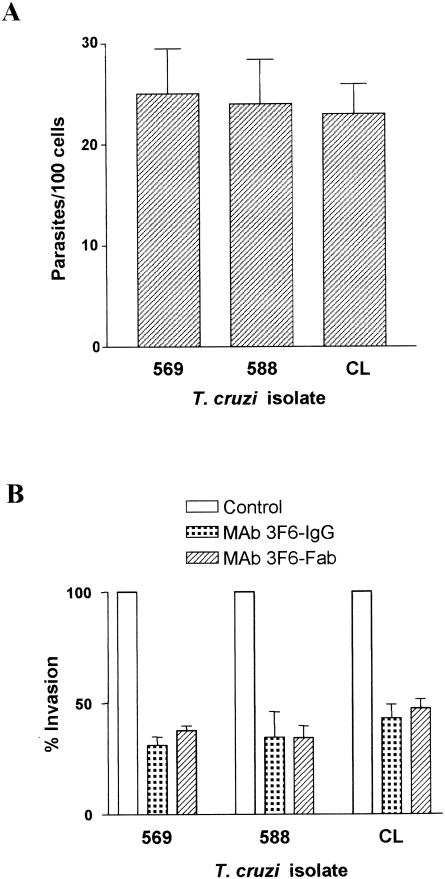
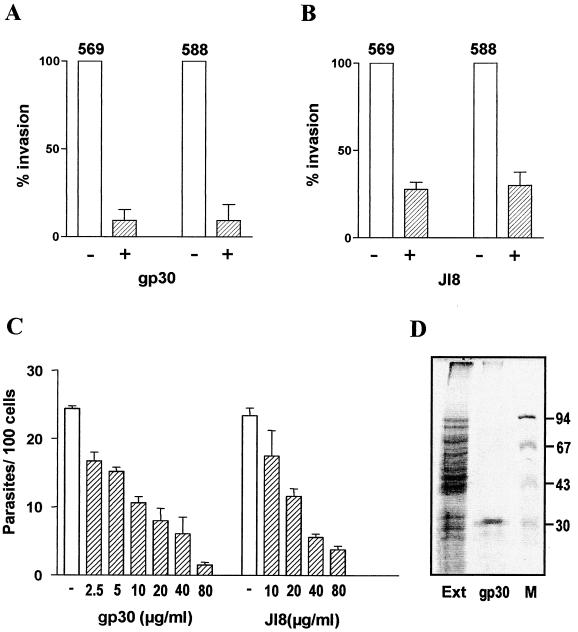
To determine whether the metacyclic forms of isolates 569 and 588 differed from the CL isolate in their ability to enter host cells, we performed cell invasion assays by incubating the parasites with HeLa cells at 37°C for 1 h. Following washes in PBS, HeLa cells were stained with Giemsa and the intracellular parasites in at least 500 cells were counted. As shown in Fig. 3, the infectivity of the three isolates was comparable and their internalization was similarly diminished when they were treated with MAb 3F6, suggesting that gp30 is involved in target cell invasion of gp82-deficient T. cruzi isolates. Compatible with this view, the native gp30 exhibited a Ca2+-signaling activity toward HeLa cells similar to that of the native gp82 (data not shown). Furthermore, penetration of metacyclic forms of isolates 569 and 588 into HeLa cells was inhibited by ca. 90% in the presence of purified gp30 at 60 μg/ml (Fig. 4A) and by ca. 70% in the presence of the recombinant gp82 at 80 μg/ml (Fig. 4B). Even at a concentration as low as 2.5 μg/ml, gp30 still displayed some inhibitory effect (Fig. 4C). Although to a lesser degree, HeLa cell entry of isolate CL metacyclic forms was also reduced by gp30, with inhibition of ca. 80% and ca. 55%, respectively, at gp30 concentrations of 60 and 30 μg/ml. As judged by the purity of gp30, determined by Coomassie blue staining of an SDS-PAGE gel (Fig. 4D), the possibility that eventual contaminant proteins contributed to the observed effect of gp30 appears to be minimal.
FIG. 3.
Host cell invasion of gp82-expressing or gp82-deficient T. cruzi isolates. (A) Metacyclic forms of isolates 569, 588, and CL were incubated with HeLa cells for 1 h at 37°C, and the intracellular parasites in at least 500 Giemsa-stained cells were counted. Values are means and standard deviations (SD) of six experiments. (B) Parasites were left untreated or treated for 30 min at room temperature with 500 μg of MAb 3F6 per ml or 250 μg of the corresponding Fab fragments per ml before being added to HeLa cells. After a 1-h incubation, the HeLa cells were washed in PBS and stained with Giemsa. The reference value of 100% was ascribed to the untreated control. Values are means and SD of three experiments.
FIG. 4.
Inhibitory effect of gp30 and recombinant gp82 on host cell invasion of gp82-deficient T. cruzi isolates. Metacyclic forms of isolates 569 and 588 were added to HeLa cells that had been left unincubated or had been preincubated for 15 min with 60 μg of native gp30 per ml (A) or with 80 μg of J18 per ml (B), the recombinant protein containing the entire gp82 sequence. After 1 h at 37°C, the intracellular parasites in a total of at least 500 Giemsa-stained cells were counted. A reference value of 100% was ascribed to the control without the T. cruzi protein. Values are the means and SD of three experiments performed in duplicate. (C) HeLa cells were preincubted with purified gp30 or J18 at the indicated concentrations before the addition of metacyclic forms of T. cruzi isolate 569. Representative results of a set of experiments are shown. Values are the means and SD of triplicate determinations. (D) SDS-PAGE gel containing the extract of T. cruzi isolate 569 (Ext), purified gp30, and molecular size markers (M), stained with Coomassie blue.
Signal transduction in gp82-deficient T. cruzi isolates during host cell invasion.
Previous studies with isolate CL have shown that target cell invasion requires the activation of the parasite protein tyrosine kinase (PTK), which is triggered by gp82 binding to its receptor (8, 16, 30). To determine the association between PTK activation and infectivity of metacyclic forms of gp82-deficient T. cruzi isolates, we examined the effect of genistein, a specific inhibitor of PTK which scarcely inhibits the enzyme activities of serine- and threonine-specific protein kinases (1). Metacyclic forms of 569 and 588 isolates were treated at 37°C for 30 min with 250 μM genistein. After being washed in PBS, control and genistein-treated parasites were incubated with HeLa cells for 30 min at 37°C. Figure 5A shows that the ability of genistein-treated parasites to invade HeLa cells was significantly diminished. In isolate CL, PTK mediates the phosphorylation of a 175-kDa protein (p175) in a manner inducible by soluble extract of HeLa cells (8). We assayed the presence of HeLa cell-inducible p175 in isolates 569 and 588. Metacyclic trypomastigotes were incubated in the absence or presence of HeLa cell extract for 20 min at 37°C. After being washed in PBS, the parasites were disrupted by adding 1% Triton X-100 solution containing phosphatase inhibitors. Whole lysates were then analyzed by Western blotting using antiphosphotyrosine antibodies. Similarly to what is seen in isolate CL, the phosphorylation levels of p175 were augmented by the parasite-host cell interaction (Fig. 5B). The differences in the intensity of p175 bands in different samples are not due to an eventual unequal loading, because we ascertained that equal amounts of parasite extracts were loaded, either by staining the SDS-PAGE gel with Coomassie blue or by staining the nitrocellulose membrane with Ponceau-S.
FIG. 5.
Effect of inhibition of parasite PTK and Ca2+ mobilization on host cell invasion by gp82-deficient T. cruzi isolates. (A) Metacyclic forms of isolates 569 and 588, pretreated (+) or not pretreated (−) with the PTK inhibitor genistein, were incubated with HeLa cells at 37°C for 30 min. After the cells were washed in PBS, the intracellular parasites in at least 500 Giemsa-stained cells were counted. The values are the means and SD of three experiments performed in duplicate. (B) Parasites were incubated for 20 min at 37°C in the absence (−) or presence (+) of HeLa cell extract, washed with PBS, and processed for immunoblotting using antiphosphotyrosine antibodies. Note the increase in p175 phosphorylation levels in parasites exposed to HeLa cell extract. (C) Metacyclic forms were either untreated or treated with 1 μM thapsigargin before being incubated with HeLa cells for 30 min. Values are the means and SD of three experiments performed in duplicate.
Ca2+ mobilization in the parasite is a requisite for cell invasion (14, 20, 28). Experiments with metacyclic forms of isolate CL have shown that HeLa cell entry is inhibited by treatment of parasites with thapsigargin, a sesquiterpene lactone that depletes intracellular Ca2+ stores in many mammalian cell types by specific inhibition of endoplasmic reticulum Ca2+-ATPase (26). We treated metacyclic trypomastigotes of isolates 569 and 588 with 1 μM thapsigargin at 37°C for 30 min before using them to seed HeLa cells. Compared to untreated controls, the ability of thapsigargin-treated parasites to invade HeLa cells was significantly diminished (Fig. 5C).
Effect of gastric mucin on host cell entry of gp82-deficient T. cruzi isolates.
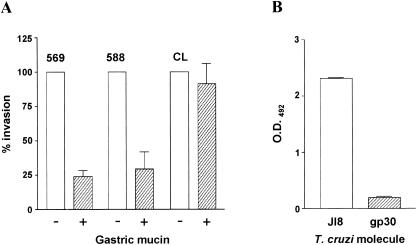
The finding that the in vivo infectivity of metacyclic forms of gp82-deficient isolates differs from that of the gp82-expressing isolate CL (Fig. 2), although no apparent difference was seen in vitro (Fig. 3A), led us to consider the possibility that mucin, the protective coat that lines gastrointestinal mucosal surfaces, could be affecting the interaction of gp82-deficient parasites with the underlying epithelial cells. In an attempt to mimic the in vivo mucosal infection, we performed HeLa cell invasion assays in the presence of gastric mucin at 20 mg/ml. The internalization of metacyclic forms of isolates 569 and 588, but not of CL, was diminished by about 70 to 75% in the presence of mucin (Fig. 6A).
FIG. 6.
Differential interaction of T. cruzi metacyclic-stage surface molecule gp30 and 82 with gastric mucin. (A) Gastric mucin at 20 mg/ml was added to HeLa cells 15 min before they were incubated with metacyclic trypomastigotes. After a 1-h incubation, the cells were washed, fixed, and stained for intracellular parasite counting. The values are the means and SD of three experiments performed in duplicate. (B) J18, the recombinant gp82, or purified gp30 at 10 μg/ml was added to microtiter plates coated with porcine gastric mucin. After being washed, the plates were sequentially incubated with anti-J18 antibody and anti-mouse immunoglobulin conjugated to peroxidase. The bound enzyme was revealed using o-phenylenediamine. The values are the means and SD of triplicate samples. O.D.492, optical density at 492 nm.
Inefficient binding of gp30 to gastric mucin.
gp82, either as the native molecule or as the recombinant protein, was found to bind to gastric mucin in vitro (17). Since this property may be associated with the efficient mucosal infection by gp82-expressing metacyclic forms of isolate CL, we examined the adhesive capacity of gp30 towards gastric mucin. Using anti-gp82 polyclonal antibodies, which cross-react with gp30, we found poor gp30 binding to mucin, in contrast to J18, the recombinant gp82 (Fig. 6B). The binding assay using anti-gp30 antibodies gave essentially the same results.
DISCUSSION
In this study, we performed the first analysis of T. cruzi isolates deficient in the expression of gp82, the metacyclic stage-specific surface molecule that appears to promote mucosal infection by mediating parasite penetration into host epithelial cells (17).
Metacyclic trypomastigotes of T. cruzi isolates 569 and 588, deficient in gp82, were found to express gp30, a surface glycoprotein recognized by MAb 3F6 (Fig. 1). Otherwise, these isolates displayed a surface profile similar to that of the gp82-expressing isolate CL. The abilities of metacyclic forms of isolates 569, 588, and CL to invade HeLa cells were similar and were inhibited similarly by MAb 3F6 (Fig. 3B). The fact that both gp30 and J18, the recombinant gp82, can significantly reduce the internalization of metacyclic forms of isolates 569 and 588 (Fig. 4), in addition to the inhibitory effect of gp30 on isolate CL infectivity, suggests that gp30 and gp82 are possibly recognized by a common target cell receptor. In this regard, it is significant that gp30 induced a Ca2+ response in HeLa cells in the same manner as gp82. It is possible that gp30 and gp82 have in common the host cell adhesion site, which in gp82 is located downstream of and contiguous to MAb 3F6-epitope (12). Target cell invasion of gp82-deficient metacyclic trypomastigotes requires parasite PTK activation (Fig. 5A) and release of Ca2+, at least in part, from thapsigargin-sensitive stores (Fig. 5C). In isolate CL metacyclic forms, Ca2+ is also mobilized in a thapsigargin-susceptible manner during cell invasion, and this Ca2+ response is associated with PTK activation induced by gp82-mediated attachment to host cells (30). We also found that, as in isolate CL, the substrate for PTK in 569 and 588 isolates is p175 (Fig. 5B), a component that is undetectable in noninfective epimastigote forms and is specifically phosphorylated on interaction of infective trypomastigotes with T. cruzi-susceptible, but not with T. cruzi-resistant, target cells (8). Taken together, these observations indicate that gp82- and gp30-expressing T. cruzi isolates use a similar mechanism to invade host cells.
We have recently shown that metacyclic forms of isolates G and CL, which belong to the two highly divergent phylogenetic lineages T. cruzi I and II, respectively (4), use distinct signal transduction pathways to enter host cells (16). In isolate G (T. cruzi I), PTK appears to play no role and the Ca2+ required for parasite internalization is released from acidic vacuoles containing a Ca2+/H+ exchange system (16), described by Docampo et al. (6). The finding that T. cruzi isolates 569 and 588, like isolate CL, use the PTK-dependent signaling pathway for host cell penetration is consistent with the fact that they are all T. cruzi II parasites.
Although the metacyclic forms of gp82-deficient and gp82-expressing T. cruzi isolates apparently use similar mechanims to invade epithelial cells, when we compared their capacity to produce systemic infection after oral challenge, we observed marked difference. In contrast to infection by gp82-expressing isolate CL, which results in high parasitemias, mice inoculated orally with gp82-deficient metacyclic forms of isolate 569 developed low parasitemias (Fig. 2). Moreover, mortality (10 to 20%) has been observed among mice inoculated with isolate CL but not among those isolated with isolate 569 or 588. It is possible that this difference is due to the differential capacity of gp82 and gp30 to bind to gastric mucin. Unlike gp82, gp30 bound to mucin very poorly (Fig. 6B). If the gp82-mediated interaction with mucin is the first step in the penetration of metacyclic trypomastigotes into the underlying epithelial cells, the gp82-deficient parasites would have difficulty in penetrating the thick mucin coat that lines the gastrointestinal mucosal surfaces. Shigella dysenteriae, for instance, whose pathogenic potential is correlated with its ability to invade and multiply within the cells of the colonic epithelium, preferentially adheres to colonic mucin (22). The observation that the presence of gastric mucin impairs HeLa cell invasion of metacyclic forms of gp82-deficient isolates, but not of gp82-expressing isolate CL (Fig. 6A), reinforces the role played by gp82 in mucosal infection. In T. cruzi developmental forms circulating in the bloodstream, the surface molecules that bind to components of the extracellular matrix would be playing a role analogous to that of the metacyclic-stage gp82. Molecules implicated in cell invasion, such as the laminin-binding Tc-85 (9) and penetrin, which has affinity for heparin, heparan sulfate and collagen (18), would help the parasites to traverse the extracellular matrices and basal laminae to reach the target cells. Overall, our results indicate that gp30 is implicated in host cell invasion of gp82-deficient metacyclic trypomastigotes but fails to promote an efficient mucosal infection due to its deficient interaction with gastric mucin.
Acknowledgments
This work was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP).
We thank Alice T. Ferreira for helping with Ca2+-signaling experiments, Marcelo R. S. Briones and Laize Tomazi for performing rRNA-based typing of T. cruzi isolates, and Renato A. Mortara for reading the manuscript.
Editor: J. M. Mansfield
REFERENCES
- 1.Akiyama, T., J. Ishida, S. Nakagawa, H. Ogawara, S. Watanabe, N. Itoh, M. Shibuya, and Y. Fukami. 1987. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J. Biol. Chem. 262:5592-5595. [PubMed] [Google Scholar]
- 2.Barbiéri, C. L., S. Giorgio, A. J. C. Merjan, and E. N. Figueiredo. 1993. Glycosphingolipid antigens of Leishmania (Leishmania) amazonensis amastigotes identified by use of a monoclonal antibody. Infect. Immun. 61:2131-2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brener, Z., and E. Chiari. 1963. Variações morfológicas observadas em diferentes amostras de Trypanosoma cruzi. Rev. Inst. Med. Trop. São Paulo 5:220-224. [PubMed] [Google Scholar]
- 4.Briones, M. R. S., R. P. Souto, B. S. Stolf, and B. Zingalez. 1999. The evolution of two Trypanosoma cruzi subgroups inferred from rRNA genes can be correlated with the interchange of American mammalian faunas in the Cenozoic and has implications to pathogenicity and host specificity. Mol. Biochem. Parasitol. 104:219-232. [DOI] [PubMed] [Google Scholar]
- 5.Coura, J. R., A. C. V. Junqueira, O. Fernandes, S. A. S. Valente, and M. A. Miles. 2002. Emerging Chagas disease in Amazonian Brazil. Trends Parasitol. 18:171-176. [DOI] [PubMed] [Google Scholar]
- 6.Docampo, R., D. A. Scott, A. E. Vercesi, and S. N. J. Moreno. 1995. Intracellular Ca2+ storage in acidocalcisomes of Trypanosoma cruzi. Biochem. J. 310:1005-1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dorta, M. L., A. T. Ferreira, M. E. M. Oshiro, and N. Yoshida. 1995. Ca2+ signal induced by Trypanosoma cruzi metacyclic trypomastigote surface molecules implicated in mammalian cell invasion. Mol. Biochem. Parasitol. 73:285-289. [DOI] [PubMed] [Google Scholar]
- 8.Favoreto, S., Jr., M. L. Dorta, and N. Yoshida. 1998. Trypanosoma cruzi 175-kDa protein tyrosine phosphorylation is associated with host cell invasion. Exp. Parasitol. 89:188-194. [DOI] [PubMed] [Google Scholar]
- 9.Giordano, R., R. Chammas, S. S. Veiga, W. Colli, and M. J. M. Alves. 1994. An acidic component of the heterogeneous Tc-85 protein family from the surface of Trypanosoma cruzi is a laminin binding glycoprotein. Mol. Biochem. Parasitol. 65:85-94. [DOI] [PubMed] [Google Scholar]
- 10.Hoft, D. F. 1996. Differential mucosal infectivity of different life stages of Trypanosoma cruzi. Am. J. Trop. Med. Hyg. 55:360-364. [DOI] [PubMed] [Google Scholar]
- 11.Hoft, D. F., P. L. Farrar, K. Kratz-Owens, and D. Shaffer. 1996. Gastric invasion by Trypanosoma cruzi and induction of protective mucosal immune responses. Infect. Immun. 64:3800-3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manque, P. M., D. Eichinger, M. A. Juliano, L. Juliano, J. E. Araya, and N. Yoshida. 2000. Characterization of the cell adhesion site of Trypanosoma cruzi metacyclic stage surface glycoprotein gp82. Infect. Immun. 68:478-484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manque, P. M., I. Neira, V. D. Atayde, E. Cordero, A. T. Ferreira, J. Franco da Silveira, M. Ramirez, and N. Yoshida. 2003. Cell adhesion and Ca2+ signaling activity in stably transfected Trypanosoma cruzi epimastigotes expressing the metacyclic stage-specific surface molecule gp82. Infect. Immun. 71:1561-1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moreno, S. N. J., J. Silva, A. E. Vercesi, and R. Docampo. 1994. Cytosolic-free calcium elevation in Trypanosoma cruzi is required for cell invasion. J. Exp. Med. 180:1535-1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mortara, R. A., S. Silva, M. F. Araguth, S. A. Blanco, and N. Yoshida. 1992. Polymorphism of the 35- and 50-kilodalton surface glycoconjugates of Trypanosoma cruzi metacyclic trypomastigotes. Infect. Immun. 60:4673-4678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neira, I., A. T. Ferreira, and N. Yoshida. 2002. Activation of distinct signal transduction pathways in Trypanosoma cruzi isolates with differential capacity to invade host cells. Int. J. Parasitol. 32:405-414. [DOI] [PubMed] [Google Scholar]
- 17.Neira, I., F. A. Silva, M. Cortez, and N. Yoshida. 2003. Involvement of Trypanosoma cruzi metacyclic trypomastigote surface molecule gp82 in adhesion to gastric mucin and invasion of epithelial cells. Infect. Immun. 71:557-561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ortega-Barria, E., and M. E. A. Pereira. 1991. A novel Trypanosoma cruzi heparin binding protein promotes fibroblast adhesion and penetration on engineered bacteria and trypanosomes into mammalian cells. Cell 67:411-421. [DOI] [PubMed] [Google Scholar]
- 19.Ramirez, M. I., R. C. Ruiz, J. E. Araya, J. Franco da Silveira, and N. Yoshida. 1993. Involvement of the stage-specific 82-kilodalton adhesion molecule of Trypanosoma cruzi metacyclic trypomastigotes in host cell invasion. Infect. Immun. 61:3636-3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruiz, R. C., S. Favoreto, Jr., M. L. Dorta, M. E. M. Oshiro, A. T. Ferreira, P. M. Manque, and N. Yoshida. 1998. Infectivity of Trypanosoma cruzi strains is associated with differential expression of surface glycoproteins with differential Ca2+ signaling activity. Biochem. J. 330:505-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Santori, F. R., M. L. Dorta, L. Juliano, M. A. Juliano, J. Franco da Silveira, R. C. Ruiz, and N. Yoshida. 1996. Identification of a domain of Trypanosoma cruzi metacyclic trypomastigote surface molecule gp82 required for attachment and invasion of mammalian cells. Mol. Biochem. Parasitol. 78:209-216. [DOI] [PubMed] [Google Scholar]
- 22.Sudha, P. S., H. Devaraj, and N. Devaraj. 2001. Adherence of Shigella dysenteriae 1 to human colonic mucin. Curr. Microbiol. 42:381-387. [DOI] [PubMed] [Google Scholar]
- 23.Tardieux, I., M. H. Nathanson, and N. W. Andrews. 1994. Role in host cell invasion of Trypanosoma cruzi-induced cytosolic free Ca2+ transients. J. Exp. Med. 179:1017-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tarentino, A. L., and F. Maley. 1974. Purification and properties of an endo-β-N-acetylglucosaminidase from Streptococcus griseus. J. Biol. Chem. 10:811-817. [PubMed] [Google Scholar]
- 25.Teixeira M. M. G., and N. Yoshida. 1986. Stage-specific surface antigens of metacyclic trypomastigotes of Trypanosoma cruzi identified by monoclonal antibodies. Mol. Biochem. Parasitol. 18:271-282. [DOI] [PubMed] [Google Scholar]
- 26.Thastrup, O., P. J. Cullen, B. K. Drobak, M. R. Hanley, and A. P. Dawson. 1990. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2+-ATPase. Proc. Natl. Acad. Sci. USA 87:2466-2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wheatley, M. 1993. Peptide mapping and the generation and isolation of sequenciable peptides from receptors, p. 213-261. In E. C. Hulme (ed.), Receptor biochemistry: a practical approach. IRL Press at Oxford University Press, Oxford, United Kingdom.
- 28.Yakubu, M. A., S. Majunder, and F. Kierszenbaum. 1994. Changes in Trypanosoma cruzi infectivity by treatment that affect calcium ion levels. Mol. Biochem. Parasitol. 66:119-125. [DOI] [PubMed] [Google Scholar]
- 29.Yoshida N., R. A. Mortara, M. F. Araguth, J. C. Gonzalez, and M. Russo. 1989. Metacyclic neutralizing effect of monoclonal antibody 10D8 directed to the 35 and 50 kilodalton surface glycoconjugates of Trypanosoma cruzi Infect. Immun. 57:1663-1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yoshida, N., S. Favoreto, Jr., A. T. Ferreira, and P. M. Manque. 2000. Signal transduction induced in Trypanosoma cruzi metacyclic trypomastigotes during the invasion of mammalian cells. Braz. J. Med. Biol. Res. 33:269-278. [DOI] [PubMed] [Google Scholar]