Abstract
Cattle may provide a suitable model for testing ways of improving tuberculosis vaccine efficacy in human infants. A vaccination and challenge study was undertaken in calves to determine the optimal time to vaccinate neonatal animals with Mycobacterium bovis bacillus Calmette-Guérin (BCG) for protection against tuberculosis and to determine whether revaccination with BCG was beneficial. Calves (10 per group) were vaccinated with BCG within 8 h of birth or at 6 weeks of age, when immune responses to antigens of environmental mycobacteria were detectable, or vaccinated at birth and revaccinated at 6 weeks. A control group was not vaccinated. BCG vaccination at birth induced strong antigen-specific gamma interferon (IFN-γ) and interleukin-2 (IL-2) responses and antigen-specific activation in CD4+, CD8+, and WC1+ γδ T-cell subsets from blood. The proportions of animals per group with macroscopic tuberculous lesions after challenge were 0/10 for BCG at birth, 1/9 for BCG at 6 weeks, 4/10 for the revaccinated group, and 10/10 for the nonvaccinated group. There was no significant difference in the levels of protection between groups vaccinated at birth or at 6 weeks, while animals vaccinated both at birth and at 6 weeks had significantly less protection than those vaccinated only at birth. The revaccinated calves that subsequently developed tuberculous lesions had significantly stronger IFN-γ and IL-2 responses to bovine purified protein derivative after the BCG booster than those in the same group that did not develop lesions. The results indicated that BCG vaccination at birth induced a high level of immunity and that the sensitization of very young animals to antigens of environmental mycobacteria by 6 weeks of age did not affect the effectiveness of BCG. However, BCG revaccination of these young animals was contraindicated.
Human tuberculosis, predominantly caused by Mycobacterium tuberculosis, continues to be a major health problem on a global scale, affecting people of all ages. The development of effective vaccination strategies and vaccines will be key to achieving true control, even with early, accurate diagnosis and effective treatment (15). The Mycobacterium bovis bacillus Calmette-Guérin (BCG) vaccine, an attenuated strain of M. bovis, was developed for control of human tuberculosis more than 70 years ago and is still the only tuberculosis vaccine available. BCG is the world's most widely used human vaccine, with an estimated 3 billion doses having been administered, yet despite its widespread use, the efficacy of BCG varies, with success rates in human trials ranging between 0 and 80% protection. Meta-analysis of human BCG vaccination trials revealed a reduction in the risk of tuberculosis with vaccination and a strong association between early life BCG vaccination and reduced risk of meningeal and miliary tuberculosis in children (12, 13). The strategy of vaccinating neonates with BCG in developing countries has well-recognized health benefits and is likely to be continued, although it is generally acknowledged that BCG vaccine efficacy against pulmonary tuberculosis is highly variable. Until new, improved tuberculosis vaccines are developed, it is crucial that the most effective ways of using BCG are discovered.
There are a number of reasons proposed for the variation in the efficacy of BCG. One of the most compelling explanations is interference with or masking of protection by environmental mycobacterial infections (4, 14). Recent studies with mice and cattle have shown that prior exposure to environmental mycobacteria reduced the efficacy of BCG in protecting against tuberculosis (5, 8). The problem remains that the highest prevalence of human tuberculosis is in Africa, Asia, and Central America, where there is a high incidence of exposure to environmental mycobacteria. These countries are in the greatest need of an effective vaccine against human tuberculosis, yet it is precisely in these places that BCG vaccine has a low efficacy against pulmonary tuberculosis. Infants in Africa as young as 2 to 4 months are likely to be exposed to environmental mycobacteria (25). The timing of BCG vaccination could be critical. It may be preferable to vaccinate infants as neonates prior to any exposure to environmental mycobacteria, but at this age the type and degree of immune response may not be optimal.
It is thought that protection against tuberculosis requires the induction of Th1 immune responses characterized by the production of gamma interferon (IFN-γ) (1). Studies with newborn mice have shown that they preferentially develop Th2-type responses following immunization and are deficient in Th1 responses (2, 3). Relatively little is known about helper T-cell responses in human neonates. One study has shown that Th1-type responses could be defective in human neonates, as cord blood-derived dendritic cells have a profound defect in the production of interleukin-12 (IL-12), a cytokine playing a central role in the differentiation of Th1 lymphocytes (16). In contrast, BCG vaccination of infants at birth induced a potent Th1-type immune response with a magnitude similar to that achieved with vaccination later in life (25, 35). However, it is difficult to assess whether this finding translates into protection against tuberculosis as there are no reliable correlates of protective immunity and protection studies dependent on natural exposure to M. tuberculosis may take many years to complete.
There are few animal models for testing tuberculosis vaccines where neonates are immunocompetent and naturally sensitized to antigens of environmental mycobacteria, a model that mimics the human situation. Tuberculosis in cattle is a good model to use for improving tuberculosis vaccine efficacy in humans as the disease has a similar pathogenesis and the kinetics of immune responses induced by vaccination and challenge can be readily measured (19). Cattle are a natural host for tuberculosis, and the causative microorganism, M. bovis, produces tuberculosis in humans and is closely related to M. tuberculosis within the tuberculosis complex. Calves are immunocompetent at birth and are naturally sensitized to antigens of environmental mycobacteria at a young age. By 6 weeks of age, calves usually show a strong immunological response to such antigens. This is not to say that the immune systems of cattle and humans or the disease caused by M. tuberculosis and M. bovis are the same. In calves less than 3 weeks of age, γδ T cells constitute about 25% of the mononuclear cells in the peripheral blood but <5% of the cells in the thymus, spleen, and lymph nodes (11). In humans, γδ T cells are evenly distributed throughout the lymphoid system and range between 0.5 and 16% of CD3+ cells in the thymus, peripheral lymphoid organs, and blood (18). Although the distribution of lesions in humans resulting from infection with M. tuberculosis and M. bovis varies, cases of pulmonary disease due to M. tuberculosis and M. bovis are indistinguishable clinically, radiographically, and pathologically (28). In this study, we used calves as a model to optimize BCG vaccination of neonates. Results for BCG vaccination within 8 h of birth were compared with results for vaccination at 6 weeks of age or vaccination at birth and revaccination at 6 weeks of age. The calves were challenged intratracheally with a low dose of virulent M. bovis at 14 to 17 weeks of age, killed 16 weeks after challenge, and examined for tuberculous lesions. Vaccine effectiveness was assessed from the presence and distribution of tuberculous lesions and the culture of M. bovis from tissues.
MATERIALS AND METHODS
Animals.
Forty Friesian-cross calves were removed from their mothers 4 to 12 h after birth and taken to a calf-rearing facility. The cows were from a tuberculosis-free accredited herd in an area of New Zealand where both farmed animals and wildlife were free of tuberculosis. The calves were born over a 4-week period and fed pooled colostrum for the first 4 weeks and then maintained on whole milk for a further 4 weeks. Meal was provided to the calves from day 1 to 10 weeks of age, until they were weaned onto a pasture-only diet. The calves were kept on wood shavings in a calf shed for the first week and on pasture thereafter. One week prior to the M. bovis challenge, the calves (13 to 16 weeks of age) were moved to a high-security containment unit, where they grazed on pasture. Animal ethics approval was granted for all animal manipulations.
Bacterial strains.
The M. bovis BCG strain, Pasteur 1173P2, was the vaccine strain, and M. bovis WAg202, originally isolated from a tuberculous possum (Trichosurus vulpecula) in New Zealand, was the challenge strain. These strains were used in previous vaccination and challenge studies with cattle (6, 7, 38). For our use, they were grown to mid-log phase in Tween albumin broth (Dubos broth base; Difco Laboratories, Detroit, Mich.) supplemented with 0.006% (vol/vol) alkalinized oleic acid, 0.5% (wt/vol) albumin fraction V, and 0.25% (wt/vol) glucose. Dilutions were made in Tween albumin broth to obtain the appropriate doses for inoculating the cattle. The number of CFU per inoculation was determined retrospectively by plating tenfold dilutions on Middlebrook 7H11 agar (Difco) supplemented with 0.5% (wt/vol) albumin, 0.2% (wt/vol) glucose, and 1% (wt/vol) sodium pyruvate.
Vaccination.
The 40 calves were randomly divided into four groups of 10 animals. The animals in groups 1 and 2 were vaccinated subcutaneously in the neck with 106 CFU of BCG within 8 h of birth. The animals in group 2 were revaccinated with BCG by using a similar route and dose at 6 weeks of age. The animals in group 3 were vaccinated subcutaneously with 106 CFU of BCG at 6 weeks of age, and the animals in group 4 served as nonvaccinated controls. One calf from group 3 died from viral pneumonia at 9 weeks of age. Heparinized blood samples and sera were collected from the calves at 0, 2, 4, 6, 9, and 12 weeks of age and used to analyze cellular and humoral immune responses. After collection, the sera were stored at −20°C until tested.
M. bovis challenge and necropsy procedure.
The calves were challenged intratracheally with 5 × 103 CFU of virulent M. bovis as previously described (6). The challenge was carried out over a 2-day period when the calves were between 14 and 17 weeks of age. Briefly, an 80-cm endotracheal tube containing a fine cannula was inserted per os into the trachea of an anesthetized animal. A 1.5-ml inoculum containing the M. bovis strain was injected through the cannula and flushed out with 2 ml of saline. Heparinized blood and sera were collected from the animals at 0, 2, 5, 10, and 15 weeks after challenge. All the cattle were killed humanely 16 weeks after challenge and necropsied. The individual performing necropsy examinations was unaware of the vaccination status of the animal. The procedures used for identifying macroscopic tuberculous lesions and processing for histopathology have been described previously (8). Samples from four thoracic lymph nodes (left and right bronchial and anterior and posterior mediastinal) were collected from all of the animals for bacterial culture and histology. Additional samples were collected from any tuberculous lesions observed in lungs or other lymph nodes or organs. For bacterial culture, the tissue samples were homogenized in a Tenbroeck grinder (Wheaton, Millville, N.J.), decontaminated in 0.75% cetylpyridinium chloride for 1 h, centrifuged at 3500 × g for 20 min, and processed for isolation of mycobacteria as described previously (6).
Antibody ELISA.
A culture filtrate was prepared from M. bovis strain AN5, the strain used for the production of purified protein derivative from M. bovis (bovine PPD) for skin testing in New Zealand. The culture filtrate was diluted to 3 μg/ml in carbonate buffer (pH 9.6); 100 μl per well was added to 96-well enzyme-linked immunosorbent assay (ELISA) plates (Maxisorb; Nunc, Roskilde, Denmark), and the plates were incubated overnight at 4°C. The antibody ELISA was carried out as described previously (39). The results were calculated as percentages by expressing the value found for the test serum as a fraction of the value for the binding of a strong-positive reference serum and multiplying by 100 (6).
IFN-γ and IL-2 assays.
Heparinized blood (1.5 ml) was dispensed into three wells of a 24-well plate 20 to 24 h after collection of the blood, and 100 μl of bovine PPD or PPD prepared from Mycobacterium avium (avian PPD; final concentration, 20 μg/ml; IFN-γ assay kit; CSL Ltd., Parkville, Victoria, Australia) or phosphate-buffered saline (PBS) was added. The whole blood cultures were incubated at 37°C for 24 h, and IFN-γ levels in the plasma supernatants were measured by using a sandwich ELISA kit (CSL Ltd.) as described previously (30). The amount of IFN-γ was calculated by reference to a standard curve prepared from the results for recombinant bovine IFN-γ (37).
The levels of IL-2 in the plasma supernatants from the whole blood cultures was assayed by using a bioassay as described previously (37). Briefly, triplicate wells containing 104 concanavalin A-stimulated 4-day lymphoblasts were incubated in 200 μl of supplemented RPMI 1640 medium (RPMI 1640 medium containing 100 U of penicillin/ml, 100 μg of streptomycin/ml, 2 mM glutamine, and 10% fetal calf serum; Gibco Invitrogen, Grand Island, N.Y.) with 1:10 dilutions of supernatant. After incubation for 24 h, 0.25 μCi of [3H]thymidine was added to each well, and the cultures were harvested 18 nb h later. The amount of tritiated thymidine incorporated was determined by using a liquid β-scintillation counter (Micro Beta, Wallac, Finland). The results were expressed as a stimulation index (SI), defined as mean counts per minute for bovine PPD plasma supernatant/mean counts per minute for PBS plasma supernatant. Addition of a monoclonal antibody against bovine IL-2 to the concanavalin A-stimulated lymphoblasts immediately before addition of the plasma supernatants has been shown to block the IL-2 bioactivity.
Real-time PCR and quantification of cytokine gene expression.
Heparinized blood was collected randomly from eight BCG-vaccinated calves and six nonvaccinated calves when the calves were 4 weeks of age. Peripheral blood mononuclear cells (PBMCs) were obtained by centrifugation of blood on a Lymphoprep 1.077 (Axis-Shield, Oslo, Norway). The purified PBMCs were cultured at a density of 2 × 106 cells/ml in supplemented RPMI 1640 medium. PBMCs (10 ml) were dispensed into two 25-cm2 cell culture flasks, and 720 μl of PBS or bovine PPD (300 μg/ml; CSL Ltd.) was added. The cultures were incubated for 20 h. Total RNA was prepared by using TRIzol (Invitrogen, Carlsbad, Calif.), and cDNA was synthesized by using oligod(T)-primed RNA (3 μg) and 200 U of SuperScript II reverse transcriptase (Invitrogen). Cytokine gene expression was measured by real-time PCR by using a GeneAmp 5700 sequence detection system and sequence detector software (Applied Biosystems, Foster City, Calif.). The primers used for analysis of IFN-γ were 5′-TGATTCAAATTCCGGTGGATG-3′ (forward primer) and 5′-TTCATTGATGGCTTTGCGC-3′ (reverse primer). The primers for IL-4 were 5′-TCGCAAGCAAGACCTGTTCTG-3′ (forward) and 5′-GCGTACTTGTGCTCGTCTTGG-3′ (reverse), and the expression of the housekeeping GAPDH (glyceraldehyde-3-phosphate dehydrogenase) gene was measured by using primers 5′-CACCATCTTCCAGGAGCGAG-3′ (forward) and 5′-CCAGCATCACCCCACTTGAT-3′ (reverse). Reactions (25 μl) were assembled in duplicate in optical 96-well reaction plates by using AmpErase uracil N-glycosylase (Applied Biosystems) and SYBR green PCR Master mix (Applied Biosystems). Quantitative analysis of cytokine expression was performed by using the comparative CT method, in which CT is the threshold cycle number (minimum number of cycles before the product can be detected). The amount of cytokine mRNA expressed in cDNA from both nonstimulated (PBS) and bovine PPD-stimulated PBMC cultures was normalized to the levels of GAPDH mRNA. Bovine PPD-specific expression of IFN-γ and IL-4 was then stated as relative quantitative values calculated as 2(ΔΔCT), where (ΔΔCT) is defined as (CT cytokine PBS − CT GAPDH PBS) − (CT cytokine bovine PPD − CT GAPDH bovine PPD).
IL-2 receptor assay.
The expression of IL-2 receptor (CD25) on T-cell subsets was used as a measure of T-cell activation for specific T-cell subsets. Heparinized blood samples were collected randomly from eight BCG-vaccinated calves and six nonvaccinated calves at 4 weeks of age. The PBMCs were purified as described above for cytokine mRNA expression. PBMCs in 2 ml of supplemented RPMI 1640 medium were dispensed into two wells of 24-well plates, 140 μl of PBS or bovine PPD (final concentration, 20 μg/ml; CSL Ltd.) was added, and the cultures were incubated at 37°C for 72 h. From each of four of the nonvaccinated calves, an additional well containing PBMCs was prepared, and 140 μl of avian PPD (final concentration, 20 μg/ml; CSL Ltd.) was added per well. The expression of IL-2 receptors on T cells was determined by cell surface double staining with antibodies against CD25 (CACT 116A-IgG1; VMRD Inc., Pullman, Wash.) and CD4 (CC8-IgG2a), CD8 (CC63-IgG2a), or WC1 γδ (CC15-IgG2a) followed by isotype-specific antibodies conjugated with phycoerythrin (for CD4, CD8, and WC1 γδ; Southern Biotechnology, Birmingham, Ala.) or with fluorescein (for CD25 staining; Pharmingen, San Diego, Calif.). The anti-CD4 and anti-CD8 antibodies were provided by Karl Walravens, Brussels, Belgium, and the anti-WC1 γδ antibody was provided by Chris Howard, Compton, United Kingdom. The stained cells were analyzed by flow cytometry with a Becton Dickinson FACsCalibur.
Tuberculin skin test.
Delayed hypersensitivity to bovine PPD was measured when the calves were approximately 12 weeks of age and at 15 weeks after the M. bovis challenge (1 week prior to slaughter). The animals were inoculated intradermally in the side of the neck with a 0.1-ml volume containing 100 μg of bovine PPD, which is used for the tuberculin skin-testing of cattle in New Zealand (AgriQuality, Upper Hutt, New Zealand). Delayed hypersensitivity responses were expressed as the difference in skin fold thickness between the time of inoculation and 72 h later.
Statistical analyses.
Analyses of antibody levels, IFN-γ and IL-2 responses, cytokine mRNA data, and bacterial counts were done by performing analysis of variance on log10-transformed data. For analysis of skin tuberculin test responses, expression of CD25 on T-cell subsets, and lung lesion scores, analysis of variance was performed on the raw data. The proportion of animals with lesions was analyzed by using Fisher's exact test with pairwise comparisons. Statistical significance for all tests was set at a P value of <0.05.
RESULTS
Immune responses to avian PPD prior to vaccination.
Blood samples collected within 24 h of birth were tested by the whole blood IFN-γ assay for responses to avian PPD. Only 2 of the 39 calves had an IFN-γ response to avian PPD of >0.15 ng/ml from whole blood cultures. The IFN-γ responses of calves that had not been vaccinated with BCG at birth (the nonvaccinated group and the group vaccinated with BCG at 6 weeks) were monitored for responses to avian PPD over the next 6 weeks. The cumulative frequency of calves with responses of >0.15 ng of IFN-γ/ml increased from 5% within 24 h of birth to 95% at 6 weeks of age. When these calves produced a high IFN-γ response to avian PPD (>0.15 ng of IFN-γ/ml) in this period, their response to bovine PPD was always lower. The mean IFN-γ responses to avian PPD increased significantly from birth to 6 weeks of age (P < 0.05), from a mean ± standard error of the mean (SEM) of 0.10 ± 0.03 ng/ml at birth to 0.68 ± 0.17 ng/ml at 6 weeks.
T-cell responses after vaccination and challenge.
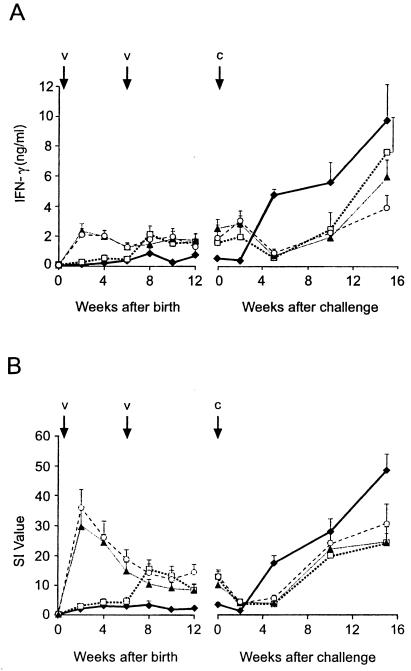
In order to determine whether there was any association between IFN-γ or IL-2 responses and protection against experimental infection with M. bovis, these cytokine responses were measured at regular intervals during the vaccine trial. Differences were observed in the IFN-γ and IL-2 responses (Fig. 1) to bovine PPD between the different groups, but the pattern of the responses for the two cytokines was similar. The two groups vaccinated with BCG at birth produced significantly higher mean peripheral blood IFN-γ and IL-2 responses to bovine PPD from 2 weeks after birth until challenge than those for the nonvaccinated group (P < 0.05). The group vaccinated with BCG only at 6 weeks of age produced significantly higher mean IFN-γ and IL-2 responses than those for the nonvaccinated group from 8 weeks after birth until challenge (P < 0.05). There were no significant differences between the mean IFN-γ and IL-2 responses of the three BCG-vaccinated groups between 8 weeks of age to challenge. After challenge, the mean IFN-γ and IL-2 responses of the nonvaccinated group were significantly lower than those of the three BCG-vaccinated groups at 2 weeks and significantly higher at 5 and 10 weeks after challenge. At 15 weeks after challenge, only the mean IL-2 response of the nonvaccinated group was significantly higher than those of the three BCG-vaccinated groups.
FIG. 1.
Levels of IFN-γ (A) and IL-2 (B) released from bovine PPD-stimulated whole blood cultures from animals vaccinated with BCG at birth (▴), at 6 weeks of age (□), and at birth and 6 weeks of age (○) and from nonvaccinated animals (⧫). IFN-γ levels are presented as mean concentrations in plasma (nanograms per milliliter), and IL-2 levels are presented as mean SIs. The bars above the data points represent SEMs. V represents BCG vaccination dates (0 and 6 weeks), and C represents the challenge with M. bovis, which was undertaken when the calves were 14 to 17 weeks of age.
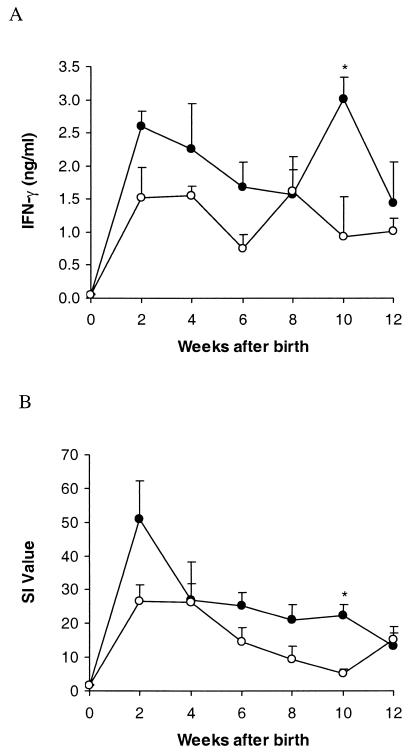
Since calves from the group revaccinated with BCG were poorly protected against development of tuberculous lesions, the T-cell responses of these calves after vaccination were analyzed to determine whether there were any links between immune responses and protection. The mean peripheral blood IFN-γ and IL-2 responses to bovine PPD after vaccination of the four animals that subsequently developed macroscopic tuberculous lesions and of those that did not develop lesions are shown in Fig. 2. The mean IFN-γ and IL-2 responses of the lesioned animals at 10 weeks after birth (4 weeks after revaccination with BCG) were significantly higher than those of the nonlesioned animals from the same group (P < 0.05). The mean IFN-γ and IL-2 responses of the lesioned animals were generally higher than those of the nonlesioned animals after challenge, but the mean IFN-γ response was significantly higher at only one time point, 5 weeks after challenge (data not shown).
FIG. 2.
Comparison of IFN-γ (A) and IL-2 (B) responses after vaccination for the animals from the group vaccinated at birth and at 6 weeks of age which subsequently developed tuberculous lesions after challenge (•) and those without lesions (○). IFN-γ and IL-2 were released from whole blood cultures stimulated with bovine PPD. IFN-γ levels are presented as mean concentrations in plasma (ng/ml), and IL-2 levels are presented as mean SIs. The bars above the data points represent SEMs. An asterisk (*) indicates an IFN-γ or IL-2 level significantly different from that for the nonvaccinated group (P < 0.05).
Cytokine mRNA expression.
Quantitative IFN-γ and IL-4 mRNA expression from PBMCs stimulated with bovine PPD was measured at 4 weeks after birth to determine whether a Th1- or Th2-type immune response was induced by vaccination with BCG. For calves vaccinated with BCG, a significantly higher expression of both IFN-γ and IL-4 mRNA was induced than for the nonvaccinated animals (P < 0.05) (Table 1).
TABLE 1.
Quantitative expression of IFN-γ and IL-4 mRNA at 4 weeks after birth in PBMCs of calves stimulated with bovine PPD
| Group (no.) | mRNA expression fora:
|
||
|---|---|---|---|
| IFN-γ | IL-4 | IFN-γ/IL-4 | |
| Nonvaccinated (6) | 8.3 ± 2.1 | 1.4 ± 0.2 | 6.7 ± 2.1 |
| BCG-vaccinatedb (8) | 339.6c ± 155.3 | 18.0c ± 4.3 | 19.0 ± 7.5 |
The results are expressed as relative quantitation values, means ± SEMs.
These calves were vaccinated with BCG at birth.
The mean was significantly greater than the mean for the nonvaccinated animals (P < 0.05).
Expression of IL-2 receptor (CD25) on T-cell subsets.
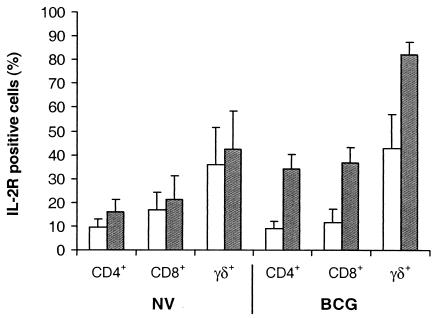
When the calves were 4 weeks of age, vaccination with BCG resulted in a significant increase in CD25 expression on CD4+, CD8+, and WC1+ γδ T cells from PBMC cultures stimulated with bovine PPD compared to that for nonvaccinated animals (P < 0.05) (Fig. 3). The increase in CD25 expression on T cells following stimulation with bovine PPD cultures was calculated as the difference in the percentage of T cells positive for expression of CD25 in bovine PPD-stimulated cultures and nonstimulated PBMC cultures. For the 14 animals sampled, the mean percentages (±SEM) of CD4+, CD8+, and WC1+ γδ T cells in the nonstimulated PBMC cultures were 20.1 ± 2.8, 16.2 ± 2.1, and 23.0 ± 3.8, respectively. One of the nonvaccinated animals tested was an avian PPD-responder in the IFN-γ assay, and this animal showed a marked increase in CD25 expression on CD4+, CD8+, and WC1+ γδ T cells from PBMC cultures stimulated with avian PPD compared to that for the nonstimulated cultures (data not shown).
FIG. 3.
Mean percentages of CD4+, CD8+, and WC1+ γδ T cells from PBMCs of nonvaccinated (NV) and BCG-vaccinated (BCG) calves expressing CD25 (IL-2 receptor) following culture with (black column) or without (white column) bovine PPD. The bars above the columns represent SEMs.
Antibody responses.
The mean antibody responses to M. bovis culture filtrate for the different vaccine groups were not significantly different at any time point, although the antibody levels changed markedly during the course of the study (data not shown). The calves acquired antibodies from the ingestion of colostrum over the first few days after birth, and antibody levels decreased rapidly between 2 and 8 weeks after birth. From challenge with M. bovis to 15 weeks after challenge, there was a significant increase in the mean antibody levels for all of the animals (P < 0.01). The cows were all free of bovine tuberculosis, and their prechallenge antibody responses were to cross-reactive antigens.
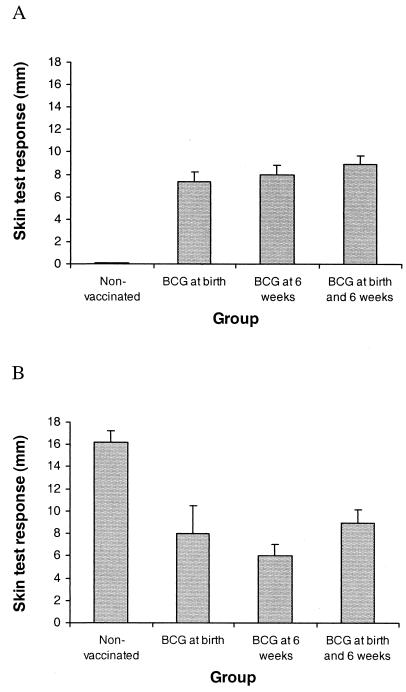
Tuberculin skin test responses.
All three BCG-vaccinated groups had mean skin test responses which were significantly greater than those for the nonvaccinated group at 12 weeks after the initial vaccination but significantly less than those for the nonvaccinated group at 15 weeks after challenge (P < 0.01) (Fig. 4). All BCG-vaccinated animals produced an increase in skin fold thickness of ≥3 mm at 12 weeks after birth, while none of the nonvaccinated animals produced a response of ≥1 mm at the same age. For the BCG-vaccinated animals, there was no correlation between the skin test response at 12 weeks after birth and susceptibility to a subsequent M. bovis challenge, in either the presence of macroscopic lesions or in culture positivity for M. bovis.
FIG. 4.
Effects of vaccination and M. bovis challenge on mean skin test responses to bovine PPD at 11 weeks after the first vaccination (A) or 15 weeks after challenge (B). Data are expressed as the increase in skin thickness (mm) between the time of inoculation and 72 h later. The bars above the columns represent SEMs.
Pathological and microbiological findings.
Following challenge with M. bovis, macroscopic tuberculous lesions were found only in the lungs and thoracic lymph nodes of the calves. The lung lesions consisted of small nodules 2 to 5 mm in diameter with yellow caseous centers, and the lymph node lesions were 1 to 35 mm in diameter with yellow calcified caseous centers. The mean number (±SEM) of lung lesions for the nonvaccinated group was 39 ± 11 (range 1 to 112). No lung lesions were found in animals from the groups vaccinated with BCG at birth or at 6 weeks. The three animals with lung lesions in the revaccinated group had 35, 43, and 80 lung lesions. A comparison of the pathological and microbiological findings for the different vaccine groups following challenge is shown in Table 2. All BCG-vaccinated groups had a significant level of protection, as shown in seven of the eight parameters displayed in Table 2, compared to that for the nonvaccinated group (P < 0.05). The vaccinated groups included a smaller proportion of animals with lung or lymph node lesions, a lower mean lesion score for the lungs or lymph nodes, a lower mean number of lesioned lymph nodes per animal, a smaller proportion of their thoracic lymph nodes culture positive for M. bovis, and a lower mean bacterial count for the thoracic lymph nodes than those for the nonvaccinated group. Even when only the lesioned lymph nodes were considered, the mean bacterial count (±SEM) for these nodes for the group revaccinated with BCG (2.809 ± 0.173 log10 CFU/g of tissue) was significantly less than that for the nonvaccinated group (3.292 ± 0.098 log10 CFU/g of tissue; P < 0.05). Comparison between the BCG-vaccinated groups showed that a single vaccination at birth induced greater protection than vaccination with BCG at birth and revaccination at 6 weeks, with significant differences in six of the eight parameters displayed in Table 2 (P < 0.05).
TABLE 2.
Pathological and microbiological findings following challenge of calves with M. bovis
| Vaccine group | Proportion of animals with:
|
Mean lung scorea | Mean LN scoreb | Mean no. of lesioned LN per animalc | Proportion of animals with cultures positive for M. bovis | Proportion of thoracic lymph node cultures positive for M. bovis | Mean bacterial count for thoracic lymph nodesd | |
|---|---|---|---|---|---|---|---|---|
| Lung lesions | LN lesions | |||||||
| Nonvaccinated | 10/10 | 10/10 | 2.40 ± 0.31 | 3.70 ± 0.15a | 3.00 ± 0.26a | 10/10 | 31/40 | 2.898 ± 0.168 |
| BCG at birth | 0/10e | 0/10e,f | 0e,f | 0e,f | 0e,f | 6/10 | 12/40e,f | 0.893 ± 0.062e,f |
| BCG at 6 weeks | 0/9e | 1/9e | 0e,f | 0.11e ± 0.26 | 0.22 ± 0.22e,f | 8/9 | 14/36e | 1.279 ± 0.136e |
| BCG at birth and at 6 weeks | 3/10e | 4/10e | 0.90 ± 0.46e | 0.50 ± 0.22e | 1.20 ± 0.49e | 9/10 | 21/40e | 1.463 ± 0.157e |
Lung lesion score: 0, no lesions; 1, 1 to 9 lesions; 2, 10 to 29 lesions; 3, 30 to 99 lesions; 4, 100 to 199 lesions.
Lymph node (LN) lesion score for the most severely affected lymph node per animal: 0, no lesions; 1, 1 to 19 small lesions (1- to 4-mm diameter); 2, ≥20 small lesions; 3, medium size lesion (5- to 9-mm diameter); 4, large lesion (≥10-mm diameter).
Mean number of lymph nodes with macroscopic tuberculous lesions per animal.
Mean bacterial count, log10 CFU/gram of tissue (when no bacteria were isolated, a count of 0.699 log10 CFU/g was recorded).
Significantly less than for the nonvaccinated group (P<0.05).
Significantly less than for the group vaccinated with BCG at birth and at 6 weeks (P>0.05).
Histologically, the tuberculous granulomata in the lung and lymph node lesions had a central necrotic area which was usually mineralized. The necrotic areas were surrounded by a wide band of granulomatous tissue containing epithelioid macrophages and lymphocytes as well as small numbers of neutrophils. The lesions were walled off by fibrosis. When lesions were found in the BCG-vaccinated animals, there was an approximately two- to threefold increase in the number of neutrophils in the granulomatous tissue of the lymph node lesions compared to that for the nonvaccinated animals. This finding was consistent for the one lesioned animal that received a single BCG vaccination at 6 weeks and the lesioned animals that were vaccinated with BCG at birth and revaccinated at 6 weeks. No difference could be seen in the numbers of neutrophils from the lung lesions in the BCG-vaccinated and nonvaccinated animals.
DISCUSSION
Infants in developing countries may be exposed to M. tuberculosis infection at a very early age, and it is important to optimize the vaccination strategy for protection of young children against tuberculosis. Factors that may influence the optimal timing of BCG vaccination include the possible immaturity of the immune system at birth and interference with the development of protective immunity by exposure to environmental mycobacteria in the months following birth. In the present study, vaccination of calves at birth or 6 weeks of age induced a high level of protection against challenge with virulent M. bovis. Although the level of protection against the development of macroscopic lesions was very high in these two BCG-vaccinated groups, vaccination did not induce sterile immunity, as M. bovis was cultured from the majority of these animals. Most of the calves vaccinated at 6 weeks of age had a preexisting cellular immune response to avian PPD, indicating immune responses to antigens of environmental mycobacteria, yet there was no significant difference in the level of protection between this group and those vaccinated at birth. Surprisingly, 4 of the 10 calves vaccinated with BCG at birth and revaccinated at 6 weeks of age developed macroscopic tuberculous lesions after challenge and this group had significantly less protection than those vaccinated only at birth. Revaccination with BCG in these young calves reduced the level of protection induced by a single vaccination.
Calves vaccinated at birth with BCG produced elevated IFN-γ and IL-2 responses from whole blood cultures stimulated with bovine PPD that were comparable to those for calves vaccinated at 5 to 8 months of age (6, 7). However, the IFN-γ response did not show the rapid decline at 4 to 6 weeks after vaccination usually seen in older calves. This finding implied that the BCG infection might be cleared more slowly in these young animals. The small rise in mean IFN-γ responses for the BCG-vaccinated animals at 2 weeks after challenge compared to those for the nonvaccinated group suggested that the M. bovis challenge induced an anamnestic immune response in the vaccinated animals.
BCG vaccination of the calves induced a mixed Th1/Th2-type immune response with increased expression of both IFN-γ and IL-4 mRNA from PBMCs stimulated with bovine PPD. Analysis of CD25 expression by flow cytometry for the different T-cell subsets indicated that BCG vaccination induced the antigen-specific activation of CD4+, CD8+, and WC1+ γδ T cells. The level of protection in the calves vaccinated at birth was higher than has been observed in our previous trials, where calves were vaccinated at 5 to 8 months of age. In seven previous trials where BCG was used in 5- to 8-month-old calves, the proportion of BCG-vaccinated calves with tuberculous lesions was approximately half the proportion of nonvaccinated calves with the lesions (9). A direct comparison within the same experiment is required to define the optimal time for vaccination.
An important finding from the present study is that natural sensitization of young calves to environmental mycobacterial antigens during their first 6 weeks of life did not influence protection subsequently induced by BCG vaccination. The high susceptibility of the nonvaccinated animals suggested that the sensitization by itself did not induce protection against tuberculosis. The present results differed from those of an earlier calf vaccination study (8) where BCG vaccination failed to induce protection in much older calves (5 to 6 months of age) that had preexisting cellular immune responses to avian PPD. The influence of environmental mycobacteria on protective immunity is likely to be complex. It has been shown that different strains of these mycobacteria can induce varied effects, with some strains masking the protective effect of BCG (29) and other strains being directly antagonistic to protection induced by BCG (5, 32). The age of the calves at vaccination may also be important in the relationship between the response to environmental mycobacterial antigens and protection induced by BCG. Many uninfected calves less than 3 months of age can produce an IFN-γ response to avian and bovine PPD as well as to specific antigens of the M. tuberculosis complex, such as ESAT-6 and CFP10 (27; G. de Lisle and B. Buddle, unpublished data). Neonatal animals are exposed to a variety of new antigens resulting in a high degree of activation of peripheral blood lymphocytes. These cells may be more easily triggered to release IFN-γ in response to mycobacterial antigens or to cross-reactive antigens than those from older animals. The response of calves 5 months or older is more likely to be a specific response to environmental mycobacteria, as it is often associated with a movement of animals to a new location or a change in feed.
Another important finding from this study was the reduced protection observed in the calves revaccinated with BCG. A possible explanation for this lack of protection in some animals may be found in the T-cell responses of revaccinated animals that subsequently developed tuberculous lesions. The lesioned animals had significantly higher IFN-γ and IL-2 responses to bovine PPD at 4 weeks after revaccination than those from the same group without lesions. The high level of immune reactivity after revaccination for those that developed tuberculous lesions indicated that this lack of protection may have resulted from an immunopathological response following challenge. Alternatively, the cells secreting high levels of IFN-γ may not have developed into long-term memory cells (40). The lack of protection associated with revaccination is in contrast to the results of Griffin et al. (17), who showed that BCG revaccination of 6- to 12-month-old deer calves, with 8 weeks between vaccinations, enhanced protection against an experimental M. bovis challenge. The difference in the protection levels may be related to the age at which the animals were initially vaccinated. The kinetics of IFN-γ responses in the present study suggested that the initial BCG infection may have been cleared more slowly in these neonatal animals and that revaccination in this situation may have triggered an inappropriate immune response.
The tuberculous lymph node lesions from the revaccinated calves and the one vaccinated calf with a lesion contained higher numbers of neutrophils than did the lesions from the nonvaccinates. This finding suggested that a more inflammatory response was mounted. A more severe pyogranulomatous response has been seen in the lungs of mice infected by aerosol with M. tuberculosis and subsequently given repeated vaccinations with BCG (34). A stronger neutrophil response has also been observed in the early development of tuberculous lesions in cattle (10). Thus, the lesions in the vaccinated animals could be interpreted as undergoing a more degenerative reaction or could be at an earlier stage of development than those in the nonvaccinated animal. Despite the increased number of neutrophils in these lesions, the mean bacterial count was significantly lower in these lesioned lymph nodes than in the lesions from the nonvaccinated animals.
Revaccination of humans with BCG remains a contentious issue. A number of studies have failed to detect any protective effects of BCG revaccination (20, 23, 31, 33). By contrast, two other studies, one in Hungary (24) and one in Poland (21), concluded that BCG revaccination might be useful. Extrapolation from the results of the present study indicates that revaccination of infants with BCG may be contraindicated. However, the time interval between vaccination and revaccination may be crucial in determining whether protection is reduced or enhanced, and the interval in the present study was relatively short (6 weeks). Further studies are required to define the boundaries of the conditions which contributed to revaccination having a detrimental effect on protection. In another situation, recombinant BCG is being considered as a vaccine vector to immunize against a range of other human diseases (26). Repeated immunizations of infants with these types of vaccines may adversely affect protection against tuberculosis.
Identification of an immune correlate of protection against tuberculosis would greatly facilitate development of an improved tuberculosis vaccine; however, finding such a correlate has proved difficult. In the present study, BCG vaccination was associated with enhanced whole blood IFN-γ and IL-2 responses as well as skin test responses to bovine PPD, but within the BCG-vaccinated groups, these responses were not correlated with protection. In fact, the IFN-γ and IL-2 responses after revaccination were more closely associated with a lack of protection. Recent BCG vaccination studies in nonhuman primates have also shown a lack of correlation between peripheral blood IFN-γ responses to PPD and protection against tuberculosis (22). Following challenge in the present study, the elevated immune responses were more closely associated with disease severity. From 5 weeks after M. bovis challenge, IFN-γ, IL-2, and skin test responses were significantly higher in the nonvaccinated group, which had the highest level of disease. Vordermeier et al. (36) recently reported that ESAT-6-specific IFN-γ production from whole blood cultures of cattle was closely correlated with the severity of disease following M. bovis challenge. In the present study, antibody responses to M. bovis culture filtrate were not influenced by BCG vaccination and increased in all groups after challenge.
The present study is the first to assess the effectiveness of BCG administered to neonatal calves to induce immune responses and protection against challenge with virulent M. bovis. The findings are important for vaccination of calves against bovine tuberculosis, as vaccination within the first 6 weeks of age induced a very high level of protection. Since calves become responsive to antigens of environmental mycobacteria at a very young age, this mimics the situation found in human infants in many countries. This responsiveness did not interfere with development of protection against tuberculosis, as a single vaccination with BCG at birth or at 6 weeks of age induced similar levels of protection. In contrast, animals vaccinated at birth and again at 6 weeks of age had significantly less protection than those vaccinated only at birth. It is possible that revaccination of human infants with BCG within 1 to 2 months of birth may result in reduced protection.
Acknowledgments
This work was supported by grants from the Sequella Global Tuberculosis Foundation and the New Zealand Ministry of Agriculture and Forestry (Policy Management).
We thank Denise Keen, Allison McCarthy, Keith Hamel, Maree Joyce, and Gary Yates for excellent technical assistance and Lilian Morrison for the statistical analyses. Thanks to Karl Walravens and Chris Howard for antibodies to the T-cell subsets and Lew Barker for helpful comments.
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Andersen, P. 2001. TB vaccines: progress and problems. Trends Immunol. 22:160-168. [DOI] [PubMed] [Google Scholar]
- 2.Barrios, C., P. Brawand, M. Berney, C. Brandt, P. H. Lambert, and C. A. Siegrist. 1996. Neonatal and early life immune responses to various forms of vaccine antigens qualitatively differ from adult responses: predominance of a Th2-biased pattern which persists after adult boosting. Eur. J. Immunol. 26:1489-1496. [DOI] [PubMed] [Google Scholar]
- 3.Beverley, P. C. L. 1997. Vaccine immunity. Immunol. Today 18:413-415. [DOI] [PubMed] [Google Scholar]
- 4.Bloom, B. R., and P. E. M. Fine. 1994. The BCG experience: implications for future vaccines against tuberculosis, p 531-557. In B. R. Bloom (ed.), Tuberculosis: pathogenesis, protection, and control. American Society for Microbiology, Washington, D.C.
- 5.Brandt, L., J. F. Cunha, A. W. Olsen, B. Chilima, P. Hirsch, R. Appelberg, and P. Andersen. 2002. Failure of the Mycobacterium bovis BCG vaccine: some species of environmental mycobacteria block multiplication of BCG and induction of protective immunity to tuberculosis. Infect. Immun. 70:672-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buddle, B. M., G. W. de Lisle, A. Pfeffer, and F. E. Aldwell. 1995. Immunological responses and protection against Mycobacterium bovis in calves vaccinated with a low dose of BCG. Vaccine 13:1123-1130. [DOI] [PubMed] [Google Scholar]
- 7.Buddle, B. M., D. Keen, A. Thomson, G. Jowett, A. R. McCarthy, J. Heslop, G. W. de Lisle, J. L. Stanford, and F. E. Aldwell. 1995. Protection of cattle from bovine tuberculosis by vaccination with BCG by the respiratory or subcutaneous route, but not by vaccination with killed Mycobacterium vaccae. Res. Vet. Sci. 59:10-16. [DOI] [PubMed] [Google Scholar]
- 8.Buddle, B. M., B. J. Wards, F. E. Aldwell, D. M. Collins, and G. W. de Lisle. 2002. Influence of sensitisation to environmental mycobacteria on subsequent vaccination against bovine tuberculosis. Vaccine 20:1126-1133. [DOI] [PubMed] [Google Scholar]
- 9.Buddle, B. M., J. M. Pollock, M. A. Skinner, and D. N. Wedlock. 2003. Development of vaccines to control bovine tuberculosis in cattle and relationship to vaccine development for other intracellular pathogens. Int. J. Parasitol. 33:555-566. [DOI] [PubMed] [Google Scholar]
- 10.Cassidy, J. P., D. G. Bryson, J. M. Pollock, R. T. Evans, F. Forester, and S. D. Neill. 1998. Early lesion formation in cattle experimentally infected with Mycobacterium bovis. J. Comp. Pathol. 119:27-44. [DOI] [PubMed] [Google Scholar]
- 11.Clevers, H., N. D. MacHugh, A. Bensaid, S. Dunlap, C. L. Baldwin, A. Kaushal, K. Iams, C. J. Howard, and W. I. Morrison. 1990. Identification of a bovine surface antigen uniquely expressed on CD4-CD8-T cell receptor γ/δ+ T lymphocytes. Eur. J. Immunol. 20:809-817. [DOI] [PubMed] [Google Scholar]
- 12.Colditz, G. A., T. F. Brewer, C. S. Berkey, M. E. Wilson, E. Burdick, H. V. Fineberg, and F. Mosteller. 1994. The efficacy of BCG vaccine in the prevention of tuberculosis. Meta-analysis of the published literature. JAMA 271:698-702. [PubMed] [Google Scholar]
- 13.Colditz, G. A., C. S. Berkey, F. Mosteller, T. F. Brewer, M. E. Wilson, E. Burdick, and H. V. Fineberg. 1995. The efficacy of bacillus Calmette-Guerin vaccination of newborns and infants in the prevention of tuberculosis: meta-analyses of published literature. Pediatrics 96:29-35. [PubMed] [Google Scholar]
- 14.Fine, P. E. M. 1995. Variation in protection by BCG: implications of and for heterologous immunity. Lancet 346:1339-1345. [DOI] [PubMed] [Google Scholar]
- 15.Ginsberg, A. M. 2002. What's new in tuberculosis vaccines? Bull. W. H. O. 80:483-488. [PMC free article] [PubMed] [Google Scholar]
- 16.Goriely, S., B. Vincart, P. Stordeur, J. Vekemans, F. Willems, M. Goldman, and D. De Wit. 2001. Deficient IL-12 (p35) gene expression by dendritic cells derived from neonatal monocytes. J. Immunol. 166:2141-2146. [DOI] [PubMed] [Google Scholar]
- 17.Griffin, J. F. T., C. G. Mackintosh, L. Slobbe, A. J. Thomson, and G. S. Buchan. 1999. Vaccine protocols to optimise the protective efficacy of BCG. Tuber. Lung Dis. 79:135-143. [DOI] [PubMed] [Google Scholar]
- 18.Groh, V., S. Porcelli, M. Fabbi, L. L. Lanier, L. J. Picker, T. Anderson, R. A. Warnke, A. K. Bhan, J. L. Strominger, and M. B. Brenner. 1989. Human lymphocytes bearing T cell receptor γ/δ are phenotypically diverse and evenly distributed throughout the lymphoid system. J. Exp. Med. 169:1277-1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hewinson, R. G., H. M. Vordermeier, and B. M. Buddle. 2003. Use of the bovine model of tuberculosis for the development of improved vaccines and diagnostics. Tuberculosis 83:119-130. [DOI] [PubMed]
- 20.Karonga Prevention Trial Group. 1996. Randomised controlled trial of single BCG, repeated BCG, or combined BCG and killed Mycobacterium leprae vaccine for the prevention of leprosy and tuberculosis in Malawi. Lancet 348:17-24. [PubMed] [Google Scholar]
- 21.Kubit, S., S. Czajka, T. Olakowski, and Z. Piasecki. 1983. Evaluation of the effectiveness of BCG vaccinations. Pediatr. Pol. 58:775-782. [PubMed] [Google Scholar]
- 22.Langermans, J. A. M., P. Andersen, D. van Soolingen, R. A. W. Vervenne, P. A. Frost, T. van der Laan, L. A. H. van Pixteren, J. van den Hombergh, S. Kroon, I. Peekel, S. Florquin, and A. W. Thomas. 2001. Divergent effect of bacillus Calmette-Guerin (BCG) vaccination on Mycobacterium tuberculosis infection in highly related macaque species: implications for primate models in tuberculosis vaccine research. Proc. Natl. Acad. Sci. USA 98:11497-11502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leung, C. C., C. M. Tam, S. L. Chan, M. Chan-Yeung, C. K. Chan, and K. C. Chang. 2001. Efficacy of the BCG revaccination programme in a cohort given BCG vaccination at birth in Hong Kong. Int. J. Tuberc. Lung Dis. 5:717-723. [PubMed] [Google Scholar]
- 24.Lugosi, L. 1987. Analysis of the efficacy of mass BCG vaccination from 1959-1983 in tuberculosis control in Hungary: multiple comparison of results. Bull. Int. Union Tuberc. Lung Dis. 62:15-34. [PubMed] [Google Scholar]
- 25.Marchant, A., T. Goetghebuer, M. O. Ota, I. Wolfe, S. J. Ceesay, D. De Groote, T. Corrah, S. Bennett, J. Wheeler, K. Huygen, P. Aaby, K. P. W. J. McAdam, and M. J. Newport. 1999. Newborns develop a Th1-type immune response to Mycobacterium bovis bacillus Calmette-Guerin vaccination. J. Immunol. 163:2249-2255. [PubMed] [Google Scholar]
- 26.Ohara, N., and T. Yamada. 2001. Recombinant BCG vaccines. Vaccine 19:4089-4098. [DOI] [PubMed] [Google Scholar]
- 27.Olsen, I., and A. K. Storset. 2001. Innate IFN-γ production in cattle in response to MPP14, a secreted protein from Mycobacterium avium ssp. paratuberculosis. Scand. J. Immunol. 54:306-313. [DOI] [PubMed] [Google Scholar]
- 28.O'Reilly, L. M., and C. J. Daborn. 1995. The epidemiology of Mycobacterium bovis infections in animals and man: a review. Tuber. Lung Dis. 76(Suppl. 1):1-46. [DOI] [PubMed] [Google Scholar]
- 29.Palmer, C. E., and M. W. Long. 1966. Effects of infection with atypical mycobacteria on BCG vaccination and tuberculosis. Am. Rev. Respir. Dis. 94:553-568. [DOI] [PubMed] [Google Scholar]
- 30.Rothel, J. S., S. L. Jones, L. A. Corner, J. C. Cox, and P. R. Wood. 1990. A sandwich enzyme immunoassay for bovine interferon-gamma and its use for the detection of tuberculosis in cattle. Aust. Vet. J. 67:134-137. [DOI] [PubMed] [Google Scholar]
- 31.Sepulveda, R. L., C. Parcha, and R. U. Sorenson. 1992. Case-control study of the efficacy of BCG immunization against pulmonary tuberculosis in young adults in Santiago, Chile. Tuber. Lung Dis. 73:372-377. [DOI] [PubMed] [Google Scholar]
- 32.Stanford, J. L., Shield, M. J., and G. A. Rook. 1981. How environmental mycobacteria may predetermine the protective efficacy of BCG. Tubercle 62:55-62. [DOI] [PubMed] [Google Scholar]
- 33.Tala-Heikkila, M. M., J. E. Tuominen, and E. O. Tala. 1998. Bacillus Calmette-Guerin revaccination questionable with low tuberculosis incidence. Am. J. Respir. Crit. Care Med. 157:1324-1327. [DOI] [PubMed] [Google Scholar]
- 34.Turner, J., E. R. Rhoades, M. Keen, J. T. Belisle, A. A. Frank, and I. M. Orme. 2000. Effective preexposure tuberculosis vaccines fail to protect when they are given in an immunotherapeutic mode. Infect. Immun. 68:1706-1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vekemans, J., A. Amedei, M. O. Ota, M. M. D'Elois, T. Goetghebuer, J. Ismaili, M. J. Newport, G. Del Prete, M. Golman, K. P. W. J. McAdam, and A. Marchant. 2001. Neonatal bacillus Calmette-Guerin vaccination induces adult-like IFN-γ production by CD4+ T lymphocytes. Eur. J. Immunol. 31:1531-1535. [DOI] [PubMed] [Google Scholar]
- 36.Vordermeier, H. M., M. A. Chambers, P. J. Cockle, A. O. Whelan, J. Simmons, and R. G. Hewinson. 2002. Correlation of ESAT-6-specific gamma interferon production with pathology in cattle following Mycobacterium bovis BCG vaccination against experimental bovine tuberculosis. Infect. Immun. 70:3026-3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wedlock, D. N., F. E. Aldwell, D. M. Collins, G. W. de Lisle, T. Wilson, and B. M. Buddle. 1999. Immune responses induced in cattle by virulent and attenuated Mycobacterium bovis strains: correlation of delayed-type hypersensitivity with ability of strains to grow in macrophages. Infect. Immun. 67:2172-2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wedlock, D. N., B. Vesosky, M. A. Skinner, G. W. de Lisle, I. M. Orme, and B. M. Buddle. 2000. Vaccination of cattle with Mycobacterium bovis culture filtrate proteins and interleukin-2 for protection against bovine tuberculosis. Infect. Immun. 68:5809-5815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wood, P. R., L. A. Corner, J. S. Rothel, J. L. Ripper, T. Fifis, B. S. McCormick, B. Francis, L. Melville, K. Small, K. De Witte, J. Tolson, T. J. Ryan, G. W. de Lisle, J. C. Cox, and S. L. Jones. 1992. A field evaluation of serological and cellular diagnostic tests for bovine tuberculosis. Vet. Microbiol. 31:71-79. [DOI] [PubMed] [Google Scholar]
- 40.Wu, C. Y., J. R. Kirman, M. J. Rotte, D. F. Davey, S. P. Perfetto, E. G. Rhee, B. L. Freidag, B. J. Hill, D. C. Douek, and R. A. Seder. 2002. Distinct lineages of TH1 cells have differential capacities for memory cell generation in vivo. Nat. Immunol. 3:852-858. [DOI] [PubMed] [Google Scholar]