Abstract
Here, we have investigated the mRNA expression of Toll-like receptor 2 (TLR-2), TLR-4, and MD-2 in spleens and livers of C3H/HeN mice (carrying wild-type TLR-4) and C3H/HeJ mice (carrying mutated TLR-4) in response to Salmonella infection. During Salmonella infections, TLR-4 is activated, leading to increased TLR-2 and decreased TLR-4 expression.
The early innate host response to infection by bacteria is triggered by host cell recognition of pathogen-associated molecular patterns through pattern recognition receptors, and key among these is the family of Toll-like receptors (TLRs). TLR-2 recognizes gram-positive bacteria, and TLR-4 responds to gram-negative bacteria. The TLR-2 complex recognizes bacterial lipoproteins, lipoteichoic acid, and peptidoglycan (1, 15, 19-21, 23), while TLR-4 in combination with MD-2 and CD14 recognizes lipopolysaccharide (LPS), the main component of the gram-negative bacterial outer membrane (16, 17, 20). Signaling through TLRs results in the production of proinflammatory mediators.
Infection of mice with Salmonella enterica serovar Typhimurium produces a systemic disease similar to human typhoid fever (2, 3). The bacterial growth rate in livers and spleens of infected animals depends on the genetic background of the host. In infections of naturally resistant mouse strains (Nramp wild type), the initial bacterial growth rate is slow enough that other host responses can be activated to arrest bacterial growth and control the infection, causing development of a plateau in the in vivo growth curve often followed by bacterial clearance. The plateau phase is essential for the control of the primary infection and depends on the release of proinflammatory mediators such as tumor necrosis factor alpha, gamma interferon, and interleukin-1 (8, 9, 12). It probably occurs locally at the level of single foci of infection (5-7). The mechanisms which are activated by a Salmonella infection in the host to induce plateau phase are largely unknown. In mice of the C3H lineage (Nramp wild type), salmonellae exhibit a slow initial growth rate. In C3H/HeN mice (HeN+), growth in spleens is controlled and a normal plateau occurs. However, in the closely related, LPS-resistant C3H/HeJ mice (HeJ−), the plateau does not occur and the bacteria continue to grow at the same slow rate until they reach approximately 108 bacteria per organ, when the mice die (4, 13, 14). These mice have a nonfunctional TLR-4 (16-18). The plateau phase may therefore be driven by a response to LPS delivered when salmonellae infect organs.
To understand the role of TLR-4 during induction of the plateau phase, we have investigated whether RNA expression for TLR-2, TLR-4, and MD-2 alters during Salmonella infection. Changes in expression levels of these important pattern recognition receptors are likely to have a profound effect on the host response to Salmonlla infection.
Functional TLR-4 is required for control of the bacterial growth rate in vivo.
Six- to eight-week-old HeJ− and HeN+ mice were obtained from Harlan Olac Laboratories. Salmonella serovar Typhimurium C5 (4) was grown overnight at 37°C as a static culture in Luria-Bertani (LB) medium. The bacteria were washed and diluted in phosphate-buffered saline (PBS) to obtain 5 × 103 CFU/ml. Two hundred microliters (103 CFU) of this bacterial suspension was used to inoculate mice via the tail vein. Appropriate dilutions of the inoculum were plated on LB agar to calculate the number of viable bacteria given to the mice. At each time point postinfection (p.i.), the spleens and livers of four mice were divided into three and samples were immediately snap-frozen in liquid nitrogen for RNA isolation, submerged in 10% (vol/vol) formalin-PBS for hematoxylin-eosin staining, or placed into 10 ml of sterile distilled water and homogenized in a Colworth Stomacher for determination of viable bacterial counts by plating out appropriate dilutions of the homogenates on LB agar. For HeN+ mice, the counts in the spleens and livers initially increased at a rate of approximately 0.5 log per day. From day 7 onward, a plateau in the in vivo growth curve at approximately log 5.5/organ was observed for the spleens. However, in the livers bacterial numbers continued to increase until day 14, the final time point of the experiments (data not shown). By day 14, bacterial growth had reached about log 8/liver and the animals were displaying clinical signs typical of mouse typhoid, which, as expected, was associated with the development of large abscesses in the liver, a common phenomenon in strains of mice such as HeN and CBA that have reduced resistance to Salmonella infection (4).
The bacterial growth rates in the spleens and livers of HeJ− mice were higher in the first few days of infection (about 0.7 log per day) compared to those for HeN+ mice, and plateau formation in the bacterial growth curves was not observed in either organ (data not shown). Seven to eight days p.i., a bacterial load of log 7.5 to 8 was reached in all organs tested, leading to severe mouse typhoid, and the remaining animals were killed. Splenomegaly and discoloration of the spleen were observed in both mouse strains over the course of infection (data not shown). At day 7 p.i., however, liver weights were markedly less for the HeJ− mice than for the HeN+ mice. These results were consistent with previous observations with these mice and suggest that a functional TLR-4 is important in controlling both the initial growth rate and the growth arrest of Salmonella leading to plateau formation in the spleen.
TLR-2 and TLR-4 expression is regulated by TLR-4.
We determined whether the expression of TLR-2, TLR-4, and MD-2 might alter during the course of infection. These mRNA levels were compared to 18S rRNA levels in spleens and livers from infected and control mice by using real-time PCR (11). Statistical analyses were performed by analysis of variance and an unpaired Student's t test, where appropriate, with PRISM (GraphPad) software.
Total RNA was prepared from homogenized organs by using the RNeasy Mini kit (QIAGEN), including on-column DNase treatment. Primers and probes were designed with the Primer Express software program (PE Applied Biosystems). The four probes were labeled with the fluorescent reporter dye 5-carboxyfluorescein (FAM) at the 5′ end and the quencher N,N,N,N′-tetramethyl-6-carboxyrhodamine (TAMRA) at the 3′ end. Reverse transcription (RT)-PCR was performed by using the TaqMan EZ RT-PCR kit (PE Applied Biosystems). Amplification and detection of specific products were performed by using the ABI PRISM 7700 Sequence Detection system (PE Applied Biosystems). Results are expressed in terms of the threshold cycle (Ct), which is the cycle at which the change in reporter dye (ΔRn) passes a significant threshold. The threshold values of ΔRn for all reactions described are shown in Table 1. To generate standard curves for the specific reactions, total RNA was extracted from stimulated RAW264.7 macrophages. To control for variations in sampling and RNA preparation, the Ct values for products specific for TLR-2, TLR-4, and MD-2 for each sample were standardized by using the Ct value of the 18S rRNA product for the same sample. All standard curve data are summarized in Table 1.
TABLE 1.
Standard curve data from real-time quantitative RT-PCRs on total RNA extracted from a stimulated RAW macrophage-like cell linea
| Target | Significance threshold for ΔRn | Dilution | Range of Ct values | R2b | Slope |
|---|---|---|---|---|---|
| 18S | 0.04 | 10−3-10−7 | 71-124 | 0.9927 | 3.3896 |
| TLR-2 | 0.04 | 10−1-10−5 | 17-33 | 0.9871 | 3.2153 |
| TLR-4 | 0.04 | 10−1-10−5 | 21-36 | 0.9951 | 3.5359 |
| MD-2 | 0.04 | 10−1-10−5 | 23-38 | 0.9963 | 3.5103 |
To generate standard curves for specific reactions for TLR-2, TLR-4, and MD-2 mRNA and 18S rRNA, total RNA extracted from stimulated RAW macrophages was serially diluted.
R2, coefficient of regression. Regression analyses of the mean values of the results for four replicate RT-PCRs for log10-diluted RNA were used.
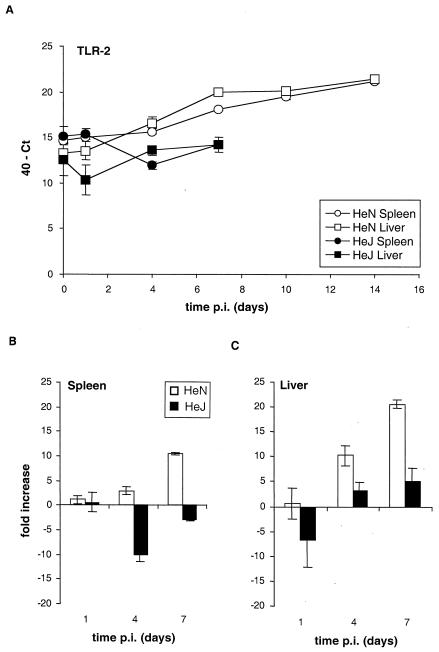
For HeN+ mice, the levels of TLR-2 mRNA increased during the course of infection in spleens and livers, attaining a 20-fold and 25-fold increase, respectively, by day 14 p.i. (Fig. 1A). Marked differences in TLR-2 mRNA expression between HeN+ and HeJ− mice were seen over the first 7 days of infection. In the HeN+ spleens, TLR-2 mRNA levels steadily increased to 10-fold by day 7 (P < 0.001). In HeJ− spleens, TLR-2 mRNA levels were transiently reduced, returning by day 7 to levels close to those for the uninfected control samples (P > 0.1) (Fig. 1B). Similar TLR-2 mRNA expression profiles were observed in liver samples (Fig. 1C). At day 7, for HeN+ mice the TLR-2 mRNA levels were 12-fold and 18-fold higher in spleens and livers, respectively, than those for HeJ− mice.
FIG. 1.
Signaling through TLR-4 controls the expression of TLR-2 mRNA in spleen and liver. Quantification of TLR-2 mRNA levels in spleen and liver from C3H/HeN and C3H/HeJ mice at various time points p.i. after intravenous inoculation with 103 CFU of Salmonella serovar Typhimurium C5 is shown. (A) The Ct values expressed were subtracted from 40 (the negative end-point). Higher values represent higher levels of TLR-2 mRNA. (B and C) The data from panel A expressed as fold changes in mRNA levels over the first 7 days of infection compared to those for the uninfected controls in spleen (B) and liver (C) for C3H/HeN and C3H/HeJ mice.
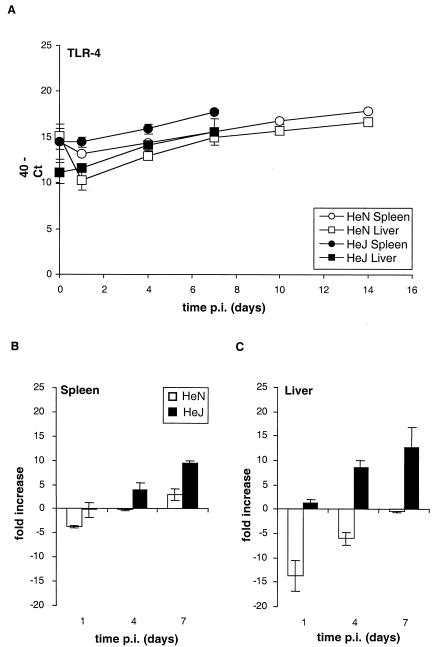
Thus, TLR-2 mRNA expression is induced in response to Salmonella serovar Typhimurium infection in both strains of mice, but the induction is much less and is delayed in the HeJ− mice, suggesting that signaling through TLR-4 is required for the optimal kinetics of increased TLR-2 mRNA expression. TLR-4 mRNA expression increased only slightly for HeN+ mice over the 14 days of infection after an initial decrease on day 1 to reach levels similar to that for uninfected animals (P > 0.1) (Fig. 2). In contrast, in Salmonella-infected HeJ− mice at day 7, TLR-4 mRNA levels were increased ninefold in spleens (P < 0.005) and 13-fold in livers (P < 0.05) compared to those for uninfected animals (Fig. 2B and C) and sevenfold in spleens and twofold in livers compared to those for HeN+ mice (Fig. 2A). This finding suggests that activation of TLR-4 leads to the suppression of its own mRNA expression during Salmonella infection. In the livers of uninfected HeJ− mice, the TLR-4 mRNA levels were significantly lower (P < 0.05) than those in uninfected HeN+ mice (Fig. 2A). MD-2 mRNA levels in both strains of mice increased by approximately sixfold in spleens and eightfold in livers over the first 7 days of infection, with no differences between the mouse strains being detectable (data not shown). Our data clearly suggest that signaling through TLR-4 does not affect levels of MD-2 mRNA expression during infection with Salmonella.
FIG. 2.
Functional TLR-4 is required for its own down regulation during the course of infection. Quantification of TLR-4 mRNA in spleen and liver from C3H/HeN and C3H/HeJ mice at various time points p.i. after intravenous inoculation with 103 CFU of Salmonella serovar Typhimurium C5 is shown. (A) The Ct values expressed were subtracted from 40 (the negative end point). Higher values represent higher levels of TLR-4 mRNA. (B and C) The data from panel A expressed as fold changes in mRNA levels over the first 7 days of infection compared to those for the uninfected controls in spleen (B) and liver (C) for C3H/HeN and C3H/HeJ mice.
Zarember and Godowski (24) used quantitative TaqMan PCR to assay a wide panoply of TLRs and associated molecules. After incubation of phorbol myristate acetate-differentiated human THP-1 macrophage-like cells with live Escherichia coli or Staphylococcus aureus for 6 h, increased mRNA expression was seen for most TLRs and Myd88, whereas MD-1 and MD-2 were not altered. The increase in expression of TLR-2 and the relatively unaltered expression levels of MD-2 in THP-1 cells in response to live E. coli infection is very similar to the expression patterns observed in our in vivo experiments with virulent Salmonella serovar Typhimurium. Zarember and Godowski focused their work on a single time point p.i. (24), but infection is a dynamic process that affects the host immune response differentially over time. Critically, our in vivo study shows that the profile of TLR expression changes as the infection process develops.
Loss of TLR-4 function affects the recruitment of polymorphonuclear leukocytes (PMNs) and macrophages and lesion formation in spleen and liver.
To confirm the role of TLR-4 in lesion formation and to assess whether the increase in TLR expression is due mainly to the infiltration of PMNs and macrophages, we analyzed the histology of spleen and liver during the course of infection. Five-micrometer-thick sections, fixed by immersion in 10% (vol/vol) formalin-PBS, embedded in paraffin wax, and then stained by hematoxylin-eosin, were prepared from all tissues sampled. Stained sections were analyzed for cell counts. These counts were performed blind, and cell types were verified by an independent observer. Five areas from each specimen were differentially counted, amounting to five sections per mouse, and thus 25 fields, that were counted per mouse per time point. At 10× magnification, the area measured was 0.1 mm2. For splenic samples, differential counts were made in red pulp only (lymphoid sheaths were not examined). When counting liver granulomata, whole samples were counted rather than a set area (since few were observed initially compared with the large numbers of splenic granulomata). Cell counts for each organ are presented as the mean of the results for 25 fields.
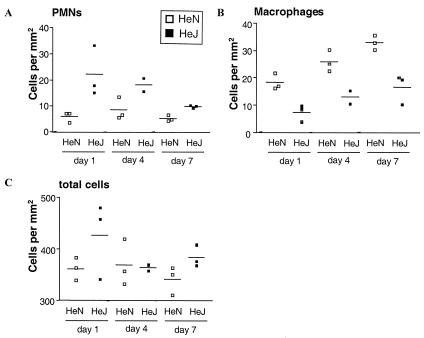
PMN and macrophage infiltration into the spleens was significantly different (P < 0.0001 and P < 0.005, respectively) for HeN+ mice than for HeJ− mice (Fig. 3A and B). On day 1 p.i., more PMNs were observed in HeJ− spleens than in HeN+ spleens (Fig. 3A), while more macrophages were observed in HeN+ spleens (Fig. 3B). In HeN+ spleens, the number of granulomata correlated with the number of macrophages (Fig. 3B and data not shown). HeN+ mice have visible lesions at earlier time points and larger numbers of granulomata (some of which are very large) than HeJ− mice, where only individual mice surviving until days 8 and 9 show granuloma formation (data not shown). In contrast, in the liver the infiltration of PMNs and macrophages is similar for both mouse strains. However, granuloma formation was observed for HeN+ mice over only the first 7 days of infection, and only HeJ− mice at days 8 and 9 showed granuloma formation (data not shown). A distinctive feature of the histology for the HeN+ mice was the presence of large abscesses, which were not observed with HeJ− mice. Additionally, with HeJ− mice the absence of plateau formation coincided with the absence of granulomata in the spleens and livers for the first 7 days of infection. These data confirm other observations that functional TLR-4 is required to control Salmonella infection. This finding is not unexpected, since the activation of this receptor initiates an innate immune response leading to the induction of proinflammatory mediators. Weinstein et al. (22) found that during Salmonella serovar Typhimurium strain TML infection in HeJ− mice, a reduced number of infectious foci were found in the livers compared to that for HeN+ mice. However, the number of granulomata was not investigated. In our experiments, we did not observe a significant difference between HeN+ and HeJ− mice in the numbers of PMNs and macrophages recruited to the liver. However, while granuloma formation was observed in HeN+ livers from day 4 onward, no granulomata were identified in HeJ− livers over the first 7 days of infection. In the spleen, we did observe a significant difference in the recruitment of PMNs and macrophages for HeJ− mice compared to that for HeN+ mice, though over this short time period there was no significant difference in splenomegaly between the two mouse strains. Again, while granulomata were identified from day 1 p.i. in HeN+ spleens, no granuloma formation was observed in HeJ− spleens over the first 7 days of infection. These findings confirm the importance of granuloma formation in host suppression of bacterial growth.
FIG. 3.
Disfunctional TLR-4 leads to changes in PMN and macrophage migration in spleen in response to Salmonella infection. Counts of PMNs (A), macrophages (B), and total cells (C) in spleens from C3H/HeJ mice over the first 7 days p.i. with 103 CFU of Salmonella serovar Typhimurium C5. The counts are expressed as the averages of 25 fields per mouse. Counts for individual animals are shown as lines.
The increase in TLR-2 mRNA levels, therefore, cannot be solely accounted for by the influx of macrophages in liver and spleen. Macrophage numbers increased only two- and fivefold compared to 10- and 20-fold increases in TLR-2 mRNA levels in spleen and liver, respectively. In contrast, the mRNA levels of MD-2, a protein also expressed in macrophages, increased only six- and eightfold in spleens and livers, respectively, during infection, significantly less than the changes seen in TLR-2 expression. This increase in TLR-2 expression is most likely due to activation by Salmonella rather than to an increase in cell numbers. Infiltration increases during infection, and the change in MD-2 mRNA levels correlates with the increase in macrophage numbers. In contrast, the increase in TLR-2 and decrease in TLR-4 mRNA levels do not correlate with cellular infiltration. As TLR-2 can also be expressed in hepatocytes (10) as well as in macrophages, it is likely that TLR-2 expression in response to Salmonella infection is regulated in a number of cell types, including hepatocytes and macrophages. The profound changes in mRNA levels seen during our infection study may not reflect changes in protein expression; hence, future studies using specific murine anti-TLR antibodies suitable for fluorescence-activated cell sorter analysis and immunohistochemistry will be necessary. Although we clearly see a TLR-4-dependent induction of TLR-2 mRNA expression, this does not necessarily mean that TLR-2 plays an important role in host defense against Salmonella infection. To define the precise role that TLR-2 plays in regulating the host response to Salmonella serovar Typhimurium, the infection study needs to be repeated in TLR-2 knockout mice.
In Salmonella infections, TLR-4 is probably activated to regulate the release of inflammatory mediators, to increase TLR-2 expression, and to reduce its own expression, which lead to the recruitment of inflammatory cells and the initiation of the appropriate responses in the spleen to control bacterial growth leading to plateau formation in the in vivo bacterial growth curve. The response in HeJ− mice, carrying dysfunctional TLR-4, is very different. In the absence of functional TLR-4, TLR-2 mRNA expression is unchanged and the levels of TLR-4 mRNA increase over the course of infection. In the absence of the appropriate TLR-4 response and possibly also via the reduced TLR-2 activity, the recruitment of inflammatory cells is impaired and bacterial growth in spleen and liver is uncontrolled. Given that TLR-4 controls the expression of TLR-2 and TLR-4 transcription during infection, it is possible that the transcription of other TLRs such as TLR-5 and TLR-9 might also be affected. We are currently investigating this hypothesis. The regulation of expression of different TLRs during bacterial infection may play a key role in initiating the appropriate immune responses to the different pathogen-associated molecular patterns recognized by the host during infection and hence in preventing death from bacterial infection.
Acknowledgments
This study was supported by Biotechnology and Biological Sciences Research Council ROPA grant number 8/9912336. C.E.B. is the recipient of a Wellcome Trust advanced fellowship.
We thank Romina Emilianus, Matthew Cable, Louise Alldridge, and Matthew Royle for assistance with the in vivo studies.
Editor: A. D. O'Brien
REFERENCES
- 1.Bulut, Y., E. Faure, L. Thomas, O. Equils, and M. Arditi. 2001. Cooperation of Toll-like receptor 2 and 6 for cellular activation by soluble tuberculosis factor and Borrelia burgdorferi outer surface protein A lipoprotein: role of Toll-interacting protein and IL-1 receptor signaling molecules in Toll-like receptor 2 signaling. J. Immunol. 167:987-994. [DOI] [PubMed] [Google Scholar]
- 2.Carter, P. B., and F. M. Collins. 1974. Growth of typhoid and paratyphoid bacilli in intravenously infected mice. Infect. Immun. 10:816-822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carter, P. B., and F. M. Collins. 1974. The route of enteric infection in normal mice. J. Exp. Med. 139:1189-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hormaeche, C. E. 1979. Natural resistance to Salmonella typhimurium in different inbred mouse strains. Immunology 37:311-318. [PMC free article] [PubMed] [Google Scholar]
- 5.Hormaeche, C. E., P. Mastroeni, A. Arena, J. Uddin, and H. S. Joysey. 1990. T cells do not mediate the initial suppression of a Salmonella infection in the RES. Immunology 70:247-250. [PMC free article] [PubMed] [Google Scholar]
- 6.Khan, S. A., P. J. Strijbos, P. Everest, D. Moss, R. Stratford, P. Mastroeni, J. Allen, S. Servos, I. G. Charles, G. Dougan, and D. J. Maskell. 2001. Early responses to Salmonella typhimurium infection in mice occur at focal lesions in infected organs. Microb. Pathog. 30:29-38. [DOI] [PubMed] [Google Scholar]
- 7.Maskell, D. J., C. E. Hormaeche, K. A. Harrington, H. S. Joysey, and F. Y. Liew. 1987. The initial suppression of bacterial growth in a salmonella infection is mediated by a localized rather than a systemic response. Microb. Pathog. 2:295-305. [DOI] [PubMed] [Google Scholar]
- 8.Mastroeni, P., A. Arena, G. B. Costa, M. C. Liberto, L. Bonina, and C. E. Hormaeche. 1991. Serum TNF alpha in mouse typhoid and enhancement of a Salmonella infection by anti-TNF alpha antibodies. Microb. Pathog. 11:33-38. [DOI] [PubMed] [Google Scholar]
- 9.Mastroeni, P., J. A. Harrison, J. A. Chabalgoity, and C. E. Hormaeche. 1996. Effect of interleukin 12 neutralization on host resistance and gamma interferon production in mouse typhoid. Infect. Immun. 64:189-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsumura, T., A. Ito, T. Takii, H. Hayashi, and K. Onozaki. 2000. Endotoxin and cytokine regulation of toll-like receptor (TLR) 2 and TLR4 gene expression in murine liver and hepatocytes. J. Interferon Cytokine Res. 20:915-921. [DOI] [PubMed] [Google Scholar]
- 11.Moody, A., S. Sellers, and N. Bumstead. 2000. Measuring infectious bursal disease virus RNA in blood by multiplex real-time quantitative RT-PCR. J. Virol. Methods 85:55-64. [DOI] [PubMed] [Google Scholar]
- 12.Muotiala, A., and P. H. Makela. 1990. The role of IFN-gamma in murine Salmonella typhimurium infection. Microb. Pathog. 8:135-141. [DOI] [PubMed] [Google Scholar]
- 13.O'Brien, A. D., D. L. Rosenstreich, I. Scher, G. H. Campbell, R. P. MacDermott, and S. B. Formal. 1980. Genetic control of susceptibility to Salmonella typhimurium in mice: role of the LPS gene. J. Immunol. 124:20-24. [PubMed] [Google Scholar]
- 14.O'Brien, A. D., D. A. Weinstein, M. Y. Soliman, and D. L. Rosenstreich. 1985. Additional evidence that the Lps gene locus regulates natural resistance to S. typhimurium in mice. J. Immunol. 134:2820-2823. [PubMed] [Google Scholar]
- 15.Ozinsky, A., K. D. Smith, D. Hume, and D. M. Underhill. 2000. Co-operative induction of pro-inflammatory signaling by Toll-like receptors. J. Endotoxin Res. 6:393-396. [PubMed] [Google Scholar]
- 16.Poltorak, A., X. He, I. Smirnova, M. Y. Liu, C. V. Huffel, X. Du, D. Birdwell, E. Alejos, M. Silva, C. Galanos, M. Freudenberg, P. Ricciardi-Castagnoli, B. Layton, and B. Beutler. 1998. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282:2085-2088. [DOI] [PubMed] [Google Scholar]
- 17.Poltorak, A., I. Smirnova, X. He, M. Y. Liu, C. Van Huffel, O. McNally, D. Birdwell, E. Alejos, M. Silva, X. Du, P. Thompson, E. K. Chan, J. Ledesma, B. Roe, S. Clifton, S. N. Vogel, and B. Beutler. 1998. Genetic and physical mapping of the Lps locus: identification of the toll-4 receptor as a candidate gene in the critical region. Blood Cells Mol. Dis. 24:340-355. (Erratum, 25:78, 1999.) [DOI] [PubMed]
- 18.Qureshi, S. T., L. Lariviere, G. Leveque, S. Clermont, K. J. Moore, P. Gros, and D. Malo. 1999. Endotoxin-tolerant mice have mutations in Toll-like receptor 4 (Tlr4). J. Exp. Med. 189:615-625. (Erratum, 189:1518.) [DOI] [PMC free article] [PubMed]
- 19.Schwandner, R., R. Dziarski, H. Wesche, M. Rothe, and C. J. Kirschning. 1999. Peptidoglycan- and lipoteichoic acid-induced cell activation is mediated by toll-like receptor 2. J. Biol. Chem. 274:17406-17409. [DOI] [PubMed] [Google Scholar]
- 20.Takeuchi, O., K. Hoshino, T. Kawai, H. Sanjo, H. Takada, T. Ogawa, K. Takeda, and S. Akira. 1999. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity 11:443-451. [DOI] [PubMed] [Google Scholar]
- 21.Takeuchi, O., T. Kawai, P. F. Muhlradt, M. Morr, J. D. Radolf, A. Zychlinsky, K. Takeda, and S. Akira. 2001. Discrimination of bacterial lipoproteins by Toll-like receptor 6. Int. Immunol. 13:933-940. [DOI] [PubMed] [Google Scholar]
- 22.Weinstein, D. L., C. R. Lissner, R. N. Swanson, and A. D. O'Brien. 1986. Macrophage defect and inflammatory cell recruitment dysfunction in Salmonella susceptible C3H/HeJ mice. Cell. Immunol. 102:68-77. [DOI] [PubMed] [Google Scholar]
- 23.Yoshimura, A., E. Lien, R. R. Ingalls, E. Tuomanen, R. Dziarski, and D. Golenbock. 1999. Cutting edge: recognition of gram-positive bacterial cell wall components by the innate immune system occurs via Toll-like receptor 2. J. Immunol. 163:1-5. [PubMed] [Google Scholar]
- 24.Zarember, K. A., and P. J. Godowski. 2002. Tissue expression of human Toll-like receptors and differential regulation of Toll-like receptor mRNAs in leukocytes in response to microbes, their products, and cytokines. J. Immunol. 168:554-561. [DOI] [PubMed] [Google Scholar]