Abstract
Antigens presented by class I major histocompatibility complex (MHC) molecules for recognition by cytotoxic T lymphocytes consist of 8–10-amino-acid-long cytosolic peptides. It is not known whether posttranslationally modified peptides are also presented by class I MHC molecules in vivo. Many different posttranslational modifications occur on cytoplasmic proteins, including a cytosolic O-β-linked glycosylation of serine and threonine residues with N-acetylglucosamine (GlcNAc). Using synthetic glycopeptides carrying the monosaccharide O-β-GlcNAc substitution on serine residues, we have shown that glycopeptides bind efficiently to class I MHC molecules and elicit a glycopeptide-specific cytotoxic T lymphocyte response in mice. In this study, we provide evidence that peptides presented by human class I MHC molecules in vivo encompass a small, significant amount of glycopeptides, constituting up to 0.1% of total peptide. Furthermore, we find that carbohydrate structures present on glycopeptides isolated from class I MHC molecules are dominated by the cytosolic O-β-GlcNAc substitution, and synthetic peptides carrying this substitution are efficiently transported by TAP (transporter associated with antigen presentation) into the endoplasmic reticulum. Thus, in addition to unmodified peptides, posttranslationally modified cytosolic peptides carrying O-β-linked GlcNAc can be presented by class I MHC molecules to the immune system.
Keywords: glycopeptides/immunology, class I histocompatibility antigens, posttranslational protein processing, antigen presentation, acetylglucosamine
The majority of peptides presented by MHC molecules for recognition by CD8+ CTLs are derived from cytosolic proteins that have been degraded in the cytosol before transport via the transporter associated with antigen presentation (TAP) into the endoplasmic reticulum (ER). Here, the peptides assemble with “empty” class I MHC H chain and β2-microglobulin (β2m) into stable class I MHC–peptide complexes, followed by their egress through the ER–Golgi apparatus to the cell surface 1.
The nature of peptides presented naturally by classical MHC molecules in normal or infected cells has been extensively studied, revealing features such as length restriction and the presence of allele-specific amino acid motifs corresponding to so-called peptide anchor residues 2. It has also been clearly demonstrated that the great majority of antigens presented by classical MHC molecules consist of unmodified peptides. There is, however, a growing number of examples of class I MHC–restricted T cells recognizing products of posttranslational modifications in vivo, e.g., glycosylation 3, deglycosylation 4, or cysteinylation 5, as well as class II MHC–restricted T cells recognizing peptides modified by glycosylation 6 7 or deamidation 8.
Previously, we have shown that class I MHC molecules efficiently bind synthetic peptides carrying the naturally occurring cytosolic type of O-β-GlcNAc (N-acetylglucosamine) glycosylation. Furthermore, we have shown that such glycopeptides are immunogenic in mice, where they induce classical class I MHC–restricted, α/β-TCR+, glycopeptide-specific CTL 9 10 11. No class I MHC reactivity has yet been identified toward a defined, naturally processed glycopeptide. Although glycopeptides have been isolated from class II MHC molecules 12, so far no glycopeptides have been identified among peptides presented by class I MHC, and it has not been investigated to what extent such modifications persist during antigen presentation of cytosolic peptide fragments in vivo.
Here, we present evidence that peptides carrying natural cytosolic posttranslational modifications act as good substrates for the TAP transporter. Furthermore, we demonstrate that peptides presented naturally by human class I MHC molecules contain a subset of glycopeptides with the cytosolic O-β-GlcNAc monosaccharide glycosylation.
Materials and Methods
Peptides.
The following peptides and O-β-GlcNAc–substituted peptides were synthesized as previously described 9: wt-S (FASGNYSAL), 417 (TVNKTERAY), 417-S (TVNKTESAY), wt-G (FAS[O-β-GlcNAc]GNYSAL) and 417-G (TVNKTES [O-β-GlcNAc]AY). The glycopeptides K1G, carrying an N-linked monosaccharide, and K2G, carrying an O-β-linked monosaccharide, have been described previously 9 10. All peptides were purified by reverse-phase (RP)-HPLC and characterized by mass spectroscopy and nuclear magnetic resonance. Before use in the TAP assay, some peptides were radiolabeled with Na-125I catalyzed by chloramine-T.
Assay for TAP Transport of Peptides across the ER Membrane.
Assays for the TAP-mediated translocation of radiolabeled, posttranslationally modified peptides across the ER membrane of human LCL721 cells were performed as described 13. T2 cells were used to demonstrate the TAP dependence of transport. Samples incubated in the absence of ATP were carried out as controls for the ATP dependence of the transport. In competition assays, iodinated peptide 417 was mixed with competitor peptide before addition to permeabilized cells. The substrate peptide 417-G carrying O-β-GlcNAc monosaccharide is not itself a ligand for Con A in the absence of an N-linked glycan, as seen from the inability to recover 417-G by Con A–Sepharose in the absence of ATP (see Fig. 1 B).
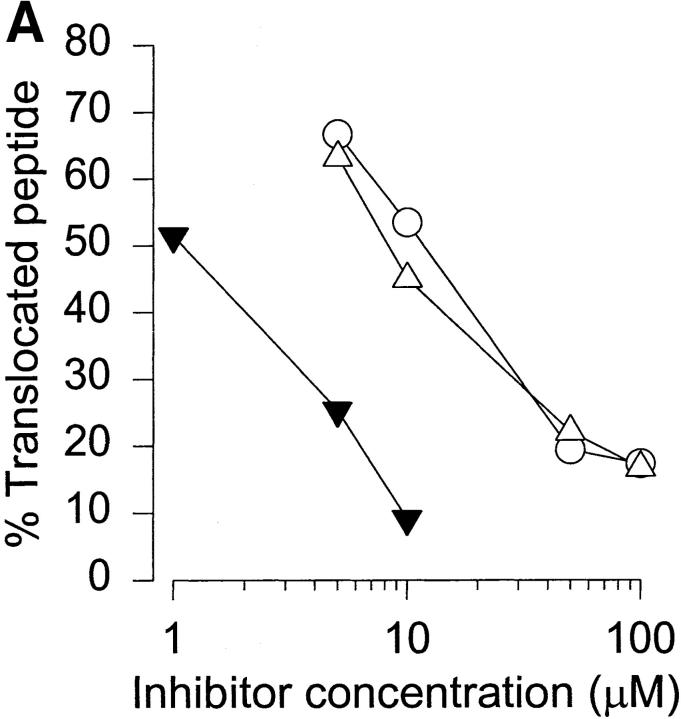
Figure 1.
TAP-mediated transport of posttranslationally modified peptides. (A) Competition of peptide 417 translocation by glycopeptide wt-G (○), unmodified peptide wt-S (▵), or index peptide 417 (▴). The amount of iodinated index peptide is expressed as a fraction of the amount recovered in the absence of competitor. (B) Direct translocation of iodinated glycopeptides was analyzed by comparing translocation of peptide 417 with a serine-substituted analogue (417-S), as well as an O-GlcNAc–glycosylated (417-G) version thereof. The results are the amounts of translocated peptide recovered expressed as a fraction of total input peptide.
Immunoaffinity Isolation of Peptides from Human Class I MHC Molecules.
MHC–peptide complexes were purified from 100 g of normal human spleen (tissue type HLA-A3, -A30, -B7, -B8, -Cw*0701, -Cw*0702; provided by Dr. M. Bunce, Churchill Hospital, Oxford, England) lysed in 1% CHAPS (3-[(3-cholamidopropyl)-dimethylammonio]-1-propane-sulfonate)-containing buffer as described 14. Affinity columns (Pharmacia HiTrap Protein A–Sepharose columns with 10 mg mAb/ml column volume) were equilibrated with lysis buffer, and the lysate (10 g of tissue/ml column volume) was passed through a Sepharose 4B precolumn, then a column containing the irrelevant H-2Db–specific mAb 28-14-8s, and finally a column conjugated with the anti–HLA class I mAb W6/32. Each column was washed extensively with lysis buffer, followed by 150 mM NaCl and 1.0 M NaCl (both with 20 mM Tris/HCl, pH 8.0) and then 20 mM Tris/HCl, pH 8.0, before elution with 0.2 M acetic acid. Acetic acid was then added to the eluate to a final concentration of 10%, and after 30 min on ice, the eluate was filtered through prewashed 5,000-daltons cut-off UFC4LCC00 ultrafiltration filters (Millipore Corp.), concentrated in a vacuum concentrator, and frozen at −80°C. The class I MHC–peptide complex affinity column eluates were analyzed for contaminating polypeptide on precast 10% NuPAGE Bis-Tris SDS-PAGE (Novex). These gels can separate polypeptides in the 2.5–200-kD molecular mass range, when using a 2-(N-morpholino)ethane sulfonic acid containing SDS running buffer (Novex). An aliquot of the column eluate corresponding to 50 μg of total protein was concentrated by vacuum centrifugation and dissolved in PBS containing NuPAGE Sample Reducing Agent and LDS Sample Buffer, according to the manufacturer's instructions (Novex), before heating and loading onto a 10 × 10 cm precast NuPAGE gel in a Novex Xcell II Mini-Cell. NuPAGE Antioxidant was added to the NuPAGE MES SDS running buffer according to the manufacturer's instructions. The gel was run at 200 V for 35 min, after which it was stained in Coomassie blue dye, destained O/N, and dried on paper using a gel dryer. SeeBlue (Novex) prestained standard molecular mass markers containing BSA (62 kD), glutamic dehydrogenase (49 kD), alcohol dehydrogenase (38 kD), carbonic anhydrase (28 kD), myoglobin (18 kD), lysozyme (14 kD), aprotinin (6 kD), and insulin (B chain; 3 kD) were used for calibration.
Galactosyltransferase Labeling of Peptides.
Before labeling with [3H] galactose (Gal) using bovine milk GlcNAcβ1-4galactosyltransferase 15, the MHC-derived peptides were desalted by RP-HPLC, lyophilized, and dissolved in water. Galactosyltransferase enzyme (50 mU) was mixed with 20 μl labeling buffer (100 mM Hepes and 50 mM MnCl2, pH 7.3), 50 μl of peptide, 20 μl of 25 mM 5′-AMP containing uridine 5′-diphosphate (UDP)-[3H]Gal (2.5 μCi; Amersham International), and water to a final volume of 200 μl. The reaction proceeded for 90 min (37°C) before termination with EDTA (0.1 M, pH 8.0).
Sensitivity of Labeled Peptides to N-glycosidase F or β-elimination.
Equal aliquots of labeled peptide were mixed with 0.7 U peptide–N-glycosidase F (Boehringer Mannheim) in sodium phosphate (pH 7.2) or β-elimination buffer (0.1 M NaOH and 1 M NaBH4, pH 13) 15 16 and incubated at 37°C for 18 h. The β-elimination reaction was neutralized with 4 M acetic acid. Using the synthetic peptides K1G (carrying an N-β-linked GlcNAc) and K2G (carrying an O-β-linked GlcNAc), the β-elimination procedure was optimized such that it resulted in the complete removal of [3H]Gal-labeled O-β-linked GlcNAc residues from K2G while leaving all [3H]Gal-labeled N-linked GlcNAc residues on K1G intact. Ovalbumin was galactosyltransferase-labeled with [3H]Gal and then shown to be efficiently deglycosylated by N-glycosidase F as a positive control for the procedure.
Analysis of Labeled Peptides by RP-HPLC.
The galactosyltransferase-labeled peptides were analyzed by RP-HPLC (3.9 mm × 15 cm, 300-Å Waters C-18 column) and an on-line radioactivity monitor (Reeve Analytical Instruments). Buffer A: 0.1% (vol/vol, unless otherwise stated) TFA in water; buffer B: 0.1% TFA in acetonitrile. The gradient was 95:5 to 50:50 buffer A/buffer B in 45 min and 50:50 to 20:80 in 2 min (flow, 1 ml/min).
Analysis of the β-Elimination Product by Size Exclusion Chromatography.
After labeling of terminal GlcNAc residues with tritiated galactose, the peptides were RP-HPLC purified to separate unincorporated, free label from peptide-bound [3H]Gal. The labeled peptides were analyzed by size exclusion chromatography on a Bio-Gel P-2 column (10 × 350 mm; separation range, 100–1,800 daltons) at a linear flow rate of 15.29 cm/h (200 μl/min) PBS before and after β-elimination. The column was calibrated with GlcNAc  , chitobiose
, chitobiose 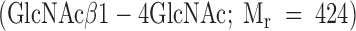 , UDP-[3H]Gal
, UDP-[3H]Gal  , K2G
, K2G 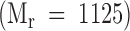 , and [3H] Gal-K2G
, and [3H] Gal-K2G 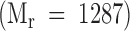 . The Vo and Vt volumes were determined with BSA
. The Vo and Vt volumes were determined with BSA 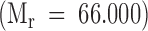 and 51Cr
and 51Cr 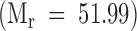 , respectively.
, respectively.
Lectin-Affinity Chromatography of Class I MHC–derived Peptides.
Peptide–MHC complexes were affinity purified as described above from a JestHom (expressing HLA-A*0201 and -B*2705; 5 × 1010 cells) cell lysate using the mAb BB7.2 to extract HLA-A*0201–peptide complexes, followed by W6/32 to extract HLA-B*2705 complexes. Subsequently, the isolated peptides were passed through a series of 1-ml lectin–agarose affinity columns (Sigma Chemical Co.) in the following sequence: (i) Con A, (ii) wheat germ agglutinin (WGA), and (iii) and Arachis hypogea lectin (peanut lectin). WGA beads were eluted with 1 M GlcNAc and the Con A beads with 50 mM α-methylmannoside. All three columns were washed with 0.1 M glycine/HCl and equilibrated in PBS before the peptide preparation was passed slowly through the columns. The columns were washed with 200 ml PBS before elution with 50 mM α-methylmannoside (Con A), 1 M GlcNAc (WGA), or 0.1 M glycine/HCl (peanut lectin).
Results and Discussion
In previous studies 9 10, we have demonstrated how peptides carrying the cytosolic type of O-linked GlcNAc glycosylation of serine and threonine residues 17 constitute a potential new group of antigenic epitopes. Our recent X-ray crystallographic analysis of these class I MHC–glycopeptide complexes show that the glycans are solvent exposed and, though mobile, are orientated in such a way as to permit specific contact with the TCR of glycopeptide-specific CTL 11.
Natural presentation of such peptides by class I MHC in vivo would require the proteolytic cleavage of O-β-GlcNAc–containing glycopeptides from cytosolic glycoproteins, followed by their transport into the ER, allowing binding to class I MHC molecules. Fig. 1 shows that peptides carrying the cytosolic O-β-GlcNAc modification are indeed substrates for TAP-mediated transport across the ER membrane. Fig. 1 A shows that the glycopeptide wt-G competed about as efficiently as wt-S for translocation of 417 (IC50 values, 12 and 8 μM for wt-S and wt-G, respectively). These values remain well within the range of those obtained with several natural immunodominant peptide epitopes 13. Assays for direct translocation of glycopeptides by TAP were carried out by adding radiolabeled peptides or glycopeptides, which contain an N-linked glycosylation sequon, to streptolysin O–permeabilized cells. In the event of TAP-mediated transport into the ER, these peptides will acquire an N-linked glycan structure, thus allowing recovery with Con A–Sepharose. Any difference in the amount of N-glycosylated peptide between permeabilized TAP-competent cells and TAP-deficient control cells (T2) is due to TAP-mediated translocation of the peptide into ER. Peptide 417, as well as 417-S, was very efficiently translocated by both human (Fig. 1 B) and murine (data not shown) TAP, with recoveries of 4–5% for LCL721, consistent with previously published data 18. The glycosylated version of 417-S (giving 417-G) was also translocated by TAP and resulted in the recovery of 40–50% of the control peptide (Fig. 1 B). These results are in accord with the finding that TAP allows translocation of peptides with side chains much longer than naturally occurring ones 19.
We next sought to determine to what extent O-GlcNAc–containing glycopeptides were present amongst peptides isolated from natural human class I MHC molecules affinity purified from human spleen lysates. A widely used method for the detection of O-β-GlcNAc on glycoproteins is based on the enzymatic transfer of [3H]Gal from UDP-[3H]Gal onto terminal GlcNAc residues in O-β-GlcNAc–containing proteins, as well as N-linked carbohydrate structures, catalyzed by galactosyltransferase 15.
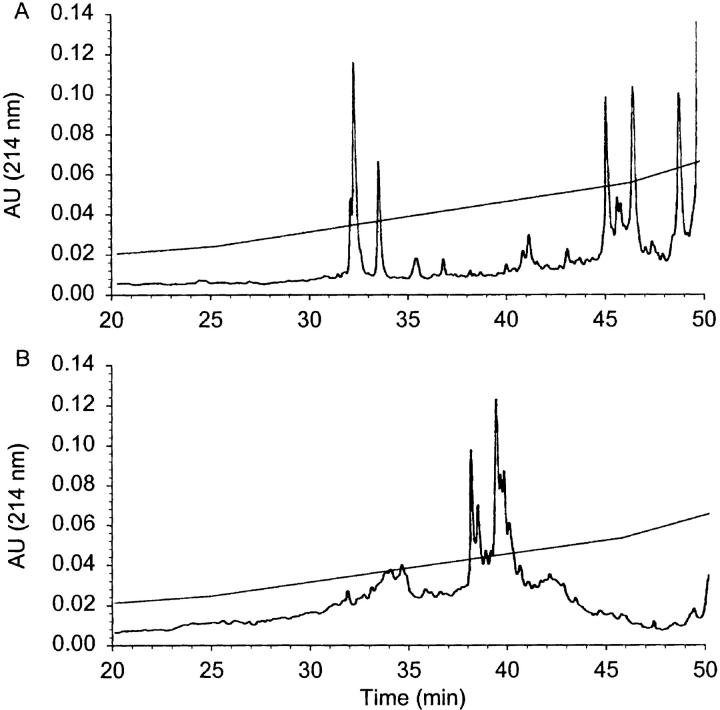
Peptides presented by normal human spleen class I MHC molecules were fractionated by RP-HPLC, and the majority of peptides eluted in a typical broad interval corresponding to 10–35% buffer B. Numerous well defined individual peaks were clearly distinguishable above a bell-shaped UV trace of heterogeneously eluting peptide material. SDS-PAGE analysis of the MHC–peptide preparation demonstrated the absence of contaminating low molecular weight proteins, which might separate with the peptides during the ultrafiltration (Fig. 2, insert). An identical aliquot of the class I MHC–derived peptides was then subjected to galactosyltransferase-mediated labeling with [3H]Gal. Fig. 2 B shows that radioactively labeled peptide material eluted between 10 and 35 min and was dominated by two to three major peaks at 20% buffer B, while also containing many minor labeled species. This result suggests that a small fraction of peptides isolated from natural class I MHC molecules was able to act as substrate for the GlcNAc-specific galactosyltransferase, strongly indicating that the pool of peptides eluted from MHC class I contain a subset of peptides with covalently linked terminal GlcNAc structures. Importantly, similar glycopeptides were not present in the negative control precolumn extract, derived using an irrelevant Ab (28-14-8s, H-2Db-specific; results not shown). This strongly supports the interpretation that the labeled glycopeptides have indeed been presented by class I MHC molecules. Based on comparisons with positive control O-β-GlcNAc–containing glycopeptides, we estimate that the amount of labeled peptide corresponds to 0.1% of peptides presented by class I MHC being O-glycosylated.
Figure 2.

RP-HPLC chromatogram of peptides extracted from human spleen class I MHC molecules before labeling with [3H]Gal. (A) The boxed inset is an SDS-PAGE analysis of the MHC–peptide preparation with molecular mass standards on the left. (B) The corresponding radioactivity trace after galactosyltransferase labeling of these peptides with [3H]Gal using bovine milk galactosyltransferase. C illustrates the sensitivity of [3H]Gal-labeled peptides to β-elimination. An aliquot of labeled peptide identical to that in B was subjected to β-elimination and analyzed by RP-HPLC.
To characterize the carbohydrate structures and their linkages on peptides from class I MHC, aliquots of the [3H]Gal-labeled peptides were treated with N-glycosidase F or weak alkali (β-elimination). N-glycosidase F cleaves N-linked carbohydrate structures, whereas only O-β-linked carbohydrate structures are susceptible to β-elimination 16. Fig. 2 C shows that the amount of [3H]Gal-labeled glycopeptides recovered after β-elimination was significantly reduced, whereas the labeled peptides were not sensitive to treatment with N-glycosidase F digestion (data not shown). This indicates that the majority of MHC-derived peptides that could be labeled by the galactosyltransferase contained O-β-linked GlcNAc residues.
Next, the radioactively labeled glycans were analyzed by size exclusion chromatography. As seen in Fig. 3 A, peptides isolated from human class I MHC and labeled with [3H]Gal using galactosyltransferase eluted in a broad peak at ∼40 min. This corresponds to the elution time of [3H]Gal-labeled K2G (Fig. 3 A, arrows), a prototype synthetic class I MHC–restricted nine–amino acid-long glycopeptide carrying one O-β-linked GlcNAc residue 10. In addition, a late-eluting peak, equivalent to the size of [3H]Gal, was found at 90 min. As the β-elimination reaction reduces any free reducing sugar to alditol, the carbohydrate reaction byproduct would be the alcohol form of the disaccharide [3H] Galβ1-4GlcNAc if peptides from MHC class I originally carried O-β-linked GlcNAc monosaccharide. Accordingly, after β-elimination, the elution by size exclusion chromatography of the glycan product from the [3H]Gal-labeled class I MHC–derived peptides corresponded accurately with the elution time of [3H]Gal-GlcNAcitol (Fig. 3 B), similar to the elution time of the O-GlcNAc–containing positive control peptide K2G β-elimination product (Fig. 3 C). More than 95% of the [3H]Gal-labeled class I MHC–derived peptides were sensitive to β-elimination, whereas <1% of the label incorporated into the N-linked glycan control peptide K1G was sensitive to the β-elimination procedure (data not shown), strongly supporting that the glycan structures present on a subset of peptides from human spleen class I MHC are dominated by the cytosolic type of O-β-linked GlcNAc glycosylation. We did not find any evidence for the presence of glycan structures other than the O-β-GlcNAc modification among peptides isolated from class I MHC molecules.
Figure 3.

The β-elimination glycan product of peptides labeled with [3H]Gal was subjected to size exclusion chromatography on a Bio-Gel P-2 column. This column separates mono- and disaccharides efficiently and distinguishes between GlcNAcβ1-4GlcNAc and Galβ1-4GlcNAc. Arrows at the top indicate the elution of compounds used to calibrate the column. (A) Elution of [3H]Gal-labeled class I MHC–derived peptides. (B) Elution of the glycan β-elimination product of [3H]Gal-labeled class I MHC–derived peptides. (C) Elution of the glycan β-elimination product of [3H]Gal-labeled positive control peptide K2G.
In a separate attempt to demonstrate that O-β-GlcNAc–modified peptides were specifically bound to MHC molecules, peptides eluted from HLA-A*0201 that had been affinity purified from 1010 JestHom cells were passed through a series of lectin affinity columns in the following order: (i) Con A (specific for high-mannose structures in N-glycosylated proteins), (ii) WGA (specific for terminal GlcNAc residues), and (iii) and Arachis hypogea lectin (specific for saccharide structures containing terminal N-acetylgalactosamine (GalNAc) residues, as they are found in the mucin-type O-linked glycosylation). The majority (99%) passed through the affinity columns (data not shown). The WGA column retained ∼1% of the peptides (Fig. 4 B), supporting the notion that O-β-GlcNAc–modified peptides are represented among natural HLA-A*0201 ligands. Markedly less peptide-like material was retained by the Con A column (Fig. 4 A), which, being first in the series, is likely to contain any nonspecific binding material. Almost no peptide was retained by the peanut lectin column (data not shown). Similar overall results were obtained for the HLA-B*2705–derived peptides, although the fraction of peptide retained by the lectin columns was significantly lower (not shown), possibly reflecting an incompatibility between the sequence requirement for peptide binding to HLA-B*2705 and the specificity requirements of the O-β-GlcNAc transferase responsible for O-β-GlcNAc glycosylation.
Figure 4.
RP-HPLC analysis of peptides extracted from HLA-A*0201 and purified by lectin affinity chromatography. A shows the eluate from the Con A–agarose column, and B shows the peptides retained by WGA–agarose specific for terminal GlcNAc residues.
The cytosolic O-β-GlcNAc modification is known to be dynamically regulated and changes reciprocally with phosphorylation in response to cellular activation 17. This study shows that steady-state, low-level presentation by class I MHC molecules of glycopeptides carrying the cytosolic type of O-β-GlcNAc modification occurs in normal cells, and it is possible that regulatory changes in O-GlcNAc glycosylation during malignancy could result in the presentation of novel glycopeptides for recognition by CTL. In addition, presentation of O-β-GlcNAc–modified proteins may be relevant during infection, as examples of cytosolic O-β-GlcNAc–modified proteins have been identified from human cytomegalovirus 20, adenovirus 21, trypanosomes 22, schistosomes 23, leishmania 24, and malaria 25. With improved techniques to detect glycopeptides among complex mixtures of peptides eluted from class I MHC molecules isolated from normal, infected, and malignant cells, it may soon be possible to identify glycoprotein antigens that can be processed to yield glycopeptide epitopes for class I MHC–restricted antigen presentation.
Acknowledgments
We are greatly indebted to Stefan Stevanovic and Hans-Georg Rammensee, Department of Immunology, University of Tübingen, Germany for invaluable input during this project.
This work was supported by the Carlsberg Foundation, the Wellcome Trust, the Danish Medical Research Council, the Novo Nordisk Foundation, the Alfred Benzon Foundation, and the Danish Cancer Society. Tim Elliott is a Wellcome Trust Senior Fellow in Basic Biomedical Science.
References
- Pamer E.G., Cresswell P. Mechanisms of MHC class I-restricted antigen processing. Annu. Rev. Immunol. 1998;16:323–358. doi: 10.1146/annurev.immunol.16.1.323. [DOI] [PubMed] [Google Scholar]
- Rammensee H.G., Bachmann J., Stevanovic S. MHC Ligands and Peptide Motifs 1997. Springer-Verlag Inc; Heidelberg, Germany: pp. 450 [Google Scholar]
- Zhao X.J., Cheung N.K. GD2 oligosaccharidetarget for cytotoxic T lymphocytes. J. Exp. Med. 1995;182:67–74. doi: 10.1084/jem.182.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skipper J.C., Hendrickson R.C., Gulden P.H., Brichard V., Van Pel A., Chen Y., Shabanowitz J., Wölfel T., Slingluff C.L., Jr., Boon T. An HLA-A2–restricted tyrosinase antigen on melanoma cells results from posttranslational modification and suggests a novel pathway for processing of membrane proteins. J. Exp. Med. 1996;183:527–534. doi: 10.1084/jem.183.2.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meadows L., Wang W., den Haan J.M., Blokland E., Reinhardus C., Drijfhout J.W., Shabanowitz J., Pierce R., Agulnik A.I., Bishop C.E. The HLA-A*0201-restricted H-Y antigen contains a posttranslationally modified cysteine that significantly affects T cell recognition. Immunity. 1997;6:273–281. doi: 10.1016/s1074-7613(00)80330-1. [DOI] [PubMed] [Google Scholar]
- Michaelsson E., Malmstrom V., Reis S., Engstrom Å., Burkhardt H., Holmdahl R. T cell recognition of carbohydrates on type II collagen. J. Exp. Med. 1994;180:745–749. doi: 10.1084/jem.180.2.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudler T., Altmann F., Carballido J.M., Blaser K. Carbohydrate-dependent, HLA class II-restricted, human T cell response to the bee venom allergen phospholipase A2 in allergic patients. Eur. J. Immunol. 1995;25:538–542. doi: 10.1002/eji.1830250235. [DOI] [PubMed] [Google Scholar]
- Molberg Ø., McAdam S.N., Körner R., Quarsten H., Kristiansen C., Madsen L., Fugger L., Scott H., Norén O., Roepstorff P. Tissue transglutaminase selectively modifies gliadin peptides that are recognized by gut-derived T cells in celiac disease. Nat. Med. 1998;4:713–717. doi: 10.1038/nm0698-713. [DOI] [PubMed] [Google Scholar]
- Haurum J.S., Arsequell G., Lellouch A.C., Wong S.Y., Dwek R.A., McMichael A.J., Elliott T. Recognition of carbohydrate by major histocompatibility complex class I–restricted, glycopeptide-specific cytotoxic T lymphocytes. J. Exp. Med. 1994;180:739–744. doi: 10.1084/jem.180.2.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haurum J.S., Tan L., Arsequell G., Frodsham P., Lellouch A.C., Moss P.A., Dwek R.A., McMichael A.J., Elliott T. Peptide anchor residue glycosylationeffect on class I major histocompatibility complex binding and cytotoxic T lymphocyte recognition. Eur. J. Immunol. 1995;25:3270–3276. doi: 10.1002/eji.1830251211. [DOI] [PubMed] [Google Scholar]
- Glithero A., Tormo J., Haurum J.S., Arsequell G., Valencia G., Edwards J., Springer S., Townsend A., Pao Y.L., Wormald M. Crystal structures of two H-2Db/glycopeptide complexes suggest a molecular basis for CTL cross-reactivity. Immunity. 1999;10:63–74. doi: 10.1016/s1074-7613(00)80007-2. [DOI] [PubMed] [Google Scholar]
- Chicz R.M., Urban R.G., Gorga J.C., Vignali D.A., Lane W.S., Strominger J.L. Specificity and promiscuity among naturally processed peptides bound to HLA-DR alleles. J. Exp. Med. 1993;178:27–47. doi: 10.1084/jem.178.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neisig A., Roelse J., Sijts A.J., Ossendorp F., Feltkamp M.C., Kast W.M., Melief C.J., Neefjes J.J. Major differences in transporter associated with antigen presentation (TAP)-dependent translocation of MHC class I-presentable peptides and the effect of flanking sequences. J. Immunol. 1995;154:1273–1279. [PubMed] [Google Scholar]
- Engelhard V.H., Appella E., Benjamin D.C., Bodnar W.M., Cox A.L., Chen Y., Henderson R.A., Huczko E.L., Michel H., Sakaguichi K. Mass spectrometric analysis of peptides associated with the human class I MHC molecules HLA-A2.1 and HLA-B7 and identification of structural features that determine binding. Chem. Immunol. 1993;57:39–62. [PubMed] [Google Scholar]
- Roquemore E.P., Chou T.Y., Hart G.W. Detection of O-linked N-acetylglucosamine (O-GlcNAc) on cytoplasmic and nuclear proteins. Methods Enzymol. 1994;230:443–460. doi: 10.1016/0076-6879(94)30028-3. [DOI] [PubMed] [Google Scholar]
- Spiro R.G. Study of the carbohydrates of glycoproteins Methods Enzymol. 28B1972. 3 43 [Google Scholar]
- Hart G.W. Dynamic O-linked glycosylation of nuclear and cytoskeletal proteins. Annu. Rev. Biochem. 1997;66:315–335. doi: 10.1146/annurev.biochem.66.1.315. [DOI] [PubMed] [Google Scholar]
- Neefjes J., Gottfried E., Roelse J., Gromme M., Obst R., Hämmerling G.J., Momburg F. Analysis of the fine specificity of rat, mouse and human TAP peptide transporters. Eur. J. Immunol. 1995;25:1133–1136. doi: 10.1002/eji.1830250444. [DOI] [PubMed] [Google Scholar]
- Gromme M., van der Valk R., Sliedregt K., Vernie L., Liskamp R., Hämmerling G.J., Koopmann J.O., Momburg F., Neefjes J. The rational design of TAP inhibitors using peptide substrate modifications and peptidomimetics. Eur. J. Immunol. 1997;27:898–904. doi: 10.1002/eji.1830270415. [DOI] [PubMed] [Google Scholar]
- Benko D.M., Haltiwanger R.S., Hart G.W., Gibson W. Virion basic phosphoprotein from human cytomegalovirus contains O-linked N-acetylglucosamine. Proc. Natl. Acad. Sci. USA. 1988;85:2573–2577. doi: 10.1073/pnas.85.8.2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caillet-Boudin M.L., Strecker G., Michalski J.C. O-linked GlcNAc in serotype-2 adenovirus fibre. Eur. J. Biochem. 1989;184:205–211. doi: 10.1111/j.1432-1033.1989.tb15008.x. [DOI] [PubMed] [Google Scholar]
- Haltiwanger R.S., Kelly W.G., Roquemore E.P., Blomberg M.A., Dong L.Y., Kreppel L., Chou T.Y., Hart G.W. Glycosylation of nuclear and cytoplasmic proteins is ubiquitous and dynamic. Biochem. Soc. Trans. 1992;20:264–269. doi: 10.1042/bst0200264. [DOI] [PubMed] [Google Scholar]
- Nyame K., Cummings R.D., Damian R.T. Schistosoma mansoni synthesizes glycoproteins containing terminal O-linked N-acetylglucosamine residues. J. Biol. Chem. 1987;262:7990–7995. [PubMed] [Google Scholar]
- Handman E., Barnett L.D., Osborn A.H., Goding J.W., Murray P.J. Identification, characterisation and genomic cloning of an O-linked N-acetylglucosamine-containing cytoplasmic Leishmania glycoprotein. Mol. Biochem. Parasitol. 1993;62:61–72. doi: 10.1016/0166-6851(93)90178-z. [DOI] [PubMed] [Google Scholar]
- Dieckmann-Schuppert A., Bause E., Schwarz R.T. Studies on O-glycans of Plasmodium- falciparum-infected human erythrocytes. Evidence for O-GlcNAc and O-GlcNAc-transferase in malaria parasites. Eur. J. Biochem. 1993;216:779–788. doi: 10.1111/j.1432-1033.1993.tb18198.x. [DOI] [PubMed] [Google Scholar]