Abstract
Trichosanthin (TCS), an active protein component isolated from a traditional Chinese medicinal herb Trichosanthes kirilowii, has been shown to inhibit HIV infection and has been applied in clinical treatment of AIDS. The recent development that chemokines and chemokine receptors play important roles in HIV infection led us to investigate the possible functional interaction of TCS with chemokines and their receptors. This study demonstrated that TCS greatly enhanced both RANTES (regulated upon activation, normal T cell expressed and secreted)– and stromal cell–derived factor (SDF)-1α–stimulated chemotaxis (EC50 ≅ 1 nM) in leukocytes (THP-1, Jurkat, and peripheral blood lymphocyte cells) and activation of pertussis toxin–sensitive G proteins (EC50 ≅ 20 nM). TCS also significantly augmented chemokine-stimulated activation of chemokine receptors CCR5 and CXCR4 as well as CCR1, CCR2B, CCR3, and CCR4 transiently expressed in HEK293 cells. A mutant TCS with 4,000-fold lower ribosome-inactivating activity showed similar augmentation activity as wild-type TCS. Moreover, flow cytometry demonstrated that the specific association of TCS to the cell membranes required the presence of chemokine receptors, and laser confocal microscopy reveals that TCS was colocalized with chemokine receptors on the membranes. The results from TCS-Sepharose pull-down and TCS and chemokine receptor coimmunoprecipitation and cross-linking experiments demonstrated association of TCS with CCR5. Thus, our data clearly demonstrated that TCS synergizes activities of chemokines to stimulate chemotaxis and G protein activation, and the effects of TCS are likely to be mediated through its interaction with chemokine receptors.
Keywords: chemokine receptors, trichosanthin, G proteins, chemotaxis, HIV
Trichosanthin (TCS),1 a 27-kd protein, is an active component extracted from the root tuber of Chinese medicinal herb Tian-Hua-Fen (Trichosanthes kirilowii) of the Cucurbitaceae family. In the classical Chinese medical reference work Compendium of Materia Medica written in the late 14th century, Tian-Hua-Fen was documented as a drug that resets menstruation and expels retained placentas, and has been used in medical practice in China for hundreds of years. In the early 1970s, TCS was isolated from T. kirilowii and has been used to terminate early and midtrimester pregnancies 1 2 and to treat ectopic pregnancies, hydatidiform moles, and trophoblastic tumors 2 3. Pharmacological studies reported that TCS is able to inactivate eukaryotic ribosomes 4 5 and to suppress the immune responses 6 7. More interestingly, TCS was shown to inhibit HIV replication in infected cells of lymphocyte and mononuclear phagocytic lineage, with no measurable toxicity in uninfected cells 8 9. In the early 1990s, TCS was applied in the treatment of patients with AIDS or AIDS-related complex in phase I and II studies 10 11 12 13. However, the underlying mechanisms of the activities of TCS are not yet well-understood.
Chemokines are a superfamily of small structurally related cytokine molecules characterized by their ability to induce leukocyte migration and related responses 14 15 16 17 18. Chemokines also play important roles in regulation of growth, and in angiogenic and developmental processes 14 19 20 21. Biological activities of chemokines are mediated by G protein–coupled chemokine receptors (GPCRs) classified as CC or CXC receptors based on the structures and types of chemokines they interact with 14 15. Of considerable interest is the recent discovery that CC chemokine receptors CCR5, CCR2B, and CCR3 and the CXC chemokine receptor CXCR4 are the essential coreceptors on the cell surface for HIV-1 fusion and infection 22 23 24 25 26 27 28. Substantial progress has been made recently in the understanding of chemokine receptor–mediated cellular signaling 29 30 31 32. Activation of chemokine receptors by chemokines induces downregulation of chemokine receptors on the cell surface 31 32. Prevention of HIV-1 infection and inhibition of HIV-1 replication by chemokines, antagonists of chemokine receptors, or mAbs to chemokine receptors, which induce downregulation of chemokine receptors and/or directly block HIV-1 interaction with the coreceptors, have been demonstrated 33 34 35 36 37. Therefore, drugs that target chemokine receptors, the coreceptors of HIV, have great potential in AIDS therapy.
This study was carried out in an attempt to understand the cellular and molecular mechanisms of the activities of TCS against HIV infection. Our results demonstrated that TCS profoundly augments the ability of different chemokines to activate a wide spectrum of chemokine receptors, leading to chemotaxis and G protein activation, and that the effect of TCS is likely to be mediated through its functional interaction with these chemokine receptors.
Materials and Methods
Materials.
Recombinant human RANTES (regulated upon activation, normal T cell expressed and secreted), macrophage inflammatory protein (MIP)-1β, and monocyte chemotactic protein (MCP)-1 were purchased from Sigma Chemical Co., and stromal cell–derived factor (SDF)-1α was from PharMingen. Native TCS was isolated from T. kirilowii. Recombinant TCS (r-TCS) and a mutant of TCS (m-TCS) were prepared as described previously 38 39 40. The homogeneity of TCS preparations used was >98%. Rabbit anti-TCS antibodies and an mAb against TCS were provided by Prof. Ming Ye (Shanghai Institute of Cell Biology). Mouse mAb 12CA5 against the influenza hemagglutinin (HA) epitope was obtained from Boehringer Mannheim. [35S]GTPγS and [3H]cAMP were purchased from Amersham Pharmacia Biotech. MEM and RPMI 1640 were from GIBCO BRL. GDP and GTPγS were from Sigma Chemical Co. CNBr-activated Sepharose 4B was from Amersham Pharmacia Biotech.
Cloning.
CCR5 was cloned as described previously 32. The full-length cDNA encoding CCR1, CCR2B, CCR3, CCR4, and CXCR4 was cloned by reverse transcription PCR and PCR from THP-1 cells (for CCR1, CCR2B, and CXCR4) or PBL cells (for CCR3 and CCR4), using specific primers designed from the published sequences (available from EMBL/GenBank/DDBJ under accession nos. L09230, U03905, U28694, X85740, and X71635). The amplified human chemokine receptor cDNA fragments were then subcloned into a modified pcDNA3 vector (Invitrogen) with the sequence of the HA epitope tag at the 5′ end of the inserted receptor sequence. The authenticity of the receptor sequences was confirmed by DNA sequencing.
Cell Culture and Transfection.
THP-1 and Jurkat cells (American Type Culture Collection) were cultured in RPMI 1640 (GIBCO BRL) supplemented with 10% heat-inactivated fetal bovine serum (FBS; GIBCO BRL), 100 U/ml penicillin, 100 μg/ml streptomycin, and 2 mM glutamine. PBMCs were obtained by the Ficoll-Hypaque method from heparinized whole blood, and PBLs were derived by serial depletion of adherent cells and maintained in RPMI 1640 supplemented with 10–15% FBS. PBLs were stimulated with 5 μg/ml phytohemagglutinin for 1 wk and maintained thereafter in the presence of IL-2. Human embryonic kidney (HEK)293 cells (American Type Culture Collection) were cultured in MEM supplemented with 10% heat-inactivated FBS, 100 U/ml penicillin, 100 μg/ml streptomycin, and 2 mM glutamine. Transient transfection of HEK293 cells was performed using 4 μg DNA/106 cells and the calcium phosphate–DNA coprecipitation method, and the transfected cells were used 48 h after transfection.
Chemotaxis Assay.
Chemotaxis was performed as described previously 41 42. In brief, cells were resuspended in RPMI 1640 containing 1 mg/ml BSA overnight in 5% CO2 at 37°C. 0.1 ml cells at 5 × 106/ml were added to the top chamber of a 24-well transwell (6.5-mm diameter, 5-μm pore size; Corning Costar) and incubated for 3 h or the time indicated at 37°C in 5% CO2. Cells passing through the membrane were collected from the lower well and counted by mixing a predetermined number of yeast with the cells and running them through a FACSCalibur™ flow cytometer (Becton Dickinson). The yeast and the cells were easily distinguishable on a side scatter vs. forward scatter plot, which allowed the calculation of the ratio of yeast to cells and the total number of cells that had migrated to the lower wells. Cell numbers were also determined using a cell counter and found to be in good agreement with the results from fluorescence-activated cell sorting.
[35S]GTPγS Binding Assay.
The assay was carried out as described 43 44. Cells were lysed in 5 mM Tris-HCl, pH 7.5, 5 mM EDTA, and 5 mM EGTA at 4°C. After the lysate was centrifuged at 30,000 g for 10 min, the membrane pellet was resuspended and aliquots containing 12 μg protein were incubated at 30°C for 1 h in 50 mM Tris-HCl, pH 7.5, 1 mM EDTA, 1 mM dithiothreitol, 5 mM MgCl2, 100 mM NaCl, 40 μM GDP, and 0.5 nM [35S]GTPγS (1,200 Ci/mmol) in the presence or absence of the agonists in a total volume of 100 μl. The reaction was terminated by adding cold PBS and filtering through GF/C filters. Radioactivity of each sample was measured in a liquid scintillation spectrophotometer. Data were means of duplicate samples. Basal binding was determined in the absence of agonists, and nonspecific binding was obtained in the presence of 10 μM GTPγS. The percentage of stimulated [35S]GTPγS binding was calculated as 100 × (cpmsample − cpmnonspecific)/(cpmbasal − cpmnonspecific).
cAMP Assay.
Cells were challenged with agonists in the presence of 10 μM forskolin (Sigma Chemical Co.) and 500 μM 1-methyl-3-isobutylxanthine (IBMX; Sigma Chemical Co.) at 37°C for 10 min. The reaction was terminated with 1 N perchloric acid and then neutralized with 2 M K2CO3. The cAMP level of each sample was determined using radioimmunoassay as described previously 45 46 47. Data were averages of duplicate samples and were presented as a percentage of control, calculated as 100 × [cAMP(forskolin + agonist) − cAMP(basal)]/[cAMP(forskolin) − cAMP(basal)]. cAMP(forskolin + agonist) is cAMP accumulation in the presence of forskolin and agonist, cAMP(basal) is cAMP accumulation in the absence of forskolin and agonist, and cAMP(forskolin) is cAMP accumulation in the presence of forskolin alone.
Flow Cytometry.
Cells were incubated with TCS (100 nM) in PBS containing 2% BSA at 4°C for 1 h and, after washing with PBS, were incubated with 12CA5 (5 μg/ml) and rabbit TCS-specific antibodies (1:1,000) in PBS containing 2% BSA at 4°C for 1 h. The presence of HA-tagged chemokine receptors and TCS on the cell surface was detected by incubation with FITC-conjugated, affinity-purified goat anti–mouse IgG (Tago) and tetramethyl-rhodamine isothiocyanate (TRITC)-conjugated goat anti–rabbit IgG (Jackson ImmunoResearch Labs). The cells were analyzed on a FACSCalibur™ flow cytometer. Basal cell fluorescence intensity was determined with cells stained with the secondary antibody alone.
Immunofluorescence Microscopy.
As described previously 48 49, cells grown on coverslips were fixed in 1% polyformaldehyde for 20 min. After incubation with TCS (100 nM) in PBS containing 2% BSA at 4°C for 1 h and washing twice with cold PBS, cells were treated with 12CA5 mAb and rabbit anti-TCS antibodies. The presence of HA-tagged chemokine receptors and TCS in the cells was then detected with FITC-conjugated, affinity-purified goat anti–mouse IgG and Texas Red–conjugated, affinity-purified goat anti–rabbit IgG (Amersham Pharmacia Biotech), respectively. In addition, control experiments with mock transfection, or in the absence of the first antibodies, or without TCS were performed. Images were recorded using a Leica TCS NT laser confocal scanning microscope.
SDS-PAGE and Silver Staining.
The experiment was performed by using a modified silver stain process. 5 μg purified protein was loaded to 12% SDS-PAGE. The gel was then prefixed with 30% ethanol and 10% acetic acid (HAc) and fixed in 30% ethanol, 0.4 M NaAc, pH 6.0, and 0.03% Na2S2O3. After washing, the gel was incubated in 0.1% AgNO3 and then in 2.5% Na2CO3 with 0.1% Na2S2O3. The reaction was terminated by incubating the gel in 10% HAc.
Immunoprecipitation, Western Blotting, and Cross-linking Experiments.
The immunoprecipitation experiment was performed as described 50 51. HEK293 cells grown in a 60-mm culture dish were lysed in 0.8 ml IP buffer (20 mM Tris-HCl, pH 7.4, 150 mM NaCl, and 0.4% digitonin) containing protease inhibitors on ice for 45 min. The lysate was centrifuged at 12,000 g for 30 min, and the supernatants were incubated with the 12CA5 antibody (0.5 μg) and protein A–Sepharose (GIBCO BRL) on ice for 2 h. TCS was then added (0.5 μg) and incubated for another 2 h. After washing with IP buffer, the immunocomplexes absorbed onto protein A–Sepharose were eluted in SDS-PAGE sample buffer (50 mM Tris-HCl, pH 7.4, 2% SDS, 5% 2-ME, 10% glycerol, and 0.01% bromophenol blue) and subjected to 10% SDS-PAGE and Western blot analysis. TCS present in the samples was detected using rabbit anti-TCS antibodies, and the presence of CCR5 on the same blot was detected using 12CA5 after stripping the antibodies off by incubating in 62.5 mM Tris-HCl, pH 6.7, 100 mM 2-ME, and 2% SDS at 70°C for 30 min.
Alternatively, the cell lysate prepared as described above was incubated with TCS-coupled Sepharose or BSA-coupled Sepharose (prepared following the manufacturer's instructions) on ice for 4 h. The supernatants were then discarded, the beads were lightly washed, and the protein absorbed onto the beads was eluted in SDS-PAGE sample buffer. SDS-PAGE and Western blot analysis were performed as described above.
The cross-linking was performed by using disuccinimidyl suberate (DSS; Pierce Chemical Co.) following the manufacturer's instructions. In brief, cells were lysed in 10 mM Hepes, pH 7.4, 5 mM EDTA, and 5 mM EGTA at 4°C. After the lysate was centrifuged at 30,000 g for 10 min, the membrane pellet was resuspended in PBS/Hepes (PBS containing 10 mM Hepes, pH 7.4). The aliquots containing 500 μg membrane protein were then incubated with or without DSS (100 μM) at room temperature for 30 min in the presence or absence of TCS (10 μg) in a total volume of 400 μl. The reaction was terminated by adding cold 1 M Tris-HCl (pH 7.4) to a final concentration of 10 mM and incubating for an additional 15 min. The samples were analyzed using Western blotting, and the cross-linked complex of TCS and chemokine receptors was detected by rabbit anti-TCS antibodies.
Statistical Analysis.
Each experimental point was performed in duplicate, and at least three independent experiments were carried out. Data are expressed as means ± SE of all determinations. Statistical significance of the experimental results was obtained by Student's t test. P < 0.05 was accepted as denoting statistical significance.
Results
TCS Enhanced Chemokine-induced Chemotaxis in THP-1 Cells.
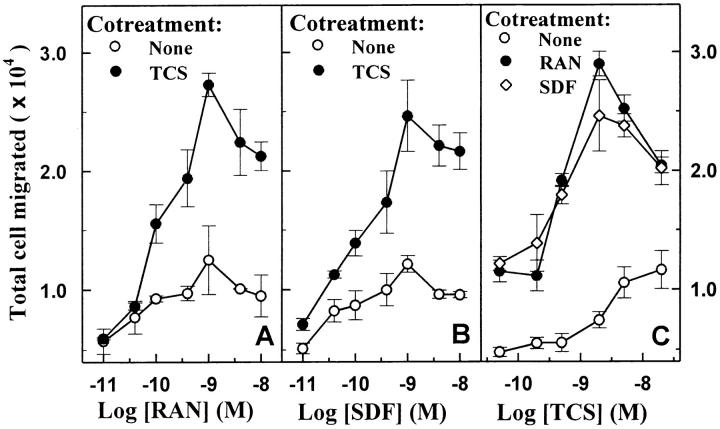
Chemotaxis is the prototypic function of chemokines, and thus serves as a biologically relevant functional in vitro assay for chemokine receptor activation 41 42. THP-1 cells are of human leukocyte origin and express functional CCR1 52, CCR5 53, and CXCR4 (Zhao, J., and G. Pei, unpublished observation), and therefore were used in the chemotaxis experiments. THP-1 cells showed a classic bell-shaped chemotactic response upon exposure to increasing concentrations of either RANTES (Fig. 1 A) or SDF-1α (Fig. 1 B), and both concentration–response curves reached maximum at 1 nM of chemokine (Fig. 1A and Fig. B). The presence of 2 nM TCS alone did not significantly affect chemotaxis (Fig. 1A–C). However, cotreatment of 2 nM TCS with RANTES or SDF-1α (0.1 nM and above) strongly increased cell migration induced by either chemokine (to ∼300% at chemokine concentrations of 1–10 nM). The concentration–effect curves of TCS on chemokine-induced chemotaxis show that a significant increase of RANTES- and SDF-1α–stimulated chemotaxis occurred at TCS concentrations of as low as 0.5 nM, and 2 nM TCS resulted in the maximal enhancement of ∼250% (Fig. 1 C).
Figure 1.
Enhancement of RANTES- and SDF-1α–induced chemotaxis by TCS in THP-1 cells. Cells were challenged with RANTES (RAN, A) or SDF-1α (SDF, B) at concentrations indicated in the presence or absence of 2 nM TCS at 37°C for 3 h, and the chemotaxis was determined as described in Materials and Methods. THP-1 cells were incubated with different concentrations of TCS (C) in the absence or presence of 1 nM RANTES or 1 nM SDF-1α at 37°C for 3 h, and the migrated cells were collected and counted as described above. Data were mean ± SE of two independent experiments performed in duplicate.
TCS Enhanced Chemokine-induced G Protein Activation in THP-1 Cells.
Our previous study demonstrated that stimulation of chemokine receptors by their agonists activates membrane-associated Gi/Go proteins using [35S]GTPγS binding assay 32. As shown in Fig. 2A and Fig. B, RANTES (agonist of both CCR1 and CCR5) and SDF-1α (agonist of CXCR4) activated membrane-associated G proteins in a concentration-dependent manner in THP-1 cells. TCS (0.2 μM) alone did not have a significant effect on [35S]GTPγS binding. But interestingly, in the presence of 0.2 μM TCS, RANTES- or SDF-1α–stimulated G protein activation increased significantly and the maximal stimulation induced by RANTES and SDF-1α increased by 150 and 200%, respectively (Fig. 2A and Fig. B). As shown in Fig. 2 C, the ability of TCS to enhance chemokine RANTES- and SDF-1α–induced G protein activation was dependent on TCS concentration (EC50 ≅ 20 nM). At 5 nM or higher concentration, TCS showed a significant enhancement effect, and in the presence of 200 nM TCS, RANTES- and SDF-1α–induced chemokine receptor stimulation increased by two- to threefold. TCS alone did not have a significant effect on basal [35S]GTPγS binding (Fig. 2 C).
Figure 2.
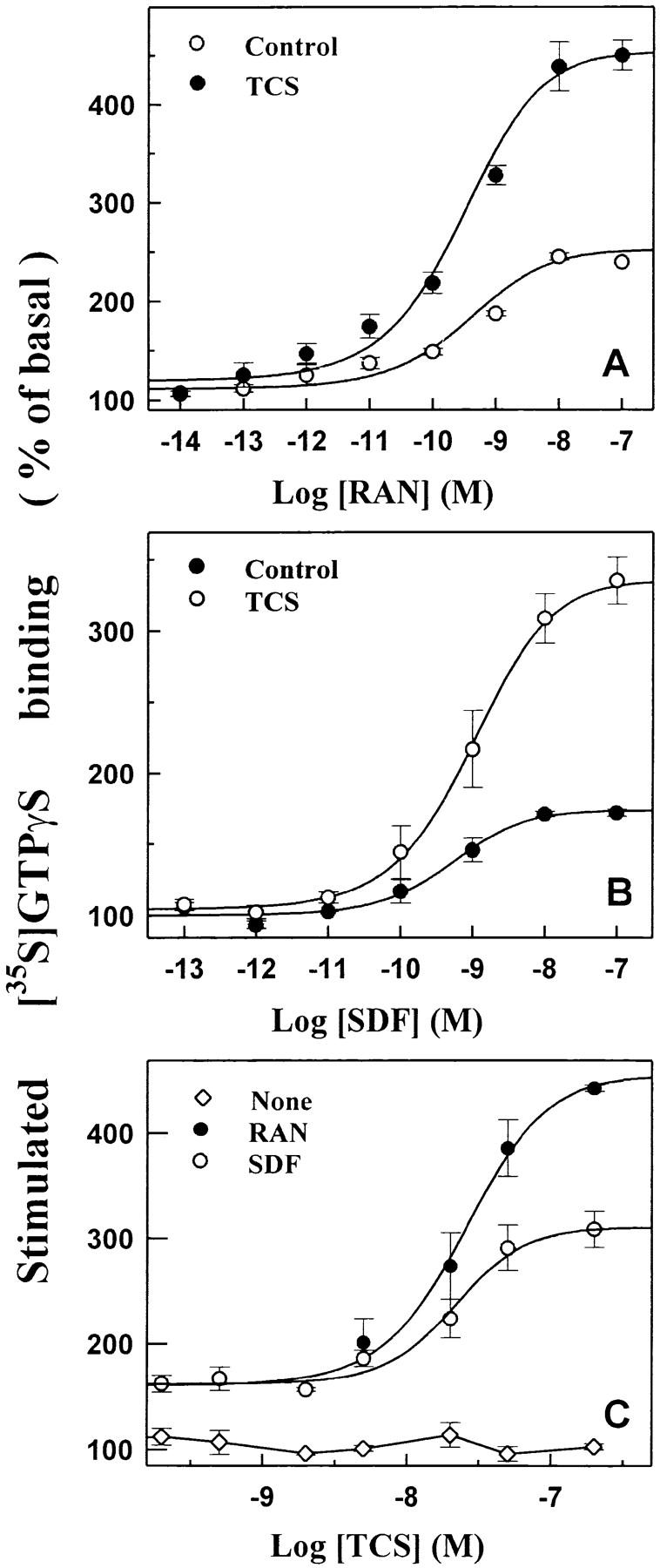
Enhancement of RANTES- and SDF-1α–stimulated G protein activation by TCS in THP-1 cells. The cell membranes were challenged with different concentrations of RANTES or SDF-1α in the absence or presence of 0.2 μM TCS at 30°C for 60 min (A and B) or with none or 10 nM RANTES or SDF-1α in the presence of different concentrations of TCS at 30°C for 60 min (C). The [35S]GTPγS binding of each sample was measured as described in Materials and Methods. Data were mean ± SE of three independent experiments performed in duplicate.
TCS Augmented Chemokine-induced Signaling in PBLs and Jurkat Cells.
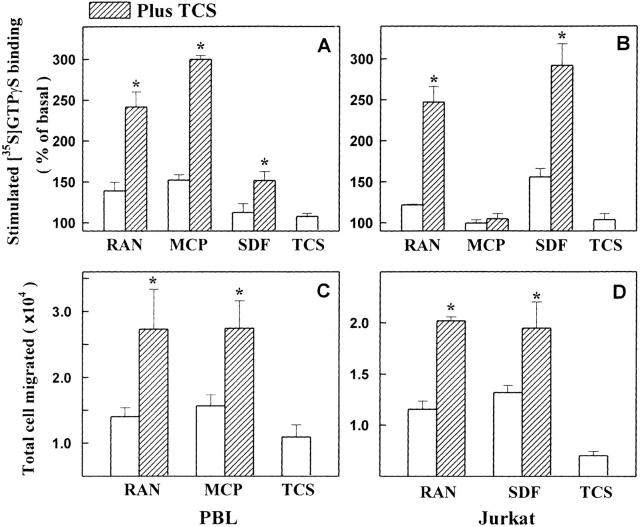
To test whether TCS can enhance the capability of chemokine to activate chemokine receptors in other cells, PBLs and Jurkat cells were used in this study. As shown in Fig. 3A and Fig. B, TCS significantly enhanced the activation of G proteins induced by RANTES, MCP-1, and SDF-1α in PBLs and by RANTES and SDF-1α in Jurkat cells, respectively. Neither MCP-1 alone nor MCP-1 plus TCS resulted in any stimulation of G protein activation in Jurkat cells that lack CCR2, the receptor of MCP-1. This indicates that the specificity of the effects of TCS relies on both chemokine and chemokine receptor. In the chemotaxis assay, TCS also increased the efficacies of RANTES and MCP-1 to induce cell migration in PBLs (Fig. 3 C) and of RANTES and SDF-1α in Jurkat cells (Fig. 3 D). These data clearly demonstrate that TCS enhances the ability of chemokines to stimulate chemokine receptors and to induce chemotaxis in leukocytes.
Figure 3.
The enhancement effects of TCS on chemokine-stimulated G protein activation and chemotaxis in PBLs and Jurkat cells. PBLs (A and C) and Jurkat cells (B and D) were stimulated with RANTES, MCP-1, or SDF-1α in the absence or presence of TCS (0.2 μM for [35S]GTPγS binding and 2 nM for chemotaxis), and [35S]GTPγS binding of cell membranes and number of cells migrated for each sample were determined as described in Materials and Methods. Data were mean ± SE of at least two independent experiments performed in duplicate.
The Effects of TCS Were Chemokine Receptor Dependent and Required G Proteins.
In HEK293 cells transiently expressing CCR5 (Fig. 4A and Fig. C) or CXCR4 (Fig. 4B and Fig. D), TCS significantly enhanced both RANTES- and SDF-1α–stimulated G protein activation. The effect of TCS in these cells was chemokine concentration and TCS concentration dependent. However, in the mock-transfected HEK293 cells, neither was chemokine-stimulated [35S]GTPγS binding observed nor did TCS show any augmentation effects when used together with RANTES or SDF-1α under the same conditions. In addition, MCP-1, an agonist of CCR2, in either the absence or presence of TCS, was not able to stimulate G protein activation in HEK293 cells transfected with CCR5 (data not shown). Chemokine receptors are able to couple to Gi and Gq proteins. Activation of chemokine receptors causes activation of membrane-associated G proteins and results in inhibition of adenylyl cyclase. As shown in Fig. 5, RANTES and SDF-1α caused inhibition of adenylyl cyclase activity in HEK293 cells transiently expressing CCR5 or CXCR4. Coapplication of TCS under such conditions considerably increased the efficacies of both chemokines to inhibit cellular cAMP production (Fig. 5). Chemokine-induced inhibition of cAMP production and the enhancement of this by TCS were abolished by pertussis toxin (data not shown). The above results further demonstrate the indispensability of both chemokines and the corresponding chemokine receptors for TCS to exert its effects.
Figure 4.
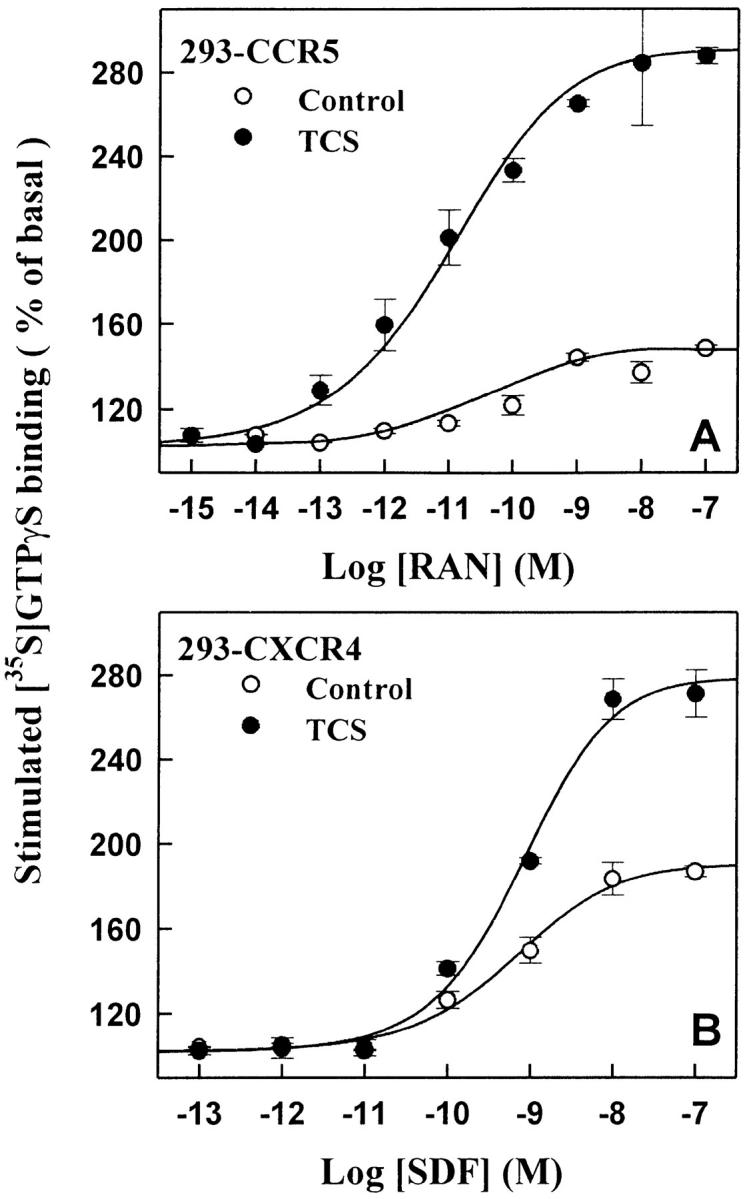
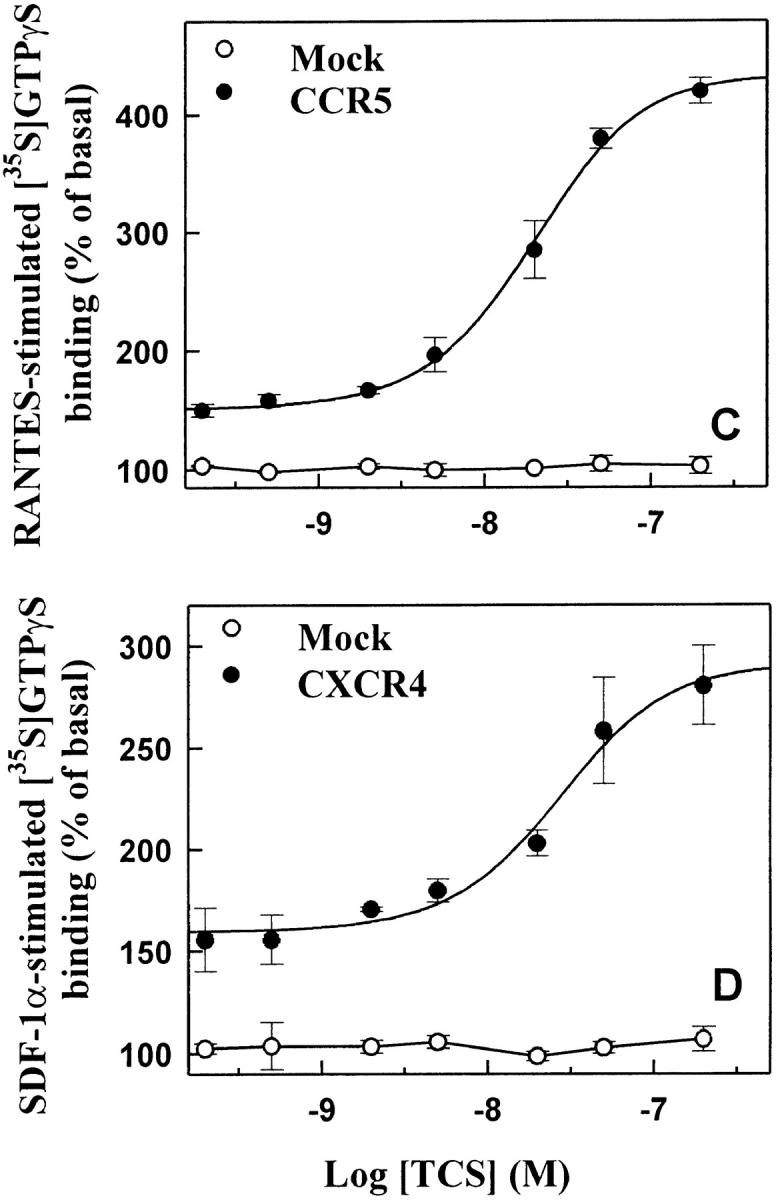
The enhancement effect of TCS in HEK293 cells expressing CCR5 or CXCR4. The cell membranes from CCR5 (A and C) or CXCR4 (B and D) transfected cells were stimulated with different concentrations of RANTES (A) or SDF-1α (B) in the absence or presence of 0.2 μM TCS, or incubated with different concentrations of TCS in the presence of 10 nM RANTES (C) or SDF-1α (D). The [35S]GTPγS binding of each sample was then measured as described in Materials and Methods. Data were mean ± SE of three independent experiments performed in duplicate.
Figure 5.

Augmentation of RANTES- or SDF-1α–induced inhibition of cAMP accumulation by TCS. HEK293 cells transfected with CCR5 (A) or CXCR4 (B) were treated by different concentrations of RANTES or SDF-1α in the presence or absence of 0.2 μM TCS at 37°C for 10 min, and the cellular cAMP level was measured as described in Materials and Methods. Data were mean ± SE of three independent experiments performed in duplicate.
The Effects of TCS Extended to Many Other Chemokine Receptors.
To test the potential effects of TCS on cellular signaling mediated by other chemokine receptors and GPCRs in addition to CCR5 and CXCR4, chemokine receptors CCR1, CCR2B, CCR3, and CCR4 and κ and δ opioid receptor were transiently expressed in HEK293 cells. As shown in Fig. 6, TCS significantly enhanced G protein activation mediated by CCR1, CCR2B, CCR3, and CCR4, but failed to enhance opioid agonist–induced G protein activation mediated by either κ (Fig. 6) or δ opioid receptor (data not shown). The above data suggest that in addition to CCR5 and CXCR4, TCS could exert its effect on other members of the chemokine receptor family, probably via a similar mechanism, but the effect of TCS is chemokine receptor specific and may not extend to other peptide Gi/Go-coupled receptors.
Figure 6.
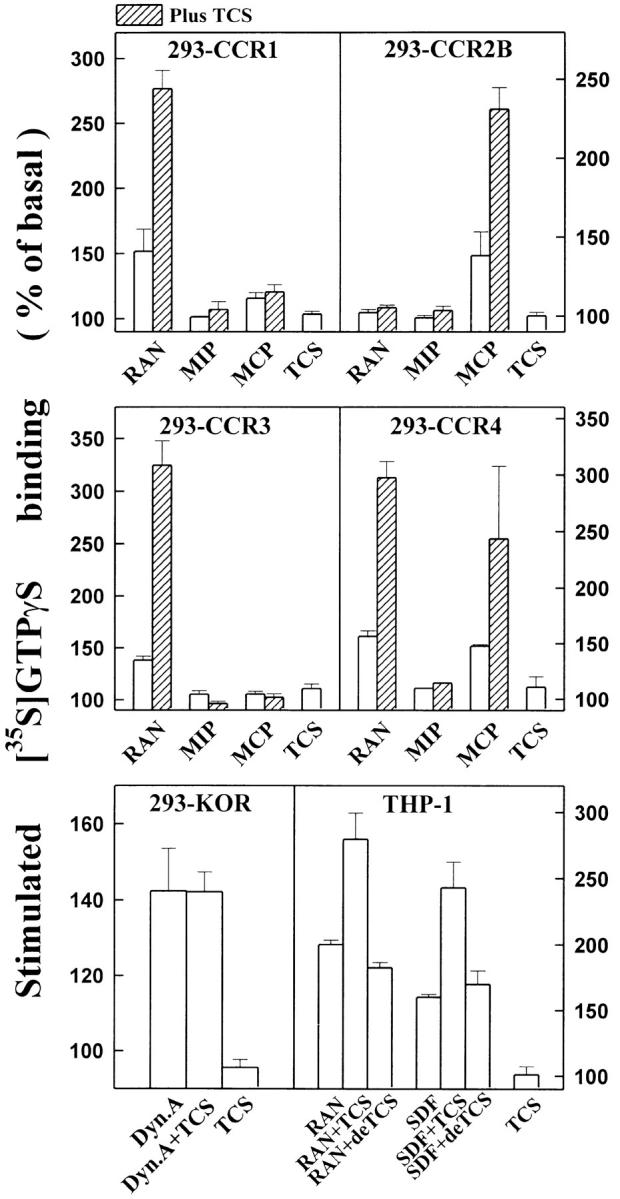
TCS enhances chemokine receptor–mediated G protein activation. Membranes from THP-1 and HEK293 cells transiently transfected with chemokine receptors CCR1, CCR2B, CCR3, or CCR4, or κ opioid receptor (KOR) were challenged with RANTES, MIP-1β, MCP-1, or SDF-1α in the absence or presence of 0.2 μM TCS or deTCS (TCS denatured at 60°C for 20 min) at 30°C for 60 min as indicated. The [35S]GTPγS binding of each sample was then measured as described in Materials and Methods. Data were mean ± SE of three independent experiments performed in duplicate.
The Effects of TCS on Chemokine Receptor Activation Were Independent of Its Ribosome-inactivating Activity.
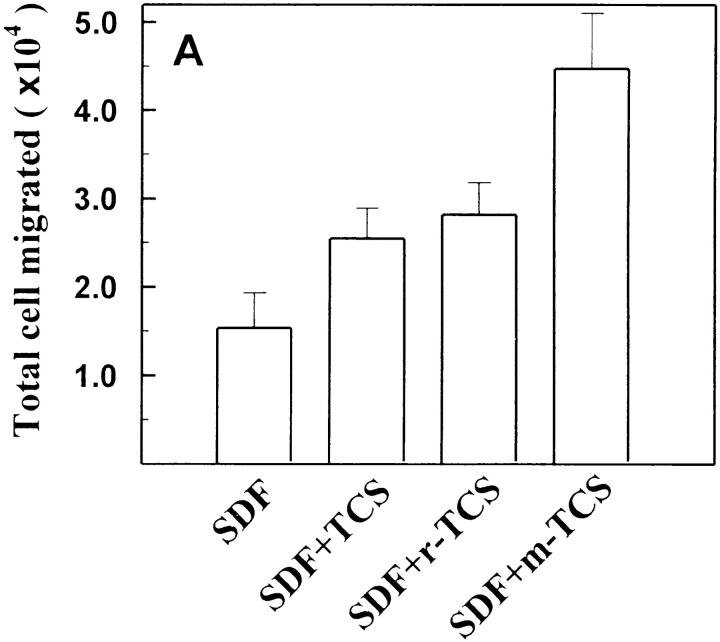
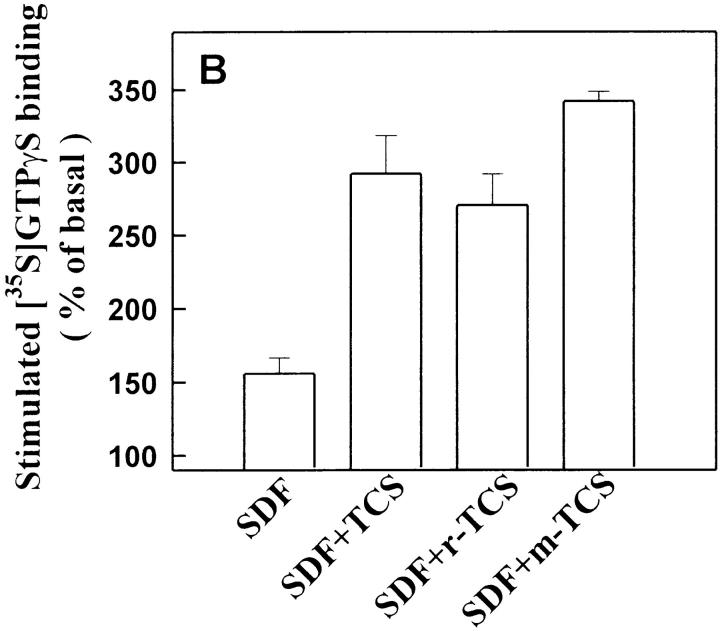
As shown in Fig. 6, after denaturation, TCS lost its ability to enhance chemokine receptor–mediated G protein activation. Furthermore, the enhancement effects of TCS were also blocked by preincubation with the purified mAb against TCS (data not shown). These experiments indicate that TCS is responsible for the observed magnification of chemokine-induced signaling. TCS was originally isolated from T. kirilowii, and the recombinant TCS with comparable activities was later successfully produced from Escherichia coli 38 39. As shown in Fig. 7r-TCS compared with native TCS conferred indistinguishable magnification effects on the chemokine-induced G protein activation and chemotaxis of leukocytes. These data argue that it is the presence of TCS, not any impurities from the preparation, that causes the observed effects on chemokine receptor activation and provides the molecular basis for structure–function studies of TCS.
Figure 7.
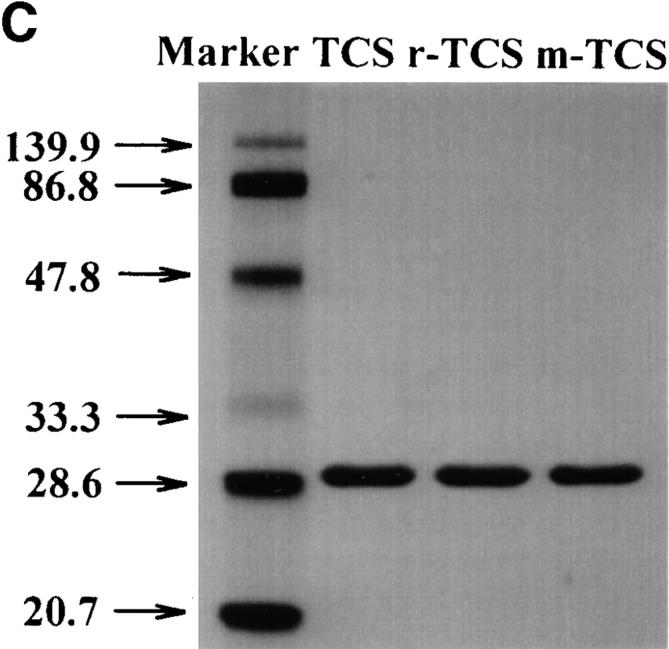
The effects of mutant TCS on chemokine receptor activation and chemotaxis. THP-1 cells were stimulated with SDF-1α (10 nM) in the absence or presence of TCS (isolated from T. kirilowii), r-TCS (recombinant TCS expressed in E. coli), or m-TCS (mutation at position 120–123) (0.2 μM for [35S]GTPγS binding and 2 nM for chemotaxis). [35S]GTPγS binding (A) and chemotaxis (B) were determined as described in Materials and Methods. Data were mean ± SE of at least two independent experiments performed in duplicate. The purities of TCS, r-TCS, and m-TCS were examined using silver staining after SDS-PAGE (C).
TCS has been identified as a type I ribosome-inactivating protein (RIP) with a wide spectrum of biological and pharmacological activities. Recent studies showed that mutation at position 120–123 (Lys-Ile-Arg-Glu to Ser-Ala-Gly-Gly) in TCS causes a 4,000-fold decrease in ribosome-inactivating activity 54, implying that this region of the TCS molecule plays a critical role in maintaining its ribosome-inactivation activity. However, this very mutant of TCS (m-TCS) showed similar, or perhaps even higher, enhancement on chemokine-induced G protein activation and chemotaxis of leukocytes compared with native TCS (Fig. 7). These results indicate that residues 120–123 of TCS required for its ribosome-inactivation activity are not essential for the enhancement effects of TCS in the chemokine receptor–mediated signaling, and suggest that the effects of TCS we observed in this study are not related to its ribosomal inactivation.
Colocalization of TCS with Chemokine Receptors on the Membrane, and Interaction of TCS with Chemokine Receptors.
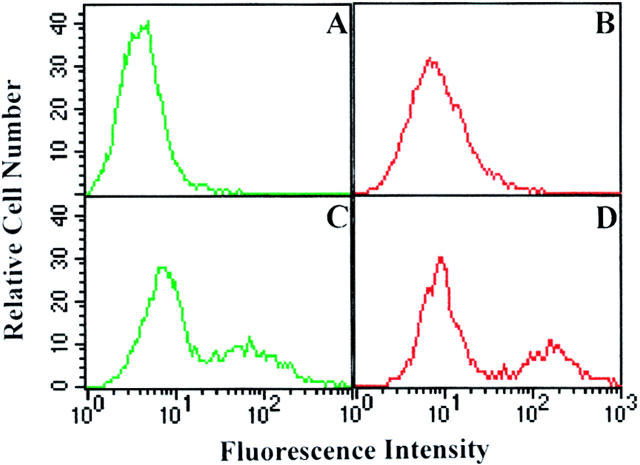
The ability of TCS to synergize chemokine-induced chemotaxis and G protein activation requires the presence of the chemokine receptors and may be accomplished through direct interaction of TCS with chemokine receptors on the membrane. To test this possibility, HEK293 cells were transiently transfected with control vector or HA-tagged CCR5 and incubated with or without 0.1 μM TCS. The HA-tagged chemokine receptors were labeled by staining the cells with 12CA5 and FITC-conjugated anti–mouse IgG, and TCS on the cell surface was detected with rabbit anti-TCS antibodies and TRITC-conjugated anti–rabbit secondary antibody. The results from flow cytometry are shown in Fig. 8 (A–D): typically, ∼30% of CCR5-transfected cells expressed CCR5 on the cell surface and were stained FITC fluorescence positive (Fig. 8 C). Strong TCS-specific TRITC fluorescence signal was detected in the CCR5-expressing cells (Fig. 8 D), and no significant positive TCS-like TRITC fluorescence was observed in the control vector–transfected cell population (Fig. 8 B). The immunofluorescence staining of TCS was also detected in the HEK293 cells expressing CXCR4 but not in the control cells (data not shown). These results indicate that the presence of chemokine receptors is a prerequisite for TCS binding to the cell surface.
Figure 8.
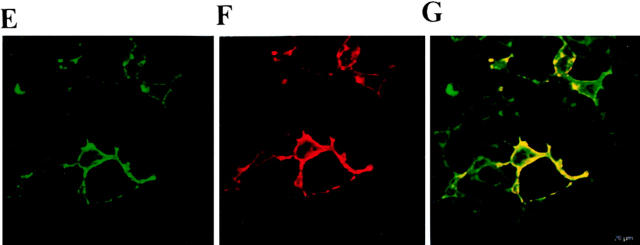
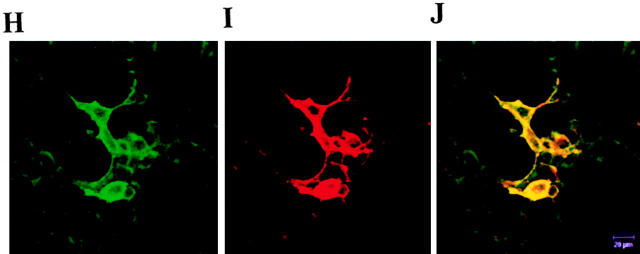
Colocalization of TCS with chemokine receptors on cell surface. The HEK293 cells transiently transfected with HA-tagged CCR5 were incubated without (A and B) or with (C and D) 0.1 μM TCS at 4°C for 1 h. The cells were then stained with 12CA5 and FITC-conjugated anti–mouse IgG for transfected CCR5 and with rabbit anti-TCS antibodies and TRITC-conjugated anti–rabbit IgG for TCS. The samples were analyzed by flow cytometry for FITC-labeled (A and C) and TRITC-labeled (B and D) fluorescence signals on the cell surface. Similarly, colocalization of TCS with chemokine receptor on the cell surface was examined by laser confocal fluorescence microscopy (E–J). The cells transiently expressing CCR5 (E–G) or CXCR4 (H–J) were stained with 12CA5/anti–mouse IgG–FITC (for expressed receptor) and anti-TCS/anti–rabbit IgG–Texas Red (for TCS); FITC (green, E and H), Texas Red (red, F and I), and FITC and Texas Red overlapping (yellow, G and J) fluorescent images from the same view were then visualized. Untransfected cells or mock-transfected cells showed negative staining under the same conditions (data not shown).
The location of TCS and chemokine receptors on the surface of the cells expressing CCR5 and CXCR4 was visualized under a laser scanning confocal fluorescence microscope after staining with anti-TCS/anti–rabbit IgG–Texas Red and 12CA5/anti–mouse IgG–FITC. The visible binding of TCS (Fig. 8F and Fig. I) was only observed on the surface of the cells expressing either CCR5 (Fig. 8 E) or CXCR4 (Fig. 8 H), and it appeared, in more detail, that TCS was localized on the cell surface at the sites where the chemokine receptor CCR5 (Fig. 8 G) or CXCR4 resides (Fig. 8 J).
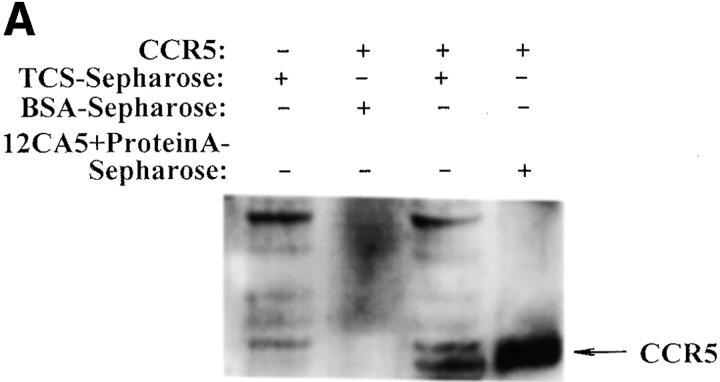
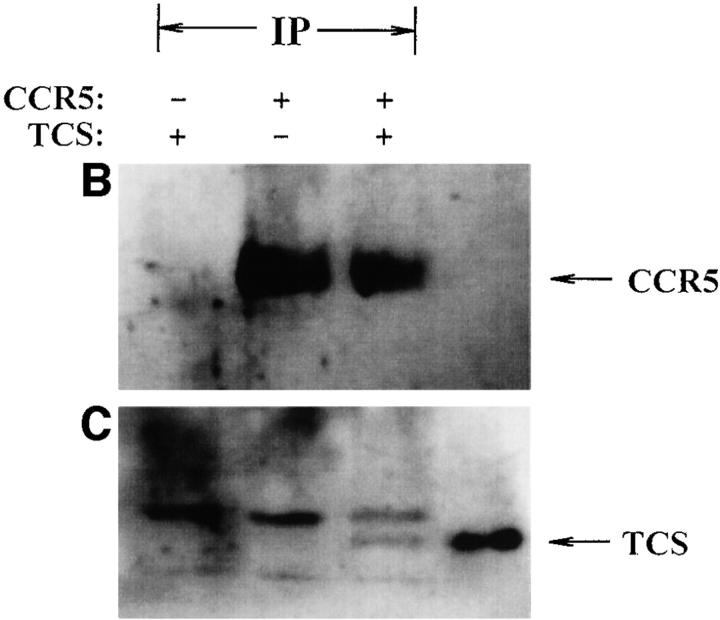
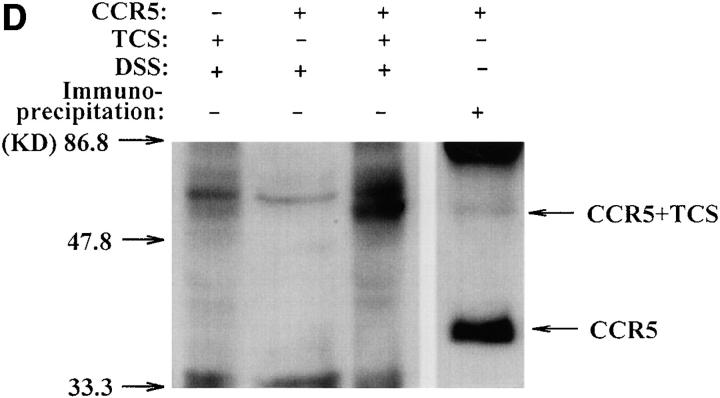
Finally, the interaction between TCS and chemokine receptors was investigated using several approaches. As shown in Fig. 9 A, chemokine receptor CCR5 in cell lysate was pulled down by the TCS-Sepharose but not by the BSA-Sepharose. Experiments done with chemokine receptor CXCR4 gave similar results (data not shown). Coimmunoprecipitation of TCS with chemokine receptors was also detected (Fig. 9B and Fig. C). In cross-linking experiments, incubation of TCS and membranes containing CCR5 with DSS resulted in an upshift of the TCS band to ∼70 kd, approximately the sum of TCS (29 kd) and CCR5 (50 kd) (Fig. 9 D). These results indicate that specific association of TCS to the cell membranes requires the presence of chemokine receptors, and that the synergic effects of TCS on chemotaxis and G protein activation induced by chemokines may be a result of direct interaction of TCS with chemokine receptors.
Figure 9.
Interaction of TCS and chemokine receptors. Lysates of mock- or CCR5-transfected HEK293 cells were incubated with TCS-Sepharose or BSA-Sepharose, and the proteins absorbed onto these Sepharose beads were analyzed with 12CA5 after Western blotting (A). Lysate from mock- or CCR5-transfected HEK293 cells was incubated with or without TCS and immunoprecipitated with 12CA5. The presence of CCR5 (B) and TCS (C) in the immunocomplexes was detected with 12CA5 and anti-TCS antibodies after Western blotting. Purified TCS (1 ng) was loaded onto gel as a control. The cross-linking experiments were performed by incubating TCS with the membrane proteins extracted from mock- or CCR5-transfected cells as described in Materials and Methods. The existence of TCS cross-linking to CCR5 was detected by rabbit anti-TCS antibodies, and immunoprecipitation-purified CCR5 (detected by anti-12CA5 antibody) was shown for comparison (D).
Discussion
TCS has been used in the clinical treatment of patients with AIDS or AIDS-related syndromes, but its underlying mechanisms are not well-understood. Recent discoveries that the chemokine receptors CCR5, CXCR4, CCR2B, and CCR3 are HIV-1 coreceptors have thrown new light on the combat against AIDS and other viral diseases. In this work, the effects of TCS on chemokine-stimulated chemotaxis and cellular signaling events and the potential interaction of TCS with the chemokine receptors were investigated. Our results demonstrated that TCS significantly enhanced the chemokine-induced leukocyte chemotaxis, and that the effect of TCS was primarily due to its ability to synergize chemokine-dependent activation of chemokine receptors and subsequent receptor-mediated signaling. Our data also revealed the specific association of TCS to the cell membranes with the expression of chemokine receptors and the colocalization and coimmunoprecipitation of TCS with chemokine receptors, suggesting the possibility of direct interaction of TCS with chemokine receptors. Furthermore, the mutant TCS, which lacks the ribosome-inactivating activity, possessed similar enhancement activity as wild-type TCS. Taken together, these results brought to light that TCS was able to functionally interact with a broad spectrum of chemokine receptors as a potent coactivator, which may be one of the mechanisms underlying application of TCS in AIDS treatment.
Although our results demonstrated that TCS functionally interacts with chemokine receptors and is colocalized with the receptors on the cell surface, it remains unclear how TCS is able to costimulate the activation of many different kinds of chemokine receptors by their corresponding chemokines (such as RANTES, SDF-1α, MIP-1β, and MCP-1). One possibility could be that TCS induces the conformational change of chemokine receptors through direct physical contact at the putative binding site that possesses a common structural feature shared by these receptors. Alternatively, TCS may exert its effects through indirect interaction with a third partner on the cell surface, forming a complex capable of interacting with chemokine receptors. Our data suggest that on these chemokine receptors, the putative association site(s) at which TCS directly or indirectly interacts appears distinct from the site(s) associated with the chemokines. It has been shown that gp120 envelope glycoproteins of human HIV-1 can physically and functionally interact with chemokine receptors 36 55 56 57, and on the receptors the association site(s) of gp120 apparently overlaps with the site(s) associated with the chemokines, since gp120 is able to displace chemokines. Very recent reports from x-ray crystal studies have revealed the structural determinants of gp120 for its binding to CCR5 58. Similar approaches will be helpful in determining the structural domain of TCS essential for its association with chemokine receptors, since x-ray structural information for TCS is already available 59 60 61.
Several members of the chemokine receptor family function in association with CD4 to permit entry and infection of HIV-1. CCR5 is a major fusion coreceptor for macrophage-tropic HIV-1 isolates, and CXCR4 is a coreceptor for the entry of T cell line–tropic HIV-1 strains. Chemokines have been shown to inhibit HIV-1 infection, though inefficiently, by interacting with chemokine receptors and thus preventing HIV-1 from using the coreceptors 62 63. However, the clinical use of excess amounts of chemokines, which induce chemotaxis and activation of leukocytes, may result in undesirable inflammatory side effects. Recently, a CCR5 antagonist from RANTES derivatives has been shown in vitro to block HIV-1 infection of macrophage and lymphocytes at nanomolar concentration 34. Searching for potent antagonists of chemokine receptors is now popularly considered and heavily pursued as one of the most promising strategies for HIV therapy. The result from this study that TCS strongly enhanced the ability of chemokines to activate their receptors may further provide another useful approach to inhibit HIV infection. One of the potential advantages of using coactivators such as TCS could be that the agents, which are not agonists or antagonists, can effectively interact with a wide spectrum of chemokine receptors and may thus promote the efficiency of various endogenous chemokines in blocking HIV infection. It is also worth mentioning that very little about the coactivator(s) of GPCRs has been reported to date. Therefore, the enhancement effects of TCS on chemokine receptor activation may offer a good working model for augmenting our understanding of GPCR activation.
Trichosanthin is a member of the type I RIPs with RNA N-glycosidase activity. It has been reported that TCS inhibits HIV replication in vitro in acutely and chronically infected lymphocytes and monocytes 9. Clinical studies also show that TCS treatment may help to prevent loss of CD4+ cells in AIDS patients failing treatment with antiretroviral agents such as zidovudine 10 and even to increase CD4+ cells in other cases 11 12. It was speculated that the anti-HIV effects of TCS might be due to its ribosome-inactivating activity. However, studies suggest that the mechanism of TCS to inhibit the replication and infection of HIV-1 is different from its activities of ribosome inactivation and immunomodulation 40 64. In addition, the undesirable side effects of TCS, which have seriously limited its clinical application, may also be related to its activity to induce unwanted cytotoxicities and allergic reactions. Our data in this study showing that TCS greatly enhances activation of chemokine receptors independent of its RIP activity may not only reveal an alternative mechanism underlying the anti-HIV effects of TCS but may also provide a mutagenesis strategy potentially to improve its therapeutical effectiveness and to reduce its side effects.
Acknowledgments
The authors wish to thank Prof. Ming Ye for providing rabbit anti-TCS antibodies, Prof. Zhi-Jiang Wu for PBL cell culturing, Dr. Guo-Xiang Wu for technical support on cloning CXCR4, Ms. Ru Zhang for technical assistance with Western blotting, Dr. Zhi-Jie Cheng for helpful discussion, and Dr. Jun Guo, Xu-Ming Zhang, and Pei-Hua Wu for their help in preparing this manuscript.
This research was supported by research grants from the National Natural Science Foundation of China (39630130, 39625015, and 39825110), the Chinese Academy of Sciences (KJ951-B1 and KY951-A1), and the German Max-Planck Society.
Footnotes
1used in this paper: DSS, disuccinimidyl suberate; FBS, fetal bovine serum; GPCR, G protein–coupled receptor; HA, hemagglutinin; HEK, human embryonic kidney; MCP, monocyte chemotactic protein; MIP, macrophage inflammatory protein; RANTES, regulated upon activation, normal T cell expressed and secreted; RIP, ribosome-inactivating protein; SDF, stromal cell–derived factor; TCS, trichosanthin
References
- Lui G., Lui F., Li Y., Yu S. A summary of 402 cases of termination of early pregnancy with crystalline preparations of trichosanthin. In: Chang H.-M., Yeung H.-W., Tso W.-W., Koo A., editors. Advances in Chinese Medicinal Materials Research. World Scientific Publishing Co. Pte., Ltd; Singapore: 1985. pp. 327–333. [Google Scholar]
- Jin Y.-C. Clinical study of trichosanthin. In: Chang H.-M., Yeung H.-W., Tso W.-W., Koo A., editors. Advances in Chinese Medicinal Materials Research. World Scientific Publishing Co. Pte., Ltd; Singapore: 1985. pp. 319–325. [Google Scholar]
- Huang Y.L. A clinical study on treatment of malignant trophoblastic neoplasia with trichosanthin (in Chinese) Chung Hsi I Chieh Ho Tsa Chih. 1987;7:154–155. [PubMed] [Google Scholar]
- Maraganore J.M., Joseph M., Bailey M.C. Purification and characterization of trichosanthin. Homology to the ricin A chain and implications as to mechanism of abortifacient activity. J. Biol. Chem. 1987;262:11628–11633. [PubMed] [Google Scholar]
- Yeung H.-W., Li W.-W., Feng Z., Barbieri L., Stirpe F. Trichosanthin, alpha-momorcharin and beta-momorcharinidentity of abortifacient and ribosome-inactivating proteins. Int. J. Pept. Protein Res. 1988;31:265–268. doi: 10.1111/j.1399-3011.1988.tb00033.x. [DOI] [PubMed] [Google Scholar]
- Leung K.-N., Yeung H.-W., Leung S.-O. The immunomodulatory and antitumor activities of trichosanthin—an abortifacient protein isolated from tian-hua-fen (Trichosanthes kirilowii) Asian Pac. J. Allergy Immunol. 1986;4:111–120. [PubMed] [Google Scholar]
- Yeung H.-W., Poon S.P., Ng T.B., Li W.W. Isolation and characterization of an immunosuppressive protein from Trichosanthes kirilowii root tubers. Immunopharmacol. Immunotoxicol. 1987;9:25–46. doi: 10.3109/08923978709035200. [DOI] [PubMed] [Google Scholar]
- McGrath M.S., Santulli S., Gaston I. Effects of GLQ223 on HIV replication in human monocyte/macrophages chronically infected in vitro with HIV. AIDS Res. Hum. Retroviruses. 1990;6:1039–1043. doi: 10.1089/aid.1990.6.1039. [DOI] [PubMed] [Google Scholar]
- McGrath M.S., Hwang K.-M., Caldwell S.E., Gaston I., Luk K.C., Wu P., Ng V.L., Crowe S., Daniels J., Marsh J. GLQ223an inhibitor of human immunodeficiency virus replication in acutely and chronically infected cells of lymphocyte and mononuclear phagocyte lineage. Proc. Natl. Acad. Sci. USA. 1989;86:2844–2848. doi: 10.1073/pnas.86.8.2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers V.S., Levin A.S., Malvino A., Waites L., Robins R.A., Baldwin R.W. A phase II study of effect of addition of trichosanthin to zidovudine in patients with HIV disease and failing antiretroviral agents. AIDS Res. Hum. Retroviruses. 1994;10:413–420. doi: 10.1089/aid.1994.10.413. [DOI] [PubMed] [Google Scholar]
- Mayer R.A., Sergios P.A., Coonan K., O'Brien L. Trichosanthin treatment of HIV-induced immune dysregulation. Eur. J. Clin. Invest. 1992;22:113–122. doi: 10.1111/j.1365-2362.1992.tb01944.x. [DOI] [PubMed] [Google Scholar]
- Byers V.S., Levin A.S., Waites L.A., Starrett B.A., Mayer R.A., Clegg J.A., Price M.R., Robins R.A., Delaney M., Baldwin R.W. A phase I/II study of trichosanthin treatment of HIV disease. AIDS. 1990;4:1189–1196. doi: 10.1097/00002030-199012000-00002. [DOI] [PubMed] [Google Scholar]
- Kahn J.O., Kaplan L.D., Gambertoglio J.G., Bredesen D., Arri C.J., Turin L., Kibort T., Williams R.L., Lifson J.D., Volberding P.A. The safety and pharmacokinetics of GLQ223 in subjects with AIDS and AIDS-related complexa phase I study. AIDS. 1990;4:1197–1204. doi: 10.1097/00002030-199012000-00003. [DOI] [PubMed] [Google Scholar]
- Baggiolini M. Chemokines and leukocyte traffic. Nature. 1998;392:565–568. doi: 10.1038/33340. [DOI] [PubMed] [Google Scholar]
- Moser B., Loetscher M., Piali L., Loetscher P. Lymphocyte responses to chemokines. Int. Rev. Immunol. 1998;16:323–344. doi: 10.3109/08830189809043000. [DOI] [PubMed] [Google Scholar]
- Proost P., Wuyts A., van Damme J. The role of chemokines in inflammation. Int. J. Clin. Lab. Res. 1996;26:211–223. doi: 10.1007/BF02602952. [DOI] [PubMed] [Google Scholar]
- Strieter R.M., Polverini P.J., Arenberg D.A., Walz A., Opdenakker G., van Damme J., Kunkel S.L. Role of C-X-C chemokines as regulators of angiogenesis in lung cancer. J. Leukocyte Biol. 1995;57:752–762. doi: 10.1002/jlb.57.5.752. [DOI] [PubMed] [Google Scholar]
- Friedland J.S. Chemokines in viral disease. Res. Virol. 1996;147:131–138. doi: 10.1016/0923-2516(96)80227-5. [DOI] [PubMed] [Google Scholar]
- Luster A.D. Chemokines—chemotactic cytokines that mediate inflammation. N. Engl. J. Med. 1998;338:436–445. doi: 10.1056/NEJM199802123380706. [DOI] [PubMed] [Google Scholar]
- Tachibana K., Hirota S., Iizasa H., Yoshida H., Kawabata K., Kataoka Y., Kitamura Y., Matsushima K., Yoshida N., Nishikawa S. The chemokine receptor CXCR4 is essential for vascularization of the gastrointestinal tract. Nature. 1998;393:591–594. doi: 10.1038/31261. [DOI] [PubMed] [Google Scholar]
- Zou Y.R., Kottmann A.H., Kuroda M., Taniuchi I., Littman D.R. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature. 1998;393:595–599. doi: 10.1038/31269. [DOI] [PubMed] [Google Scholar]
- Furci L., Polo S., Lusso P. D8+ T lymphocyte-derived chemokines and other HIV-suppressive factorsmini-review. J. Chemother. 1998;10:146–149. doi: 10.1179/joc.1998.10.2.146. [DOI] [PubMed] [Google Scholar]
- Wagner L., Yang O.O., Garcia-Zepeda E.A., Ge Y., Kalams S.A., Walker B.D., Pasternack M.S., Luster A.D. Beta-chemokines are released from HIV-1-specific cytolytic T-cell granules complexed to proteoglycans. Nature. 1998;391:908–911. doi: 10.1038/36129. [DOI] [PubMed] [Google Scholar]
- Littman D.R. Chemokine receptorskeys to AIDS pathogenesis? Cell. 1998;93:677–680. doi: 10.1016/s0092-8674(00)81429-4. [DOI] [PubMed] [Google Scholar]
- Horuk R. Chemokines beyond inflammation. Nature. 1998;393:524–525. doi: 10.1038/31116. [DOI] [PubMed] [Google Scholar]
- Clapham P.R., Weiss R.A. Immunodeficiency viruses. Spoilt for choice of co-receptors. Nature. 1997;388:230–231. doi: 10.1038/40758. [DOI] [PubMed] [Google Scholar]
- Thornhill M.H., Li J. Chemokines and chemokine receptorsthe key to understanding AIDS. Oral Dis. 1997;3:3–8. doi: 10.1111/j.1601-0825.1997.tb00002.x. [DOI] [PubMed] [Google Scholar]
- Alkhatib G., Combadiere C., Broder C.C., Feng Y., Kennedy P.E., Murphy P.M., Berger E.A. CC CKR5a RANTES, MIP-1alpha, MIP-1beta receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–1958. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- Raport C.J., Gosling J., Schweickart V.L., Gray P.W., Charo I.F. Molecular cloning and functional characterization of a novel human CC chemokine receptor (CCR5) for RANTES, MIP-1beta, and MIP-1alpha. J. Biol. Chem. 1996;271:17161–17166. doi: 10.1074/jbc.271.29.17161. [DOI] [PubMed] [Google Scholar]
- Haribabu B., Richardson R.M., Fisher I., Sozzani S., Peiper S.C., Horuk R., Ali H., Snyderman R. Regulation of human chemokine receptors CXCR4. Role of phosphorylation in desensitization and internalization. J. Biol. Chem. 1997;272:28726–28731. doi: 10.1074/jbc.272.45.28726. [DOI] [PubMed] [Google Scholar]
- Aramori I., Zhang J., Ferguson S.S., Bieniasz P.D., Cullen B.R., Caron M.G. Molecular mechanism of desensitization of the chemokine receptor CCR-5receptor signaling and internalization are dissociable from its role as an HIV-1 co-receptor. EMBO (Eur. Mol. Biol. Organ.) J. 1997;16:4606–4616. doi: 10.1093/emboj/16.15.4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J., Ma L., Wu Y.-L., Wang P., Hu W., Pei G. Chemokine receptor CCR5 functionally couples to inhibitory G proteins and undergoes desensitization. J. Cell. Biochem. 1998;71:36–45. doi: 10.1002/(sici)1097-4644(19981001)71:1<36::aid-jcb4>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Alkhatib G., Locati M., Kennedy P.E., Murphy P.M., Berger E.A. HIV-1 coreceptor activity of CCR5 and its inhibition by chemokinesindependence from G protein signaling and importance of coreceptor downmodulation. Virology. 1997;234:340–348. doi: 10.1006/viro.1997.8673. [DOI] [PubMed] [Google Scholar]
- Simmons G., Clapham P.R., Picard L., Offord R.E., Rosenkilde M.M., Schwartz T.W., Buser R., Wells T.N.C., Proudfoot A.E. Potent inhibition of HIV-1 infectivity in macrophages and lymphocytes by a novel CCR5 antagonist. Science. 1997;276:276–279. doi: 10.1126/science.276.5310.276. [DOI] [PubMed] [Google Scholar]
- Arenzana-Seisdedos F., Virelizier J.L., Rousset D., Clark-Lewis I., Loetscher P., Moster B., Baggiolini M. HIV blocked by chemokine antagonist. Nature. 1996;383:400. doi: 10.1038/383400a0. [DOI] [PubMed] [Google Scholar]
- Wu L., LaRosa G., Kassam N., Gordon C.J., Heath H., Ruffing N., Chen H., Humblias J., Samson M., Parmentier M. Interaction of chemokine receptor CCR5 with its ligandsmultiple domains for HIV-1 gp120 binding and a single domain for chemokine binding. J. Exp. Med. 1997;186:1373–1381. doi: 10.1084/jem.186.8.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amara A., Gall S.L., Schwartz O., Salamero J., Montes M., Loetscher P., Baggiolini M., Virelizier J.L., Arenzana-Seisdedos F. HIV coreceptor downregulation as antiviral principleSDF-1α–dependent internalization of the chemokine receptor CXCR4 contributes to inhibition of HIV replication. J. Exp. Med. 1997;186:139–146. doi: 10.1084/jem.186.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P.C., Yung M.H., Zhu R.H., Ho W.K., Ng T.B., Yeung H.W. Cloning of trichosanthin cDNA and its expression in Escherichia coli . Gene. 1991;97:267–272. doi: 10.1016/0378-1119(91)90061-f. [DOI] [PubMed] [Google Scholar]
- Zhu R.H., Ng T.B., Yeung H.W., Shaw P.C. High level synthesis of biologically active recombinant trichosanthin in Escherichia coli . Int. J. Pept. Protein Res. 1992;39:77–81. doi: 10.1111/j.1399-3011.1992.tb01558.x. [DOI] [PubMed] [Google Scholar]
- Casellas P., Dussossoy D., Falasca A.I., Barbieri L., Guillemot J.C., Ferrara P., Bolognesi A., Cenini P., Stirpe F. Trichokirin, a ribosome-inactivating protein from the seeds of Trichosanthes kirilowii Maximowicz. Purification, partial characterization and use for preparation of immunotoxins. Eur. J. Biochem. 1988;176:581–588. doi: 10.1111/j.1432-1033.1988.tb14317.x. [DOI] [PubMed] [Google Scholar]
- Campbell J.J., Qin S., Bacon K.B., Mackay C.R., Butcher E.C. Biology of chemokine and classical chemoattractant receptorsdifferential requirements for adhesion-triggering versus chemotactic responses in lymphoid cells. J. Cell Biol. 1996;134:255–266. doi: 10.1083/jcb.134.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosling J., Monteclaro F.S., Atchison R.E., Arai H., Tsou C.L., Goldsmith M.A., Charo I.F. Molecular uncoupling of C-C chemokine receptor 5-induced chemotaxis and signal transduction from HIV-1 coreceptor activity. Proc. Natl. Acad. Sci. USA. 1997;94:5061–5066. doi: 10.1073/pnas.94.10.5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Z.J., Fan G.H., Zhao J., Zhang Z., Wu Y.L., Jiang L.Z., Zhu Y., Pei G., Ma L. Endogenous opioid receptor-like receptor in human neuroblastoma SK-N-SH cellsactivation of inhibitory G protein and homologous desensitization. Neuroreport. 1997;8:1913–1918. doi: 10.1097/00001756-199705260-00024. [DOI] [PubMed] [Google Scholar]
- Traynor J.R., Nahouaki S.R. Modulation by μ-opioid agonists of guanosine-5′-O-3(3-[35S]thio)triphosphate binding to membranes from human neuroblastoma SH-SY5Y cells. Mol. Pharmacol. 1995;47:848–854. [PubMed] [Google Scholar]
- Cai Y.C., Zhang Y., Wu Y.L., Pei G. δ Opioid receptor in neuronal cells undergoes acute and homologous desensitization. Biochem. Biophys. Res. Commun. 1996;219:342–347. doi: 10.1006/bbrc.1996.0235. [DOI] [PubMed] [Google Scholar]
- Cai Y.C., Ma L., Fan G.H., Zhao J., Jiang L.Z., Pei G. Activation of N-methyl-D-aspartate receptor attenuates acute responsiveness of delta-opioid receptors. Mol. Pharmacol. 1997;51:583–587. doi: 10.1124/mol.51.4.583. [DOI] [PubMed] [Google Scholar]
- Ma L., Cheng Z.J., Fan G.H., Cai Y.C., Jiang L.Z., Pei G. Functional expression, activation and desensitization of opioid receptor-like receptor ORL1 in neuroblastoma × glioma NG108-15 hybrid cells. FEBS Lett. 1997;403:91–94. doi: 10.1016/s0014-5793(97)00031-8. [DOI] [PubMed] [Google Scholar]
- Zhu X., Ding L., Pei G. Carboxyl terminus of mitosin is sufficient to confer spindle pole localization. J. Cell. Biochem. 1997;66:441–449. [PubMed] [Google Scholar]
- Zhao J., Pei G., Huang Y.L., Zhong F.M., Ma L. Carboxyl terminus of delta opioid receptor is required for agonist-dependent receptor phosphorylation. Biochem. Biophys. Res. Commun. 1997;238:71–76. doi: 10.1006/bbrc.1997.7242. [DOI] [PubMed] [Google Scholar]
- Cai G., Wang H.-Y., Gao E., Horwitz J., Snyder D.L., Pelleg A., Roberts J., Friedman E. Reduced adenosine A1 receptor and G alpha protein coupling in rat ventricular myocardium during aging. Circ. Res. 1997;81:1065–1071. [PubMed] [Google Scholar]
- Damaj B.B., McColl S.R., Neote K., Songqing N., Ogborn K.T., Hebert C.A., Naccache P.H. Identification of G-protein binding sites of the human interleukin-8 receptors by functional mapping of the intracellular loops. FASEB J. 1996;10:1426–1434. doi: 10.1096/fasebj.10.12.8903513. [DOI] [PubMed] [Google Scholar]
- Proudfoot A.E., Power C.A., Hoogewerf A., Montjovent M.O., Borlat F., Wells T.N. Characterization of the RANTES/MIP-1 alpha receptor (CC CKR-1) stably transfected in HEK 293 cells and the recombinant ligands. FEBS Lett. 1995;376:19–23. doi: 10.1016/0014-5793(95)01235-x. [DOI] [PubMed] [Google Scholar]
- Moriuchi H., Moriuchi M., Fauci A.S. Nuclear factor-kappa B potently up-regulates the promoter activity of RANTES, a chemokine that blocks HIV infection. J. Immunol. 1997;158:3483–3491. [PubMed] [Google Scholar]
- Nie H., Cai X., He X., Xu L., Ke X., Ke Y.B., Tam S.C. Position 120-123, a potential active site of trichosanthin. Life Sci. 1998;62:491–500. doi: 10.1016/s0024-3205(97)01145-4. [DOI] [PubMed] [Google Scholar]
- Cocchi F., DeVico A.L., Garzino-Demo A., Cara A., Gallo R.C., Lusso P. The V3 domain of the HIV-1 gp120 envelope glycoprotein is critical for chemokine-mediated blockade of infection. Nat. Med. 1997;3:367–368. doi: 10.1038/nm1196-1244. [DOI] [PubMed] [Google Scholar]
- Weissman D., Rabin R.L., Arthos J., Rubbert A., Dybul M., Swofford R., Venkatesan S., Farber J.M., Fauci A.S. Macrophage-tropic HIV and SIV envelope proteins induce a signal through the CCR5 chemokine receptor. Nature. 1997;389:981–985. doi: 10.1038/40173. [DOI] [PubMed] [Google Scholar]
- Madani N., Kozak S.L., Kavanaugh M.P., Kabat D. gp120 envelope glycoproteins of human immunodeficiency viruses competitively antagonize signaling by coreceptors CXCR4 and CCR5. Proc. Natl. Acad. Sci. USA. 1998;95:8005–8010. doi: 10.1073/pnas.95.14.8005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzuto C.D., Wyatt R., Hernandez-Ramos N., Sun Y., Kwong P.D., Hendrickson W.A., Sodroski J. A conserved HIV gp120 glycoprotein structure involved in chemokine receptor binding. Science. 1998;280:1949–1953. doi: 10.1126/science.280.5371.1949. [DOI] [PubMed] [Google Scholar]
- Pan K.Z., Fu Z.J., Lin Y.J., Zhou K.L., Dodson E., Chen Z.W., Ye X.M. Crystallographic refinement of trichosanthin at 2.6Å resolution. Sci. China B. 1992;35:1203–1213. [PubMed] [Google Scholar]
- Pan K.Z., Lin Y.J., Zhou K.J., Fu Z.J., Chen M.H., Huang D.R., Huang D.H. The crystal and molecular structure of trichosanthin at 2.6 Å resolution. Sci. China. B. 1993;36:1069–1081. [PubMed] [Google Scholar]
- Gao B., Ma X.Q., Wang Y.P., Chen S.Z., Wu S., Dong Y.C. Refined structure of trichosanthin at 1.73 Å resolution. Sci. China B. 1994;37:59–73. [PubMed] [Google Scholar]
- Cocchi F., DeVico A.L., Garzino-Demo A., Arya S.K., Gallo R.C., Lusso P. Identification of RANTES, MIP-1 alpha, and MIP-1 beta as the major HIV-suppressive factors produced by CD8+ T cells. Science. 1995;270:1811–1815. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- Paul W.E. Can the immune response control HIV infection? Cell. 1995;82:177–182. doi: 10.1016/0092-8674(95)90304-6. [DOI] [PubMed] [Google Scholar]
- Pinching A.J. Early trials of GLQ223/trichosanthinwhat do they show? AIDS. 1990;4:1289–1291. [PubMed] [Google Scholar]