Telomeres are unique protein–DNA structures that comprise the termini of eukaryotic linear chromosomes (for review see references 1, 2). Telomeric DNA does not contain protein-encoding genes but rather consists of G-rich hexanucleotide repeats that in vertebrate cells are (TTAGGG)n sequences. Based on studies initially carried out in yeast and other single cell organisms, it appears that telomere functions include the stabilization and protection of chromosomal ends from events such as illegitimate recombination, the determination of chromosomal localization within the nucleus, and the regulation of cellular replicative capacity. It is this last function, the role of telomeres in regulation of replicative capacity, that has received particular attention in studies of cellular senescence and organismal aging.
A pivotal finding in the understanding of somatic cell biology was the observation that normal somatic cells have a finite replicative life span 3. That is, they are capable of a finite number of cell divisions, after which they undergo what has been termed replicative senescence and are incapable of further cell division. The mechanism underlying the replicative clock that monitors this process has evoked considerable attention, and it is in this context that telomere function has been of particularly intense interest. The most widely accepted paradigm relating telomere function to cellular aging and replicative senescence is based on the observation that in normal somatic cells telomeres shorten with each cell division 4 5. This telomere shortening has been attributed to the primer requirement for DNA synthesis during chromosomal replication, and results in incomplete replication and a loss of terminal telomeric repeats with each cell division 6. Telomeres thus shorten progressively with successive cell divisions, and telomere length in a somatic cell may thus reflect the replicative history of that cellular lineage. In principle, this is a potentially powerful tool for the analysis of cell division under physiologic circumstances in which it is otherwise very difficult to monitor in vivo clonal expansion. For this reason, measurement of telomere length has been widely used to analyze lineage or precursor–product relationships and rates of cell division.
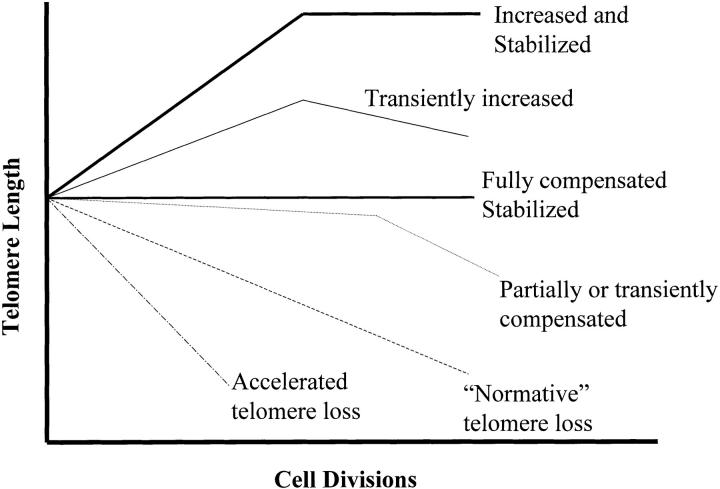
However, in interpreting the significance of changes in telomere length, it is critical to consider the multiple factors that may influence the net length of telomeres at any point in time. The starting point for telomere length in somatic cells is the length of telomeres in germ line cells of the individual or species, a parameter that is and must be strictly conserved in order to maintain viability of the species. From this starting point, multiple factors can act to change telomere length during somatic development. A loss of terminal telomeric repeats appears to occur as a result of incomplete DNA replication during cell division, as noted above. The rate of this loss, as inferred from in vitro analysis of cultured human fibroblasts or lymphocytes, has been repeatedly measured as 50–100 bp per cell division 7 (Fig. 1, “Normative” telomere loss). However, this rate of loss is not necessarily a constant function of cell division, and a number of circumstances have been identified in which there are apparent deviations from this normative rate of telomere loss. Important in this respect, mechanisms have been identified that are capable of increasing telomere length and therefore of compensating for the telomere shortening that would otherwise occur as a consequence of cell division and chromosomal replication. The telomere lengthening mechanism that has been most widely studied is that mediated by telomerase, a unique ribonucleoprotein enzyme that mediates RNA-dependent synthesis of telomeric repeats in species ranging from yeast to human (for reviews see references 2, 8). High levels of telomerase activity are expressed in cells of the germ line and appear to be critical in maintaining stable telomere length and thereby conserving the capacity for essentially limitless replication in highly proliferative male and female germ cells (Fig. 1, Fully compensated). Analysis of human cell populations initially detected telomerase activity in germ line cells and in most malignant transformed cells, but not in normal somatic cells 9. However, more recently it has been established that some normal somatic cells do, under certain conditions, express significant and at times very high levels of telomerase 10 11 12 13. Therefore, telomerase activity as well as telomerase-independent mechanisms for telomere extension must be considered as possible determinants of overall telomere length dynamics.
Figure 1.
Alternative relationships between cell division and changes in telomere length. As noted in the text, the relationship between cell division and net alteration in telomere length is not a constant one. Under specific in vivo or in vitro conditions, distinct patterns of telomere length change can predominate.
A striking example of the apparent failure of a simple paradigm relating telomere shortening to cell division and lineage differentiation is provided by the in vivo B lymphocyte germinal center (GC) response to antigen challenge. Findings from a number of laboratories support a model in which naive B cells, identified by cell surface phenotype, differentiate to GC centroblast and centrocyte populations, which in turn differentiate further to generate memory B cells 14. GC B cells undergo a remarkable sequence of events in which Ig diversity is generated by somatic hypermutation, followed by exceptional levels of clonal expansion and antigen-mediated selection. This process can proceed through multiple cycles to ultimately generate a mature, hypermutated, high affinity antigen-specific Ig repertoire. It might have been expected that the differentiation of naive B cells into GC B cells and subsequently into memory cells would be accompanied by progressive telomere shortening, reflecting the cell division that occurs during this differentiation. However, in marked contrast to this expectation, it was in fact observed that telomeres of human tonsil GC B cells are consistently longer than those of either precursor naive B cells or progeny memory B cells, suggesting that telomere length can actually increase under physiologic conditions during a period of active cell division 15 (Fig. 1, Transiently increased). Parallel measurements of telomerase activity demonstrated a very high level of telomerase in GC B cells, in contrast to low or undetectable levels in naive and memory B cells, suggesting that telomere elongation during GC B cell differentiation might be mediated by telomerase. The conclusion that telomere length can increase during normal somatic cell differentiation, even in the face of extensive cell division, was strongly supported by studies of inter-species crosses between Mus musculus and M. spretus mice. In these studies, it was observed that short M. spretus telomeres lengthen substantially during the generation of an adult mouse from a single progenitor cell 16 (Fig. 1, Increased and Stabilized).
At present it is impossible to directly measure in vivo the number of cell divisions that accumulate in the history of a somatic cell. For this reason, estimates derived from measurements of telomere length have been received with interest. However, it is possible to study more directly the relationship of cell division and telomere length in vitro. When these studies were carried out with human fibroblasts, telomeres were found to shorten at a relatively constant rate (50–100 bp/population doubling) throughout the replicative life span up to the point of senescence 7 (Fig. 1, “Normative” telomere loss). It is relevant that human fibroblasts remain telomerase negative throughout this culture period. Initial studies of human T lymphocytes similarly noted a loss of telomeres at approximately the same rate per population doubling 17 18. However, when telomere length was more closely monitored during T cell cultures, it was observed that the rate of telomere shortening is not uniform. During early stages of culture, when most of the overall cell division occurs, there is little or no shortening of telomeres; that is, cell division occurs without measurable telomere loss 19 20. In later stages of culture, when population expansion is minimal, a high rate of telomere loss per population doubling is observed 19 20 (Fig. 1, Partially or transiently compensated). When telomerase activity was measured in these T cell cultures, a high level of telomerase was induced in the early stage of culture, correlating with maintenance of telomere length, with subsequently lower levels of telomerase activity expressed during the period of rapid telomere loss late in culture. Conditions have also been described in which telomeric repeats are lost at an unusually rapid rate during cell division. For example, fibroblasts cultured at increased oxygen concentration appear to undergo telomere shortening at an accelerated rate (>500 bp/cell division) 21. These in vitro results reinforce the caution that telomere shortening, in vivo or in vitro, may not correlate in a straightforward or quantitative manner with a cell's replicative history.
With attention thus paid to the complex factors that can influence telomere length, how can measurements of telomere length be used to assess “aging” or replicative history of somatic cell populations such as those of the hematopoietic lineage? The earliest studies of in vivo telomere length in mammalian cells focused on adult human skin fibroblasts and concluded that telomere length decreased as a function of donor age 4 5. Similar telomere shortening with age was observed in peripheral blood mononuclear populations 22 and in naive and memory CD4+ and CD8+ peripheral T cells 18. Although initial reports found a relatively linear shortening of telomeres as a function of age, more recent analyses have identified a more complex pattern. In a study of adults and children, Frenck et al. found that telomere shortening occurs at a rapid rate (>1 kb/yr) in peripheral blood cells of young children, then at a reduced rate between the ages of 4 and 20 yr, and at a relatively constant and intermediate rate through the remainder of adult life 23. Rufer et al., in this issue of The Journal of Experimental Medicine, have extended this analytic approach by measuring telomere length in peripheral blood cells of >500 subjects ranging in age from 0 to 90 yr, using a flow cytometric technique developed for this purpose 24. This technique of flow fluorescence in situ hybridization (flow FISH) uses a fluorochrome-conjugated probe specific for the telomeric TTAGGG sequence. This probe is used to stain the denatured telomeres of fixed cells, followed by flow cytometric analysis to quantitate the bound probe, and thereby the total telomere content of individual cells. The authors carry out a commendably thorough validation of this technique in comparison with telomere length quantitation by other techniques. Important advantages of this technique include the ability to carry out single cell analyses and the relative ease of its applicability to analysis of multiple cell populations. As all techniques, it also has its disadvantages or limitations. Because flow FISH measures only the total telomere signal in a cell, it does not provide information concerning telomere length of individual chromosomes and cannot, for example, indicate whether telomeres on a subset of chromosomes are critically short, a circumstance that may be functionally critical for a cell. In contrast, the technique of quantitative FISH (Q-FISH) developed by Lansdorp et al. 25, or even the conventional analysis of telomere length by Southern blot analysis, is capable of providing information concerning the distribution of individual telomere lengths. The flow FISH technique would be particularly useful if it were possible to employ simultaneous cell surface staining to analyze telomere length in subsets of heterogeneous populations, but it appears that this is not yet technically feasible.
Rufer et al. find that in granulocytes as well as T lymphocytes telomere length decreases as a function of age. Consistent with and extending the previous report of Frenck et al., the rate of telomere loss as a function of age is greatest in early childhood for granulocytes as well as for naive and memory CD4+ and CD8+ T cell subpopulations (1,000–3,000 bp/yr). In each of these populations, the rate of telomere shortening then continues at a much lower rate of loss (30–60 bp/yr) for the remainder of life. It is telling that these two reports, from Frenck et al. and Rufer et al., favor different interpretations of their very similar findings. Frenck et al. suggest that the difference in rate of telomere loss during early childhood and later life most probably reflects a difference in the rate of telomere loss per cell division during different stages of life. In contrast, Rufer et al. favor a model in which each postnatal division of somatic cells results in “more or less constant losses of telomere repeats.” In the context of this latter model, the more rapid rate of telomere loss observed in early childhood is interpreted as reflecting a correspondingly rapid rate of cellular turnover during this time. Thus, although the study of telomere dynamics has been informative for a variety of molecular, cellular, and physiologic issues, the complexity of telomere length regulation makes it extremely difficult to draw definitive conclusions concerning cellular turnover on the basis of telomere length alone.
In addition to serving as a potential index of replicative history for somatic cells, telomere length may also reflect the residual replicative capacity of cells, and in this capacity would be an important biomarker. Indeed, a correlation has been demonstrated between telomere length of human fibroblasts and their capacity for cell division before reaching replicative senescence 7. A similar correlation has been reported between telomere length and capacity for cell division in naive and memory subpopulations of CD4+ human T cells 18. Recently, the relationship between telomere length and the capacity for cell division was supported by the demonstration that transfecting telomerase negative and “mortal” human fibroblasts or retinal epithelial cells with cDNA encoding a telomerase catalytic subunit resulted in expression of telomerase activity, maintenance of telomere length, and apparently unlimited replicative capacity 26. Constitutive expression of telomerase in these cells was thus capable of altering the relationship between telomere length and both replicative history and replicative capacity.
Further insight into the relationship between telomere length and cellular function in vivo has come from studies of mice rendered deficient in telomerase by disruption of the gene encoding the telomerase RNA template component 27 28 29 30. Mice of the species M. musculus have extremely long telomeres, and a line of mice deficient in telomerase was initially able to reproduce with apparently normal function of multiple biological systems. However, after four to six generations of breeding of homozygous telomerase-deficient mice, progressive telomere shortening was detected. At this point, both males and females were sterile and, with aging, revealed decreased life expectancy as well as defects in wound healing, the capacity for bone marrow hematopoietic reconstitution, and other organ systems, traits that can be easily related to a diminished capacity for replication in both germ line and somatic cells. Although these findings suggest that telomere shortening can have functional consequences on somatic and germ line function in vivo, a number of critical questions remain to be addressed. Primary among these are the questions of whether telomere shortening plays a functional role in human aging, whether manipulation of telomere length can impact favorably on adverse consequences of aging, and whether bypassing normal constraints on telomere length maintenance or telomerase expression can be accomplished without increased risk of malignant transformation or other unanticipated consequences.
Acknowledgments
The author wishes to acknowledge Karen Hathcock for her thoughtful reading and comments during preparation of this commentary.
References
- Blackburn E.H. Structure and function of telomeres. Nature. 1991;350:569–573. doi: 10.1038/350569a0. [DOI] [PubMed] [Google Scholar]
- Greider C.W. Telomere length regulation. Annu. Rev. Biochem. 1996;65:337–365. doi: 10.1146/annurev.bi.65.070196.002005. [DOI] [PubMed] [Google Scholar]
- Hayflick L. The limited in vitro lifetime of human diploid cell strains. Exp. Cell Res. 1965;37:614–636. doi: 10.1016/0014-4827(65)90211-9. [DOI] [PubMed] [Google Scholar]
- Harley C.B., Futcher A.B., Greider C.W. Telomeres shorten during ageing of human fibroblasts. Nature. 1990;345:458–460. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- Hastie N.D., Dempster M., Dunlop M.G., Thompson A.M., Green D.K., Allshire R.C. Telomere reduction in human colorectal carcinoma and with ageing. Nature. 1990;346:866–868. doi: 10.1038/346866a0. [DOI] [PubMed] [Google Scholar]
- Watson J. Origin of concatemeric T7 DNA. Nat. New Biol. 1972;239:197–201. doi: 10.1038/newbio239197a0. [DOI] [PubMed] [Google Scholar]
- Allsopp R.C., Vaziri H., Patterson C., Goldstein S., Younglai E.V., Futcher A.B., Greider C., Harley C.B. Telomere length predicts replicative capacity of human fibroblasts. Proc. Natl. Acad. Sci. USA. 1992;89:10114–10118. doi: 10.1073/pnas.89.21.10114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn E.H. Telomerases. Annu. Rev. Biochem. 1992;61:113–129. doi: 10.1146/annurev.bi.61.070192.000553. [DOI] [PubMed] [Google Scholar]
- Shay J.W., Wright W.E. Telomerase activity in human cancer. Curr. Opin. Oncol. 1996;8:66–71. doi: 10.1097/00001622-199601000-00012. [DOI] [PubMed] [Google Scholar]
- Broccoli D., Young J.W., de Lange T. Telomerase activity in normal and malignant hematopoietic cells. Proc. Natl. Acad. Sci. USA. 1995;92:9082–9086. doi: 10.1073/pnas.92.20.9082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiyama K., Hirai Y., Kyoizumi S., Akiyama M., Hiyama E., Piatyszek M.A., Shay J.W., Ishioka S., Yamakido M. Activation of telomerase in human lymphocytes and hematopoietic progenitor cells. J. Immunol. 1995;155:3711–3715. [PubMed] [Google Scholar]
- Buchkovich K.J., Greider C.W. Telomerase regulation during entry into the cell cycle in normal human T cells. Mol. Biol. Cell. 1996;7:1443–1454. doi: 10.1091/mbc.7.9.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng, N., B.L. Levine, C.H. June, and R.J. Hodes. 1996. Regulated expression of telomerase activity in human T lymphocyte development and activation. J. Exp. Med. 2471–2479. [DOI] [PMC free article] [PubMed]
- Kelsoe G. Life and death in germinal centers (redux) Immunity. 1996;4:107–111. doi: 10.1016/s1074-7613(00)80675-5. [DOI] [PubMed] [Google Scholar]
- Weng N.P., Granger L., Hodes R.J. Telomere lengthening and telomerase activation during human B cell differentiation. Proc. Natl. Acad. Sci. USA. 1997;94:10827–10832. doi: 10.1073/pnas.94.20.10827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L., Hathcock K.S., Hande P., Lansdorp P.M., Seldin M.F., Hodes R.J. Identification of a chromosome locus that regulates telomere length in mice. Proc. Natl. Acad. Sci. USA. 1998;95:8648–8653. doi: 10.1073/pnas.95.15.8648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaziri H., Schachter F., Uchida I., Wei L., Zhu X., Effros R., Cohen D., Harley C.B. Loss of telomeric DNA during aging of normal and trisomy 21 human lymphocytes. Am. J. Hum. Genet. 1993;52:661–667. [PMC free article] [PubMed] [Google Scholar]
- Weng N., Levine B.L., June C.H., Hodes R.J. Human naive and memory T lymphocytes differ in telomeric length and replicative potential. Proc. Natl. Acad. Sci. USA. 1995;92:11091–11094. doi: 10.1073/pnas.92.24.11091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodnar A.G., Kim N.W., Effros R.B., Chiu C.-P. Mechanism of telomerase induction during T cell activation. Exp. Cell Res. 1996;228:58–64. doi: 10.1006/excr.1996.0299. [DOI] [PubMed] [Google Scholar]
- Weng N., Palmer L., Levine B.L., Lane H.C., June C.H., Hodes R.J. Tales of tailsregulation of telomere length and telomerase activity during lymphocyte development, differentiation, activation, and aging. Immunol. Rev. 1997;160:43–54. doi: 10.1111/j.1600-065x.1997.tb01026.x. [DOI] [PubMed] [Google Scholar]
- Von Zglinicki T., Saretzki G., Docke W., Lotze C. Mild hyperoxia shortens telomeres and inhibits proliferation of fibroblasts. Exp. Cell Res. 1995;220:186–193. doi: 10.1006/excr.1995.1305. [DOI] [PubMed] [Google Scholar]
- Slagboom P.E., Droog S., Boomsma D.I. Genetic determination of telomere size in humansa twin study of three age groups. Am. J. Hum. Genet. 1994;55:876–882. [PMC free article] [PubMed] [Google Scholar]
- Frenck R.W., Blackburn E.H., Shannon K.M. The rate of telomere sequence loss in human leukocytes varies with age. Proc. Natl. Acad. Sci. USA. 1998;95:5607–5610. doi: 10.1073/pnas.95.10.5607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rufer N., Brummendorf T.H., Kolvraa S., Bischoff C., Christensen K., Wadsworth L., Schulzer M., Lansdorp P.M. Telomere fluorescence measurements in granulocytes and T lymphocyte subsets point to a high turnover of hematopoietic stem cells and memory T cells in early childhood. J. Exp. Med. 1999;190:157–167. doi: 10.1084/jem.190.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansdorp P.M., Verwoerd N.P., van de Rijke F.M., Dragowska V., Little M.-T., Dirks R.W., Raap A.K., Tanke H.J. Heterogeneity in telomere length of human chromosomes. Hum. Mol. Genet. 1996;5:685–691. doi: 10.1093/hmg/5.5.685. [DOI] [PubMed] [Google Scholar]
- Bodnar A.G., Ouellette M., Frolkis M., Holt S.E., Chiu C.-P., Morin G.B., Harley C.B., Shay J.W., Lichtsteiner S., Wright W.E. Extension of life-span by introduction of telomerase into normal human cells. Science. 1998;279:349–352. doi: 10.1126/science.279.5349.349. [DOI] [PubMed] [Google Scholar]
- Blasco M.A., Lee H.-W., Hande M.P., Samper E., Lansdorp P.M., DePinho R.A., Greider C.W. Telomerase shortening and tumor formation by mouse cells lacking telomerase RNA. Cell. 1997;91:25–34. doi: 10.1016/s0092-8674(01)80006-4. [DOI] [PubMed] [Google Scholar]
- Lee H.-W., Blasco M.A., Gottlieb G.J., Horner J.W., Greider C.W., DePinho R.A. Essential role of mouse telomerase in highly proliferative organs. Nature. 1998;392:569–574. doi: 10.1038/33345. [DOI] [PubMed] [Google Scholar]
- Rudolph K.L., Chang S., Lee H.-W., Blasco M., Gottlieb G.J., Greider C., DePinho R.A. Longevity, stress response, and cancer in aging telomerase-deficient mice. Cell. 1999;96:701–712. doi: 10.1016/s0092-8674(00)80580-2. [DOI] [PubMed] [Google Scholar]
- Herrera E., Samper E., Martin-Caballero J., Flores J.M., Lee H.-W., Blasco M.A. Disease states associated with telomerase deficiency appear earlier in mice with short telomeres. EMBO (Eur. Mol. Biol. Organ.) J. 1999;18:2950–2960. doi: 10.1093/emboj/18.11.2950. [DOI] [PMC free article] [PubMed] [Google Scholar]