Abstract
The pathogenesis of Acanthamoeba keratitis begins when Acanthamoeba trophozoites bind specifically to mannosylated glycoproteins upregulated on the surfaces of traumatized corneal epithelial cells. When Acanthamoeba castellanii trophozoites are grown in methyl-α-d-mannopyranoside, they are induced to secrete a novel 133-kDa protein that is cytolytic to corneal epithelial cells. Clinical isolates of Acanthamoeba spp., and not the soil isolates, were proficient at producing a mannose-induced protein (MIP-133) and generating disease in Chinese hamsters. The purified protein was efficient at killing corneal epithelial cells, the first mechanistic barrier, by inducing apoptosis in a caspase 3-dependent pathway. Subsequent steps in pathogenesis require the amoebae to penetrate and degrade collagen. Only the clinical isolates tested were efficient at migrating through a collagenous matrix in vitro, presumably by MIP-133 degradation of both human type I and human type IV collagen. A chicken anti-MIP-133 antiserum effectively bound to the protein and blocked collagenolytic activity, migration, and cytopathic effects (CPE) against corneal cells in vitro. Chinese hamsters orally immunized with MIP-133 displayed a >30% reduction in disease. Immunoglobulin A isolated from immunized animals bound MIP-133 and blocked CPE on corneal cells in vitro. Animals induced to generate severe chronic infections displayed significant reductions in disease symptoms upon oral immunization postinfection. These data suggest that MIP-133 production might be necessary to initiate corneal disease and that it may play an important role in the subsequent steps of the pathogenic cascade of Acanthamoeba keratitis. Furthermore, as antibodies produced both prior to and after infection reduced clinical symptoms of disease, the protein may represent an important immunotherapeutic target for Acanthamoeba keratitis.
Acanthamoeba keratitis is a very painful, sight-threatening corneal infection caused by several species of free-living pathogenic amoebae (22, 40). Acanthamoeba spp. are ubiquitous in nature and have been isolated from a wide variety of environments, including swimming pools, hot tubs, lakes, soil, dust, drinking fountains, eyewash stations, and the nasopharyngeal mucosae of healthy individuals (3, 4, 6, 12, 19, 27, 30, 39, 41). Despite the wide distribution of amoebae, this disease is largely restricted to contact lens wearers who have experienced some sort of trauma to their corneal epithelium (3, 5, 24, 32, 33, 40). Moreover, induction of the disease in experimental animals requires corneal abrasion prior to exposure to Acanthamoeba trophozoites (9, 26, 33).
Characteristic symptoms of Acanthamoeba keratitis include a ring-like corneal infiltrate, epithelial destruction, disproportionately severe ocular pain, and resistance to antimicrobial agents (2, 20, 29). Treatment of the disease is very demanding, consisting of hourly applications of brolene, polyhexamethylene biguanide, or chlorhexidine for several weeks. Even with such therapies, Acanthamoeba spp. can cause severe damage to the corneal epithelium and stroma, resulting in the need for corneal transplantation (2).
Very little is known about how Acanthamoeba can penetrate the ocular defense and enter the stromal layer of the cornea. Upon abrasion of the corneal surface, the corneal epithelium expresses elevated concentrations of mannose glycoproteins, to which the amoebae can adhere with high affinity (11, 25, 42). Subsequently, the amoebae penetrate and destroy the corneal epithelium, perforate Bowman's membrane, and gain entry into the underlying stroma. Both Bowman's membrane and the stroma are primarily comprised of collagen.
Recent studies by our laboratory have shown that when Acanthamoeba castellanii is stimulated with methyl-α-d-mannopyranoside, the amoebae secrete a new 133-kDa protein (MIP-133) that is highly cytolytic to both human and hamster corneal epithelial cells in vitro (10, 16). Secretion of this protein is mannose specific, as neither lactose nor galactose induces the production of the protein. Moreover, the cytolytic activity against corneal epithelial cells is eliminated when the MIP-133 protein is incubated with serine protease inhibitors (10).
In this study, we examined the correlation of this mannose-induced protein with known pathogenic and nonpathogenic strains of Acanthamoeba, the mechanism behind the cytolytic activity, and the possible role of the protein in the subsequent steps in the pathogenic cascade of Acanthamoeba keratitis. Furthermore, we investigated how antibodies may defend against the actions of the protein and whether the protein can be effectively used as an immunogen against disease.
MATERIALS AND METHODS
Animals.
Chinese hamsters were purchased from Cytogen Research and Development (West Bury, Mass.). All animals used were from 4 to 6 weeks of age, and all corneas were examined before experimentation to exclude animals with preexisting corneal defects. Animals were handled in accordance with the Association of Research in Vision and Ophthalmology Statement on the Use of Animals in Ophthalmic and Vision Research.
Amoebae and cell lines.
All Acanthamoeba species were originally obtained from the American Type Culture Collection, Manassas, Va. A. castellanii (ATTC 30868), Acanthamoeba polyphaga (ATTC 30461), and Acanthamoeba rhysodes (ATTC 50368) were isolated from human corneas. Acanthamoeba culbertsoni (ATTC 30171) was originally isolated from a diseased human kidney. Acanthamoeba hatchetti (ATTC 30730), Acanthamoeba astronyxis (ATTC 30137), and A. castellanii strain neff (ATTC 30010) were isolated from soil. The chosen species represent all three of the different subgroups of Acanthamoeba based on morphology, isoenzyme analysis, and serology (38). Amoebae were grown as axenic cultures in peptone-yeast extract-glucose (PYG) at 35°C with constant agitation.
To examine whether the Acanthamoeba spp. produced the MIP-133 protein, trophozoites were grown in 200 ml of PYG either with or without 100 mM methyl-α-d-mannopyranoside (Sigma Chemical Co., St. Louis, Mo.) on a shaker incubator set at 125 rpm and at 35°C (10). Initial cultures were seeded with a total of 107 trophozoites. Individual supernatants from cultures at mid-log phase were collected, centrifuged, filter sterilized, 10-fold concentrated, and immediately examined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis using 4 to 15% ready gels (Bio-Rad, Hercules, Calif.) or used for detection of the MIP-133 protein by enzyme-linked immunosorbent assay (ELISA).
Human corneal epithelial (HCE) cells were a generous gift from Sherry Ward of the Gillette Company (Gillette Medical Evaluation Laboratories, Gaithersburg, Md.). Cells were cultured in keratinocyte growth medium with a Bullet kit (catalog number CC-3111; Clonetics, Walkersville, Md.).
Contact lens preparation.
Contact lenses were prepared from Spectra/Por dialysis membrane tubing (Spectrum Medical Industries, Los Angeles, Calif.) with a 3-mm-diameter trephine prior to heat sterilization. Lenses were placed in sterile 96-well microtiter plates (Costar, Cambridge, Mass.) and incubated with 3 × 106 A. castellanii trophozoites at 35°C for 24 h. Attachment of amoebae to the lenses was verified microscopically before infection.
In vivo corneal infections.
Acanthamoeba keratitis was induced as described previously (9, 13, 34). Briefly, the Chinese hamsters were anesthetized with ketamine (100 mg/kg of body weight; Fort Dodge Laboratories, Fort Dodge, Iowa), injected peritoneally. Prior to manipulation, the corneas were anesthetized by topical application of Alcain (Alcon Laboratories, Fort Worth, Tex.). Approximately 25% of the cornea was abraded with a sterile cotton applicator before the amoeba-laden lens was placed onto the center of the cornea. The eyelids were then closed by tarsorrhaphy with 6-0 Ethilon sutures (Ethicon, Somerville, N.J.). The contact lenses were removed 3 to 4 days postinfection, and the corneas were visually inspected by microscopy for severity of disease. Visual inspections were recorded daily as indicated. The infections were scored on a scale of 0 to 5 based on the following parameters: corneal infiltration, corneal neovascularization, and corneal ulceration. The pathology was recorded as follows: 0 indicates no pathology, 1 indicates that <10% of the cornea was involved, 2 indicates a 10 to 25% involvement, 3 indicates 25 to 50% involvement, 4 indicates 50 to 75% involvement, and 5 indicates 75 to 100% involvement as described previously (17). In Chinese hamsters, Acanthamoeba keratitis resolves at approximately 3 weeks. At this time, there is a conspicuous absence of corneal opacity, edema, epithelial defects, and stromal necrosis and inflammation.
MIP-133 isolation.
The MIP-133 protein was purified as stated previously (10). Briefly, 10-fold-concentrated supernatants from A. castellanii cultures grown with 100 mM methyl-α-d-mannopyranoside, were analyzed by SDS-4 to 15% PAGE Ready Gels (Bio-Rad) under both reducing and nonreducing conditions. Supernatants were taken from trophozoites at mid-log phase.
For fast protein liquid chromatography, culture supernatants of mannose-stimulated A. castellanii trophozoites were concentrated 10-fold with Ultrafree-15 centrifuge concentrators with a molecular cutoff of 5 kDa (Millipore, Bedford, Mass.). Samples were centrifuged at 3,000 × g for 20 min and passed in 0.5-ml volumes over a Superdex 200 (Amersham Pharmacia Biotech, Piscataway, N.J.) column with phosphate-buffered saline (PBS) (pH. 7.2). Fractions were collected every 0.5 ml and examined by SDS-4 to 15% PAGE, fractions containing the mannose-induced cytolytic protein were pooled and concentrated 10-fold, and the buffer was exchanged three times with 10 mM Tris buffer (pH 8.0) with Ultrafree 0.5 concentrators. Two-hundred-microliter samples containing 1 mg of protein were applied to a DEAE ionic-exchange column by using 10 mM Tris buffer, pH 8.0 (buffer A). Adsorbed protein was eluted by using a gradient of 10 mM Tris buffer, pH 8.0, with 1 M NaCl (buffer B). Fractions were examined for the ∼133-kDa protein by SDS-4 to 15% PAGE. An initial 15% buffer B step removed contaminating proteins, and the ∼133-kDa protein was eluted between 15 to 30% buffer B run at 0.1 ml/min. Fractions containing the ∼133-kDa protein were pooled and concentrated 10-fold and washed three times with PBS (pH 7.2) to exchange the buffer.
Apoptosis assay.
The experiment was carried out by using a TACSAnnexin V-fluorescein isothiocyanate kit (R&D Systems) according to the manufacturer's instructions. Briefly, HCE cells were grown to ∼90% confluence in 24-well plates. The cells were then incubated with either 1.5 μg of MIP-133, 20 μM Z-DEVD-FMK caspase 3 inhibitor (BD Pharmingen, San Diego, Calif.), 20 μM Z-FA-FMK control inhibitor (BD Pharmingen), or staurosporine (3 μg/ml; Sigma) as a positive control for apoptosis for 18 h. Then the cells were trypsinized, washed twice with PBS, and stained with Annexin V (1 μl) and propidium iodide (10 μl) for 15 min, and apoptosis was quantified by flow cytometry at a 488-nm wavelength. All subsequent incubation steps were performed on ice, and centrifugation steps were performed at 4°C. For each sample, 10,000 ungated events were acquired and the results were analyzed with CellQuest software (BD Biosciences, Franklin Lakes, N.J.). The results are expressed as percentages of cells that were apoptotic.
Migration assays.
The migration assays were performed with 24-well transwell plates (6.5-mm diameter, 3.0-μm pore size; Costar, Corning Inc., Corning, N.Y.). The top chamber membrane was coated with 100 μl of Matrigel (Collaborative Biomedical Products, Bedford, Mass.) that had been diluted 1:3 in Hanks' balanced salt solution. Excess Matrigel was removed after 10 min, and the membranes were allowed to dry at room temperature. Acanthamoeba trophozoites (105) were then placed in the top chamber in 100 μl of PYG. Plates were incubated at 37°C for 2 h, and acanthamoebae were then counted in the bottom chamber by light microscopy (magnification, ×100). Inhibition assays involved incubating A. castellanii trophozoites with either a 1:75 or a 1:100 dilution of chicken anti-MIP-133 antiserum or chicken preimmune serum, 1.0 mM phenylmethylsulfonyl fluoride (PMSF; Sigma), or 10 μM cystatin (Sigma) at 37°C for 30 min prior to their addition to the upper chambers. All experiments were performed in triplicate.
Collagen digestion assay.
Collagen assays were performed in 96-well plates. In separate plates, 10-μg samples of human collagen types I (Sigma) and IV (USBiological, Swampscott, Mass.) were added and incubated at 37°C until the plates were dry. Total volumes were 50 μl in PBS. Plates were then washed three times with PBS and then incubated with either 15.6 μg of MIP-133 or PBS or 0.1 mg of Clostridium histolyticum collagenase (Sigma) at 37°C for 24 or 72 h. Sample wells were then washed three times with PBS, incubated with a 1:500 dilution of mouse anti-collagen type IV immunoglobulin G (IgG) or a 1:2,000 dilution of mouse anti-collagen type I IgG (Sigma) as the primary antibody and goat anti-mouse IgG-horse radish peroxidase (HRP) (Southern Biotechnology Associates, Inc., Birmingham, Ala.) as the secondary antibody. Plates were read at an optical density (OD) at 405 nm in a Molecular Devices (Menlo Park, Calif.) microplate reader. Inhibition studies involved coincubating the MIP-133 protein with the chicken anti-MIP-133 antiserum diluted 1:75. All experiments were performed in triplicate.
Western blot analysis and ELISA.
Ninety-six-well assay plates were coated with 50 μg of MIP-133 overnight in carbonate buffer. Plates were washed four times with PBS containing 0.05% Tween 20 (wash buffer; Sigma) and then blocked with 0.5% bovine serum albumin in PBS for 1 h at room temperature. All subsequent antibodies were diluted with blocking buffer and incubated at room temperature. Chicken anti-MIP-133 antiserum (Aveslabs, Tigard, Oreg.) was added at either a 1:50, 1:75, or 1:100 dilution for 1 h and washed. HRP-conjugated goat anti-chicken IgY (Aveslabs) was added at a 1:10,000 dilution and incubated for 1 h. Plates were developed by adding 1.0 mM 2,2′-azinobis(3-ethyl-benzthiazoline-6-sulfonic acid) (Sigma) containing 0.003% H2O2 and incubated for 30 min at room temperature. After development, 100 μl of 10% SDS (Sigma) was added per well prior to growth being read at an OD at 405 nm with a microplate reader.
Western blot analysis was carried out by conventional techniques. Briefly, 30 μg of 10-fold-concentrated crude supernatant taken from A. castellanii trophozoites grown in mannose was resolved by using 4 to 25% ready gels. Gels were transferred to Trans-Immun-Blot PVDF membranes (Bio-Rad) with a Bio-Rad minitransfer apparatus. Blots were blocked in 5% dry milk in PBS (blocking buffer) overnight at 4°C prior to the addition of a 1:200 dilution of chicken anti-MIP-133 antiserum for 1 h at room temperature. After 1 h, membranes were washed in PBS and incubated in a 1:2,000 dilution of rabbit anti-chicken IgG-alkaline phosphatase (Sigma) in blocking buffer. After 1 h, the membrane was washed and developed with Nitro Blue Tetrazolium-BCIP (5-bromo-4-chloro-3-indolylphosphate) stock solution (Roche Diagnostics, Mannheim, Germany) as recommended by the manufacturer.
Assay for CPE.
The MIP-133 protein was added at 1.5, 7.8, and 15.6 μg of protein (in 25 μl of PBS) to 96-well plates with confluent monolayers of HCE cells and incubated for 18 h at 35°C. Each well contained 200 μl of the respective required growth medium. Inhibition experiments involved incubating the protein samples with either a 1:75 dilution of chicken anti-MIP-133 antiserum or of a chicken preserum control and a 1:50 dilution of pooled enteric washes from immunized hamsters or from control (PBS)-immunized animals. Additional control wells consisted of untreated confluent cells. Following incubation, all wells were washed three times with their respective growth medium and stained with Giemsa stain (Shandon, Inc., Pittsburgh, Pa.). After being stained, the wells were washed three times with PBS (pH 7.2) and solubilized in 0.1 ml of 5% SDS in PBS and the OD was read at 590 nm in a Molecular Devices microplate reader. Percentages of cytopathic effects (CPE) were calculated according to the following formula: percent CPE = 100 − [(OD of experimental well − OD of supernatant alone/OD of control cells alone) × 100]. Assays were performed in triplicate.
Oral immunizations.
Animals received 1 ml of 0.1 M sodium carbonate (pH 9.6; Sigma) by lavage tube prior to administration of either 100 μg of MIP-133 (in 100 μl of PBS) plus 10 μg of cholera toxin (Sigma), 200 μg of MIP-133 plus 20 μg of cholera toxin, or 400 μg of MIP-133 plus 40 μg of cholera toxin. Immunizations were administered once a week for 4 weeks prior to infection with A. castellanii. Control groups included animals immunized with equivalent doses of cholera toxin alone and untreated animals prior to infection.
Collection of Chinese hamster IgA secretions.
Enteric washes were collected as stated previously (14). Briefly, the enteric washes were collected from Chinese hamsters after the animals were anesthetized with ketamine and euthanized by cervical dislocation. Approximately 20 cm of the small intestine was removed, and 10 ml of PBS was injected through the intestinal section with an 18-gauge needle. The enteric washes were collected and centrifuged at 700 × g to remove sediments. Protease inhibitor cocktail tablets (Boehringer Mannheim, Indianapolis, Ind.) were added to the pooled enteric washes at one tablet per 10 ml. The wash was stored at −80°C until used.
IgA ELISA.
Ninety-six-well assay plates were coated with 50 μg of MIP-133 overnight in carbonate buffer. Plates were washed four times with PBS containing 0.05% Tween 20 (wash buffer; Sigma) and then blocked with 0.5% bovine serum albumin in PBS for 1 h at room temperature. All subsequent antibodies were diluted in blocking buffer and incubated at room temperature. Enteric washes were added at a dilution of 1:2 for 1 h and then washed. Rabbit anti-Chinese hamster IgA hyperimmune serum (15) was then added to a 1:2 dilution and incubated for 2 h. Plates were washed, and a 1:1,000 dilution of goat anti-rabbit IgG-HRP (Santa Cruz Biotechnology) was added. Plates were developed by adding 1.0 mM 2,2′-azinobis(3-ethyl-benzthiazoline-6-sulfonic acid) (Sigma) containing 0.003% H2O2 and incubated for 30 min at room temperature. After development, 100 μl of 10% SDS (Sigma) was added per well prior to growth being read on a microplate reader at 405 nm.
Preparation of clodronate liposomes.
Multilamellar liposomes were prepared as described earlier (35, 36). Briefly, 8 mg of cholesterol and 86 mg of phosphatidylcholine (Lipoid GmbH, Ludwigshafen, Germany) were dissolved in 10 ml of chloroform in a round-bottomed flask. After low-vacuum rotary evaporation at 37°C, a thin film was then dispersed by gentle rotation for 10 min in PBS for the preparation of PBS containing liposomes. Liposomes were washed twice by centrifugation in PBS at 100,000 × g for 30 min and resuspended in 4 ml of PBS that contained 20 mg of clodronate (Roche Diagnostics). Each 100 μl of the clodronate liposome suspension contained 1 mg of clodronate. Clodronate liposomes and PBS liposomes were stored at 4°C and used within 7 days of preparation. Clodronate liposomes were tested for in vitro toxicity against macrophages prior to use.
Liposome-treated animals.
Both clodronate and PBS containing liposomes were administered via subconjunctival injection on days −8, −6, −4, and −2 of infection. Fifty microliters of the liposome preparation was injected in four quadrants of the eye encircling the entire conjunctiva. Acanthamoeba infections were performed via contact lens placement as mentioned above.
Animals were orally immunized on days 5, 12, 19, and 26 days postinfection with 400 μg of the MIP-133 protein as mentioned above.
Statistics.
Statistical analyses of all data except clinical scores were performed by using unpaired Student's t tests. Clinical-severity scores were analyzed by the Mann-Whitney test.
RESULTS
Correlation of MIP-133 production to pathogenic and nonpathogenic strains of Acanthamoeba.
A. castellanii has been shown to produce the MIP-133 protein when it was grown in the presence of methyl-α-d-mannopyranoside (10). However, it was important to determine whether there was a correlation between production of the protein and the ability of the amoebae to generate disease.
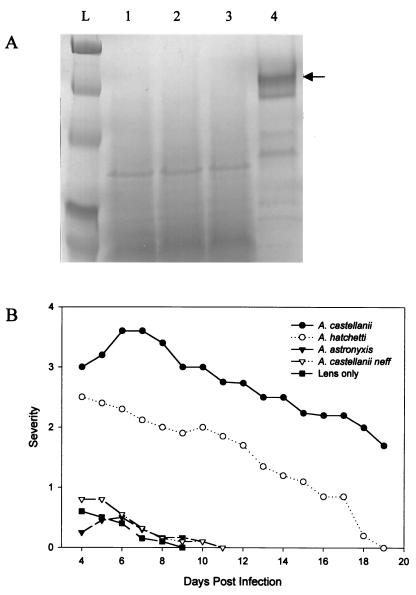
When grown in mannose, none of the Acanthamoeba soil isolates tested produced MIP-133 at levels detectable by SDS-PAGE (Fig. 1A). A. castellanii, the positive control, produced significant amounts of the MIP-133 protein. Moreover, when tested in vivo, all three of the soil isolates produced either no disease or significantly less disease than that produced by the standard ocular isolate of A. castellanii (Fig. 1B). Both A. astronyxis and A. castellanii neff produced symptoms similar to those of the sterile contact lens control animals, while A. hatchetti produced a mild infection. The MIP-133 protein was not detectable in lysates made from the three soil isolates (data not shown).
FIG. 1.
Correlation between MIP-133 production and the ability of Acanthamoeba soil isolates to cause disease. (A) Acanthamoeba spp. were grown in PYG containing 100 mM methyl-α-d-mannopyranoside. Supernatants were collected at mid-log phase, filter sterilized, concentrated 10-fold, and analyzed by SDS-PAGE. Lanes: L, molecular weight ladder; 1, A. hatchetti; 2, A. astronyxis; 3, A. castellanii neff; 4, A. castellanii (clinical isolate). The arrow points at ∼133 kDa. (B) Ability of Acanthamoeba spp. to cause keratitis in Chinese hamsters. Animals were infected with Acanthamoeba-laden lenses as described in Materials and Methods. The results are representative of three separate experiments (eight hamsters in each group).
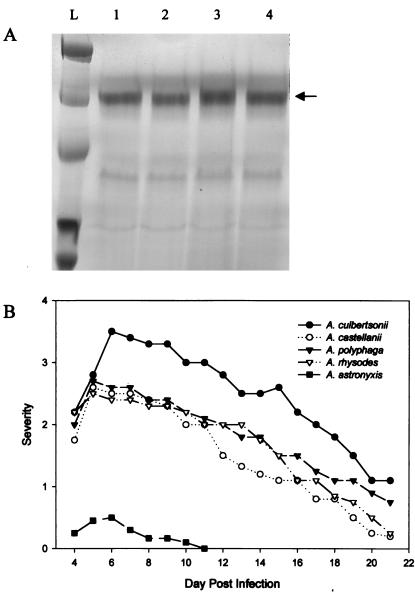
By contrast, the clinical isolates of Acanthamoeba produced the MIP-133 protein when they were grown in mannose (Fig. 2A). All three clinical isolates tested produced levels similar to those produced by A. castellanii (Fig. 2B). Additionally, when tested in vivo, the clinical isolates produced severe Acanthamoeba keratitis.
FIG. 2.
Correlation between MIP-133 production and the ability of clinical isolates of Acanthamoeba to cause disease. (A) Acanthamoeba spp. were grown in PYG containing 100 mM methyl-α-d-mannopyranoside. Supernatants were collected at mid-log phase, filter sterilized, concentrated 10-fold, and analyzed by SDS-PAGE. Lanes: L, molecular weight ladder; 1, A. culbertsoni; 2, A. polyphaga; 3, A. rhysodes; 4, A. castellanii. The arrow points at ∼133 kDa. (B) Ability of Acanthamoeba spp. to cause keratitis in Chinese hamsters. Animals were infected with Acanthamoeba-laden lenses as described in Materials and Methods. The results are representative of three separate experiments (eight hamsters in each group).
Mechanism of cell death in vitro.
Though the MIP-133 protein is effective at killing both human and hamster corneal epithelial cells in vitro (10), it is unknown how the protein kills the corneal epithelial cells.
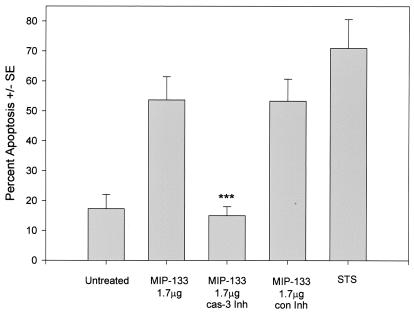
When incubated with fluorescein-encapsulated liposomes, neither 1.5, 7.8, nor 15.6 μg of the MIP-133 protein was able to perforate the liposomes (data not shown). However, when examined by Annexin V flow cytometry, the protein was found to cause cell death by inducing apoptosis (Fig. 3). MIP-133-treated HCE cells displayed a threefold increase in apoptosis over the level in untreated cells. Incubations with a caspase 3 inhibitor completely eliminated the apoptosis caused by the MIP-133 protein. By contrast, the control inhibitor did not reduce apoptosis. Staurosporine, known to induce apoptosis, produced a nearly fourfold increase in apoptosis.
FIG. 3.
Effects of caspase 3 inhibition of MIP-133-mediated apoptosis. HCE cells were treated with 1.7 μg of MIP-133 either alone or with a 20 μM concentration of the caspase 3 inhibitor Z-DEVD-FMK (cas-3 Inh), or a 20 μM concentration of the caspase 3 control inhibitor Z-FA-FMK (con Inh). HCE cells were examined by Annexin V flow cytometry as described in Materials and Methods. Additional controls included untreated cells, and 3 μg of staurosporine (STS) per ml served as a positive control. Bars and error bars represent the means ± standard errors (SE) of results of triplicate experiments. ***, significantly different from the value for the untreated controls (P < 0.001).
Migration assay and collagen degradation.
Although the MIP-133 protein correlated with clinical disease and cytotoxic activity against corneal epithelial cells, it was important to determine how the protein might play a role in the pathogenic cascade of Acanthamoeba keratitis.
All three of the soil isolates tested were ineffectual in migrating through the collagenous Matrigel (number of migrating amoebae, 10 to 18). By contrast, all of the clinical isolates were capable of migrating through the Matrigel at levels approximately equal to those of the original standard ocular isolate of A. castellanii (number of migrating amoebae, 60 to 84).
Matrigel is comprised of many components, with collagen (type A) being only one of the components. However, both Bowman's membrane and the stroma are comprised almost entirely of collagen (types IV and I, respectively).
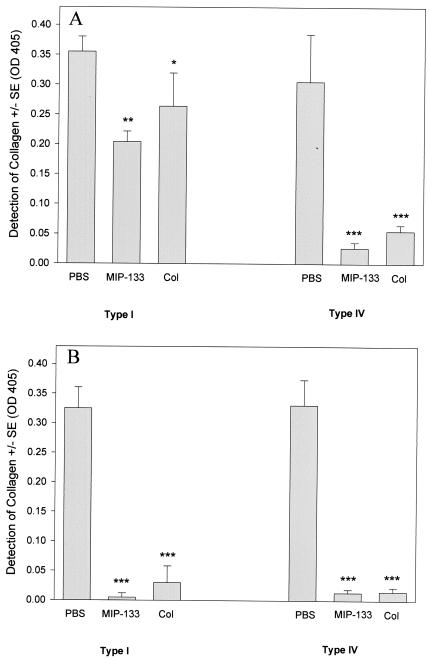
The MIP-133 protein was efficient at degrading both human type I and human type IV collagen (Fig. 4). At 24 h, type IV collagen was more efficiently degraded than type I. However, by 72 h, both forms of collagen were nearly completely degraded. Degradation of collagen was similar to that of the collagenase control at both the 24 and 72 h time points tested. The PBS control did not show lytic ability against either collagen.
FIG. 4.
Collagenolytic activity of the MIP-133 protein. Ten micrograms of human collagen types I and IV were incubated and dried onto 96-well plates. Wells were treated with either 15.6 μg of the MIP-133 protein, PBS, or 0.1 mg of collagenase (Col) for 24 (A) and 72 (B) h. Sample wells were then washed three times and incubated with mouse anti-collagen type IV IgG or mouse anti-collagen type I IgG as the primary antibody, followed by goat anti-mouse IgG-HRP, as described in Materials and Methods. Plates were developed and read at an OD at 405 nm. Bars and error bars represent the means ± SE of triplicate experiments. *, **, and ***, significantly different from values for PBS-treated controls (P < 0.05, P < 0.01, and P < 0.001, respectively).
Neutralization of MIP-133 and inhibition of trophozoite migration by anti-MIP-133 antibody.
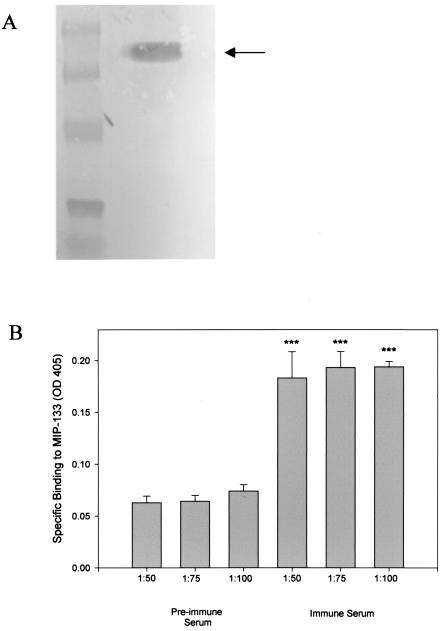
The specificity of the chicken anti-MIP-133 antiserum was confirmed by Western blotting (Fig. 5A). Preimmune serum did not bind to the mannose-stimulated protein (data not shown). Moreover, ELISA analysis indicated specific binding of the anti-MIP-133 antiserum to the MIP-133 protein at 1:50, 1:75, and 1:100 dilutions (Fig. 5B). As before, there was no ELISA evidence that the preimmune serum bound to the MIP-133 protein. Additionally, neither the preimmune nor the anti-MIP-133 serum bound to the surfaces of the Acanthamoeba trophozoites (data not shown).
FIG. 5.
Specific binding of the chicken anti-MIP-133 antiserum. (A) Western blotting. Crude supernatants taken from A. castellanii trophozoites grown in mannose were resolved by SDS-PAGE and transferred to PVDF membranes. Membranes were first incubated with chicken anti-MIP-133 antiserum, followed by rabbit anti-chicken IgG-alkaline phosphatase, as described in Materials and Methods. The membranes were then washed and developed with Nitro Blue Tetrazolium-BCIP stock solution. The arrow indicates the 133-kDa band. (B) ELISA. Ninety-six-well plates were coated with 50 μg of MIP-133 and allowed to dry. After drying, wells were blocked and incubated with a 1:50, 1:75, or 1:100 dilution of either the chicken anti-MIP-133 antiserum or preimmune chicken antiserum. Wells were then washed and incubated with goat anti-chicken IgY-HRP. ELISA plates were developed and read at an OD at 405 nm. Bars and error bars represent the means ± SE of results from triplicate experiments. ***, significantly different from values obtained with preimmune serum at the same dilutions (P < 0.001).
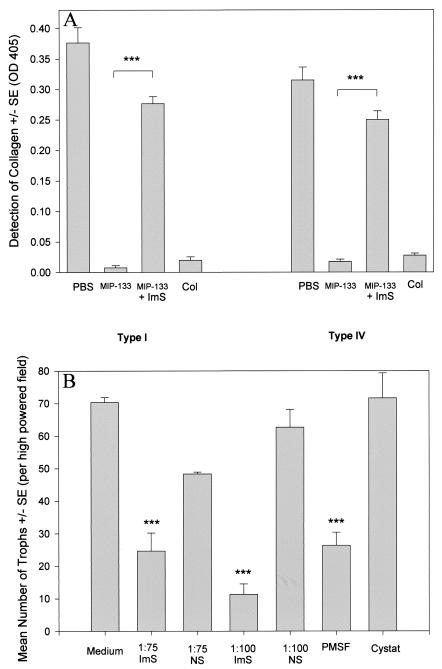
Accordingly, we tested the ability of anti-MIP-133 antiserum to neutralize the collagenolytic activity of MIP-133 (Fig. 6A). Antiserum treatment reduced the degradation of type I collagen by approximately 75% and type IV collagen by 80%. Degradation in the collagenase control was not significantly different from that after the MIP-133 treatments.
FIG. 6.
Anti-MIP-133 inhibition of MIP-133 collagenolytic activity and trophozoite migration. (A) ELISA. Ten-microgram samples of human collagen types I and IV were incubated and dried onto 96-well plates. Wells were treated with either 15.6 μg of the MIP-133 protein, 15.6 μg of the MIP-133 protein coincubated with a 1:75 dilution of chicken anti-MIP-133 antiserum (ImS), PBS, or 0.1 mg of collagenase (Col) for 72 h. Sample wells were then washed three times and incubated with mouse anti-collagen type IV IgG or mouse anti-collagen type I IgG as the primary antibody, followed by goat anti-mouse IgG-HRP, as described in Materials and Methods. Plates were then read at an OD at 405 nm. ***, significantly different from values from MIP-133 treatments (P < 0.001). (B) Migration assay. A. castellanii trophozoites (105) were placed in the upper chambers of 3.0-μm-pore-size transwells coated with Matrigel. Trophozoites were treated with 1:75 and 1:100 dilutions of either the chicken anti-MIP-133 antiserum (ImS) or the preimmune normal serum control (NS). Additional controls included 1.0 mM PMSF and 10 μM cystatin (Cystat). After 2 h, trophozoites in the bottom chamber were counted. Bars and error bars indicate means ± SE of results from 10 random high-powered fields (magnification, ×100). ***, significantly different from values obtained with the medium control or the normal serum control (P < 0.001).
A. castellanii trophozoites incubated with the anti-MIP-133 antiserum displayed a significant decrease in their ability to migrate through the Matrigel matrix (Fig. 6B). Trophozoites incubated with the serine protease inhibitor PMSF displayed similar reductions in their ability to migrate through the Matrigel. However, trophozoites incubated with the cysteine protease inhibitor cystatin did not show reduced migration.
Inhibition of CPE.
The MIP-133 protein has been shown to be highly cytotoxic for HCE cells in vitro. Being able to block this killing with an antibody might be a therapeutic option for mitigating disease.
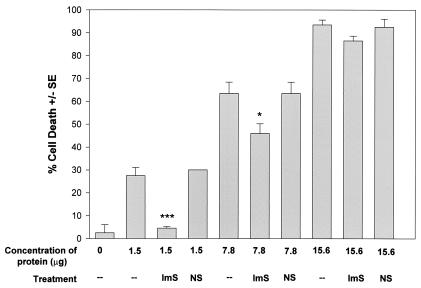
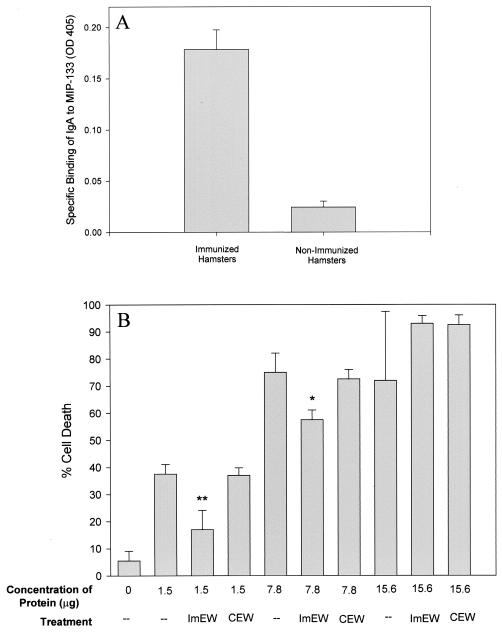
Figure 7 shows that the anti-MIP-133 antiserum was effective at blocking the cytotoxic activity of the MIP-133 protein. The cytotoxic effect of the 1.5-μg dose was completely eliminated, while the effect of the 7.8-μg dose was blocked by approximately 25%. The higher dosage of 15.6 μg was not inhibited by the antiserum. The preimmune serum control did not significantly reduce the cytolytic ability of the protein at any of the three doses tested.
FIG. 7.
Inhibition of MIP-133-mediated CPE against HCE cells. MIP-133 protein samples were adjusted to 1.5, 7.8, and 15.6 μg of protein in 25 μl of PBS before addition to HCE cells in 96-well microtiter plates for 18 h. Protein samples were either used alone, or coincubated with a 1:75 dilution of chicken anti-MIP-133 antiserum (ImS) or the normal serum control (NS). All final volumes were 200 μl. CPE were assessed spectrophotometrically. Each bar and error bar show the mean ± SE of triplicate counts. * and ***, significantly different from values for untreated controls (P < 0.05 and P < 0.001, respectively).
Clinical disease following MIP-133 immunizations.
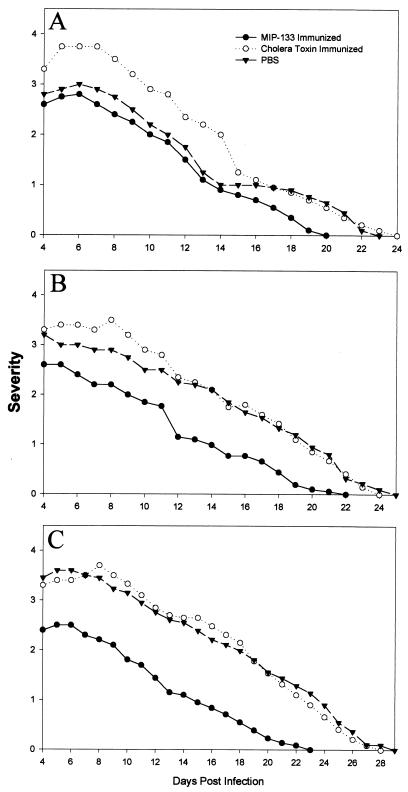
We have previously shown that oral immunizations with antigens conjugated to neutralized cholera toxin is an effective method of inducing mucosal antibody responses and the appearance of IgA antibodies in the tears and enteric washes of Chinese hamsters (13, 15). Therefore, Chinese hamsters were immunized with either 100, 200, or 400 μg of MIP-133 conjugated with neutralized cholera toxin. The results of a typical experiment are shown in Fig. 8 and demonstrate that oral immunization with 400 μg of MIP-133 reduced the severity of corneal infection by 30% and shortened the duration of the disease by 5 days (P < 0.01). Immunization with lower doses of MIP-133 or with cholera toxin alone did not significantly affect the course of disease.
FIG. 8.
Effect of oral immunization with the MIP-133 protein on Acanthamoeba keratitis. Hamsters were orally immunized with either 100 (A), 200 (B), or 400 (C) μg of the MIP-133 protein once a week for 4 weeks prior to infection with A. castellanii-infected lenses as described in Materials and Methods. Lenses were removed 4 days postinfection, and corneas were evaluated for clinical severity at the times indicated. At all time points, the animals immunized with 400 μg of MIP-133 (C) displayed infections significantly different (P < 0.01) than those of the cholera toxin and PBS control groups. The results shown are representative of three separate experiments for each treatment (eight hamsters in each treatment group per experiment).
Inhibition of CPE by enteric washes collected from immunized hamsters.
Enteric washes from orally immunized Chinese hamsters were tested by ELISA for the presence of mucosal IgA antibodies specific for the MIP-133 protein. Figure 9A shows that the pooled enteric washes from the immunized hamsters bound to the MIP-133 protein. By contrast, enteric washes taken from the PBS-treated animals did not specifically bind to the MIP-133 protein.
FIG. 9.
Inhibition of MIP-133-mediated CPE by mucosal anti-MIP-133 antibodies from immunized Chinese hamsters. (A) ELISA. Ninety-six-well plates were coated with 50 μg of MIP-133 and allowed to dry in carbonate buffer. After drying, wells were blocked and incubated with a 1:2 dilution of pooled enteric wash from immunized hamsters. Wells were then washed and incubated with a 1:2 dilution of rabbit anti-Chinese hamster IgA hyperimmune serum, washed, and further incubated with a 1:1,000 dilution of goat anti-rabbit IgG-HRP. ELISA plates were developed and read at an OD at 405 nm. (B) Inhibition of CPE. MIP-133 protein samples were adjusted to 1.5, 7.8, and 15.6 μg of protein in 25 μl of PBS before addition to HCE cells in 96-well microtiter plates for 18 h. Protein samples were either used alone or coincubated with a 1:50 dilution of pooled IgA enteric wash from immunized hamsters (ImEW) or of control enteric wash from PBS-immunized animals (CEW). All final volumes were 200 μl. CPE were assessed spectrophotometrically. Each bar and error bar show the mean ± SE of triplicate counts. * and **, significantly different from values for untreated controls (P < 0.05 and P < 0.01, respectively).
The capacity of the mucosal anti-MIP-133 antibodies to neutralize the CPE of MIP-133 was tested in vitro. The results of a typical experiment are shown in Fig. 9B and demonstrate that anti-MIP-133 mucosal antibody preparations significantly inhibited the cytopathic activity of MIP-133 (P < 0.01). Enteric washes from PBS-immunized animals did not inhibit cytopathic activity at any of the doses tested.
Oral immunization with MIP-133 mitigates chronic Acanthamoeba keratitis.
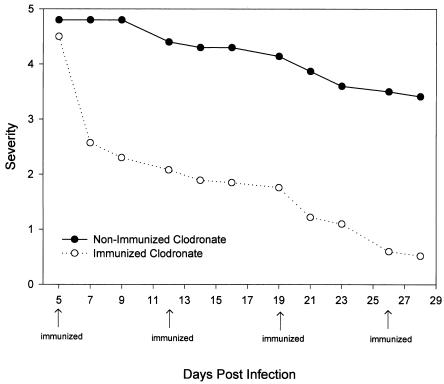
Acanthamoeba keratitis is an acute infection in Chinese hamsters that is self-limiting due to the rapid and effective response of the innate system, especially the conjunctival macrophages (7, 35). However, subconjunctival injection of liposomes containing the macrophagicidal drug clodronate removes periocular macrophages and results in a chronic infection that resembles the clinical course of Acanthamoeba keratitis in humans. Accordingly, we wished to determine if mucosal immunization with MIP-133 would affect a form of Acanthamoeba keratitis that mimicks the human counterpart in terms of its severity, persistence, and chronicity. Oral immunization with MIP-133 produced a remarkable mitigation of corneal disease in animals pretreated with clodronate-containing liposomes (Fig. 10). By contrast, infected animals treated with clodronate-containing liposomes and orally immunized with cholera toxin alone displayed clinical disease that was not significantly different from that of animals treated with clodronate-containing liposomes (data not shown).
FIG. 10.
Effects of oral immunization in animals with chronic, progressive Acanthamoeba keratitis. Chinese hamsters were treated with clodronate-encapsulated liposomes administered via subconjunctival injection on days −8, −6, −4, and −2 prior to infection with A. castellanii-infected lenses. Hamsters were then either left untreated or orally immunized with 400 μg of the MIP-133 protein on days 5, 12, 19, and 26 postinfection. Lenses were removed 5 days postinfection, and corneas were evaluated for clinical severity at the times indicated. All time points of the immunized clodronate-treated animals displayed infections that were significantly different (P < 0.001) from those of the nonimmunized clodronate group after day 7 postinfection. The results shown are representative of two separate experiments for each treatment (eight hamsters in each group).
DISCUSSION
The purposes of this study were to determine (i) the correlation between MIP-133 production and pathogenicity, (ii) the pathogenic mechanisms of MIP-133, (iii) the mechanism of MIP-133-induced corneal cell death, and (iv) if neutralization of MIP-133 affects the clinical course of Acanthamoeba keratitis.
The results show that production of the MIP-133 protein correlates with an amoeba's ability to cause disease. All clinical isolates produced MIP-133 and caused significant disease in Chinese hamsters. In contrast, three soil isolates did not produce the MIP-133 protein and also did not produce severe eye disease. However, A. hatchetti did consistently generate a mild disease, even though the strain did not produce the MIP-133 protein. It is uncertain what factor(s) may be responsible for this mild disease. A. castellanii neff has been shown by genetic analysis to be an avirulent strain, and our study further provides evidence that the strain is deficient in both producing disease and MIP-133 production (8). The correlation of MIP-133 production and the ability to cause disease may provide a valuable tool in further classifying the known pathogenic strains.
Other pathogenic amoebae, such as Entamoeba histolytica, have been shown to secrete cytolytic peptides in response to binding to glycoproteins (17, 21, 31, 37). These cytolytic peptides, called amoebapores, bind to the cell surface and cause cell death by generating pores in the lipid bilayer (18). However, unlike E. histolytica, the MIP-133 protein does not perforate the lipid bilayers. In contrast, the MIP-133 protein was found to be effective at activating a caspase 3-dependent apoptosis pathway in HCE cells. How the protein interacts with the cell surface to cause apoptosis is still unknown.
All of the pathogenic strains of Acanthamoeba tested were efficient at migrating through a Matrigel. The soil isolates were very inefficient at penetrating the Matrigel layer. This further correlates pathogenicity with the ability to penetrate an extracellular matrix. This ability to penetrate beyond the epithelial cell layer may represent an important hurdle in an organism's ability to generate disease in vivo. Therefore, we examined the ability of the MIP-133 protein to degrade an artificial Bowman's membrane (type IV collagen) and human stroma (type I collagen). Our results show that the MIP-133 protein is efficient at degrading both forms of collagen, with type IV collagen being almost completely degraded by 24 h. As penetration of Bowman's membrane and dissolution of the stroma represent the sequential steps in the pathogenic cascade, production of MIP-133 may correlate with an organism's ability to kill the outer corneal epithelial cells, penetrate Bowman's membrane, and gain entry into and dissolve the corneal stroma.
The role of MIP-133 in multiple phases of the pathogenic cascade of Acanthamoeba keratitis suggests that neutralizing this molecule might prove beneficial. Accordingly, an antiserum against MIP-133 was raised and was found to block the cytopathic and proteolytic activities of this protein. In addition, the anti-MIP-133 antiserum also inhibited trophozoite invasion of Matrigel, which was used as a model of the corneal basement membrane. Importantly, the antiserum blocked 80% of the MIP-133-induced CPE on corneal epithelial cells.
Based on these in vitro findings, we surmised that the MIP-133 protein might be used as an effective immunogen in vivo. Orally immunized Chinese hamsters have been shown to produce significantly high levels of IgA antibody that can be readily detected in both tear secretions and enteric washes (14). Since the tears continuously bathe the ocular surface, the anti-MIP-133 IgA antibodies have direct and prolonged contact with trophozoites and their products. By contrast, parental immunization does not elicit significant accumulation of antibodies in the tears (23, 28). Repeated intramuscular immunization with MIP-133 failed to provide any evidence of protection against Acanthamoeba keratitis in Chinese hamsters (data not shown). Tear IgA in human patients may be vital to protecting against the disease, as patients with Acanthamoeba keratitis have been shown to possess significantly lower levels of Acanthamoeba-specific IgA than the normal control population (1). Animals given four oral immunizations prior to Acanthamoeba infection generated protection in a dose-dependent fashion. Oral immunizations with 400 μg of MIP-133 provided a >30% reduction in the severity of the disease in vivo. Furthermore, enteric washes from the animals treated with 400 μg of MIP-133 displayed high levels of IgA specific for binding to the MIP-133 protein. These IgA washes also effectively neutralized nearly 50% of the MIP-133 cytolytic activity against HCE cells.
Although oral immunization prior to corneal exposure to Acanthamoeba trophozoites was effective in reducing disease, it is not a realistic paradigm for managing patients. Therefore, we employed a more relevant model in which we tested the efficacy of oral immunization with MIP-133 administered after the corneal infections had been established. In order to produce a chronic form of Acanthamoeba keratitis in Chinese hamsters, it is necessary to deplete the conjunctival macrophage population with a macrophagicidal drug, clodronate (35). The clodronate-treated animals developed a chronic form of Acanthamoeba keratitis. However, repeated oral immunizations with MIP-133 conjugated with the mucosal adjuvant, cholera toxin, produced a dramatic amelioration in corneal lesions and an eventual resolution of the disease. By contrast, animals treated with cholera toxin alone displayed corneal disease that was indistinguishable from that of the nonimmunized animals.
We are currently investigating the possible role of host cell surface molecules and their subsequent activation of caspase molecules upstream from caspase 3. As many caspase pathways are routed through caspase 3, it is likely that more than one caspase may be activated following MIP-133 binding to a host cell receptor. Furthermore, we are currently sequencing the protein in an effort to clone the protein.
The results offer glimmers of hope for immunotherapy for persistent, drug-resistant Acanthamoeba keratitis. It will be important to enhance the immunogenicity of MIP-133 and to optimize the oral immunization protocol to obtain higher mucosal antibody titers. It may also be possible to directly apply anti-MIP-133 monoclonal antibodies to the corneas of Acanthamoeba keratitis patients as a means of neutralizing the pathogenic disease.
Acknowledgments
This work was supported by Pubic Health Service grant EY09756 from the National Institutes of Health, NIAID molecular microbiology training grant AI07520, and an unrestricted grant from Research to Prevent Blindness, Inc., New York, N.Y.
We thank Elizabeth Mayhew for assistance with flow cytometry analysis and Bob Ritter for his assistance with protein purification and scientific discussions. Roche Diagnostics graciously provided clodronate for these experiments.
Editor: J. M. Mansfield
REFERENCES
- 1.Alizadeh, H., S. Apte, M. S. El-Agha, L. Li, M. Hurt, K. Howard, H. D. Cavanagh, J. P. McCulley, and J. Y. Niederkorn. 2001. Tear IgA and serum IgG antibodies against Acanthamoeba in patients with Acanthamoeba keratitis. Cornea 20:622-627. [DOI] [PubMed] [Google Scholar]
- 2.Alizadeh, H., J. Y. Niederkorn, and J. P. McCulley. 1996. Acanthamoeba keratitis, p. 1062-1071. In J. S. Pepose, G. N. Holland, and K. R. Wilhelmus (ed.), Ocular infection and immunity. Mosby, St. Louis, Mo.
- 3.Auran, J. D., M. B. Starr, and F. A. Jacobiec. 1987. Acanthamoeba keratitis. Cornea 6:2-26. [PubMed] [Google Scholar]
- 4.Brown, T. J., R. T. M. Cursons, and E. A. Keys. 1982. Amoeba from antarctic soil and water. Appl. Environ. Microbiol. 44:491-493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control. 1987. Acanthamoeba keratitis in soft-contact-lens wearers—United States. Morb. Mortal. Wkly. Rep. 36:397-398, 403-404. [PubMed] [Google Scholar]
- 6.Cerva, L., C. Serbus, and V. Skocil. 1973. Isolation of limax amoeba from the nasal mucosa of man. Folia Parasitol. 20:97-103. [PubMed] [Google Scholar]
- 7.He, Y. G., J. Y. Niederkorn, J. P. McCulley, G. L. Stewart, D. R. Meyer, R. Silvany, and J. Dougherty. 1990. In vivo and in vitro collagenolytic activity of Acanthamoeba castellanii. Investig. Ophthalmol. Vis. Sci. 31: 2235-2240. [PubMed] [Google Scholar]
- 8.Howe, D. K., M. H. Vodkin, R. J. Novak, G. Visvesvara, and G. L. McLaughlin. 1997. Identification of two genetic markers that distinguish pathogenic and nonpathogenic strains of Acanthamoeba spp. Parasitol. Res. 83:345-348. [DOI] [PubMed] [Google Scholar]
- 9.Hurt, M., S. Apte, H. Leher, K. Howard, J. Niederkorn, and H. Alizadeh. 2001. Exacerbation of Acanthamoeba keratitis in animals treated with anti-macrophage inflammatory protein 2 or antineutrophil antibodies. Infect. Immun. 69:2988-2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hurt, M., J. Niederkorn, and H. Alizadeh. 2003. Effects of mannose on Acanthamoeba castellanii proliferation and cytolytic ability to corneal epithelial cells. Investig. Ophthalmol. Vis. Sci. 44:3424-3431. [DOI] [PubMed] [Google Scholar]
- 11.Jaison, P. L., Z. Cao, and N. Panjwani. 1998. Binding of Acanthamoeba to [corrected] mannose-glycoproteins of corneal epithelium: effect of injury. Curr. Eye Res. 17:770-776. [PubMed] [Google Scholar]
- 12.Kingston, D., and D. C. Warhurst. 1969. Isolation of amoebae from the air. J. Med. Microbiol. 2:27-36. [DOI] [PubMed] [Google Scholar]
- 13.Leher, H., K. Kinoshita, H. Alizadeh, F. L. Zaragoza, Y. G. He, and J. Y. Niederkorn. 1998. Impact of oral immunization with Acanthamoeba antigens on parasite adhesion and corneal infection. Investig. Ophthalmol. Vis. Sci. 39:2337-2343. [PubMed] [Google Scholar]
- 14.Leher, H., F. Zaragoza, S. Taherzadeh, H. Alizadeh, and J. Y. Niederkorn. 1999. Monoclonal IgA antibodies protect against Acanthamoeba keratitis. Exp. Eye Res. 69:75-84. [DOI] [PubMed] [Google Scholar]
- 15.Leher, H. F., H. Alizadeh, W. M. Taylor, A. S. Shea, R. S. Silvany, F. Van Klink, M. J. Jager, and J. Y. Niederkorn. 1998. Role of mucosal IgA in the resistance to Acanthamoeba keratitis. Investig. Ophthalmol. Vis. Sci. 39:2666-2673. [PubMed] [Google Scholar]
- 16.Leher, H. F., R. E. Silvany, H. Alizadeh, J. Huang, and J. Y. Niederkorn. 1998. Mannose induces the release of cytopathic factors from Acanthamoeba castellanii. Infect. Immun. 66:5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leippe, M. 1997. Amoebapores. Parasitol. Today 13:178-183. [DOI] [PubMed] [Google Scholar]
- 18.Leippe, M., S. Ebel, O. L. Schoenberger, R. D. Horstmann, and H. J. Muller-Eberhard. 1991. Pore-forming peptide of pathogenic Entamoeba histolytica. Proc. Natl. Acad. Sci. USA 88:7659-7663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lyons, T. B., and R. Kapur. 1977. Limax amoeba in public swimming pools of Albany, Schenectady, and Ransselear Counties, New York: their concentrations, correlations, and significance. Appl. Environ. Microbiol. 33:551-555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mathers, W. D., J. E. Sutphin, R. Folberg, P. A. Meier, R. P. Wenzel, and R. G. Elgin. 1996. Outbreak of keratitis presumed to be caused by Acanthamoeba. Am. J. Ophthalmol. 121:207-208. [DOI] [PubMed] [Google Scholar]
- 21.McCoy, J. J., B. J. Mann, and W. A. Petri, Jr. 1994. Adherence and cytotoxicity of Entamoeba histolytica or how lectins let parasites stick around. Infect. Immun. 62:3045-3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCulley, J. P., H. Alizadeh, and J. Y. Niederkorn. 1995. Acanthamoeba keratitis. CLAO J. 21:73-76. [PubMed] [Google Scholar]
- 23.Mestecky, J. 1987. The common mucosal immune system and current strategies for induction of immune responses in external secretions. J. Clin. Immunol. 7:265-276. [DOI] [PubMed] [Google Scholar]
- 24.Morlet, N., G. Duguid, C. Radford, M. Matheson, and J. Dart. 1997. Incidence of Acanthamoeba keratitis associated with contact lens wear. Lancet 350:414. [DOI] [PubMed] [Google Scholar]
- 25.Morton, L. D., G. L. McLaughlin, and H. E. Whiteley. 1991. Effects of temperature, amebic strain, and carbohydrates on Acanthamoeba adherence to corneal epithelium in vitro. Infect. Immun. 59:3819-3822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Niederkorn, J. Y., H. Alizadeh, H. F. Leher, and J. P. McCulley. 1999. The immunobiology of Acanthamoeba keratitis. Springer Semin. Immunopathol. 21:147-160. [DOI] [PubMed] [Google Scholar]
- 27.Paszko-Kolva, C., H. Yamamoto, M. Shahamat, T. K. Sawyer, G. Morris, and R. R. Colwell. 1991. Isolation of amoeba and Pseudomonas and Legionella spp. from eyewash stations. Appl. Environ. Microbiol. 57:163-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peppard, J. V., R. V. Mann, and P. C. Montgomery. 1988. Antibody production in rats following ocular-topical or gastrointestinal immunization: kinetics of local and systemic antibody production. Curr. Eye Res. 7:471-481. [DOI] [PubMed] [Google Scholar]
- 29.Pettit, D. A., J. Williamson, G. A. Cabral, and F. Marciano-Cabral. 1996. In vitro destruction of nerve cell cultures by Acanthamoeba spp.: a transmission and scanning electron microscopy study. J. Parasitol. 82:769-777. [PubMed] [Google Scholar]
- 30.Rivera, F., F. Medina, P. Ramirez, J. Alcocer, G. Vilaclara, and E. Robles. 1984. Pathogenic and free-living protozoa cultured from the nasopharyngeal and oral regions of dental patients. Environ. Res. 33:428-440. [DOI] [PubMed] [Google Scholar]
- 31.Stanley, S. L. 2001. Pathophysiology of amoebiasis. Trends Parasitol. 17:280-285. [DOI] [PubMed] [Google Scholar]
- 32.Stehr-Green, J. K., T. M. Baily, and G. S. Visvesvara. 1989. The epidemiology of Acanthamoeba keratitis in the United States. Am. J. Ophthalmol. 107:331-336. [DOI] [PubMed] [Google Scholar]
- 33.Van Klink, F., H. Alizadeh, Y. G. He, J. A. Mellon, R. E. Silvany, J. P. McCulley, and J. Y. Niederkorn. 1993. The role of contact lenses, trauma, and Langerhans cells in the Chinese hamster model of Acanthamoeba keratitis. Investig. Ophthalmol. Vis. Sci. 34:1937-1944. [PubMed] [Google Scholar]
- 34.Van Klink, F., H. F. Leher, M. J. Jager, H. Alizadeh, W. Taylor, and J. Y. Niederkorn. 1997. Systemic immune response to Acanthamoeba keratitis in the Chinese hamster. Ocular Immunol. Inflamm. 5:234-244. [DOI] [PubMed] [Google Scholar]
- 35.Van Klink, F., W. M. Taylor, H. Alizadeh, M. J. Jager, N. Van Rooijen, and J. Y. Niederkorn. 1996. The role of macrophages in Acanthamoeba keratitis. Investig. Ophthalmol. Vis. Sci. 37:1271-1281. [PubMed] [Google Scholar]
- 36.Van Rooijen, N. 1989. The liposome-mediated macrophage ‘suicide' technique. J. Immunol. Methods 124:1-6. [DOI] [PubMed] [Google Scholar]
- 37.Vines, R. R., G. Ramakrishnan, J. B. Rogers, L. A. Lockhart, B. J. Mann, and W. A. Petri, Jr. 1998. Regulation of adherence and virulence by the Entamoeba histolytica lectin cytoplasmic domain, which contains a beta2 integrin motif. Mol. Biol. Cell 9:2069-2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Visvesvara, G. S. 1991. Classification of Acanthamoeba. Rev. Infect. Dis. 13(Suppl. 5):S369-S372. [DOI] [PubMed] [Google Scholar]
- 39.Visvesvara, G. S., S. S. Mirra, F. H. Brandt, D. M. Moss, H. M. Mathews, and A. J. Martinez. 1983. Isolation of two strains of Acanthamoeba castellanii from human tissue and their pathogenicity and isoenzyme profiles. J. Clin. Microbiol. 6:1405-1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Visvesvara, G. S., and J. K. Stehr-Green. 1990. Epidemiology of free-living amoeba infections. J. Protozool. 37:25s-33s. [DOI] [PubMed] [Google Scholar]
- 41.Wang, S. S., and H. A. Feldman. 1967. Isolation of Hartmannella species from human throats. N. Engl. J. Med. 277:1174-1179. [DOI] [PubMed] [Google Scholar]
- 42.Yang, Z. T., Z. Y. Cao, and N. Panjwani. 1997. Pathogenesis of Acanthamoeba keratitis: carbohydrate-mediated host-parasite interactions. Infect. Immun. 65:439-445. [DOI] [PMC free article] [PubMed] [Google Scholar]