Abstract
We demonstrated previously that a single injection of recombinant human macrophage colony-stimulating factor (rhM-CSF) is sufficient for osteoclast recruitment and survival in osteopetrotic (op/op) mice with a deficiency in osteoclasts resulting from a mutation in M-CSF gene. In this study, we show that a single injection of recombinant human vascular endothelial growth factor (rhVEGF) can similarly induce osteoclast recruitment in op/op mice. Osteoclasts predominantly expressed VEGF receptor 1 (VEGFR-1), and activity of recombinant human placenta growth factor 1 on osteoclast recruitment was comparable to that of rhVEGF, showing that the VEGF signal is mediated through VEGFR-1. The rhM-CSF–induced osteoclasts died after injections of VEGFR-1/Fc chimeric protein, and its effect was abrogated by concomitant injections of rhM-CSF. Osteoclasts supported by rhM-CSF or endogenous VEGF showed no significant difference in the bone-resorbing activity. op/op mice undergo an age-related resolution of osteopetrosis accompanied by an increase in osteoclast number. Most of the osteoclasts disappeared after injections of anti-VEGF antibody, demonstrating that endogenously produced VEGF is responsible for the appearance of osteoclasts in the mutant mice. In addition, rhVEGF replaced rhM-CSF in the support of in vitro osteoclast differentiation. These results demonstrate that M-CSF and VEGF have overlapping functions in the support of osteoclastic bone resorption.
Keywords: osteoclasts, vascular endothelial growth factor, vascular endothelial growth factor receptor, macrophage colony-stimulating factor, osteopetrosis
Mice homozygous for the recessive mutation, osteopetrosis (op), on chromosome 3 have a severe deficiency of osteoclasts, monocytes, and macrophages in various organs 1 2 3. The deficiency is caused by the absence of functional macrophage colony-stimulating factor (M-CSF or CSF-1) as a result of a single basepair insertion within the coding region of the M-CSF gene 4 and can be cured by injections of recombinant human (rh)M-CSF 5 6 7 8. Direct action of M-CSF on osteoclast lineage cells is demonstrated by the expression of the receptor for M-CSF, c-Fms, in osteoclasts both in vitro 9 and in vivo 10. These findings indicate that M-CSF plays an essential role in the differentiation of osteoclasts, as well as macrophages in some organs, under physiological conditions.
However, severe osteopetrosis in op/op mice is evident only during their youth and is progressively corrected in association with an increase of osteoclasts 1 2 11. We found that when injected at high doses (>5 μg/mouse), only a single injection of rhM-CSF is sufficient to induce a synchronous wave of osteoclast recruitment, survival, and active bone resorption for a prolonged period in op/op mice 12 13. These observations have suggested the presence of other regulatory factor(s) that are responsible for osteoclastic bone resorption in the absence of M-CSF. In this context, controversial data have been reported on the effects of GM-CSF on the cure of osteopetrosis in op/op mice, whereas several in vitro studies have suggested a role of this cytokine in osteoclast differentiation 14 15 16. Wiktor-Jedrzejczak et al. 17 and Nilsson et al. 18 reported that GM-CSF can correct macrophage deficiencies but fails to resolve osteopetrosis. Very recently, Myint et al. 19 reported that GM-CSF and/or IL-3 at low doses can induce osteoclast development in op/op mice.
c-Fms is one of the eight members of the platelet-derived growth factor receptor (PDGFR) family 20. As specific receptors for vascular endothelial growth factor (VEGF), two receptor tyrosine kinases of the PDGFR family, VEGFR-1/Flt-1 and VEGFR-2/KDR/Flk-1, as well as neuropilin-1, have been identified 21. In contrast to endothelial cells which express all of the VEGFRs, monocyte/macrophage lineage cells predominantly express VEGFR-1 21 22 23. VEGFR-1 mediates chemotactic response of the cells to VEGF or placenta growth factor 1 (PlGF-1), which shows high homology to VEGF and is expressed in umbilical vein endothelia and placenta 21 22 23 24 25 26.
Taking note of the close lineage relationship between macrophages and osteoclasts, we show in this study that VEGF can fully compensate for the deficiency of M-CSF in op/op mice in osteoclastic bone resorption, manifesting a unique type of redundancy in cytokine signaling by using different ligand–receptor combinations. Furthermore, we present evidence that endogenously produced VEGF is responsible for the age-related resolution of osteopetrosis in op/op mice.
Materials and Methods
Mice.
op/op mice and their normal littermates (+/?) were raised in our animal facility as described previously 6 12. Mice of op/op genotype were identified at 11 d of age by the absence of incisor eruption. Male ddY mice were obtained from Saitama Experimental Animals Supply (Sugito, Saitama, Japan).
Injection of Cytokines, Antibody, and/or VEGFR-1/Fc Chimeric Protein into op/op Mice.
5 μg of either rhM-CSF (Austral Biologicals), rhVEGF165 (Genzyme Corp.), rhVEGF121 (Genzyme Corp.), or rhPlGF-1 (R&D Systems) was intraperitoneally injected into 12-d-old op/op mice, and the mice were killed 3 or 7 d after the injection. AFS98 rat anti–mouse c-Fms mAb 27 was intraperitoneally injected at a dosage of 750 μg/mouse into mutant mice both 2 h before and 24 h after the cytokine injection, and the mice were killed 3 d after cytokine injection.
In another series of experiments, op/op mice were pretreated with a single injection of rhM-CSF at 12 d of age. Starting at 4 d after the pretreatment, 5 μg of either VEGFR-1/Fc chimeric protein (R&D Systems) and/or rhM-CSF was intraperitoneally injected six times at 12-h intervals. The mice were killed 7 d after the pretreatment. As a control for the chimeric protein, human IgG1 (ICN Pharmaceuticals) was injected similarly as above. rhM-CSF alone or together with VEGFR-1/Fc was also consecutively injected six times at 12-h intervals into the mutant mice starting at 12 d of age without pretreatment. Mice were killed 3 d after the onset of the treatment.
Five consecutive injections of 100 μg of goat anti–mouse VEGF polyclonal antibody (R&D Systems) at 12-h intervals were given to 2-mo-old op/op mice. As a control, goat IgG (Santa Cruz Biotechnology) was injected similarly as above. The last group of mice received a single dose of 5 μg rhVEGF165. All of these mice were killed 3 d after the onset of the treatments.
Histological Observations.
op/op mice were anesthetized with ether and perfused with 4% periodate-lysine-paraformaldehyde fixative solution (pH 7.4) through descending aorta. Femurs were decalcified in 10% EDTA (pH 7.0) for 4 d and embedded in paraffin. Longitudinal sections (7 μm thick) of the median portion of whole femurs were stained for tartrate-resistant acid phosphatase (TRAP) activity as described previously 12 13 and counterstained with hematoxylin. TRAP-positive cells with two or more nuclei were counted as osteoclasts. Some sections were stained by Mallory's azan staining.
Immunohistochemical Staining for VEGFRs.
Femurs of 2–3-wk-old +/? or op/op mice were fixed and embedded in paraffin as described above. Sections (5 μm thick) of the femurs were immunohistochemically stained with rabbit anti–mouse VEGFR-1 polyclonal antibody (Santa Cruz Biotechnology) or AVAS12 rat anti–mouse VEGFR-2 mAb 28, using Vectastain Elite ABC kits (Vector Laboratories), and counterstained with hematoxylin. Normal rabbit IgG (Santa Cruz Biotechnology) and rat IgG2a (Santa Cruz Biotechnology) were used as controls for the polyclonal and monoclonal antibodies, respectively.
In Vitro Generation of Osteoclasts.
rhVEGF165 and rhM-CSF were dissolved in fetal bovine serum (FBS) at concentrations of 2 μg/ml and 400 ng/ml, respectively. Wells of 96-well plates were coated with 5 μl of either of the cytokine solutions or FBS and air dried for 30 min. Bone marrow cells obtained from tibias and femurs of 5–8-wk-old male ddY mice were passed through a Sephadex G-10 (Amersham Pharmacia Biotech) column as described by Ly and Mishell 29. Nonadherent cells were plated at a density of 105 cells/well into the cytokine-coated wells and cultured with α-MEM supplemented with 15% FBS in the absence or presence of 100 ng/ml of recombinant human receptor activator of nuclear factor κB ligand (rhRANKL; PeproTech) for 7 d. The final concentrations of rhVEGF165 and rhM-CSF were 100 and 20 ng/ml, respectively. The cultures were fixed with 4% paraformaldehyde and stained for TRAP as described above. The nonadherent bone marrow cells were also inoculated onto dentine slices with a diameter of 5 mm, placed in the wells of 24-well plates, similarly as described above, and cultured for 7 d. The slices were examined by backscattered electron microscopy as described previously 30.
Results and Discussion
To examine whether VEGF can compensate for the absence of functional M-CSF in op/op mice in the support of osteoclast recruitment, we first injected either rhM-CSF, rhVEGF165, rhVEGF121, or rhPlGF-1 into 12-d-old op/op mice. As shown in Table , a single 5-μg injection of any of these factors was sufficient for the osteoclast recruitment in the mutant mice, although the number of osteoclasts recruited by rhVEGFs or rhPlGF-1 was 60–70% of that by rhM-CSF. The antagonistic anti–c-Fms mAb, AFS98 27, decreased osteoclast recruitment by rhM-CSF to ∼25%, but not that by rhVEGFs or rhPlGF-1, confirming that c-Fms mediates the response of osteoclast precursor cells to M-CSF, but not the response to VEGFs or PlGF-1.
Table 1.
Capacity of rhM-CSF, rhVEGFs, and rhPlGF-1 to Recruit Osteoclasts in op/op Mice
| Cytokine | AFS98 | No. of osteoclasts/section |
|---|---|---|
| (mean ± SD) | ||
| None | − | 3 ± 2 |
| rhM-CSF | − | 60 ± 6 |
| rhM-CSF | + | 14 ± 9 |
| rhVEGF165 | − | 42 ± 1 |
| rhVEGF165 | + | 43 ± 7 |
| rhVEGF121 | − | 37 ± 4 |
| rhPlGF-1 | − | 37 ± 2 |
| rhPlGF-1 | + | 35 ± 2 |
Cytokines were injected at a single dosage of 5 μg into 12-d-old op/op mice, and mice were killed 3 d after the injection. AFS98 anti–c-Fms mAb was injected at a dosage of 750 μg/mouse both 2 h before and 24 h after the cytokine injection. Osteoclasts in the longitudinal sections of the median portion of whole femurs were counted. Results represent the mean ± SD of six sections from three mice.
As shown in Fig. 1 A, osteoclasts were strongly stained with rabbit anti–mouse VEGFR-1 polyclonal antibody, whereas endothelial cells were weakly positive for VEGFR-1. In contrast, osteoclasts were not stained with AVAS12 anti–mouse VEGFR-2 mAb 28, while endothelial cells were positively stained for VEGFR-2 (Fig. 1 B). Neither normal rabbit IgG (Fig. 1 C) nor rat IgG2a (data not shown) stained any cell types. rhM-CSF–induced osteoclasts in op/op mice showed the same staining pattern as described above (data not shown). These results demonstrate that osteoclasts predominantly express VEGFR-1, in a manner similar to monocyte/macrophage lineage cells 22 23. VEGF121 does not bind neuropilin-1 21. PlGF-1 binds VEGFR-1, but not VEGFR-2 or neuropilin-1 21 22 23 24 25. The results that both rhVEGF121 and rhPlGF-1 showed activities comparable to rhVEGF165 in the support of osteoclast recruitment (Table ) confirm that the response of osteoclast precursor cells to VEGF is mediated by VEGFR-1.
Figure 1.
Immunohistochemical staining of femur sections for VEGFRs. Longitudinal sections of femurs of 3-wk-old +/? mice were stained with either anti–VEGFR-1 polyclonal antibody (A), AVAS12 anti–VEGFR-2 mAb (B), or rabbit IgG (C). Arrowheads indicate osteoclasts, and arrows indicate endothelial cells. Original magnifications: ×238.
Next, we examined the capacity of VEGF and M-CSF to support the survival of mature osteoclasts by neutralizing VEGF endogenously produced in op/op mice with injections of VEGFR-1/Fc chimeric protein. Consistent with our previous observations 12 13, osteoclast number reached a plateau at 3 d after a single rhM-CSF injection and was maintained up to day 7 (Table and Table ). Consecutive injections of the chimeric protein at 12-h intervals during days 4–6 decreased osteoclasts to ∼25%, whereas injections of human IgG1 did not affect osteoclast number (Table ). In contrast, when rhM-CSF was injected together with VEGFR-1/Fc, osteoclast number increased to the levels observed in mice consecutively injected with rhM-CSF alone. These results indicate that survival of osteoclasts recruited after a single rhM-CSF injection was supported by endogenously produced VEGF in op/op mice and that M-CSF can support the survival of mature osteoclasts without the help of VEGF.
Table 2.
Effect of Injections of VEGFR-1/Fc Chimeric Protein on the Survival of rhM-CSF–recruited Osteoclasts in op/op Mice
| Treatment | No. of osteoclasts/section |
|---|---|
| (mean ± SD) | |
| None | 59 ± 9 |
| VEGFR-1Fc | 15 ± 5 |
| Human IgG1 | 65 ± 9 |
| VEGFR-1/Fc and rhM-CSF | 87 ± 9 |
| rhM-CSF | 81 ± 8 |
op/op mice were pretreated with a single injection of 5 μg rhM/CSF at 12 d of age. 5 μg each of VEGFR-1/Fc and/or rhM-CSF or 5 μg human IgG1 was consecutively injected six times at 12-h intervals into the mice during 16–18 d of age. Osteoclasts in the longitudinal sections of the median portion of whole femurs were counted. Results represent the mean ± SD of six sections from three mice.
We also examined the bone resorption in the femurs of op/op mice that had received either a single rhM-CSF injection only or consecutive injections of VEGFR-1/Fc and rhM-CSF in addition to the single rhM-CSF injection. Osteoclasts in the former group of mice are thought to perform their functions with the support of endogenous VEGF, whereas those in the latter rely on exogenous rhM-CSF. As reported previously 12, resorption of a massive amount of bone trabeculae and replacement with bone marrow in femurs were apparent by 7 d after a single rhM-CSF injection (Fig. 2A and Fig. B). Bone resorption was also similarly observed in the latter group of mice (Fig. 2 C). These observations show that both M-CSF and VEGF can support the bone-resorbing function of osteoclasts.
Figure 2.
Resorption of bone trabeculae in the femurs of op/op mice by osteoclasts with the support of endogenous VEGF or exogenous rhM-CSF. The treatment was started at 12 d of age, and the mice were killed at 19 d of age. Longitudinal sections of femurs were stained by Mallory's azan. Each micrograph represents a group of femurs from three animals. (A) No injection; (B) a single injection of 5 μg rhM-CSF at 12 d of age; (C) a single injection of 5 μg rhM-CSF at 12 d of age, and six consecutive injections of 5 μg each of VEGFR-1/Fc chimeric protein and rhM-CSF at 12-h intervals during 16 and 18 d of age. Original magnifications: ×20.
The above finding that VEGF is endogenously produced at levels sufficient for the survival of mature osteoclasts and expression of their functions prompted us to confirm that rhM-CSF can induce osteoclast recruitment without the help of endogenous VEGF. As shown in Table , twice the number of osteoclasts were recruited by multiple injections of rhM-CSF compared with a single injection. Concomitant injections of VEGFR-1/Fc with rhM-CSF did not affect osteoclast recruitment. These results are the first unequivocal demonstration of the capacity of M-CSF to support in vivo osteoclast differentiation.
Table 3.
Effect of VEGFR-1/Fc Injection on Osteoclast Recruitment by rhM-CSF in op/op Mice
| Treatment | No. of injections | No. of osteoclasts/section |
|---|---|---|
| (mean ± SD) | ||
| None | 0 | 3 ± 2 |
| rhM-CSF | 1 | 56 ± 9 |
| rhM-CSF | 6 | 108 ± 11 |
| rhM-CSF and VEGFR-1/Fc | 6 | 101 ± 7 |
The treatments of op/op mice began at 12 d of age. They received either a single injection of 5 μg rhM-CSF or six consecutive injections of 5 μg rhM-CSF alone or together with 5 μg VEGFR-1/Fc at 12-h intervals, and were killed at 3 d after the onset of the treatments. Osteoclasts in the longitudinal sections of the median portion of whole femurs were counted. Results represent the mean ± SD of six sections from three mice.
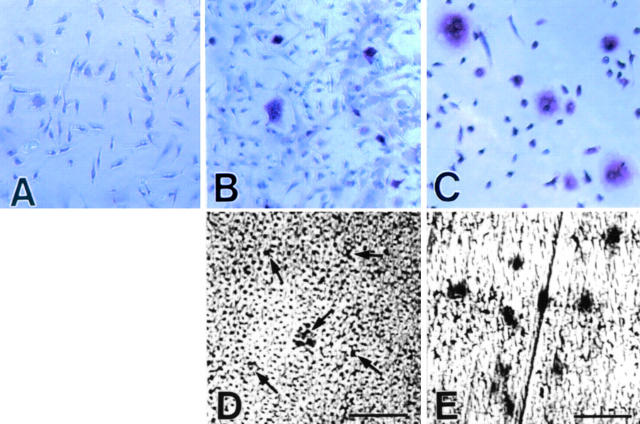
It became clear that M-CSF supports osteoclast differentiation in cooperation with osteoclast differentiation factor (ODF)/osteoprotegerin ligand (OPGL)/TNF-related activation-induced cytokine (TRANCE)/RANKL 31 32. We examined whether rhVEGF165 can replace rhM-CSF in osteoclast generation in in vitro culture of nonadherent bone marrow cells. Consistent with previous observations 31 32, no TRAP-positive cells appeared in the presence of rhM-CSF or rhRANKL alone (data not shown). rhVEGF165 alone also failed to support the osteoclast differentiation (Fig. 3 A). A combination of rhVEGF165 and rhRANKL supported the generation of TRAP-positive cells (Fig. 3 B), although the cells were significantly smaller in size than those generated in the presence of rhM-CSF and rhRANKL (Fig. 3 C). Consequently, the osteoclasts supported by rhVEGF165 and rhRANKL formed smaller resorption lacunae than those supported by rhM-CSF and rhRANKL (Fig. 3D and Fig. E). These results demonstrate that VEGF can indeed support osteoclast differentiation in cooperation with ODF/OPGL/TRANCE/RANKL.
Figure 3.
Ability of VEGF to support in vitro generation of osteoclasts. Nonadherent bone marrow cells were cultured in the presence of rhVEGF165 alone (A), rhVEGF165 and rhRANKL (B and D), or rhM-CSF and rhRANKL (C and E) in the wells of 96-well plates (A–C) or on dentine slices (D and E) for 7 d. The cultures were either stained for TRAP activity (A–C) or examined by backscattered electron microscopy (D and E). Arrows in D indicate small resorption lacunae. Original magnifications (A–C): ×25. Bars (D and E): 50 μm.
Finally, we examined whether progressive correction of osteopetrosis with age accompanied by an increase of osteoclasts in op/op mice 1 2 11 is due to endogenously produced VEGF. As shown in Fig. 4 A, a significantly larger number of small osteoclasts with 2–3 nuclei was observed in the femurs of 2-mo-old op/op mice (28 ± 1 osteoclasts/section) compared with those of 2-wk-old mutants (Table and Table ), even though the size of the femur sections of the older animals was ∼1.6 times larger than that of younger ones. In addition, TRAP-positive mononuclear cells were frequently observed in the marrow space. Five consecutive injections of 100 μg goat anti-VEGF polyclonal antibody at 12-h intervals significantly decreased osteoclast number (4 ± 2 osteoclasts/section; Fig. 4 B). Injections of goat IgG did not affect osteoclast number (data not shown). VEGFR-1/Fc injections according to the protocol applied to 2-wk-old mutant mice (Table ) failed to show any noticeable effect on osteoclast number (data not shown). A single injection of 5 μg rhVEGF165 induced further recruitment of osteoclasts (64 ± 5 osteoclasts/section; Fig. 4 C), indicating that VEGF levels in the femurs of 2-mo-old op/op mice are still insufficient to recruit osteoclasts at maximum level. These results demonstrate that VEGF is responsible for the spontaneous osteoclast recruitment in the absence of functional M-CSF in op/op mice. Changes in osteoclast number with the age and difference in the amount of VEGFR-1/Fc required to neutralize endogenous VEGF activity in 2-wk- and 2-mo-old animals suggest higher levels of VEGF production in older mutant mice, although the possibility that sensitivity of osteoclast precursors to VEGF changes with age cannot be ruled out.
Figure 4.

Dependence of osteoclasts in the femurs of 2-mo-old op/op mice on endogenously produced VEGF. The mice were killed 3 d after the onset of the treatment. Longitudinal sections of femurs were stained for TRAP activity and counterstained with hematoxylin. Each micrograph represents a group of femurs from three animals. Arrows in B indicate mononuclear TRAP-positive cells. (A) No injection; (B) five consecutive injections of 100 μg anti-VEGF polyclonal antibody at 12-h intervals; (C) a single injection of 5 μg rhVEGF165. Original magnifications: ×103.
This study demonstrates that M-CSF and VEGF can play almost entirely overlapping roles in osteoclastic bone resorption. The presence of either of the cytokines was sufficient to support all the processes of osteoclastic bone resorption, i.e., the differentiation of osteoclasts and their survival and active bone resorption, representing a unique type of redundancy of cytokine signaling. However, osteoclasts generated in vitro with the support of rhVEGF165 and rhRANKL were significantly smaller in size and formed smaller resorption lacunae compared with those supported by rhM-CSF and rhRANKL. Osteoclasts observed in 2-mo-old op/op mice had only two to three nuclei. Nevertheless, our data indicated that progressive correction of osteopetrosis in op/op mice is due to endogenously produced VEGF.
It has been well established that VEGF is a key regulator of vasculogenesis 21. Osteoclastic bone resorption and concomitant bone marrow formation are closely associated with active neovascularization 8 33, and osteoblasts have been reported to produce VEGF 34. Our results indicate that VEGF is produced in op/op mice at levels sufficient for the survival and functioning of mature osteoclasts, but not for their recruitment at maximal levels. The finding that mice lacking a single VEGF allele die in utero with aberrant blood vessel formation in the yolk sac and embryo indicates that VEGF is produced at threshold levels for endothelial cell proliferation 35 36. Furthermore, mice expressing the VEGFR-1 lacking the tyrosine kinase domain 26 had no appreciable abnormality in osteoclastic bone resorption (M. Shibuya, The University of Tokyo, personal communication). Therefore, M-CSF seems to play a dominant role in osteoclastic bone resorption under physiological conditions.
Macrophages from mice with kinase-deficient VEGFR-1 exhibit a defect in their migratory response to VEGF 26. The common feature of predominant expression of VEGFR-1 in monocytes and macrophages 21 22 23 and in osteoclasts may provide further support for the view of shared origin of these cells. We found previously that multiple injections of rhM-CSF are required for macrophage recruitment in the femurs of op/op mice 12 13. In the present study, we also failed to find any sign of macrophage recruitment in the femurs after a single injection of rhVEGFs or rhPlGF-1 (data not shown). These observations may suggest that macrophage lineage cells are less sensitive to M-CSF, VEGFs, and PlGF-1 compared with osteoclast precursors or more probably that macrophage precursors are more strictly dependent on the continuous presence of M-CSF.
The function of VEGFR-1 as a mediator of mitogenic response of endothelial cells to VEGF has yet to be clearly identified, although unequivocal evidence for such a role of VEGFR-2 has accumulated 21. The phenotypes of the mice with VEGFR-1 deficiency 37 and those expressing kinase-deficient VEGFR-1 29 strongly suggest the role of VEGFR-1 in the negative regulation of endothelial growth in embryonic angiogenesis. Therefore, it is of interest to compare the VEGFR-1 signaling in osteoclasts and their precursor cells with that in endothelial cells.
Acknowledgments
We wish to thank Drs. M. Shibuya and S.-I. Hayashi for valuable information and suggestions, and Drs. S. Yamada and Y. Ishiduka for encouragement. We also thank Drs. S. Kawasoko, K. Suzuki, and M. Kawahara for technical assistance, and K. Yamashita for secretarial assistance.
References
- Marks S.C., Jr., Lane P.W. Osteopetrosis, a new recessive skeletal mutation on chromosome 12 of the mouse. J. Hered. 1976;67:11–18. doi: 10.1093/oxfordjournals.jhered.a108657. [DOI] [PubMed] [Google Scholar]
- Marks S.C., Jr. Morphological evidence of reduced bone resorption in osteopetrotic (op) mice. Am. J. Anat. 1982;163:157–167. doi: 10.1002/aja.1001630205. [DOI] [PubMed] [Google Scholar]
- Wiktor-Jedrzejczak W., Ahmed A., Szczylik C., Skelly R.R. Hematological characterization of congenital osteopetrosis in op/op mouse. J. Exp. Med. 1982;156:1516–1527. doi: 10.1084/jem.156.5.1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H., Hayashi S., Kunisada T., Ogawa M., Nishikawa S., Okumura H., Sudo T., Shultz L.D., Nishikawa S.-I. The murine mutation osteopetrosis is in the coding region of the macrophage colony stimulating factor gene. Nature. 1990;345:442–444. doi: 10.1038/345442a0. [DOI] [PubMed] [Google Scholar]
- Felix R., Cecchini M.G., Fleisch H. Macrophage colony-stimulating factor restores in vivo bone resorption in the op/op osteopetrotic mouse. Endocrinology. 1990;127:2592–2594. doi: 10.1210/endo-127-5-2592. [DOI] [PubMed] [Google Scholar]
- Kodama H., Yamasaki A., Nose M., Niida S., Ohgame Y., Abe M., Kumegawa M., Suda T. Congenital osteoclast deficiency in osteopetrotic (op/op) mice is cured by injections of macrophage colony-stimulating factor. J. Exp. Med. 1991;173:269–272. doi: 10.1084/jem.173.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiktor-Jedrzejczak W., Urbanowska E., Aukerman S.L., Pollard J.W., Stanley E.R., Ralph P., Ansari A.A., Sell K.W., Szperl M. Correction by CSF-1 of defects in the osteopetrotic op/op mouse suggests local, developmental, and humoral requirements for this growth factor. Exp. Hematol. 1991;19:1049–1054. [PubMed] [Google Scholar]
- Sundquist K.T., Cecchini M.G., Marks S.C., Jr. Colony-stimulating factor-1 injections improve but do not cure skeletal sclerosis in osteopetrotic (op) mice. Bone. 1995;16:39–46. [PubMed] [Google Scholar]
- Kodama H., Nose M., Niida S., Yamasaki A. Essential role of macrophage colony-stimulating factor in the osteoclast differentiation supported by stromal cells. J. Exp. Med. 1991;173:1291–1294. doi: 10.1084/jem.173.5.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofstetter W., Wetterwald A., Cecchini M.C., Felix R., Fleisch H., Mueller C. Detection of transcripts for the receptor for macrophage colony-stimulating factor, c-fms, in murine osteoclasts. Proc. Natl. Acad. Sci. USA. 1992;89:9637–9641. doi: 10.1073/pnas.89.20.9637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begg S.K., Radley J.M., Polland J.W., Chisholm O.T., Stanley E.R., Bertoncello I. Delayed hematopoietic development in osteopetrotic (op/op) mice. J. Exp. Med. 1993;177:237–242. doi: 10.1084/jem.177.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama H., Yamasaki A., Abe M., Niida S., Hakeda Y., Kawashima H. Transient recruitment of osteoclasts and expression of their function in osteopetrotic (op/op) mice by a single injection of macrophage colony-stimulating factor. J. Bone Miner. Res. 1993;8:45–50. doi: 10.1002/jbmr.5650080107. [DOI] [PubMed] [Google Scholar]
- Niida S., Amizuka N., Hara F., Ozawa H., Kodama H. Expression of Mac-2 antigen in the preosteoclast and osteoclast identified in the op/op mouse injected with macrophage colony-stimulating factor. J. Bone Miner. Res. 1994;9:873–881. doi: 10.1002/jbmr.5650090613. [DOI] [PubMed] [Google Scholar]
- MacDonald B.R., Mundy G.R., Clark S., Wang E.A., Kuehl T.J., Stanley E.R., Roodman D. Effects of recombinant CSF-GM and highly purified CSF-1 on the formation of multinucleated cells with osteoclast characteristics in long-term bone marrow cultures. J. Bone Miner. Res. 1986;1:227–233. doi: 10.1002/jbmr.5650010210. [DOI] [PubMed] [Google Scholar]
- Kurihara N., Suda T., Miura Y., Nakauchi H., Kodama H., Hiura K., Hakeda Y., Kumegawa M. Generation of osteoclasts from isolated hematopoietic progenitor cells. Blood. 1989;74:1295–1302. [PubMed] [Google Scholar]
- Takahashi N., Udagawa N., Akatsu T., Tanaka H., Shionome M., Suda T. Role of colony-stimulating factors in osteoclast development. J. Bone Miner. Res. 1991;6:977–985. doi: 10.1002/jbmr.5650060912. [DOI] [PubMed] [Google Scholar]
- Wiktor-Jedrzejczak W., Urbanowska E., Szperl M. Granulocyte-macrophage colony-stimulating factor corrects macrophage deficiencies, but not osteopetrosis, in the colony-stimulating factor-1-deficient op/op mouse. Endocrinology. 1994;134:1932–1935. doi: 10.1210/endo.134.4.8137761. [DOI] [PubMed] [Google Scholar]
- Nilsson S.K., Lieschke G.J., Garcia-Wijnen C.C., Williams B., Tzelepis D., Hodgson G., Grail D., Dunn A.R., Bertoncello I. Granulocyte-macrophage colony-stimulating factor is not responsible for the correction of hematopoietic deficiencies in the maturing op/op mouse. Blood. 1995;86:66–72. [PubMed] [Google Scholar]
- Myint Y.Y., Miyakawa K., Naito M., Shultz L.D., Oike Y., Yamamura K.-I., Takahashi K. Granulocyte/macrophage colony-stimulating factor and interleukin-3 correct osteopetrosis in mice with osteopetrosis mutation. Am. J. Pathol. 1999;154:553–566. doi: 10.1016/s0002-9440(10)65301-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo K., Hiratsuka S., Subbalakshmi E., Matsushime H., Shibuya M. Genomic organization of the flt-1 gene encoding for vascular endothelial growth factor (VEGF) receptor-1 suggests an intimate evolutionary relationship between the 7-Ig and the 5-Ig tyrosine kinase receptors. Gene. 1998;208:297–305. doi: 10.1016/s0378-1119(98)00006-7. [DOI] [PubMed] [Google Scholar]
- Neufeld G., Cohen T., Gengrinovitch S., Poltorak Z. Vascular endothelial growth factor (VEGF) and its receptors. FASEB J. 1999;13:9–22. [PubMed] [Google Scholar]
- Berleon B., Sozzani S., Shou D., Weich H.A., Mantovani A., Marmé D. Migration of human monocytes in response to vascular endothelial growth factor (VEGF) is mediated via the VEGF receptor flt-1. Blood. 1996;87:3336–3343. [PubMed] [Google Scholar]
- Clauss M., Weich H., Breier G., Knies U., Röckl W., Waltenberger J., Risau W. The vascular endothelial growth factor receptor Flt-1 mediates biological activities. Implication for a functional role of placenta growth factor in monocyte activation and chemotaxis. J. Biol. Chem. 1996;271:17629–17634. doi: 10.1074/jbc.271.30.17629. [DOI] [PubMed] [Google Scholar]
- Park J.E., Chen H.H., Winer J., Houck K.A., Ferrara N. Placenta growth-factor. Potentiation of vascular endothelial growth-factor bioactivity, in vitro and in vivo, and high affinity binding to Flt-1 but not to Flk-1/KDR. J. Biol. Chem. 1994;169:25646–25654. [PubMed] [Google Scholar]
- Sawano A., Takahashi T., Yamaguchi S., Aonuma M., Shibuya M. Flt-1 but not KDR/Flk-1 tyrosine kinase is a receptor for placenta growth factor, which is related to vascular endothelial growth factor. Cell Growth Differ. 1996;7:213–221. [PubMed] [Google Scholar]
- Hiratsuka S., Minowa O., Kuno J., Noda T., Shibuya M. Flt-1 lacking the tyrosine kinase domain is sufficient for normal development and angiogenesis in mice. Proc. Natl. Acad. Sci. USA. 1998;95:9349–9354. doi: 10.1073/pnas.95.16.9349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudo T., Nishikawa S., Ogawa M., Kataoka H., Ohno N., Izawa A., Hayashi S.-I., Nishikawa S.-I. Hierarchical relation of c-kit and c-fms in intramarrow production of CFU-M. Oncogene. 1995;11:2469–2476. [PubMed] [Google Scholar]
- Kataoka H., Takakura N., Nishikawa S., Tsuchida K., Kodama H., Kunisada T., Risau W., Kita T., Nishikawa S.-I. Expression of PDGF receptor alpha, c-Kit and Flk1 genes clustering in mouse chromosome 5 define distinct subsets of nascent mesodermal cells. Dev. Growth Differ. 1997;39:729–740. doi: 10.1046/j.1440-169x.1997.t01-5-00009.x. [DOI] [PubMed] [Google Scholar]
- Ly I.A., Mishell R.I. Separation of mouse spleen cells by passage through column of Sephadex G-10. J. Immunol. Methods. 1974;5:239–247. doi: 10.1016/0022-1759(74)90108-2. [DOI] [PubMed] [Google Scholar]
- Amano H., Yamada S., Felix R. Colony-stimulating factor-1 stimulates the fusion process in osteoclasts. J. Bone Miner. Res. 1998;13:846–853. doi: 10.1359/jbmr.1998.13.5.846. [DOI] [PubMed] [Google Scholar]
- Yasuda H., Shima N., Nakagawa N., Yamaguchi K., Kinosaki M., Mochizuki S.-I., Tomoyasu A., Yano K., Goto M., Murakami A. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc. Natl. Acad. Sci. USA. 1998;95:3597–3602. doi: 10.1073/pnas.95.7.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey D.L., Timms E., Tan H.L., Kelley M.J., Dunstan C.R., Burgess T., Elliott R., Colombero A., Elliott G., Scully S. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165–176. doi: 10.1016/s0092-8674(00)81569-x. [DOI] [PubMed] [Google Scholar]
- Hamilton W.J., Mossman H.W. Hamilton, Boyd and Mossman's Human Embryology 4th ed 1976. The Macmillan Press Ltd; London: pp. 526–547 [Google Scholar]
- Wang D.S., Miura M., Demura H., Sato K. Anabolic effects of 1,25-dihydroxyvitamin D3 on osteoclasts are enhanced by vascular endothelial growth factor produced by osteoclasts and by growth factors produced by endothelial cells. Endocrinology. 1997;138:2953–2962. doi: 10.1210/endo.138.7.5275. [DOI] [PubMed] [Google Scholar]
- Carmeliet P., Ferreira V., Breier G., Pollefeyt S., Kieckens L., Gertsenstein M., Fahrig M., Vandenhoeck A., Harpal K., Eberhardt C. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380:435–439. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- Ferrara N., Carver-Moore K., Chen H., Dowd M., Lu L., O'Shea K.S., Powell-Braxton L., Hillan K.J., Moore M.W. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 1996;380:439–442. doi: 10.1038/380439a0. [DOI] [PubMed] [Google Scholar]
- Fong G.H., Rossant J., Gertsenstein M., Breitman M.L. Role of the Flt-1 receptor tyrosine kinase in regulating the assembly of vascular endothelium. Nature. 1995;376:66–70. doi: 10.1038/376066a0. [DOI] [PubMed] [Google Scholar]