Abstract
Interaction between a T cell receptor (TCR) and various ligands, i.e., anti-TCR antibodies, superantigens, peptides, or altered peptide ligands in the context of major histocompatibility complex (MHC) molecules can trigger different T helper cell (Th) effector functions. Herein, we studied the T cell response induced by a soluble, dimeric peptide/MHC class II chimera, namely hemagglutinin (HA)110-120/I-Edαβ/Fcγ2a (DEF). We have previously demonstrated that the soluble DEF molecule binds stably and specifically to HA110-120–specific TCRs expressed by a T cell hybridoma. Administration of DEF in vivo induced differentiation of resting and activated peptide-specific T cells toward a Th2 response, as indicated by the increase of interleukin (IL)-4, IL-10, and specific immunoglobulin (Ig)G1 antibodies and decrease of IL-2, specific IgG2a antibodies, and cytotoxic T lymphocyte activity. In contrast to HA110-120 peptide presented by the DEF molecule to T cells, the nominal synthetic peptide induced a predominant Th1 response, and the PR8 virus–derived HA110-120 peptides induced a mixed Th1/Th2 response. Independent of antigen processing, soluble DEF was almost 2 logs more potent in stimulating cognate T cells than the nominal peptide. Polarization of cognate T cells toward the Th2 response occurred upon interaction of soluble DEF with TCR and CD4 molecules followed by early activation of p56lck and ZAP-70 tyrosine kinases, and negative signaling of the signal transducer and activator of transcription (STAT)4 pathway of Th1 differentiation. DEF-like molecules may provide a new tool to study the mechanisms of signaling toward Th2 differentiation and may also provide a potential immunotherapeutic approach to modulate autoreactive T cells toward protective Th2 immune responses.
Keywords: Th2 differentiation, peptide/MHC II chimera, STAT proteins
Recent investigations on the functionality of CD4 T cell–derived Th1 and Th2 responses have disclosed new information on the pathogenesis of several infectious and autoimmune diseases. The Th1 response is highly protective against intracellular parasites 1 2 and acute allograft rejection 3 4, whereas the Th2 response protects against organ-specific autoimmune diseases such as thyroiditis, insulin-dependent diabetes mellitus, multiple sclerosis, and Crohn's disease 5. Although the genetic mechanisms responsible for Th1 or Th2 development remain elusive, the environmental factors, i.e., route of antigen administration, dose of antigen, type of APCs, and the type of adjuvants have been shown to play a critical role 6.
The primum movens toward Th differentiation depends on the type of cytokines present in the microenvironment 7. The IL-4 required for Th2 differentiation can be provided by a subset of CD4 NK1.1+ cells 8, naive CD4 T cells after stimulation with IL-6 produced by APCs 9, or progesterone 4. In a like manner, the IFN-γ required for Th1 development can be provided by a subset of NK cells, CD8 T cells, or naive CD4 T cells upon stimulation with IL-12 secreted by dendritic cells, macrophages, and neutrophils 10. Binding of cytokines to their cognate receptors induces phosphorylation-mediated activation of multiple signaling molecules, including the signal transducers and activators of transcription (STATs)1 11. Activated STATs rapidly translocate to the nucleus as homodimers or heterodimers and bind to cis-acting elements of the cytokine promoter genes with concurrent gene activation and augmentation of cytokine production. Whereas signaling of IL-12Rβ1β2 complex by IL-12 induces STAT4-dependent IFN-γ secretion with consequent Th1 differentiation 12, signaling of IL-4Rα by IL-4 induces STAT6-dependent IL-4 secretion with consequent Th2 differentiation 13.
Among several experimental approaches aimed at modulating T cell responses, such as immobilized anti-TCR mAb 14 or altered peptide ligands 15, the peptide/MHC molecules have been considered an attractive alternative. MHC class II molecules extracted from cell membranes and loaded in vitro with autoreactive peptides were able to ameliorate allergic encephalomyelitis and myasthenia gravis in animal models 16 17. Recently, several variants of peptides covalently linked to MHC class II molecules have been genetically engineered 18 19 20 21. Soluble monomers and plastic-immobilized dimers of peptide/MHC II chimeras were shown to activate cognate T cells 18 20 21. To our knowledge, no data are available relative to the immunomodulatory effects of soluble multimeric forms of peptide/MHC II chimeras. Herein, we provide evidence that a genetically engineered, soluble dimeric peptide/MHC class II/Fc chimera (DEF) can polarize resting and activated CD4 T cells toward Th2 response after interaction with TCR and CD4 molecules and subsequent negative regulation of the STAT4 pathway of Th1 differentiation.
Materials and Methods
Mice.
BALB/c mice were obtained from The Jackson Laboratory. Transgenic (Tg) BALB/c mice express the 14.3d TCR-α/β specific for hemagglutinin (HA)110-120 in the context of I-Ed class II molecules.
Antigens.
The CD4 T cell epitope HA110-120 of influenza virus PR8/A/8/34 HA 22 and NP147-154 of influenza virus PR8/A/8/34 23 were prepared by solid phase Fmoc technology and purified by reverse phase HPLC on a C2/C18 column (Amersham-Pharmacia Biotech). Purity of the synthetic peptides was assessed by amino acid sequencing in the Protein Core Facility at Mount Sinai School of Medicine. The DEF molecule consists of the I-Edα and I-Edβ extracellular domains that were dimerized through a murine Fcγ2a fragment at the COOH termini of I-Edβ chain 20. The HA110-120 (SFERFEIFPKE) CD4 T cell epitope of HA of influenza virus A/PR/8/34 was covalently linked at the NH2 terminus of I-Edβ chains as previously described and designated DEF 20. Recombinant DEF protein was produced in the baculovirus/SF9 insect cell system and purified by affinity chromatography on goat anti–γ2a–Sepharose column as described 20. MOPC 173 myeloma cells secrete an IgG2a that was used in experiments as an isotype control for the Fcγ2a portion of the DEF molecule. A BSA–HA110-120 conjugate was prepared using the heterobifunctional cross-linker 1-ethyl-3-(3-dimethyl aminopropyl) carbodiimide (Imject Immunogen EDC Conjugation kit; Pierce Perstorp Biotech Co.) according to the manufacturer's instructions. The conjugate was purified by size exclusion chromatography on a Superose-6 column (Amersham-Pharmacia Biotech). Influenza A/PR/8/34 virus was used for immunization of BALB/c mice and for measurement of the antiviral isotypes in these mice by RIA. The virus was grown in the allantoic fluid of chicken eggs and then purified on sucrose gradient according to standard procedures. Chemicals were purchased from Sigma Chemical Co. unless noted otherwise.
Cells.
TCR-HA (TCR recognizing HA110-120 peptide in context of I-Ed class II molecules) T cells were purified from the spleens of Tg mice by Ficoll-Hypaque gradient centrifugation followed by two passages on nylon wool columns (Unisorb T&B; Nycomed Pharmaceuticals) and incubation for 2 h at 37°C in plastic dishes. The nonadherent cells were collected and measured for purity by FACS™ analysis (Becton Dickinson) using 2C11 anti-CD3∈ mAb (American Type Culture Collection [ATCC]) conjugated with PE and 6.5.2 clonotypic anti–TCR-HA mAb conjugated with FITC. Such preparations contained 95% CD3+ cells, of which 32% expressed the TCR-HA transgene. P-815 is a mouse mastocytoma cell line expressing MHC class I H-2d and lacking class II molecules (ATCC). 14-3-1 T cell hybridoma (TcH) expresses the 14.3d TCR-α/β that recognizes HA110-120 peptide in the context of class II I-Ed molecules 24.
T Cell Proliferation Assays.
The proliferative response of T cells upon incubation with various antigens was measured by a thymidine incorporation assay. In brief, splenic cells or purified TCR-HA T cells (5 × 105) were incubated for 72 h at 37°C in the presence or absence of various antigens. Tritiated thymidine (1 μCi) was added for the last 24 h, cells were harvested on filter papers (Skatron Inc.), and radioactivity was measured in a β-scintillation chamber (Amersham-Pharmacia Biotech).
CTL Assays.
Spleen cells from PR8-immunized BALB/c mice treated or untreated with antigens on days 7, 8, and 9 after immunization were collected on day 10 after immunization and then stimulated for 5 d in vitro with NP147-154 peptide (2 μg/2 × 106 cells/ml) and then incubated for 4 h at 37°C in 96-well V-bottomed plates at various ratios with P-815 target cells labeled with 51Cr and coated with NP147-154 peptide (20 μg/106 cells/ml). Cell culture supernatants were measured for the amount of 51Cr release in a gamma counter (Amersham-Pharmacia Biotech), and the results were computed as percentage of cytotoxicity in triplicate samples for each E/T ratio as follows: (experimental release − spontaneous release)/(maximum release − spontaneous release) × 100. Noncoated P-815 target cells were used to determine 51Cr spontaneous release. Maximum 51Cr release refers to the amount of 51Cr liberated from noncoated target cells upon lysis with 5% Triton X-100.
FACS™ Analyses.
106 cells in 1% PBS/BSA were singly or doubly stained with various mAbs conjugated with fluorochrome (1–2 μg/106 cells/ml; PharMingen) for 30 min on ice, washed with 3% BSA in PBS, and then fixed with 1% paraformaldehyde in PBS. The fluorescence intensity was analyzed in an EPICS PROFILE II Analyzer (Coulter Corp.).
Treatment of Mice with DEF Molecules.
The effects of DEF were determined in vivo using BALB/c mice inoculated with 3.0 × 107 TCR-HA Tg T cells that were injected 24 h later with 130 μg, i.v. of DEF or MOPC 173 protein control (single dose injection) or with 390 μg of DEF or MOPC 173 (three injections at 1-d intervals) in 200 μl PBS. Spleen cells (106) were isolated 1 and 6 d after the last injection. The proliferative response to HA110-120 peptide and the amount of IL secreted in culture supernatants were determined by [3H]TdR incorporation and ELISA (Cytoscreen Immunoassay; Biosource International, Inc.), respectively. To analyze DEF specificity in vivo, BALB/c mice were immunized i.p. with 100 μg of OVA in PBS. 9 d later, mice were injected intravenously at 24-h intervals with three 130-μg doses of either DEF or MOPC 173 protein, and spleen cells were collected 3 d after the last injection to measure the proliferative response and cytokine production upon in vitro challenge with OVA (10 μg/ml). The effect of DEF on Ab response was investigated in BALB/c mice previously immunized with 5 μg, i.p. of live influenza virus PR8/A/8/34 in PBS and then injected on days 7, 8, and 9 after immunization with 130 μg, i.v. of DEF or PBS. Blood samples were collected on days 0, 7, 8, 9, and 14 after immunization, and the titers of anti-PR8 isotypes were determined by RIA on individual mice sera. In brief, 96-well plates coated with 5 μg/ml PR8 virus were incubated for 2 h at 37°C with serum samples diluted 1:100 with 1% BSA in PBS. Plates were washed with PBS/0.05% Tween 20 and incubated for 2 h at 37°C with 50 μl of rabbit anti–mouse isotypes (mouse isotyping kit; Bio-Rad Labs.), washed with PBS/0.05% Tween 20, and then incubated for 2 h at room temperature with 125I-goat anti–rabbit IgG Ab (5 × 104 cpm/well). Bound Abs were measured as cpm in a gamma counter (Pharmacia LKB).
Detection of Phosphorylated Proteins.
Phosphorylation of p56lck, ZAP-70, STAT4, and STAT6 proteins was analyzed in total cell lysates. To detect phosphorylated p56lck and ZAP-70, 2.0 × 107 TcH cells were treated for 5 min with 5 μg/ml soluble DEF or medium alone. DEF was then removed by centrifugation, and the cell pellets were lysed for 30 min on ice with 2 ml of RIPA buffer, pH 7, containing 1% NP-40, 10 mM Tris, 150 mM NaCl, 5 mM NaF, 2 mM Na3VO4, 5 mM Na pyrophosphate, and a cocktail of protease inhibitors (Complete™; Boehringer Mannheim). To detect phosphorylation of STAT4 and STAT6, TCR-HA Tg spleen cells (6.0 × 107) were incubated for 48 h at 37°C with 5 μg/ml of HA110-120 peptide, soluble DEF, anti-CD3∈ mAb 2C11, or clonotypic anti–TCR-HA 6.5.2 mAb. T cells were purified to >95% CD3+ T cells as described and stimulated for 30 min at 37°C with either mouse recombinant IL-4 (40 ng/ml−1) or IL-12 (1 ng/ml−1) (Sigma Chemical Co.). Furthermore, TcH or TCR-HA Tg cells were washed with PBS and lysed with RIPA buffer, and 100 μl of total cellular lysates (0.2 mg protein) was immunoprecipitated overnight at 4°C with 10 μg of one of the following rabbit Abs: anti-p56lck (2102-G), specific for the COOH terminus; anti–ZAP-70 (LR), specific for an amino acid sequence mapping within the “linker” region of ZAP-70 in the case of TcH; anti-STAT4 (C-20), specific for a region at the COOH terminus of mouse origin; or anti-STAT6 (M-200), specific for the amino acid region 280–480 of mouse origin (Santa Cruz Biotechnology) in the case of purified TCR-HA T cells. The immunoprecipitates were incubated for 2 h at room temperature with 20 μl of 50% slurry of agarose–protein A/G (Santa Cruz Biotechnology). The agarose–protein A/G–Ab–protein complexes were washed twice with lysis buffer and then boiled for 5 min in 1% SDS buffer and 5% 2-ME. Samples were electrophoresed in a 10–15% gradient of polyacrylamide, electrotransferred onto PVDF (polyvinylidene difluoride) membranes, blocked with 3% BSA, and probed with RC20 antiphosphotyrosine mAb–horseradish peroxidase (HRP) conjugate (1:10,000; Transduction Labs.). Bound Ab–enzyme conjugates were revealed by chemiluminescence using the Supersignal® substrate for HRP (Pierce Chemical Co.) as indicated by the manufacturer. To identify the protein of interest, PVDF membranes developed with RC20–HRP conjugate were stripped with 0.1% SDS/1% guanidine hydrochloride for 30 s and reprobed for 2 h at room temperature with 10 μg/ml of rabbit Abs specific for p56lck, ZAP-70, STAT4, or STAT6. Membranes were washed with 0.1 M Tris/HCl/PBS/0.05% Tween 20, pH 7.5, and bound Abs were revealed by chemiluminescence using goat anti–rabbit IgG Ab–HRP conjugate (Santa Cruz Biotechnology).
Results
Soluble DEF Is a Potent Th2 Stimulatory Molecule.
We have previously shown that a soluble, dimeric HA110-120–I-Ed/Fc chimera (DEF) binds stably and specifically to HA110-120–specific 14.3d TCR-α/β expressed by 14-3-1 TcH cells 20. Herein, we investigated the T cell response to soluble DEF molecules. Using a protocol aimed at determining the immunopotency of HA110-120 peptide expressed by various protein carriers 25, we found that soluble DEF is ∼88 times more potent in stimulating cognate T cells than the nominal peptide. Thus, half-maximal proliferation of spleen cells from naive TCR-HA Tg mice as determined by [3H]thymidine incorporation assay was obtained with 4.5 nM peptide carried by DEF or with 400 nM synthetic peptide. Inhibition of antigen processing with 50 μM chloroquine did not significantly reduce the stimulatory capacity of HA110-120 synthetic peptide (12%) and DEF (17%) (data not shown). This was expected in the case of HA110-120 peptide, because stimulation of T cells by synthetic peptides does not require processing.
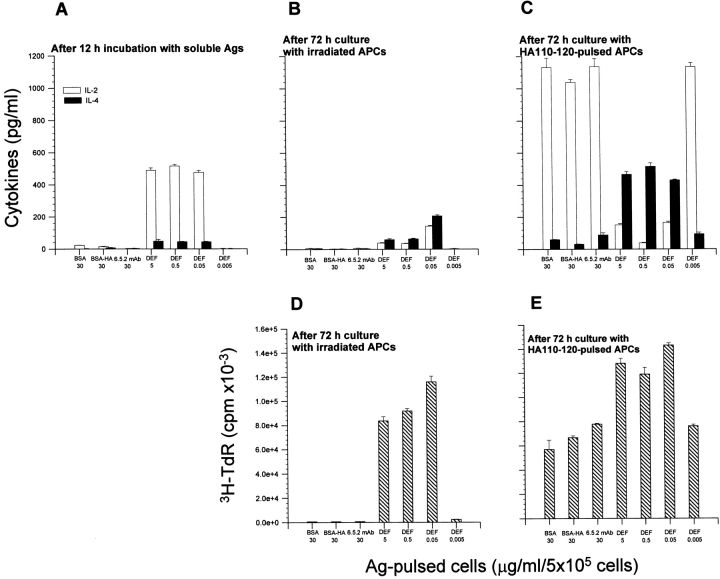
As antigen processing did not significantly affect the stimulatory capacity of soluble DEF, we next analyzed the nature of DEF interaction with the cognate T cells using a two-step in vitro culture system. In the first step, purified resting T cells were incubated for 12 h with various doses of soluble DEF, BSA–HA110-120 conjugate, soluble anti-TCR clonotypic 6.5.2. mAb, or medium alone. Only T cells treated with soluble DEF secreted IL-2 (213–510 pg/ml; Fig. 1 A). Lack of IL-2 secretion upon incubation with BSA–HA110-120 conjugate in the purified T cell preparation confirmed the absence of APCs, which are required for the processing of conjugate and presentation of peptides to T cells. It is noteworthy that the same magnitude of IL-2 secretion was observed with 0.05–5 μg/ml DEF but not with 0.005 μg/ml DEF. Also, lack of IL-2 secretion upon incubation with a soluble clonotypic anti-TCR mAb revealed its inability to stimulate T cells (Fig. 1 A). Removal of antigens from the cell culture and addition of irradiated syngeneic APCs (2,200 rads) for 72 h in the second step of the assay augmented IL-4 secretion and fostered proliferation only in 5–0.05 μg/ml of DEF-pretreated T cells. In contrast, T cells pretreated with BSA–HA110-120 conjugate, clonotypic anti-TCR mAb, or the lowest dose of DEF (0.005 μg/ml) did not secrete cytokines and did not proliferate (Fig. 1B and Fig. D). Addition of HA110-120–pulsed APCs to the cultures enhanced proliferation (Fig. 1 E) and secretion of IL-4 up to two to three times only in DEF-pretreated T cells (Fig. 1 C). Under the same culture conditions, T cells pretreated with BSA–HA110-120 conjugate, clonotypic anti-TCR mAb, or 0.005 μg/ml DEF secreted mainly IL-2 and proliferated (Fig. 1C and Fig. E). These results indicate that the interaction of resting T cells with soluble DEF, but not with a soluble clonotypic anti-TCR mAb, induced efficient IL-4–dependent proliferation. IL-4 is a potent mitogen in T cells via IL-4 nuclear activating factor 26. As little as 0.005 μg/ml DEF was unable to trigger a Th2 response, as no proliferative response or cytokine production was detected at this dose. Most likely, the Th1 response obtained in T cells treated with 0.005 μg/ml DEF and then challenged with HA110-120–pulsed APCs was the result of further stimulation with HA110-120–pulsed APCs.
Figure 1.
Effects of soluble DEF on the proliferative and cytokine responses of the purified resting TCR-HA T cells. Purified TCR-HA Tg T cells (5 × 105) were incubated for 12 h at 37°C with BSA–HA110-120 (30 μg/ml) conjugate, 6.5.2 anti-TCR clonotypic mAb, and various amounts of soluble DEF. (A) Cytokine production (pg/ml) of T cells after 12-h incubation with Ags in the absence of peptide and APCs. (B and D) cytokine production and proliferative response, respectively, of T cells pretreated with Ags as in A and then cultured for 72 h in the presence of irradiated (2,200 rads) naive BALB/c spleen cells as APCs (105). (C and E) Cytokine production and proliferative response, respectively, of T cells pretreated with Ags as in A and then cultured for 72 h in the presence of irradiated (2,200 rads) naive BALB/c spleen cells as APCs (105) and HA110-120 peptide (5 μg/ml). Values represent the mean of triplicate samples ± SD.
To determine the effect of soluble DEF on resting T cells in vivo, BALB/c mice were inoculated with TCR-HA T cells from nonimmunized Tg mice and then injected with 390 μg, i.v. of DEF or MOPC 173 as a control administered at 24-h intervals as 130-μg injections. Spleen cells from mice injected with DEF proliferated upon challenge in vitro with HA110-120 peptide (Fig. 2 A) and showed the following kinetics of cytokine secretion: IL-2 followed an ascending pattern in control animals, while descending on day 3 in all DEF-treated mice (Fig. 2 B); IL-4 was barely detected on days 2 and 3 in control animals and significantly elevated on day 2 in all DEF-treated animals (Fig. 2 C), and IL-10 was considerably increased in DEF-treated mice on day 3 as compared with the control mice (Fig. 2 D). Similar results were obtained with a single dose of DEF (130 μg), although the magnitude of the proliferative response and cytokine production was slightly lower (data not shown). The results clearly indicate that a quite wide range of DEF doses (5–0.05 μg/ml) was able to induce differentiation of resting, peptide-specific T cells toward a Th2 phenotype.
Figure 2.
T cell proliferative response and cytokine production of spleen cells from mice injected with DEF. BALB/c mice adoptively transferred with TCR-HA Tg T cells (3.0 × 107) were injected three times at 24-h intervals with 130 μg/ml DEF or MOPC 173 protein control. 24 h after the last injection, spleen cells (5 × 105) were collected and challenged in vitro with HA110-120 peptide (5 μg/ml), and thymidine incorporation was determined in individual mice (A). Values represent the mean of triplicate wells ± SD (cpm). In the same culture system, the levels of IL-2, IL-4, and IL-10 were determined by ELISA 2 and 3 d after challenge with peptide (B, C, and D, respectively). Values are expressed in pg/ml for individual mice. The percentage of TCR-HA T cells in spleen cells was determined by FACS™ analysis using 6.5.2–FITC conjugate as previously described 20. Animals injected with MOPC 173 protein control showed 30% of TCR-HA T cells in mouse no. 1 and 33% in mouse no. 2, and in those injected with DEF, 44% in mouse no. 1, 43% in mouse no. 2, 41% in mouse no. 3, and 37% in mouse no. 4.
We next analyzed the fate of activated T cells upon interaction with soluble DEF. TCR-HA T cells stimulated with HA peptide and then restimulated with DEF or HA peptide showed a strong proliferative response, but only restimulation with DEF reversed the IL-2/IL-4 ratio, with an order of magnitude of ∼7 in favor of IL-4 (Table ). When BALB/c mice were immunized intraperitoneally with live influenza PR8 virus and then injected intravenously with DEF or MOPC 173 protein control, the HA110-120–specific proliferative response in mice treated with DEF was accompanied by a 30-fold increase in IL-4 production as compared with control mice (Table ). These data demonstrate that DEF polarized HA110-120–activated T cells into proficient IL-4 producers. As revealed in BALB/c mice immunized with OVA and then treated with DEF or MOPC 173 protein control, DEF-induced Th2 differentiation was restricted to HA110-120–specific T cells. The spleen cells from OVA-immunized mice that were or were not treated with DEF mounted a vigorous proliferative response to OVA in vitro and secreted comparable amounts of IL-2 and IL-4 (data not shown).
Table 1.
The Proliferative Response and Cytokine Production by Activated TCR-HA Tg T Cells Restimulated In Vitro with Soluble DEF or HA110-120 Peptide
| Restimulation with antigen | [3H]TdR | IL-2 | IL-4 | IL-2/IL-4 ratio |
|---|---|---|---|---|
| cpm | pg/ml | pg/ml | ||
| Nil | 59,469 ± 5,859 | 841 ± 12 | 35 ± 3 | 24.0 |
| HA110-120 (5 μg/ml) | 90,844 ± 5,019 | 2,331 ± 143 | 111 ± 8 | 21.4 |
| DEF (5 μg/ml) | 68,627 ± 4,706 | 774 ± 9 | 134 ± 10 | 5.7 |
TCR-HA Tg splenic cells (106/ml) were activated for 24 h in vitro with HA110-120 peptide, washed, and reincubated with HA110-120 peptide or DEF. Values indicate the thymidine incorporation (cpm ± SD) and the amounts of cytokines (pg/ml ± SD) determined 3 d after restimulation in vitro with Ags.
Table 2.
The Proliferative Response and Cytokine Production by Spleen Cells from Mice Immunized with Influenza PR8 Virus and Injected with DEF
| Mice immunized with PR8 virus and injected with | [3H]thymidineincorporation | IL-2 | IL-4 | IL-2/IL-4 ratio |
|---|---|---|---|---|
| cpm | pg/ml | pg/ml | ||
| MOPC 173 | 10,685 ± 546 | 147 ± 12 | 29 ± 7 | 4.7 |
| DEF | 26,945 ± 687 | 31 ± 4 | 960 ± 22 | 0.033 |
Spleen cells were collected from BALB/c mice immunized with live influenza PR8 virus and treated with DEF or MOPC 173 protein control. Spleen cells (106/ml) were challenged in vitro with HA110-120 peptide, and the thymidine incorporation (cpm ± SD) and amount of cytokines (pg/ml ± SD) was determined after 3 d culture. Background of proliferation was 1,267 ± 657 cpm.
Together, these data indicate that soluble, dimeric peptide/MHC II/Fc molecule (DEF) can polarize resting and activated peptide-specific T cells toward the Th2 immune response.
DEF Induces Th2 Bystander Effects In Vivo.
The recruitment and differentiation of CD8 T cells depends greatly on the availability of cytokines secreted by CD4 Th1 cells, particularly IL-2. We thus investigated the extent to which DEF-induced Th2 response in vivo may affect the cytolytic function of CD8 T cells. BALB/c mice immunized with live PR8 virus and then treated with DEF showed a 40% reduction of CTL activity against target cells coated with NP147-154 peptide (Fig. 3 A). NP147-154 is a dominant CD8 T cell epitope of the nucleoprotein of influenza virus A/PR/834 in H-2d mice 23. Reduced CTL activity was correlated with a double bystander effect: IL-2 deprivation and increase of IL-4 production. Th1-derived IL-2 is the major growth factor required for differentiation and activation of CD8 T cells 27. Indeed, we previously found that depletion of CD4 T cells in naive TCR-HA Tg mice resulted in lack of differentiation of CTLs 28. Increase of IL-4 after DEF treatment could also deprive CD8 T cells of IL-2, as it is known that IL-4 and IL-10 are potent suppressors of IL-2 synthesis 29.
Figure 3.
Effect of DEF on CTL and Ab responses. (A) NP147-154–specific CTL activity from mice immunized with live PR8 influenza virus and treated or untreated with DEF on days 7, 8, and 9 after immunization. Spleen cells collected on day 10 after immunization were stimulated in vitro for 5 d with NP147-154 peptide, and CTL activity was measured against P18 target cells coated with NP147-154 synthetic peptide. The percentage of cytotoxicity was determined in four mice and corresponds to the mean of triplicate samples (cpm) ± SD. (B) IgG2a and IgG1 anti-PR8 Ab titers in BALB/c mice previously immunized with live influenza PR8 virus and then injected three times with 130 μg of DEF or PBS on days 7, 8, and 9 after immunization. Values represent the mean cpm measured in triplicate blood samples ± SD collected from individual mice on days 0, 7, and 14 after immunization.
Th1 cells mediate IgG2a, and Th2 cells mediate IgG1 Ab response 30. We thus sought to analyze the effect of DEF-induced Th2 polarization on the IgG1 and IgG2a Ab responses in BALB/c mice immunized with live influenza PR8 virus. BALB/c mice immunized with live PR8 virus develop a mixed Th1/Th2-mediated Ab response 31. The HA of influenza PR8 virus bears major B cell epitopes 32 and the HA110-120 T cell immunodominant epitope that is recognized by CD4 T cells in the context of I-Ed class II molecules 22. Mice immunized with PR8 virus and treated with DEF developed higher IgG1 and lower IgG2a anti-PR8 responses than the control mice (Fig. 3 B). This can be explained by a double bystander effect mediated by elevated IL-4: enhanced transcription of γ1 constant region in the germline and inhibition of Th1-mediated IgG2a synthesis 30. It is likely that DEF inhibited virus-specific CTL activity in PR8-immunized BALB/c mice by a double bystander effect (IL-2 deprivation and IL-10–induced suppression), whereas it had no apparent bystander effect on the proliferative response of T cells nor on the cytokine profile in OVA-immunized BALB/c mice. This can be explained by a very low frequency of HA-specific T cells in OVA-immunized mice, demonstrating once more that DEF targeting ability is specific to T cells recognizing HA110-120 peptide in association with I-Edαβ class II molecules.
Interaction of Soluble DEF with Cognate T Cells Depends on both TCR and CD4 Molecules.
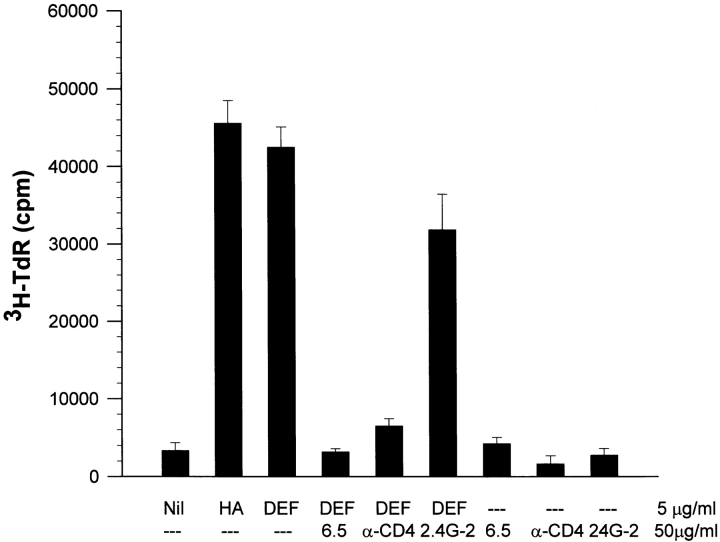
We previously demonstrated that DEF binds stably and specifically to 14.3d TCR-α/β, which recognizes HA110-120 peptide in the context of class II I-Edαβ molecules, and this binding was efficiently inhibited by anti-TCR clonotypic 6.5.2 mAb 20. We also showed that soluble DEF binds to FcγRIIβ on the surfaces of APCs 20. Herein, we investigated the role of DEF on the HA110-120–specific response upon its interaction with TCR, CD4, and FcγRIIb. Blocking of TCR with clonotypic 6.5.2 mAb or CD4 with GK1.5 mAb inhibited DEF-induced proliferation of TCR-HA Tg spleen cells. We and others have found that soluble 6.5.2 and GK1.5 mAbs have no effect on signaling T cells 20 33. Therefore, inhibition of DEF-induced proliferation by these Abs was the result of hindering DEF access to TCR and CD4 molecules. In contrast, blocking of FcγRIIβ on APCs with 2.4G-2 mAb showed a weak inhibitory effect on DEF-induced proliferation (Fig. 4). This indicated that the stimulation of T cells by soluble DEF molecules depends on its interaction with TCR and CD4 molecules and is little influenced by the binding of DEF to FcγR on APCs.
Figure 4.
Effects of various blocking Abs on DEF-induced proliferative response of cognate T cells. TCR-HA spleen cells (2 × 105) from naive Tg mice were incubated with various blocking Abs (50 μg/ml), isotype control Abs (data not shown), or medium alone. 30 min later, soluble DEF or HA110-120 synthetic peptide (5 μg/ml) was added to the cultures, and thymidine incorporation was determined after 72 h as described in the text. Values represent the mean of cpm measured in triplicate wells ± SD.
Interaction of Soluble DEF with TCR and CD4 Molecules Triggers Early Biochemical Events in Cognate T Cells.
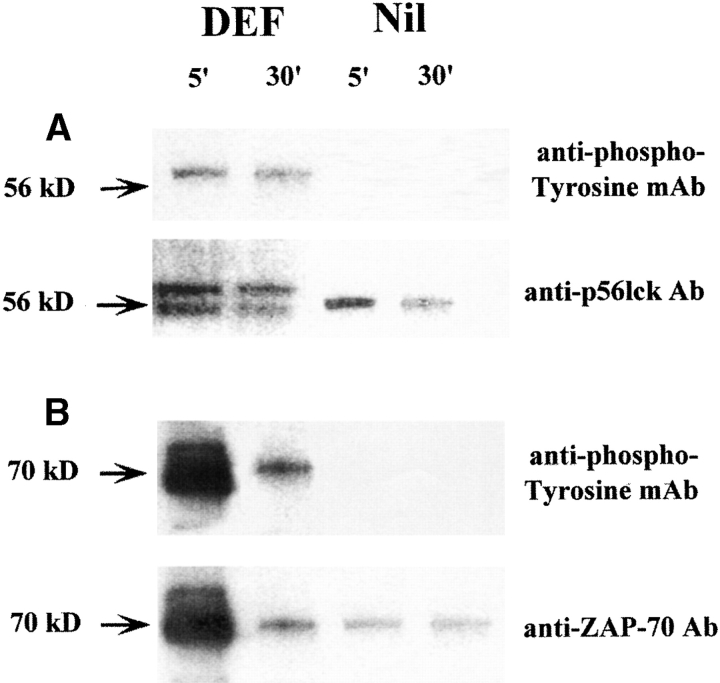
To analyze the nature of the interaction between soluble DEF and TCR/CD4, we investigated the phosphorylation of p56lck and ZAP-70 tyrosine kinases. p56lck associates with the cytoplasmatic domain of CD4 molecules 34 and undergoes autophosphorylation upon engagement of CD4 and CD3–TCR complex with the peptide/MHC ligands expressed on APCs 35. Phosphorylated p56lck translocates to the ITAM (immune receptor tyrosine-based activation motifs) of the CD3/TCR ζ chain and mediates recruitment and phosphorylation of ZAP-70, a tyrosine kinase expressed exclusively in T cells 35. Phosphorylation of p56lck and ZAP-70 is critical for triggering downstream stimulatory events in T cells 36. We found that 5-min exposure of 14-3-1 TcH to soluble DEF sufficed for phosphorylation of p56lck and ZAP-70, which persisted 30 min after treatment (Fig. 5). The results demonstrated that interaction of soluble DEF with the cognate TCR and CD4 molecules induced early transductional events in T cells.
Figure 5.
Phosphorylation of p56lck and ZAP-70 tyrosine kinases in T cells treated with DEF. TcH (2.0 × 107) were incubated for 5 min with 5 μg/ml of soluble DEF or medium alone (Nil), and total cellular lysates were electrophoretically separated as described. (A, top) Western blot of the immunoprecipitates developed with rabbit anti–p56lck Ab followed by RC20 antiphosphotyrosine mAb–HRP conjugate; bottom, the same membrane stripped and reprobed with rabbit anti–p56lck Ab followed by goat anti–rabbit IgG–HRP conjugate. The double band indicates the presence of both phosphorylated (upper band) and nonphosphorylated (lower band) p56lck in the lysates of cells treated with soluble DEF but not with medium alone. (B, top) Western blot of the immunoprecipitates obtained with rabbit anti–ZAP-70 Ab and developed with RC20 antiphosphotyrosine mAb; bottom, the same membrane stripped and reprobed with rabbit anti–ZAP-70 Ab followed by goat anti–rabbit IgG–HRP conjugate.
Interaction of Soluble DEF with TCR and CD4 Molecules Induces Negative Regulation of STAT4 Protein.
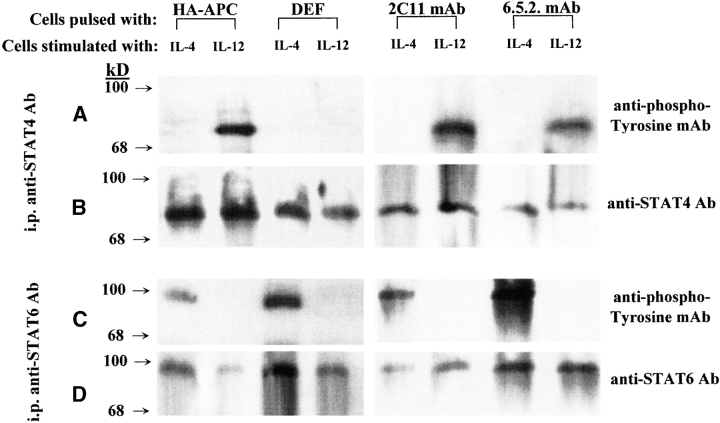
Among the signaling pathways mediated through the cytokine receptors, activation of the cytosolic latent STATs can rapidly modulate expression of genes responsible for Th differentiation 37. We investigated the extent to which critical STAT proteins involved in Th1 and Th2 differentiation may be affected upon stimulation with DEF. Naive TCR-HA Tg splenocytes were first incubated for 48 h with soluble DEF, HA110-120–pulsed APCs, soluble anti-CD3 mAb, or clonotypic anti–TCR-HA mAb. The phosphorylation-mediated activation of STAT4 and STAT6 proteins has been determined in the purified preparation of T cells, shortly after stimulation with IL-12 and IL-4, respectively. IL-12–induced phosphorylation of STAT4 was impaired in T cells treated with DEF but not in cells treated with other TCR ligands. Although not phosphorylated, STAT4 showed unaltered molecular size, indicating the lack of its proteolytic degradation (Fig. 6). In contrast, IL-4–induced phosphorylation of STAT6 was not affected in cells treated with any of the TCR ligands, including DEF. These results suggest that DEF-induced negative regulation of the STAT4 pathway of Th1 differentiation may have been potentiated by the STAT6 pathway of Th2 differentiation. A predominant Th1 response in cells treated with HA110-120–pulsed APCs may have been the result of the antagonist effect of STAT4 on the STAT6 pathway rather than a negative regulation of STAT6 pathways, because both STAT4 and STAT6 proteins were phosphorylated in these cells. STAT4 was shown to activate a genetic program able to antagonize the STAT6 pathway of Th2 differentiation, whereas no antagonistic effect of STAT6 on the STAT4 pathway has yet been described 12.
Figure 6.
Phosphorylation of STAT4 and STAT6 in T cells treated with DEF. TCR-HA Tg splenic cells were incubated for 48 h with HA110-120–pulsed APCs, soluble DEF, anti-CD3 mAb, or anti–TCR-HA clonotypic mAb. T cells were purified and stimulated with IL-4 or IL-12, and total cellular lysates were immunoprecipitated with rabbit anti–STAT4 or rabbit anti–STAT6 Abs as described. Samples were electrophoretically separated, electrotransferred onto PVDF membranes, and developed with RC20 antiphosphotyrosine mAb–HRP conjugate (A and C). To confirm STAT4 and STAT6 identity and to ensure that approximately equal amounts of proteins were present in the samples analyzed, membranes were stripped and reprobed with rabbit anti–STAT4 or anti–STAT6 Abs, and bound Abs were revealed by chemiluminescence using goat anti–rabbit IgG–HRP conjugate (B and D).
Discussion
In this study, we provide evidence that a soluble dimeric HA110-120–MHC class II molecule built on a Fcγ2a scaffold (DEF) can induce in vitro and in vivo differentiation of resting and activated peptide-specific T cells toward the Th2 response.
Gajewsky and colleagues proposed a model of T cell differentiation in which naive T cells secreting IL-2 upon primary stimulation in the absence of costimulation either undergo Th1 development or develop into Th0 cells secreting both IL-2 and IL-4 33 38. Further stimulation of Th0 cells in the presence of costimulation induces Th1 differentiation, whereas stimulation in the absence of costimulation induces a defect in IL-2 but not IL-4 production, leading to Th2 differentiation. Similarly, stimulation of naive HA-specific T cells with soluble DEF in the absence of APC-derived costimulatory signals induced IL-2 secretion and further differentiation toward the Th0 phenotype. In contrast, we found that the presence of APC-derived costimulatory signals augmented polarization of Th0 cells toward the Th2 rather than the Th1 phenotype. This may be explained by different stimuli used to prime the naive T cells, i.e., anti-CD3 mAb, which stimulates an alternative pathway of T cell activation, versus DEF, which stimulates the TCR pathway of T cell activation. At the time of this writing, the extent to which costimulation may have contributed during this stage of differentiation is under investigation. We also found that the presence of HA-pulsed APCs enhanced the IL-4 secretion of DEF-induced Th2 cells. This is consistent with the results of McKnight et al., demonstrating that stimulation of Th2 cells with peptide/MHC class II complexes on APCs enhanced IL-4 secretion 39. The authors showed that IL-4 secretion by the Th2 cells does not require costimulation. It is thus likely that increased IL-4 secretion detected in our system was induced mainly by the interaction of APC-derived HA/MHC complexes with HA-specific Th2 cells, rather than APC-derived costimulatory signals.
Goldstein and colleagues showed that the requirement for costimulation in activating naive CD8 T cells by MHC class I/peptide ligands can be circumvented by potent signaling of TCRs 40 41. Using a costimulation-free system, Boniface et al. demonstrated that the potency of TCR signaling by soluble peptide/MHC class II ligands parallels the increase in valency from two to four, whereas the monovalent forms are inefficient in stimulating T cells 42. The monomeric peptide/MHC class II molecules failed to activate T cells at 20,000 times higher concentration than the tetramers, despite the fact that TCR occupancy by the monomeric form was significantly higher. The authors suggested that multivalency-mediated TCR cross-linking, rather than occupancy of individually engaged TCRs, may play an essential role in triggering potent TCR signals by peptide/MHC class II complexes. We found that independent of antigen processing but in the presence of APC-derived costimulation, soluble bivalent DEF was 88 times more potent in stimulating T cells than the HA/MHC complexes expressed on APCs.
Soluble DEF induced a potent Th2 differentiation over a large range of doses in vitro (5–0.05 μg/ml) as well as in vivo (130 and 390 μg). For the in vivo experiments, MOPC 173 (γ2a) was used as specificity control for DEF bearing the exon encoding Cγ2a. Although MOPC 173 is a reasonable control, the ideal specificity control would be a DEF-like molecule in which an irrelevant peptide such as hen lysozyme 108–116 43 is linked to the β1 exon. Preparation of such a construct is underway in our laboratory, to be used in future experiments aimed at evaluating the effect of DEF in autoimmune diseases.
Hamad et al. showed that various doses of a plastic-immobilized dimeric peptide/MHC class II chimera similar to DEF induced a Th1-like response 21. Data suggest that not only valency but also the physical form of a dimeric HA/MHC ligand may dictate the outcome in Th effector functions. This may account for a different topology of TCR binding. Dimeric TCR ligands are able to cross-link TCR molecules, and presumably their soluble form can cross-link a larger number of TCRs, whereas surface immobilization can limit this process. High numbers of peptide/MHC TCR complexes can aggregate in large clusters by diffusion on the cell surface. Thus, different degrees of TCR clustering as induced by soluble versus immobilized forms of dimeric ligands may differentially signal the development of T cells.
The role of quantitative versus qualitative signaling of T cells at various stages of differentiation upon interaction with TCR ligands is controversial. It has been hypothesized that the nature of TCR ligation dictates conformational changes in the cytoplasmatic domains of the CD3/TCR complex 44, which in turn favors the engagement of different transductional pathways able to direct the development of T cells toward various effector functions 45. We found that the same HA110-120 CD4 T cell epitope of PR8 influenza virus induced different T cell responses: (a) a mixed Th1/Th2 response upon immunization with live PR8 virus, (b) a predominant Th1 response upon challenge with synthetic peptide in the context of I-Edαβ class II molecules on APCs, and (c) a Th2 response when the peptide was presented in the context of a soluble, dimeric peptide/MHC class II/Fc chimeric molecule. A soluble clonotypic anti–TCR-HA mAb was unable to stimulate the cognate T cells, suggesting that some conformational constraints cannot trigger productive signaling of T cells.
DEF-induced Th2 differentiation depended on the interaction of DEF with TCR and CD4 molecules. Viola et al. showed that Ab-mediated blocking of CD4 molecules precluded T cell activation 46. Consistent with this report, we found that interaction of soluble DEF with CD4 and TCR molecules was significantly inhibited by Ab-mediated blocking of CD4 and TCR molecules. Brown et al. showed that CD4 molecules preferentially potentiate Th2 differentiation 47. Although CD4 does not significantly stabilize the interaction of multimeric peptides/MHC class II chimeras with TCRs, the CD4 molecules are able to enhance the threshold of T cell activation 21 48. This is because signaling of the p56lck tyrosine kinase causes colocalization with CD4, which then rapidly undergoes autophosphorylation and translocates to the ITAM units on the ξ invariant chains of the CD3/TCR complex, where it mediates phosphorylation of ZAP-70 tyrosine kinase 35. We found that interaction of soluble DEF with TCR and CD4 molecules induced early phosphorylation of both p56lck and ZAP-70 tyrosine kinases. Both p56lck and ZAP-70 kinases are critical for triggering downstream events required for productive signaling of T cells.
Phosphorylation-mediated activation of STAT4 is a critical event for the development of Th1 cells 12, and phosphorylation-mediated activation of STAT6 is required for the development of Th2 cells 13. In contrast to various TCR ligands, such as HA110-120/MHC complexes expressed by APCs, anti-CD3 mAbs, and clonotypic anti-TCR mAbs, soluble DEF preferentially induced Th2 differentiation, which was correlated to negative regulation of the STAT4 protein. STAT4 could not be phosphorylated in T cells treated with DEF, although its structural integrity was unaltered. Lack of STAT4 phosphorylation may account for several mechanisms, including increased activity of protein tyrosine phosphatases, as previously demonstrated by Haspel et al. in the case of STAT1 protein 49. Phosphatases may rapidly dephosphorylate STAT4 or alternatively may dephosphorylate the docking site of STAT4 on IL-12R generated by the recruitment of JAK and Tyk upon stimulation with IL-12. Docking of STAT4 on the IL-12R chain is essential for its phosphorylation. Other mechanisms that may be accounted for by lack of STAT4 phosphorylation are competition for the STAT4 docking site by small proteins of the CIS/SOCS/JAB/SSSI family 50, which may have been upregulated upon DEF interaction with T cells, and downregulation or loss of IL-12R as the only docking site for STAT4 protein. Disregulation and immunodeficiency of IL-12R and/or IFN-γ-Rα chain leading to ineffective Th1 responses has been described in individuals with severe mycotic and bacterial infections 51 as well as in various types of carcinomas 52. Whether DEF-induced negative regulation of STAT4 may account for one of these regulatory mechanisms remains to be investigated.
DEF polarization toward a Th2 response was also detected in activated, peptide-specific T cells, in vitro as well as in mice preimmunized with live PR8 virus. Our working hypothesis is that the peptide- or PR8 virus–induced Th1 response was significantly diminished by negative regulation of STAT4, with compensatory augmentation of the STAT6 pathway of Th2 differentiation. The Th2 response induced by DEF was specific for TCR-HA T cells in vitro as well as in vivo. DEF-like molecules may provide a tool to dissect the intimate mechanisms of Th differentiation and perhaps lead to the development of more efficient immunotherapeutics in autoimmune diseases.
Acknowledgments
We thank Dr. Caton for the 6.5.2 anti-TCR clonotypic mAb and Dr. Harald von Boehmer for 14-3-1 TcH and TCR-HA transgenic mice.
This work received financial support from The Juvenile Diabetes Foundation International (grant 1-1999-272 to S. Casares), Alliance Pharmaceutical Corporation, San Diego, CA (grant GCO#87-009MI to C. Bona), and Mount Sinai School of Medicine (grant GCO#0247-3631 to T.-D. Brumeanu).
Footnotes
1used in this paper: HA, hemagglutinin; HRP, horseradish peroxidase; STATs, signal transducers and activators of transcription; TcH, T cell hybridoma; Tg, transgenic
References
- Daugelat S., Kauffman S.H.E. Role of Th1 and Th2 cells in bacterial infections. Chem. Immunol. 1996;63:66–69. [PubMed] [Google Scholar]
- Romagnani S. The Th1/Th2 paradigm. Immunol. Today. 1997;18:263–266. doi: 10.1016/s0167-5699(97)80019-9. [DOI] [PubMed] [Google Scholar]
- Wegman T.G., Lin H., Guilbert L., Mosmann T.R. Bidirectional cytokine interactions in the maternal-fetal relationshipis successful pregnancy a Th2 phenomenon? Immunol. Today. 1993;14:353–356. doi: 10.1016/0167-5699(93)90235-D. [DOI] [PubMed] [Google Scholar]
- Piccinni M.-P., Giudizi M.-G., Biagiotti R., Beloni L., Giannarini L., Sampognaro S., Parronchi P., Manetti R., Annunziato F., Livi C. Progesterone favors the development of human T helper cells producing Th2-type cytokines and promotes both IL-4 production and membrane CD30 expression in established Th1 cell clones. J. Immunol. 1995;155:128–133. [PubMed] [Google Scholar]
- Romagnani S. The Th1/Th2 Paradigm in Disease. R.G. Landes Company, Austin/Springer-Verlag; New York, New York: 1997. [Google Scholar]
- Constant S.L., Bottomly K. Induction of Th1 and Th2 CD4+ T cell responsesthe alternative approaches. Annu. Rev. Immunol. 1997;15:297–322. doi: 10.1146/annurev.immunol.15.1.297. [DOI] [PubMed] [Google Scholar]
- Paul W.E., Seder R.A. Lymphocyte responses and cytokines. Cell. 1994;76:241–251. doi: 10.1016/0092-8674(94)90332-8. [DOI] [PubMed] [Google Scholar]
- Yoshimoto T., Bendelac A., Watson C., Hu-Li J., Paul W.E. Role of NK.1.1+ T cells in a Th2 response and immunoglobulin E production. Science. 1995;270:1845–1847. doi: 10.1126/science.270.5243.1845. [DOI] [PubMed] [Google Scholar]
- Rincon M., Anguita J., Nakamura T., Fikring E., Flavell R.A. Interleukin (IL)-6 directs the differentiation of IL-4. J. Exp. Med. 1997;185:461–469. doi: 10.1084/jem.185.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson N.G., Szabo S.J., Weber-Nordt R.M., Zong Z., Schreiber R.D., Darnell J.E., Jr., Murphy K.M. Interleukin 12 signaling in T helper type 1 (Th1) cells involves tyrosine phosphorylation of signal transducer and activation of transcription (Stat)3 and Stat4. J. Exp. Med. 1995;181:1755–1762. doi: 10.1084/jem.181.5.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihle J.N. Cytokine receptor signalling. Nature. 1995;377:591–594. doi: 10.1038/377591a0. [DOI] [PubMed] [Google Scholar]
- Kaplan M.H., Sun Y.L., Hoey T., Grusby M.J. Impaired IL-12 responses and enhanced development of Th2 cells in STAT4-deficient mice. Nature. 1996;382:174–177. doi: 10.1038/382174a0. [DOI] [PubMed] [Google Scholar]
- Kaplan M.H., Schindler U., Smiley S.T., Grusby M.J. Stat6 is required for mediating responses to IL-4 and for development of Th2 cells. Immunity. 1996;4:313–319. doi: 10.1016/s1074-7613(00)80439-2. [DOI] [PubMed] [Google Scholar]
- Nau G.J., Moldwin R.L., Lancki D.W., Kim D.K., Fitch F.W. Inhibition of IL-2-driven proliferation of murine T lymphocyte clones by supraoptimal levels of immobilized anti-T cell receptor monoclonal antibody. J. Immunol. 1987;139:114–122. [PubMed] [Google Scholar]
- Sloan-Lancaster J., Allen P.M. Significance of T cell stimulation by altered peptide ligands in T cell biology. Curr. Opin. Immunol. 1995;7:103–109. doi: 10.1016/0952-7915(95)80035-2. [DOI] [PubMed] [Google Scholar]
- Sharma S.D., Nag B., Su X.-M., Green D., Spack E., Clark B.R., Sriram S. Antigen-specific therapy of experimental allergic encephalomyelitis by soluble class II major histocompatibility complex-peptide complexes. Proc. Natl. Acad. Sci. USA. 1991;88:11465–11469. doi: 10.1073/pnas.88.24.11465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spack E., McCutcheon M., Corbelleta N., Nag B., Passmore D., Sharma S.D. Induction of tolerance in experimental autoimmune myasthenia gravis with solubilized MHC class-II acetylcholine receptor peptide complexes. J. Autoimmunity. 1995;8:787–807. doi: 10.1016/s0896-8411(95)80018-2. [DOI] [PubMed] [Google Scholar]
- Kozono H., White J., Clements J., Marrack P., Kappler J. Production of soluble MHC class II proteins with covalently bound single peptides. Nature. 1994;369:151–154. doi: 10.1038/369151a0. [DOI] [PubMed] [Google Scholar]
- Rhode P.R., Burkhardt M., Jiao J., Siddiqui A.H., Huang G.P., Wong H.C. Single-chain MHC class II molecules induce T-cell activation and apoptosis. J. Immunol. 1996;157:4885–4891. [PubMed] [Google Scholar]
- Casares S., Bona C.A., Brumeanu T.-D. Engineering and characterization of a murine MHC class II-immunoglobulin chimera expressing an immunodominant CD4+ T viral epitope. Protein Eng. 1997;10:1295–1301. doi: 10.1093/protein/10.11.1295. [DOI] [PubMed] [Google Scholar]
- Hamad A.R., O'Herrin S., Lebowitz M.S., Srikishann A., Bieler J., Schneck J., Pardoll D. Potent T cell activation with dimeric peptide major histocompatibility complex class II ligandthe role of CD4 coreceptor. J. Exp. Med. 1998;188:1633–1640. doi: 10.1084/jem.188.9.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberman A.M., Moller A.M., McCreedy C.D., Gerhard W.V. A large degree of functional diversity exists among helper T-cells specific for the same antigenic site of influenza hemagglutinin. J. Immunol. 1990;145:3087–3094. [PubMed] [Google Scholar]
- Taylor J.M., Davey J., Howland K., Rothbard J.B., Askonas B.A. Class I MHC molecules rather than other mouse genes dictate influenza epitope recognition by cytotoxic cells. Immunogenetics. 1987;26:267–272. doi: 10.1007/BF00346521. [DOI] [PubMed] [Google Scholar]
- Weber S., Trannecker A., Olivery F., Gerhard W.V., Karjalainen K. Specific low-affinity recognition of MHC complex plus peptide by soluble T-cell receptor. Nature. 1992;356:793–796. doi: 10.1038/356793a0. [DOI] [PubMed] [Google Scholar]
- Brumeanu T.-D., Casares S., Harris P.E., Dehazya P., Wolf I., Bona C.A. Immunopotency of a viral peptide assembled on the carbohydrate moieties of immunoglobulins. Nat. Biotechnol. 1996;14:722–725. doi: 10.1038/nbt0696-722. [DOI] [PubMed] [Google Scholar]
- Kotanides H., Moczygemba M., White M.F., Reich N.C. Characterization of interleukin-4 nuclear activated factor/STAT and its activation independent of the insulin receptor substrate proteins. J. Biol. Chem. 1995;270:19481–19486. doi: 10.1074/jbc.270.33.19481. [DOI] [PubMed] [Google Scholar]
- Waldmann T., Tagaya Y., Bamford R. Interleukin-2, interleukin-15, and their receptors. Int. Rev. Immunol. 1998;16:205–226. doi: 10.3109/08830189809042995. [DOI] [PubMed] [Google Scholar]
- Bot A., Casares S., Bot S., von Boehmer H., Bona C.A. Cellular mechanisms involved in protection against influenza virus infection in transgenic mice expressing a TCR specific for class-II hemagglutinin peptide in CD4+ and CD8+ T cells. J. Immunol. 1998;160:4500–4507. [PubMed] [Google Scholar]
- Seder R.A., Paul W.E. Acquisition of lymphokine-producing phenotype by CD4+ T cells. Annu. Rev. Immunol. 1995;12:635–673. doi: 10.1146/annurev.iy.12.040194.003223. [DOI] [PubMed] [Google Scholar]
- Coffman R.L., Seymour B.W., Lebman D.A., Hirachi D.D., Christiansen J.A., Shrader B., Cherwinski H.M., Savelkoul H.F.J., Finkelman F.D., Bond M.W. The role of T cell products in mouse B cell differentiation and isotype regulation. Immunol. Rev. 1988;102:5–28. doi: 10.1111/j.1600-065x.1988.tb00739.x. [DOI] [PubMed] [Google Scholar]
- Allan W., Tabi Z., Clearly A., Doherty P.C. Cellular events in the lymph node and lung of mice infected with influenza. Consequences of depleting CD4+ T cells. J. Immunol. 1990;144:3980–3986. [PubMed] [Google Scholar]
- Caton A.J., Brownlee J.C., Yewdell J.W., Gerhard W.V. The antigenic structure of influenza virus A/PR8/34 hemagglutinin (H1 subtype) Cell. 1982;31:417–427. doi: 10.1016/0092-8674(82)90135-0. [DOI] [PubMed] [Google Scholar]
- Gajewski T.F., Lancki D.W., Stack R., Fitch F.W. Anergy of Th0 helper T lymphocytes induces downregulation of Th1 characteristics and a transition to a Th2-like phenotype. J. Exp. Med. 1994;179:481–491. doi: 10.1084/jem.179.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veillete A., Bookman M.A., Horak E.M., Bolen J.B. The CD4 and CD8 T cell surface antigens are associated with the internal membrane tyrosine-protein kinase p56lck. Cell. 1988;55:301–308. doi: 10.1016/0092-8674(88)90053-0. [DOI] [PubMed] [Google Scholar]
- Iwashima M., Irving B.A., van Oers N.S.C., Chan A.C., Weiss A. Sequential interactions of the TCR with two distinct cytoplasmic tyrosine kinases. Science. 1994;263:1136–1139. doi: 10.1126/science.7509083. [DOI] [PubMed] [Google Scholar]
- Janeway C.A., Jr. The T cell receptor as a multicomponent signalling machineCD4/CD8 coreceptors and CD45 in T cell activation. Annu. Rev. Immunol. 1992;10:645–674. doi: 10.1146/annurev.iy.10.040192.003241. [DOI] [PubMed] [Google Scholar]
- Horvath C.M., Darnell J.E. The state of STATsrecent developments in the study of signal transduction to the nucleus. Curr. Opin. Cell Biol. 1997;9:30–239. doi: 10.1016/s0955-0674(97)80067-1. [DOI] [PubMed] [Google Scholar]
- Gajewski T.F., Pinnas M., Wong T., Fitch F.W. Murine Th1 and Th2 clones proliferate optimally in response to distinct antigen presenting cell populations. J. Immunol. 1991;146:1750–1758. [PubMed] [Google Scholar]
- McKnight J.A., Perez V.L., Shea C.M., Gray G.S., Abbas A.K. Costimulator dependence of lymphokine secretion by naive and activated CD4+ T lymphocytes from TCR transgenic mice. J. Immunol. 1994;152:5220–5225. [PubMed] [Google Scholar]
- Goldstein J., Mostowsky H., Tung J., Hon H., Brunswick M., Kozlowski S. Naive alloreactive CD8 T cells are activated by purified major histocompatibility complex class I and antigenic peptide. Eur. J. Immunol. 1997;27:871–878. doi: 10.1002/eji.1830270411. [DOI] [PubMed] [Google Scholar]
- Goldstein J.S., Chen T., Brunswick M., Mostowsky H., Kozlowski S. Purified MHC class I and peptide complexes activate naive CD8+ T cells independently of the CD28/B7 and LFA-1/ICAM-1 costimulatory interactions. J. Immunol. 1998;160:3180–3187. [PubMed] [Google Scholar]
- Boniface J.J., Rabinowitz J.D., Wulfing C., Hampl J., Reich Z., Altman J.D., Kantor R., Beeson C., McCornell H.M., Davis M.M. Initiation of signal transduction through the T cell receptor requires multivalent engagement of peptide/MHC ligands. Immunity. 1998;9:459–466. doi: 10.1016/s1074-7613(00)80629-9. [DOI] [PubMed] [Google Scholar]
- Adorini, L. 1992. Immunosuppression by MHC class II blockade in T lymphocytes: structure, functions and choices. F. Cellada and B. Pernis, editors. Plenum Press, NY. pp. 187–194.
- Weiss A., Littman D.R. Signal transduction by lymphocyte antigen receptors. Cell. 1994;76:263–274. doi: 10.1016/0092-8674(94)90334-4. [DOI] [PubMed] [Google Scholar]
- Constant S., Pfeiffer C., Pasqualini T., Bottomly K. Extent of T cell receptor ligation can determine the functional differentiation of naive CD4 T cells. J. Exp. Med. 1995;182:1591–1596. doi: 10.1084/jem.182.5.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viola A., Salio M., Tuosto L., Linkert S., Acuto O., Lanzavecchia A. Quantitative contribution of CD4 and CD8 to T cell antigen receptor serial triggering. J. Exp. Med. 1997;186:1775–1779. doi: 10.1084/jem.186.10.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D.R., Moskowitz N.H., Killen N., Reiner S.L. A role for CD4 in peripheral T cell differentiation. J. Exp. Med. 1997;186:101–107. doi: 10.1084/jem.186.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford F., Kozono H., White J., Marrack P., Kappler J. Detection of antigen-specific T cells with multivalent soluble class II MHC covalent peptide complexes. Immunity. 1998;8:675–682. doi: 10.1016/s1074-7613(00)80572-5. [DOI] [PubMed] [Google Scholar]
- Haspel R.L., Salditt-Georgief M., Darnell J.E., Jr. The rapid inactivation of nuclear tyrosine phosphorylated Stat1 depends upon a protein tyrosine phosphatase. EMBO (Eur. Mol. Biol. Organ.) J. 1996;15:6262–6268. [PMC free article] [PubMed] [Google Scholar]
- Starr R., Willson T.A., Viney E.M., Murray L.J.L., Rayner J.R., Jenkins B.J., Gonda T.J., Alexander W.S., Metcalf D., Nicola N.A. A family of cytokine-inducible inhibitors of signaling. Nature. 1997;387:917–921. doi: 10.1038/43206. [DOI] [PubMed] [Google Scholar]
- Altare F., Durandy A., Lammas D., Emile J.-F., Lamhamedi S., Diest F., Drysdale P., Jouanguy E., Doffinger R., Bernaudin F. Impairment of mycobacterial immunity in human interleukin-12 receptor deficiency. Science. 1998;280:1432–1435. doi: 10.1126/science.280.5368.1432. [DOI] [PubMed] [Google Scholar]
- Bach E.A., Aquel M., Schreiber R.D. The IFN-γ receptora paradigm for cytokine receptor signaling. Annu. Rev. Immunol. 1997;15:563–591. doi: 10.1146/annurev.immunol.15.1.563. [DOI] [PubMed] [Google Scholar]