Abstract
We compared the pathogenicity of intimin-negative non-O157:H7 Shiga toxin (Stx)-producing Escherichia coli (STEC) O91:H21 and O104:H21 strains with the pathogenicity of intimin-positive O157:H7 and O157:H− strains in neonatal pigs. We also examined the role of Stx2d-activatable genes and the large hemolysin-encoding plasmid of O91:H21 strain B2F1 in the pathogenesis of STEC disease in pigs. We found that all E. coli strains that made wild-type levels of Stx caused systemic illness and histological lesions in the brain and intestinal crypts, whereas none of the control Stx-negative E. coli strains evoked comparable central nervous system signs or intestinal lesions. By contrast, the absence of intimin, hemolysin, or motility had little impact on the overall pathogenesis of systemic disease during STEC infection. The most striking differences between pigs inoculated with non-O157 STEC strains and pigs inoculated with O157 STEC strains were the absence of attaching and effacing intestinal lesions in pigs inoculated with non-O157:H7 strains and the apparent association between the level of Stx2d-activatable toxin produced by an STEC strain and the severity of lesions.
Serotype O157:H7 Shiga toxin (Stx)-producing Escherichia coli (STEC) strains cause the majority of sporadic and multiperson outbreaks of bloody diarrhea in the United States (15). However, non-O157 STEC strains were the source of three such outbreaks of bloody diarrhea (1) and an apparent cluster of three cases of hemolytic-uremic syndrome (HUS) (5, 26) and may be responsible for 20% (2) or even 50% (13) of all STEC infections. Up to 10% of patients with hemorrhagic colitis due to infection with an O157 strain develop HUS, a sequela that includes serious kidney damage. The risk of HUS for patients infected with non-O157 STEC is not as clear, but it could be as high as that for patients infected with O157 strains (29).
STEC can be grouped by the array of potential virulence factors that they express. All STEC strains produce one or more Stxs. There are two main groups of Stxs (also called verotoxins), Stx1 and Stx2, which have the same enzymatic activity and general structure but are not cross-neutralized by heterologous antisera (34). The Stx2 group contains variants that include Stx2c (39), Stx2d-activatable (whose toxicity is increased by elastase from intestinal mucus [31]) (23, 43), Stx2d-nonactivatable (36), Stx2e (associated with edema disease in weaned pigs [48]), and Stx2f (isolated from feral pigeons and from a child with diarrhea [33, 38]).
A number of STEC strains have the same general constellation of pathogenic factors as the O157:H7 isolates. These strains not only produce Stxs but also have the capacity to cause attaching and effacing (A/E) lesions in the intestines of animals or in tissue culture models (8). A/E lesion formation is mediated by products of the locus of enterocyte effacement (LEE) found in enterohemorrhagic E. coli O157 strains and some non-O157 STEC strains, such as all O26:H11 strains and most O111:H− strains (20). The LEE is also found in enteropathogenic E. coli and in some non-E. coli species (27). Intimin, the product of the eae gene (located in, and sometimes used as a marker for, the LEE pathogenicity island), is required for both the adherence of O157:H7 strain 86-24 to HEp-2 cells and the colonization of neonatal pigs and calves by strain 86-24 (8, 9, 46). However, the factor(s) responsible for colonization by non-O157 strains that lack intimin is not as well established, although a few candidate adhesins have been identified (10, 35, 41). Most STEC strains harbor a large (∼90-kb) hemolysin-encoding plasmid. There is conflicting evidence about whether this plasmid is required for virulence (19, 21, 47).
Intimin-negative STEC strains that produce the Stx2d-activatable variant of Stx2 (30) have low 50% lethal doses (LD50s) (101 to 102 CFU) in a streptomycin-treated CD-1 mouse model of STEC infection, whereas O157:H7 strains consistently have LD50s of 1010 CFU or greater in CD-1 mice (24, 30, 47). One such Stx2d-activatable variant producer, B2F1, is an O91:H21 strain associated with the development of HUS in a child (18). The essential role of the Stx2d-activatable variant in the pathogenesis of B2F1 in streptomycin-treated mice was demonstrated by the finding that toxin mutants of B2F1 are attenuated in this mouse model (24) and the finding that passive administration of monoclonal antibodies that recognize Stx2 or Stx2 variants (e.g., Stx2d-activatable variants) protects mice from the otherwise lethal B2F1 infection (43). B2F1 has two copies of the gene for the Stx2d-activatable variant, one of which is phage encoded and expressed at low levels (43). Another strain that produces an Stx2d-activatable variant is 3024-94, an O104:H21 strain associated with an outbreak of bloody diarrhea in Montana (4, 12). This strain appears to contain only one copy of the gene for the potent Stx2d-activatable toxin (Melton-Celsa, unpublished observations).
The low oral LD50s of the intimin-negative non-O157 STEC strains B2F1 and 3024-94 for streptomycin-treated mice (30), along with the linkage of these strains to human cases of HUS and hemorrhagic colitis, respectively, led us to hypothesize that these isolates would be virulent in neonatal pigs. In this study, we tested this theory by comparing the pathogenicity of these intimin-negative isolates with that of STEC O157 strains. We also evaluated the role of each of the copies of the Stx2d-activatable gene and of the large hemolysin-encoding plasmid of strain B2F1 in the pathogenesis of STEC disease.
MATERIALS AND METHODS
Bacteriologic strains and inocula.
The bacterial strains used in this study are described in Table 1. All STEC strains used were streptomycin-resistant derivatives of the original clinical isolate. The control E. coli strain, 123, is resistant to nalidixic acid. Stock inocula were prepared and stored as previously described (7). The STEC strain B2F1 used in this study has two copies of stx2d-activatable, which are referred to below as stx2d1-activatable and stx2d2-activatable for clarity. The plasmid-cured derivative of B2F1 no longer produces detectable hemolytic activity (40).
TABLE 1.
E. coli strains used in this study
| Group | Strain | Serotype | eae gene | stx genotype | Source | Reference(s) |
|---|---|---|---|---|---|---|
| A | 86-24 | O157:H7 | + | stx2 | P. Tarr | 8, 28 |
| B | E32511/HSC/L | O157:H− | + | stx2c | 17 | |
| C | 3024-94 | O104:H21 | − | stx2d-activatable | CDCa | 4 |
| D | B2F1 | O91:H21 | stx2d1-activatable | M. Karmali | 18 | |
| stx2d2-activatable | 30 | |||||
| E | B2F1 (plasmid cured) | O91:H21 | − | stx2d1-activatable | A. D. O'Brien | 40 |
| stx2d2-activatable | ||||||
| F | B2F1 Stx2d2-activatable+ | O91:H21 | − | stx2d1-activatable::cat | A. D. O'Brien | 43 |
| stx2d2-activatable | ||||||
| G | B2F1 Stx2d1-activatable+ | O91:H21 | − | stx2d1-activatable | A. D. O'Brien | 43 |
| stx2d2-activatable::cat | ||||||
| H | 123 (control) | O43:H28 | − | NAb | 7, 32 |
CDC, Centers for Disease Control and Prevention.
NA, not applicable.
Bacteriologic examination.
The numbers of an inoculated strain recovered from tissues and feces of pigs were determined on sorbitol MacConkey agar containing 100 μg of streptomycin per ml (strain 86-24 and strain E32511/HSC/L), MacConkey agar containing 100 μg of streptomycin per ml (eae-negative STEC strains), or MacConkey agar containing 20 μg of nalidixic acid per ml (strain 123). Samples from which the inoculated strain were not recovered were recorded as having <103 CFU/g of tissue. Selected sorbitol-negative isolates (strains 86-24 and E32511/HSC/L) were tested for O157:H7 antigen by the latex agglutination assay (7). Selected coliforms were tested for O91 antigen (strain B2F1 and its derivatives), O104 antigen (strain 3024-94), or O43 antigen (strain 123) by performing a colony blot immunoassay with appropriate O-specific sera (8).
Histologic studies.
Tissues were fixed in neutral buffered 10% formalin for 24 to 48 h, embedded in paraffin, sectioned, and stained with hematoxylin and eosin. The periodic acid-Schiff reaction was used to detect microvascular damage in the brain. Samples of colon from selected animals were prepared for electron microscopy studies (7).
Immunoperoxidase staining with O-specific antibodies.
Inoculum-type bacteria in formalin-fixed intestinal tissues were identified by indirect immunoperoxidase (horseradish peroxidase) staining with goat anti-O157:H7 antibody (7) or by using rabbit anti-O91, anti-O104, or anti-O43 antibody (E. coli Reference Center, Pennsylvania State University, University Park, Pa.) as the primary antibody and biotinylated goat anti-rabbit antibody (Vector Laboratories, Inc., Burlingame, Calif.) the as the secondary antibody.
Pathogenicity in neonatal pigs.
Sixty-one cesarean-derived, colostrum-deprived (CDCD) <8-h-old pigs from seven litters, which had received 20 ml of normal swine serum intraperitoneally shortly after birth, were inoculated via a stomach tube with 1010 CFU of the following strains: O157:H7 strain 86-24 (group A; 15 pigs from six litters); O157:H− strain E32511/HSC/L (group B; 5 pigs from two litters); O104:H21 strain 3024-94 (group C; 6 pigs from two litters); O91:H21 strain B2F1 (group D; 9 pigs from five litters); B2F1 (plasmid cured) (group E; 4 pigs from 2 litters); B2F1stx2d1-activatable::cat, designated B2F1 Stx2d2-activatable+ (group F; 7 pigs from 4 litters); B2F1stx2d2-activatable::cat, designated B2F1 Stx2d1-activatable+ (group G; 7 pigs from four litters); and control E. coli strain 123 (group H; 8 pigs from five litters). The inoculated animals were maintained in individual plastic cages and fed a diet consisting of autoclaved SPF-LAC (Borden, Elgin, Ill.) or Esbilac (Pet Ag, Hampshire, Ill.) as previously described (7). The pigs were observed every 4 to 8 h for signs of clinical disease and were euthanized with sodium pentobarbital 31 to 69 h after inoculation. The pigs were euthanized as soon as central nervous system (CNS) signs were noted.
At necropsy, sections from the ileum and cecum were collected aseptically and frozen at −80°C for bacteriological culture. Sections of terminal ileum, cecum, colon (complete coil to provide several crosscuts in one section), distal colon, brain (medulla oblongata and brain stem), kidney, and other parenchymal tissues were collected for histopathologic examination and immunoperoxidase staining (intestinal tissues only).
Disclaimer.
Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture.
RESULTS
Clinical signs.
CNS signs were the most striking clinical observations in pigs inoculated with STEC. As shown in Table 2, one or more pigs in all groups except group H (control strain 123) showed CNS signs that ranged from mild (tremors, depression, poor appetite) to severe (seizures, ataxia, recumbency, paddling). The CNS signs were severe (e.g., extreme weakness in rear legs, lateral recumbency, seizures, paddling) in approximately one-third of the pigs that developed CNS signs. Most CNS signs appeared between 41 and 50 h postinfection (pi), but three pigs (one each in groups A, E, and F) showed severe CNS signs by 31 to 32 h pi. One other pig in group F died between observations at 32 and 39 h pi. The CNS signs were milder and less common in pigs in group G (B2F1 Stx2d1-activatable+) than in the other STEC groups. Diarrhea was uncommon (Table 2) and occurred only in pigs inoculated with STEC belonging to serotype O157 or O104 (groups A, B, and C). Thirteen STEC-inoculated pigs that did not have diarrhea had liquid feces in the rectum at necropsy. None of the control pigs (group H) had diarrhea or liquid contents in the rectum.
TABLE 2.
Results for neonatal CDCD pigs after inoculation with STEC or control E. coli
| Bacteria and Serotype | Group | Inoculum bacteria | Duration (h) | No. of pigs tested | No. of pigs with:
|
Bacterial counts (log10 CFU/g of tissue) in:
|
||||
|---|---|---|---|---|---|---|---|---|---|---|
| Diarrhea | CNS signs | CNS lesionsa | Adherent bacteriab | Ileum | Cecum | |||||
| STEC | ||||||||||
| O157 | A | 86-24 | 32-49 | 15 | 3 | 12 | 8 | 15c | 7.6d | 8.3 |
| B | E32511/HSC/L | 46-69 | 5 | 2 | 2 | 5 | 5c | 6.8 | 7.1 | |
| O104 | C | 3024-94 | 42-48 | 6 | 2 | 3 | 5 | 6 | 6.5 | 7.0 |
| O91 | D | B2F1 | 41-50 | 9 | 0 | 5 | 5 | 9 | 6.1 | 8.2 |
| E | B2F1 (plasmid cured) | 31-44 | 4 | 0 | 4 | 2 | 4 | 6.1 | 8.6 | |
| F | B2F1Stx2d2-activatable+ | 32-46 | 7e | 0 | 4e | 1 | 6e | 6.4e | 8.2e | |
| G | B2F1Stx2d1-activatable+ | 43-47 | 7 | 0 | 1 | 0 | 7 | 7.7 | 8.3 | |
| Control | ||||||||||
| O43 | H | 123 | 41-68 | 8 | 0 | 0 | 0 | 8 | 6.7 | 7.5 |
Medulla oblongata-brain stem sections from all pigs were investigated.
Adherent inoculum bacteria were identified by immunoperoxidase staining with appropriate O-specific antisera as described in Materials and Methods.
A/E bacteria stained with anti-E. coli O157:H7 antibody by immunoperoxidase staining.
Mean values. For each site, mean STEC strain counts were compared with mean control strain counts by using the two-sample t test and a significance level of 0.05. Bacterial counts that differed significantly from control counts are indicated by boldface type.
No clinical, bacteriogical, or histological data were obtained for one pig that was found dead at 39 h pi.
Macroscopic lesions.
Colonic edema (22 pigs) and subcutaneous edema (19 pigs) were seen in all STEC groups except group G (B2F1 Stx2d1-activatable+), but they occurred in only one of the six pigs in group C (3024-94). An increase in clear fluid was noted in the meninges of some O157-infected pigs (four pigs in group A and one pig in group B) and O91-infected pigs (four pigs in group E and one pig in group F), and this was interpreted to be edema. Gall bladder edema was seen in one pig each in groups A and E. No macroscopic lesions were seen in any of the controls (group H).
Bacterial counts.
The mean numbers of inoculum-type bacteria recovered from the ileum and cecum of pigs in each inoculation group are shown in Table 2. Groups A (86-24) and G (B2F1 Stx2d1-activatable+) had significantly higher levels of inoculum-type bacteria in the ileum than the control group had, but the bacterial counts for the other STEC groups were similar to those for the control group. All groups had >107 CFU of inoculum-type bacteria/g of cecal tissue. STEC groups A, D, E, F, and G had significantly higher numbers of inoculum-type bacteria in the cecum than the controls (group H) had.
Microscopic lesions.
Histologic lesions were frequently found in brain and intestinal tissues and sometimes were also found in kidney tissues of pigs inoculated with STEC but not in any of the control pigs. The findings for specific anatomical sites were as follows.
(i) Brain.
Lesions were seen in pigs in all groups that were infected with STEC except group G (B2F1 Stx2d1-activatable+). No brain lesions were seen in any of the control pigs (group H). The lesions in group C pigs (3024-94) were subtler than those in the pigs belonging to the other groups (Table 2). Perivascular edema and perivascular accumulations of protein droplets, which stained positive with periodic acid-Schiff stain, were seen around arterioles, capillaries, and venules in sections of brain from affected animals. These lesions resembled those which were described previously for CDCD and suckling pigs experimentally infected with O157:H7 strain 86-24 (the strain used for group A in this experiment) (6) but were less severe than those in suckling piglets.
(ii) Intestine.
Irregular layers of E. coli control strain 123 were identified with the O43 antiserum on stained mucosal surfaces of colonic and cecal tissues from all pigs inoculated with this control strain. Seven of the eight control pigs had no morphological lesions in any of the intestinal sites sampled, but mild to moderate edema and mild to moderate infiltration of mononuclear cells were observed in sections of the cecum and colon from one control pig.
In contrast, histologic lesions were evident in the intestines of all pigs inoculated with an STEC strain. Such lesions were more common in the cecum and colon than in the ileum. The type (described below) and distribution of these lesions varied with the inoculum strain (Table 3). Within inoculum groups, the degree and intensity of lesions differed from animal to animal and with the duration of exposure. Intestinal lesions were seen as early as 31 h pi but were more extensive and more pronounced in pigs necropsied at later times after inoculation.
TABLE 3.
Intestinal crypt lesions in neonatal CDCD pigs 31 to 69 h after inoculation with STEC or control E. coli
| Group | Inoculum bacteria | No. of pigs | Proportion of pigs witha:
|
|||||
|---|---|---|---|---|---|---|---|---|
| Irregular goblet cellsb
|
Shift of goblet cellsb
|
Crypt activationb
|
||||||
| Cecum | Colon | Cecum | Colon | Cecum | Colon | |||
| A | 86-24 | 15 | +++ | +++ | +++c | ++ | 0 | ++ |
| B | E32511/HSC/L | 5 | +++ | +++ | +++ | +++ | 0 | 0 |
| C | 3024-94 | 6 | ++ | +++ | 0 | 0 | +++ | +++ |
| D | B2F1 | 9 | +++ | +++ | 0 | +++ | +++ | 0 |
| E | B2F1 (plasmid cured) | 4 | ++ | ++ | ++ | ++ | 0 | 0 |
| F | B2F1 Stx2d2-activatable+ | 6 | +++ | +++ | +++ | +++ | 0 | 0 |
| G | B2F1 Stx2d1-activatable+ | 7 | +++ | +++ | 0 | 0 | +++ | + |
| H | 123 (control) | 8 | ++ | 0 | 0 | 0 | 0 | 0 |
+++, lesions in 100% of the pigs; ++, lesions in 50 to 70% of the pigs; +, lesions in <20% of the pigs; 0, no lesions in any of the pigs.
The three types of lesions observed in cecal and colonic crypts of STEC-infected pigs are described in the text.
Cecum morphological data were available for 9 of the 15 pigs in this group.
Inoculum-type bacteria, identified by immunoperoxidase staining with appropriate O-specific antiserum, were present in the lumen and were adherent to mucosal surfaces in cecal and colonic sections from pigs in all groups. Inoculum-type bacteria were less frequently seen in the ileum and, when found, were more often associated with the follicular associated epithelium (FAE) of the domes than with other epithelial surfaces and were sometimes associated with FAE cells sloughing off from the domes.
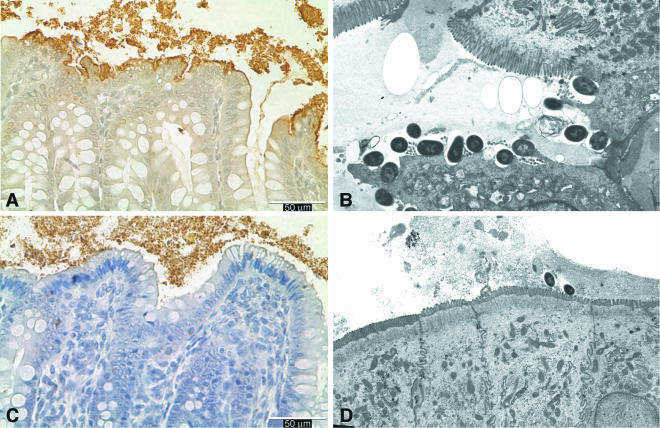
A/E lesions were found only in pigs inoculated with an O157 strain (groups A and B). These lesions occurred predominantly in the large intestine and contained O157+ bacteria (Fig. 1A and B). In pigs inoculated with a non-O157 STEC strain (groups C to G), inoculum-type bacteria were found closely adherent to colonic and cecal surfaces (Fig. 1C and D). Bacterial adherence was associated with irregular vacuolated cells that contained fine to coarse droplets (Fig. 1A and C).
FIG. 1.
(A and C) Horseradish peroxidase-stained sections of colon from a neonatal pig necropsied 49 h after inoculation with STEC O157:H7 strain 86-24 (A) or from a pig necropsied 41 h after inoculation with strain B2F1 (C), showing typical immunostained colonies of O157:H7+ A/E bacteria (A) and adherent O91+ bacteria (C) on the mucosal surface. (B and D) Electron micrographs showing intimately attached bacteria and effaced microvilli in the cecum from a pig necropsied 18 h after inoculation with strain 86-24 (B) (magnification, approximately ×6,000) and adherent bacteria and intact villi on the surface of absorptive epithelial cells in the colon from a pig necropsied 43 h after inoculation with strain B2F1 (D) (magnification, approximately ×3,500).
Histologic lesions were seen in crypts of cecal and colonic tissues from all pigs inoculated with an STEC strain. No histologic lesions were seen in colonic tissues from any of the control pigs, but irregular goblet cells (see below) were seen in cecal tissues from four of eight control pigs. The type and distribution of intestinal lesions varied with the inoculum strain. We grouped the kinds of lesions observed in the crypts of the cecum and colon of STEC-infected pigs into three categories (Table 3 and Fig. 2). Definitions of these three categories are given below.
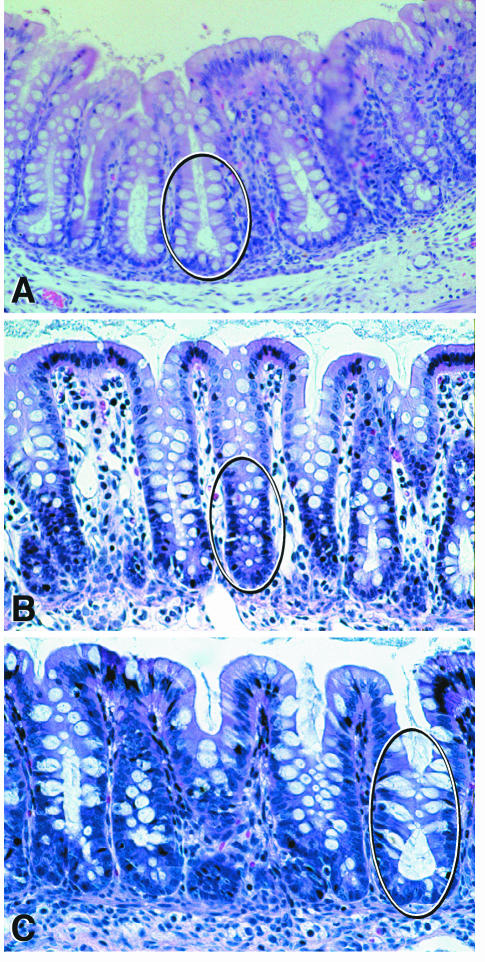
FIG. 2.
Hemotoxylin- and eosin-stained sections of the large intestine from neonatal pigs. (A) Normal distribution of goblet cells in crypts of the cecum (circled) from a pig necropsied 48 h after inoculation with E. coli strain 123 (control). (B) Shift of goblet cells in the colon of a pig 31 h after inoculation with strain B2F1 (plasmid cured). Crypts were irregular and contained immature crypt cells that stained dark blue and irregularly distributed goblet cells with a reduced mucus content (circled). (C) Crypt activation in the cecum from a pig necropsied 46 h after inoculation with B2F1. Dilated crypts contained intensely stained immature cells and ballooning goblet cells with bulging mucus droplets oriented towards the lumen. Note the mucus streaming out of crypt openings (circled).
(a) Category 1: irregular goblet cells.
Category 1 included irregularly shaped and distributed goblet cells that were often extensively dilated. These types of cells were seen in crypts of colonic samples from all pigs in all STEC groups (Fig. 2B) and in cecal samples from all but two pigs in the STEC groups (they were not seen in two pigs in group C). Irregular goblet cells were not seen in colonic samples from any of the control pigs but were seen in cecal tissues from four of the eight pigs in the control group.
(b) Category 2: shift of goblet cells.
In category 2 (Fig. 2B), goblet cells in the base of crypts were replaced by immature cells that stained dark blue with hematoxylin and eosin and contained large nuclei. Goblet cells in mid-crypt areas were irregularly distributed. There was mild to moderate mononuclear infiltration of the lamina propria, and in some areas there was an increase in eosinophils and occasional intraepithelial cells. Neutrophils were a rare component of the infiltrating cells. As shown in Table 2, a shift of goblet cells was seen in cecal and colonic tissues from pigs in groups A and B (O157 strains) and groups E and F [B2F1 (plasmid cured) and B2F1 Stx2d2-activatable+] and in colonic tissues but not cecal tissues from pigs in group D (B2F1). This shift was not seen in groups C, G, and H (3024-94, B2F1 Stx2d1-activatable+, and control strains, respectively).
Category 3: crypt activation.
In category 3 (Fig. 2C), some goblet cells in mid-crypt areas were extremely dilated and irregular, and accumulations of mucus and bulging mucus droplets were oriented towards the lumen. Several crypts were dilated, and there was mucus streaming out of crypt openings. Crypts contained intensely stained immature cells. A few neutrophils were seen infiltrating the lamina propria in crosscuts of colonic sections. Crypt activation was seen at all sites at which a shift of goblet cells was not seen (i.e., in cecal sections from pigs in groups C, D, and G and in colonic sections from pigs in groups C and G). Crypt activation was not seen in colonic samples in which a shift of goblet cells was seen (groups B, D, F, and G).
(iii) Kidney.
Focal tubular necrosis and vascular damage were noted in some of the pigs inoculated with STEC (groups A, B, C, E, and G) but not in any of the controls (group H). Electron microscopic and morphometric investigations to further characterize these lesions are in progress.
DISCUSSION
Several conclusions about the pathogenesis of STEC in the neonatal pig model can be derived from the observations made in this study. First, intimin is not required for the pathogenicity of non-O157 STEC in CDCD pigs. Systemic (apparently Stx-mediated) disease was evident in all groups of animals inoculated with STEC whether the isolate had intimin or not. The results for strain B2F1 obtained in this study differ from the previous finding that B2F1, like an eae mutant of O157:H7 strain 86-24, is not pathogenic for CDCD pigs (8). This discrepancy can be explained by differences in the duration of the experiments. Pigs in the previous study were necropsied 18 h after they were inoculated with strain B2F1; clinical CNS signs in the present study appeared between 31 and 50 h after inoculation.
The presence of CNS damage in all groups of pigs inoculated with STEC strains strongly suggests that Stx was translocated across the intestinal mucosa whether the strain colonized the mucosa by A/E-mediated mechanisms or not. However, STEC strains such as B2F1 and 3024-94 that do not cause A/E lesions may deliver toxin in a different way than STEC strains that produce the products of the LEE. The presence of large numbers of B2F1 and 3024-94 cells in the large intestine is consistent with the hypothesis that toxin is absorbed through the mucus and the intestinal epithelial barrier. Furthermore, the observation that inoculum bacteria covered the FAE cells of ileal domes in two of the animals suggests that penetration or entry of toxin might begin in the lower small intestine.
A second conclusion is that STEC-induced mucosal damage is not dependent on intimin. Mucosal lesions were seen in the large intestines of all pigs inoculated with either eae+ or eae STEC strains. These lesions occurred both in cells on the surface of the intestine and in crypts in the cecum and colon. A similar absence of goblet cells and discharge of mucus has been seen in STEC-inoculated ligated colonic loops in calves (37). The presence of STEC-induced changes in porcine intestinal mucus suggests that porcine mucus, like human mucus and mouse mucus (30), may activate Stx2d-activatable variants. Indeed, preliminary studies suggested that porcine mucus did activate Stx2d2-activatable variants (data not shown). The observation that there were STEC-induced changes in mucus, mucus release, and crypt architecture in the porcine cecum and colon in these short-term experiments suggests that there should be further investigation to ascertain the importance of mucus, crypt cells, and potential early damage of the intestinal barrier in STEC-mediated disease.
The type of crypt lesions which we saw in the piglets inoculated with STEC strains resembled those described for mice infected with LEE-positive Citrobacter rodentium (25). Perhaps the lesions that were seen in pigs 31 to 69 h after STEC inoculation were a very early phase of a more severe hyperplasia like that which occurs in mice 2 to 3 weeks after they are inoculated with C. rodentium. Because there are homologous virulence factors in the LEE pathogenicity islands of C. rodentium and LEE-positive STEC, it is possible that these organisms use similar mechanisms to induce colonic lesions in mice and piglets. Our observation that intimin-negative STEC strains produced similar colonic lesions in piglets indicates that factors other than LEE-encoded proteins induce development of these lesions.
A third conclusion is that classic inflammatory responses are not common in early STEC disease in CDCD pigs. Inflammatory cells were rarely seen in any of the tissue sections from STEC-inoculated animals. However, it is possible that the damage to the mucus and the crypts observed in these experiments in fact reflected very early events in an inflammatory response. To address this question, the levels of inflammatory mediators early in infection need to be determined.
A fourth conclusion is that nonmotile STEC O157:H strains, like O157:H7 strains that are motile, can cause disease in neonatal pigs. The O157:H strain E32511/HSC/L (eae+ stx2c+) colonized neonatal pigs and produced disease and lesions that were indistinguishable from those produced by STEC O157:H7 strain 86-24.
A fifth conclusion is that the large plasmid of strain B2F1 does not appear to contain genes that are required for the development of STEC disease in CDCD pigs. All four of the piglets inoculated with the plasmid-cured derivative of strain B2F1 were colonized, displayed signs of CNS disease, and had CNS and intestinal lesions that were indistinguishable from those caused by strain B2F1.
A sixth conclusion is that all of the types of Stx2 produced by the strains used in this study cause similar systemic disease and CNS lesions in neonatal CDCD pigs. More than one-half of the pigs inoculated with an STEC strain developed CNS signs and had histologic lesions in the brain (Table 2). Vascular lesions in the brains and intestines were similar in pigs inoculated with STEC strains that produced Stx2, Stx2c, or Stx2d-activatable toxin. These lesions resembled those previously described for gnotobiotic pigs (14, 45), CDCD pigs (8), and suckling pigs (6) and early stages of cerebrospinal angiopathy lesions in weaned pigs with edema disease (3, 22). Approximately one-third of the STEC-inoculated pigs in this study had tubular and vascular lesions in the kidneys that were similar to, but more subtle than, those observed in gnotobiotic pigs at 3 to 33 days after inoculation with STEC (16). Electron microscopic and morphometric investigations to characterize these lesions are ongoing.
A final conclusion is that different types of Stx2 cause different types of lesions. In spite of the similarities of the findings for the STEC-challenged animals, there were inoculum-associated differences in the types and distribution of intestinal lesions (Table 3). For example, whereas intimin-positive O157 strains caused A/E lesions, the intimin-negative non-O157 strains (including the control strain) formed bacterial layers on the intestinal mucosal surfaces of CDCD pigs. This observation suggests that STEC and other E. coli strains that lack intimin may produce other adhesins that facilitate bacterial colonization of the large intestine. Such nonintimin adhesins (like the bundle-forming pili of intimin-positive enteropathogenic E. coli strains that promote initial attachment of some strains to intestinal epithelium) could also be involved in the adherence of intimin-positive STEC strains. Several putative nonintimin adhesins have been described, such as long polar fimbriae (Lpf) and the Vibrio cholerae immunoglobulin A homologue adhesin (Iha) in STEC O157:H7 (42, 44) and Lpf and STEC autoagglutinating adhesin (Saa) in non-O157:H7 STEC (10, 35).
Another difference involved the intensities of vascular lesions caused by different types of Stx2. Thus, the vascular damage in all six pigs inoculated with strain 3024-94 (group C) was less intense than that in the pigs in the other groups. These pigs, which were necropsied 42 to 48 h pi, might have developed more intense lesions if they had been exposed to the toxin longer. In support of the hypothesis that 3024-94 might have been as virulent as the other STEC if the pigs had been necropsied at a later time is the fact that strain 3024-94 has a longer mean time to death than B2F1 in the streptomycin-treated mouse model (Melton-Celsa, unpublished observations).
Although similar vascular alterations were seen in 8 of 16 pigs inoculated with non-O157:H7 strains, including strains 3024-94, B2F1, B2F1 (plasmid cured), and B2F1 Stx2d2-activatable+, these lesions were not seen in the seven animals inoculated with the B2F1 mutant that produced only Stx2d1-activatable toxin (group G). The lack of vascular lesions might be explained by the fact that B2F1 Stx2d1-activatable+ produces lower levels of toxin than the parental strain B2F1 or B2F1 Stx2d2-activatable+ produces (11). The hypothesis that B2F1 produces higher levels of Stx2d2-activatable toxin in vivo, as well as in vitro, is supported by the observation that B2F1 Stx2d2-activatable+ is more virulent than B2F1 Stx2d1-activatable+ in both mice (11) and pigs (this study).
In summary, the absence of intimin in non-O157 STEC had little impact on the overall pathogenesis of systemic disease during STEC infection of neonatal pigs. The most striking differences between groups of animals were the presence of A/E lesions in the pigs inoculated with the intimin-positive strains and the apparent association between the level of Stx2d-activatable toxin and the severity of CNS disease and lesions in pigs inoculated with the intimin-negative non-O157 strains. The most surprising finding in this study was the ubiquity of mucus and crypt lesions observed for all STEC groups, an observation that suggests that Stx plays a critical role in this type of intestinal damage. Perhaps the equivalent crypt damage caused by non-O157 strain B2F1 and the O157 strains is a consequence of a balance of the virulence factors produced by the two types of STEC strains: the more potent activatable toxin is made by the less adherent B2F1 strain, while the less potent toxin is produced by the O157 strains that form the highly damaging A/E lesions.
Acknowledgments
This work was supported in part by grant 97-35201-4578 from the U.S. Department of Agriculture and by Public Health Service grant AI20148-20 from the National Institutes of Health to Alison D. O'Brien.
We thank M. H. Inbody, R. W. Morgan, R. J. Spaete, and B. K. Wheeler for technical assistance; NADC Audiovisual Services and S. L. Johnson for assistance with preparation of the manuscript; and H. S. Hurd and M. V. Palmer for their critical reviews of the manuscript.
Editor: B. B. Finlay
REFERENCES
- 1.Banatvala, N., M. M. Debeukelaer, P. M. Griffin, T. J. Barrett, K. D. Greene, J. H. Green, and J. G. Wells. 1996. Shiga-like toxin-producing Escherichia coli O111 and associated hemolytic-uremic syndrome: a family outbreak. Pediatr. Infect. Dis. J. 15:1008-1011. [DOI] [PubMed] [Google Scholar]
- 2.Banatvala, N., P. M. Griffin, K. D. Greene, T. J. Barrett, W. F. Bibb, J. H. Green, and J. G. Wells. 2001. The United States National Prospective Hemolytic Uremic Syndrome Study: microbiologic, serologic, clinical, and epidemiologic findings. J. Infect. Dis. 183:1063-1070. [DOI] [PubMed] [Google Scholar]
- 3.Bertschinger, H., and J. Pohlenz. 1983. Bacterial colonization and morphology of the intestine in porcine Escherichia coli enterotoxemia (edema disease). Vet. Pathol. 20:99-110. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. 1995. Outbreak of acute gastroenteritis attributable to Escherichia coli serotype O104:H21—Helena, Montana 1994. Morb. Mortal. Wkly. Rep. 44:501-503. [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. 2000. Escherichia coli O111:H8 outbreak among teenage campers—Texas, 1999. Morb. Mortal. Wkly. Rep. 49:321-324. [PubMed] [Google Scholar]
- 6.Dean-Nystrom, E. A., J. Pohlenz, H. W. Moon, and A. D. O'Brien. 2000. Escherichia coli O157:H7 causes more severe systemic disease in suckling piglets than in colostrum-deprived neonatal piglets. Infect. Immun. 68:2356-2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dean-Nystrom, E. A., B. T. Bosworth, W. C. Cray, Jr., and H. W. Moon. 1997. Pathogenicity of Escherichia coli O157:H7 in the intestines of neonatal calves. Infect. Immun. 65:1842-1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dean-Nystrom, E. A., B. T. Bosworth, H. W. Moon, and A. D. O'Brien. 1998. Escherichia coli O157:H7 requires intimin for enteropathogenicity in calves. Infect. Immun. 66:4560-4563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Donnenberg, M. S., S. Tzipori, M. L. McKee, A. D. O'Brien, J. Alroy, and J. B. Kaper. 1993. The role of the eae gene of enterohemorrhagic Escherichia coli in intimate attachment in vitro and in a porcine model. J. Clin. Investig. 92:1418-1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doughty, S., J. Sloan, V. Bennett-Wood, M. Robertson, R. M. Robins-Browne, and E. L. Hartland. 2002. Identification of a novel fimbrial gene cluster related to long polar fimbriae in locus of enterocyte effacement-negative strains of enterohemorrhagic Escherichia coli. Infect Immun. 70:6761-6769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dytoc, M. T., A. Ismaili, D. J. Philpott, R. Soni, J. L. Brunton, and P. M. Sherman. 1994. Distinct binding properties of eaeA-negative verocytotoxin-producing Escherichia coli of serotype O113:H21. Infect. Immun. 62:3494-3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feng, P., S. D. Weagant, and S. R. Monday. 2001. Genetic analysis for virulence factors in Escherichia coli O104:H21 that was implicated in an outbreak of hemorrhagic colitis. J. Clin. Microbiol. 39:24-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fey, P. D., R. S. Wickert, M. E. Rupp, T. J. Safranek, and S. H. Hinrichs. 2000. Prevalence of non-O157:H7 Shiga toxin-producing Escherichia coli in diarrheal stool samples from Nebraska. Emerg. Infect. Dis. 6:530-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Francis, D. H., R. A. Moxley, and C. Y. Andraos. 1989. Edema disease-like brain lesions in gnotobiotic piglets infected with Escherichia coli serotype O157:H7. Infect. Immun. 57:1339-1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Griffin, P. M. 1998. Epidemiology of Shiga toxin-producing Escherichia coli infections in humans in the United States, p. 15-22. In J. B. Kaper and A. D. O'Brien (ed.), Escherichia coli O157:H7 and other Shiga toxin-producing E. coli strains. American Society for Microbiology Press, Washington, D.C.
- 16.Gunzer, F., I. Hennig-Pauka, K. H. Waldmann, R. Sandhoff, H. J. Grone, H. H. Kreipe, A. Matussek, and M. Mengel. 2002. Gnotobiotic piglets develop thrombotic microangiopathy after oral infection with enterohemorrhagic Escherichia coli. Am. J. Clin. Pathol. 118:364-375. [DOI] [PubMed] [Google Scholar]
- 17.Hii, J. H., C. Gyles, T. Morooka, M. A. Karmali, R. Clarke, S. De Grandis, and J. L. Brunton. 1991. Development of verotoxin 2- and verotoxin 2 variant (VT2v)-specific oligonucleotide probes on the basis of the nucleotide sequence of the B cistron of VT2v from Escherichia coli E32511 and B2F1. J. Clin. Microbiol. 29:2704-2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ito, H., A. Terai, H. Kurazono, Y. Takeda, and M. Nishibuchi. 1990. Cloning and nucleotide sequencing of Vero toxin 2 variant genes from Escherichia coli O91:H21 isolated from a patient with the hemolytic uremic syndrome. Microb. Pathog. 8:47-60. [DOI] [PubMed] [Google Scholar]
- 19.Junkins, A. D., and M. P. Doyle. 1989. Comparison of adherence properties of Escherichia coli O157:H7 and a 60-megadalton plasmid-cured derivative. Curr. Microbiol. 19:21-27. [Google Scholar]
- 20.Kaper, J. B., L. J. Gansheroff, M. R. Wachtel, and A. D. O'Brien. 1998. Attaching-and-effacing intestinal histopathology and the locus of enterocyte effacement, p. 163-182. In J. B. Kaper and A. D. O'Brien (ed.), Escherichia coli O157:H7 and other Shiga toxin-producing E. coli strains. American Society for Microbiology Press, Washington, D.C.
- 21.Karch, H., J. Heesemann, R. Laufs, A. D. O'Brien, C. O. Tacket, and M. M. Levine. 1987. A plasmid of enterohemorrhagic Escherichia coli O157:H7 is required for expression of a new fimbrial antigen and for adhesion to epithelial cells. Infect. Immun. 55:455-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kausche, F. M., E. A. Dean, L. H. Arp, J. E. Samuel, and H. W. Moon. 1992. An experimental model for subclinical edema disease (Escherichia coli enterotoxemia) manifest as vascular necrosis in pigs. Am. J. Vet. Res. 53:281-287. [PubMed] [Google Scholar]
- 23.Kokai-Kun, J. F., A. R. Melton-Celsa, and A. D. O'Brien. 2000. Elastase in intestinal mucus enhances the cytotoxicity of Shiga toxin type 2d. J. Biol. Chem. 275:3713-3721. [DOI] [PubMed] [Google Scholar]
- 24.Lindgren, S. W., A. R. Melton, and A. D. O'Brien. 1993. Virulence of enterohemorrhagic Escherichia coli O91:H21 clinical isolates in an orally infected mouse model. Infect. Immun. 61:3832-3842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luperchio, S. A., and D. B. Schauer. 2001. Molecular pathogenesis of Citrobacter rodentium and transmissible murine colonic hyperplasia. Microbes Infect. 3:333-340. [DOI] [PubMed] [Google Scholar]
- 26.McCarthy, T. A., N. L. Barrett, J. L. Hadler, B. Salsbury, R. T. Howard, D. W. Dingman, C. D. Brinkman, W. F. Bibb, and M. L. Cartter. 2001. Hemolytic-uremic syndrome and Escherichia coli O121 at a lake in Connecticut, 1999. Pediatrics 108:E59. [DOI] [PubMed] [Google Scholar]
- 27.McDaniel, T. K., K. G. Jarvis, M. S. Donnenberg, and J. B. Kaper. 1995. A genetic locus of enterocyte effacement conserved among diverse enterobacterial pathogens. Proc. Natl. Acad. Sci. USA 92:1664-1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McKee, M. L., A. R. Melton-Celsa, R. A. Moxley, D. H. Francis, and A. D. O'Brien. 1995. Enterohemorrhagic Escherichia coli O157:H7 requires intimin to colonize the gnotobiotic pig intestine and to adhere to HEp-2 cells. Infect. Immun. 63:3739-3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mead, P. S., L. Slutsker, V. Dietz, L. F. McCaig, J. S. Bresee, C. Shapiro, P. M. Griffin, and R. V. Tauxe. 1999. Food-related illness and death in the United States. Emerg. Infect. Dis. 5:607-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Melton-Celsa, A. R., S. C. Darnell, and A. D. O'Brien. 1996. Activation of Shiga-like toxins by mouse and human intestinal mucus correlates with virulence of enterohemorrhagic Escherichia coli O91:H21 isolates in orally infected, streptomycin-treated mice. Infect. Immun. 64:1569-1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Melton-Celsa, A. R., H. F. Kokai-Kun, and A. D. O'Brien. 2002. Activation of Shiga toxin type 2d (Stx2d) by elastase involves cleavage of the C-terminal two amino acids of the A2 peptide in the context of the appropriate B pentamer. Mol Microbiol. 43:207-215. [DOI] [PubMed] [Google Scholar]
- 32.Moon, H. W., D. K. Sorensen, and J. H. Sautter. 1968. Experimental enteric colibacillosis in piglets. Can. J. Comp. Med. 32:493-497. [PMC free article] [PubMed] [Google Scholar]
- 33.Morabito, S., G. Dell'Omo, U. Agrimi, H. Schmidt, H. Karch, T. Cheasty, and A. Caprioli. 2001. Detection and characterization of Shiga toxin-producing Escherichia coli in feral pigeons. Vet. Microbiol. 82:275-283. [DOI] [PubMed] [Google Scholar]
- 34.O'Brien, A. D., and R. K. Holmes. 1987. Shiga and Shiga-like toxins. Microbiol. Rev. 51:206-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paton, A. W., P. Srimanote, M. C. Woodrow, and J. C. Paton. 2001. Characterization of Saa, a novel autoagglutinating adhesin produced by locus of enterocyte effacement-negative Shiga-toxigenic Escherichia coli strains that are virulent for humans. Infect. Immun. 69:6999-7009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pierard, D., G. Muyldermans, L. Moriau, D. Stevens, and S. Lauwers. 1998. Identification of new verocytotoxin type 2 variant B-subunit genes in human and animal Escherichia coli isolates. J. Clin. Microbiol. 36:3317-3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sandhu, K. S., and C. L. Gyles. 2002. Pathogenic Shiga toxin-producing Escherichia coli in the intestine of calves. Can. J. Vet. Res. 66:65-72. [PMC free article] [PubMed] [Google Scholar]
- 38.Schmidt, H., J. Scheef, S. Morabito, A. Caprioli, L. H. Wieler, and H. Karch. 2000. A new Shiga toxin 2 variant (Stx2f) from Escherichia coli isolated from pigeons. Appl. Environ. Microbiol. 66:1205-1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schmitt, C. K., M. L. McKee, and A. D. O'Brien. 1991. Two copies of Shiga-like toxin II-related genes common in enterohemorrhagic Escherichia coli strains are responsible for the antigenic heterogeneity of the O157:H- strain E32511. Infect. Immun. 59:1065-1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scott, M. E., A. R. Melton-Celsa, and A. D. O'Brien. 2003. Mutations in hns reduce the adherence of Shiga toxin-producing E. coli O91:H21 strain B2F1 to human colonic epithelial cells and increase the production of hemolysin. Microb. Pathog. 34:155-159. [DOI] [PubMed] [Google Scholar]
- 41.Srimanote, P., A. W. Paton, and J. C. Paton. 2002. Characterization of a novel type IV pilus locus encoded on the large plasmid of locus of enterocyte effacement-negative Shiga-toxigenic Escherichia coli strains that are virulent for humans. Infect. Immun. 70:3094-3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tarr, P. I., S. S. Bilge, J. C. Vary, Jr., S. Jelcic, R. L. Haabeeb, T. R. Ward, M. R. Baylor, and T. E. Besser. 2000. Iha: a novel Escherichia coli O157:H7 adherence-conferring molecule encoded on a recently acquired chromosomal island of conserved structure. Infect. Immun. 68:1400-1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Teel, L. D., C. K. Schmitt, A. R. Melton-Celsa, and A. D. O'Brien. 2002. One of two copies of the gene for the activatable Shiga toxin type 2d in Escherichia coli O91:H21 strain B2F1 is associated with an inducible bacteriophage. Infect. Immun. 70:4282-4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Torres, A. G., J. A. Giron, N. T. Perna, V. Burland, F. R. Blattner, F. Avelino-Flores, and J. B. Kaper. 2002. Identification and characterization of lpfABCC′DE, a fimbrial operon of enterohemorrhagic Escherichia coli O157:H7. Infect Immun. 70:5416-5427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tzipori, S., C. W. Chow, and H. R. Powell. 1988. Cerebral infection with Escherichia coli 0157:H7 in humans and gnotobiotic piglets. J. Clin. Pathol. 41:1099-1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tzipori, S., F. Gunzer, M. S. Donnenberg, L. de Montigny, J. B. Kaper, and A. Donohue-Rolfe. 1995. The role of the eaeA gene in diarrhea and neurological complications in a gnotobiotic piglet model of enterohemorrhagic Escherichia coli infection. Infect. Immun. 63:3621-3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wadolkowski, E. A., J. Burris, and A. D. O'Brien. 1990. Mouse model for colonization and disease caused by enterohemorrhagic Escherichia coli O157:H7. Infect. Immun. 58:2438-2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weinstein, D. L., M. P. Jackson, J. E. Samuel, R. K. Holmes, and A. D. O'Brien. 1988. Cloning and sequencing of a Shiga-like toxin type II variant from an Escherichia coli strain responsible for edema disease of swine. J. Bacteriol. 170:4223-4230. [DOI] [PMC free article] [PubMed] [Google Scholar]