According to WHO statistics, in 1996 875,000 new cases of colorectal cancer were diagnosed and 495,000 deaths were attributed to it worldwide 1. It is the third most common cause of cancer deaths and its incidence and mortality rates continue to rise.
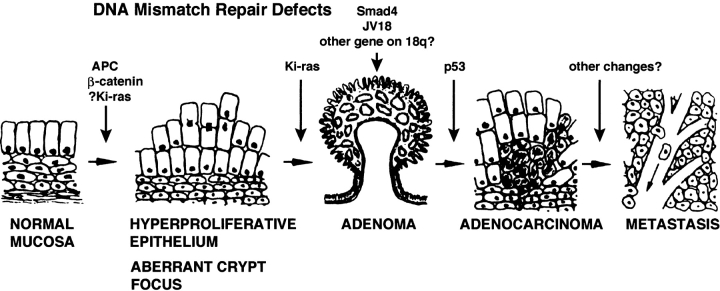
Most colorectal cancers develop via a characteristic series of pathological steps (Fig. 1). Colorectal epithelial cells acquire abnormal growth and morphological characteristics and form an adenoma, a tumor mass often protruding into the lumen of the colon or rectum. In time, these lesions enlarge and a subset of cells can acquire additional abnormal growth behaviors, which allows them to invade into the bowel wall and metastasize. At this point, the tumors are classified as adenocarcinomas and can be lethal. In the course of this typical multistep process, somatic mutations develop in key genes in the cells that comprise these lesions, such as in: the adenomatous polyposis coli (APC) and p53 tumor suppressor genes; the Ki-ras oncogene; and various genes that mediate DNA mismatch repair 2. The mutational events presumably drive the evolution of the pathological lesions and their neoplastic and malignant behavior.
Figure 1.
Colorectal carcinogenesis. Normal epithelial cells acquire abnormal growth and morphological characteristics and form an aberrant crypt focus, which enlarges to become an adenoma. With time, adenomas increase in size and can become adenocarcinomas, which have the ability to invade and metastasize. Presumably driving this process are somatic mutations that develop in the designated key genes in the colonocytes that make up these lesions; abnormalities in DNA mismatch repair mechanisms can also contribute. Aspirin and other NSAIDs inhibit this process, perhaps at several distinct points. Figure is adapted from reference 2.
Considerable evidence supports the view that aspirin (ASA) and other nonsteroidal antiinflammatory drugs (NSAIDs) prevent colorectal cancer 3. A combination of epidemiological, animal, and basic studies make a compelling case that regular use of these compounds lowers the risk for the development of colorectal cancer, as well as adenomas. Recently, sulindac was also shown to eliminate aberrant crypt foci in the colorectum of patients who have had adenomatous polyps. These lesions are similar to adenomas but are not yet visible to the naked eye, and probably grow to become adenomas 4. Overall, the weight of the evidence indicates that NSAIDs are the preeminent colorectal cancer chemopreventive agents. It is of paramount importance, then, to identify their mechanism of action in the chemoprevention of colorectal cancer.
NSAIDs inhibit the catalytic activity of cyclooxygenase (COX, more properly called prostaglandin H synthase, or PGHS), and this is thought to be the predominant mechanism by which they act as analgesic, antipyretic, and antiinflammatory agents (Fig. 2). Reports indicating that ASA and other NSAIDs inhibit colon carcinogenesis provided the impetus for a series of studies showing that the levels of both PGE2 and COX-2 are augmented in human colon cancer (for review see reference 3). As a result, it generally has been assumed that the antineoplastic effects of NSAIDs were dependent upon inhibition of COX activity and PG synthesis. Yet evidence is mounting that NSAIDs produce some of their clinical and experimental cancer chemopreventive effects via mechanisms that are independent of COX inhibition.
Figure 2.
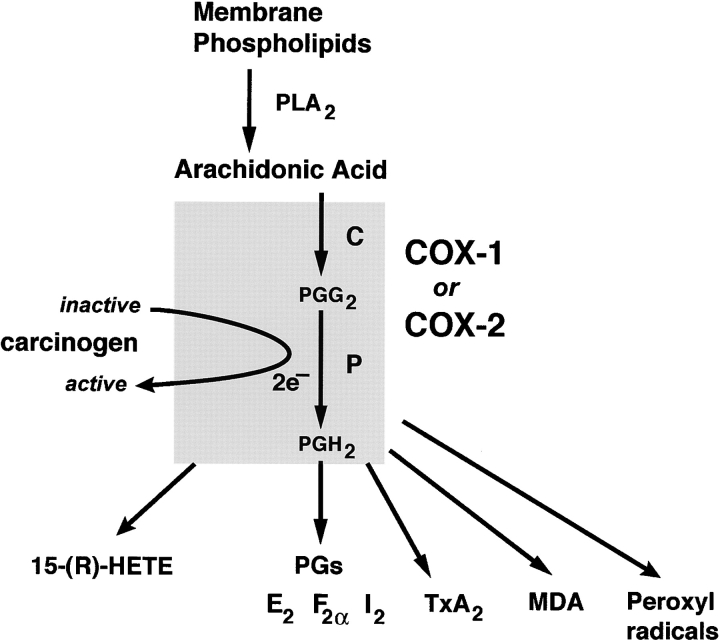
Arachidonic acid metabolism by COX isoenzymes. Phospholipase A2 (PLA2) releases arachidonic acid (AA) from membrane phospholipids, which is in turn converted by either COX-1 or COX-2 to PGG2 (C, cyclooxygenase catalytic activity of COX) and then to PGH2 (P, peroxidase catalytic activity of COX). PGH2 is converted to either PGs (e.g., E2, F2α, I2, D2); thromboxane A2 (TxA2); or malondialdehyde (MDA). MDA is a direct-acting mutagen and carcinogen and can be produced without COX by direct lipid peroxidation. AA can be converted directly to 15-(R)-HETE by both COX isoenzymes. COX-1 is constitutively expressed in most tissues, whereas COX-2 is induced by cytokines, growth factors, tumor promoters, or other agents after the initiation of specific physiological events. Compounds other than PGG2, e.g., procarcinogenic hydroperoxides, can serve as substrates for the peroxidase activity of both COX enzymes. Inactive carcinogens serving as electron acceptors can also become activated by this activity. The COX isoenzymes are also involved in the formation of peroxyl radicals that can activate procarcinogens. Figure is adapted from reference 3.
In this issue of The Journal of Experimental Medicine, Zhang et al. provide additional compelling evidence for COX-independent chemopreventive effects of NSAIDs 5. They accomplish this by demonstrating that NSAID-induced inhibition of cell transformation, inhibition of cell proliferation, and initiation of apoptosis are not dependent on the expression of COX isozymes. By genetic manipulation of mouse embryos, they developed fibroblasts in which COX-1 or COX-2 or both COX-1 and COX-2 mRNAs and proteins were absent. The resulting three types of cell lines were equally capable of undergoing oncogenic transformation induced by ras and SV-40 oncogenes. In addition, the proliferation of transformed versions of these three cell types was inhibited and apoptosis was stimulated equally by various NSAIDs. Both non-COX-selective NSAIDs (i.e., sulindac, ibuprofen, piroxicam, and indomethacin) and the selective COX-2 inhibitor, NS-398 produced these effects in all three types of cell lines. Thus, targeted disruption of COX genes does not affect these NSAID-induced events related to transformation and cell turnover kinetics.
A few years ago, we demonstrated that NSAIDs inhibited the proliferation and induced apoptosis in human colon cancer cell lines 6 7. Since this effect was obtained in medium supplemented with serum rich in PGs, we pursued this observation further to assess its dependence upon PGs and COX. In due course, a colon cancer cell line, HCT-15, which was intrinsically deficient in COX-1 and COX-2 expression and failed to produce any prostanoids even upon stimulation with arachidonic acid, mellitin, and the calcium ionophore A23187 was identified 8. NSAIDs had effects on proliferation and apoptosis in these cells similar to those in cells that express both COX enzymes and produce several PGs, such as HT-29. These observations, reinforced by subsequent studies demonstrating that compounds with meager COX inhibitory activity (e.g., sulindac sulfone, and salicylic acid [SA]) also inhibit cell proliferation and induce apoptosis in colonocytes, led to the hypothesis that COX-independent mechanisms account for at least some of the chemopreventive actions of NSAIDs 3. A trivial explanation for our results and those of Zhang et al. is that there exist additional undiscovered COX isoforms that compensate for the loss of the known cyclooxygenases. However, this is practically untenable, because COX-1 and COX-2 null fibroblasts and HCT-15 cells do not produce PGs, a defining characteristic of COX enzymes; as well, HCT-15 cells do not produce PGs even upon stimulation. Regardless of this, the evidence should and already has aroused suspicion that NSAIDs may induce their chemopreventive effects by targeting other proteins or pathways, beyond the cyclooxygenases. Because of the probable importance of these proteins or pathways in preventing neoplasia, many laboratories are now actively working to identify them.
Several selected observations regarding the antineoplastic actions of NSAIDs are briefly reviewed below. They are broadly classified into three major categories according to their dependence on COX inhibition: (i) COX-dependent or COX-related; (ii) COX-independent; and (iii) uncertain, i.e., those that do not appear to involve COX, although their COX independence is not yet proven (Table ).
Table 1.
Mechanisms Influenced by NSAIDS That May Contribute to Their Antineoplastic Effects
| COX-dependent | COX-independent | COX dependence unclear |
|---|---|---|
| Cell turnover | Cell turnover | Tumor immunity |
| proliferation/apoptosis | proliferation/apoptosis | Myc transcription |
| Carcinogen formation | Cell transformation | PPAR activation |
| Angiogenesis | DNA repair | |
| Angiogenesis | ||
| Ras signal transduction | ||
| MAP kinase activation | ||
| NF-κB activation |
The table shows the cellular or molecular mechanisms influenced by NSAIDs that may contribute to their antineoplastic effects, classified in relation to their dependence on inhibition of cyclooxygenase catalytic activity.
COX-dependent Effects
For the antineoplastic effects of NSAIDs to be exerted through COX inhibition, COX and PGs must contribute substantially to colorectal carcinogenesis. Evidence that eicosanoids and COX isozymes are important in colorectal cancer development includes: (a) PGE2 levels are elevated in colorectal tumors; (b) eicosanoids, including PGs, stimulate proliferation and reduce apoptosis in colonocytes; (c) COX-2 is upregulated in colorectal tumors, more frequently in cancers than adenomas; and (d) COX-2 contributes to colorectal tumorigenesis in APC knockout mice 9 where APCΔ716(+/−)COX-2(−/−) mice develop fewer intestinal tumors than do APCΔ716(+/−)COX-2(−/+) mice, which in turn bear fewer tumors than do APCΔ716(+/−)COX-2(+/+) mice (for review see reference 3). Aside from producing several eicosanoids, COX may promote carcinogenesis by activating carcinogens via its peroxidase activity, which can operate on substrates other than PGG2; or by producing either malondialdehyde, a direct-acting mutagen, or peroxyl radicals (see Fig. 2).
Angiogenesis, which is important in carcinogenesis, is related to PGs and COX. PGE1 and COX-2 induce angiogenesis 10 and NSAIDs inhibit it 11. Clearly, inhibition of angiogenesis could explain the ability of NSAIDs to regress adenomas in familial adenomatous polyposis (FAP) patients and to inhibit colorectal adenocarcinoma formation. However, like most if not all of the mechanisms discussed here, it has yet to be formally demonstrated in vivo that angiogenesis mediates the chemopreventive effects of NSAIDs in colorectal cancer. It is noteworthy that sulindac sulfone also inhibits angiogenesis 12. This raises the possibility of PG- or COX-independent mechanisms of angiogenesis as well.
Whether COX or eicosanoids are necessary or essential for colorectal carcinogenesis has not been fully assessed. Clearly, some colorectal cancers develop without overexpressing COX or producing high levels of PGs. However, this still does not rule out a central role for COX enzymes, at least for most colorectal cancers.
COX-independent Effects
Cell Turnover.
A potentially important means by which NSAIDs prevent colorectal neoplasia is to affect cell turnover in the colorectal epithelium. Cell death and renewal are critical for the regulation of the structural integrity of all tissues. The growth rate of a tissue or a tumor is determined by the rate of proliferation and counterbalanced by the rates of cell loss by apoptosis or necrosis of the cells that comprise them. We and others have shown that several NSAIDs, including SA, ASA, sulindac (and its metabolites sulindac sulfide and sulindac sulfone), indomethacin, piroxicam, naproxen, as well as selective COX-2 inhibitors, retard the proliferation and induce apoptosis in colon cancer cells 3 13.
Human studies assessing the effects of NSAIDs on colonocytic proliferation have generated conflicting results, with sulindac reported either to show no effect upon proliferation or to reduce it 3. Apoptosis is suppressed in sporadic colorectal adenomas and carcinomas and in the flat mucosa or adenomas of patients with FAP. In FAP patients, sulindac normalizes apoptosis in normal rectal mucosal colonocytes while reducing the size and number of their adenomas (for review see reference 3). Thus, it is possible, but not yet fully substantiated, that cell kinetic effects play a major role in the antineoplastic effects of these compounds.
As we have shown previously, and as is now described in additional detail by Zhang et al., these events in vitro are clearly not dependent upon the expression of COX isozymes 5 8. NSAIDs inhibit cell proliferation by inducing cell cycle quiescence in colonocytes, in part by reducing the levels of several key molecules that catalyze transitions through the various phases of the cell division cycle 3 6 7. However, the detailed molecular pathways that induce quiescence have yet to be fully elucidated.
Several groups contend that they have identified mechanisms by which NSAIDs induce PG- or COX-independent apoptosis. Preliminary reports claim that sulindac sulfone induces this form of cell death by inhibiting cGMP-dependent phosphodiesterase 5 14. Others have shown that NSAID treatment of colon cancer cells generates the proapoptotic lipid, ceramide 15. By blocking the biosynthesis of prostanoids, NSAIDs increase the intracellular levels of arachidonic acid, which activates neutral sphingomyelinase, which in turn converts sphingomyelin to ceramide. Strictly speaking, this is a PG-independent mechanism, but, as presented, this model of ceramide formation is dependent upon COX inhibition and therefore may not explain the apoptosis induced by sulindac sulfone or SA, nor that induced in COX null cells, like HCT-15 or those generated by Zhang et al.
Cell Transformation.
Aside from Zhang et al., other groups have also reported that NSAIDs inhibit cell transformation. Dong et al. showed that salicylates inhibited phorbol ester (TPA)-induced transformation of mouse epidermal JB6 cells 16. Hermann et al. demonstrated that sulindac sulfide inhibited transformation of primary rat embryo fibroblasts by activated H-ras and SV40 T antigen or other transformation-inducing stimuli 17. In both cases, transformation was inhibited at drug concentrations below those required to inhibit cell proliferation or cell viability. Dong et al. proposed that AP-1 activation is important for this effect of salicylates. This process appeared to be independent of PG inhibition, as indomethacin could not inhibit TPA-induced transformation, nor AP-1 activity. Mitogen-activated protein (MAP) kinase was shown not to be involved in this process. Hermann et al. proposed that sulindac sulfide inhibits oncogenic cell transformation by directly inhibiting Ras signaling. They showed that sulindac sulfide binds noncovalently to Ras and inhibits Ras-dependent Raf binding and Raf activation without affecting its GTPase or GTP binding activity.
Apart from its role in cell transformation, Ras influences many pathways potentially important for the chemopreventive activity of NSAIDs, and thus it may be a pivotal target molecule integrating several disparate pathways influenced by NSAIDs. Inhibition of Ras signaling may also explain the effects of NSAIDs on proliferation and apoptosis at higher drug concentrations. Ras inhibition may link the effect of NSAIDs to NF-κB and MAP kinase activity (see below) 18. Modulation of Ras activity by NSAIDs may also relate to the eicosanoid pathway, as arachidonic acid and PGs regulate Ras regulatory proteins such as p120GAP and NF-1GAP 19.
DNA Repair.
Both ASA and nonsalicylate NSAIDs reduce microsatellite instability in colon cancer cell lines deficient in DNA mismatch repair (i.e., HCT-116, HCT-15, SW48, and LoVo cells) 20. Mismatch repair–deficient cells with mutations in hMLH1, hMSH2, or hMSH6, but not with a hPMS2 mutation, die selectively by apoptosis in response to NSAID treatment. Given that HCT-15 cells were used in this study, which we found to be deficient in COX expression 8, this effect is also likely to be COX independent. However, Rüschoff et al. 20 did not directly confirm the absence of COX isozymes or PG production in the cells they used. Patients with hereditary nonpolyposis colorectal cancer have germline mutations in DNA mismatch repair genes. Therefore, it was speculated that NSAIDs could prevent colon cancer in most of these patients. However, the clinical efficacy of NSAIDs in this particular high-risk group remains unknown.
MAP Kinases.
SA activates p38 MAP kinase and induces apoptosis in FS-4 fibroblasts, and both are inhibited by the p38 MAP kinase inhibitor SB-203580. p38MAP kinase activation may also be important for inhibition of nuclear factor (NF)-κB by SA 21.
NF-κB.
ASA and SA, but not indomethacin, inhibit NF-κB activation 21 22. There is evidence that these compounds bind to and inactivate IκB kinase β, which in turn prevents the degradation of IκB and the subsequent translocation of NF-κB to the nucleus, where it activates the transcription of a variety of genes 22. Depending on the cell type and the circumstances, NF-κB augments or inhibits apoptosis. Inhibition of the proliferation of ras-transformed rat fibroblasts by ASA may also be related to inhibition of NF-κB activation 18.
To show that activation of MAP kinases or inhibition of NF-κB activation are COX-independent processes, Yin et al. demonstrated that each is influenced exclusively by salicylates, and not by indomethacin 22. However, indomethacin and other nonsalicylate NSAIDs prevent colon neoplasias and regress adenomas in FAP patients. As a result, it is unlikely that these two processes, as delineated in these experiments, play a major role in the chemopreventive effects of NSAIDs.
Role of COX Unclear
The observations that follow relate to processes that do not appear related to PGs or COX inhibition. However, since they have not been examined in detail in cells that lack COX enzymes or PG production, their relationship to COX and/or arachidonic acid metabolism remains uncertain.
Myc/src Oncoproteins.
Lu et al. found that, in addition to apoptosis, Myc was markedly induced in serum-starved chicken embryo fibroblasts after activation of pp60v-src and NSAID treatment. Apoptosis was markedly inhibited by transfection of antisense myc 23. Similar results were found in other cancer cell lines.
Peroxisomal Proliferator-activated Receptors.
Peroxisomal proliferator-activated receptors (PPARs) are a group of nuclear hormone receptor protein transcription factors that, when stimulated, induce differentiation of fibroblasts to adipocytes. PPAR-γ receptors are expressed in the colon and to an even greater degree in colon tumors 24. Activation of PPARs in colon cancer cells reduces their growth and induces differentiation in vitro 25. Interestingly, indomethacin and selected, but not all, NSAIDs bind to and activate PPAR-γ receptors 26. However, piroxicam, which regresses colorectal polyps in humans and animals, does not bind PPAR-γ as effectively as other NSAIDs 26 and PPAR-γ activators, such as troglitazone, increase colon tumors in APCMin mice 27 28. These latter data suggest it is unlikely that PPAR activation is important for the chemoprevention of colorectal cancer by NSAIDs.
Tumor Immunity.
There is ample indirect evidence for a role of NSAIDs, and by extension of COX enzymes, in immune phenomena related to various cancers. A clearly studied case concerns their role in the expression of HLAs, which is altered in many cancers and frequently downregulated in colon cancer 3. Such abnormalities may adversely affect the clinical course of cancer and the outcome of T cell–based immunotherapy.
NSAIDs may boost mechanisms of tumor immune surveillance; tumors are hypothesized to escape from immune-mediated destruction by thwarting mechanisms that detect tumor-associated antigens. Class I and II HLA antigens, participating in antigen presentation, may be critical to this process. The role of NSAIDs in these processes has been assessed in animal models of colon cancer and in cell culture systems. Piroxicam upregulates the expression of MHC genes in the colonic mucosa of rats treated with a carcinogen 3. PGE2 reduces the transcription of HLA class II molecules and NSAIDs can increase it 29. That such effects occur in the presence of PG-rich serum in cell culture systems indicates, along with additional evidence (Rigas, B., unpublished observations), that this is probably another COX-independent effect of NSAIDs. That NSAIDs can induce the expression of the suppressed HLA genes in colorectal neoplasias suggests that these versatile compounds may restore the ability of the immune system to eliminate transformed cells.
As this brief overview demonstrates, multiple processes with their attendant molecular pathways have been proposed to mediate the chemopreventive effects of NSAIDs (Table ). The NSAIDs, highly protein bound molecules, probably interact with and inhibit the function of many proteins and, perhaps, other macromolecules. To complicate matters, it is not clear whether the key in vitro effects of NSAIDs that were discussed earlier, such as cell transformation, cell growth, or angiogenesis, are at all relevant to colorectal cancer chemoprevention.
Some of the data reviewed here indicate that COX inhibition by NSAIDs is indeed required for their chemopreventive effect. However, another body of data, including that of Zhang et al. in this issue 5, make the equally strong case that COX inhibition is not required for certain presumed chemopreventive effects of NSAIDs. This apparent inconsistency is not merely of theoretical interest, but has important implications for the rational design of strategies for colon chemoprevention and for assessing the relative significance of each mechanism in carcinogenesis. In all likelihood this is not a contradiction at all; rather, NSAIDs bring about their chemopreventive effects in the colon through both COX-dependent and -independent mechanisms. Indeed, we have proposed a model that assumes both mechanisms operate to produce the clinical antineoplastic effects of NSAIDs, in which COX-dependent and -independent pathways modulate different stages of the multistep process of colon carcinogenesis or different events regulating each stage 30. The multiplicity of action of NSAIDs, if confirmed, could in fact explain their high degree of effectiveness in colon cancer prevention in humans.
The great challenge will be to determine which of these or other yet unknown mechanisms produce the remarkable anticancer effect of NSAIDs, as well as the relative contribution of each. If and when these key questions are worked out, then a great deal will have been learned about colorectal carcinogenesis. In addition, new tools will have been collected to identify new compounds that hold promise to prevent colorectal cancer more safely and effectively than the conventional NSAIDs.
Acknowledgments
We thank the Arthur and Rochelle Belfer Foundation and the National Institutes of Health (grant CA73298) for funding.
References
- WHO . The World Health Report. World Health Organization; Geneva, Switzerland: 1997. [Google Scholar]
- Kinzler K.W., Vogelstein B. Lessons from hereditary colorectal cancer. Cell. 1996;87:159–170. doi: 10.1016/s0092-8674(00)81333-1. [DOI] [PubMed] [Google Scholar]
- Shiff S.J., Rigas B. Nonsteroidal anti-inflammatory drugs and colorectal cancerevolving concepts of their chemopreventive actions. Gastroenterology. 1997;113:1992–1998. doi: 10.1016/s0016-5085(97)99999-6. [DOI] [PubMed] [Google Scholar]
- Takayama T., Katsuki S., Takahashi Y., Ohi M., Nojiri S., Sakamaki S., Kato J., Kogawa K., Miyake H., Niitsu Y. Aberrant crypt foci of the colon as precursors of adenoma and cancer. N. Engl. J. Med. 1998;339:1277–1284. doi: 10.1056/NEJM199810293391803. [DOI] [PubMed] [Google Scholar]
- Zhang X., Morham S.G., Langenbach R., Young D.A. Malignant transformation and antineoplastic actions of nonsteroidal antiinflammatory drugs (NSAIDs) on cyclooxygenase-null embryo fibroblasts. J. Exp. Med. 1999;190:451–459. doi: 10.1084/jem.190.4.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiff S.J., Qiao L., Tsai L.-L., Rigas B. Sulindac sulfide, an aspirin-like compound, inhibits proliferation, causes cell cycle quiescence, and induces apoptosis in HT-29 colon adenocarcinoma cells. J. Clin. Invest. 1995;96:491–503. doi: 10.1172/JCI118060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiff S.J., Koutsos M.I., Qiao L., Rigas B. Nonsteroidal antiinflammatory drugs inhibit the proliferation of colon adenocarcinoma cellseffects on cell cycle and apoptosis. Exp. Cell Res. 1996;222:179–188. doi: 10.1006/excr.1996.0023. [DOI] [PubMed] [Google Scholar]
- Hanif R., Pittas A., Feng Y., Koutsos M.I., Staiano-Coico L., Shiff S.J., Rigas B. Effects of nonsteroidal antiinflammatory drugs on proliferation and on induction of apoptosis in colon cancer cells by a prostaglandin-independent pathway. Biochem. Pharmacol. 1996;52:237–245. doi: 10.1016/0006-2952(96)00181-5. [DOI] [PubMed] [Google Scholar]
- Oshima M., Dinchuk J.E., Kargman S.L., Oshima H., Hancock B., Kwong E., Trzaskos J.M., Evans J.F., Taketo M.M. Suppression of intestinal polyposis in Apc Δ716 knockout mice by inhibition of cyclooxygenase 2 (COX-2) Cell. 1996;87:803–809. doi: 10.1016/s0092-8674(00)81988-1. [DOI] [PubMed] [Google Scholar]
- Tsujii M., Kawano S., Tsuji S., Sawaoka H., Hori M., DuBois R.N. Cyclooxygenase regulates angiogenesis induced by colon cancer cells. Cell. 1998;93:705–716. doi: 10.1016/s0092-8674(00)81433-6. [DOI] [PubMed] [Google Scholar]
- Peterson H.I. Tumor angiogenesis inhibition by prostaglandin synthetase inhibitors. Anticancer Res. 1986;6:251–253. [PubMed] [Google Scholar]
- Skopinska-Rózewska E., Piazza G.A., Sommer E., Pamukcu R., Barcz E., Filewska M., Kupis W., Caban R., Rudzinski P., Bogdan J. Inhibition of angiogenesis by sulindac and its sulfone metabolite (FGN-1)a potential mechanism for their antineoplastic properties. Int. J. Tissue React. 1998;20:85–89. [PubMed] [Google Scholar]
- Elder D.J., Halton D.E., Hague A., Paraskeva C. Induction of apoptotic cell death in human colorectal carcinoma cell lines by a cyclooxygenase-2 (COX-2)-selective nonsteroidal anti-inflammatory drugindependence from COX-2 protein expression. Clin. Cancer Res. 1997;3:1679–1683. [PubMed] [Google Scholar]
- Piazza G.A., Li H., Liu L., Sperl G., Pamukcu R., Thompson W.J., Ahnen D.J. Cyclic GMP (CG) phosphodiesterase (PDE) inhibitiona novel mechanism for the antineoplastic properties of exisulind. Gastroenterology. 1999;116:A485. [Google Scholar]
- Chan T.A., Morin P.J., Vogelstein B., Kinzler K.W. Mechanisms underlying nonsteroidal antiinflammatory drug-mediated apoptosis. Proc. Natl. Acad. Sci. USA. 1998;95:681–686. doi: 10.1073/pnas.95.2.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Z., Huang C., Brown R.E., Ma W.Y. Inhibition of activator protein 1 activity and neoplastic transformation by aspirin. J. Biol. Chem. 1997;272:9962–9970. doi: 10.1074/jbc.272.15.9962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann C., Block C., Geisen C., Haas K., Weber C., Winde G., Möröy T., Müller O. Sulindac sulfide inhibits Ras signaling. Oncogene. 1998;17:1769–1776. doi: 10.1038/sj.onc.1202085. [DOI] [PubMed] [Google Scholar]
- Ljungdahl S., Shoshan M.C., Linder S. Inhibition of the growth of 12V-ras-transformed rat fibroblasts by acetylsalicylic acid correlates with inhibition of NF-κB. Anti-Cancer Drugs. 1997;8:62–66. doi: 10.1097/00001813-199701000-00008. [DOI] [PubMed] [Google Scholar]
- Han J.-W., McCormick F., Macara I.G. Regulation of ras-GAP and the neurofibromatosis-1 gene product by eicosanoids. Science. 1991;252:576–579. doi: 10.1126/science.1902323. [DOI] [PubMed] [Google Scholar]
- Rüschoff J., Wallinger S., Dietmaier W., Bocker T., Brockhoff G., Hofstadter F., Fishel R. Aspirin suppresses the mutator phenotype associated with hereditary nonpolyposis colorectal cancer by genetic selection. Proc. Natl. Acad. Sci. USA. 1998;95:11301–11306. doi: 10.1073/pnas.95.19.11301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwenger P., Alpert D., Skolnik E.Y., Vilcek J. Activation of p38 mitogen-activated protein kinase by sodium salicylate leads to inhibition of tumor necrosis factor-induced IκB α phosphorylation and degradation. Mol. Cell. Biol. 1998;18:78–84. doi: 10.1128/mcb.18.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin M.J., Yamamoto Y., Gaynor R.B. The anti-inflammatory agents aspirin and salicylate inhibit the activity of IκB kinase-β. Nature. 1998;396:77–80. doi: 10.1038/23948. [DOI] [PubMed] [Google Scholar]
- Lu X., Fairbairn D.W., Bradshaw W.S., O'Neill K.L., Ewert D.L., Simmons D.L. NSAID-induced apoptosis in Rous sarcoma virus-transformed chicken embryo fibroblasts is dependent on v-src and c-myc and is inhibited by bcl-2. Prostaglandins. 1997;54:549–568. doi: 10.1016/s0090-6980(97)00125-1. [DOI] [PubMed] [Google Scholar]
- DuBois R.N., Gupta R., Brockman J., Reddy B.S., Krakow S.L., Lazar M.A. The nuclear eicosanoid receptor, PPARγ, is aberrantly expressed in colonic cancers. Carcinogenesis. 1998;19:49–53. doi: 10.1093/carcin/19.1.49. [DOI] [PubMed] [Google Scholar]
- Sarraf P., Mueller E., Jones D., King F.J., DeAngelo D.J., Partridge J.B., Holden S.A., Chen L.B., Singer S., Fletcher C., Spiegelman B.M. Differentiation and reversal of malignant changes in colon cancer through PPAR-gamma. Nat. Med. 1998;4:1046–1052. doi: 10.1038/2030. [DOI] [PubMed] [Google Scholar]
- Lehmann J.M., Lenhard J.M., Oliver B.B., Ringold G.M., Kliewer S.A. Peroxisome proliferator-activated receptors alpha and gamma are activated by indomethacin and other non-steroidal anti-inflammatory drugs. J. Biol. Chem. 1997;272:3406–3410. doi: 10.1074/jbc.272.6.3406. [DOI] [PubMed] [Google Scholar]
- Saez E., Tontonoz P., Nelson M.C., Alvarez J.G., Ming U.T., Baird S.M., Thomazy V.A., Evans R.M. Activators of the nuclear receptor PPAR-gamma enhance colon polyp formation. Nat. Med. 1998;4:1058–1061. doi: 10.1038/2042. [DOI] [PubMed] [Google Scholar]
- Lefebvre A.-M., Chen I., Desreumaux P., Najib J., Fruchart J.-C., Geboes K., Briggs M., Heyman R., Auwerx J. Activation of the peroxisome proliferator-activated receptor gamma promotes the development of colon tumors in C57BL/6J-APCMIN/+mice. Nat. Med. 1998;4:1053–1057. doi: 10.1038/2036. [DOI] [PubMed] [Google Scholar]
- Arvind P., Papavassiliou E.D., Tsioulias G.J., Qiao L., Lovelace C.I.P., Rigas B. PGE2 down-regulates the expression of HLA-DR antigen in human colon adenocarcinoma cell lines. Biochemistry. 1995;34:5604–5609. doi: 10.1021/bi00016a035. [DOI] [PubMed] [Google Scholar]
- Rigas B., Shiff S.J. Is inhibition of cyclooxygenase required for the chemopreventive effect of NSAIDs in colon cancer? A model reconciling the current contradiction. Med. Hypotheses. 1999;In press doi: 10.1054/mehy.1999.0023. [DOI] [PubMed] [Google Scholar]