Abstract
In this paper, we test the hypothesis that triggering of a second T cell receptor (TCR) expressed on diabetogenic T cells might initiate the onset of diabetes. A cross between two TCR-transgenic strains, the BDC2.5 strain that carries diabetogenic TCRs and the A18 strain that carries receptors specific for C5, was set up to monitor development of diabetes after activation through the C5 TCR. F1 BDC2.5 × A18 mice developed diabetes spontaneously beyond 3–4 mo of age. Although their T cells express both TCRs constitutively, the A18 receptor is expressed at extremely low levels. In vitro activation of dual TCR T cells followed by adoptive transfer into neonatal or adult F1 mice resulted in diabetes onset and death within 10 d after transfer. In contrast, in vivo immunization of F1 mice with different forms of C5 antigen not only failed to induce diabetes but protected mice from the spontaneous onset of diabetes. We propose that antigenic stimulation of cells with low levels of TCR produces signals inadequate for full activation, resulting instead in anergy.
Keywords: diabetes, T cell receptor, anergy, T lymphocytes, islets
The existence of T cells with two TCR-α chains is a consequence of gene rearrangement at the α chain locus, which only terminates once an α/β TCR is expressed that allows positive selection of the cell 1. Therefore, T cells expressing two TCRs composed of the same β chain paired with two different α chains are not infrequent 2 3 4. We have previously shown that expression of a second receptor in a transgenic model can rescue T cells with receptors for self-antigen from thymic deletion, provided that these are expressed at low levels 5 6. Such rescued cells could not be activated through their self-antigen–specific receptors, but once activated via the second TCR, they were capable of autoreactive effector function in vitro. This posed the question of whether dual receptor–expressing T cells might be able to initiate autoimmune disease. To test such a hypothesis in vivo, we made use of an experimental diabetes model. The BDC2.5 TCR-transgenic mouse strain expresses diabetogenic TCRs on a NOD background 7. These mice have been shown to spontaneously develop diabetes, albeit with late onset, irrespective of the presence of other MHC molecules, e.g., H-2E, which in nontransgenic NOD mice prevent diabetes 8. It is not known what triggers the onset of islet destruction, but the presence of a second TCR is not essential, as BDC2.5 mice on a SCID background develop diabetes even earlier than transgenic mice with a diverse repertoire 9. Animal models of autoimmune diseases provide evidence that additional contributing factors, e.g., viral or bacterial infections, play an ill-defined role in triggering the disease in genetically susceptible animals 10 11 12. We crossed BDC2.5 transgenic mice with the A18 transgenic strain, which expresses C5-specific TCRs 5, so that T cells in the F1 hybrid would constitutively express both receptors. Immunization of these mice with C5 served to test whether onset of diabetes could be triggered via stimulation of the second TCR.
Materials and Methods
Transgenic Mice.
A18 TCR-transgenic Rag1−/−C5−/− mice on an A/J background 5 (TCR specific for epitope 106–121 of mouse complement C5 presented by H2-Ek) were crossed with the BDC2.5 strain 7 recognizing an unidentified peptide from islet cells presented with H-2Ag7, which we received from Drs. Diane Mathis and Christophe Benoist. These mice are Rag+/+ C5−/− and have been backcrossed onto a NOD background for 16 generations. Both parental strains were heterozygous for TCR expression so that the resulting F1 mice expressed either the A18 receptor alone (F1 A18+), the BDC2.5 receptor alone (F1 BDC+), or both receptors (F1 dual TCR).
Flow Cytometry.
Lymph node cell suspensions were stained with PE-conjugated anti-CD4 (PharMingen), FITC-conjugated anti-Vβ8.3 13 specific for the A18 TCR β chain, and biotinylated anti-Vβ4 (PharMingen) specific for the BDC2.5 TCR β chain, followed by streptavidin Red 670 (GIBCO BRL).
Cell Cultures.
Cells were stimulated in round-bottomed 96-well plates (2 × 105 cells/well) with dendritic cells from bone marrow cultures with GM-CSF (2 × 104/well). Culture medium was IMDM (Sigma Chemical Co.) supplemented with 5% FCS, 5 × 10−5 M 2-ME, 2 × 10−3 M l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin. After culture for 48 h, aliquots of supernatants were removed and tested for IL-2 content in serial dilutions on IL-2–dependent CTLL cells. Bone marrow–derived dendritic cells were generated as previously described 14 15 with some modifications 16. In brief, 5 × 106 bone marrow cells were cultured in petri dishes (9-cm diameter; Nunc, Inc.) in 10 ml culture medium containing 10% supernatant of Ag8653 myeloma cells transfected with murine GM-CSF cDNA (≈ 25 U/ml). On day 4 of culture, nonadherent cells, mostly granulocytes, were removed. Loosely adherent cells were transferred onto a second dish on day 6 of culture. From day 6 to 10, these transferred cells were used as a source of dendritic cells. Islet antigen for stimulation of BDC2.5 was prepared by subcellular fractionation of beta-tumor cells as previously described 17.
Transfer of In Vitro–activated Spleen Cells.
Spleen cells from dual or single TCR–expressing F1 mice were depleted of B cells with sheep anti–mouse IgG–coupled Dynabeads (Dynal, Inc.) and cultured at 2 × 105/ml with 2 × 104/ml dendritic cells in 25-ml culture flasks (Falcon Labware). 48 h later, cells were collected, washed, and injected intravenously into host mice.
Measurement of Cytokine Production in Cells from Pancreatic Draining Lymph Nodes.
Cell suspensions from pancreatic lymph nodes (2 × 105/well) were prepared by digestion with a cocktail of 1.6 mg/ml Collagenase (Worthington CLS4) and 0.1% DNase (Sigma Chemical Co.) for 30 min at 37°C. 2 × 105 lymph node cells per well were then cultured with different doses of C5 protein, and supernatants were removed after 48 h (for IL-2 and IL-10 measurements) or 72 h for measurement of IFN-γ production. IL-2 production was determined by a bioassay with the IL-2–dependent CTLL line, whereas IL-10 and IFN-γ production was measured in a sandwich ELISA using pairs of antibodies for each cytokine (PharMingen cytokine kits).
Measurement of Blood Glucose Levels.
Diabetes was assessed by weekly measurements of venous blood glucose concentration using BM-Test 1-44 strips and Reflolux S glucometer (Boehringer Mannheim). Animals were considered diabetic after at least two consecutive measurements >12 mM. Onset of diabetes was then dated from the first of the sequential (glucose or diabetic) measurements. After sustained hyperglycemia, mice were killed to prevent prolonged discomfort.
Results
T Cells from BDC2.5 × A18 F1 Mice Express Both TCRs.
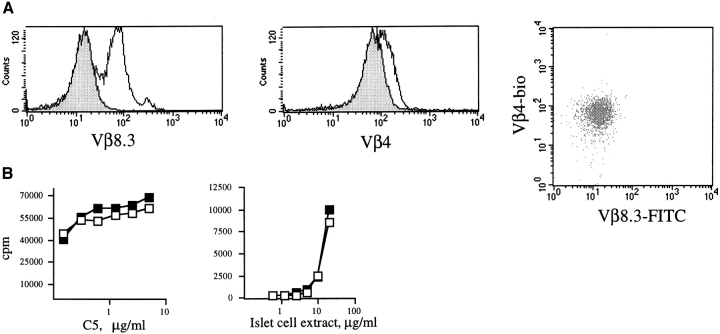
The BDC2.5 TCR-transgenic strain recognizes an unidentified peptide from islet cells presented by H-2Ag7, whereas the A18 TCR-transgenic strain recognizes a peptide from the mouse complement component C5. Both strains are C5−/− and all mice are Rag+, as the BDC2.5 strain has not been crossed onto a Rag−/− background. BDC2.5 and A18 mice, heterozygous for expression of their transgenic TCRs, were crossed to obtain F1 offspring transgenic for the A18 TCR (F1 A18+), the BDC2.5 TCR (F1 BDC+), or both (F1 dual TCR). T cells from all F1 mice were analyzed for expression of Vβ4 and Vβ8.3 (the BDC and A18 TCR-β chains respectively). As shown in the Fig. 1 histogram overlays gated for CD4 T cells, expression of the BDC Vβ4 chain is only slightly lower in dual TCR F1 mice compared with F1 mice expressing the parental BDC2.5 TCR alone, whereas expression of the A18 Vβ8.3 chain is 10-fold lower in dual TCR F1 mice compared with F1 mice transgenic for the A18 TCR only.
Figure 1.
(A) Spleen cells were triple stained with anti-Vβ8.3–FITC, anti-Vβ4–biotin, and anti-CD4–PE. Expression of TCR β chains characteristic for A18 (Vβ.8.3, left panel) or BDC2.5 (Vβ4, center panel) on gated CD4+ spleen cells. The histograms show an overlay of TCR β chain expression on CD4 T cells expressing a single TCR (open histogram) or both TCRs (shaded histogram). The dot plot shows staining for both TCRs on gated CD4 T cells. (B) IL-2 secretion by spleen cells from F1 mice transgenic for one TCR only (▪) or both TCRs (□). Spleen cells (2 × 105/well) were stimulated with A/J dendritic cells (104/well) and C5 protein (left) or dendritic cells from BDC2.5 and islet cell extract (right). The figure shows [3H]thymidine incorporation by triplicate cultures of IL-2–dependent CTLL cells. Error bars were very small and are omitted from the figure for sake of clarity.
T Cells from BDC2.5 × A18 F1 Mice Respond to Islet Antigen and C5.
As there are no clonotype-specific antibodies available for the BDC2.5 or A18 TCRs, the constitutive expression of both receptors cannot formally be verified by FACS™ analysis. We therefore compared T cell responses from BDC2.5 and A18 mice as well as the F1 strain expressing both TCRs after stimulation with either C5 protein or a preparation of islet cell membranes that contained the as yet unidentified antigen recognized by the BDC2.5 TCR. As shown in Fig. 1 B, T cells from dual TCR–expressing mice can respond to both islet cell antigen and C5, indicating that both transgenic TCRs on their surfaces are functional.
Dual TCR–expressing F1 Mice Develop Diabetes Spontaneously.
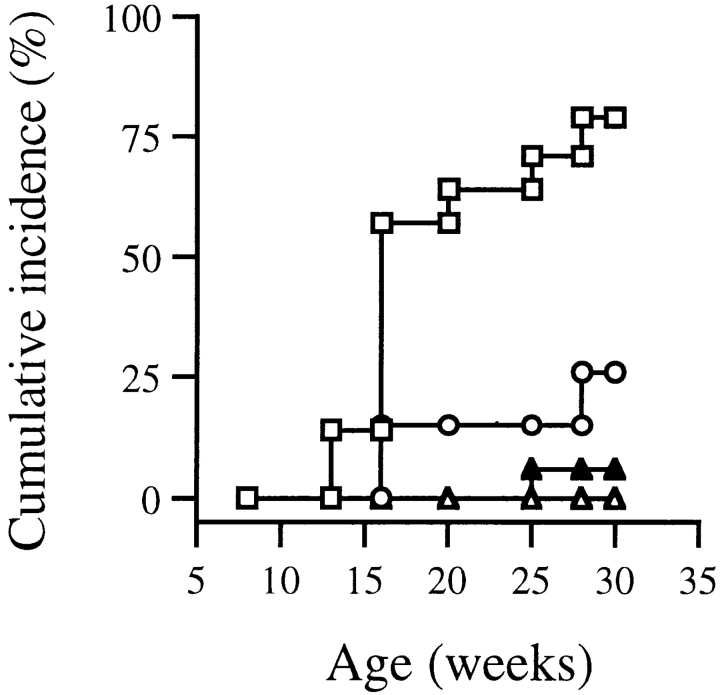
The spontaneous incidence of diabetes in BDC2.5 mice and F1 mice with A18 was determined in a cohort of mice left untreated for 9 mo. Whereas the parental BDC2.5 strain had a very low incidence of spontaneous diabetes, the dual TCR–expressing F1 mice became diabetic from 3–4 mo of age. The incidence of diabetes was higher in F1 mice transgenic for both the BDC2.5 and A18 receptors compared with F1 mice expressing the BDC2.5 receptor alone. F1 mice transgenic for only the A18 receptor never developed diabetes (Fig. 2).
Figure 2.
The cumulative incidence of diabetes is shown for a cohort of F1 dual TCR mice (transgenic for both TCR, □, 28 mice), F1 A18+ mice (transgenic for the A18 receptor only, ▵, 10 mice), F1 BDC+ (transgenic for the BDC2.5 receptor only, ○, 10 mice), and the parental BDC2.5 strain (▴, 13 mice). Animals were considered diabetic after two consecutive measurements >12 mM blood glucose.
Transfer of In Vitro–activated Dual TCR T Cells Causes Diabetes in Adoptive Hosts.
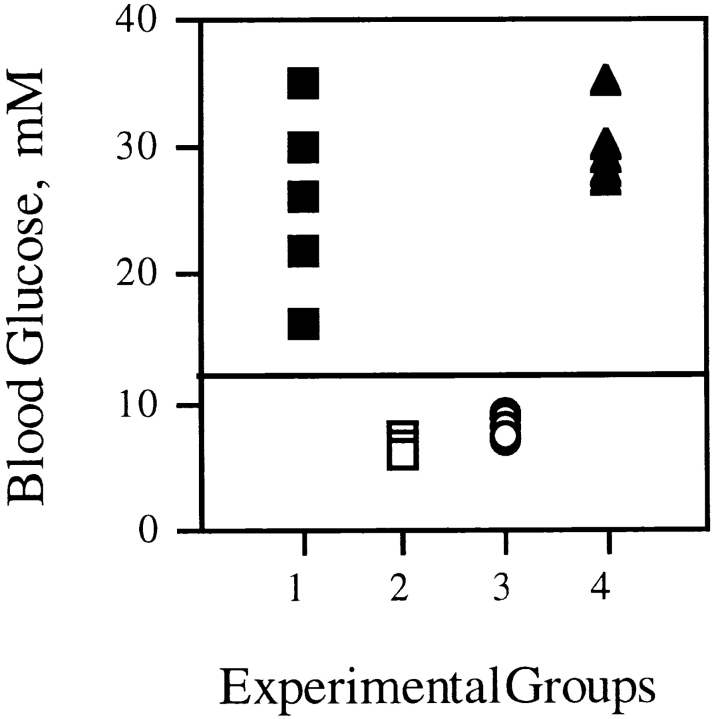
A standard protocol for testing the diabetogenic potential of T cells is transfer of activated T cells into neonatal recipients. Spleen cells from BDC2.5 × A18 F1 mice transgenic for either both TCRs or only the A18 TCR were activated in vitro with H-2E–expressing A/J dendritic cells and C5 protein and injected into F1 A18+ neonates 48 h later; these adoptive host mice were transgenic for only the A18 TCR and therefore never developed spontaneous diabetes themselves. Neonatal mice injected with C5-activated T cells from dual TCR–expressing mice developed fulminant diabetes within 1 wk after transfer (Fig. 3). In contrast, injection of C5-activated F1 A18+ cells, which express only the C5-specific TCR, did not result in diabetes. Similarly, the injection of C5-activated dual TCR T cells resulted in rapid onset of diabetes upon transfer into adult F1 A18+ mice. These data indicate that activation of the second—in this case C5-specific—TCR could indeed activate the diabetogenic potential of dual TCR–expressing T cells.
Figure 3.
Blood glucose levels in neonatal BDC2.5 × A18 F1 mice (transgenic for the A18 TCR only) after adoptive transfer of 107 T cells from C5-activated adult dual TCR–expressing F1 mice (▪, 5 mice). Control groups received an equivalent number of C5-stimulated T cells from F1 mice transgenic for the A18 TCR only (○, 4 mice) or nonactivated T cells from dual TCR mice (□, 3 mice). Adult BDC2.5 × A18 (transgenic only for the A18 TCR) recipients for 107 C5-activated T cells from dual TCR–expressing mice are shown as ▴ (5 mice). Each symbol represents one mouse.
Immunization of Dual TCR–expressing F1 Mice Does Not Induce Diabetes but Instead Protects against Spontaneous Onset of Diabetes.
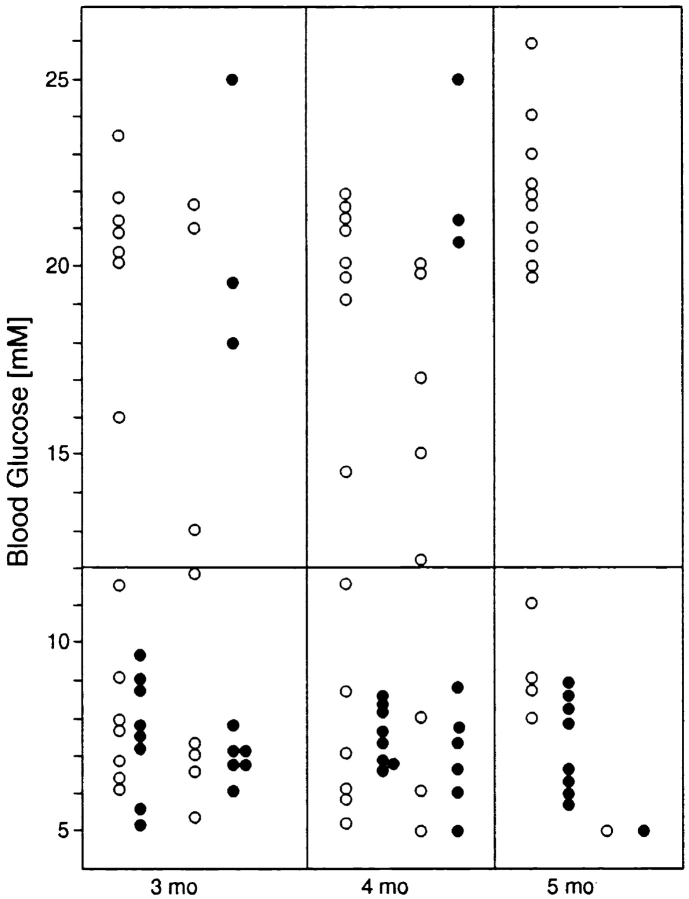
Given that C5 activation in vitro was able to induce the diabetogenic potential of dual TCR–expressing T cells, we proceeded to test this phenomenon in vivo. F1 dual TCR mice were immunized between 6–8 wk of age with C5 protein or C5 peptide either in CFA or with PBS. Control mice received CFA or PBS alone. In contrast to the results we obtained after in vitro activation of T cells, immunization with C5 protein or peptide did not result in onset of diabetes. Other immunization protocols used were subcutaneous injection with Escherichia coli expressing a C5 fusion protein with maltose binding protein, which has previously been shown to be a powerful immunogen; scarification of ear skin with a DNA construct encoding C5; and intravenous injection of dendritic cells pulsed with C5 peptide. We did not observe induction of diabetes under any of these protocols (data not shown). On the contrary, it emerged that mice immunized with various forms of C5 antigen were protected from spontaneous onset of diabetes. Fig. 4 summarizes data from a cohort of mice immunized with either C5 protein or C5 peptide in CFA (mice pooled) compared with control mice that received PBS or CFA alone, showing blood glucose levels for both groups at different ages. Only three mice in the immunized group became diabetic, whereas the majority of the control mice were diabetic by 4 mo of age.
Figure 4.
Blood glucose levels in A18 × BDC2.5 dual TCR mice that were either immunized with C5 protein or peptide (•) or treated with PBS or CFA alone (○). Immunization took place at 4–6 wk of age. Each circle represents a single mouse, and circles are spaced in four columns for each month of age to make it easier to follow the development of individual mice. For the last time point at 5 mo, not all mice were still available, as a proportion of mice had been killed for in vitro experiments.
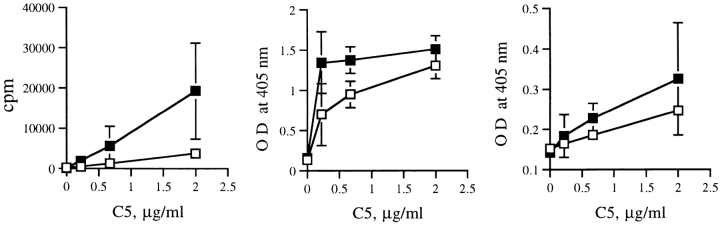
Cells from lymph nodes draining the pancreas were analyzed for cytokine secretion after in vitro restimulation with C5. It was evident that cells from immunized F1 dual TCR mice produced less IL-2 and IFN-γ than those of control F1 dual TCR mice (Fig. 5). There was no difference in IL-10 production, and IL-4 secretion (data not shown) was not detectable.
Figure 5.
Cytokine production of pancreatic lymph node cells from immunized (□) or control (▪) dual TCR mice aged 8–10 wk. Left, IL-2 production assessed by proliferation of the IL-2–dependent CTLL line; center, IFN-γ production and right, IL-10 production, both measured in ELISA.
Discussion
In this paper, we tested the hypothesis that T cells expressing two TCRs might be involved in triggering the onset of autoimmune disease. We pursued this hypothesis, which was first proposed by Padovan et al. 3, because of our finding that dual TCR T cells that had escaped from thymic deletion due to low expression of the self-specific TCR could be activated for autoreactive effector function by triggering through the second, nonself-specific TCR 6. There was no in vivo correlate for the autoreactivity demonstrated in vitro, and we attributed this to the presence of systemic and high levels of self-antigen in the periphery for this model. The choice of the BDC2.5 TCR-transgenic strain for testing the hypothesis in vivo was prompted by the description that this diabetogenic TCR is not subjected to negative selection in the thymus, the autoantigen, albeit not identified on the molecular level, is restricted to pancreas islet cells, autoimmune disease has a delayed onset in these mice, and the introduction of H-2E molecules is not protective as in the nontransgenic NOD strain 8.
T cells in the periphery of genetically susceptible individuals carrying receptors specific for autoantigen expressed in the pancreas normally should not cause any harm given the recirculation characteristics of naive T cells, which exclude their access to peripheral tissues 18. Therefore, the crucial question is how potentially diabetogenic T cells get activated to allow them to enter the pancreas and cause destruction. Several possibilities exist. For instance, dendritic cells might carry antigens from the pancreas to draining lymph nodes. This scenario took place in a transgenic model in which ovalbumin, exclusively expressed by islet cells, was cross-presented by bone marrow–derived APCs, resulting in activation of ovalbumin-specific transgenic CD8 T cells in lymph nodes draining the pancreas but not in other lymphoid sites 19. Similarly, activated T cells from BDC2.5 mice were found only in the islets and draining lymph nodes, suggesting transport of islet antigens to this site 20. However, dendritic cells do not constitutively engage in this form of presentation, termed cross-presentation, but appear to require the donor cell to undergo apoptotic death 21. It is debatable, therefore, whether antigens expressed by cells in the pancreas (unless they are secreted) are constitutively processed by dendritic cells and transported to lymph nodes, although it may be possible that individuals genetically susceptible to diabetes have a higher baseline rate of apoptosis in the pancreas, which might support such a mechanism.
An alternative explanation for how diabetogenic T cells may be activated takes into account that the onset of many autoimmune diseases is correlated with microbial infections, proposing either molecular mimicry, i.e., cross-reactive recognition of peptides shared by pathogens and auto-antigens 12 22 23 or bystander activation by inflammatory cytokines released in the course of immune responses to pathogens 24 25 26 27. Activation of a second, e.g., pathogen-specific TCR on potentially autoreactive T cells could be another way of involving pathogens in the induction of disease.
The results we obtained with mice expressing a diabetogenic TCR from the BDC2.5 TCR-transgenic strain together with the C5-specific TCR A18 indicate that activation of dual TCR cells by stimulation with C5 in vitro and transfer into neonatal or adult BDC2.5 × A18 F1 mice indeed results in rapid development of diabetes. However, immunization in vivo did not give the same results. On the contrary, we observed that immunized mice were protected from the spontaneous onset of diabetes, which occurs with high frequency in this strain combination. This suggests that T cells within the immunized mice exerted a regulatory influence on otherwise diabetogenic T cells newly emerging from the thymus. Although the underlying mechanisms of protection and regulation remain elusive, there are a number of points worth considering.
First, the expression of the C5 TCR, as indicated by staining with anti-Vβ8.3 antibody, was drastically reduced in dual TCR F1 mice compared with F1 mice carrying only the A18 transgene. Because we do not have clonotypic or Vα-specific antibodies for either receptor, we cannot formally exclude the possibility that this is due to preferential pairing of the BDC Vβ chain with the A18 Vα chain. However, these cells have reasonable reactivity to C5 stimulation, indicating that they must express the correct TCR. In several other TCR combinations, many of which were on a Rag−/− background, the presence of a second TCR resulted in reduction of the levels of the C5 TCR, even if there was no negative selection pressure from the presence of C5 6. The only exception was a dual TCR combination with the H-Y specific, H-2Ek–restricted A1 TCR 28 in which both receptors were expressed at equivalent levels (our unpublished data); this combination was also the only one in which both TCRs were expressed in the same construct under control of the human CD2 promoter. We assume that the overall level of TCRs on T cells is adjusted during thymic development, but it is not clear what factors are dictating the relative TCR levels. Positive selection in the thymus and subsequent survival signals in the periphery may provide signals that allow high surface expression of a TCR. It is interesting to note that dual TCR expression by thymocytes was prominent in immature subpopulations but much rarer in mature single positive thymocytes 29; however, the latter frequently expressed a second TCR intracellularly 30.
Irrespective of low C5 TCR expression, in vitro stimulation with C5 efficiently activated dual TCR T cells from the BDC2.5 × A18 F1 mice. However, in vitro activation, compared with immunization in vivo, is likely to be artificially optimized. Supplementation with highly efficient dendritic cells as APCs as well as optimal contact in close proximity to APC and antigen might allow strong stimulation even if the expression of TCR is reduced, whereas it may be difficult to achieve in vivo. T cells may be driven into different response modes depending on receptor levels and stimulus strength 31. In several experimental models 32 33 34, a phenotype of apparent ‘anergy’ is coupled with regulatory activity. Although we have no direct evidence for such a phenomenon in our system, the finding that protection from diabetes takes place despite the presence of a thymus that would continue to export new T cells suggests that an active mechanism is operative.
Thus, although in principle the starting hypothesis is not incorrect, it seems that the physiological behavior of dual TCR T cells cannot be predicted in simple terms. Although it has been argued that dual TCR–expressing T cells are immunologically less effective than single TCR cells 35, this does not seem to preclude functional activity. For instance, T cells expressing low levels of autoreactive receptors together with unidentified additional receptors were capable of initiating autoimmune responses in vivo 36, whereas using the BDC2.5 strain on different MHC backgrounds, Lühder et al. 37 describe a protective effect of MHC class II molecules that exerts itself through selection of T cells with additional TCRs. In our experimental system, BDC2.5 mice crossed to A18 were not protected from diabetes by the presence of MHC alleles of the A/J strain; in fact, the F1 combination had a far higher incidence of diabetes than the parental BDC2.5 strain. Instead, protection was induced by in vivo stimulation of the C5-specific TCR. It is unclear at the time of this writing what the underlying mechanisms for protection are. IL-10 has been invoked as a ‘suppressive’ cytokine 38, but in our study we could not detect any significant differences in IL-10 production between cells from immunized and protected dual TCR mice, whereas there was a significant reduction in IL-2 and IFN-γ secretion in the former group. NOD mice can be protected from diabetes by many types of immunostimulation, including nonspecific (e.g., CFA) stimulation 39 40 41, and perturbations of the cytokine milieu have been suggested as the underlying cause for this effect. It is difficult to rule out subtle changes in the internal cytokine milieu that are not detected in the in vitro assays, but CFA, which protects nontransgenic NOD mice, had no such effect in the dual TCR-transgenic mice (Fig. 4).
Although the frequency of T cells with two functionally relevant TCRs may be low 42 43 under physiological conditions, it does exist 44. T cells expressing additional TCRs are not obligatory for development of autoimmune disease, but as our and other data show, they may contribute to it, either by exacerbating or downmodulating the onset of autoimmune destruction 36 37. Differential expression levels of TCR provide a way for modulation of antigenic signals 45, and evidence is accumulating that suboptimal signals drive cells into alternative response modes rather than just leaving them unresponsive, so that they may be able to regulate the degree and mode of activation of other T cells.
Acknowledgments
We would like to thank Drs. Diane Mathis and Christophe Benoist for making the BDC2.5 strain available to us.
Gianluca Fossati was supported by grant RD96/0001290 from the British Diabetic Association, and Anne Cooke is supported by the Wellcome Trust and Medical Research Council.
References
- Borgulya P., Kishi H., Uematsu Y., von Boehmer H. Exclusion and inclusion of α and β T cell receptor alleles. Cell. 1992;69:529–537. doi: 10.1016/0092-8674(92)90453-j. [DOI] [PubMed] [Google Scholar]
- Heath W.R., Carbone F.R., Bertolino P., Kelly J., Cose S., Miller J.F. Expression of two T cell receptor α chains on the surface of normal murine T cells. Eur. J. Immunol. 1995;25:1617–1623. doi: 10.1002/eji.1830250622. [DOI] [PubMed] [Google Scholar]
- Padovan E., Casorati G., Dellabona P., Meyer S., Brockhaus M., Lanzavecchia A. Expression of two T cell receptor α chainsdual receptor T cells. Science. 1993;262:422–424. doi: 10.1126/science.8211163. [DOI] [PubMed] [Google Scholar]
- Simpson E., Chandler P., Sponaas A., Millrain M., Dyson P.J. T cells with dual antigen specificity in T cell receptor transgenic mice rejecting allografts. Eur. J. Immunol. 1995;25:2813–2817. doi: 10.1002/eji.1830251015. [DOI] [PubMed] [Google Scholar]
- Zal T., Volkmann A., Stockinger B. Mechanisms of tolerance induction in major histocompatibility complex class II–restricted T cells specific for a blood-borne self-antigen. J. Exp. Med. 1994;180:2089–2099. doi: 10.1084/jem.180.6.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zal T., Weiss S., Mellor A., Stockinger B. Expression of a second receptor rescues self-specific T cells from thymic deletion and allows activation of autoreactive effector function. Proc. Natl. Acad. Sci. USA. 1996;93:9102–9107. doi: 10.1073/pnas.93.17.9102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz J.D., Wang B., Haskins K., Benoist C., Mathis D. Following a diabetogenic T cell from genesis through pathogenesis. Cell. 1993;74:1098–1100. doi: 10.1016/0092-8674(93)90730-e. [DOI] [PubMed] [Google Scholar]
- Nishimoto H., Kikutani H., Yamamura K.I., Kishimoto T. Prevention of autoimmune insulitis by expression of I-E molecules in NOD mice. Nature. 1987;328:432–434. doi: 10.1038/328432a0. [DOI] [PubMed] [Google Scholar]
- Kurrer M.O., Pakala S.V., Hanson H.L., Katz J.D. B cell apoptosis in T cell-mediated autoimmune diabetes. Proc. Natl. Acad. Sci. USA. 1997;94:213–218. doi: 10.1073/pnas.94.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha A.A., Lopez M.T., McDevitt H.O. Autoimmune diseasethe failure of self tolerance. Science. 1990;248:1380–1388. doi: 10.1126/science.1972595. [DOI] [PubMed] [Google Scholar]
- Wekerle H. The viral triggering of autoimmune disease. Nat. Med. 1998;4:770–771. doi: 10.1038/nm0798-770. [DOI] [PubMed] [Google Scholar]
- Zhao Z.-S., Granucci F., Yeh L., Schaffer P., Cantor H. Molecular mimicry by herpes simplex virus-type 1autoimmune disease and viral infection. Science. 1998;279:1344–1347. doi: 10.1126/science.279.5355.1344. [DOI] [PubMed] [Google Scholar]
- Förster I., Hirose R., Arbeit J.M., Clausen B.E., Hanahan D. Limited capacity for tolerization of CD4+ T cells specific for a pancreatic beta cell neo-antigen. Immunity. 1995;2:573–585. doi: 10.1016/1074-7613(95)90002-0. [DOI] [PubMed] [Google Scholar]
- Scheicher C., Mehlig M., Zecher R., Reske K. Dendritic cells from mouse bone marrowin vitro differentiation using low doses of recombinant granulocyte-macrophage colony-stimulating factor. J. Immunol. Methods. 1992;154:253–264. doi: 10.1016/0022-1759(92)90199-4. [DOI] [PubMed] [Google Scholar]
- Inaba K., Inaba M., Romani N., Aya H., Deguchi M., Ikehara S., Muramatsu S., Steinman R.M. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J. Exp. Med. 1992;176:1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockinger B., Hausmann B. Functional recognition of in vivo processed self antigen. Int. Immunol. 1994;6:247–254. doi: 10.1093/intimm/6.2.247. [DOI] [PubMed] [Google Scholar]
- Bergmann B., Haskins K. Islet-specific T-cell clones from the NOD mouse respond to beta-granule antigen. Diabetes. 1994;43:197–203. doi: 10.2337/diab.43.2.197. [DOI] [PubMed] [Google Scholar]
- Mackay C.R. Homing of naive, memory and effector lymphocytes. Curr. Opin. Immunol. 1993;5:423–427. doi: 10.1016/0952-7915(93)90063-x. [DOI] [PubMed] [Google Scholar]
- Kurts C., Kosaka H., Carbone F.R., Karamalis F., Miller J.F., Heath W.R. Class I–restricted cross-presentation of exogenous self-antigens leads to deletion of autoreactive CD8+ T cells. J. Exp. Med. 1997;186:239–245. doi: 10.1084/jem.186.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höglund P., Mintern J., Waltzinger C., Heath W., Benoist C., Mathis D. Initiation of autoimmune diabetes by developmentally regulated presentation of islet cell antigens in the pancreatic lymph nodes. J. Exp. Med. 1999;189:331–339. doi: 10.1084/jem.189.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert M.L., Sauter B., Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature. 1998;392:86–89. doi: 10.1038/32183. [DOI] [PubMed] [Google Scholar]
- Wucherpfennig K.W., Strominger J.L. Molecular mimicry in T cell-mediated autoimmunityviral peptides activate human T cell clones specific for myelin basic protein. Cell. 1995;80:695–705. doi: 10.1016/0092-8674(95)90348-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldstone M.B.A. Molecular mimicry and autoimmune disease. Cell. 1987;50:819–820. doi: 10.1016/0092-8674(87)90507-1. [DOI] [PubMed] [Google Scholar]
- Heath W.R., Allison J., Hoffmann M.W., Schonrich G., Hammerling G., Arnold B., Miller J.F. Autoimmune diabetes as a consequence of locally produced interleukin-2. Nature. 1992;359:547–549. doi: 10.1038/359547a0. [DOI] [PubMed] [Google Scholar]
- Horwitz M.S., Bradley L.M., Harbertson J., Krahl T., Lee J., Sarvetnick N. Diabetes induced by Coxsackie virusinitiation by bystander damage and not molecular mimicry. Nat. Med. 1998;4:781–785. doi: 10.1038/nm0798-781. [DOI] [PubMed] [Google Scholar]
- Röcken M., Urban J.F., Shevach E.M. Infection breaks T-cell tolerance. Nature. 1992;359:79–82. doi: 10.1038/359079a0. [DOI] [PubMed] [Google Scholar]
- Sinha A.A., Lopez M.T., McDevitt H.O. Autoimmune diseasesthe failure of self tolerance. Science. 1990;248:1380–1388. doi: 10.1126/science.1972595. [DOI] [PubMed] [Google Scholar]
- Zelenika D., Adams E., Mellor A., Simpson E., Chandler P., Stockinger B., Waldmann H., Cobbold S.P. Rejection of H-Y disparate skin grafts by monospecific CD4+ T helper 1(Th1) and T helper 2 (Th2) cellsno requirement for CD8+ T cells or B cells. J. Immunol. 1998;161:1868–1874. [PubMed] [Google Scholar]
- Alam S.M., Crispe I.N., Gascoigne N.R.J. Allelic exclusion of mouse T cell receptor α chains occurs at the time of thymocyte TCR up-regulation. Immunity. 1995;3:449–458. doi: 10.1016/1074-7613(95)90174-4. [DOI] [PubMed] [Google Scholar]
- Alam S.M., Gascoigne N.R.J. Posttranslational regulation of TCR Vα allelic exclusion during T cell differentiation. J. Immunol. 1998;160:3883–3890. [PubMed] [Google Scholar]
- Valitutti S., Müller S., Dessing M., Lanzavecchia A. Different responses are elicited in cytotoxic T lymphocytes by different levels of T cell receptor occupancy. J. Exp. Med. 1996;183:1917–1921. doi: 10.1084/jem.183.4.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buer J., Lanoue A., Franzke A., Garcia C., von Boehmer H., Sarukhan A. Interleukin 10 secretion and impaired effector function of major histocompatibility complex class II–restricted T cells anergized in vivo. J. Exp. Med. 1998;187:177–183. doi: 10.1084/jem.187.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobbold S.P., Adams E., Marshall S.E., Davies J.D., Waldmann H. Mechanisms of peripheral tolerance and suppression induced by monoclonal antibodies to CD4 and CD8. Immunol. Rev. 1996;149:5–33. doi: 10.1111/j.1600-065x.1996.tb00897.x. [DOI] [PubMed] [Google Scholar]
- Groux H., O'Garra A., Bigler M., Rouleau M., Antonenko S., de Vries J.E., Roncarolo M.G. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–742. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- Blichfeldt E., Munthe L.A., Rotnes J.S., Bogen B. Dual T cell receptor T cells have a decreased sensitivity to physiological ligands due to reduced density of each T cell receptor. Eur. J. Immunol. 1996;26:2876–2884. doi: 10.1002/eji.1830261211. [DOI] [PubMed] [Google Scholar]
- Sarukhan A., Garcia C., Lanoue A., von Boehmer H. Allelic inclusion of T cell receptor α genes poses an autoimmune hazard due to low-level expression of autospecific receptors. Immunity. 1998;8:563–570. doi: 10.1016/s1074-7613(00)80561-0. [DOI] [PubMed] [Google Scholar]
- Lühder F., Katz J., Benoist C., Mathis D. Major histocompatibility complex class II molecules can protect from diabetes by positively selecting T cells with additional specificities. J. Exp. Med. 1998;187:379–387. doi: 10.1084/jem.187.3.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groux H., Bigler M., de Vries J.E., Roncarolo M.G. Interleukin-10 induces a long-term antigen-specific anergic state in human CD4+ T cells. J. Exp. Med. 1996;184:19–29. doi: 10.1084/jem.184.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadelain M.W., Qin H.Y., Lauzon J., Singh B. Prevention of type I diabetes in NOD mice by adjuvant immunotherapy. Diabetes. 1990;39:583–589. doi: 10.2337/diab.39.5.583. [DOI] [PubMed] [Google Scholar]
- Shehadeh N.N., LaRosa F., Lafferty K.J. Altered cytokine activity in adjuvant inhibition of autoimmune diabetes. J. Autoimmun. 1993;6:291–300. doi: 10.1006/jaut.1993.1025. [DOI] [PubMed] [Google Scholar]
- Ulaeto D., Lacy P.E., Kipnis D.M., Kanagawa O., Unanue E.R. A T-cell dormant state in the autoimmune process of nonobese diabetic mice treated with complete Freund's adjuvant. Proc. Natl. Acad. Sci. USA. 1992;89:3927–3931. doi: 10.1073/pnas.89.9.3927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardardottir F., Baron J.L., Janeway C.A. T cells with two functional antigen-specific receptors. Proc. Natl. Acad. Sci. USA. 1995;92:354–358. doi: 10.1073/pnas.92.2.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason D. Allelic exclusion of α chains in TCRs. Int. Immunol. 1994;6:881–885. doi: 10.1093/intimm/6.6.881. [DOI] [PubMed] [Google Scholar]
- Padovan E., Giachino C., Cella M., Valitutti S., Acuto O., Lanzavecchia A. Normal T lymphocytes can express two different T cell receptor β chainsimplications for the mechanism of allelic exclusion. J. Exp. Med. 1995;181:1587–1591. doi: 10.1084/jem.181.4.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girgis L., Davis M.M., Fazekas de St. Groth B. The avidity spectrum of T cell receptor interactions accounts for T cell anergy in a double transgenic model. J. Exp. Med. 1999;189:265–278. doi: 10.1084/jem.189.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]