Abstract
Addition of lipopolysaccharide (LPS) to cells in the form of LPS–soluble (s)CD14 complexes induces strong cellular responses. During this process, LPS is delivered from sCD14 to the plasma membrane, and the cell-associated LPS is then rapidly transported to an intracellular site. This transport appears to be important for certain cellular responses to LPS, as drugs that block transport also inhibit signaling and cells from LPS-hyporesponsive C3H/HeJ mice fail to exhibit this transport. To identify the intracellular destination of fluorescently labeled LPS after its delivery from sCD14 into cells, we have made simultaneous observations of different organelles using fluorescent vital dyes or probes. Endosomes, lysosomes, the endoplasmic reticulum, and the Golgi apparatus were labeled using Texas red (TR)–dextran, LysoTracker™ Red DND-99, DiOC6(3), and boron dipyrromethane (BODIPY)–ceramide, respectively. After 30 min, LPS did not colocalize with endosomes, lysosomes, or endoplasmic reticulum in polymorphonuclear leukocytes, although some LPS-positive vesicles overlapped with the endosomal marker, fluorescent dextran. On the other hand, LPS did appear to colocalize with two markers of the Golgi apparatus, BODIPY–ceramide and TRITC (tetramethylrhodamine isothiocyanate)–labeled cholera toxin B subunit. We further confirmed the localization of LPS in the Golgi apparatus using an epithelial cell line, HeLa, which responds to LPS–sCD14 complexes in a CD14-dependent fashion: BODIPY–LPS was internalized and colocalized with fluorescently labeled Golgi apparatus probes in live HeLa cells. Morphological disruption of the Golgi apparatus in brefeldin A–treated HeLa cells caused intracellular redistribution of fluorescent LPS. These results are consistent with the Golgi apparatus being the primary delivery site of monomeric LPS.
Keywords: lipopolysaccharide, endosomes, Golgi apparatus, retrograde transport
Recent work has shown that LPS is delivered to the membranes of responsive cells by the action of the lipid transport proteins, LPS binding protein (LBP)1 and CD14 1. These proteins transport LPS from micelles to the cell surface by a two-step mechanism. First, LBP catalyzes the transfer of LPS monomers from micelles to a binding site on soluble (s)CD14 and, in a second step, LPS monomers are transferred from LPS–sCD14 complexes to the plasma membranes of cells 2 3 4 5. These two steps, binding of LPS monomers to CD14 and their release into the membrane, are necessary for sensitive inflammatory responses.
We have recently shown 6 that after binding to the plasma membrane, monomeric bacterial LPS presented to PMNs as LPS–sCD14 complexes is rapidly delivered to an intracellular site. This intracellular movement of LPS appears to be necessary for certain cellular responses, as agents that block vesicular transport (lowering temperature to 19°C or addition of wortmannin or cytochalasin D) also block the integrin-mediated adhesion response of neutrophils to LPS 6. None of these conditions blocks cellular responses to the alternative stimuli, PMA or formyl peptide. Moreover, cells from LPS-hyporesponsive (Lpsd) mice exhibit defective vesicular transport of LPS and ceramide to a perinuclear site 7, and LPS antagonists block the transport of LPS to a perinuclear site 8.
As responses to LPS appear to involve its transport to a defined intracellular location, we have sought the identity of this site. To do this, we observed in parallel the location of fluorescently labeled LPS and the location of vital stains for known vesicular compartments in neutrophils and an epithelial cell line. We show here that most of the internalized boron dipyrromethane (BODIPY)–LPS accumulates in the Golgi apparatus.
Materials and Methods
Reagents.
Aprotinin, defatted (DF)–BSA, and PMA were purchased from Sigma Chemical Co. Pyrogen-free human serum albumin (HSA) was obtained from Centeon, Armour, and Berring Pharmaceutical Co. TNF-α was purchased from Genzyme Corp. The mAbs used were 26ic (anti-CD14; reference 9), 3C10 (anti-CD14; reference 10), and 44a (anti-CD11b; reference 11), available from American Type Culture Collection (ATCC), and were purified from ascites fluid by chromatography on protein G. The anti-CD14 mAb MY4 was purchased from Coulter Immunology. Dulbecco's PBS, DME, AIM-V serum-free medium, FCS, penicillin, and streptomycin were purchased from BioWhittaker. Brefeldin A (BFA) was purchased from Epicentre Technologies. TRITC-labeled cholera toxin B subunit (TRITC–CTB) and unlabeled LPS from Salmonella minnesota R595 (LPS) were purchased from List Biological Labs. LysoTracker™ Red DND-99, BODIPY 558/568–BFA, DiOC6(3), TR–dextran, BODIPY–fluorescein-like (FL) ceramide, BODIPY 558/568 ceramide, TR–Con A, and TR–transferrin (Tf) were purchased from Molecular Probes, Inc.
BODIPY–LPS was prepared using LPS from S. minnesota using BODIPY–FL and BODIPY–558/568 amine labeling kits (BODIPY–LPS; Molecular Probes, Inc.) as previously described 12. The ratio of BODIPY/LPS molecules was estimated at 1:5. A 1:1 complex of BODIPY–C5-ceramide with DF–BSA was prepared as described 13. The complex (5 μM) was prepared in acid-buffered Eagle's MEM, pH 7.4, without color indicator. Recombinant human sCD14 was purified from conditioned medium of Schneider-2 insect cells transfected with cDNA encoding human CD14 and was provided by Dr. R. Thieringer (Merck Research Laboratories).
To deliver LPS as a monomer, preformed complexes of BODIPY–LPS with sCD14 were used. To prepare LPS–sCD14 complexes, 100 μg/ml sCD14 was incubated with 5 μg/ml LPS for 16 h at 37°C. Previous work has shown that under these conditions, all of the LPS forms stoichiometric complexes with monomeric sCD14 and that these complexes efficiently stimulate cells and deliver LPS to the membrane 2 5.
Cells.
Heparinized blood was obtained by venipuncture from human healthy volunteers and PMN were purified on neutrophil isolation medium (Cardinal Associates, Inc.) according to the manufacturer's directions. Cells were suspended in HAP buffer (Dulbecco's PBS with 0.5 mg/ml HSA, 0.3 U/ml aprotinin, and 3 mM glucose). Human epithelial HeLa cell line was obtained from ATCC and were cultured in DME supplemented with 10% heat-inactivated FCS, 100 IU/ml penicillin, and 100 μg/ml streptomycin.
Stimulation and Assay of IL-8 Production.
HeLa cells were plated in 96-well plates at a density of 100,000 cells/well 24 h before stimulation. The cells were washed extensively with AIM-V serum-free medium and then incubated with various stimuli in AIM-V medium containing 0.5 mg/ml of HSA. After 5 h, the supernatants were collected and stored at −20°C. Samples were assayed for the presence of IL-8 using a commercially available human IL-8 ELISA kit (Endogen, Inc.). Results are the mean values of triplicate wells ± SEM.
Confocal Scanning Laser Microscopy.
Purified PMN were washed in HAP buffer and plated on glass microslides (Carlson Scientific, Inc.) for 20 min on ice and then exposed to LPS (100 ng/ml) and fluorescent probes for organelles for 30 min at 37°C. LPS was added complexed to sCD14. After the incubation, cells were washed in HAP and examined live by confocal microscopy. HeLa cells (106 cells/ml) were washed in DME containing 0.5 mg/ml HSA, incubated with the fluorescent markers at 37°C, and processed for microscopy.
Analysis of BFA treatment was performed using HeLa cells incubated with TRITC–CTB to visualize the Golgi complex. BFA (1 μg/ml) was added after labeling of cells for 1 h at 37°C. Cells were viewed unfixed with a confocal laser scanning system.
Confocal scanning laser microscopy was performed using a Nikon microscope equipped with a ×100 objective (NA 1.4) and Bio-Rad MRC 600 or MRC 1024 instrumentation. A dual wavelength laser was used to excite green (BODIPY or FITC) and red (BODIPY 558/568, TRITC, or TR) fluorochromes at 488 and 568 nm spectral line of an Ar-Kr laser, respectively. The fluorescence signals from the two fluorochromes were recorded sequentially. Confocal images presented were single optical sections. Images were analyzed using NIH image 1.6 (National Institutes of Health) and LaserSharp (Bio-Rad Labs.) software and were processed for presentation with Adobe Photoshop 3.0 (Adobe Systems, Inc.) and Corel Draw 6.0 (Corel Corp.).
Results
LPS Does Not Concentrate in Lysosomes.
Lysosomes are acidic vesicles rich in hydrolytic enzymes and represent the site of degradation of extracellular macromolecules internalized by pinocytosis or phagocytosis. It has been shown that LPS aggregates move at least transiently into an acidic intracellular compartment of PMN, and deacylation by acyloxyacyl hydrolase occurs over several hours 14 15. We have determined if LPS is internalized into lysosomes using a fluorescent, freely permeant probe with a high selectivity for acidic organelles, LysoTracker™ Red DND-99 16. PMN were incubated with BODIPY–LPS (presented as BODIPY–LPS–sCD14) for 30 min at 37°C in the presence of LysoTracker™ Red DND-99, and live cells were observed by confocal microscopy. As previously reported, LPS appeared in a perinuclear area in a punctate or tubular pattern (Fig. 1 A; references 6 and 7). In contrast, the fluorescent lysosomes were distributed throughout the cytoplasm of PMN, consistent with the numerous azurophilic granules contained in these cells. The compartment containing the internalized LPS was readily distinguishable from lysosomes. We cannot exclude the possibility that a small amount of BODIPY–LPS might be localized within lysosomes. However, our methods could not distinguish LPS fluorescence above background autofluorescence in positions coincident with lysosomes. According to these observations, the vesicular transport from the plasma membrane does not appear to deliver LPS to lysosomes.
Figure 1.
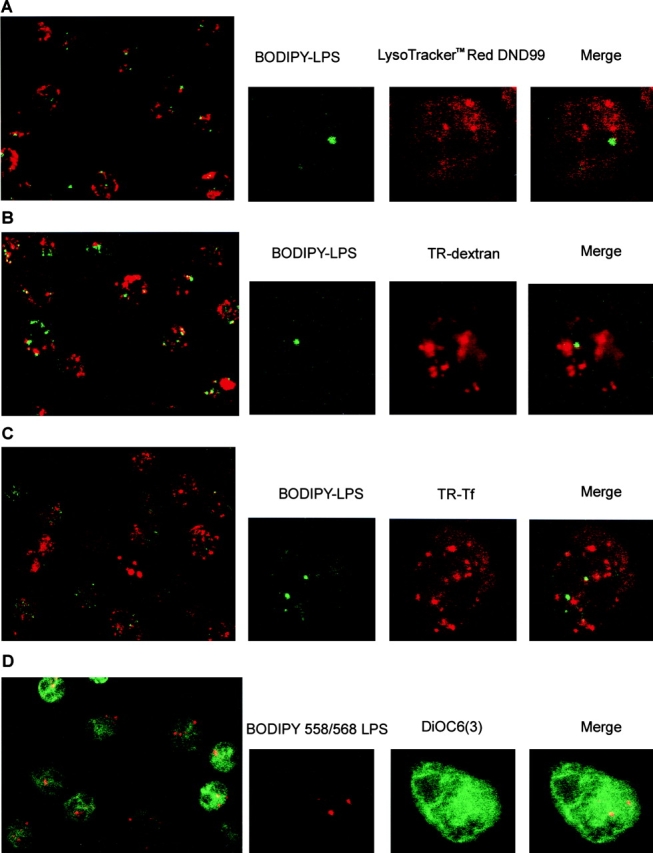
Intracellular distribution of LPS and organelle markers in neutrophils. Different markers of organelles were incubated with BODIPY–LPS–sCD14 for 30 min at 37°C and processed for microscopy. LPS, visualized in green (A–C, E–H) or in red (D), is concentrated in vesicles or tubules. Optical sections of single fluorescently labeled neutrophils are shown in the smaller panels: left, fluorescence for LPS alone; center, the organelle marker alone; and right, a merged image of both. A merged image of a lower power field is shown in the large panels. (A) Labeling in red of lysosomes with LysoTracker™ Red DND-99 (50 nM) shows lysosomes to be distributed throughout the cytoplasm in a punctate pattern. Merging magnified areas of optical sections of fluorescently double-labeled neutrophils shows that LPS vesicles do not overlap with the labeling pattern of lysosomes. (B) Labeling with TR–dextran (1 mg/ml) reveals endosomes. Although LPS vesicles appear in different sites than endosomes, some can be found associated with endosomes. (C) Labeling in red with TR–Tf (2 μg/ml) shows endosomes of the recycling pathway. Merging magnified areas of optical sections of fluorescently double-labeled neutrophils shows that LPS vesicles do not overlap with the labeling pattern of Tf. (D) When BODIPY–LPS labeling is compared with the ER labeling in green performed with DiOC6(3), LPS fluorescence was distinct from the ER pattern. (E) Similar results were observed with TR–BFA, a second ER stain. (F) Con A stains ER and the cis-Golgi apparatus. Double-labeled neutrophils with BODIPY–LPS–sCD14 and TR–Con A (2 μg/ml) show that LPS vesicles partially overlap with structures labeled with Con A. When the patterns of BODIPY–ceramide–BSA (0.5 μM) labeling (G) or TRITC–CTB (0.1 μg/ml) labeling (H) are compared with the pattern of BODIPY–LPS–sCD14 labeling, the brightly labeled LPS vesicles colocalize with the Golgi complex markers.
LPS Does Not Concentrate in Specific Granules.
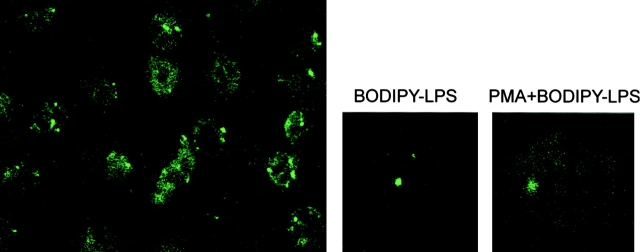
Activation of PMN with PMA or TNF-α induces fusion of specific granules with the cell membrane and upregulation of extracellular matrix receptors (laminin receptor, vitronectin receptor, and CD11b/CD18 antigens) on the cell surface, leading to leukocyte adhesion and extravasation 17. PMA-stimulated PMN release the contents of both their specific and azurophilic granules. When PMN were incubated with both PMA and BODIPY–LPS–sCD14 complexes, the autofluorescence of the cells was increased (Fig. 2). However, no modification in the punctate pattern of LPS fluorescence was observed, suggesting that LPS does not accumulate in specific granules.
Figure 2.
Intracellular distribution of LPS in neutrophils. Optical sections of fluorescently labeled neutrophils are shown. PMN were incubated with both PMA (10 ng/ml) and BODIPY–LPS–sCD14 complexes (left and right) or BODIPY–LPS–sCD14 complexes only (center) for 30 min at 37°C. Comparing magnified cells of optical sections of neutrophils in the presence or absence of PMA shows that vesicular LPS labeling was not affected by the released specific granules, although the autofluorescence of PMN was increased with PMA stimulation.
LPS Does Not Concentrate in Endosomes.
Endosomes are a structurally diverse population of vacuoles and tubules serving as sorting intermediates along both the biosynthetic and endocytic pathways, accumulating intraluminal membrane as they mature. Fluorescent dextran, an hydrophilic polysaccharide with poly-(α-d-1,6-glucose) linkages, is rapidly taken up by an endocytic process and moves to early and late endosomes 18. We have studied the internalization of a fluid-phase marker (TR–dextran) and LPS in neutrophils. After 30 min at 37°C, TR–dextran appeared in an heterogeneous assortment of internalized vesicles as expected (Fig. 1 B). BODIPY–LPS, on the other hand, was concentrated in an organelle distinct from TR–dextran (Fig. 1 B), suggesting that LPS is not localized in late endosomes after 30 min. Nevertheless, there was partial colocalization of LPS vesicles with dextran, as manifested by the punctate yellow staining in the merged images in some cells. This partial colocalization was detected most often near the plasma membrane, suggesting that LPS may traffic via endosomes containing dextran. Vesicular traffic involves several steps with distinct kinetics, and intermingling of endocytic markers with LPS after sequential endocytic uptake may be dependent on the length of incubation.
To further resolve the localization of LPS in endosomes, we examined the intracellular transport of endocytosed LPS in PMN using Tf to probe the recycling pathway. Fluorescent Tf (TR–Tf), which reaches sorting and recycling endosomes via clathrin-coated pits 19, was localized in a compartment clearly different from the structure containing BODIPY–LPS. After 30 min of incubation at 37°C, TR–Tf was in fact preferentially excluded from the regions labeled with BODIPY–LPS–sCD14 (Fig. 1 C). These observations indicate that the trafficking and sorting of LPS in neutrophils may occur in endosomes containing dextran and but not Tf.
LPS Does Not Concentrate in the Endoplasmic Reticulum.
The endoplasmic reticulum (ER) is the largest endomembrane system within eukaryotic cells and performs a wide variety of functions, including calcium uptake and release, lipid and protein synthesis, protein translocation, folding, glycosylation, concentration, and export to the Golgi complex. In live cells, the flattened membranous sacs of the ER can be stained with DiOC6(3), a lipophilic fluorescent dye 20. AS DiOC6(3) fluoresces green, we performed colocalization studies using LPS labeled with a BODIPY probe that fluoresces in the red part of the spectrum (BODIPY 558/568–LPS). Green or red staining thus illustrates the respective distribution of DiOC6(3)– and BODIPY 558/568–LPS. Fluorescent LPS was observed in vesicular structures distinct from the ER (Fig. 1 D), suggesting that there was no direct transport of LPS to the ER from the cell surface. We have confirmed the absence of LPS in ER using a second marker. The fluorescently labeled fungal metabolite BFA intensely stains the ER at concentrations that have no discernible effects on intracellular transport or other cellular functions 21. After incubation of PMN with BODIPY– BFA, LPS was observed in vesicular structures clearly distinct from those stained with BFA (Fig. 1 E).
Con A binds with high affinity to immature structures that terminate in glucosyl, mannosyl, or mannosyl and N-acetyl glucosaminyl residues. Con A can label internal structures by uptake and trafficking and stains the rough ER and the dilated cisternae of the cis-Golgi apparatus side 22. Fig. 1 F depicts confocal images of PMN that were treated with TR–Con A and BODIPY–LPS–sCD14 for 30 min at 37°C. There was partial colocalization of LPS with Con A in most of the cells, as manifested by the punctate yellow staining in the merged images. As there was no colocalization of LPS with the previous ER probes, these observations were consistent with the finding that LPS concentrates in the proximal side of the Golgi complex.
LPS Colocalizes with Golgi Apparatus Markers.
The Golgi apparatus can be selectively stained with fluorescent ceramide, which tends to associate preferentially with the trans-Golgi complex 23. After rapid transport to the Golgi apparatus, ceramide is metabolized to sphingomyelin, glucosylceramide, and further glycosphingolipids, suggesting that the pattern of distribution of fluorescently labeled ceramide may change over time in live cells. To stain cells with this probe, PMN were incubated first with BODIPY–LPS–sCD14 complexes for 20 min at 37°C and then BODIPY–ceramide–BSA was added for 5 min at room temperature. Cells were washed and subsequently warmed for 2 min at 37°C. Confocal microscopy studies showed that the punctate pattern of labeled cells was very similar with LPS or ceramide and that the brightly labeled LPS vesicles colocalized with ceramide fluorescence (Fig. 1 G). This strong colocalization suggests that LPS accumulates in the Golgi region.
Various glycolipid-binding toxins are internalized from the cell surface to the Golgi complex, and we have used CTB as marker of the Golgi apparatus 24. CT consists of a pentameric B subunit, which binds with high affinity to ganglioside GM1, and an A subunit, which stimulates adenylate cyclase, resulting in the elevation of cAMP. CTB is internalized by vesicular transport from the plasma membrane to the Golgi apparatus and persists in this compartment. As previously observed with ceramide, the punctate pattern of TRITC–CTB was similar to that seen for fluorescent LPS. Merging magnified areas of optical sections of fluorescently double-labeled PMN confirmed that the brightly labeled LPS vesicles colocalized with fluorescent CTB (Fig. 1 H). The specific Golgi subcompartment(s) containing LPS (or CTB) could not be identified, as they cannot be resolved by confocal fluorescence microscopy.
Quantification of LPS with Lysosomal and Golgi Apparatus Markers in Neutrophils.
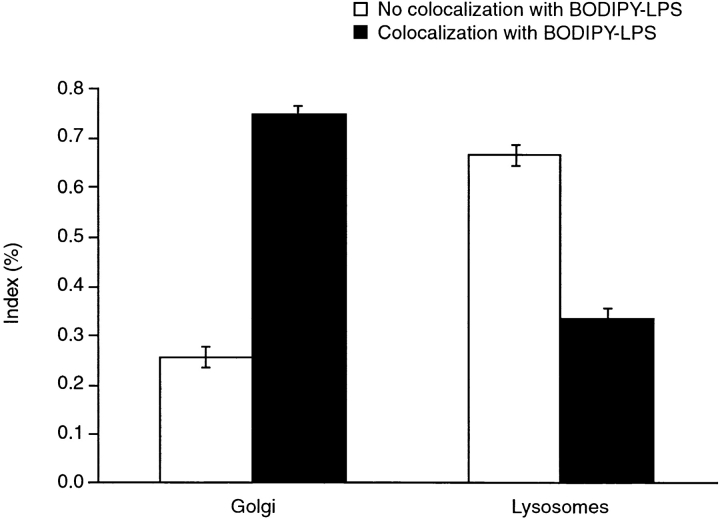
Transport of LPS was quantified by counting fluorescent LPS vesicles, which overlap with specific probes of the intracellular compartment. Using LysoTracker™ Red DND-99 and TRITC–CTB as probes of lysosomes and the Golgi apparatus, respectively, we observed that 74% of LPS vesicles colocalized with CTB probe in neutrophils (Fig. 3). In contrast, most LPS vesicles failed to colocalize with the lysosomal marker. These results suggest that, as seen in Fig. 1, most LPS is directed to the Golgi apparatus.
Figure 3.
Quantification of LPS with lysosomal and Golgi apparatus markers in neutrophils. Cells were labeled in the presence of either LysoTracker™ Red DND-99 or TRITC–CTB for 30 min at 37°C and processed for microscopy. 15–40 fields of live cells per experiment were observed, with an average of 5–20 cells per field. All green (LPS-containing) vesicles in the field were visually assigned as either not colocalized or colocalized with the red Golgi apparatus or lysosomal marker. Each experiment included data on 101–245 vesicles. Data is presented as the mean ± range for two experiments.
HeLa Cells Respond to LPS–sCD14 Complexes.
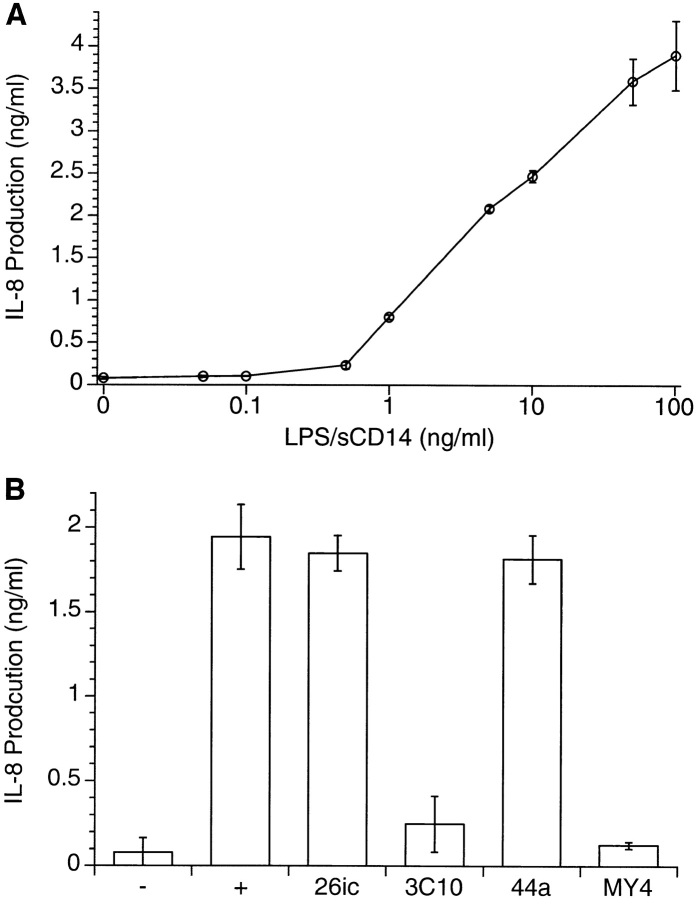
Although LPS appeared to be transported to the Golgi complex, which is also stained with CTB and ceramide, it was possible that the probes were in separate compartments that were unresolvable using confocal microscopy. This is a particular concern in the centers of the cells, where many organelles are concentrated. It is even more of an issue with neutrophils, which are small (12–14 μm in diameter) and have compact, spheroidal and juxtanuclear Golgi complexes. In contrast, epithelial cells exhibit an extensive, loose, and perinuclear Golgi apparatus, forming a heterogeneous ribbon-like structure connected to several networks of anastomosed membranous tubules or trans-Golgi network (TGN). The Golgi structure has been thoroughly investigated in epithelial HeLa cells, which are 100–140 μm in diameter 25 26, and we therefore used this cell type to confirm the localization of LPS within the cell. HeLa cells take up a low amount of BODIPY–LPS–sCD14 after 30 min at 37°C, as detected by FACScan™ (Becton Dickinson; data not shown). Although the uptake of LPS from LPS–sCD14 complexes is relatively low, HeLa cells do respond to LPS–sCD14 complexes by secretion of IL-8 (Fig. 4 A). This secretion was LPS dose– and CD14-dependent, as the cytokine response was completely inhibited by two neutralizing anti-CD14 mAbs, 3C10 27 and MY4 (Fig. 4 B). IL-8 production was not inhibited by two control mAbs, 26ic, which recognizes CD14 without inhibiting binding of LPS or responses towards LPS 4 28, and 44a, which binds CD11b molecules 11.
Figure 4.
IL-8 secretion by HeLa cells in response to LPS–sCD14 complexes. (A) Monolayers of HeLa cells were incubated for 5 h with different concentrations of LPS–sCD14 complexes, and the supernatants were assayed for IL-8 production by ELISA. Results are means ± SD of triplicate determinations of a representative experiment repeated three times. (B) CD14-dependent IL-8 secretion in response to LPS–sCD14 complexes. HeLa cells were incubated with LPS–sCD14 in the presence or absence of mAbs (10 μg/ml) for 5 h, and the supernatants were assayed for IL-8 production by ELISA. Results are means ± SD of triplicate determinations of a representative experiment repeated two times.
Internalized LPS Colocalizes with Golgi Apparatus Markers in HeLa Cells.
We have tested the ability of HeLa cells to internalize LPS. Cells were labeled for 60 min at 37°C with BODIPY–LPS and washed before observation by confocal microscopy. Fluorescent LPS was detected in a collection of elongate and punctate structures consistent with the location of the Golgi (Fig. 5). Cells contained either a compact juxtanuclear reticulum, a structure characteristic of the Golgi apparatus, or dispersed tubulovesicular membranes, suggesting mitotic disassembly, fragmentation, and redistribution of the structure in living HeLa cells.
Figure 5.
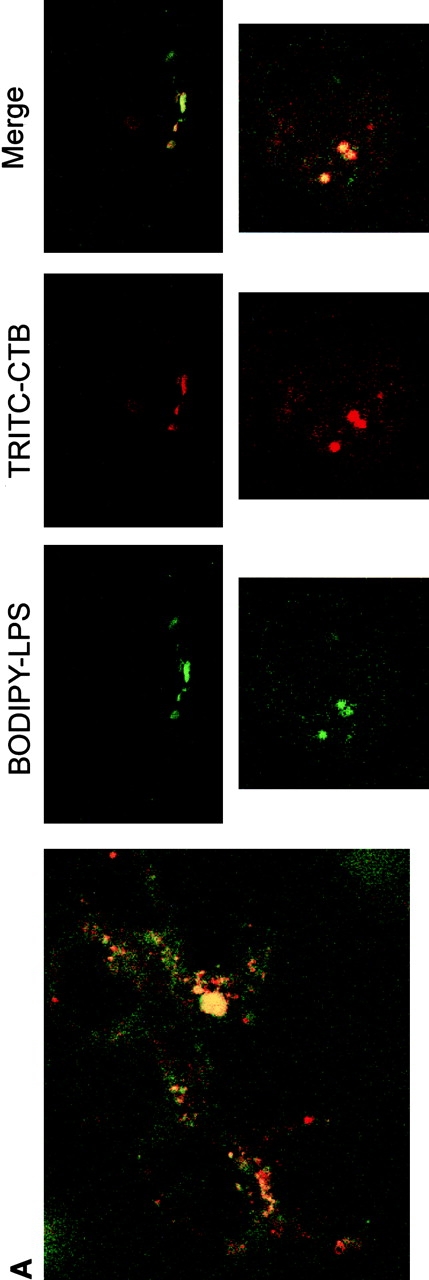
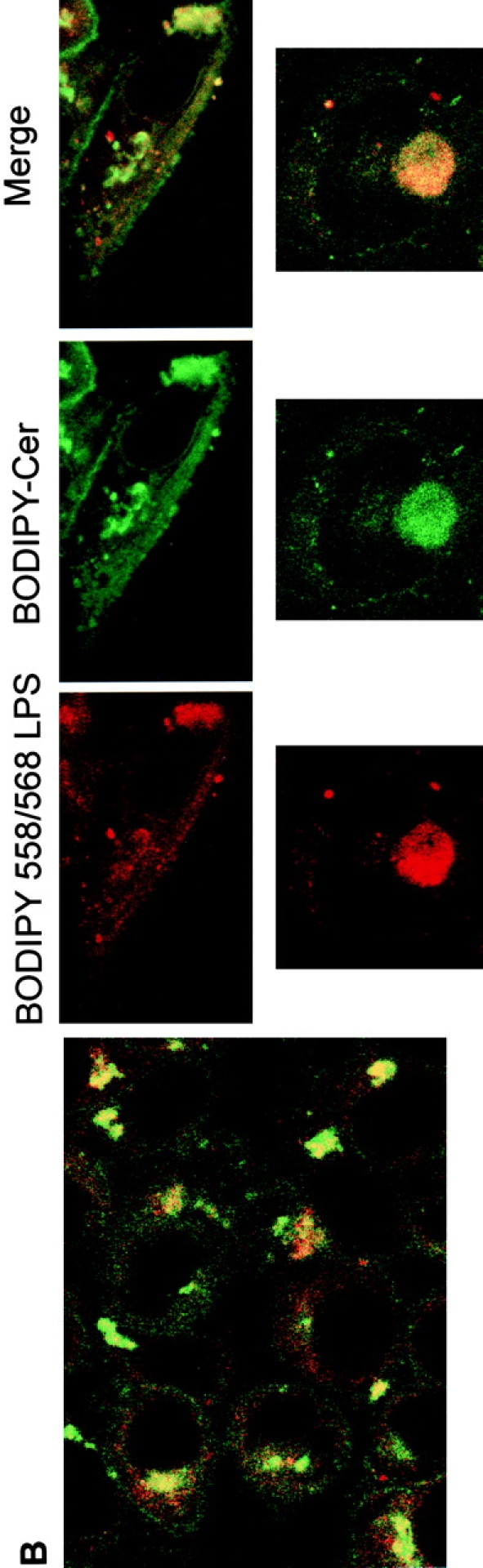
Intracellular distribution of LPS in HeLa cells. Optical sections of fluorescently labeled cells are shown. HeLa cells were incubated simultaneously with BODIPY–LPS and TRITC–CTB for 60 min at 37°C in DME with HSA and then processed for confocal microscopy. (A) LPS (green) is internalized and appears in a perinuclear area. Double-labeled HeLa cells reveal that BODIPY–LPS colocalizes with TRITC–CTB in the perinuclear area. (B) Cells were examined for staining of LPS (red) and ceramide (green). Merging the images revealed colocalization of LPS with ceramide (yellow), indicating that LPS colocalizes with Golgi apparatus markers.
We analyzed in parallel the location of LPS and Golgi apparatus labels. We observed that the distribution of LPS overlapped with that of both CTB–FITC and BODIPY–ceramide (Fig. 5a and Fig. b), confirming that most of the internalized BODIPY–LPS accumulated in the Golgi apparatus. It should be noted that dots of fluorescent LPS not overlapping with Golgi apparatus probes were also detected, suggesting that a fraction of internalized LPS may reside in endosomes, resembling the pattern of BODIPY–LPS observed in PMN (Fig. 1 B). These results are consistent with the Golgi apparatus being the primary delivery site of LPS by endocytic membrane movement from the plasma membrane. These observations suggest that the trafficking and sorting of LPS in HeLa cells follows the same general scheme as in PMN: LPS internalization may occur through endosomes, initially accessible to the fluid phase marker dextran, and is further directed into vesicular/tubular parts of the Golgi apparatus.
BFA Causes Redistribution of BODIPY–LPS and Golgi Apparatus Marker.
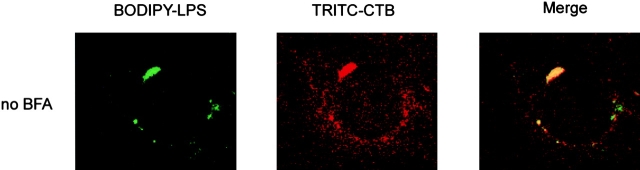
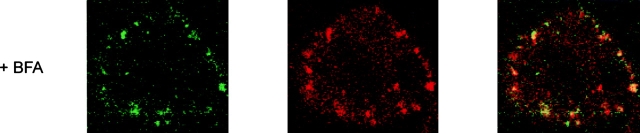
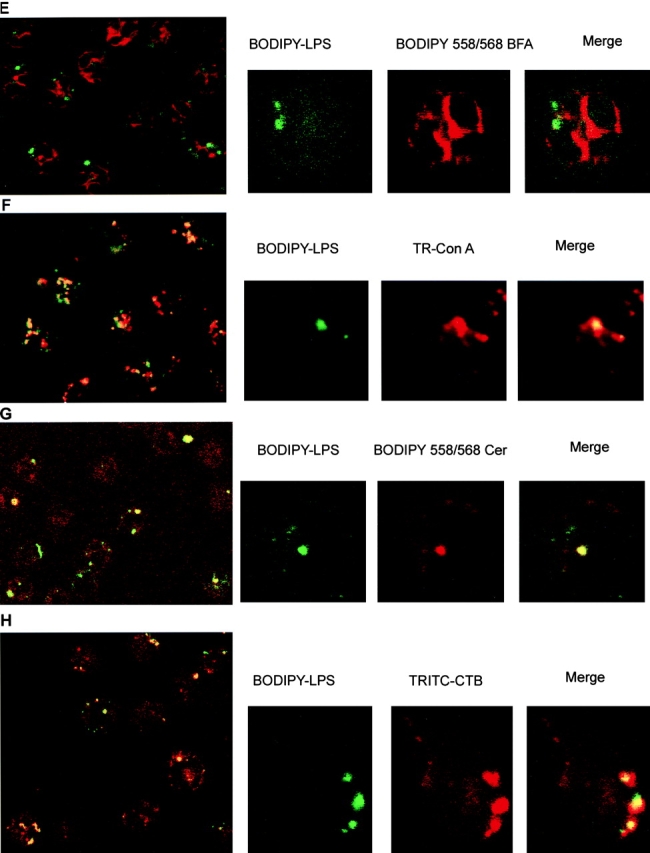
Treatment of cells with BFA is known to interfere with coat assembly and results in retrograde merging of Golgi complex membranes with the ER 29. BFA blocks membrane export out of the ER and inhibits vesicle formation. This is due to BFA's inhibition of nucleotide exchange onto ADP ribosylation factor, a low-molecular-mass GTP-binding protein that prevents assembly of cytosolic coat proteins (including COP I components) onto target membranes. At the same time, extensive retrograde transport of Golgi complex components to the ER mediated by growth of Golgi tubules occurs with BFA, leading to the dilation of the ER and the complete loss of Golgi apparatus structure. In this study, we investigated the effect of BFA-induced retrograde membrane transport from Golgi apparatus to ER on LPS vesicles. HeLa cells were first labeled with both LPS and CTB probes for 1 h at 37°C and washed and then incubated with BFA for 1 h at 37°C before observation by confocal microscopy. In BFA-treated cells, the fluorescent LPS probe was found in scattered/fragmented cytoplasmic patches (Fig. 6). The disassembly of the Golgi complex was visualized by the dispersed CTB fluorescence after BFA treatment. Fluorescent LPS frequently colocalized or was located in proximity to the dispersed CTB-positive structures. Our finding indicates that BFA induced disassembly of the Golgi complex and, in parallel, redistribution of LPS vesicles. These results confirm our conclusion that LPS localizes in the Golgi compartment.
Figure 6.
Localization of LPS in HeLa cells is disrupted by BFA. HeLa cells were incubated with BODIPY–LPS and TRITC–CTB for 60 min at 37°C in DME with HSA, washed, and warmed in the absence or presence of BFA (1 μg/ml) for 60 min at 37°C before examination by confocal microscopy. LPS (green) colocalizes with CTB (red) and appears in a perinuclear area before BFA treatment (yellow on merge panels). Upon BFA exposure, however, BODIPY–LPS and TRITC–CTB redistribute to scattered/fragmented cytoplasmic structures.
Discussion
Monomeric LPS May Move to the Golgi Apparatus by Retrograde Transport.
Here we show that biologically active LPS, delivered to cell membranes as monomers, rapidly colocalizes with vital stains of the Golgi apparatus. Furthermore, the LPS localization was disrupted after BFA-induced disruption of the Golgi complex. The colocalization was observed in two cell types (PMN and HeLa cells, Fig. 1G and Fig. H and Fig. 5a and Fig. b), suggesting that trafficking of LPS to the Golgi complex is not unique to leukocytes and may be a general property of cells.
A major function of the ER and Golgi apparatus is to package proteins for export, and membranes of these organelles must therefore move with their protein cargo toward the plasma membrane. Retrieval of the vesicular membrane is achieved by the process of “retrograde transport,” and we hypothesize that LPS utilizes this process to achieve movement to the Golgi complex. The pathway of retrograde transport involves the endosomal compartment 30 31 and, in keeping with this hypothesis, we have observed that LPS shows partial overlap with the endosomal marker, dextran (Fig. 1 B). It is interesting to note that certain bacterial toxins such as cholera toxin, diphtheria toxin, Pseudomonas exotoxin A, Shiga toxin, or plant toxins such as ricin also utilize retrograde transport to reach their target intracellular compartments 24 30. Surface-bound toxin enters cells by endocytosis, with the precise endocytic route depending on the nature of the receptor. The toxins are then carried on membranes that recycle between the plasma membrane and the TGN and between the TGN and the ER. After reaching the ER, the toxins cross the membrane and introduce a toxic enzyme into the cytoplasm. In a similar fashion, retrograde transport of LPS (endotoxin) via sorting endosomes may be responsible for its Golgi complex localization and perhaps its biological activity as well.
As BFA disrupts the Golgi apparatus, it would be of interest to determine if it affects LPS signaling. Unfortunately, BFA is also a potent inhibitor of protein secretion and, therefore, we could not evaluate a potential effect of BFA treatment on cytokine production in response to LPS. Furthermore, previous work 32 has shown that BFA treatment strongly enhances nuclear factor (NF)-κB translocation in HeLa cells, thus precluding a study on LPS-induced NF-κB activation in BFA-treated cells.
Two Fates for Internalized LPS with Opposite Biological Consequences.
We wish to stress that the process observed here with monomeric LPS differs critically from that studied by a range of prior authors using LPS aggregates or whole Gram-negative bacteria 33 34 35 36 37 38 39 40. Indeed, it appears that there are two distinct routes of LPS trafficking with two distinct biological outcomes: monomeric LPS is delivered to the Golgi apparatus, a process associated with cell stimulation, whereas LPS aggregates are delivered to lysosomes, a process not associated with cell stimulation.
Delivery of LPS Aggregates to Lysosomes.
LPS aggregates and whole bacteria are clearly delivered to lysosomes 38 41 42. Like other particulates, LPS aggregates and whole bacteria are carried as cargo in the lumen of endosomes or phagosomes. Delivery to lysosomes results in degradation of LPS, and the action of one particular lysosomal enzyme (acyloxyacyl hydrolase; reference 14) results in the transformation of LPS to a form that antagonizes the cell-stimulating capacity of complete LPS 43. Receptors for IgG on macrophages are known to deliver antigen to lysosomes, and anti-LPS antibodies are known to hasten clearance and decrease cellular responses to LPS 44. Finally, recent work indicates that during uptake of LPS aggregates, mCD14 accompanies LPS into the cell 45.
Delivery of LPS Monomers to the Golgi Apparatus.
sCD14 delivers LPS to cells not as aggregates but as monomers. In contrast to aggregates, endocytosis of monomeric LPS occurs after dissociation from mCD14 45, and it appears that this LPS is not carried in the lumen of a vesicle but rather as part of the membrane bilayer. Monomeric LPS is clearly not transported to lysosomes (Fig. 1 A) but moves instead to the Golgi complex. Finally, it is clear that LPS delivered as a monomer is biologically active. Responses to LPS are optimized when LPS is delivered as monomers with CD14 (references 2 and 4 and Le Grand, C.B., N. Lamping, T. Sugiyama, S.D. Wright, and R. Thieringer, manuscript submitted for publication) or when fused with the plasma membrane after incorporation into a viral envelope 46.
The studies reported here use LPS–sCD14 complexes designed to optimize the efficacy of LPS in cell stimulation and have resulted in predominant delivery of LPS to the Golgi complex. At the opposite pole, LPS may be delivered to cells in complex with IgG in such a way as to minimize cell stimulation 44 and presumably maximize delivery to lysosomes. We wish to stress that neither condition closely mimics infection. It appears likely that during phagocytosis of a live Gram-negative bacterium, the bulk of LPS will be delivered as vesicular cargo to the lysosome, and a smaller fraction of LPS may fuse with the plasma membrane, either by diffusion or the action of the lipid transfer proteins LBP and CD14. Under these circumstances, the bulk of LPS may be destroyed in the lysosomes, while the cells may simultaneously receive an optimal stimulus from LPS that has fused with the membrane.
Role of Internalization in Cell Stimulation.
As described in the Introduction, several lines of evidence suggest that internalization of LPS may be necessary for at least some types of signal transduction by LPS. The precise mechanism by which Golgi apparatus internalization affects signal transduction, however, is not clear at the time of this writing. Two hypotheses may be entertained. In the first, LPS may have its effects through modulation or interruption of vesicular transport per se. It is clear that the lipid composition of membranes may direct their fate. Transformation of sphingomyelin to ceramide in the plasma membrane causes vesiculation 47, and after traffic to the Golgi complex, ceramide may be converted to glucosyl ceramide which, in turn, is brought back to the plasma membrane 48. LPS differs from endogenous lipids in that it cannot be metabolically transformed in the Golgi complex and may thereby interrupt traffic. In support of this hypothesis, accumulation of unfolded proteins in the ER is known to initiate a stress response called the “ER stress response” 49 or “unfolded protein response” 50. Activation of NF-κB 51 is a well known signal induced by LPS and has been reported to occur during ER stress 52. A second hypothesis is that proteins involved in sensing infection (pattern recognition receptors) may be located in the Golgi apparatus or may need to move to the Golgi apparatus to signal. An example of the latter case is seen in the proteins that sense cholesterol concentration 53. SCAP (SREBP cleavage-activating protein) senses cholesterol concentration and initiates a cascade of events that activates transcription of genes for cholesterol synthesis. In the presence of adequate levels of cholesterol, SCAP does not initiate this cascade, and recent data suggests that under these conditions it is sequestered in a pre-Golgi compartment in an endo H–sensitive form. Low cholesterol levels trigger both translocation to the Golgi apparatus and initiate the steps leading to cholesterol synthesis. Mutations in SCAP delete in parallel the ability to both move to the Golgi complex and initiate cholesterol synthesis. In this regard, recent studies have implicated both Toll-like receptor (TLR)2 and TLR4 as proteins involved in signaling by LPS 54 55 56 57 58 59. The subcellular distribution of TLR protein before and after LPS stimulation will be of substantial interest.
Whether or not responses are initiated by either of the mechanisms described above, it is now increasingly clear that LPS is recognized not as a free monomer but in the context of its packing in a membrane. E5531 is a synthetic LPS antagonist that resembles LPS but blocks its action in cells 60. A mirror image of E5531 was synthesized by inversion of all 13 optically active sites in the molecule, and the enantiomeric form was an equally active antagonist (Christ, W.J. 1998. Advances in synthetic LPS antagonists. Oral presentation at Fifth Conference of the International Endotoxin Society, Santa Fe, NM). This observation argues against the recognition of LPS by a stoichiometric interaction with a stereospecific binding site and rather suggests that the colligative properties of LPS may play a key role in recognition. In keeping with this view, we have observed that the ability of another LPS antagonist (Rhodobacter sphaeroides [Rs]LPS) to stimulate cells is dramatically altered by membrane-active agents. The antagonist activity of RsLPS may be transformed to agonist by the addition of the membrane-active agent, chlorpromazine, suggesting an important role for membrane packing in recognition of LPS. From these considerations, we are directed to seek recognition proteins that sense LPS in a bilayer or the properties of a bilayer containing LPS. The LPS trafficking process we have observed here represents a cellular response to LPS as part of a membrane and contributes to an emerging picture of key steps in the innate recognition of LPS.
Acknowledgments
We gratefully acknowledge the gift of recombinant sCD14 and fluorescently labeled LPS preparations from Dr. R. Thieringer. We thank Paul Fischer and Jeanine Regenthal for assistance in cytometry facility. We thank Drs. P. Detmers, N. Lamping, R. Thieringer, and T. Vasselon for helpful discussion or critical reading of this manuscript.
Footnotes
1used in this paper: BODIPY, boron dipyrromethane; CTB, cholera toxin B subunit; DF, defatted; ER, endoplasmic reticulum; HSA, human serum albumin; LBP, LPS binding protein; Lpsd, LPS hyporesponsive; s, soluble; SCAP, SREBP (sterol regulatory element–binding proteins) cleavage–activating protein; Tf, transferrin; TGN, trans-Golgi network; TLR, Toll-like receptor; TR, Texas red; TRITC, tetramethylrhodamine isothiocyanate
References
- Wright S.D. Innate recognition of microbial lipids. In: Gallin J.I., Snyderman R., Fearon D., Haynes B., Nathan C., editors. In Inflammation: Basic Principles and Clinical Correlates. Raven Press; New York, New York: 1999. [Google Scholar]
- Hailman E., Vasselon T., Kelley M., Busse L.A., Hu M.C.-T., Lichenstein H.S., Detmers P.A., Wright S.D. Stimulation of macrophages and neutrophils by complexes of lipopolysaccharide and soluble CD14. J. Immunol. 1996;156:4384–4390. [PubMed] [Google Scholar]
- Wurfel M.M., Hailman E., Wright S.D. Soluble CD14 acts as a shuttle in the neutralization of lipopolysaccharide (LPS) by LPS-binding protein and reconstituted high density lipoprotein. J. Exp. Med. 1995;181:1743–1754. doi: 10.1084/jem.181.5.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hailman E., Lichenstein H.S., Wurfel M.M., Miller D.S., Johnson D.A., Kelley M., Busse L.A., Zubrowski M.M., Wright S.D. Lipopolysaccharide (LPS)-binding protein accelerates the binding of LPS to CD14. J. Exp. Med. 1994;179:269–277. doi: 10.1084/jem.179.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasselon T., Pironkova R., Detmers P.A. Sensitive responses of leukocytes to LPS require a protein distinct from CD14 at the cell surface. J. Immunol. 1997;159:4498–4505. [PubMed] [Google Scholar]
- Detmers P.A., Thieblemont N., Vasselon T., Pironkova R., Miller D.S., Wright S.D. Potential role of membrane internalization and vesicle fusion in adhesion of neutrophils in response to lipopolysaccharide and TNF. J. Immunol. 1996;157:5589–5596. [PubMed] [Google Scholar]
- Thieblemont N., Wright S.D. Mice genetically hyporesponsive to lipopolysaccharide (LPS) exhibit a defect in endocytic uptake of LPS and ceramide. J. Exp. Med. 1997;185:2095–2100. doi: 10.1084/jem.185.12.2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thieblemont N., Thieringer R., Wright S.D. Innate immune recognition of bacterial lipopolysaccharidedependence on interactions with membrane lipids and endocytic movement. Immunity. 1998;8:771–777. doi: 10.1016/s1074-7613(00)80582-8. [DOI] [PubMed] [Google Scholar]
- Todd R.F., Van Agthoven A., Schlossman S.F., Terhorst C. Structural analysis of differentiation antigens Mo1 and Mo2 on human monocytes. Hybridoma. 1982;1:329–337. doi: 10.1089/hyb.1.1982.1.329. [DOI] [PubMed] [Google Scholar]
- Van Voorhis W.C., Steinman R.M., Hair L.S., Luban J., Witmer M.D., Koide S., Cohn Z.A. Specific antimononuclear phagocyte monoclonal antibodies. Application to the purification of dendritic cells and the tissue localization of macrophages. J. Exp. Med. 1983;158:126–145. doi: 10.1084/jem.158.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dana N., Styrt B., Griffin J.D., Todd R.F., Klempner M.S., Arnaout M.A. Two functional domains in the phagocyte membrane glycoprotein Mo1 identified with monoclonal antibodies. J. Immunol. 1986;137:3259–3263. [PubMed] [Google Scholar]
- Yu B., Wright S.D. Catalytic properties of lipopolysaccharide (LPS) binding protein. Transfer of LPS to soluble CD14. J. Biol. Chem. 1996;271:4100–4105. doi: 10.1074/jbc.271.8.4100. [DOI] [PubMed] [Google Scholar]
- Pagano R.E., Martin O.C. A series of fluorescent N-acetylsphingosinessynthesis, physical properties, and studies in cultured cells. Biochemistry. 1989;27:4439–4445. doi: 10.1021/bi00412a034. [DOI] [PubMed] [Google Scholar]
- Munford R.S., Hall C.L. Detoxification of bacterial lipopolysaccharides (endotoxins) by a human neutrophil enzyme. Science. 1986;234:203–205. doi: 10.1126/science.3529396. [DOI] [PubMed] [Google Scholar]
- Munford R.S., Hall C.L. Uptake and deacylation of bacterial lipopolysaccharides by macrophages from normal and endotoxin-hyporesponsive mice. Infect. Immun. 1985;48:464–473. doi: 10.1128/iai.48.2.464-473.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Via L.E., Fratti R.A., McFalone M., Pagan-Ramos E., Deretic D., Deretic V. Effects of cytokines on mycobacterial phagosome maturation. J. Cell Sci. 1998;111:897–905. doi: 10.1242/jcs.111.7.897. [DOI] [PubMed] [Google Scholar]
- Singer I.I., Scott S., Kawka D.W., Kazazis D.M. Adhesomesspecific granules containing receptors for laminin, C3bi/fibrinogen, fibronectin, and vitronectin in human polymorphonuclear leukocytes and monocytes. J. Cell Biol. 1989;109:3169–3182. doi: 10.1083/jcb.109.6.3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlin R.D., Oliver J.M. Surface functions during mitosis. II. Quantification of pinocytosis and kinetic characterization of the mitotic cycle with a new fluorescence technique. J. Cell Biol. 1980;85:660–671. doi: 10.1083/jcb.85.3.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh R.N., Gelman D.L., Maxfield F.R. Quantification of low density lipoprotein and transferrin endocytic sorting HEp2 cells using confocal microscopy. J. Cell Sci. 1994;107:2177–2189. doi: 10.1242/jcs.107.8.2177. [DOI] [PubMed] [Google Scholar]
- Sabnis R.W., Deligeorgiev T.G., Jachak M.N., Dalvi T.S. DiOC6(3)a useful dye for staining the endoplasmic reticulum. Biotech. Histochem. 1997;72:253–258. doi: 10.3109/10520299709082249. [DOI] [PubMed] [Google Scholar]
- Deng Y., Bennink J.R., Kang H.C., Haugland R.P., Yewdell J.W. Fluorescent conjugates of brefeldin A selectively stain the endoplasmic reticulum and Golgi complex of living cells. J. Histochem. Cytochem. 1995;43:907–915. doi: 10.1177/43.9.7543914. [DOI] [PubMed] [Google Scholar]
- Winqvist L., Eriksson L.C., Dallner G. Interaction of lectins with proteins of the endoplasmic reticulum and Golgi system of rat liver. J. Cell Sci. 1979;39:101–116. doi: 10.1242/jcs.39.1.101. [DOI] [PubMed] [Google Scholar]
- Lipsky N.G., Pagano R.E. A vital stain for the Golgi apparatus. Science. 1985;228:745–747. doi: 10.1126/science.2581316. [DOI] [PubMed] [Google Scholar]
- Bastiaens P.I.H., Majoul I.V., Verveer P.J., Soling H.D., Jovin T.M. Imaging the intracellular trafficking and state of the AB5 quaternary structure of cholera toxin. EMBO (Eur. Mol. Biol. Organ.) J. 1996;15:4246–4253. [PMC free article] [PubMed] [Google Scholar]
- Shima D.T., Cabrera-Poch N., Pepperkok R., Warren G. An ordered inheritance strategy for the Golgi apparatusvisualization of mitotic disassembly reveals a role for the mitotic spindle. J. Cell Biol. 1998;141:955–966. doi: 10.1083/jcb.141.4.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jesch S.A., Linstedt A.D. The Golgi and endoplasmic reticulum remain independent during mitosis in HeLa cells. Mol. Biol. Cell. 1998;9:623–635. doi: 10.1091/mbc.9.3.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juan T.S., Kelley M.J., Johnson D.A., Busse L.A., Hailman E., Wright S.D., Lichenstein H.S. Soluble CD14 truncated at amino acid 152 binds lipopolysaccharide (LPS) and enables cellular response to LPS. J. Biol. Chem. 1995;270:1382–1387. doi: 10.1074/jbc.270.3.1382. [DOI] [PubMed] [Google Scholar]
- Wright S.D., Ramos R.A., Tobias P.S., Ulevitch R.J., Mathison J.C. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990;249:1431–1433. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- Lippincott-Schwartz J., Yuan L.C., Bonifacino J.S., Klausner R.D. Rapid redistribution of Golgi proteins into the ER in cells treated with brefeldin Aevidence for membrane cycling from Golgi to ER. Cell. 1989;56:801–813. doi: 10.1016/0092-8674(89)90685-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandvig K., van Deurs B. Endocytosis, intracellular transport, and cytotoxic action of Shiga toxin and ricin. Physiol. Rev. 1996;76:949–966. doi: 10.1152/physrev.1996.76.4.949. [DOI] [PubMed] [Google Scholar]
- Schapiro F.B., Lingwood C., Furuya W., Grinstein S. pH-independent retrograde targeting of glycolipids to the Golgi complex. Am. J. Physiol. 1998;274:C319–C332. doi: 10.1152/ajpcell.1998.274.2.C319. [DOI] [PubMed] [Google Scholar]
- Pahl H.L., Baeuerle P.A. A novel signal transduction pathway from the endoplasmic reticulum to the nucleus is mediated by transcription factor NF-κB. EMBO (Eur. Mol. Biol. Organ.) J. 1995;14:2580–2588. doi: 10.1002/j.1460-2075.1995.tb07256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D.C., Ulevitch R.J. The effects of bacterial endotoxins on host mediation systems. A review. Am. J. Pathol. 1978;93:526–617. [PMC free article] [PubMed] [Google Scholar]
- Ruiter D.J., van der Meulen J., Brouwer A., Hummel M.J., Mauw B.J., van der Ploeg J.C., Wisse E. Uptake by liver cells of endotoxin following its intravenous injection. Lab. Invest. 1981;45:38–45. [PubMed] [Google Scholar]
- Kang Y.H., Dwivedi R.S., Lee C.H. Ultrastructural and immunocytochemical study of the uptake and distribution of bacterial lipopolysaccharide in human monocytes. J. Leukoc. Biol. 1990;48:316–332. doi: 10.1002/jlb.48.4.316. [DOI] [PubMed] [Google Scholar]
- Risco C., Carrascosa J.L., Bosch M.A. Uptake and subcellular distribution of Escherichia coli lipopolysaccharide by isolated rat type II pneumocytes. J. Histochem. Cytochem. 1991;39:607–615. doi: 10.1177/39.5.2016511. [DOI] [PubMed] [Google Scholar]
- Kang Y.H., Lee C.H., Monroy R.L., Dwivedi R.S., Odeyale C., Newball H.H. Uptake, distribution and fate of bacterial lipopolysaccharides in monocytes and macrophagesan ultrastructural and functional correlation. Electron Microsc. Rev. 1992;5:381–419. doi: 10.1016/0892-0354(92)90016-j. [DOI] [PubMed] [Google Scholar]
- Gegner J.A., Ulevitch R.J., Tobias P.S. Lipopolysaccharide (LPS) signal transduction and clearance. Dual roles for LPS binding protein and membrane CD14. J. Biol. Chem. 1995;270:5320–5325. doi: 10.1074/jbc.270.10.5320. [DOI] [PubMed] [Google Scholar]
- Kitchens R.L., Munford R.S. CD14-dependent internalization of bacterial lipopolysaccharide (LPS) is strongly influenced by LPS aggregation but not by cellular responses to LPS. J. Immunol. 1998;160:1920–1928. [PubMed] [Google Scholar]
- Kitchens R.L., Wang P., Munford R.S. Bacterial lipopolysaccharide can enter monocytes via two CD14-dependent pathways. J. Immunol. 1998;161:5534–5545. [PubMed] [Google Scholar]
- Hampton R.Y., Golenbock D.T., Penman M., Krieger M., Raetz C.R. Recognition and plasma clearance of endotoxin by scavenger receptors. Nature. 1991;352:342–344. doi: 10.1038/352342a0. [DOI] [PubMed] [Google Scholar]
- Grunwald U., Fan X., Jack R.S., Workalemahu G., Kallies A., Stelter F., Schutt C. Monocytes can phagocytose gram-negative bacteria by a CD14-dependent mechanism. J. Immunol. 1996;157:4119–4125. [PubMed] [Google Scholar]
- Kitchens R.L., Ulevitch R.J., Munford R.S. Lipopolysaccharide (LPS) partial structures inhibit responses to LPS in a human macrophage cell line without inhibiting LPS uptake by a CD14-mediated pathway. J. Exp. Med. 1992;176:485–494. doi: 10.1084/jem.176.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner J.D., Heumann D., Gerain J., Weinbreck P., Grau G.E., Glauser M.P. Association between protective efficacy of anti-lipopolysaccharide (LPS) antibodies and suppression of LPS-induced tumor necrosis factor α and interleukin 6. Comparison of O side chain–specific antibodies with core LPS antibodies. J. Exp. Med. 1990;171:889–896. doi: 10.1084/jem.171.3.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasselon T., Hailman E., Thieringer R., Detmers P.A. Internalization of monomeric lipopolysaccharide occurs after transfer out of cell surface CD14. J. Exp. Med. 1999;190:509–521. doi: 10.1084/jem.190.4.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkstra J., Bron R., Wilschut J., de Haan A., Ryan J.L. Activation of murine lymphocytes by lipopolysaccharide incorporated in fusogenic, reconstituted influenza virus envelopes (virosomes) J. Immunol. 1996;157:1028–1036. [PubMed] [Google Scholar]
- Zha X., Pierini L.M., Leopold P.L., Skiba P.J., Tabas I., Maxfield F.R. Sphingomyelinase treatment induces ATP-independent endocytosis. J. Cell Biol. 1998;140:39–47. doi: 10.1083/jcb.140.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Meer G., Stelzer E.H., Wijnaendts-van-Resandt R.W., Simons K. Sorting of sphingolipids in epithelial (Madin-Darby canine kidney) cells. J. Cell Biol. 1987;105:1623–1635. doi: 10.1083/jcb.105.4.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox J.S., Shamu C.E., Walter P. Transcriptional induction of genes encoding endoplasmic reticulum resident proteins requires a transmembrane protein kinase. Cell. 1993;73:1197–1206. doi: 10.1016/0092-8674(93)90648-a. [DOI] [PubMed] [Google Scholar]
- Sidrauski C., Chapman R., Walter P. The unfolded protein responsean intracellular signalling pathway with many surprising features. Trends Cell Biol. 1998;8:245–249. doi: 10.1016/s0962-8924(98)01267-7. [DOI] [PubMed] [Google Scholar]
- Sen R., Baltimore D. Inducibility of kappa immunoglobulin enhancer-binding protein NF-kappa B by a posttranslational mechanism. Cell. 1986;47:921–928. doi: 10.1016/0092-8674(86)90807-x. [DOI] [PubMed] [Google Scholar]
- Pahl H.L., Baeuerle P.A. Endoplasmic-reticulum-induced signal transduction and gene expression. Trends Cell Biol. 1997;7:50–55. doi: 10.1016/S0962-8924(96)10050-7. [DOI] [PubMed] [Google Scholar]
- Brown M.S., Goldstein J.L. The SREBP pathwayregulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell. 1997;89:331–340. doi: 10.1016/s0092-8674(00)80213-5. [DOI] [PubMed] [Google Scholar]
- Yang R.B., Mark M.R., Gray A., Huang A., Xie M.H., Goddard A., Wood W.I., Gurney A.L., Godowski P.J. Toll-like receptor-2 mediates lipopolysaccharide-induced cellular signalling. Nature. 1998;395:284–288. doi: 10.1038/26239. [DOI] [PubMed] [Google Scholar]
- Kirschning C.J., Wesche H., Merrill Ayres T., Rothe M. Human Toll-like receptor 2 confers responsiveness to bacterial lipopolysaccharide. J. Exp. Med. 1998;188:2091–2097. doi: 10.1084/jem.188.11.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medzhitov R., Preston-Hurlburt P., Janeway C.A., Jr. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–397. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- Poltorak A., He X., Smirnova I., Liu M.Y., Huffel C.V., Du X., Birdwell D., Alejos E., Silva M., Galanos C. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr micemutations in tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- Qureshi S.T., Lariviere L., Leveque G., Clermont S., Moore K.J., Gros P., Malo D. Endotoxin-tolerant mice have mutations in Toll-like receptor 4 (tlr4) J. Exp. Med. 1999;189:615–625. doi: 10.1084/jem.189.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S.D. Toll, a new piece in the puzzle of innate immunity. J. Exp. Med. 1999;189:605–609. doi: 10.1084/jem.189.4.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christ W.J., Asano O., Robidoux A.L., Perez M., Wang Y., Dubuc G.R., Gavin W.E., Hawkins L.D., McGuinness P.D., Mullarkey M.A. E5531, a pure endotoxin antagonist of high potency. Science. 1995;268:80–83. doi: 10.1126/science.7701344. [DOI] [PubMed] [Google Scholar]