Abstract
T cell receptor α chain–deficient (TCR-α−/−) mice are known to spontaneously develop inflammatory bowel disease (IBD). The colitis that develops in these mice is associated with increased numbers of T helper cell (Th)2-type CD4+TCR-ββ (CD4+ββ) T cells producing predominantly interleukin (IL)-4. To investigate the role of these Th2-type CD4+ββ T cells, we treated TCR-α−/− mice with anti–IL-4 monoclonal antibody (mAb). Approximately 60% of TCR-α−/− mice, including those treated with mock Ab and those left untreated, spontaneously developed IBD. However, anti–IL-4 mAb–treated mice exhibited no clinical or histological signs of IBD, and their levels of mucosal and systemic Ab responses were lower than those of mock Ab–treated mice. Although TCR-α−/− mice treated with either specific or mock Ab developed CD4+ββ T cells, only those treated with anti–IL-4 mAb showed a decrease in Th2-type cytokine production at the level of mRNA and protein and an increase in interferon γ–specific expression. These findings suggest that IL-4–producing Th2-type CD4+ββ T cells play a major immunopathological role in the induction of IBD in TCR-α−/− mice, a role that anti–IL-4 mAb inhibits by causing Th2-type CD4+ββ T cells to shift to the Th1 type.
Keywords: inflammatory bowel disease, T cell receptor α chain–deficient mice, interleukin 4, mucosal immunity, pathogenic T cell, Th2-induced colitis
The etiopathogenesis of inflammatory bowel disease (IBD),1 a chronic and relapsing inflammation of the gastrointestinal tract, remains poorly understood. In an effort to elucidate the molecular and cellular mechanisms behind IBD 1 2 3 4 5 6 7 8, researchers in the last six years have used several specific gene-manipulated animal models, including TCR α chain–deficient (TCR-α−/−) mice, TCR-β−/− mice, MHC class II–deficient mice, IL-2–deficient mice, IL-10–deficient mice, IL-7 transgenic mice, Gαi2-deficient mice, and severe combined immunodeficiency (SCID) mice restored with CD45RBhiCD4+ T cells. Of interest here are the TCR-α−/− mice, which spontaneously develop chronic colitis, a condition similar to ulcerative colitis in humans 2 9 10. About 60% of TCR-α−/− mice develop chronic diarrhea and anorectal prolapse under specific pathogen-free conditions at ∼3–4 mo of age. Tissue sections from the diseased colons of these animals show infiltration of inflammatory cells and elongation of crypts. In addition, higher levels of Ab are found in the mucosa-associated tissues (e.g., mesenteric lymph nodes [MLNs], Peyer's patches, and intestinal lamina propria [LP]) as well as in the systemic lymphoid organs (e.g., spleen [SP]) of these animals 9 10.
Our own previous studies and those of other investigators have shown that CD4+TCR-α−/β+ (CD4+βdim) T cells develop in the mucosal and peripheral lymphoid tissues of TCR-α−/− mice with IBD 9 10 11 12. These CD4+βdim T cells are considered to be pathogenic for the induction of IBD in this mouse model, since depletion of CD4+βdim T cells by anti–TCR-β mAb prevents the onset of IBD 10. The pathogenic nature of these CD4+βdim T cells is also evidenced by the lesser severity of IBD seen in TCR-β−/− mice, from which CD4+βdim T cells are absent 2. Our previous study showed that CD4+βdim T cells produce a predominance of IL-4, suggesting a potential role for Th2-type responses in the development of IBD 10. However, the association between IL-4–producing Th2-type CD4+βdim T cells and IBD is still poorly understood. Since our most recent study has provided strong evidence that these CD4+βdim T cells use a ββ homodimeric form of TCR, this subset of T cells will be designated here as ββ T cells in order to reflect the unique characteristics of these pathologic lymphocytes 13.
In this study, TCR-α−/− mice were treated with either anti–IL-4 mAb or mock Ab. In treated TCR-α−/− mice, Ab responses in the serum and fecal extracts were strongly suppressed and cytokine production from CD4+ββ T cells was shifted from the Th2 to the Th1 type. In addition, morphological and histological studies showed no obvious signs of IBD in TCR-α−/− mice treated with anti–IL-4 mAb. These findings provide new evidence that Th2-type CD4+ββ T cells can induce IBD. Conversion from a dominant Th2 to a Th1 environment resulted in the prevention of IBD in TCR-α−/− mice.
Materials and Methods
Mice.
TCR-α−/− mice with a background of C57BL/6 were obtained from The Jackson Laboratory. The mice were originally generated by a targeted disruption of TCR genes in embryonic stem cells 2. They were housed in the Experimental Animal Facility at the Research Institute for Microbial Diseases, Osaka University, under specific pathogen-free conditions and received sterilized food and autoclaved distilled water ad libitum.
Anti–IL-4 mAb Treatment.
In this study, a standard protocol was used for mAb in vivo treatment 10. TCR-α−/− mice from 6 to 25 wk of age were intraperitoneally injected with rat anti–mouse IL-4 mAb (BVD4-1D11, 1 mg/mouse; PharMingen) or rat IgG2b (R35-38, 1 mg/mouse; PharMingen) as mock Ab in 250 μl of PBS once a week. These treatments did not produce any signs of serum sickness.
Detection of Abs by ELISA.
Levels of IgA, IgG, and IgM Ab in serum and fecal extracts at 25 wk of age were examined by ELISA as described previously 14. In brief, ELISA plates (Nunc) were coated with 100 μl of 5 μg/ml goat anti–mouse Ig (H+L) (Southern Biotechnology Associates) in PBS and were incubated for 16 h at 4°C. Serial twofold dilutions of sera or fecal extracts were added (100 μl/well). The fecal extracts were the supernatants obtained after fresh feces were mixed with PBS containing 0.5% sodium azide (100 mg wet weight of feces/ml) and then centrifuged 15. After 2 h incubation at 37°C, unbound Abs were removed, and alkaline phosphatase–conjugated goat or rat anti–mouse α, γ, and μ chains (Southern Biotechnology Associates) were added. The plates were incubated at 37°C for 2 h and developed with p-nitrophenyl phosphate (1 mg/ml; Wako) in 10 mM diethanolamine buffer (pH 9.6). Ab concentrations were calculated from the standard curve by using purified mouse IgA, IgG, and IgM (Southern Biotechnology Associates). In addition, subclass–specific IgG Ab titers were also analyzed by using alkaline phosphatase–conjugated goat anti–mouse IgG1, IgG2a, IgG2b, and IgG3 (Southern Biotechnology Associates) as detection Ab. Further, murine IgG1, IgG2a, IgG2b, and IgG3 antibodies (Southern Biotechnology Associates) were used for the generation of a standard curve.
Preparation of Cell Suspensions.
Mice anesthetized with ketamine (Sigma Chemical Co.) were killed at 25 wk of age. The SPs and MLNs were aseptically removed, and single-cell suspensions were prepared by a standard mechanical procedure. Mononuclear cells from the LP of the colon were dissociated using type IV collagenase (Sigma Chemical Co.) to obtain single-cell preparations as described previously 16.
Enzyme-linked Immunospot.
Total IgA, IgG, and IgM Ab–forming cells in SP, MLNs, and colonic LP were analyzed by an enzyme-linked immunospot (ELISPOT) assay as described previously 10. Nitrocellulose microtiter plates (Millipore Co.) were coated with 100 μl of anti–mouse IgA, IgG, or IgM (Southern Biotechnology Associates) at a concentration of 5 μg/ml in PBS. For the detection of Ab-producing cells, alkaline phosphatase–conjugated anti–mouse IgA, IgG, or IgM (1 μg/ml; Southern Biotechnology Associates) was added and then visualized with a substrate. The substrate used was 5-bromo-4-chloro-3-indolyl phosphate (Wako)/nitroblue tetrazolium (Wako) in alkaline phosphatase buffer (100 mM Tris-HCl [pH 9.5] containing 100 mM NaCl and 5 mM MgCl2).
Flow Cytometric Analysis and Cell Sorting.
For the analysis of the profile of CD4+ββ T cells by flow cytometry, PE-conjugated anti-CD4 (anti-L3T4; RM4-5) mAb and FITC-conjugated anti–TCR-β mAb (H57-597) were obtained from PharMingen. Single-cell suspensions of lymphocytes (106/sample) were then stained with optimal concentrations of PE-conjugated anti-CD4 mAb and FITC-conjugated anti–TCR-β mAb. These samples were subjected to flow cytometric analysis by using a FACScan™ (Becton Dickinson). Data were analyzed by using CellQuest software (Becton Dickinson). For the analysis of the cytokine profile, CD4+ββ T cells were purified by FACS Vantage™ (Becton Dickinson).
Reverse-transcription PCR.
Cytokine production by purified CD4+ββ T cells from colonic LP was analyzed by modified cytokine-specific reverse-transcription (RT)-PCR as described 17 18. To isolate RNA from the CD4+ββ T cells purified by flow cytometry, TRIzol reagent (GIBCO BRL) was used. Purified RNA was reverse transcribed into cDNA using Superscript II reverse transcriptase (GIBCO BRL) and DIG DNA Labeling Mix® (Boehringer Mannheim), which incorporates DIG-labeled dUTP every 20–25 nucleotides during reverse transcription. The DIG-labeled, synthesized cDNA and a series of diluted DIG-labeled control cDNA (Boehringer Mannheim) were dot blotted onto the nucleic acid transfer membrane (Amersham Pharmacia Biotech) and cross-linked by UV cross-linker (Spectronics). The membrane was subjected to blocking by 1% Blocking reagent® (Boehringer Mannheim) in 0.15 M NaCl, 0.1 M maleic acid for 30 min, followed by an additional 30-min incubation with 7.5 U/liter of alkaline phosphatase–conjugated anti-DIG Abs (Boehringer Mannheim) in 1% Blocking reagent® (Boehringer Mannheim). The membrane was then incubated with 1% chemiluminescent substrate for alkaline phosphatase, CDPstar™ (Tropix), in 100 mM Tris-HCl, 100 mM NaCl, 50 mM MgCl2. The developed chemiluminescent signals on the membrane were exposed to an imaging screen for 18 h, and then characterized by an image analyzer (Molecular Imager® system; Bio-Rad Laboratories). The images on the screen were extracted by a laser scanner, and the number of synthesized cDNA samples were quantitated using the image analyzer. PCR amplification of 10 ng of cDNA for each sample was performed with the GeneAmp PCR System 9700 (Perkin-Elmer Cetus). The cytokine-specific primers and amplification protocols used have been described previously 18. The amplified products were separated by electrophoresis in 1.8% agarose gel and were visualized with ethidium bromide (1 μg/ml).
Cytokine ELISA.
Lymphocytes (106/well) isolated from colonic LP of anti–IL-4 mAb– or mock Ab–treated mice were stimulated in vitro with precoated purified anti–mouse CD3∈ mAb (145-2C11, 10 μg/ml; PharMingen) or anti–mouse TCR-β (H57-597, 10 μg/ml; PharMingen) in 24-well microplates for 72 h. After the incubation period, the supernatants were collected to analyze Th1 (IFN-γ) and Th2 cytokine (IL-4 and IL-6) production. Cytokine synthesis in the culture supernatants was analyzed by using the Biotrak™ ELISA system (Amersham Pharmacia Biotech) according to the manufacturer's protocol.
Histological Analysis.
Tissue samples were fixed in 4% paraformaldehyde in PBS for 4 h, embedded in paraffin, and sectioned at a thickness of 5 μm. Sections were stained by the conventional hematoxylin and eosin staining method. The periodic acid–Schiff–alcian blue procedure was also performed in order to stain goblet cells 19.
Statistical Analysis.
Data were statistically analyzed by the Student's t test.
Results
Anti–IL-4 mAb Treatment Blocked Aberrant Ig Production in TCR-α−/− Mice.
As increased levels of Abs are one of the immunological features of TCR-α−/− mice with IBD 10, we sought to determine and compare the levels of serum and fecal IgA, IgG, and IgM Abs in anti–IL-4 mAb– and mock Ab–treated TCR-α−/− mice at 25 wk of age by using ELISA. Serum as well as fecal Ab titers were increased in mock Ab–treated TCR-α−/− mice (Fig. 1 A). The levels of Ab titers in these mice were comparable to those of untreated mice, as observed in previous reports 9 10. However, the levels of IgA, IgG, and IgM Abs in serum and fecal extracts were significantly decreased in TCR-α−/− mice treated with anti–IL-4 mAb (P < 0.01; Fig. 1 A). When IgG subclass Ab titers of TCR-α−/− mice treated with anti–IL-4 mAb were examined by ELISA, levels of IgG1 and IgG2b were found to have decreased and those of IgG2a to have increased significantly (P < 0.01; Fig. 1 B).
Figure 1.
Comparison of Ig levels in serum and fecal extracts of TCR-α−/− mice treated with anti–IL-4 mAb (hatched bars) or rat IgG2b (mock Ab, black bars). (A) The levels of IgA, IgG, and IgM Abs in serum and fecal extracts were analyzed by ELISA. (B) The levels of IgG subclass Ab were also analyzed by ELISA. Data represent the mean ± SEM from eight mice per group. *Significantly different from each other (P < 0.01) by Student's t test.
Inhibition of B Cell Development in TCR-α−/− Mice by Anti–IL-4 mAb Treatment.
To further confirm the reduction of Ab production at the cellular base, mononuclear cells were isolated from systemic and mucosal tissues of TCR-α−/− mice treated with anti–IL-4 mAb and mock Ab for subsequent ELISPOT assay. The numbers of Ab-forming cells were increased in the systemic lymphoid (e.g., SP) as well as in mucosa-associated tissues (e.g., MLNs, colonic LP) of TCR-α−/− mice treated with mock Ab (Fig. 2). On the other hand, numbers of IgA, IgG, and IgM Ab–forming cells from TCR-α−/− mice treated with anti–IL-4 mAb were significantly decreased both in the systemic lymphoid and mucosa-associated tissues (P < 0.01; Fig. 2).
Figure 2.
Enumeration of Ab-producing cells in systemic and mucosal lymphoid tissues from mice treated with anti–IL-4 mAb (hatched bars) or mock Ab (black bars). Mononuclear cells isolated from SP, MLNs, and colonic LP (LPL) of TCR-α−/− mice treated with anti–IL-4 mAb or rat IgG2b (mock Ab) were examined by isotype-specific ELISPOT. Data represent the mean ± SEM from five mice per group of three separate experiments. *Significantly different from each other (P < 0.01) by Student's t test.
Anti–IL-4 mAb Did Not Influence the Development of CD4+ββ T Cells.
Since the administration of anti–IL-4 mAb inhibited Ab production in TCR-α−/− mice (Fig. 1 and Fig. 2), we next used flow cytometry to assess the influence of mAb treatment on the development of CD4+ββ T cells. A subset of CD4+ββ T cells costained with PE-conjugated anti-CD4 mAb (RM4-5) and FITC-conjugated anti-TCR-β (H57-597) was detected in the mucosal and peripheral tissues of mock Ab–treated TCR-α−/− mice. Surprisingly, a similar frequency of CD4+ββ T cells also developed in TCR-α−/− mice treated with anti–IL-4 mAb (Fig. 3). Additionally, the number of total lymphocytes in colonic LP obtained by dissociation with collagenase showed no statistical change between the two groups of mice (Mock Ab, 4.4 ± 0.8 × 106 cells/mouse; and anti–IL-4 mAb, 4.0 ± 0.6 × 106 cells/mouse). Further, mice treated with anti–IL-4 mAb did not show obvious clinical signs of IBD (see section below). These findings show that anti–IL-4 mAb treatment did not influence the development of CD4+ββ T cells.
Figure 3.
Flow cytometric analysis of CD4+ββ T cells in the colonic LP of TCR-α−/− mice treated with/without anti–IL-4 mAb. Mononuclear cells from the colonic LP of TCR-α−/− mice treated with anti–IL-4 mAb or mock Ab were isolated and costained with anti-CD4 (L3T4) and anti–TCR-β (H57-597) mAbs. Flow cytometric analysis was performed by FACScan™.
Anti–IL-4 mAb Treatment Altered the Cytokine Profile of CD4+ββ T Cells from Dominant Th2 to Th1 Type.
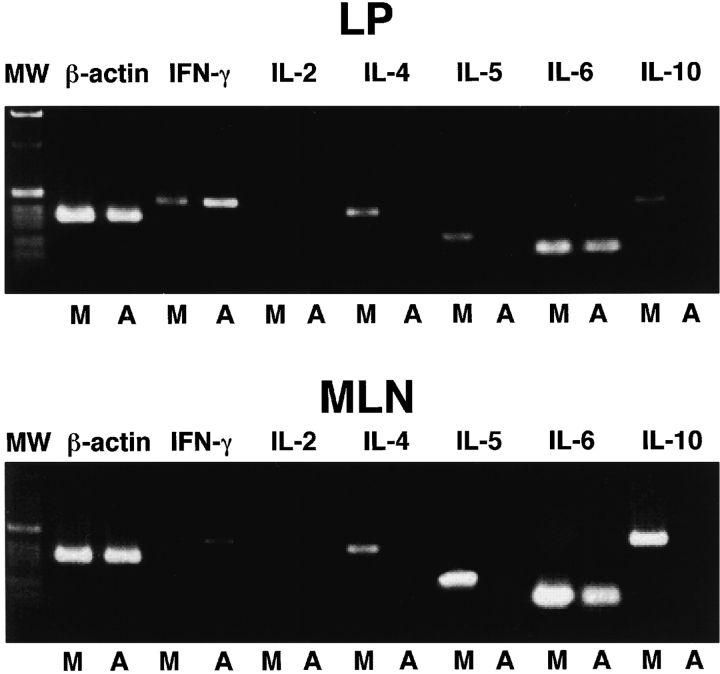
As the development of aberrant CD4+ββ T cells was not affected by treatment with anti–IL-4 mAb (Fig. 3), we next analyzed the cytokine profile of CD4+ββ T cells isolated from TCR-α−/− mice treated with anti–IL-4 mAb or mock Ab. The CD4+ββ T cells were isolated from the colonic LP of TCR-α−/− mice by FACS Vantage™, and the profile of Th1 and Th2 cytokine expression was examined by cytokine-specific RT-PCR. Our previous study showed that CD4+ββ T cells could be considered to be of the Th2 phenotype, since a cytokine-specific ELISPOT assay showed that they produced IL-4 but not Th1 cytokines 10. This pattern of Th2-type cytokine production was also confirmed by cytokine-specific RT-PCR, since CD4+ββ T cells isolated from the colonic LP of mock Ab–treated TCR-α−/− mice expressed mRNA specific for IL-4, IL-5, IL-6, and IL-10, but not for Th1-type cytokines (Fig. 4). Conversely, in the TCR-α−/− mice treated with anti–IL-4 mAb, the pattern of cytokine production was significantly changed; namely, specific messages for Th2-type cytokines such as IL-4, IL-5, and IL-10 were not detected, whereas those for Th1-type cytokines (e.g., IFN-γ) were upregulated (Fig. 4). In addition, the analysis of CD4+ββ T cells isolated from the MLNs of IL-4–specific mAb–treated mice resulted in the alteration of cytokine expression from a dominant Th2 to a Th1 type. The reduction of Th2-type cytokine production and the enhancement of Th1-type cytokine production in mice treated with anti–IL-4 mAb were further confirmed at the protein level through ELISA analysis of secreted Th1 and Th2 cytokines (Fig. 5). Thus, in vitro stimulation of colonic lymphocytes isolated from anti–IL-4 mAb–treated mice with anti-CD3∈ or anti–TCR-β resulted in the decrease of Th2 cytokines (e.g., IL-4 and IL-6) and the increase of IFN-γ synthesis. In contrast, treatment with mock Ab resulted in high levels of Th2 cytokine production. These results demonstrate that a shift from a Th2- to Th1-type response was induced in anti–IL-4 mAb–treated TCR-α−/− mice.
Figure 4.
Cytokine-specific mRNA expression by CD4+ββ T cells in the mucosal compartment of TCR-α−/− mice treated with or without anti–IL-4 mAb. CD4+ββ T cells in the MLNs and colonic LP of TCR-α−/− mice treated with anti–IL-4 mAb (A) or mock Ab (M) were purified by flow cytometry, and cytokine-specific mRNA expression was analyzed by Th1 and Th2 cytokine-specific RT-PCR. The far left column (MW) shows a 1-kb DNA ladder.
Figure 5.
Cytokine production by colonic lymphocytes isolated from mice with anti–IL-4 mAb (hatched bars) or mock Ab (black bars) treatment. Colonic lymphocytes were stimulated in vitro with precoated anti-CD3∈ mAb (a-CD3) or anti–TCR-β mAb (a-TCRβ) for 72 h. Culture supernatants were then collected and cytokine production was analyzed by cytokine ELISA. Data represent the mean ± SEM from two mice per group of three different experiments.
Alteration of the Aberrant Immune Response by Anti–IL-4 mAb Treatment Resulted in the Prevention of Mucosal Inflammation in TCR-α−/− Mice.
We next investigated the effect of anti–IL-4 mAb on the induction of IBD in the TCR-α−/− mice. Approximately 60% of TCR-α−/− mice treated with mock Ab or left untreated were observed to develop the morphological changes that have been reported previously to be typical of IBD, including anorectal prolapse, diarrhea, and the weight loss and hunched posture characteristic of wasting syndrome 2 10. In contrast, the mice treated with anti–IL-4 mAb showed no significant weight loss (Fig. 6). Histological examination of the intestinal LP of TCR-α−/− mice treated with anti–IL-4 mAb showed more elongation of epithelial villi and markedly less infiltration of inflammatory cells than in that of mice treated with mock Ab (Fig. 7). Because a reduction in the number of goblet cells has also been reported in the colon of humans with IBD 20, we stained tissue sections prepared from the colon of anti–IL-4 mAb– and mock Ab–treated TCR-α−/− mice with alcian blue in order to detect goblet cells. As in the colons of humans suffering from IBD, the number of goblet cells in colons of mock Ab–treated mice was reduced. In contrast, the number of goblet cells was almost normal in the colon of TCR-α−/− mice treated with anti–IL-4 mAb (Fig. 8). These findings suggest that the inhibition of the Th2-type response by anti–IL-4 mAb treatment results in the prevention of mucosal inflammation in TCR-α−/− mice.
Figure 6.
Body weight of TCR-α−/− mice treated with/without anti–IL-4 mAb treatment. Body weight of TCR-α−/− mice treated with anti–IL-4 mAb (BVD4-1D11) or mock Ab (R35-38) was measured weekly for 25 wk. Data represent the mean ± SEM of eight mice per group.
Figure 7.


Histological analysis of TCR-α−/− mice treated with or without anti–IL-4. Rectums of TCR-α−/− mice treated with anti–IL-4 mAb or mock Ab were resected and routine histology, including fixing with formalin, embedding in paraffin, and staining with hematoxylin and eosin, was performed.
Figure 8.


Histological analysis of goblet cells in the intestinal epithelium of TCR-α−/− mice treated with or without anti–IL-4. Rectums of TCR-α−/− mice treated with anti–IL-4 mAb or mock Ab were resected and the periodic acid–Schiff–alcian blue procedure was performed, which includes fixing with formalin, embedding in paraffin, and staining with periodic acid–Schiff–alcian blue.
Discussion
The mucosal immune system consists of several immune components, including α/β and γ/δ T cells, IgA B cells, macrophages, dendritic cells, and epithelial cells, that form a molecular and cellular inter/intranet that provides a protective barrier against pathogens and environmental antigens in the gut 21 22 23. Under normal conditions, the mucosal immune system also properly regulates the intestinal mucosa. However, in those suffering from IBD, the mucosal immune system, and more particularly the T cell–dependent regulatory system, is disrupted 1 3. In TCR-α−/− mice, CD4+ββ T cells are considered to play a key role in the induction of IBD 9 10 13.
The results of this study demonstrated two new and important points regarding the role of Th1- and Th2-type cells in the development of IBD. In all murine IBD models used, including specific gene-manipulated and hapten-induced mice, a common observation was that the enhancement of Th1-type activity was associated with disease development 24 25 26. However, previous studies have posited that Th2-like responses are involved in the development of colon inflammation in human ulcerative colitis 27 28 29 30 31. Our study directly demonstrates that CD4+ββ T cells isolated from the inflamed colon possess characteristics of Th2-type cells based on their cytokine profile at the mRNA and protein levels (Fig. 4 and Fig. 5). Further, it was shown that alteration of the cytokine profile from a Th2 to a Th1 type by treatment with anti–IL-4 mAb resulted in the prevention of colitis in TCR-α−/− mice (Fig. 4 and Fig. 5). The decrease of IL-4–dependent IgG1 and IgG2b Ab responses further confirmed that anti–IL-4 mAb treatment inhibited Th2-type responses (Fig. 1 A). These two important findings suggest that aberrant mucosal Th2-type cells are involved in the development of chronic inflammation in the intestinal tract. Two very recent studies support this conclusion by demonstrating that Th2-type CD4+ T cells play a major role in the development of hapten-induced murine colitis 32 33.
IL-4 initially received attention as an enhancer of DNA synthesis in mouse B lymphocytes 34. Since then, extensive molecular and immunological investigations have established IL-4 as a biologically potent and essential cytokine for B cell development and responses, including those of IgE and IgG1 35. Further, this cytokine has been shown to play an essential role in the generation of Th2-type from Th0-type cells 35. Although IL-4 is considered to be a hallmark cytokine for Th2-type T cells, it should be noted that other immunologically competent cells such as mast cells, basophils, γ/δ T cells, and NK1.1+ T cells can produce this cytokine 35 36 37 38 39 40. NK1.1+CD4+ T cells have been shown to be a particularly important source of IL-4 37. Histological analysis with alcian blue and flow cytometric analysis using mAb specific to NK1.1 detected only a small number of mast cells and NK1.1+ T cells in TCR-α−/− mice (data not shown). Although our previous and present results clearly demonstrate that CD4+ββ T cells are the primary source for the production of IL-4 in TCR-α−/− mice with IBD, it is still possible that a minor population of NK1.1+CD4+ T cells provides an alternative source of the cytokine. When mucosal γ/δ T cells were isolated from the intestinal tract of TCR-α−/− mice with IBD for the analysis of cytokine production, no specific message for IL-4 was detected (data not shown). Therefore, the population of CD4+ββ T cells is most likely the major source of IL-4.
In regard to the increased B cell responses in these diseased TCR-α−/− mice, Th2 cytokines produced by CD4+ββ T cells are responsible for the activation of auto- and food antigen–specific IgG, IgE, and IgA Ab production pathways 10. IL-4 is a well-known switch factor for IgE and IgG1 induction 35. In addition to auto- and food antigen-specific IgG1 and IgE responses, IgA Ab responses were also upregulated in TCR-α−/− mice with IBD 10. A similar pattern of response was also observed in TCR-α−/− mice treated with mock Ab. The increased B cell responses in TCR-α−/− mice with IBD could be explained by our present finding that these CD4+ββ T cells were capable of producing IL-5, IL-6, and IL-10 in addition to IL-4 (Fig. 4). These Th2-type cytokines further support the production of Abs, including those of the IgA isotype, in TCR-α−/− mice. In fact, studies using both murine and human experimental systems have shown that IL-5, IL-6, and IL-10 are IgA-enhancing cytokines 20 21. When TCR-α−/− mice were treated with anti–IL-4 mAb, the levels of all the isotypes of Abs were significantly decreased in their serum and fecal extracts. Inasmuch as our results demonstrated that anti–IL-4 mAb treatment altered the cytokine profile of CD4+ββ T cells from a Th2 to a Th1 type (Fig. 4 and Fig. 5), it is most likely that an abrogation of Th2 cytokine synthesis resulted in the reduction of B cell development.
It is not yet known whether Abs against auto- and food antigens play a pathological role, much less by what means they might do so. Several reports have provided evidence that the levels of autoantibodies (autoAbs, e.g., anti-goblet cell, antitropomyosin, and anticolon autoAbs) were increased in the colon of humans suffering from IBD and have suggested that these autoAbs are the causative pathogens for the destruction of mucosal tissues by Ab-dependent cell-mediated cytotoxicity 40 41 42 43 44. Therefore, one possible mechanism for the prevention of IBD by anti–IL-4 mAb could be the reduction of autoAb-producing B cell responses. However, a separate study demonstrated that the disease process accelerated in double knockout mouse, which lacked both Ig μ and TCR α chains and which were generated by crossing TCR-α−/− and Ig-μ–deficient mice 45. It also showed that, in some cases, the removal of B cells worsened the symptoms of colitis. The results of that study suggest that Abs play a protective rather than a pathological role in the development of mucosal inflammation. It remains to be examined whether aberrant B cells and their derived Abs are involved in the initiation of the inflammatory process in IBD.
IL-4 has been shown to directly regulate intestinal epithelial cell functions. For example, IL-4 can regulate the growth of epithelial cells. It is also capable of disrupting the barrier function of the intestinal epithelium, of enhancing the adherence of neutrophils to the epithelia, and of upregulating transepithelial neutrophil migration 46. Overproduction of IL-4 in the intestinal epithelium may disrupt immunological homeostasis between the mucosal immune system and environmental antigens, including gut lumen microorganisms, allowing recruitment of inflammatory cells for the initiation of disease. Therefore, a second possible mechanism for the prevention of colitis development by mAb treatment might be the direct inhibition of IL-4 effects on the intestinal epithelial cells.
IL-4–specific mAb treatment directly inhibited the development of Th2-type aberrant CD4+ββ T cells, which are thought to be directly involved in the induction of IBD. For example, Th2-dominated conditions such as those characteristic of IFN-γ−/− mice have been shown to be conducive to the development of a delayed-type hypersensitivity reaction 47 48, and Th2-type cells are thought to contribute to the development of autoimmune diseases 49 50. Finally, a recent study from another group provided direct evidence that IL-4 produced by Th2-type CD4+ββ T cells was associated with the development of murine colitis by showing the reduction of disease incidence in mice of the TCR-α−/− and IL-4−/− double knockout models 51.
In summary, this study has demonstrated that the pathological ability of CD4+ββ T cells to induce IBD is inhibited by their alteration from a Th2 to a Th1 type. It has also shown that the treatment of TCR-α−/− mice with anti–IL-4 mAb resulted in the blockage of colitis formation.
Acknowledgments
This work was supported by a Grant-in-Aid from the Ministry of Education, Science, Sports and Culture and the Ministry of Health and Welfare of Japan, the Organization for Pharmaceutical Safety and Research, and the Taisho Pharmaceutical Co., Japan.
Footnotes
1used in this paper: CD4+βdim, CD4+TCR-α−/β+; CD4+ββ, CD4+TCR-ββ; ELISPOT, enzyme-linked immunospot; IBD, inflammatory bowel disease; LP, lamina propria; MLN, mesenteric lymph node; RT, reverse-transcription; SP, spleen
References
- Powrie P. T cells in inflammatory bowel diseaseprotective and pathogenic roles. Immunity. 1995;3:171–174. doi: 10.1016/1074-7613(95)90086-1. [DOI] [PubMed] [Google Scholar]
- Mombaerts P., Mizoguchi E., Grusby M.J., Glimcher L.H., Bhan A.K., Tonegawa S. Spontaneous development of inflammatory bowel disease in T cell receptor mutant mice. Cell. 1993;75:274–282. doi: 10.1016/0092-8674(93)80069-q. [DOI] [PubMed] [Google Scholar]
- Strober W., Ehrhardt R.O. Chronic intestinal inflammationan expected outcome in cytokine or T cell receptor mutant mice. Cell. 1993;75:203–205. doi: 10.1016/0092-8674(93)80062-j. [DOI] [PubMed] [Google Scholar]
- Sadlack B., Merz H., Schimpl A., Feller A.C., Horak I. Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell. 1993;75:253–261. doi: 10.1016/0092-8674(93)80067-o. [DOI] [PubMed] [Google Scholar]
- Watanabe M., Ueno Y., Yajima T., Okamoto S., Hayashi T., Yamazaki M., Iwao Y., Ishii H., Habu S., Uehira M. Interleukin 7 transgenic mice develop chronic colitis with diseased interleukin 7 protein accumulation in the colonic mucosa. J. Exp. Med. 1998;187:389–402. doi: 10.1084/jem.187.3.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn R., Jöhler J., Rennick D., Rajewsky K., Müller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- Rudolph U., Finegold M.J., Rich S.S., Harriman G.R., Srinvasan Y., Brabet P., Boulay G., Bradley A., Birnbaumer L. Ulcerative colitis and adenocarcinoma of the colon in Gαi2-deficient mice. Nat. Genet. 1995;10:143–150. doi: 10.1038/ng0695-143. [DOI] [PubMed] [Google Scholar]
- Morrissey P.J., Charrier K., Braddy S., Liggitt D., Watson J.D. CD4+ T cells that express high levels of CD45RB induce wasting disease when transferred into congenic severe combined immunodeficient mice. Disease development is prevented by cotransfer of purified CD4+ T cells. J. Exp. Med. 1993;178:237–244. doi: 10.1084/jem.178.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizoguchi A., Mizoguchi E., Chiba C., Spiekermann G.M., Tonegawa S., Nagler-Anderson C., Bhan A.K. Cytokine imbalance and autoantibody production in T cell receptor-α mutant mice with inflammatory bowel disease. J. Exp. Med. 1996;183:847–856. doi: 10.1084/jem.183.3.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi I., Kiyono H., Hamada S. CD4+ T-cell population mediates development of inflammatory bowel disease in T-cell receptor α chain-deficient mice. Gastroenterology. 1997;112:1876–1886. doi: 10.1053/gast.1997.v112.pm9178680. [DOI] [PubMed] [Google Scholar]
- Dianda L., Gulbranson-Judge A., Pao W., Hayday A.C., MacLennan I.C.M., Owen M.J. Germinal center formation in mice lacking αβ T cells. Eur. J. Immunol. 1996;26:1603–1607. doi: 10.1002/eji.1830260729. [DOI] [PubMed] [Google Scholar]
- Viney J.L., Dianda L., Roberts S.J., Wen L., Mallick C.A., Hayday A.C., Owen M.J. Lymphocyte proliferation in mice congenitally deficient in T-cell receptor αβ+ T cells. Proc. Natl. Acad. Sci. USA. 1994;91:11948–11952. doi: 10.1073/pnas.91.25.11948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi I., Iijima H., Katashima R., Itakura M., Kiyono H. Clonal expansion of CD4+TCRββ+ T cells in TCR α-chain-deficient mice by gut-derived antigens. J. Immunol. 1999;162:1843–1850. [PubMed] [Google Scholar]
- deVos T., Dick T.A. A rapid method to determine the isotype and specificity of coproantibodies in mice infected with Trichinella or fed cholera toxin. J. Immunol. Methods. 1991;141:285–288. doi: 10.1016/0022-1759(91)90155-9. [DOI] [PubMed] [Google Scholar]
- Takahashi I., Marinaro M., Kiyono H., Jackson R.J., Nakagawa I., Fujihashi K., Hamada S., Bost K.L., McGhee J.R. Mechanisms for mucosal immunogenicity and adjuvancy of Escherichia coli labile enterotoxin. J. Infect. Dis. 1996;173:627–635. doi: 10.1093/infdis/173.3.627. [DOI] [PubMed] [Google Scholar]
- Aicher W., Fujihashi K., Yamamoto M., Kiyono H., Pitts A.M., McGhee J.R. Effects of the lpr/lpr mutation on T and B cell populations in the lamina propria of small intestine, a mucosal effector site. Int. Immunol. 1992;4:959–968. doi: 10.1093/intimm/4.9.959. [DOI] [PubMed] [Google Scholar]
- Brenner C.A., Tam A.W., Nelson P.A., Engleman E.G., Suzuki N., Fry K.E., Larrick J.W. Message amplification phenotyping (MAPPing)a technique to simultaneously measure multiple mRNAs from small numbers of cells. Biotechniques. 1989;7:1096–1103. [PubMed] [Google Scholar]
- Fujihashi K., Yamamoto M., McGhee J.R., Beagley K.W., Kiyono H. Function of αβ TCR+ intestinal intraepithelial lymphocytesTh1- and Th2-type cytokine production by CD4+CD8− and CD4+CD8+ T cells for helper activity. Int. Immunol. 1993;5:1473–1481. doi: 10.1093/intimm/5.11.1473. [DOI] [PubMed] [Google Scholar]
- Yamabayashi S. Periodic acid-Schiff-alcian blue; a method for the differential staining of glycoproteins. Histochem. J. 1987;19:565–571. doi: 10.1007/BF01687364. [DOI] [PubMed] [Google Scholar]
- Evans C.M., Phillips A.D., Walker-Smith J.A., MacDonald T.T. Activation of lamina propria T cells induces crypt epithelial proliferation and goblet cell depletion in cultured human fetal colon. Gut. 1992;33:230–235. doi: 10.1136/gut.33.2.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mestecky J., McGhee J.R. Immunoglobulin A (IgA)molecular and cellular interactions involved in IgA biosynthesis and immune response. Adv. Immunol. 1987;40:153–245. doi: 10.1016/s0065-2776(08)60240-0. [DOI] [PubMed] [Google Scholar]
- Kiyono H., Bienenstock J., McGhee J.R., Ernst P.B. The mucosal immune systemfeatures of inductive and effector sites to consider in mucosal immunization and vaccine development. Reg. Immunol. 1992;4:54–62. [PubMed] [Google Scholar]
- Mowat A.M., Viney J.L. The anatomical basis of intestinal immunity. Immunol. Rev. 1997;156:145–166. doi: 10.1111/j.1600-065x.1997.tb00966.x. [DOI] [PubMed] [Google Scholar]
- Elson, C.O., K.W. Beagley, A.T. Sharmanov, K. Fujihashi, H. Kiyono, G.S. Tennyson, Y. Cong, C.A. Black, B.W. Ridwan, and J.R. McGhee. 1996. Hapten-induced model of murine inflammatory bowel disease. 157:2174–2185. [PubMed]
- Bregenholt S., Claesson M.H. Increased intracellular Th1 cytokines in scid mice with inflammatory bowel disease. Eur. J. Immunol. 1998;28:379–389. doi: 10.1002/(SICI)1521-4141(199801)28:01<379::AID-IMMU379>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Powrie F., Carlino J., Leach M.W., Mauze S., Coffman R.L. A critical role for transforming growth factor-β but not interleukin 4 in the suppression of T helper type 1–mediated colitis by CB45RBlowCD4+ T cells. J. Exp. Med. 1996;183:2669–2674. doi: 10.1084/jem.183.6.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuss I., Neurath M., Boirivant M., Klein J.S., de la Motte C., Strong S.A., Fiocchi C., Strober W. Disparate CD4+ lamina propria (LP) lymphokine secretion profiles in inflammatory bowel disease. Crohn's disease LP cells manifest increased secretion of IFN-γ, whereas ulcerative colitis LP cells manifest increased secretion of IL-5. J. Immunol. 1996;157:1261–1270. [PubMed] [Google Scholar]
- Sartor R.B. Cytokines in intestinal inflammationpathophysiological and clinical considerations. Gastroenterology. 1994;106:533–539. doi: 10.1016/0016-5085(94)90614-9. [DOI] [PubMed] [Google Scholar]
- Breese E., Braegger C.P., Corrigan C.J., Walker-Smith J.A., MacDonald T.T. Interleukin-2- and interferon-γ-secreting T cells in normal and diseased human intestinal mucosa. Immunology. 1993;78:127–131. [PMC free article] [PubMed] [Google Scholar]
- Niessner M., Volk B.A. Altered Th1/Th2 cytokine profiles in the intestinal mucosa of patients with inflammatory bowel disease as assessed by quantitative reversed transcribed polymerase chain reaction (RT-PCR) Clin. Exp. Immunol. 1995;101:428–435. doi: 10.1111/j.1365-2249.1995.tb03130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parronchi P., Romagnani P., Annuziato F., Sampognaro S., Becchio A., Giannarini L., Maggi E., Pupilli C., Tonelli F., Romagnani S. Type I T helper cell predominance and interleukin-12 expression in the gut of patients with Crohn's disease. Am. J. Pathol. 1997;150:823–832. [PMC free article] [PubMed] [Google Scholar]
- Dohi T., Fujihashi K., Rennert P.D., Iwatani K., Kiyono H., McGhee J.R. Hapten-induced colitis is associated with colonic patch hypertrophy and T helper cell 2–type responses. J. Exp. Med. 1999;189:1169–1180. doi: 10.1084/jem.189.8.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boirivant M., Fuss I.J., Chu A., Strober A. Oxazolone colitisa murine model of T helper cell type 2 colitis treatable with antibodies to interleukin 4. J. Exp. Med. 1998;188:1929–1939. doi: 10.1084/jem.188.10.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard M., Farrar J., Hilfiker M., Johnson B., Takatsu K., Hamaoka T., Paul W.E. Identification of a T cell–derived B cell growth factor distinct from interleukin 2. J. Exp. Med. 1982;155:914–923. doi: 10.1084/jem.155.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul W.E. Interleukin-4a prototypic immunoregulatory lymphokine. Blood. 1991;77:1859–1870. [PubMed] [Google Scholar]
- Bendelac A., Rivera M.N., Park S.-H., Roark J.H. Mouse CD1-specific NK1 T cellsdevelopment, specificity and function. Annu. Rev. Immunol. 1997;15:535–562. doi: 10.1146/annurev.immunol.15.1.535. [DOI] [PubMed] [Google Scholar]
- Yoshimoto T., Paul W.E. CD4+NK1.1+ T cells promptly produced IL-4 in response to in vivo challenge with anti-CD4. J. Exp. Med. 1994;179:1285–1295. doi: 10.1084/jem.179.4.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Garra A. Cytokines induce the development of functionally heterogenous T helper cell subsets. Immunity. 1998;8:275–283. doi: 10.1016/s1074-7613(00)80533-6. [DOI] [PubMed] [Google Scholar]
- Ferrick D.A., Schrenzel M.D., Mulvania T., Hsieh B., Ferlin W.G., Lepper H. Differential production of interferon-gamma and interleukin-4 in response to Th1- and Th2-stimulating pathogens by gamma delta T cells in vivo. Nature. 1995;373:255–257. doi: 10.1038/373255a0. [DOI] [PubMed] [Google Scholar]
- Zuany-Amorim C., Ruffié C., Hailé S., Vargaftig B.B., Pereira P., Pretolani M. Requirement for γδ T cells in allergic airway inflammation. Science. 1998;280:1265–1267. doi: 10.1126/science.280.5367.1265. [DOI] [PubMed] [Google Scholar]
- Brantzaeg P. Autoimmunity and ulcerative colitiscan two enigmas make sense together? Gastroenterology. 1995;109:307–322. doi: 10.1016/0016-5085(95)90298-8. [DOI] [PubMed] [Google Scholar]
- Geng X., Biancone L., Dai H.H., Lin J.J.-C., Yoshizaki N., Dasgupta A., Pallone F., Das K.M. Tropomyosin isoforms in intestinal mucosaproduction of autoantibodies to tropomyosin isoforms in ulcerative colitis. Gastroenterology. 1998;114:912–922. doi: 10.1016/s0016-5085(98)70310-5. [DOI] [PubMed] [Google Scholar]
- Folwaczny C., Noehl N., Tschöp K., Endres S.P., Heldwein W., Loeschke K., Fricke H. Goblet cell autoantibodies in patients with inflammatory bowel disease and their first-degree relatives. Gastroenterology. 1997;113:101–106. doi: 10.1016/s0016-5085(97)70085-4. [DOI] [PubMed] [Google Scholar]
- Hibi T., Aiso S., Ishikawa M., Watanabe M., Yoshida T., Tsuru S., Tsuchiya M. Anti-colon antibody and lymphocytopenia antibody in ulcerative colitis. Clin. Exp. Immunol. 1982;49:75–80. [PMC free article] [PubMed] [Google Scholar]
- Mizoguchi A., Mizoguchi E., Smith R.N., Preffer F.I., Bhan A.K. Suppressive role of B cells in chronic colitis of T cell receptor α mutant mice. J. Exp. Med. 1997;186:1749–1756. doi: 10.1084/jem.186.10.1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colgan S.P., Resnick M.B., Parcos C.A., Delp-Archer C., McGuirk D., Bacarra A.E., Weller P.F., Madara J.L. IL-4 directly modulates function of a model human intestinal epithelium. J. Immunol. 1994;153:2122–2129. [PubMed] [Google Scholar]
- Cooper A.M., Dalton D.K., Stewart T.A., Griffin J.P., Russel D.G., Orme I.M. Disseminated tuberculosis in interferon γ gene–disrupted mice. J. Exp. Med. 1993;178:2243–2247. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kweon M.-N., Fujihashi K., VanCott J.L., Higuchi K., Yamamoto M., McGhee J.R., Kiyono H. Lack of orally induced systemic unresponsiveness in IFN-γ knockout mice. J. Immunol. 1998;160:1687–1693. [PubMed] [Google Scholar]
- Mavalia C., Scaletti C., Romagnani P., Carrosso A.M., Pignone A., Emmi L., Pupilli C., Pizzolo G., Maggi E., Romagnani S. Type 2 helper T-cell predominance and high CD30 expression in systemic sclerosis. Am. J. Pathol. 1997;151:1751–1758. [PMC free article] [PubMed] [Google Scholar]
- Lafaille J.J., de Keere F.V., Hsu A.L., Baron J.L., Haas W., Raine C.S., Tonegawa S. Myelin basic protein–specific T helper 2 (Th2) cells cause experimental autoimmune encephalomyelitis in immunodeficient hosts rather than protect them from the disease. J. Exp. Med. 1997;186:307–312. doi: 10.1084/jem.186.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizoguchi A., Mizoguchi E., Bhan A.K. The critical role of interleukin 4 but not interferon gamma in the pathogenesis of colitis in T-cell receptor α mutant mice. Gastroenterology. 1999;116:320–326. doi: 10.1016/s0016-5085(99)70128-9. [DOI] [PubMed] [Google Scholar]