Abstract
The importance of B7 costimulation in regulating T cell expansion and peripheral tolerance suggests that it may also play a significant regulatory role in the development of autoimmune disease. It is unclear whether B7 costimulation is involved only in the expansion of autoreactive T cells in the periphery, or if it is also required for effector activation of autoreactive T cells in the target organ for mediating tissue injury and propagating autoimmune disease. In this study, the role of B7–CD28 costimulation and the relative importance of B7 costimulators for the induction and effector phases of experimental autoimmune encephalomyelitis (EAE) induced by myelin oligodendrocyte glycoprotein (MOG) peptide were examined. Wild-type, B7-1/B7-2–deficient mice, or CD28-deficient C57BL/6 mice were immunized with MOG 35-55 peptide. Mice lacking both B7-1 and B7-2 or CD28 showed no or minimal clinical signs of EAE and markedly reduced inflammatory infiltrates in the brain and spinal cord. However, mice lacking either B7-1 or B7-2 alone developed clinical and pathologic EAE that was comparable to EAE in wild-type mice, indicating overlapping functions for B7-1 and B7-2. Resistance to EAE was not due to a lack of induction of T helper type 1 (Th1) cytokines, since T cells from B7-1/B7-2−/− mice show reduced proliferative responses, but greater interferon γ production compared with T cells from wild-type mice. To study the role of B7 molecules in the effector phase of the disease, MOG 35-55–specific T lines were adoptively transferred into the B7-1/B7-2−/− and wild-type mice. Clinical and histologic EAE were markedly reduced in B7-1/B7-2−/− compared with wild-type recipient mice. These results demonstrate that B7 costimulation has critical roles not only in the initial activation and expansion of MOG-reactive T cells, but also in the effector phase of encephalitogenic T cell activation within the central nervous system.
Keywords: B7, costimulation, knockout mouse, autoimmunity, experimental autoimmune encephalomyelitis
The B7-CD28/CTL-associated molecule 4 (CTLA-4)1 costimulatory pathway plays a pivotal role in regulating T cell activation and peripheral tolerance and is a promising therapeutic target for treating inflammatory autoimmune diseases. The B7 family of costimulatory molecules, B7-1 (CD80) and B7-2 (CD86), provide the major costimulatory signal for augmenting and sustaining T cell responses via ligation of the CD28 receptor. B7–CD28 interactions regulate T cell growth, survival, and differentiation. The B7 family of costimulators also bind a second higher affinity receptor on T cells, CTLA-4 (CD152). B7–CTLA-4 interactions provide a critical downregulatory signal for T cell activation, which may regulate autoreactive T cells in the periphery 1.
Studies using B7 antagonists have demonstrated the importance of this pathway in the pathogenesis of autoimmune disease. B7 blockade can prevent the development of experimental autoimmune encephalomyelitis (EAE), a T cell–mediated autoimmune disease that shares many clinical and histological features with multiple sclerosis (MS). Several studies using SJL mice have shown that EAE in this strain can be blocked by administering antibodies to B7-1 but not B7-2 2 3 4. Such results led to the suggestion that B7-1 is the more important costimulatory molecule in EAE. However, whether B7-1 is also important in the induction of disease in other EAE-susceptible strains and whether the effect of anti-B7 mAbs was due to B7-1 blockade or activation of B7-1–expressing cells has not been established. One of the studies suggested that B7-1 versus B7-2 blockade may differentially affect T cell differentiation and Th1/Th2 cytokine balance 3, whereas another study showed that B7-1 becomes a dominant costimulatory molecule during EAE 4. Therefore, the mechanisms by which B7-1 blockade protect against induction of EAE remain unclear.
B7 costimulation is potentially important in regulating T cell function at several stages of EAE. These include the initial activation, expansion, and differentiation of T cells within peripheral lymphoid tissues, homing and entry into the central nervous system (CNS), and reactivation of autoreactive T cells within the CNS that mediate tissue injury. To further dissect the relative importance of B7 costimulation for the induction and effector phases of EAE, we induced EAE in C57BL/6 mice lacking B7 costimulators (B7-1/B7-2−/−) or CD28 (CD28−/−). We compared EAE induced by active immunization with myelin oligodendrocyte glycoprotein (MOG) 35-55 peptide or adoptive transfer of MOG 35-55–specific T cells. Our results demonstrate that B7 costimulation plays a critical role not only in the induction phase of the autoimmune response when T cells become activated in the periphery, but also in the effector phase when potentially pathogenic T cells infiltrate the target organ and mediate disease.
Materials and Methods
Mice
B7-1−/−, B7-2−/−, and B7-1/B7-2−/− mice were generated by gene targeting as described previously 5 6. B7-deficient mice were backcrossed from the 129/S4SvJae background to the C57BL/6 background for at least six generations before use in experiments, and were genotyped as described previously 5 6. CD28−/− mice were purchased from The Jackson Laboratory. Mice used for controls were either wild-type or heterozygous littermates or C57BL/6 mice purchased from The Jackson Laboratory. Brigham and Women's Hospital and Harvard Medical School are accredited by the American Association of Laboratory Animal Care (AALAC). Mice were cared for in accordance with institutional guidelines in a pathogen-free animal facility.
MOG Peptide
MOG 35-55 (MEVGWYRSPFSRVVHLYRNGK) and MOG 92-106 (DEGGYTCFFRDHSYQ) were synthesized by Dr. David Teplow at the Biopolymer Facility (Center for Neurological Diseases, Brigham and Women's Hospital) on an Applied Biosystems 430A peptide synthesizer using fluorenylmethoxycarbonyl (F-MOC) chemistry. The peptides were >90% pure, as determined by HPLC.
Induction and Assessment of EAE
By Active Immunization.
Groups of 6–8-wk-old female mice (three to six per group) were immunized with 100 μg of MOG 35-55 emulsified 1:1 in CFA supplemented with 400 μg of Mycobacterium tuberculosis H37RA (Difco Laboratories) in the two flanks subcutaneously. 200 ng of pertussis toxin (List Biological Laboratories) was injected intravenously on the day of immunization and 2 d later.
By Adoptive Transfer.
Wild-type mice were immunized with 100 μg of MOG 35-55 in CFA (Difco Laboratories). 10 d later, draining lymph nodes were harvested, and lymphocytes were cultured with 30 μg of MOG 35-55 and 20 ng of murine rIL-12 (Genetics Institute). After 4 d of culture, cells were washed and resuspended in PBS for transfer. Wild-type or B7-1/B7-2−/− recipients received 107 cells intravenously, as well as 200 ng of pertussis toxin immediately after cell transfer and 2 d later.
Mice were observed daily for clinical signs of EAE up to 35 d after immunization or cell transfer, and scored on a scale of 0–5: 0, no disease; 1, limp tail; 2, hind limb weakness; 3, hind limb paralysis; 4, hind and fore limb paralysis; and 5, moribund state. Mean clinical score was calculated by averaging the scores of all mice in each group, including animals that did not develop EAE.
Histology
Brains and spinal cords were removed and fixed in 10% formalin. Paraffin-embedded sections were stained with Luxol fast blue–hematoxylin and eosin for light microscopy. Inflammatory foci were counted in the meninges and parenchyma by an unbiased observer as described previously 7.
Proliferative Response of T Cells to MOG Antigens
Mice were immunized subcutaneously with 100 μg of MOG 35-55 emulsified 1:1 in CFA (Difco Laboratories). Draining lymph nodes were harvested 10 d later, and single cell suspensions were prepared. Whole lymph node cells were cultured in 96-well plates at 5 × 105 cells/well with a range of concentrations of MOG 35-55 or MOG 92-106 peptide in HL-1 media (BioWhittaker). Plates were pulsed with [3H]thymidine (New England Nuclear) at 1 μCi/well on day 3 of culture for the final 18 h. Mean incorporation of thymidine in DNA was measured in triplicate wells by liquid scintillation counting (model LS 5000; Beckman Instruments).
Cytokine ELISA
Lymph node cells were prepared and cultured as above with media alone or 50 μg of MOG 35-55 peptide. Supernatants were harvested at 24, 48, and/or 72 h of culture. Cytokines (IL-2, IFN-γ, TNF-α, IL-4, and IL-10) were determined by sandwich ELISA, as described 8. mAb pairs and recombinant cytokine standards were purchased from PharMingen. The following mAb pairs were used: IL-2 (JES6-1A12, capture; JES6-5H4 detection); IFN-γ (R4-6A2 capture; XMG1.2 detection); IL-4 (BVD4-1D11 capture; BVD6-24G2 detection); IL-10 (JES5-2A5 capture; SXC-1 detection); and TNF-α (G281-2626 capture; MP6-XT3 detection).
Results
Mice Lacking Both B7-1 and B7-2 or CD28 Are Resistant to EAE Induced by Immunization with MOG 35-55.
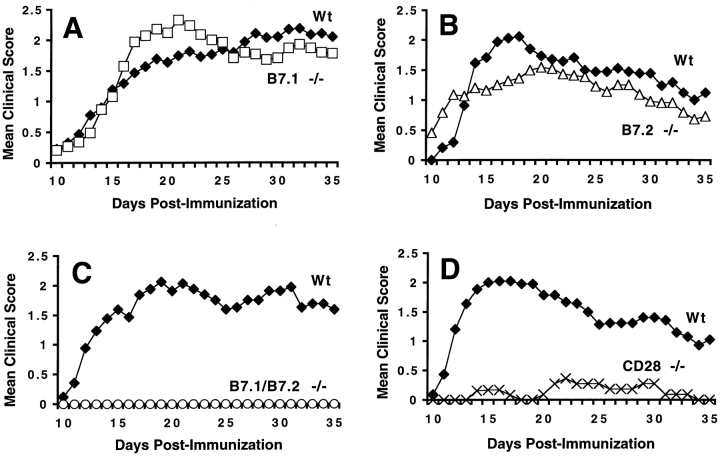
To examine the role of the B7–CD28 costimulatory pathway in the initiation of MOG-induced EAE, we immunized wild-type C57BL/6 mice and C57BL/6 mice lacking CD28 or either B7-1 or B7-2 or both B7 costimulators with MOG 35-55 and observed the mice for clinical signs of EAE. Mice deficient in either B7-1 or B7-2 developed EAE with comparable severity and time course to wild-type mice (Fig. 1A and Fig. B). EAE incidence, time of onset, and maximal clinical score were not significantly different among wild-type, B7-1−/−, and B7-2−/− mice (Table ). All three groups had >90% clinical disease incidence. EAE onset occurred 13–16 d after immunization and reached maximal severity at a clinical score of 2.5–3.0 (severe hind limb weakness or paralysis). These results indicate that the presence of either B7-1 or B7-2 alone is sufficient to provide the costimulation necessary to support the development of MOG-induced EAE in C57BL/6 mice.
Figure 1.
EAE disease course in C57BL/6 B7-1−/− mice (A; n = 34), B7-2−/− mice (B; n = 22), B7-1/B7-2−/− mice (C; n = 18), and CD28−/− mice (D; n = 13). Filled diamonds in A–D, wild-type (Wt) mice. Mice were immunized with 100 μg of MOG 35-55 subcutaneously and injected twice with 200 ng of pertussis toxin intravenously. Mice were observed daily and scored as described in Materials and Methods. Data are pooled from three to five experiments and represent mean clinical score of each group plotted against time.
Table 1.
EAE in Mice Lacking B7 Costimulators or CD28 Induced by Active Immunization
| Clinical EAE | Histological EAE | ||||||
|---|---|---|---|---|---|---|---|
| Incidence | Day of onset | Mean maximal score | Incidence | Meningeal foci | Parenchymal foci | Total foci | |
| mean ± SE | mean ± SE | mean ± SE | mean ± SE | mean ± SE | |||
| Wild-type | 27/29 | 15.8 ± 0.9 | 3.0 ± 0.2 | 18/21 | 21.7 ± 7.0 | 15.7 ± 5.0 | 37.4 ± 11.9 |
| (93.1%) | (85.7%) | ||||||
| B7-1−/− | 31/34 | 14.6 ± 0.5 | 2.9 ± 0.1 | 21/23 | 14.2 ± 3.4 | 13.0 ± 3.2 | 27.2 ± 6.4 |
| (91.2%) | (91.3%) | ||||||
| B7-2−/− | 21/22 | 13.9 ± 1.0 | 2.4 ± 0.2 | 21/21 | 29.1 ± 5.4 | 9.4 ± 2.0 | 38.9 ± 7.0 |
| (95.5%) | (100.0%) | ||||||
| B7-1/B7-2−/− | 0/18 | NA | NA | 7/14 | 12.8 ± 7.3 | 0.6 ± 0.4 | 13.3 ± 7.4 |
| (0.0%) | (50.0%) | P < 0.001 | P < 0.03 | ||||
| P < 0.0001 | P = 0.05 | ||||||
| CD28−/− | 2/13 | 17.5 ± 1.4 | 2.0 ± 0.0 | 6/11 | 2.9 ± 1.3 | 1.5 ± 0.6 | 4.4 ± 1.8 |
| (15.4%) | (54.5%) | P < 0.01 | P < 0.01 | P < 0.01 | |||
| P < 0.0001 | |||||||
In contrast, mice lacking both B7-1 and B7-2 were strikingly resistant to EAE induction (Fig. 1 C). In 3 separate experiments involving 18 B7-1/B7-2−/− mice, none of the B7-1/B7-2−/− mice developed clinical signs of EAE during the 35-d observation period, whereas 90% of wild-type littermates developed EAE. Similar to the B7-1/B7-2−/− mice, mice lacking CD28 were also resistant to EAE induction (Fig. 1 D). Only 2 of 13 (15%) CD28−/− mice developed clinical signs of EAE. In these two CD28−/− mice, the time of onset of EAE was delayed, and the maximal disease score was also less than in wild-type mice (Table ).
B7-1/B7-2−/− Mice and CD28−/− Mice Show Less CNS Inflammation after MOG Peptide Immunization Than Wild-type Mice.
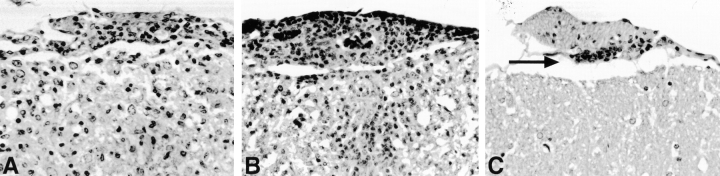
The brain and spinal cords of wild-type, B7−/−, and CD28−/− mice were examined histologically at the end of the clinical observation period (day 35 after immunization). Wild-type, B7-1−/−, and B7-2−/− mice had similar numbers of inflammatory lesions in the meninges and CNS parenchyma (Fig. 2A and Fig. B). More than 80% of mice in each of these groups demonstrated inflammatory lesions. In contrast, EAE inflammatory foci were observed in approximately half of B7-1/B7-2−/− and CD28−/− mice, and the numbers of foci were significantly reduced in both groups (Table ). Thus, the lack of clinical disease in B7-1/B7-2−/− mice was accompanied by a marked reduction in CNS inflammation. Strikingly, in the seven B7-1/B7-2−/− mice that exhibited inflammatory foci (Table ), these foci were limited almost exclusively to the leptomeninges (Fig. 2 C). Thus, the absence of both B7-1 and B7-2 resulted in a disproportionate reduction of parenchymal compared with meningeal infiltrates.
Figure 2.
CNS histopathology. Representative fields from the anterior lumbar spinal cord of wild-type (A), B7-2−/− (B), and B7-1/B7-2−/− (C) mice from day 35 after immunization. In A and B, there are many mononuclear inflammatory cells in the leptomeninges and infiltrating the parenchyma. In C, there is a small meningeal inflammatory focus (arrow), but the parenchyma is not infiltrated. All are stained with Luxol fast blue–hematoxylin and eosin. Original magnifications: 280×.
B7-1/B7-2−/− T Cells Are Primed to MOG and Secrete Abundant IFN-γ.
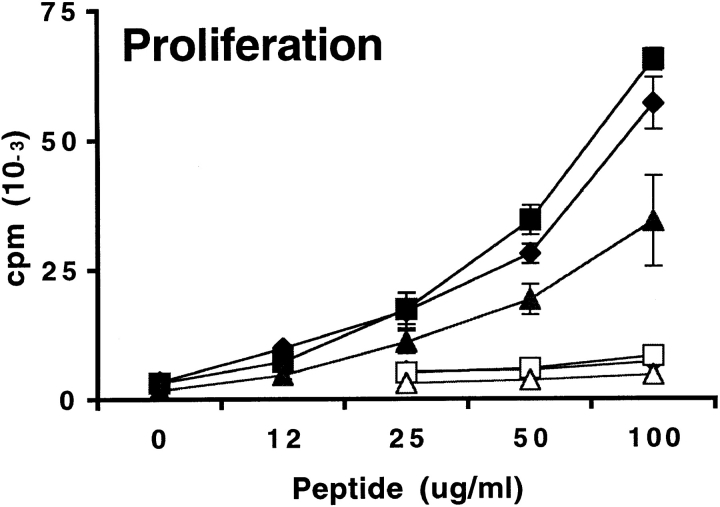
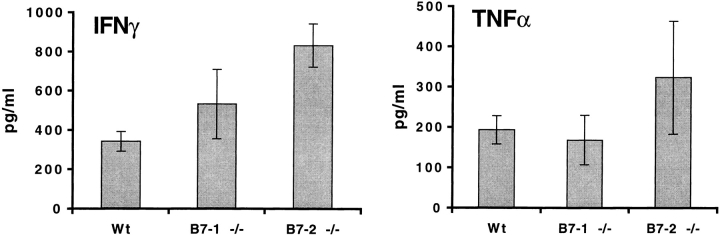
To determine whether the phenotype of responding T cells to MOG 35-55 was altered in mice lacking either or both B7 costimulators, we restimulated draining lymph node cells with MOG 35-55 in vitro and measured proliferative responses and cytokine secretion. For these studies, draining lymph nodes were obtained 10 d after immunization with MOG 35-55. Proliferation and cytokine production were evaluated on days 1, 2, and 3 of in vitro culture using a range of peptide concentrations. As shown in Fig. 3, the proliferative responses of draining lymph node cells obtained from immunized wild-type and B7-1−/− mice were comparable. No consistent or significant differences in IFN-γ and TNF-α production were observed in B7-1−/− lymph node cultures compared with wild-type. Although the proliferative responses of B7-2−/− lymph node cells were modestly reduced at high peptide concentrations, they produced more IFN-γ than wild-type cells (Fig. 3). IL-2, IL-4, and IL-10 were below detection limit for all cultures. The modest differences in T cell proliferation and cytokine production in B7-1−/− and B7-2−/− lymph node cultures are similar to the minimal variations in clinical and histological manifestations of EAE among wild-type, B7-1−/−, and B7-2−/− mice.
Figure 3.
Proliferation and cytokine production of lymph node cells from B7-1−/− and B7-2−/− mice challenged with MOG 35-55. Mice were immunized with 100 μg of MOG 35-55 in CFA. 10 d later, draining lymph node cells from wild-type (Wt, diamonds), B7-1−/− (squares), or B7-2−/− (triangles) mice were restimulated in vitro with a titration of MOG 35-55 (filled symbols) or MOG 92-106 (open symbols) as control. Culture supernatants were collected after 48 h from cultures stimulated with 50 μg/ml of MOG 35-55 or with media alone. Cytokines were measured by sandwich ELISA, and background levels from media controls were subtracted. Data are representative of eight independent experiments.
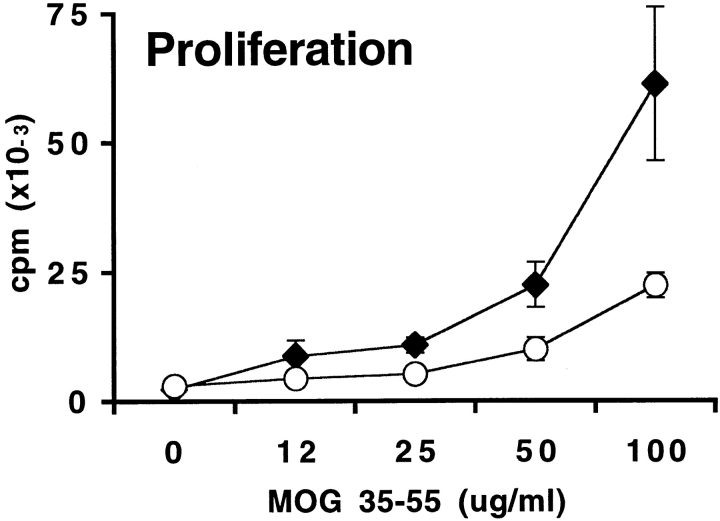
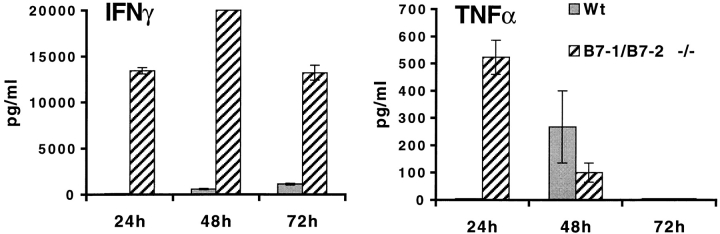
In contrast, EAE-resistant B7-1/B7-2−/− mice exhibited markedly impaired proliferative responses to MOG 35-55 compared with wild-type mice (Fig. 4). Even at the highest peptide concentration examined, the proliferation of B7-1/B7-2−/− lymph node cells was only 25% of wild-type levels. Despite the reduced proliferation, B7-1/B7-2−/− lymphocytes produced significantly increased levels of IFN-γ (>30-fold increase) with faster kinetics. Although IFN-γ was detectable in wild-type cultures only after 48 h of stimulation, IFN-γ was present at high levels in B7-1/B7-2−/− cultures by 24 h. IFN-γ production by B7-1/B7-2−/− cells remained much higher than wild-type throughout the 3-d culture period. TNF-α production was also increased in B7-1/B7-2−/− cultures. However, in contrast to IFN-γ, B7-1/B7-2−/− lymphocytes secreted higher levels of TNF-α only in the first 24 h of culture. By 48 h, TNF-α was detectable in wild-type cultures while TNF-α production in B7-1/B7-2−/− cultures had declined. IL-2, IL-4, and IL-10 were below detection limits. These results suggest that B7-1/B7-2−/− T cells are primed to MOG 35-55 in vivo, but that the expansion of these T cells is impaired. Furthermore, the increased levels of IFN-γ and TNF-α in B7-1/B7-2−/− lymphocyte cultures indicate that EAE resistance in these mice is not likely to be due to a defect in Th1 cytokine production.
Figure 4.
Proliferation and cytokine production of lymph node cells from B7-1/B7-2−/− mice challenged with MOG 35-55. Mice were immunized with 100 μg of MOG 35-55 in CFA. 10 d later, draining lymph node cells from wild-type (diamonds) or B7-1/B7-2−/− (circles) mice were restimulated in vitro with a range of MOG 35-55 peptide concentrations. Supernatants were collected after 24, 48, and 72 h from cultures stimulated with 50 μg/ml of MOG 35-55 or with media alone. Cytokines from wild-type (Wt, solid bars) and B7-1/B7-2−/− (hatched bars) cultures were measured by sandwich ELISA, and background levels from media controls were subtracted. Data are representative of eight independent experiments.
B7-1/B7-2−/− Mice Show Markedly Reduced EAE after Adoptive Transfer of Wild-type MOG-specific T Cells.
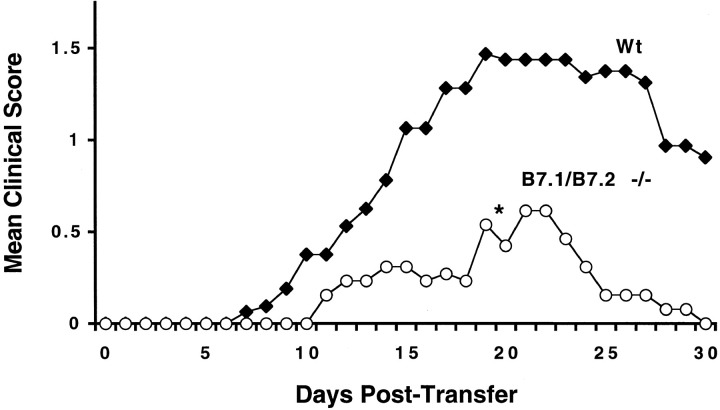
To evaluate whether B7 costimulation has a role in the effector phase of EAE, wild-type MOG 35-55–specific T cells were adoptively transferred into wild-type and B7-1/B7-2−/− mice. B7-1/B7-2−/− mice developed less severe EAE upon adoptive transfer compared with wild-type (Fig. 5). EAE onset was delayed in B7-1/B7-2−/− mice. Severity and duration of clinical disease in B7-1/B7-2−/− mice was also significantly reduced. Histologic evaluation demonstrated that the B7-1/B7-2−/− recipient mice had fewer CNS inflammatory infiltrates compared with wild-type mice, with a greater reduction of parenchymal compared with meningeal foci (Table ). Thus, B7 costimulation is required both for the initiation and/or expansion of T cells in the peripheral immune compartment and for the effector phase of EAE to mediate tissue injury and enable disease progression.
Figure 5.
EAE induction in wild-type and B7-1/B7-2−/− mice by adoptive transfer of wild-type T cells. Wild-type mice were immunized with MOG 35-55, and draining lymph nodes were harvested 10 d later. Lymphocytes were cultured in MOG 35-55 and IL-12 for 4 d and then transferred into wild-type (Wt, n = 16) and B7.1/B7.2−/− (n = 13) recipients. Data are pooled from two experiments and represent mean clinical score plotted against time. B7-1/B7-2−/− mice developed significantly less severe disease compared with wild-type. *P < 0.05 by the Mann-Whitney test.
Table 2.
EAE in Wild-type and B7-1/B7-2−/− Mice Induced by Adoptive Transfer of Wild-type MOG-specific T Cells
| Clinical EAE | Histological EAE | |||||||
|---|---|---|---|---|---|---|---|---|
| Incidence | Day of onset | Mean maximal score | Duration (d) | Incidence | Meningeal foci | Parenchymal foci | Total foci | |
| mean ± SE | mean ± SE | mean ± SE | mean ± SE | mean ± SE | mean ± SE | |||
| Wild-type | 10/16 | 14/16 | ||||||
| (62.5%) | 12.0 ± 0.8 | 2.5 ± 0.2 | 19.0 ± 1.0 | (87.5%) | 40.3 ± 9.0 | 32.9 ± 8.9 | 76.3 ± 9.4 | |
| B7-1/B7-2−/− | 8/13 | 16.0 ± 1.7 | 1.3 ± 0.1 | 8.5 ± 1.5 | 11/13 | 11.4 ± 3.3 | 2.2 ± 0.7 | 13.6 ± 3.8 |
| (61.5%) | P < 0.05 | P < 0.001 | P < 0.001 | (84.6%) | P < 0.001 | P < 0.01 | P < 0.001 | |
Discussion
In this study, we have examined the role of B7–CD28 costimulation and its relative importance for the induction and effector phases of MOG-induced EAE using mice deficient in B7 costimulators or CD28. We have demonstrated that: (a) mice lacking either B7-1 or B7-2 develop EAE comparably to wild-type mice; (b) mice lacking both B7-1 and B7-2 or CD28 are highly resistant to EAE induction; (c) resistance to EAE is not due to a lack of Th1 cytokine production; and (d) B7 costimulatory molecules are critical for the effector phase of EAE, since EAE is markedly reduced in B7-1/B7-2−/− recipients after adoptive transfer of MOG-specific wild-type T cells. Taken together, these results indicate that the B7–CD28 costimulatory pathway is required for the development of MOG 35-55–induced EAE in C57BL/6 mice. B7 costimulators have critical roles both in the initial activation and expansion of MOG-reactive T cells and in their activation within the target organ.
Our studies with mice lacking either B7-1 or B7-2 alone demonstrate that B7-1 and B7-2 have overlapping roles in MOG-induced EAE. This contrasts with EAE studies in SJL mice in which in vivo administration of anti–B7-1 antibodies reduced disease whereas anti–B7-2 antibodies either had no effect or exacerbated disease 2 3 4. Since the timing and level of B7-1 expression is unchanged in B7-2−/− mice and, likewise, B7-2 expression is unaffected in B7-1−/− mice (our unpublished data), the difference in conclusions drawn from the two systems is not likely to be attributable to alterations in B7 expression in B7-1– or B7-2–deficient mice. Several factors may have confounded the results of antibody blocking studies. These include Fc receptor–mediated effects, inadequate penetration of antibodies in vivo, and varying affinity of the antibodies to their ligand. Genetic backgrounds may also influence the respective roles of B7-1 and B7-2 in the induction of EAE. The relative importance of B7-1 and B7-2 may differ between MOG-induced EAE in C57BL/6 mice and proteolipid protein (PLP) or myelin basic protein (MBP)-induced EAE in SJL mice. Induction and level of B7-1 and B7-2 expression may differ on various genetic backgrounds. Genetic analyses have identified chromosomal regions containing the CD28/CTLA-4 and B7-1/B7-2 loci as important for the development of EAE 9. Further studies are needed to determine whether polymorphisms within the CD28/CTLA-4 or B7-1/B7-2 loci lead to altered expression of these genes or to altered protein function. Such differences in the B7-CD28/CTLA-4 costimulatory pathway on different genetic backgrounds could contribute to the relative role of B7-1 versus B7-2 or CD28 in the pathogenesis of EAE and other autoimmune diseases.
Cytokines play a key role in initiating, propagating, and regulating tissue-specific autoimmune injury. The role of cytokines in regulating EAE is complex. Although there is evidence that Th1 cells that produce proinflammatory cytokines (IFN-γ and TNF-α) are critical for the induction of EAE, direct administration of anti–IFN-γ in vivo enhanced EAE 10 11. And although intrathecal injection of IFN-γ induced local inflammation 12, systemic administration of IFN-γ suppressed EAE development 13. Recent studies using cytokine- or cytokine receptor–deficient mice suggest that rather than promoting CNS inflammation, IFN-γ and TNF-α may be important in limiting the extent and duration of EAE. Mice lacking IFN-γ or IFN-γ receptor develop more severe MOG-induced EAE than wild-type littermates 14 15 16. TNF-α−/− mice develop severe MOG-induced EAE with extensive inflammation, demyelination, and high mortality 17 18 19. Our results show that although B7-1/B7-2−/− mice are resistant to MOG-induced EAE, peripheral T cells from these mice produce increased levels of IFN-γ and TNF-α. Whether resistance to EAE in B7-1/B7-2−/− mice is due to lack of expansion of encephalitogenic precursors or to excessive production of IFN-γ and TNF-α is not clear. However, our observations of increased IFN-γ or TNF-α by B7-1/B7-2−/− T cells and EAE resistance in B7-1/B7-2−/− mice are consistent with studies that report exacerbated EAE in mice lacking IFN-γ or TNF-α. 14 15 16 17 18 19. Our data are also consistent with studies demonstrating a major defect in Th2 but not Th1 cytokine production in the absence of both B7-1 and B7-2 or CD28 20 21.
Our results suggest that impaired T cell expansion could, in part, be responsible for EAE resistance in B7-1/B7-2−/− mice, since it appears that MOG-specific T cells are primed in these mice but that their expansion is markedly reduced. However, in addition to controlling T cell expansion, B7 molecules may also be important in regulating migration of encephalitogenic T cells into the CNS and/or their reactivation within the CNS. The greater proportionate reduction of parenchymal, as opposed to meningeal, infiltrates in B7- and CD28-deficient mice compared with those in wild-type mice also suggests that B7 interactions may affect inflammatory cell homing to different CNS compartments. Furthermore, the adoptive transfer experiments demonstrate for the first time that, in addition to playing a key role in the initial expansion and differentiation of T cells, B7 costimulation is required for mediating inflammation during the effector phase of EAE. The demonstration of a key role of B7 costimulation in the effector phase of EAE has important therapeutic implications, since most autoimmune diseases are diagnosed after initial responses to the autoantigens. Our results provide impetus for testing B7 blockade as a therapeutic approach in the effector phase of human autoimmune diseases.
Acknowledgments
We thank Baolin Chang and John Burgess for outstanding technical support.
This work was supported by National Multiple Sclerosis Society grants RG2779-A1 to A.H. Sharpe and RG2571-C7 to V.K. Kuchroo, National Institutes of Health grants R01 NS35685 and P01 AI39671 to V.K. Kuchroo and A.H. Sharpe, and National Institutes of Health grant NS26773 to R.A. Sobel.
Footnotes
1used in this paper: CNS, central nervous system; CTLA-4, CTL-associated molecule 4; EAE, experimental autoimmune encephalomyelitis; MOG, myelin oligodendrocyte glycoprotein
References
- McAdam A.J., Schweitzer A.N., Sharpe A.H. The role of B7 co-stimulation in activation and differentiation of CD4+ and CD8+ T cells. Immunol. Rev. 1998;165:231–247. doi: 10.1111/j.1600-065x.1998.tb01242.x. [DOI] [PubMed] [Google Scholar]
- Racke M.K., Scott D.E., Quigley L., Gray G.S., Abe R., June C., Perrin P. Distinct roles for B7-1 (CD80) and B7-2 (CD86) in the initiation of experimental allergic encephalomyelitis. J. Clin. Invest. 1995;96:2195–2203. doi: 10.1172/JCI118274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchroo V., Das M.P., Brown J.A., Ranger A.M., Zamvil S.S., Sobel R.A., Weiner H.L., Nabavi N., Glimcher L.H. B7-1 and B7-2 costimulatory molecules activate differentially the Th1/Th2 developmental pathwaysapplication to autoimmune disease therapy. Cell. 1995;80:707–718. doi: 10.1016/0092-8674(95)90349-6. [DOI] [PubMed] [Google Scholar]
- Miller S.D., Vanderlugt C.L., Lenschow D.J., Pope J.G., Karandikar N.J., Dal Canto M.C., Bluestone J.A. Blockade of CD28/B7-1 interaction prevents epitope spreading and clinical relapses of murine EAE. Immunity. 1995;3:739–745. doi: 10.1016/1074-7613(95)90063-2. [DOI] [PubMed] [Google Scholar]
- Borriello F., Sethna M.P., Boyd S.D., Schweitzer A.N., Tivol E.A., Jacoby D., Strom T.B., Simpson E.M., Freeman G.J., Sharpe A.H. B7-1 and B7-2 have overlapping, critical roles in immunoglobulin class switching and germinal center formation. Immunity. 1997;6:303–313. doi: 10.1016/s1074-7613(00)80333-7. [DOI] [PubMed] [Google Scholar]
- Freeman G.J., Borriello F., Hodes R.J., Reiser H., Hathcock K.S., Laszlo G., McKnight A.J., Kim J., Du L., Lombard D.B. Uncovering of functional alternative CTLA-4 counter-receptor in B7-deficient mice. Science. 1993;262:907–909. doi: 10.1126/science.7694362. [DOI] [PubMed] [Google Scholar]
- Sobel R.A., Tuohy V.K., Lu Z., Laursen R., Lees M.B. Acute allergic encephalomyelitis in SJL/J mice induced by a synthetic peptide of myelin proteolipid protein. J. Neuropathol. Exp. Neurol. 1989;49:468–479. doi: 10.1097/00005072-199009000-00002. [DOI] [PubMed] [Google Scholar]
- Crowther J.R. ELISA. Theory and practice. Methods Mol. Biol. 1995;42:1–218. doi: 10.1385/0-89603-279-5:1. [DOI] [PubMed] [Google Scholar]
- Encinas J.A., Lees M.B., Sobel R.A., Symonowicz C., Greer J.M., Shovlin C.L., Weiner H.L., Seidman C.E., Seidman J.G., Kuchroo V.K. Genetic analysis of susceptibility to experimental autoimmune encephalomyelitis in a cross between SJL/J and B10.S mice. J. Immunol. 1996;157:2186–2192. [PubMed] [Google Scholar]
- Billiau A., Heremans H., Vandekerckhove F., Dijkmans R., Sobis H., Meulepas E., Carton H. Enhancement of experimental allergic encephalomyelitis in mice by antibodies against IFN-gamma. J. Immunol. 1988;140:1506–1510. [PubMed] [Google Scholar]
- Duong T.T., St J., Louis J.J., Gilbert F.D., Finkelman, Strejan G.H. Effect of anti-interferon-gamma and anti-interleukin-2 monoclonal antibody treatment on the development of actively and passively induced experimental allergic encephalomyelitis in the SJL/J mouse. J. Neuroimmunol. 1992;36:105–115. doi: 10.1016/0165-5728(92)90042-j. [DOI] [PubMed] [Google Scholar]
- Sethna M.P., Lampson L.A. Immune modulation within the brainrecruitment of inflammatory cells and increased major histocompatibility antigen expression following intracerebral injection of interferon-gamma. J. Neuroimmunol. 1991;34:121–132. doi: 10.1016/0165-5728(91)90121-m. [DOI] [PubMed] [Google Scholar]
- Voorthuis J.A., Uitdehaag B.M., De Groot C.J., Goede P.H., van der Meide P.H., Dijkstra C.D. Suppression of experimental allergic encephalomyelitis by intraventricular administration of interferon-gamma in Lewis rats. Clin. Exp. Immunol. 1990;81:183–188. doi: 10.1111/j.1365-2249.1990.tb03315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferber I.A., Brocke S., Taylor-Edwards C., Ridgway W., Dinisco C., Steinman L., Dalton D., Fathman C.G. Mice with a disrupted IFN-gamma gene are susceptible to the induction of experimental autoimmune encephalomyelitis (EAE) J. Immunol. 1996;156:5–7. [PubMed] [Google Scholar]
- Krakowski M., Owens T. Interferon-gamma confers resistance to experimental allergic encephalomyelitis. Eur. J. Immunol. 1996;26:1641–1646. doi: 10.1002/eji.1830260735. [DOI] [PubMed] [Google Scholar]
- Willenborg D.O., Fordham S., Bernard C.C., Cowden W.B., Ramshaw I.A. IFN-gamma plays a critical down-regulatory role in the induction and effector phase of myelin oligodendrocyte glycoprotein-induced autoimmune encephalomyelitis. J. Immunol. 1996;157:3223–3227. [PubMed] [Google Scholar]
- Liu J., Marino M.W., Wong G., Grail D., Dunn A., Bettadapura J., Slavin A.J., Old L., Bernard C.C. TNF is a potent anti-inflammatory cytokine in autoimmune-mediated demyelination. Nat. Med. 1998;4:78–83. doi: 10.1038/nm0198-078. [DOI] [PubMed] [Google Scholar]
- Frei K., Eugster H.P., Bopst M., Constantinescu C.S., Lavi E., Fontana A. Tumor necrosis factor α and lymphotoxin α are not required for induction of acute experimental autoimmune encephalomyelitis. J. Exp. Med. 1997;185:2177–2182. doi: 10.1084/jem.185.12.2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korner H., Riminton D.S., Strickland D.H., Lemckert F.A., Pollard J.D., Sedgwick J.D. Critical points of tumor necrosis factor action in central nervous system autoimmune inflammation defined by gene targeting. J. Exp. Med. 1997;186:1585–1590. doi: 10.1084/jem.186.9.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rulifson I.C., Sperling A.I., Fields P.E., Fitch F.W., Bluestone J.A. CD28 costimulation promotes the production of Th2 cytokines. J. Immunol. 1997;158:658–665. [PubMed] [Google Scholar]
- Schweitzer A.N., Sharpe A.H. Studies using antigen-presenting cells lacking expression of both B7-1 (CD80) and B7-2 (CD86) show distinct requirements for B7 molecules during priming versus restimulation of Th2 but not Th1 cytokine production. J. Immunol. 1998;161:2762–2771. [PubMed] [Google Scholar]