Abstract
Chemokines regulate a number of biological processes, including trafficking of diverse leukocytes and proliferation of myeloid progenitor cells. SHP-1 (Src homology 2 domain tyrosine phosphatase 1), a phosphotyrosine phosphatase, is considered an important regulator of signaling for a number of cytokine receptors. Since specific tyrosine phosphorylation of proteins is important for biological activities induced by chemokines, we examined the role of SHP-1 in functions of chemokines using viable motheaten (mev/mev) mice that were deficient in SHP-1. Chemotactic responses to stromal call–derived factor 1 (SDF-1), a CXC chemokine, were enhanced with bone marrow myeloid progenitor cells as well as macrophages, T cells, and B cells from mev/mev versus wild-type (+/+) mice. SDF-1–dependent actin polymerization and activation of mitogen-activated protein kinases were also greater in mev/mev versus +/+ cells. In contrast, immature subsets of mev/mev bone marrow myeloid progenitors were resistant to effects of a number of chemokines that suppressed proliferation of +/+ progenitors. These altered chemokine responses did not appear to be due to enhanced expression of CXCR4 or lack of chemokine receptor expression. However, expression of some chemokine receptors (CCR1, CCR2, CCR3, and CXCR2) was significantly enhanced in mev/mev T cells. Our results implicate SHP-1 involvement in a number of different chemokine-induced biological activities.
Keywords: chemokine, Src homology 2 domain tyrosine phosphatase 1, viable motheaten mice, chemotaxis, myelosuppression, stromal cell–derived factor 1
The control of cell migration and proliferation involves a dynamic equilibrium between positive and negative signals that sets a threshold of responsiveness to extracellular stimuli. Migration and proliferation of hematopoietic cells is controlled by a number of cytokines and chemokines. Chemokines are small (<10 kD) chemotactic cytokines that have a number of additional biological functions 1 2 3 4 5. In addition to guiding leukocyte trafficking, chemokines regulate proliferation of hematopoietic progenitor cells, suppress or enhance angiogenesis, activate and differentiate lymphocytes, and inhibit HIV infection. The role of chemokines in hematopoiesis and trafficking of hematopoietic progenitor cells (HPCs)1 has been emphasized by the severely abnormal phenotype of mice deficient in a CXC chemokine, stromal cell–derived factor 1 (SDF-1), or its receptor, CXCR4 6 7 8. These mutant mice were not viable, had low numbers of B cell progenitors in fetal liver and bone marrow, and had low numbers of myeloid progenitors in the bone marrow but normal numbers in the fetal liver, implying the involvement of SDF-1 in trafficking and differentiation of HPCs. SDF-1 induces chemotaxis of mature leukocytes such as lymphocytes and monocytes 9 and their immature progenitor cells 10 11 12, but not of granulocytes 9. Although information on chemokine-induced signaling pathways has been accumulating, it is still in its infancy, and biological effects for many of these intracellular molecules in these pathways have not been evaluated. It has been reported that SDF-1 and other chemokines induce signaling processes involving receptor-associated heterotrimeric GTPases, phospholipase C, calcium mobilization, phosphatidylinositol-3 kinase (PI-3K), small GTPases, and diverse protein tyrosine kinases 13 14 15 16.
Motheaten (me) and viable motheaten (mev) mice are defective in expression and activity of the Src homology 2 domain tyrosine phosphatase 1 (SHP-1) due to mutations in the SHP-1 gene 17 18 19. SHP-1 is a cytoplasmic phosphatase that is involved in the regulation of cellular activation, proliferation, and survival 20 21 22. Expression of SHP-1 is largely restricted to hematopoietic cells. SHP-1 is a negative regulator of signaling through diverse receptors, including c-fms; the receptor for CSF 1; c-Kit, the receptor for steel factor; the killer cell inhibitory receptor; the erythropoietin receptor; CD22; and the B cell and T cell antigen receptors (for review see references 20–22). SHP-1 mutant mice are defective in hematopoiesis and suffer from severe combined immunodeficiency and systemic autoimmunity due to aberrant regulation of hematopoiesis and activation of leukocytes, exhibiting various defects. These include characteristic skin and foot lesions, patchy loss of hair, slow growth, short life span, splenomegaly, lymphadenopathy, progressive thymic involusion, neutrophilia, lymphopenia, pneumonitis, elevated serum immunoglobulins, absence of terminal deoxynucleotidyl transferase-positive (TdT+) cells, and immune complex deposition in the skin and kidneys 23.
Since tyrosine phosphorylation is important in chemokine signaling processes, we investigated whether the phosphotyrosine phosphatase SHP-1 played a role in regulating chemokine responses using immature and mature hematopoietic cells from mev/mev and wild-type (+/+) littermate mice. We found that SHP-1–deficient hematopoietic progenitor cells and mature leukocytes from the mev/mev mice demonstrated altered biological responses to chemokines. This reveals a novel function of SHP-1 as an intermediary factor for chemokine-induced signaling and biological activities.
Materials and Methods
Mice.
Homozygous viable motheaten (mev) mice (C57BL/6J-mev strain) used in this study were from The Jackson Laboratory, propagated by heterozygote (+/mev) sibling matings. Wild-type (+/+), +/mev, and homozygous (mev/mev) mice were genotyped by PCR. The mev allele was detected (35 cycles; 1 min at 94°C, 1 min at 63°C, 1 min at 72°C) using two primers: 5′ GGT GGA GAA GAA AGG CCG GGA 3′ (mev–specific forward primer) and 5′ TAT CAG GCT TGG CAG CAA AC 3′ (common reverse primer). The wild-type allele was detected (35 cycles; 1 min at 94°C, 1 min at 64°C, 1 min at 72°C) using primers 5′ GGT GGA GAA AGG CCG GGT 3′ (wild-type SHP-1–specific forward primer) and 5′ TAT CAG GCT TGG CAG CAA AC 3′ (common reverse primer). Sex- and age-matched adult mev/mev mice and +/+ littermates (∼4 wk old) were used in this study.
Cell Isolation.
Total mouse bone marrow cells were aspirated from two femurs of each mouse. Total bone marrow cells were used for chemotaxis and suppression assays of HPCs. Total splenocytes were prepared by crushing spleens through iron meshes. Low density spleen mononuclear cells were recovered after density-cut on Lympholyte-M (Cedarlane Labs.). CD4+ T cells were isolated from the low density splenocytes by staining with biotin-labeled antibodies to CD4 (clone L3T4; PharMingen) followed by positively selecting the stained CD4+ T cells using streptavidin beads and magnets (Miltenyi Biotech). Isolated CD4+ T cells were ≥95% pure by CD4 expression.
Assays of HPC Proliferation and Cycling.
Total bone marrow cells were plated at 5 × 104, and total splenocytes were plated at 5 × 105/ml in 1.0% methylcellulose culture medium containing growth factors (30% vol/vol fetal bovine serum [Hyclone], 1 U/ml recombinant human erythropoietin [Amgen Biologicals], 50 ng/ml recombinant murine steel factor [Immunex Corp.], 5% vol/vol pokeweed mitogen mouse spleen cell–conditioned medium [24], and 0.1 mM hemin [Eastman-Kodak Co.]). Colonies derived from granulocyte-macrophage (CFU-GM), erythroid (BFU-E), and multipotential (CFU-GEMM) progenitor cells were scored after 7 d of incubation in a humidified environment at 5% CO2 and lowered (5%) O2 as previously described 24.
The percentage of progenitors in S-phase was estimated by the high specific activity tritiated thymidine kill technique 24. Absolute numbers of progenitors per organ were calculated based on the number of viable, total nucleated cells per femur or spleen, and on the number of colonies scored per number of cells plated.
For assessment of suppression of CFU-GM, total bone marrow cells were plated in the presence of the 1.0% methylcellulose culture medium containing growth factors as noted above in the absence or presence of control medium, or purified recombinant preparations of murine TNF-α or IFN-γ, each at 10 ng/ml, or 100 ng/ml of various human chemokines (MIP-1α, MCP-1, exodus, SLC, TECK, MIP-4, CKβ-11, IL-8, IP-10, MIG, ENA78, GCP-2, lymphotactin, MCP-4 fractalkine, and MIP-1β). These cytokine/chemokine concentrations have previously been shown to be optimal for inhibition of colony formation by bone marrow progenitor cells from mice of different strains. IFN-γ, TNF-α, and most chemokines were purchased from R&D Systems, except for CKβ-11, which was purified from Chinese hamster ovary cells 25.
Quantitative Chemotaxis Assay of HPCs and Various Mature Leukocytes.
Quantitative chemotaxis assays using transwells (6.5-mm diameter, 5-μm pore size, polycarbonate membrane; Costar) have been described previously 11 12. For chemotaxis of HPCs, 5 × 105 total bone marrow cells were used for each chemotaxis chamber. SDF-1 (obtained from Dr. Ian Clark-Lewis, University of British Columbia, Vancouver, Canada) was added to the lower chamber at various concentrations. Chemotaxis was allowed for 4 h. Input cells and cells migrating to the lower chamber were collected, and assayed for colony forming cells in methylcellulose culture containing the growth factors noted above. Cells from two transwells were combined to obtain enough numbers of HPCs for triplicated colony assays. Cells were plated at 5 × 104 per 1 ml methylcellulose culture medium. After 7 d, colonies deriving from CFU-GM were differentially counted using standard techniques under an inverted microscope 24.
Chemotaxis of mouse leukocytes has been described previously 12. 5 × 105 low density mononuclear cells were used for input cells for chemotaxis of B and T lymphocytes and bone marrow macrophages. Chemotaxis was allowed for 3 h at 37°C. Input cells and cells migrating to the lower chambers were collected. For collection of bone marrow macrophages, cells attached to the bottom of culture plates were detached by incubating in 5 mM EDTA/PBS (pH 7.4) for 20 min followed by extensive pipetting. Input cells and cells migrating to the lower chamber were stained with antibodies (anti-CD11b/Mac-1–TRIcolor and anti-F4/80–FITC for bone marrow macrophages; anti-B220–PE or anti-IgM–PE, anti-CD4–Cychrome and anti-CD8–FITC for splenic lymphocytes) and analyzed by FACscan® (Becton Dickinson) in a time-based manner for 20 s. Anti-CD4–Cychrome (clone L3T4), anti-CD8a–FITC (clone Ly-2), anti-B220 (clone RA3-6B2), and IgM-PE (clone Igh-6b) were purchased from PharMingen. Anti-F4/80–FITC was purchased from Serotec. Anti-Mac-1–TRIcolor was purchased from Caltag Labs. Migration of each subset was normalized for numbers of each subset of input cells.
Assay of Chemokine-induced Actin Polymerization.
Actin polymerization assays were performed as previously described by others 26 with some modification. Cells were resuspended in RPMI 1640 medium supplemented with 0.1% BSA at 106 cells/ml. SDF-1 was added at various concentrations (2, 20, 200, and 2,000 ng/ml) to the cell solution, and at a peak time point (25 s after treatment with SDF-1), 0.1 ml of FITC-labeled phalloidin solution (4 × 10−7 M FITC-labeled phalloidin, 0.5 mg/ml 1-α-lysophosphatidylcholine, and 18% formaldehyde in PBS, all from Sigma Chemical Co.) was added to 0.4 ml of cell solution to stain and fix cells. Cells were incubated for 10 min at 37°C, pelleted, and resuspended in 0.5 ml of 2% paraformaldehyde solution. Mean fluorescence was measured by FACscan®.
Mitogen-activated Protein Kinase and SHP-1 Phosphorylation Assays.
Spleen CD4+ T lymphocytes from wild-type and mev/mev mice were suspended in RPMI 1640 medium with 10% FCS and preincubated at 37°C for 10 min. The kinase assay was carried out as previously reported 27. In brief, 5 × 106 cells were stimulated with SDF-1 at 2 μg/ml for 60 s and lysed in RIPA buffer. 250 μg of cell lysate was immunoprecipitated with anti–Erk 1 polyclonal antibody and protein A–Sepharose 4B beads. The immunocomplex was then washed twice with HNTG (20 mM Hepes, pH 7.5, 150 mM NaCl, 0.1% Triton X-100, 10% glycerol, and 1 mM Na3VO4) and once with kinase buffer (20 mM Hepes, pH 7.5, 10 mM MgCl2, 2 mM 2-ME, and 1 mM Na3VO4). The reaction was carried out in a mixture containing 20 μl of kinase buffer, 1 mg/ml myelin basic protein, 80 μM ATP, and 0.075 μCi/μl [γ-32P]ATP at 30°C for 10 min. The beads were spun down, and 18 μl of supernatant was spotted onto P81 Whatman paper. The papers were then washed five times with 180 mM phosphoric acid and once with ethanol, then air dried and counted on a Beckman scintillation counter. For SHP-1 phosphorylation and association of SHP-1 with PI-3K, anti–SHP-1 polyclonal antibody (Santa Cruz Biotechnology) was used to immunoprecipitate SHP-1 from cell lysates of splenic CD4+ T cells or murine FDCP-1 cells activated as described above. Immunoprecipitates were resolved in SDS-PAGE and blotted with antiphosphotyrosine antibodies (Upstate Biotechnology, Inc.) and then with anti–SHP-1 antibody or anti–PI-3K P85 (Upstate Biotechnology, Inc.) as previously described 27 28.
RNase Protection Assays of Chemokine Receptor Expression.
Total RNA was isolated from various sources of cells, including bone marrow (total bone marrow cells) and spleen (low density mononuclear cells and isolated CD4+ T cells) using Trizol solution (GIBCO BRL). Multiprobe RNase protection template sets mCR5 (CCR1, CCR1b, CCR3, CCR4, CCR5, CCR2, L32, and GAPDH) and mCR6 (CXCR2, CXCR4, CXCR5, L32, and GAPDH) were purchased from PharMingen. High specific activity RNA probes were in vitro transcribed in the presence of [α-32P]UTP (3,000 Ci/mmol; 10 mCi/ml; Amersham Life Science) using T7 RNA polymerase included in the in vitro transcription kit (PharMingen) according to manufacturer's instructions. Probes and total RNA were allowed to hybridize and treated with RNAse A and T1, and followed by proteinase K treatment using RPA kit (PharMingen). The protected RNAs from the RNAse treatment were resolved on 8 M urea/5% acrylamide sequencing gels. Filters were dried, exposed on X-ray film (X-OMAT; Eastman-Kodak Co.), and analyzed for band radioactivity using a phospho-image analyzer (Molecular Image Analyzer; Bio-Rad). Each band's radio intensity was normalized for L32 internal control, and averaged expression levels from three to four independent experiments were plotted in Fig. 5 C.
Figure 5.

Expression of chemokine receptors in mev/mev mice compared with +/+ mice. Freshly isolated bone marrow cells (BM), spleen mononuclear cells (Spleen), and CD4+ T cells (purity >95%) from +/+ and mev/mev mice were analyzed for expression of chemokine receptor mRNA by RNase protection assay. L32 and GAPDH were included as internal controls to normalize expression of chemokine receptors in different mice and cell types. Expression of CXC chemokine receptors is shown in A and that of CC chemokine receptors is shown in B. Each band's radiointensity was measured by phosphor-image analyzer (Molecular Image Analyzer; Bio-Rad) to quantitate expression of chemokine receptors. Normalized expression levels of chemokine receptors for L32 internal control (expression of chemokine receptor/expression of L32) from three independent experiments were averaged, and are shown in C.
Statistical Analysis.
Student's t test was used to analyze data for significance. P < 0.05 was considered significant.
Results
Enhanced Chemotaxis of mev/mev HPCs toward SDF-1.
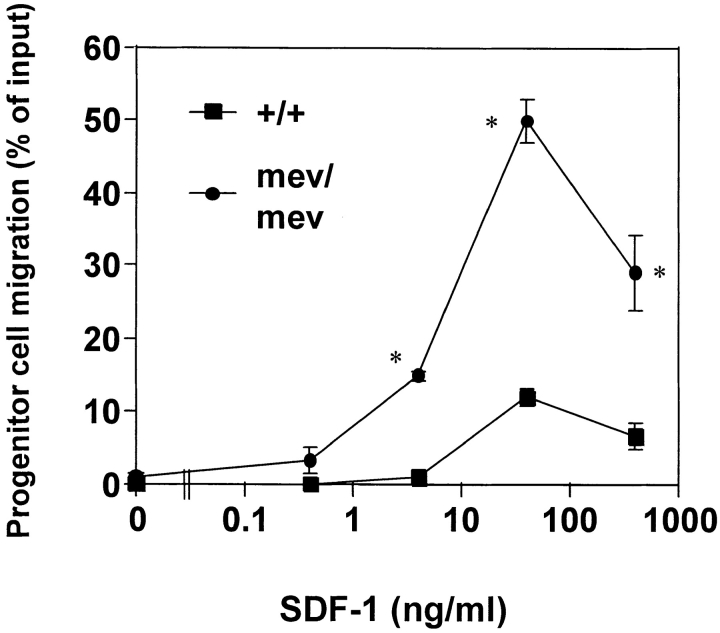
SDF-1, expressed ubiquitously in many organs including bone marrow and lymphoid tissues, induces chemotaxis of HPCs. It has been suggested that SDF-1 is a chemoattractant that induces the homing and retaining of progenitor cells in bone marrow 11 29. Thus far, SDF-1 is the only chemokine shown to induce chemotaxis of early stage myeloid and lymphoid progenitors. We examined the chemotactic responsiveness of CFU-GM (progenitor cells for granulocytes and macrophages) in bone marrow of mev/mev mice and +/+ littermates. The background migration of CFU-GM in +/+ mice and mev/mev mice was very low (Fig. 1). In response to SDF-1, a typical bell-shaped chemotactic response was observed for +/+ progenitors (Fig. 1). Bone marrow CFU-GM from mev/mev mice demonstrated a much higher chemotaxis rate (>50%) than their +/+ counterparts (slightly >10%). Also, mev/mev progenitors began to migrate at lower concentrations of SDF-1 than +/+ progenitors, demonstrating an increased sensitivity of mev/mev CFU-GM to the chemotactic effect of SDF-1.
Figure 1.
Enhanced chemotaxis of mev/mev myeloid progenitors to SDF-1. Bone marrow myeloid progenitor cells (CFU-GM, progenitors of granulocytes and macrophages) were examined for their ability to transmigrate from the upper chamber towards SDF-1 at indicated concentrations in the lower chamber. After chemotaxis, myeloid progenitors in input and progenitors migrating to the lower chamber were assayed by methylcellulose colony assay. CFU-GM migration was normalized for the number of input CFU-GM to obtain CFU-GM migration rate (% of input ± SD). *Significant differences were observed between +/+ and mev/mev cells (P < 0.05). Results are from one experiment representative of five independent experiments.
SHP-1 Deficiency Makes Myeloid Progenitor Cells Resistant to Chemokines that Suppress Proliferation.
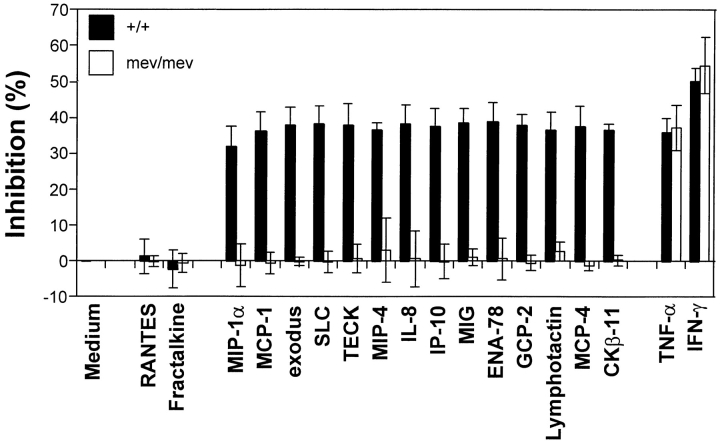
The suppressive activity of chemokines for proliferation of immature subsets of HPCs is a distinct biological activity from their chemotactic activity. Thus far, 21 chemokines that cross the CC, CXC, and C subfamilies have demonstrated such suppressive activity 30. Among these chemokines, we tested 14 myelosuppressive chemokines (MIP-1α, MCP-1, exodus, SLC, TECK, MIP-4, CKβ-11, IL-8, IP-10, MIG, ENA78, GCP-2, lymphotactin, and MCP-4) for their effects on colony formation of bone marrow CFU-GM. These chemokines cross the CC, CXC, and C families of chemokines. As controls, we included two previously determined nonsuppressive chemokines (fractalkine, a CX3C member, and MIP-1β, a CC member). As shown in Fig. 2, all 14 suppressive chemokines, but not the 2 nonsuppressive chemokines at an optimal concentration (100 ng/ml), significantly inhibited proliferation of +/+ littermate control CFU-GM. In contrast, none of the tested chemokines inhibited the proliferation of mev/mev myeloid progenitor cells (Fig. 2). As additional controls for suppression, TNF-α and IFN-γ, previously shown to be suppressors of HPC proliferation 31, were assessed. In contrast to the chemokines, TNF-α and IFN-γ demonstrated equally significant suppressive activities for bone marrow CFU-GM from both mev/mev and +/+ mice.
Figure 2.
Resistance of mev/mev progenitor cells to myelosuppressive chemokines. Bone marrow cells from wild-type and mev mice were plated into methylcellulose culture medium in the presence of either PBS, 100 ng/ml chemokines, or 10 ng/ml TNF-α or IFN-γ. Colony formation of CFU-GM was assessed after 7 d of incubation. Results shown are the average (mean ± SD) for four separate experiments.
The suppressive activities of chemokines are directly related to the percentage of HPCs in the S-phase of the cell cycle. The higher the percentage of HPCs in S-phase, the greater the inhibition by chemokines 2. Therefore, we examined the cycling status (percentage in S-phase of the cell cycle) of CFU-GM, BFU-E, and CFU-GEMM in the bone marrow of mev/mev and +/+ control mice as estimated by high specific activity tritiated thymidine kill assay 24. CFU-GM, BFU-E, and CFU-GEMM in the bone marrows of mev/mev mice were more actively proliferating (a greater percentage of HPCs in S-phase) than were these cells in +/+ mice (Table ). Thus, the inability of CFU-GM, BFU-E, and CFU-GEMM in the marrow of mev/mev mice to respond to inhibition by chemokines could not be attributed to lack of cycling HPCs. The difference in cycling of CFU-GM, BFU-E, and CFU-GEMM in mev/mev and +/+ spleens was far greater than that of bone marrow progenitors demonstrating the especially enhanced proliferation states of mev/mev progenitors in spleen (Table ).
Table 1.
Cycling Status and Absolute Numbers of Myeloid Progenitor Cells in Marrow and Spleen of +/+ and mev/mev Mice
| Cycling rates of progenitors (% in S phase) | Absolute No. progenitors (× 10−3)/organ | Nucleated cellularity | |||||
|---|---|---|---|---|---|---|---|
| CFU-GM | BFU-E | CFU-GEMM | CFU-GM | BFU-E | CFU-GEMM | ||
| Marrow | × 10−6 | ||||||
| +/+ | 27 ± 4 | 39 ± 3 | 38 ± 2 | 23.2 ± 2.6 | 2.8 ± 0.2 | 1.7 ± 0.2 | 22 ± 1.3 |
| mev/mev | 54 ± 3 | 53 ± 6 | 59 ± 6 | 24.6 ± 2.2 | 2.4 ± 0.5 | 1.5 ± 0.2 | 14 ± 0 |
| P value | <0.001 | <0.04 | <0.004 | NS (<0.03) | NS (>0.05) | NS (>0.05) | <0.001 |
| Spleen | |||||||
| +/+ | 10 ± 10 | 0 ± 0 | 0 ± 0 | 6.9 ± 2.1 | 3.5 ± 1.2 | 1.1 ± 0.3 | 166 ± 14 |
| mev/mev | 56 ± 2 | 55 ± 3 | 54 ± 4 | 57.8 ± 11.8 | 14.8 ± 4.8 | 6.4 ± 1.5 | 279 ± 25 |
| P value | <0.001 | <0.001 | <0.001 | <0.002 | <0.001 | <0.02 | <0.002 |
Results are given as mean ± SEM for 10 +/+ and 10 mev/mev mice. Each mouse was assessed individually from a total of five separate experiments.
Since myeloid progenitor cells from mev/mev mice were more active in chemotaxis to SDF-1, and were not inhibited by a number of suppressive chemokines, it would be expected that the spleen and bone marrow of these mice would have more blood cells. In this regard, splenomegaly and abnormal hematopoiesis (excessive erythropoiesis and myelopoiesis) in the spleens of mev mice have been previously reported by others 32. Thus we also examined the absolute numbers of myeloid progenitor cells in spleen and bone marrow (Table ). Spleens from mev/mev mice had far greater numbers of myeloid progenitors (8.4 times more CFU-GM, 4.2 times more BFU-E, and 5.8 times more CFU-GEMM per organ) than did those from +/+ mice. Although the absolute numbers of progenitors in the marrows of mev/mev and +/+ mice were similar, the frequency of myeloid progenitors in marrow from mev/mev mice was higher than in +/+ mice, as the nucleated cellularity of mev/mev mice was significantly decreased (perhaps due to the smaller size of mev/mev mice).
Enhanced Motility and Chemotaxis of mev/mev T Cells, B Cells, and Macrophages from Bone Marrow and Spleen to SDF-1.
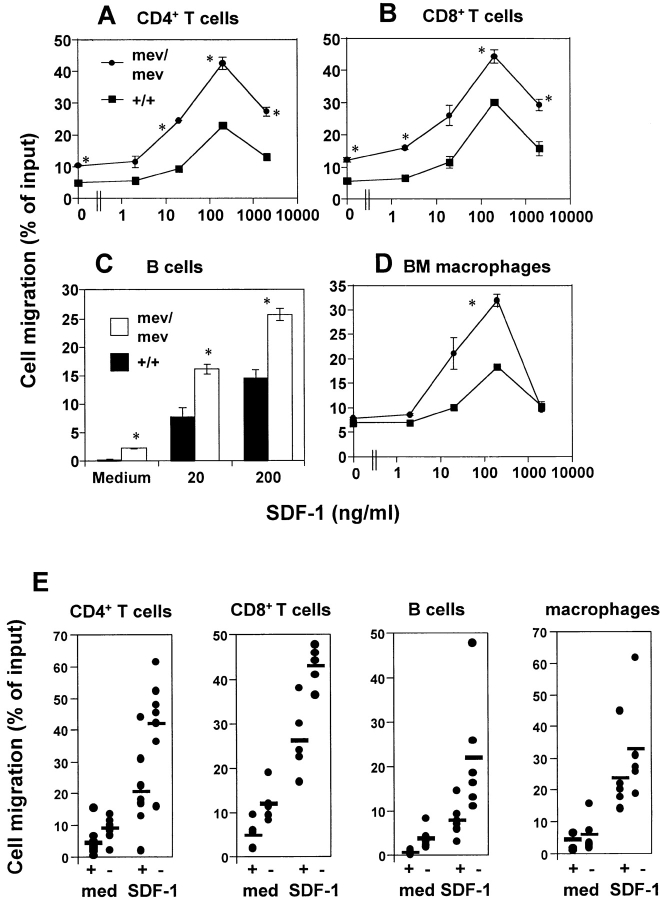
To determine whether mature leukocyte populations have any altered chemotactic responses, we tested leukocytes in spleen and bone marrow from mev/mev and +/+ mice. Spleen lymphocytes, including CD4+ T cells, CD8+ T cells, B220+ B cells, and bone marrow Mac-1+F4/80+ macrophages were tested for their chemotactic response to SDF-1. We observed a twofold greater basal motility (an ability to transmigrate through a porous membrane independently of chemoattractants) in mev/mev CD4+ T cells than in their +/+ counterparts (Fig. 3A and Fig. E). In response to SDF-1, significantly more mev/mev CD4+ spleen T cells migrated than did +/+ CD4+ T cells. Splenic CD8+ T cells from mev/mev mice demonstrated a similar enhancement in chemotaxis to SDF-1 at all concentrations tested (Fig. 3B and Fig. E). B220+ B cells in spleen (Fig. 3C and Fig. E) and bone marrow F4/80+ macrophages (Fig. 3D and Fig. E) from mev/mev mice also showed significantly enhanced chemotaxis to SDF-1. However, in response to another chemokine, SLC, that is a more efficacious chemoattractant than SDF-1 for murine CD4+ T cells 12, no enhancement of CD4+ T cell chemotaxis has been observed, demonstrating the differential effect of SHP-1 on chemotaxis to chemokines (Fig. 3 F).
Figure 3.
Enhanced chemotaxis of mev/mev T cells (spleen CD4+ T cells and CD8+ T cells), spleen B220+ B cells, and bone marrow macrophages in response to SDF-1. Numbers of cells migrating to the lower chamber containing SDF-1 at the indicated concentrations were expressed as a percentage of input cells in the upper chamber at the start time of chemotaxis. Input cells and cells migrated to the lower chambers were quantitatively phenotyped by staining with mAbs (see Materials and Methods) followed by flow cytometric analysis. Data are expressed as the mean (± difference) of percentage of cell migration obtained from duplicated points (A, B, C, and D). *Significant differences were observed between the +/+ and mev/mev cells (P < 0.05 for A, B, C, and D). Results are from one representative experiment of four to seven similar experiments. In E, basal migration to medium (med) and chemotaxis to SDF-1 at 200 ng/ml of +/+ (+) and mev/mev (−) cells from a number of experiments are shown. The horizontal bars represent mean values. In F, another chemokine, SLC, was used along with SDF-1 to examine chemotaxis of spleen CD4+ T cells from +/+ and mev/mev mice to a different chemokine. P values for differences in basal migration and chemotaxis between +/+ and mev/mev cells respectively are 0.003 and 0.0006 for CD4+ T cells (n = 10), 0.00006 and 0.0032 for CD8+ T cells (n = 6), 0.01 and 0.015 for B cells (n = 7), and 0.025 (chemotaxis) for macrophages (n = 6).
SDF-1-induced Actin Polymerization and Mitogen-activated Protein Kinase Activation in me v/me v Cells.
Chemokines bind and activate G protein–coupled receptors followed by activation of various signaling molecules, leading to reorganization of actin cytoskeletal structures. Chemokine-dependent actin polymerization is an important event for cell motility, cell polarization, and formation of membrane structures such as uropods. The latter is implicated in adhesion to other cells and extracellular matrix proteins. To evaluate changes in intracellular events, we compared the basal and chemokine-induced levels of polymerized actin (F-actin) in cells from mev/mev and +/+ mice. The basal levels of F-actin in splenic lymphocytes (Fig. 4 A) and more specifically in splenic CD4+ T cells (Fig. 4 B) from mev/mev mice were greatly increased when compared with their +/+ counterparts (Fig. 4A and Fig. B). When treated with SDF-1 at various concentrations, +/+ splenic lymphocytes and CD4+ T cells increased cellular F-actin in a dose-dependent manner (Fig. 4). Splenocytes and CD4+ T cells from the mev/mev mice demonstrated a similar trend of actin polymerization to +/+ counterparts in response to SDF-1. The overall F-actin content of mev/mev cells was greater than that of +/+ cells after activation with SDF-1, demonstrating greater cellular activity of actin polymerization.
Figure 4.

Enhanced actin polymerization and MAPK activity of mev/mev cells in response to SDF-1. Spleen lymphocytes (A) and spleen CD4+ T cells (B, purity >95%) were untreated or activated with SDF-1 at the indicated concentrations to induce actin polymerization. Peak levels of F-actin at 25 s after activation were monitored by FACScan® after FITC-phalloidin staining of polymerized actin. Results shown are representative of three different experiments each. For C, isolated CD4+ T cells (5 × 106) were treated with SDF-1 (2,000 ng/ml) or control buffer (C) for 60 s. MAPKs were immunoprecipitated from 250 μg of cell lysates and kinase activity was measured with myelin basic protein as a substrate. Results from three independent experiments are averaged (± SD) and shown in cpm (left) and induction fold from the basal level of each group (right). *Significant difference was observed between +/+ and mev/mev cells (P < 0.036).
Mitogen-activated protein kinase (MAPK) is activated in response to SDF-1 in a CXCR4-overexpressed cell line 15. We examined whether this downstream pathway of SDF-1 signaling was altered in mev/mev cells. MAPK activity in wild-type spleen CD4+ T cells was consistently induced in response to SDF-1 to 1.5-fold of basal level (Fig. 4 C). In mev/mev CD4+ T cells, MAPK activity was induced to 2.5 fold of basal level, demonstrating an enhanced activation of MAPK. SHP-1 was constitutively tyrosine-phosphorylated at a low level, and did not get more phosphorylated in response to SDF-1 in splenic T cells or in a growth factor–dependent mouse cell line, FDCP-1, which underwent chemotaxis in response to SDF-1 (data not shown). We also observed that there was no induced association of SHP-1 with a known positive signaling factor, PI-3K, in response to SDF-1 in FDCP-1 cells (data not shown).
Expression of Chemokine Receptors in me v/me v and +/+ Mice.
Expression of chemokine receptors is tightly regulated depending on the types, and states of activation and differentiation of cells. We examined the possibility that the altered biological activities of chemokines might be attributed to changes in receptor expression in mev/mev versus +/+ cells. We performed RNase protection assays to measure transcripts of various mouse chemokine receptors, including the CXC chemokine receptors CXCR2, CXCR4, and CXCR5, and the CC chemokine receptors CCR1, CCR1b, CCR2, CCR3, CCR4 and CCR5 in cells from bone marrow and spleen. Expression of CXCR4, the receptor for SDF-1, did not change in mev/mev versus +/+ cells from bone marrow, spleen, and CD4+ T cells (Fig. 5A and Fig. c). Expression of CXCR2, a receptor for IL-8, GCP-2, GRO-α, -β, -γ, ENA-78, and NAP-2, was enhanced in leukocytes from bone marrow and spleen, and in spleen CD4+ T cells. The enhancement of expression of CXCR2 in mev/mev CD4+ T cells and splenocytes was especially notable (Fig. 5 A). Expression of CXCR5, the receptor for BLC/BCA-1, was detected at high levels in +/+ splenocytes, and at low levels in mev/mev splenocytes. B cells were the major expression source of CXCR5 in +/+ splenocytes (data not shown), and the drastic decrease of CXCR5 expression appears to be due to deficiency of B cells in mev/mev spleen. In this regard, numbers of B220+ or IgM+ B cells were heavily reduced in mev/mev spleen. B cells made up 40–50% of +/+ versus <15% of mev/mev total splenocytes. Expression of CCR1, a receptor for MIP-1α and RANTES, was greatly enhanced in mev/mev CD4+ T cells and splenocytes versus their +/+ counterparts. Expression of CCR2 (a receptor for MCP-1 to MCP-5) was enhanced in mev/mev CD4+ T cells versus +/+ cells. Expression of CCR3 (a receptor for eotaxin-1 and -2 and MCP-2 and -4), CCR4 (a receptor for TARC and MDC), and CCR5 (a receptor for MIP-1α and -1β and RANTES) was very low in +/+ and mev/mev CD4+ T cells and total bone marrow cells.
Discussion
In this paper we present evidence that SHP-1 is a novel regulator of chemokine-mediated biological effects. Two major biological activities, chemotaxis and suppression of proliferation, have been examined in this study. SHP-1–deficient immature and mature hematopoietic cells manifest enhanced chemotaxis to a CXC chemokine SDF-1. Actin polymerization and MAPK activation in SHP-1–deficient cells were also hyperresponsive to SDF-1. In contrast, the deficiency of SHP-1 abolished the sensitivity of immature progenitors to suppression by chemokines.
Cellular responses to chemokines are presumably regulated by equilibrium of a number of positive and negative signaling factors. It has been reported that chemokine-mediated biological activities involve several positive factors including PI-3K 15, mitogen-activated protein kinases (Erk 1 and Erk 2) 15 33, adenylate cyclase 34, and Janus kinase 2 (JAK2) 13. As a negative signaling factor, serine/threonine kinases such as β-adrenergic receptor kinase 2 can act as a desensitization factor for chemokine receptor–mediated signaling 35 36. SHP-1 negatively regulates a number of signaling pathways by dephosphorylating proteins on specific tyrosine residues. So far, the putative substrates for SHP-1 include receptors such as IL-3R 37, B cell CD22 receptor 38, B cell receptor Ig-α subunit 39, killer cell inhibitory receptor 40, platelet endothelial cell adhesion molecule-1 41, IL-2Rβ 42, and CD72 43, as well as other intracellular proteins such as JAK1 and JAK3 42, JAK2 44, ZAP-70 45, and p56lck 46. Chemokine-induced biological activities can be inhibited by specific tyrosine kinase inhibitors such as genistein (a general tyrosine kinase inhibitor) 14 47, tyrphostin B42 (a specific JAK2 kinase inhibitor) 13, and PD98059 (an inhibitor of the Erk pathways) 48, suggesting the importance of tyrosine phosphorylation in chemokine-induced signaling. The effectiveness of these inhibitors depends on cell types, chemokines, and types of biological effects. For future study, it will be important to identify the target protein(s) of SHP-1 that can modify chemokine responses.
The observations of a dichotomy in response of mev/mev cells to chemokines was of interest. Although mev/mev cells are enhanced in chemotaxis (hyperresponse), they are resistant to myelosuppressive chemokines in proliferation (hyporesponse). Chemotactic activity and myelosuppressive effect are very distinct biological activities in terms of kinetics, cell target specificity, and cellular signaling machinery. Specifically, 21 chemokines out of 34 that had been tested previously have myelosuppressive activity, inhibiting the proliferation of immature subsets of bone marrow cells that are responsive to stimulation by combinations of growth factors 30. In contrast, only three chemokines, SDF-1, SLC, and CKβ-11, out of many that were analyzed, have been demonstrated to have chemotactic activities for human myeloid progenitors 10 11 49 50. SDF-1 is a chemoattractant for CFU-GM, BFU-E, and CFU-GEMM 10 11, whereas SLC and CKβ-11 are mainly chemotactic for the macrophage progenitors (CFU-M), a subset of CFU-GM 49 50. Chemokine-induced chemotaxis occurs quickly and depends on chemokine gradients, whereas inhibition of myeloid progenitor cells by chemokines is dependent on concentrations rather than gradients of chemokines. Chemotaxis involves cell motility and cytoskeletal machinery to migrate, including reorganization of actin structures, adhesion to and detachment from substratum, and termination of movement by desensitization. On the other hand, inhibition of myeloid cell proliferation by chemokines presumably requires regulation of cell cycle and mitogenic signaling machinery. Although further studies on the differential signaling for these two biological processes are required, our results suggest that the mechanism of SHP-1 in regulation of suppression is probably different from that of chemotaxis. It is possible that SHP-1 dephosphorylates different target signaling proteins that are directly involved in or indirectly set optimal conditions for signaling for chemotaxis and suppression. The inability of mev/mev myeloid progenitor cells to respond to chemokines is reminiscent of the insensitivity of malignant myeloid progenitor cells from some patients with leukemia to suppression by chemokines 2 51.
The activity of chemokines can be regulated in two ways in the cells: modification of chemokine receptor expression and/or signaling pathways. In this study, we examined whether SHP-1 modulates chemokine receptor expression. Expression of CXCR4, the receptor for SDF-1, did not change in mev/mev versus +/+ cells. Thus it does not appear that the enhanced chemotactic response to SDF-1 can be attributed to enhanced CXCR4 expression. In bone marrow cells from mev/mev mice, we observed similar or slightly enhanced expression of many chemokine receptors, including those that are shown to bind many of the myelosuppressive chemokines. Thus lack of suppression of mev/mev progenitors in response to chemokines is probably not due to loss of chemokine receptor expression. Leukocytes in mev/mev mice show similar profiles of chemokine receptor expression to that of activated cells. In mev/mev CD4+ T cells, expression of a number of chemokine receptors (especially CXCR2, CCR2, CCR1, CCR2, and CCR3) is much higher than in the +/+ counterparts. It is difficult, at this time, to pinpoint the direct cause for the enhanced expression of some chemokine receptors in mev/mev T cells. However, there are several possibilities: (a) absence of SHP-1 directly induces expression of these chemokine receptors; (b) absence of SHP-1 activates cells and activated cells express more chemokine receptors; and/or (c) the pathological environment in mev/mev (e.g., autoimmunity) induces expression of cytokines that in turn drive the activation of T cells and induction of chemokine receptors. Taken together, our results suggest that SHP-1 deficiency in me mice alters myeloid progenitor and mature leukocyte responses to chemokines.
Acknowledgments
This work was supported by U.S. Public Health Service grants RO1 DK53674, R01 HL56416, and R01 HL54037; by a project in P01 HL 53586 from the National Institutes of Health (NIH) to H.E. Broxmeyer; and NIH RO1 CA78606, R29 GM53660, and American Cancer Society RPG-98-273-01-TBE grants to G.-S. Feng.
C.H. Kim's present address is Dept. of Pathology, L235, Stanford University School of Medicine, Stanford, CA 94304.
Footnotes
1used in this paper: CFU-GM, colony forming unit-granulocyte and macrophage; HPC, hematopoietic progenitor cell; JAK, Janus kinase; MAPK, mitogen-activated protein kinase; me, motheaten; mev, viable motheaten; SDF-1, stromal cell–derived factor 1; SHP-1, Src homology 2 domain tyrosine phosphatase 1
References
- Baggiolini M., Dewald B., Moser B. Human chemokinesan update. Annu. Rev. Immunol. 1997;15:675–705. doi: 10.1146/annurev.immunol.15.1.675. [DOI] [PubMed] [Google Scholar]
- Broxmeyer H.E., Cooper S., Hague N., Benninger L., Sarris A., Cornetta K., Vadhan-Raj S., Hendrie P., Mantel C. Human chemokinesenhancement of specific activity and effects in vitro on normal and leukemic progenitors and a factor-dependent cell line and in vivo in mice. Ann. Hematol. 1995;71:235–246. doi: 10.1007/BF01744373. [DOI] [PubMed] [Google Scholar]
- Kim C.H., Broxmeyer H.E. Chemokinessignal lamps for trafficking of T- and B-cells for development and effector function. J. Leukocyte Biol. 1999;65:6–15. doi: 10.1002/jlb.65.1.6. [DOI] [PubMed] [Google Scholar]
- Luster A.D. Chemokines—chemotactic cytokines that mediate inflammation. N. Engl. J. Med. 1998;12:436–445. doi: 10.1056/NEJM199802123380706. [DOI] [PubMed] [Google Scholar]
- Rollins B.J. Chemokines. Blood. 1997;90:909–928. [PubMed] [Google Scholar]
- Nagasawa T., Hirota S., Tachibana K., Takakura N., Nishikawa S., Kitamura Y., Yoshida N., Kikutani H., Kishimoto T. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature. 1996;382:635–638. doi: 10.1038/382635a0. [DOI] [PubMed] [Google Scholar]
- Tachibana K., Hirota S., Iizasa H., Yoshida H., Kawabata K., Kataoka Y., Kitamura Y., Matsushima K., Yoshida N., Nishikawa S.-i. The chemokine receptor CXCR4 is essential for vascularization of the gastrointestinal tract. Nature. 1998;393:591–594. doi: 10.1038/31261. [DOI] [PubMed] [Google Scholar]
- Zou Y.-R., Kottmann A.H., Kuroda M., Taniuchi I., Littman D.R. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature. 1998;393:595–599. doi: 10.1038/31269. [DOI] [PubMed] [Google Scholar]
- Bleul C.C., Fuhlbrigge R.C., Casasnovas J.M., Aiuti A., Springer T.A. A highly efficacious lymphocyte chemoattractant, stromal cell-derived factor 1 (SDF-1) J. Exp. Med. 1996;184:1101–1109. doi: 10.1084/jem.184.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiuti A., Webb I.J., Bleul C., Springer T., Gutierrez Ramos J.C. The chemokine SDF-1 is a chemoattractant for human CD34+ hematopoietic progenitor cells and provides a new mechanism to explain the mobilization of CD34+ progenitors to peripheral blood. J. Exp. Med. 1997;185:111–120. doi: 10.1084/jem.185.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C.H., Broxmeyer H.E. In vitro behavior of hematopoietic progenitor cells under the influence of chemoattractantsSDF-1, steel factor and the bone marrow environment. Blood. 1998;91:100–110. [PubMed] [Google Scholar]
- Kim C.H., Pelus L.M., White J.R., Broxmeyer H.E. Differential chemotactic behavior of developing T cells in response to thymic chemokines. Blood. 1998;91:4434–4443. [PubMed] [Google Scholar]
- Mellado M., Rodriguez-Frade J.M., Aragay A., del Real G., Martin A.M., Vila-Coro A.J., Serrano A., Mayor F., Jr., Martinez A.C. The chemokine monocyte chemotactic protein 1 triggers Janus kinase 2 activation and tyrosine phosphorylation of the CCR2B receptor. J. Immunol. 1998;161:805–813. [PubMed] [Google Scholar]
- Suzuki M., Hirai K., Kitani S., Takaishi T., Kihara H., Kasahara T., Ito K., Morita Y. Pharmacologic study of basophil histamine release induced by monocyte chemotactic protein-1 with kinase inhibitors. Int. Arch. Allergy Immunol. 1996;111:18–22. doi: 10.1159/000237339. [DOI] [PubMed] [Google Scholar]
- Ganju R.K., Brubaker S.A., Meyer J., Dutt P., Yang Y., Qin S., Newman W., Groopman J.E. The alpha-chemokine, stromal cell-derived factor-1alpha, binds to the transmembrane G-protein-coupled CXCR-4 receptor and activates multiple signal transduction pathways. J. Biol. Chem. 1998;273:23169–23175. doi: 10.1074/jbc.273.36.23169. [DOI] [PubMed] [Google Scholar]
- Laudanna C., Campbell J.J., Butcher E.C. Role of Rho in chemoattractant-activated leukocyte adhesion through integrins. Science. 1996;271:981–983. doi: 10.1126/science.271.5251.981. [DOI] [PubMed] [Google Scholar]
- Shultz L.D., Schweitzer P.A., Rajan T.V., Yi T., Ihle J.N., Matthews R.J., Thomas M.L., Beier D.R. Mutations at the murine motheaten locus are within the hematopoietic cell protein-tyrosine phosphatase (Hcph) gene. Cell. 1993;73:1445–1454. doi: 10.1016/0092-8674(93)90369-2. [DOI] [PubMed] [Google Scholar]
- Kozlowski M., Mlinaric-Rascan I., Feng G.S., Shen R., Pawson T., Siminovitch K.A. Expression and catalytic activity of the tyrosine phosphatase PTP1C is severely impaired in motheaten and viable motheaten mice. J. Exp. Med. 1993;178:2157–2163. doi: 10.1084/jem.178.6.2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsui H.W., Siminovitch K.A., de Souza L., Tsui F.W. Motheaten and viable motheaten mice have mutations in the haematopoietic cell phosphatase gene. Nat. Genet. 1993;4:124–129. doi: 10.1038/ng0693-124. [DOI] [PubMed] [Google Scholar]
- Plas D.R., Thomas M.L. Negative regulation of antigen receptor signaling in lymphocytes. J. Mol. Med. 1998;76:589–595. doi: 10.1007/s001090050254. [DOI] [PubMed] [Google Scholar]
- Siminovitch K.A., Neel B.G. Regulation of B cell signal transduction by SH2-containing protein-tyrosine phosphatases. Semin. Immunol. 1998;10:329–347. doi: 10.1006/smim.1998.0125. [DOI] [PubMed] [Google Scholar]
- Healy J.I., Goodnow C.C. Positive versus negative signaling by lymphocyte antigen receptors. Annu. Rev. Immunol. 1998;16:645–670. doi: 10.1146/annurev.immunol.16.1.645. [DOI] [PubMed] [Google Scholar]
- Shultz L.D., Rajan T.V., Greiner D.L. Severe defects in immunity and hematopoiesis caused by SHP-1 protein-tyrosine-phosphatase deficiency. Trends Biotechnol. 1997;15:302–307. doi: 10.1016/S0167-7799(97)01060-3. [DOI] [PubMed] [Google Scholar]
- Cooper S., Broxmeyer H.E. Measurement of interleukin-3 and other hematopoietic growth factors, such as GM-CSF, G-CSF, M-CSF, and erythropoietin and the potent co-stimulating cytokines steel factor and Flt3 ligand Curr. Prot. Immunol. 18Suppl.1996. 64.1–6.4.12. [DOI] [PubMed] [Google Scholar]
- Kim C.H., Pelus L.M., White J.R., Applebaum E., Johanson K., Broxmeyer H.E. CK beta-11/macrophage inflammatory protein-3 beta/EBI1-ligand chemokine is an efficacious chemoattractant for T and B cells. J. Immunol. 1998;160:2418–2424. [PubMed] [Google Scholar]
- Howard T.H., Meyer W.H. Chemotactic peptide modulation of actin assembly and locomotion. J. Cell Biol. 1984;98:1265–1271. doi: 10.1083/jcb.98.4.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu C.-K., Shi Z.-Q., Shen R., Tsai F.-Y., Orkin S.H., Feng G.-S. A deletion mutation in the SH2-N domain of Shp-2 severely suppresses hematopoietic cell development. Mol. Cell. Biol. 1999;17:5499–5507. doi: 10.1128/mcb.17.9.5499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anzai N., Gotoh A., Shibayama H., Broxmeyer H.E. Modulation of integrin function in hematopoietic progenitor cells by CD43 engagementpossible involvement of protein tyrosine kinase and phospholipase C-gamma. Blood. 1999;93:3317–3326. [PubMed] [Google Scholar]
- Peled A., Petit I., Kollet O., Magid M., Ponomaryov T., Byk T., Nagler A., Ben-Hur H., Many A., Shultz L. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science. 1999;283:845–848. doi: 10.1126/science.283.5403.845. [DOI] [PubMed] [Google Scholar]
- Broxmeyer H.E., Kim C.H. Regulation of hematopoiesis in a sea of chemokine family members with a plethora of redundant activities. Exp. Hematol. 1999;27:1113–1123. doi: 10.1016/s0301-472x(99)00045-4. [DOI] [PubMed] [Google Scholar]
- Broxmeyer H.E. Myelosuppressive cytokines and peptides. In: Whetton T., Gordon T., editors. In Blood Cell Biochemistry, Vol 7: Hematopoietic Growth Factors. Plenem; London, UK: 1996. pp. 121–150. [Google Scholar]
- Shultz L.D., Coman D.R., Bailey C.L., Beamer W.G., Sidman C.L. “Viable motheaten,” a new allele at the motheaten locus. I. Pathology. Am. J. Pathol. 1984;116:179–192. [PMC free article] [PubMed] [Google Scholar]
- Shyamala V., Khoja H. Interleukin-8 receptors R1 and R2 activate mitogen-activated protein kinases and induce c-fos, independent of Ras and Raf-1 in Chinese hamster ovary cells. Biochemistry. 1998;37:15918–15924. doi: 10.1021/bi9811415. [DOI] [PubMed] [Google Scholar]
- del Pozo M.A., Sanchez-Mateos P., Nieto M., Sanchez-Madrid F. Chemokines regulate cellular polarization and adhesion receptor redistribution during lymphocyte interaction with endothelium and extracellular matrix. Involvement of cAMP signaling pathway. J. Cell Biol. 1995;131:495–508. doi: 10.1083/jcb.131.2.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franci C., Gosling J., Tsou C.L., Coughlin S.R., Charo I.F. Phosphorylation by a G protein-coupled kinase inhibits signaling and promotes internalization of the monocyte chemoattractant protein-1 receptor. Critical role of carboxyl-tail serines/threonines in receptor function. J. Immunol. 1996;157:5606–5612. [PubMed] [Google Scholar]
- Aramori I., Ferguson S.S., Bieniasz P.D., Zhang J., Cullen B., Cullen M.G. Molecular mechanism of desensitization of the chemokine receptor CCR-5receptor signaling and internalization are dissociable from its role as an HIV-1 co-receptor. EMBO (Eur. Mol. Biol. Org.) J. 1997;16:4606–4616. doi: 10.1093/emboj/16.15.4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi T., Mui A.L., Krystal G., Ihle J.N. Hematopoietic cell phosphatase associates with the interleukin-3 (IL-3) receptor beta chain and down-regulates IL-3-induced tyrosine phosphorylation and mitogenesis. Mol. Cell. Biol. 1993;13:7577–7586. doi: 10.1128/mcb.13.12.7577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law C.L., Sidorenko S.P., Chandran K.A., Zhao Z., Shen S.H., Fischer E.H., Clark E.A. CD22 associates with protein tyrosine phosphatase 1C, Syk, and phospholipase C-gamma(1) upon B cell activation. J. Exp. Med. 1996;183:547–560. doi: 10.1084/jem.183.2.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pani G., Kozlowski M., Cambier J.C., Mills G.B., Siminovitch K.A. Identification of the tyrosine phosphatase PTP1C as a B cell antigen receptor-associated protein involved in the regulation of B cell signaling. J. Exp. Med. 1995;181:2077–2084. doi: 10.1084/jem.181.6.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg K.L., Carlberg K., Rohrschneider L.R., Siminovitch K.A., Stanley E.R. The major SHP-1-binding, tyrosine-phosphorylated protein in macrophages is a member of the KIR/LIR family and an SHP-1 substrate. Oncogene. 1998;17:2535–2541. doi: 10.1038/sj.onc.1202203. [DOI] [PubMed] [Google Scholar]
- Hua C.T., Gamble J.R., Vadas M.A., Jackson D.E. Recruitment and activation of SHP-1 protein-tyrosine phosphatase by human platelet endothelial cell adhesion molecule-1 (PECAM-1). Identification of immunoreceptor tyrosine-based inhibitory motif-like binding motifs and substrates. J. Biol. Chem. 1998;273:28332–28340. doi: 10.1074/jbc.273.43.28332. [DOI] [PubMed] [Google Scholar]
- Migone T.S., Cacalano N.A., Taylor N., Yi T., Waldmann T.A., Johnston J.A. Recruitment of SH2-containing protein tyrosine phosphatase SHP-1 to the interleukin 2 receptor; loss of SHP-1 expression in human T-lymphotropic virus type I-transformed T cells. Proc. Natl. Acad. Sci. USA. 1998;95:3845–3850. doi: 10.1073/pnas.95.7.3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Nadler M.J., Brennan L.A., Gish G.D., Timms J.F., Fusaki N., Jongstra-Bilen J., Tada N., Pawson T., Wither J. The B-cell transmembrane protein CD72 binds to and is an in vivo substrate of the protein tyrosine phosphatase SHP-1. Curr. Biol. 1998;8:1009–1017. doi: 10.1016/s0960-9822(07)00421-6. [DOI] [PubMed] [Google Scholar]
- Klingmuller U., Lorenz U., Cantley L.C., Neel B.G., Lodish H.F. Specific recruitment of SH-PTP1 to the erythropoietin receptor causes inactivation of JAK2 and termination of proliferative signals. Cell. 1995;80:729–738. doi: 10.1016/0092-8674(95)90351-8. [DOI] [PubMed] [Google Scholar]
- Plas D.R., Johnson R., Pingel J.T., Matthews R.J., Dalton M., Roy G., Chan A.C., Thomas M.L. Direct regulation of ZAP-70 by SHP-1 in T cell antigen receptor signaling. Science. 1996;272:1173–1176. doi: 10.1126/science.272.5265.1173. [DOI] [PubMed] [Google Scholar]
- Lorenz U., Ravichandran K.S., Burakoff S.J., Neel B.G. Lack of SHPTP1 results in src-family kinase hyperactivation and thymocyte hyperresponsiveness. Proc. Natl. Acad. Sci. USA. 1996;93:9624–9629. doi: 10.1073/pnas.93.18.9624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sozzani S., Zhou D., Locati M., Rieppi M., Proost P., Magazin M., Vita N., van Damme J., Mantovani A. Receptors and transduction pathways for monocyte chemotactic protein-2 and monocyte chemotactic protein-3. Similarities and differences with MCP-1. J. Immunol. 1994;152:3615–3622. [PubMed] [Google Scholar]
- Ragozzino D., Giovannelli A., Mileo A.M., Limatola C., Santoni A., Eusebi F. Modulation of the neurotransmitter release in rat cerebellar neurons by GRO beta. Neuroreport. 1998;9:3601–3606. doi: 10.1097/00001756-199811160-00011. [DOI] [PubMed] [Google Scholar]
- Kim C.H., Pelus L.M., White J.R., Broxmeyer H.E. MIP-3 beta/ELC/CKb-11 is a chemoattractant with a specificity for macrophage progenitors among myeloid progenitor cells. J. Immunol. 1998;161:2580–2585. [PubMed] [Google Scholar]
- Kim C.H., Broxmeyer H.E. SLC/Exodus2/6Ckine induces chemotaxis of hematopoietic progenitor cellsDifferential activity of ligands of CCR7, CXCR3, or CXCR4 in chemotaxis vs. suppression of progenitor proliferation. J. Leukocyte Biol. 1999;In press doi: 10.1002/jlb.66.3.455. [DOI] [PubMed] [Google Scholar]
- Eaves C.J., Cashman J.D., Wolpe S.D., Eaves A.C. Unresponsiveness of primitive chronic myeloid leukemia cells to macrophage inflammatory protein 1 alpha, an inhibitor of primitive normal hematopoietic cells. Proc. Natl. Acad. Sci. USA. 1993;90:12015–12019. doi: 10.1073/pnas.90.24.12015. [DOI] [PMC free article] [PubMed] [Google Scholar]