Abstract
Paracoccidioidomycosis (PCM) is a systemic granulomatous mycosis whose agent is Paracoccidioides brasiliensis. In the culture supernatant, the fungus expresses glycoproteins of from 13 to 148 kDa. A cell surface glycoprotein of 43 kDa is the major antigenic component of P. brasiliensis. Another expressed glycoprotein, gp70, is recognized by 96% of sera from PCM patients and is able to induce lymphoproliferation. Since, little is known about this glycoprotein, we produced monoclonal antibodies (MAbs) against gp70 to isolate the molecule from total fungus extracts and to investigate its possible role in the pathogenesis of PCM. Using these MAbs, it was observed by confocal microscopy that gp70 is located mainly in the intracellular compartment of the fungus, although it was also detected in the culture supernatant. Based on observations showing that gp43 has a down-regulatory effect on mouse peritoneal macrophages, we tested the effects of gp70 on their phagocytic ability. Purified gp70 was able to inhibit the activity of macrophages through the mannose receptors and also through the Fc receptors; the latter effect was not observed with gp43. gp70 inhibits NO and H2O2 liberation by peritoneal macrophages in vitro, as does gp43. Results obtained with gp43 led us to hypothesize that gp70 could act as an escape mechanism for fungal establishment in primary infections. To corroborate this hypothesis, we analyzed the effect of passive immunization of mice during infection with P. brasiliensis using anti-gp70 MAbs. This treatment almost completely abolished granuloma formation in the lungs, suggesting that the protein facilitates fungal establishment and progression of lesions in primary infection.
Paracoccidioidomycosis (PCM) is a deep systemic granulomatous mycosis whose etiological agent is Paracoccidioides brasiliensis, a dimorphic fungus that grows in the mycelial phase at room temperature and in the yeast phase at 37°C or in infected tissues. This disease is one of the most prevalent human systemic mycoses in Latin America (6) and can lead to significant mortality rates in the absence of specific therapy or in immunodepressed individuals (45). The infection probably starts by inhalation of fungal propagules that undergo differentiation into yeast cells, the infective form of P. brasiliensis, in the lung (35). Once established, it induces the formation of characteristic granulomatous lesions in the lungs and lymph nodes. These lesions are rich in viable fungi that can disseminate to virtually all organs and tissues (38). Two main clinical forms of the disease are recognized by the International Committee of PCM (13), the unifocal or multifocal chronic form (AC) and the acute or subacute form (AF). The chronic form of PCM, which accounts for >90% of cases, mostly in adult males, progresses slowly and can take months to years to develop fully. Adult PCM primarily afflicts the lungs, leading to significant morbidity due to gross impairment of lung function. Subsequently, the disease can disseminate to other organs and tissues, forming secondary lesions in the mucous membranes, skin, lymph nodes, and adrenal glands that characterize the more advanced type of PCM. By contrast, the juvenile type of PCM, which develops within weeks to months, is more severe, leading to significant mortality rates due primarily to reticuloendothelial system organ hypertrophy. There is a pronounced sex bias in P. brasiliensis infection, with a male-to-female ratio in adult infections of up to 78:1, and the mechanism underlying this process is thought to involve hormonal regulation (43).
In the culture supernatant, the fungus presents a complex structure of proteins and glycoproteins whose molecular masses range from 13 to 148 kDa. A 43-kDa surface glycoprotein is considered the main antigenic component, since it is recognized by 100% of PCM patients' sera (49, 50). gp43 has been shown to bind laminin, a protein component of the extracellular matrix of mammalian tissues, and laminin-coated yeasts have increased ability to invade and destroy tissues (52). It has recently been demonstrated in our laboratory that gp43 down regulates lymphoproliferation and mouse peritoneal macrophage functions, such as phagocytosis, via mannose receptors, liberation of free radicals, and the microbicidal activity of the cells for P. brasiliensis (21).
Another glycoprotein expressed by the fungus is gp70. It was demonstrated that only the fraction-bound extract to ConA was reactive with sera of patients with PCM, and gp70 was one of its components (41). It was found that gp70 is recognized by 96% of the sera from the same patients, and during chemotherapy of 72 patients, 55 (76%) showed a much-reduced reaction. Also, some of the predominant components of this molecule are polysaccharides (51). Healthy persons previously infected by Histoplasma capsulatum reacted against gp70 but not gp43 (12). Both gp70 and gp43 also induce lymphoproliferative responses when tested with lymphocytes from PCM patients (3). gp70 was also detected, along with gp43, in the urine of patients exhibiting the acute form of PCM (44). However, despite its likely importance, this fungal component of P. brasiliensis has not yet been purified and carefully studied.
The pathophysiology of PCM is far from completely understood. Nevertheless, it is well established that macrophages constitute one of the primary mechanisms that arrest microbial invasion. It has been demonstrated that activated macrophages may have a central role in host resistance to systemic mycoses, such as coccidioidomycosis (1, 2), histoplasmosis (53), blastomycosis (10), and PCM (7, 9). Microscopic studies showed that P. brasiliensis is able to multiply intracellularly in peritoneal and pulmonary resident macrophages, indicating that they are not fungicidal for ingested fungi (8). In contrast, lymphokine-activated macrophages were found to be fungicidal for ingested P. brasiliensis (7). Identification of the fungal surface molecules that mediate the interaction with macrophage receptors is certainly important for a understanding of the host-invader interplay. However, the interaction between P. brasiliensis components and macrophages is not yet fully understood.
Cell-mediated immunity is generally acknowledged to provide important host defense against most fungal infections. The role of antibody-mediated immunity in host resistance is less certain (17), despite considerable evidence that administration of some monoclonal antibodies (MAbs) can modify the course of infection in mice by certain pathogenic fungi, such as Cryptococcus neoformans and Candida albicans (18, 20, 27, 29, 46).
In the present study, MAbs were produced against gp70 in order to isolate the molecule from total fungus extracts and to investigate its influence on the phagocytic abilities of mouse peritoneal macrophages. The effect of passive immunization of mice before infection with P. brasiliensis using the generated anti-gp70 MAbs was also analyzed. Treatment of mice by simultaneous injection of two MAbs directed to gp70 epitopes almost abolished lung infection. As this molecule also down regulates mouse peritoneal macrophage functions in vitro, we propose here that MAbs, by blocking the inhibitory effect of gp70 on phagocytes and probably on other immune effector cells, may facilitate the clearance of the fungi from lung tissues, thus aborting infection.
MATERIALS AND METHODS
Fungal strains.
P. brasiliensis strains SS and 113 were maintained by frequent subculturing on Sabouraud glucose agar (Difco BRL Products, Gaithersburg, Md.). Yeast forms were grown at 35°C and subcultured every 5 days. All experiments described below were performed with both fungal strains, except the assay in vivo, in which a highly virulent isolate (Pb18) was used. To ensure the maintenance of its virulence, this isolate was used only after three passages in mice by intraperitoneal inoculation.
Preparation of fungal antigens.
Yeast forms of P. brasiliensis were grown on Sabouraud glucose agar at 35°C for 3 days and transferred to 50 ml of TOM medium prepared in our laboratory with 6.1 g of yeast extract, 16.1 g of dextrose, 15 g of casein peptone, 0.31 g of K2HPO4, 0.12 g of MgSO4 · 7 H2O, 0.006 g of MnSO4 · H2O, 0.006 g of NaCl, and 0.006 g of FeSO4 for 1,000 ml of distilled H2O at 37°C on a rotating shaker as previously described (41). This preinoculum was cultivated for 3 days and transferred into Fernbach flasks containing 500 ml of the same medium. After 10 days of incubation at 37°C, the cells were killed by the addition of 0.2 g of thimerosal per liter; the suspension was filtered through filter paper, and the resulting filtered material represented the crude exoantigen.
Animals.
Male and female BALB/c mice, 6 to 10 weeks old, provided by the animal facilities of the Federal University of São Paulo, São Paulo, Brazil, were used throughout this study.
SDS-PAGE and immunoblot analysis.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed on vertical slab gels of 10% acrylamide according to the method of Laemmli (32) and always under reducing conditions. Glycoproteins were stained with Coomassie brilliant blue, silver nitrate, or periodic acid-Schiff. Immunoblotting was performed as described elsewhere (48).
Immunization protocols.
In the first schedule of immunization, 6-week-old BALB/c mice were intraperitoneally injected every 2 weeks for 4 months with macerated polyacrylamide gel containing 70-kDa glycoprotein (gp70). In a second schedule, mice were subcutaneously immunized every 2 weeks with 50 μg of gp70 (purified through MAbs obtained in the first fusion) in phosphate-buffered saline (PBS), incorporated in Freund's complete adjuvant for the first injection and in incomplete Freund's adjuvant for the subsequent injections. Injections were always made in four different sites in the axillary and inguinal regions in a final volume of 100 μl per site. Before each immunization, the mice were bled through the ocular plexus, and the serum was separated by centrifugation and stored at −20°C. Final immunization was done intravenously 2 days before cell fusion.
Fusion protocols.
Cells of the murine myeloma line sp2 were fused with spleen cells from immunized mice according to the method of Lopes and Alves (34). For the first fusion, hybridomas were distributed in 48-well plates (Costar Corp., Cambridge, Mass.), and 10 days postfusion, the colonies were screened by immunoblotting, as described below. For the second fusion, hybridomas were distributed in 96-well plates (Costar), and the screening was done by enzyme immunoassay (EIA) as described elsewhere (42). After cell cloning by limiting dilution and expanding of the positive clones, large amounts of antibodies were obtained from the ascites fluid induced in BALB/c mice injected with Pristane (Sigma) before the inoculation of hybridoma cells.
Antibody screening by immunoblotting.
Exoantigens were fractionated by SDS-PAGE with 10% acrylamide under reducing conditions. The proteins were then transferred by electrophoresis to nitrocellulose membranes. Fragments of nitrocellulose membranes containing gp70 were cut in small pieces and distributed in 48-well plates (Costar). After free sites were blocked with PBS containing 5% skim milk, 200 μl of supernatants from the hybridoma cultures was added to each well. After 1 h of incubation at room temperature, the wells were washed five times for 5 min each time with PBS containing 0.1% Tween 20 (Sigma) and treated with affinity-purified peroxidase-conjugated goat anti-mouse immunoglobulin (Ig) (Bio-Rad) for 1 h at room temperature. The wells were washed five times with PBS containing 0.1% Tween 20. The presence of reactive IgG was tested by the addition of 4-chloro-naftol in 50 mM Tris buffer, pH 6.8, metanol, and H2O2 and terminated with distilled water.
gp70 purification.
Exoantigen of P. brasiliensis strains SS and 113, prepared separately as described above, was used as the antigen source. Purification of gp70 was performed by affinity chromatography in a Sepharose CNbr column coupled with MAb 1A7C5F11 (from now on called C5F11) directed to gp70 epitopes. This MAb was purified from both culture supernatants and ascites fluids by affinity chromatography in a protein G column. Ig isotyping was performed with a mouse antibody isotyping kit (Gibco) according to the manufacturer's instructions.
The gp70 concentration was determined by the Bradford method (4). All steps of gp70 purification were monitored by SDS-PAGE.
ELISA.
Enzyme-linked immunosorbent assays (ELISAs) were carried out as described elsewhere (42). Briefly, plates were coated with 1 μg of purified gp70/ml in PBS, 50 μl per well, for 1 h at 37°C. Blocking of free sites on plastic was done with 5% PBS- milk for 1 h at 37°C. Anti-gp70 MAbs were added in various concentrations (5, 10, 20, and 40 μg/ml in PBS; 50 μl per well). After 1 h, the plates were incubated with anti-mouse IgG (horseradish peroxidase conjugated in PBS; 50 μl per well) (Bio-Rad). Between the incubation steps, the wells were washed three times with PBS- 0.1% Tween 20- 0.7% gelatin. After incubation for 1 h at 37°C and washing of the wells, substrate solution (1 mg of O-phenylenediamine in 5 ml of 0.1 M citrate-phosphate buffer [pH 5.0] plus 10 μl of 30% H2O2) was added to each well, and the reaction was interrupted by the addition of 50 μl of 2 N H2SO4 per well. Optical densities were read in a Multiskan MCC/340 II EIA reader at 492 nm.
Recognition of gp70 by MAbs.
To investigate whether gp70 was recognized only when purified or also in living fungi, an EIA was devised. Live fungi were bound to the plastic substrate through panning by human polyclonal anti-P. brasiliensis serum. On the side, purified gp70 was directly bound to the surface. After washing and quenching of the plastic surface as described above, the reaction was developed with mouse anti-gp70 MAbs.
Inhibition assay.
In order to ascertain the natures of gp70 epitopes to which MAbs were directed, whether carbohydrate or protein, polyvinyl plates were coated with purified gp70 and incubated with 20 μg of anti-gp70 MAbs/ml after being previously incubated for 1 h under constant agitation with 25, 50, and 100 mM solutions of mannose, galactose, glucose, and α-methyl-d-mannopyranoside. After this incubation period, the concentrations of IgG in these preparations were determined as previously described.
IEF.
Isoelectric focusing (IEF) was performed with the Pharmacia LKB (Uppsala, Sweden) PhastSystem apparatus. One microgram of affinity-purified gp70 from P. brasiliensis SS and/or 113 was submitted to IEF analysis using PhastGel IEF (Pharmacia) in a pH range of 3 to 9 according to the manufacturer's instructions. Standard markers for isoelectric point (pI) determination of proteins in the same pH range (Pharmacia) were used. gp70 was developed by silver staining (PhastGel Silver kit; Pharmacia).
Confocal microscopy.
Yeast forms of P. brasiliensis from 5-day-old cultures grown in yeast extract-peptone-dextrose broth medium were fixed for 60 min with 0.5 ml of 3.5% formaldehyde in PBS and washed as before. The pellets were resuspended in 0.1 ml of PBS, and 10 μl of each cell suspension was applied to the microwells of fluorescence slides.
The cells were incubated with 50 mM amonium choride for 60 min, followed by 1 h of incubation with 0.1% Triton X-100 diluted in PBS- 0.2% gelatin. Anti-gp70 MAbs were added at a concentration of 50 μg/ml diluted in PBS-0.2% gelatin and incubated for 2 h. Finally, fluorescein-conjugated secondary antibody was added together with DAPI (4′,6′-diamidino-2-phenylindole)-stained nuclei for 60 min of incubation. All steps were followed by constant washing with PBS. The slides were mounted with 5% buffered glycerol to minimize bleaching. Images of DAPI-stained cells were observed in a Bio-Rad 1024 UV confocal system attached to a Zeiss Axiovert 100 microscope, using a 40× numerical-aperture 1.2 plan-apochromatic differential interference contrast water immersion objective. All images were collected by Kalman averaging at least every eight frames (512 by 512 pixels), using an aperture (pinhole) of 2 mm.
Peritoneal macrophages.
Peritoneal macrophages were used as representatives of nonspecific response. Any differences from alveolar macrophages could not be checked due to the very small amount of gp70 available. Cells were collected from the abdominal cavities of BALB/c mice by repeated washing with 5 ml of fresh RPMI 1640. Peritoneal macrophages from all of the mice were pelleted by centrifugation at 390 × g rpm for 4 min and then pooled. The peritoneal macrophages were resuspended in R10 medium and distributed in wells of tissue culture plates. The cultures were incubated at 37°C in 5% CO2 for 1 h. Nonadherent cells were removed by aspiration, and RPMI 1640 with 10% (vol/vol) heat-inactivated fetal bovine serum (R10) was added to the adherent monolayers.
For the phagocytic assays, 5 × 105 peritoneal macrophages were resuspended in 0.5 ml of R10 and dispensed in 24-well tissue culture plates (Costar) containing round glass coverslips in the bottom. Adherent monolayers were rinsed with 0.5 ml of R10. The macrophages were maintained in culture for 72 h.
To determine NO and H2O2 liberation by these cultures, 2 × 105 peritoneal cells in 0.15 ml of R10 were distributed in wells of a 96-well tissue culture plate. Adherent cell monolayers were rinsed with 0.05 ml of R10, and the macrophages were maintained in culture for 48 h.
Phagocytic assays.
Phagocytic assays were done with macrophages maintained in culture for 72 h. gp70 dissolved in R10 medium at different concentrations (25, 50, and 100 μg/ml) was added to macrophage cultures. After 5 min of incubation with gp70, zymosan particles (10 mg/ml) were added to the cultures. After 60 min, the coverslips were vigorously washed with PBS and fixed in 2% glutaraldehyde. These preparations were analyzed under phase-contrast microscopy.
The same protocol was used to evaluate the influence of gp70 on the uptake of sheep red blood cells previously opsonized with subagglutinating doses of rabbit IgG anti-sheep red blood cells at 37°C for 1 h. The preparations were stained by Hema 3 (24) and analyzed by optical microscopy. The viability of the macrophages was always checked by trypan blue exclusion.
An average of 200 phagocytic cells were counted in the control group to represent 100%.
NO determination.
Peritoneal macrophage cultures as described above were incubated with different concentrations of gp70 (0.125, 0.25, 0.5, and 1 μg/ml), and after 48 h, the concentration of NO2 in the culture supernatant was measured with the Griess reagent (1% sulfanilamide, 0.1% naphthylethylenediamine dihydrochloride, 25% H3PO4). All assays were done in triplicate. The concentration of gp70 used was the result of previous experiments in our laboratory.
H2O2 determination.
Peritoneal-macrophage cultures as described above were stimulated with 10 ng of lipopolysaccharide (LPS)/ml and 10 IU of gamma interferon (IFN-γ) or unstimulated and were treated with 1 μg of gp70/ml or left untreated. After 2 days, a phenol red solution (PBS, 5 mM dextrose, 50 μg of horseradish type II, 0.28 mM phenol red) was added to the cells for 1 h at 37°C, and the reaction was stopped by the addition of 10 μl of 1 N NaOH solution. A standard curve for the determination of the H2O2 concentration in the experimental tests was constructed using H2O2 solution varying from 0.5 to 4 nmol. Absorbance was measured at 620 nm in a microtiter plate reader (MR 4000; Dynatech Chantilly).
Passive immunization.
Five groups of eight male BALB/c mice each (8 to 10 weeks old) were used. All groups were infected intratracheally with the Pb18 strain of P. brasiliensis, as previously described (16), and received different treatments by the intravenous route. The first group received 100 μl of PBS, the second group received 100 μg of an irrelevant MAb (anti-CEA [carcinoembryonic antigen]), the third group received 100 μg of MAb 1B7D6 (from now on called B7D6), the fourth group received 100 μg of MAb C5F11, and the fifth group received 100 μg each of MAbs B7D6 and C5F11. To ensure the maintenance of the concentrations of antibodies in serum throughout the infection, the MAbs were administered 3 days before and every 3 days after infection over a period of 45 days.
CFU determination.
Mice of each experimental group were sacrificed after 45 days of infection, and the numbers of viable microorganisms in the lungs, liver, and spleen were determined by the CFU method as previously described (3). The results of CFU studies were confirmed by two identical protocols.
Histopathology.
Fragments of lungs, livers, and spleens from mice of the different experimental groups were fixed in 10% formalin for 24 h and embedded in paraffin. Tissue sections (5 μm thick) were obtained and stained with hematoxylin and eosin.
Statistics.
Statistical comparisons were made by analysis of variance and the Tukey-Kramer test. All values were reported as the mean ± standard error of the mean.
RESULTS
MAb generation and antigen purification.
After cloning and selection, five stable MAbs that specifically recognized gp70 from P. brasiliensis were obtained. MAbs 1A7C5F5F11 and 2B8A6D2 were of the IgG1 isotype, and MAbs 1E6B1F4, 1E6B8F3, and 2E7A2C4 were of the IgM isotype (all κ light chains). The IgG1 MAb C5F11 was coupled to Sepharose 4B CNbr and used to isolate the antigen by affinity chromatography. gp70 was purified from exoantigens produced by strains SS and 113 (Fig. 1A and B).
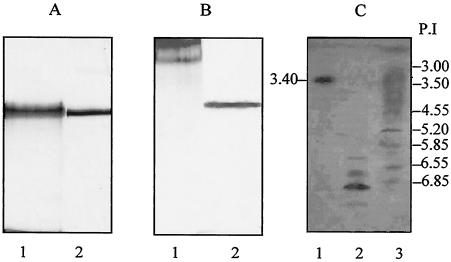
FIG. 1.
Silver-stained gel from SDS-PAGE demonstrating bovine serum albumin (67 kDa) used as standard (lanes 1) and purified gp70 (lanes 2). (A) Periodic acid-Schiff staining showing exoantigen (lane 1) and purified gp70 (lane 2). (B) IEF comparing gp70 and gp43. Lane 1, the pI of gp70 is ∼3.00; lane 2, four isoforms of gp43 are seen; lane 3, pI standards.
In order to investigate whether gp70 expressed different isoforms, an IEF assay was carried out. The results showed that the isolated protein presented a single isoform with a pI of 3.4. Conversely, gp43, used here for comparison, has several isoforms with pIs ranging from 5.85 to 6.8 (Fig. 1C).
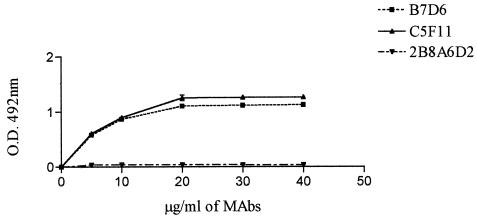
In another set of fusion experiments, one MAb directed to epitopes of gp70, B7D6, was obtained and characterized as IgG1. The binding of MAbs to gp70 was evaluated by EIA (Fig. 2).
FIG. 2.
ELISAs of MAbs showing different levels of reactivity with gp70. The negative control (5% skim milk and unrelated MAb) and positive control (mouse polyclonal anti-gp70 serum) are not shown. MAb 2B8A6D2 does not react in ELISA, probably because it recognizes a different spatial conformation of gp70.
gp70 was also recognized by the MAbs in live fungi adhering to the plastic surface by human polyclonal anti-P. brasiliensis serum (data not shown).
Inhibition assays.
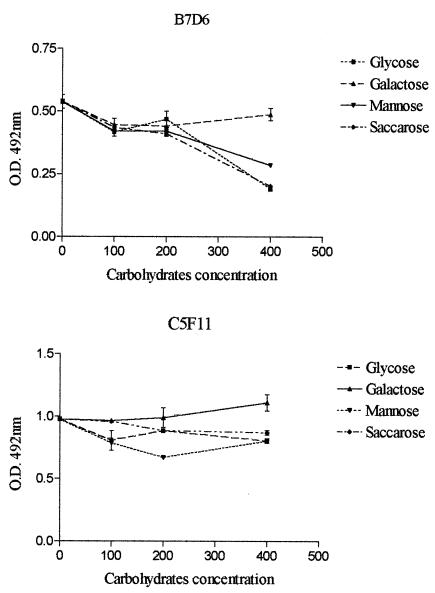
To analyze whether anti-gp70 MAbs bind the 70-kDa glycoprotein through epitopes composed of peptides or carbohydrates, inhibition assays were performed. The results showed that MAb C5F11 binds to a peptide epitope, whereas MAb B7D6 is partially inhibited by carbohydrates (Fig. 3).
FIG. 3.
Plates were coated with gp70 and incubated with B7D6 (A) and C5F11 (B), previously incubated for 1 h with carbohydrates.
Confocal microscopy.
Using two anti-gp70 antibodies, B7D6 and C5F11, confocal microscopy was employed to determine the localization of gp70 in the fungus in the yeast phase. It was observed that gp70 was present in the intracellular milieu (Fig. 4).
FIG. 4.
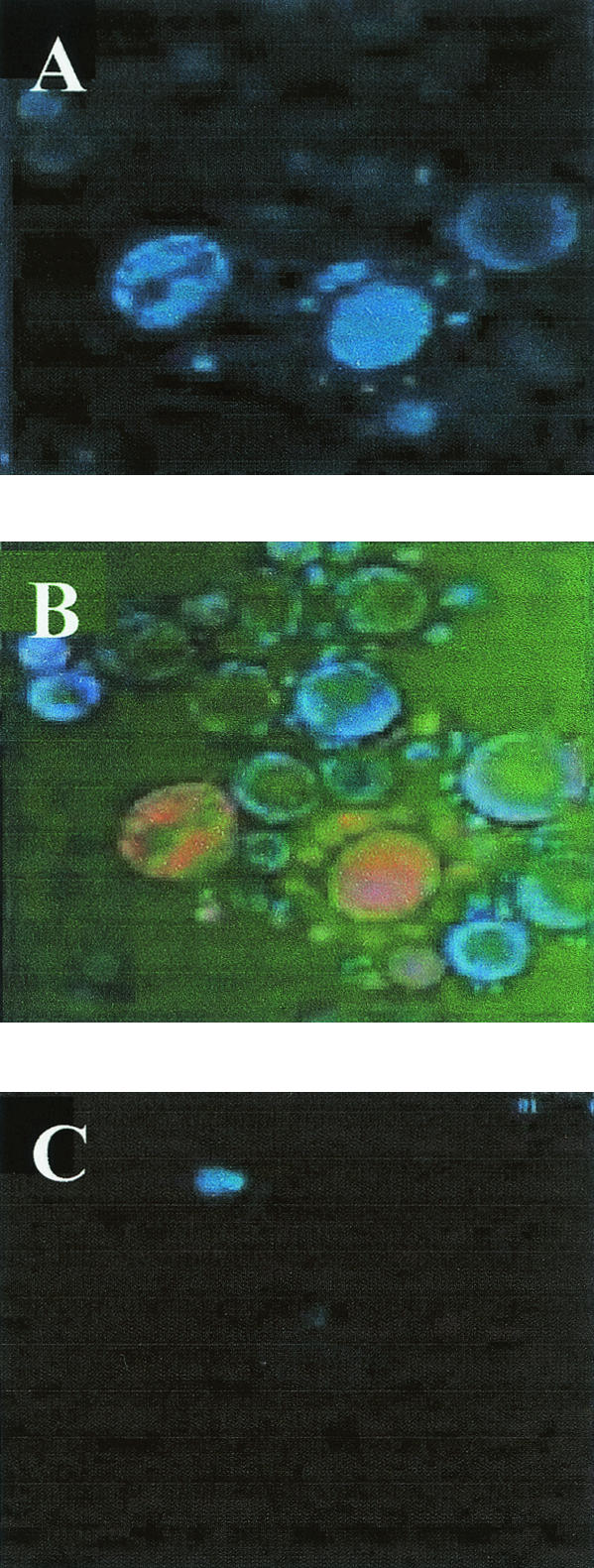
Confocal microscopy with anti-gp70 MAb revealed with mouse anti-IgG-fluorescein isothiocyanate (FITC). (A) gp70 labeling. (B) Double staining. Nuclei labeled with DAPI are blue, and gp70 is orange. (C) Negative control; absence of gp70 using only anti-mouse- FITC.
Inhibition of phagocytosis in vitro.
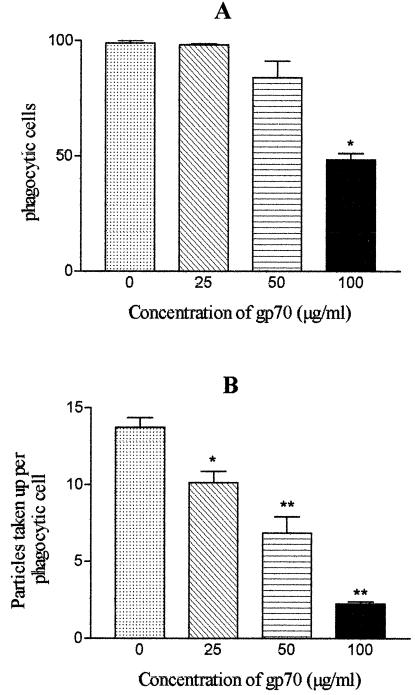
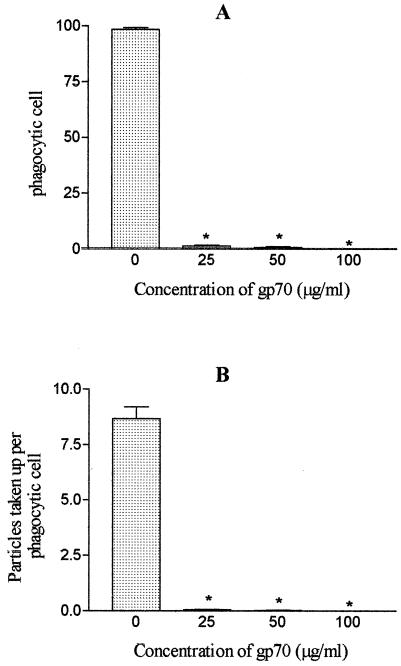
To investigate whether gp70 influences phagocytosis through the mannose receptor, zymosan particles (10 mg/ml) were added to peritoneal-macrophage cultures previously supplied with different concentrations of gp70 (25, 50, and 100 μg/ml). The results showed that purified gp70 inhibited phagocytosis of zymosan particles in a dose-dependent manner (Fig. 5). The maximal inhibition of phagocytic activity was obtained with 100 μg of gp70/ml.
FIG. 5.
Influences of different gp70 concentrations on phagocytosis of zymosan particles (10 mg/ml) by BALB/c mouse peritoneal macrophages. (A) Percentages of phagocytic cells (100% corresponds to 200 phagocytic cells). *, significantly different (P < 0.001) from controls. (B) Number of particles taken up per phagocytic cell. *, P < 0.05; **, P < 0.001 (significantly different from control). The error bars indicate standard errors.
As shown in Fig. 6, gp70 also influences phagocytosis through the Fc receptor. At a concentration of 25 μg of gp70/ml, a marked inhibition in the uptake of opsonized sheep red blood cells was observed.
FIG. 6.
Influences of different gp70 concentrations on phagocytosis of opsonized sheep red blood cells by BALB/c mouse peritoneal macrophages. (A) Percentages of phagocytic cells (100% corresponds to 200 phagocytic cells). (B) Number of particles taken up per phagocytic cell. *, statistically significant (P < 0.001) compared to controls. The error bars indicate standard errors.
NO and H2O2 release.
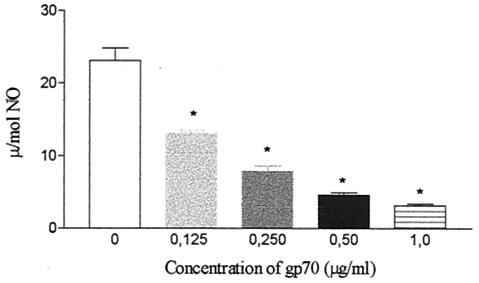
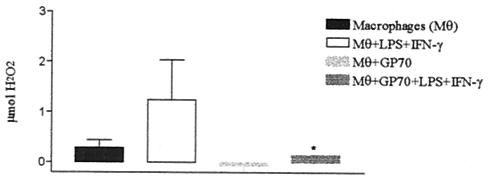
The influence of gp70 on NO and H2O2 release by cultured macrophages was investigated. The results showed that gp70 inhibited basal NO production by nonactivated peritoneal macrophages, with an initial concentration of 0.125 μg/ml (Fig. 7). Similarly, gp70 also inhibited H2O2 production by peritoneal macrophages activated or not with LPS and IFN-γ (Fig. 8).
FIG. 7.
NO release by BALB/c mouse peritoneal macrophages in culture supernatants after 2 days of treatment with gp70. *, significantly different (Ρ < 0.001) from control. The error bars indicate standard errors.
FIG. 8.
H2O2 release by BALB/c mouse peritoneal macrophage culture supernatants after 2 days with or without stimulation with 10 ng of LPS/ml and 10 IU of IFN-γ and with or without treatment with 1 μg of gp70/ml. *, significant (Ρ < 0.001) compared with control. The error bars indicate standard errors.
Passive immunization.
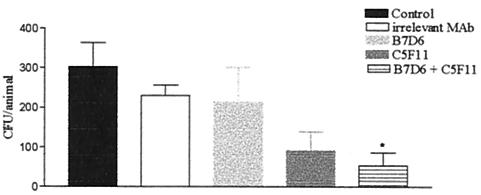
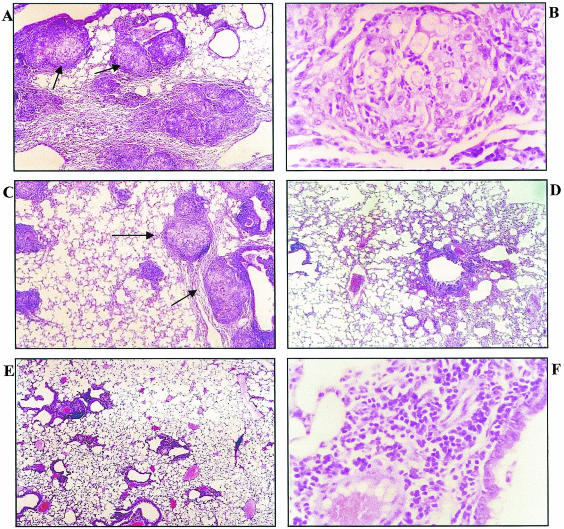
To evaluate whether administration of IgG1 anti-gp70 MAbs could alter the course of PCM, BALB/c mice were infected intratracheally with P. brasiliensis and intravenously treated with anti-gp70 MAbs. After 45 days of infection, the average time for the establishment of infection by yeast cells, the number of CFU in the lungs of animals treated with MAb B7D6 was similar to that in controls. Reduction in the number of CFU in mice treated with MAb C5F11 was observed, but a significant decrease in the number of CFU was obtained only with the group that received concomitant administration of both MAbs C5F11 and B7D6 (Fig. 9). Histopathology of lungs from infected and treated animals confirmed the CFU results. The lungs of control animals presented an inflamed parenchyma with a large cellular infiltrate, mainly of the mononuclear type, and well-organized granulomas with great numbers of viable yeast cells, most of them within the cytoplasm of phagocytic cells. Similar results were observed in the lungs of mice treated with an irrelevant MAb. The lungs of animals that received MAb B7D6 presented a minor reduction in parenchyma inflammation and granulomas. Mice treated with C5F11 showed well-preserved parenchymas with large peribronchial cellular infiltrates and did not present yeast cells or granuloma formation. The lung parenchymas of the animals treated with both the antibodies B7D6 and C5F11 were similar to those of the C5F11 group, but moderate peribronchial cellular infiltrate was not regularly observed (Fig. 10).
FIG. 9.
CFU from lungs of BALB/c mice after 45 days of infection with 106 cells of P. brasiliensis strain Pb18 and intravenous treatment with anti-gp70 MAbs (100 μg) or irrelevant MAb. *, significant (Ρ < 0.05) compared to controls. The error bars indicate standard errors.
FIG. 10.
Histopathology of lung sections of P. brasiliensis-infected BALB/c mice 45 days after infection. (A) Control mice show well-organized granulomas (arrows) and a high number of yeast cells (magnification, ×100). (B) Granulomatous lesion from control group showing intact fungi and typical macrophages and epithelial cells (magnification, ×400). (C) Mice treated with MAb B7D6 show smaller, well-organized granulomas (arrows) with a high number of yeast cells (magnification, ×100). (D and E) Mice treated with MAb C5F11 (D) and mice treated concomitantly with both MAbs C5F11 and B7D6 (E) show absence of both yeast cells and granulomas (magnification, ×400). (F) Nongranulomatous lung from both MAb-treated mice, predominantly composed of a mononuclear infiltrate in which fungi are not seen (magnification, ×100).
The histopathology and CFU of livers and spleens of all treated mice, including control animals, did not show the presence of fungus (data not shown).
DISCUSSION
The agent of PCM, P. brasiliensis, like many other eukaryotic parasites, expresses several antigenic molecules that may be recognized by antibodies produced by human patients or raised in immunized laboratory animals. The chemical natures of these components and their participation in the pathophysiology of the fungus are poorly known, since very few molecules have been isolated in pure form and subsequently characterized.
gp43 is the main purified diagnostic specific antigen when used in liquid phase serologic tests (6), since it is recognized by 100% of PCM patients' sera (49, 50). MAbs against gp43 have been used in a capture immunoassay for the specific detection of antibodies in patients with PCM (11). gp43 has been shown to bind laminin, a protein component of the extracellular matrix of mammalian tissues, and laminin-coated yeasts have increased ability to invade and destroy tissues. Based on these data, gp43 on the surfaces of yeast cells has been proposed to act as a laminin receptor (52). A T-cell epitope in gp43 has been mapped to a 15-residue sequence, and immunization with the corresponding oligopeptide elicited a protective response against virulent P. brasiliensis (47). Furthermore, DNA-based vaccination using the gp43 gene led to protective immunity against P. brasiliensis in BALB/c mice (40). It has recently been demonstrated in our laboratory that gp43 down regulates mouse peritoneal-macrophage functions, such as phagocytosis, via mannose receptors, liberation of free radicals, and the microbicidal activity of these cells for P. brasiliensis (21).
In addition to gp43, another glycoprotein, gp70, is recognized by 96% of the sera from untreated patients with PCM (12). Although it cross-reacts with sera from patients with other mycoses by ELISA, it seems to be a valuable marker of active PCM in immunoblotting reactions. gp70 and gp43 induce lymphoproliferative responses when tested with lymphocytes from PCM patients (3), and gp70 is also detected, along with gp43, in the urine of patients (44). Otherwise, little is known about the 70-kDa glycoprotein, and the purpose of the present work was to attempt to better understand its role in PCM.
We have produced and characterized six MAbs against gp70, three of which had their isotypes established as IgG1(κ) and the others as IgM(κ). We noticed that the IgG1 MAb C5F11 reacted with peptide sequences of gp70, while the IgG1 MAb B7D6 could be inhibited by carbohydrates. Immunoblotting assays revealed that all MAbs reacted with gp70 and in EIAs, and only one of the MAbs (2B8A6D2) did not recognize gp70. This antibody probably binds to different configurations of the glycoprotein, although some variations in sensitivity may depend on the assay used.
gp70 was successfully purified from exoantigen, as seen by silver-stained SDS-PAGE or periodic acid-Schiff staining. We also observed that gp70 has only one isoform, with an acid pI of 3.4, thus differing from gp43, which has several isoforms with basic pIs ranging from 6.85 to 5.8. Confocal microscopy analysis showed that gp70 is predominantly located in the intracellular compartment of the fungus, although a small amount was also detected in the culture supernatant. The same epitope of gp70 could be detected in both live fungi and purified protein, showing that despite being mainly a cytoplasmic protein, it is also expressed on the fungus surface (data not shown). Use of purified antigens led to an increased titer of antibodies, as detected by ELISA in hybridoma culture supernatants and mouse ascites fluid, suggesting that their use in serologic tests could increase the sensitivity for antibody detection, and thus, that gp70 could represent a possible target for immunodiagnostic studies.
These MAbs could also be useful for the detection of the epitopes involved in the interaction of the antigen with cells and components of the biological system being studied. The main targets of this investigation were macrophages, the first line of the host's defense. The mononuclear phagocytic system constitutes an important effector mechanism in the natural and adaptive immune responses against several pathogens. It has been suggested that macrophages play a fundamental role in resistance to P. brasiliensis (31). This function depends upon their state of activation, which leads to microbicidal activity.
Previous studies have demonstrated that murine peritoneal cells activated by IFN-γ exert a fungicidal effect on both the yeast and conidial forms of P. brasiliensis (5, 14, 15). Based on these findings, in this study we tested the effect of gp70 on the phagocytic ability of mouse peritoneal macrophages. It was demonstrated that purified gp70, at from 25 to 100 μg/ml, was able to inhibit phagocytosis of zymosan particles in a dose-dependent fashion. This inhibition probably occurred because the predominant components of gp70 are polysaccharides, which bind to mannose receptors on the surfaces of macrophages, thus blocking the binding of zymosan particles and consequently their phagocytosis. gp43 has a similar effect on the inhibition of phagocytosis of zymosan particles by macrophages (21).
We also observed that gp70 inhibited phagocytosis of opsonized sheep red blood cells, although not in a dose-dependent manner. This result supports the notion that gp70 inhibits the activity of phagocytic macrophages not only through the mannose receptor but also through the Fc receptor, in which it differs from gp43, which does not have the latter effect (21).
Macrophages activated by IFN-γ, tumor necrosis factor alpha, or LPS produce two kinds of reactive products that characterize their cytotoxic activity: reactive oxygen intermediates and reactive nitrogen intermediates. Nitric oxide, one of the most important reactive nitrogen intermediates, is responsible for the cytotoxic effect exerted on a variety of microorganisms, including parasites such as Schistosoma mansoni (30), and several fungi, such as C. neoformans (22) and H. capsulatum (33, 37). Hydrogen peroxide is one of the most important reactive oxygen intermediates. Since gp70 inhibits phagocytosis, we analyzed the effect of this glycoprotein in the liberation of these products, and we noticed that, like gp43, the 70-kDa glycoprotein decreases the release of both NO and H2O2 by macrophages (21).
The use of vaccines that stimulate humoral or cellular immunity can theoretically elicit protection against fungal pathogens. There is conclusive evidence that humoral immunity can modify the course of infection of some pathogenic fungi. C. neoformans has a polysaccharide capsule which is essential for its virulence, and MAbs reactive against it can protect against experimental cryptococcosis (19, 23, 36). It was also demonstrated that an IgG3 MAb was not protective in various mouse models of cryptococcal infection, while an IgG1 MAb significantly prolonged survival (39, 54). However, when the nonprotective IgG3 MAb was switched to IgG1, an increase of antibody protective efficacy occurred.
For another fungus, C. albicans, antibodies to cell wall polysaccharides were protective against experimental disseminated candidiasis (25, 28). Also, it has been shown that the efficiency of antibody protection depends on epitope specificity (26). These studies demonstrate a complex relationship among the efficacy of antibody protection, epitope specificity, and isotype.
In this paper, we analyzed the effect of passive immunization of mice infected with P. brasiliensis using the generated anti-gp70 IgG1 MAbs. A significant reduction in the number of CFU in the lungs was observed when MAbs C5F11 and B7D6 were administered concomitantly, thus confirming results seen with CFU. Histopathology of lung sections showed improvement of the infection with a reduction in the number of fungus particles and granulomas within the organ.
Despite gp43 being considered the main antigenic component of P. brasiliensis, the effect of passive immunization using the anti-gp43 IgG2a MAbs did not produce similar results (unpublished data).
The nature of the mechanisms which underlie the abortion of lung infection in mice treated with MAbs directed to gp70 epitopes is a complex issue. Nevertheless, two non-mutually exclusive hypotheses could be put forward. First, antibodies could be reacting with gp70 on the fungus surface, thus facilitating phagocytosis and killing of the fungal particles. Second, by neutralizing the macrophage-down-regulatory effect of the protein, macrophages could be free to eliminate the parasite. In both proposals, these mechanisms would work in the beginning of the infection, since we are far from understanding the mechanisms which determine the progression of infection in PCM and other types of infectious diseases. Negative results obtained with MAbs directed to gp43 could be due to the existing isoforms of the molecule, impairment in the neutralization of its effects, or opsonization of the fungal particles.
Antibodies have been considered the hallmarks of a more severe disease in PCM, while protection has always been attributed to cellular immune response. Here, it is shown for the first time that antibodies by themselves can abrogate establishment and development of the disease. Although these mechanisms have not yet been discovered, it is possible to suppose that vaccination with gp70 or its active fragments could provide an armamentarium, not yet available, to control the incidence of PCM in areas of endemicity.
Acknowledgments
This work was supported by grants from Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) and from Conselho Nacional de Pesquisa e Desenvolvimento Científico e Tecnológico (CNPq).
We are grateful to Renato A. Mortara for guidance during the execution of confocal microscopy and to Creuza Rosa de Oliveira and Geowá Pereira dos Santos for technical assistance.
Editor: T. R. Kozel
REFERENCES
- 1.Beaman, L. 1987. Fungicidal activation of murine macrophages by recombinant gamma interferon. Infect. Immun. 55:2951-2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beaman, L., E. Benjamini, and D. Pappagianis. 1983. Activation of macrophages by lymphokines: enhancement of phagosome-lysosome fusion and killing ofCoccidioides immitis. Infect. Immun. 39:1201-1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benard, G., M. J. Mendes-Giannini, M. Juvenale, E. T. Miranda, and A. J. Duarte. 1997. Immunosuppression in paracoccidioidomycosis: T cell hyporesponsiveness to two Paracoccidioides brasiliensis glycoproteins that elicit strong humoral immune response. J. Infect. Dis. 175:1263-1267. [DOI] [PubMed] [Google Scholar]
- 4.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 5.Brummer, E. 1994. Interaction of Paracoccidiodes brasiliensis with host defense cells, p. 213-223. In M. Franco, C. S. Lacaz, A. M. Restrepo, and G. Del Negro (ed.), Paracoccidioidomycosis. CRC Press, Boca Raton, Fla.
- 6.Brummer, E., E. Castaneda, and A. Restrepo. 1993. Paracoccidioidomycosis: an update. Clin. Microbiol. Rev. 6:89-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brummer, E., L. H. Hanson, A. Restrepo, and D. A. Stevens. 1988. In vivo and in vitro activation of pulmonary macrophages by IFN-gamma for enhanced killing of Paracoccidioides brasiliensis or Blastomyces dermatitidis. J. Immunol. 140:2786-2789. [PubMed] [Google Scholar]
- 8.Brummer, E., L. H. Hanson, A. Restrepo, and D. A. Stevens. 1989. Intracellular multiplication of Paracoccidioides brasiliensis in macrophages: killing and restriction of multiplication by activated macrophages. Infect. Immun. 57:2289-2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brummer, E., L. H. Hanson, and D. A. Stevens. 1988. Gamma-interferon activation of macrophages for killing of Paracoccidioides brasiliensis and evidence for nonoxidative mechanisms. Int. J. Immunopharmacol. 10:945-952. [DOI] [PubMed] [Google Scholar]
- 10.Brummer, E., C. J. Morrison, and D. A. Stevens. 1985. Recombinant and natural gamma-interferon activation of macrophages in vitro: different dose requirements for induction of killing activity against phagocytizable and nonphagocytizable fungi. Infect. Immun. 49:724-730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Camargo, Z. P., J. L. Gesztesi, E. C. Saraiva, C. P. Taborda, A. P. Vicentini, and J. D. Lopes. 1994. Monoclonal antibody capture enzyme immunoassay for detection of Paracoccidioides brasiliensis antibodies in paracoccidioidomycosis. J. Clin. Microbiol. 32:2377-2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Camargo, Z. P., C. Unterkircher, and L. R. Travassos. 1989. Identification of antigenic polypeptides of Paracoccidioides brasiliensis by immunoblotting. J. Med. Vet. Mycol. 27:407-412. [PubMed] [Google Scholar]
- 13.Camargo, Z. P., L. E. Cano. 1994. Humoral immunity, p. 187-201. In M. Franco, C. S. Lacaz, A. M. Restrepo, and G. Del Negro (ed.), Paracoccidioidomycosis. CRC Press, Boca Raton, Fla.
- 14.Cano, L. E., E. Brummer, D. A. Stevens, and A. Restrepo. 1992. Fate of conidia of Paracoccidioides brasiliensis after ingestion by resident macrophages or cytokine-treated macrophages. Infect. Immun. 60:2096-2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cano, L. E., B. Gomez, E. Brummer, A. Restrepo, and D. A. Stevens. 1994. Inhibitory effect of deferoxamine or macrophage activation on transformation of Paracoccidioides brasiliensis conidia ingested by macrophages: reversal by holotransferrin. Infect. Immun. 62:1494-1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cano, L. E., L. M. Singer-Vermes, C. A. Vaz, M. Russo, and V. L. Calich. 1995. Pulmonary paracoccidioidomycosis in resistant and susceptible mice: relationship among progression of infection, bronchoalveolar cell activation, cellular immune response, and specific isotype patterns. Infect. Immun. 63:1777-1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Casadevall, A. 1995. Antibody immunity and invasive fungal infections. Infect. Immun. 63:4211-4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Devi, S. J. 1996. Preclinical efficacy of a glucuronoxylomannan-tetanus toxoid conjugate vaccine of Cryptococcus neoformans in a murine model. Vaccine 14:841-844. [DOI] [PubMed] [Google Scholar]
- 19.Dromer, F., J. Charreire, A. Contrepois, C. Carbon, and P. Yeni. 1987. Protection of mice against experimental cryptococcosis by anti-Cryptococcus neoformans monoclonal antibody. Infect. Immun. 55:749-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feldmesser, M., and A. Casadevall. 1997. Effect of serum IgG1 to Cryptococcus neoformans glucuronoxylomannan on murine pulmonary infection. J. Immunol. 158:790-799. [PubMed] [Google Scholar]
- 21.Flavia Popi, A. F., J. D. Lopes, and M. Mariano. 2002. GP43 from Paracoccidioides brasiliensis inhibits macrophage functions. An evasion mechanism of the fungus. Cell Immunol. 218:87-94. [DOI] [PubMed] [Google Scholar]
- 22.Granger, D. L., J. B. Hibbs, Jr., J. R. Perfect, and D. T. Durack. 1990. Metabolic fate of l-arginine in relation to microbiostatic capability of murine macrophages. J. Clin. Investig. 85:264-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Graybill, J. R., M. Hague, and D. J. Drutz. 1981. Passive immunization in murine cryptococcosis. Sabouraudia 19:237-244. [DOI] [PubMed] [Google Scholar]
- 24.Greenlee, J. E., and P. M. Keeney. 1982. Immunofluorescent labelling of K-papovavirus antigens in glycol methacrylate embedded material: a method for studying infected cell populations by fluorescence microscopy and histological staining of adjacent sections. Stain Technol. 57:197-205. [DOI] [PubMed] [Google Scholar]
- 25.Han, Y., and J. E. Cutler. 1995. Antibody response that protects against disseminated candidiasis. Infect. Immun. 63:2714-2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Han, Y., T. Kanbe, R. Cherniak, and J. E. Cutler. 1997. Biochemical characterization of Candida albicans epitopes that can elicit protective and nonprotective antibodies. Infect. Immun. 65:4100-4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Han, Y., R. P. Morrison, and J. E. Cutler. 1998. A vaccine and monoclonal antibodies that enhance mouse resistance to Candida albicans vaginal infection. Infect. Immun. 66:5771-5776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Han, Y., M. H. Riesselman, and J. E. Cutler. 2000. Protection against candidiasis by an immunoglobulin G3 (IgG3) monoclonal antibody specific for the same mannotriose as an IgM protective antibody. Infect. Immun. 68:1649-1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han, Y., M. A. Ulrich, and J. E. Cutler. 1999. Candida albicans mannan extract-protein conjugates induce a protective immune response against experimental candidiasis. J. Infect. Dis. 179:1477-1484. [DOI] [PubMed] [Google Scholar]
- 30.James, S. L., and J. Glaven. 1989. Macrophage cytotoxicity against schistosomula of Schistosoma mansoni involves arginine-dependent production of reactive nitrogen intermediates. J. Immunol. 143:4208-4212. [PubMed] [Google Scholar]
- 31.Kashino, S. S., A. Fazioli Rdos, M. Moscardi-Bacchi, M. Franco, L. M. Singer-Vermes, E. Burger, and V. L. Calich. 1995. Effect of macrophage blockade on the resistance of inbred mice to Paracoccidioides brasiliensis infection. Mycopathologia 130:131-140. [DOI] [PubMed] [Google Scholar]
- 32.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 33.Lane, T. E., G. C. Otero, B. A. Wu-Hsieh, and D. H. Howard. 1994. Expression of inducible nitric oxide synthase by stimulated macrophages correlates with their antihistoplasma activity. Infect. Immun. 62:1478-1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lopes, J. D., and M. J. M. Alves. 1983. Production of monoclonal antibodies by somatic cells hybridization, p. 386-398. In C. M. Moreal (ed.), Genes and parasites. A laboratory manual. Rio de Janeiro, Brazil.
- 35.McEwen, J. G., V. Bedoya, M. M. Patino, M. E. Salazar, and A. Restrepo. 1987. Experimental murine paracoccidioidomycosis induced by the inhalation of conidia. J. Med. Vet. Mycol. 25:165-175. [DOI] [PubMed] [Google Scholar]
- 36.Mukherjee, J., M. D. Scharff, and A. Casadevall. 1992. Protective murine monoclonal antibodies to Cryptococcus neoformans. Infect. Immun. 60:4534-4541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakamura, L. T., B. A. Wu-Hsieh, and D. H. Howard. 1994. Recombinant murine gamma interferon stimulates macrophages of the RAW cell line to inhibit intracellular growth of Histoplasma capsulatum. Infect. Immun. 62:680-684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Negroni, R. 1993. Paracoccidioidomycosis (South American blastomycosis, Lutz's mycosis). Int. J. Dermatol. 32:847-859. [DOI] [PubMed] [Google Scholar]
- 39.Nussbaum, G., R. Yuan, A. Casadevall, and M. D. Scharff. 1996. Immunoglobulin G3 blocking antibodies to the fungal pathogen Cryptococcus neoformans. J. Exp. Med. 183:1905-1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pinto, A. R., R. Puccia, S. N. Diniz, M. F. Franco, and L. R. Travassos. 2000. DNA-based vaccination against murine paracoccidioidomycosis using the gp43 gene from Paracoccidioides brasiliensis. Vaccine 18:3050-3058. [DOI] [PubMed] [Google Scholar]
- 41.Puccia, R., S. Schenkman, P. A. Gorin, and L. R. Travassos. 1986. Exocellular components of Paracoccidioides brasiliensis: identification of a specific antigen. Infect. Immun. 53:199-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Puccia, R., and L. R. Travassos. 1991. 43-kilodalton glycoprotein from Paracoccidioides brasiliensis: immunochemical reactions with sera from patients with paracoccidioidomycosis, histoplasmosis, or Jorge Lobo's disease. J. Clin. Microbiol. 29:1610-1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Restrepo, A. 1997. Fungal interactions, p. 125-133. In D. A. Stevens (ed.), Hormonal influences in the host-interplay with Paracoc cidioides brasiliensis. National Foundation for Infectious Disease, Columbia.
- 44.Salina, M. A., M. A. Shikanai-Yasuda, R. P. Mendes, B. Barraviera, and M. J. Mendes Giannini. 1998. Detection of circulating Paracoccidioides brasiliensis antigen in urine of paracoccidioidomycosis patients before and during treatment. J. Clin. Microbiol. 36:1723-1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.San-Blas, G., A. Restrepo, K. Clemons, D. A. Stevens, F. San-Blas, R. Puccia, L. R. Travassos, J. I. Figueroa, A. J. Hamilton, M. A. Bartholomew, et al. 1992. Paracoccidioidomycosis. J. Med. Vet. Mycol. 30(Suppl. 1):59-71. [DOI] [PubMed] [Google Scholar]
- 46.Sanford, J. E., D. M. Lupan, A. M. Schlageter, and T. R. Kozel. 1990. Passive immunization against Cryptococcus neoformans with an isotype-switch family of monoclonal antibodies reactive with cryptococcal polysaccharide. Infect. Immun. 58:1919-1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Taborda, C. P., M. A. Juliano, R. Puccia, M. Franco, and L. R. Travassos. 1998. Mapping of the T-cell epitope in the major 43-kilodalton glycoprotein of Paracoccidioides brasiliensis which induces a Th-1 response protective against fungal infection in BALB/c mice. Infect. Immun. 66:786-793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Towbin, H., T. Staehelin, and J. Gordon. 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA 76:4350-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Travassos, L. R. 1994. Immunochemistry of Paracoccidioides brasiliensis antigens, p. 67-86. In M. Franco, C. S. Lacaz, A. M. Restrepo, and G. Del Negro (ed.), Paracoccidioidomycosis. CRC Press, Boca Raton, Fla.
- 50.Travassos, L. R., R. Puccia, P. Cisalpino, C. Taborda, E. G. Rodrigues, M. Rodrigues, J. F. Silveira, and I. C. Almeida. 1995. Biochemistry and molecular biology of the main diagnostic antigen of Paracoccidioides brasiliensis. Arch. Med. Res. 26:297-304. [PubMed] [Google Scholar]
- 51.Unterkircher, C. S. 1989. Antigenos de Paracoccidioides brasiliensis e sua aplicacao no diagnostico sorologico da Paracoccidioidomicose. Doutorado. Federal University of Sao Paulo, Sao Paulo, Brazil.
- 52.Vicentini, A. P., J. L. Gesztesi, M. F. Franco, W. de Souza, J. Z. de Moraes, L. R. Travassos, and J. D. Lopes. 1994. Binding of Paracoccidioides brasiliensis to laminin through surface glycoprotein gp43 leads to enhancement of fungal pathogenesis. Infect. Immun. 62:1465-1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu-Hsieh, B., A. Zlotnik, and D. H. Howard. 1984. T-cell hybridoma-produced lymphokine that activates macrophages to suppress intracellular growth of Histoplasma capsulatum. Infect. Immun. 43:380-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yuan, R., A. Casadevall, G. Spira, and M. D. Scharff. 1995. Isotype switching from IgG3 to IgG1 converts a nonprotective murine antibody to Cryptococcus neoformans into a protective antibody. J. Immunol. 154:1810-1816. [PubMed] [Google Scholar]