Abstract
The variation in sequence and length in the C-terminal region among members of the unique PE (Pro-Glu) and PPE (Pro-Pro-Glu) protein families of Mycobacterium tuberculosis is a likely source of antigenic variation, giving rise to the speculation that these protein families could be immunologically important. Based on in silico analysis, we selected a hypothetical open reading frame (ORF) encoding a protein belonging to the PPE family and having epitopes with predictably higher antigenic indexes. Reverse transcriptase PCR using total RNA extracted from in vitro-cultured M. tuberculosis H37Rv generated an mRNA product corresponding to this gene, indicating the expression of this ORF (Rv2430c) at the mRNA level. Recombinant protein expressed in Escherichia coli was used to screen the sera of M. tuberculosis-infected patients, as well as those of clinically healthy controls (n = 10), by enzyme-linked immunosorbent assay. The panel of patient sera comprised sera from fresh infection cases (category 1; n = 32), patients with relapsed tuberculosis (category 2; n = 30), and extrapulmonary cases (category 3; n = 30). Category 2 and 3 sera had strong antibody responses to the PPE antigen, equal to or higher than those to other well-known antigens, such as Hsp10 or purified protein derivative (PPD). However, a higher percentage of patients belonging to category 1, as opposed to clinically healthy controls, showed stronger antibody response against the PPE protein when probed with anti-immunoglobulin M (IgM) (71 versus 37.5%) or anti-IgG (62.5 versus 28.12%). Our results reveal that this PPE ORF induces a strong B-cell response compared to that generated by M. tuberculosis Hsp10 or PPD, pointing to the immunodominant nature of the protein.
About 10% of the genome of Mycobacterium tuberculosis codes for the PE and PPE families of proteins (7), which are glycine rich and are exclusive to M. tuberculosis. The 69 members of the PPE protein family have a conserved N-terminal domain that comprises ∼180 amino acids followed by C-terminal segments that vary markedly in sequence and length. These proteins fall into three groups, one of which constitutes the MPTR class characterized by the presence of multiple tandem copies of the motif Asn-X-Gly-X-Gly-Asn-X-Gly. The second subgroup contains a characteristic well-conserved motif, Gly-X-X-Ser-Val-Pro-X-X-Trp, around position 350. The proteins in the third group are unrelated except for the presence of the common PPE domain. The subcellular locations of a few PPE proteins are known (6, 25), and in only one case (7), that of a lipase (Rv3097), has a function been suggested. There are few studies supporting the notion that PE and PPE proteins could be of functional importance (7, 23). It is widely speculated that they could be responsible for generating antigenic variation (1, 4, 6, 8, 12, 27). However, the effects the PPE family proteins, unique in their protein sequences and possible structure, may have on the immune system have not been well documented. Furthermore, a qualitative and quantitative immune response to PPE proteins in a clinical setting has not been shown. Since 180 amino acid residues in the N-terminal regions of PPE proteins are conserved, it is interesting to speculate that the variation in sequence and length in the C-terminal region could represent a source of antigenic variability.
Based on in silico analysis and DNA microarray expression data (24), we selected an ORF, Rv2430c, whose product displays a high antigenic index and evaluated its importance in eliciting immune responses in a panel of human sera obtained from three well-classified categories of patients: (i) those reporting TB for the first time, (ii) those presenting with a relapse of TB, and (iii) those with extrapulmonary cases. Clinically healthy human sera were used as controls to compare the immunological responses to the protein. Enzyme-linked immunosorbent assay (ELISA) using the recombinant protein showed good specificity and sensitivity, suggesting that the product of this PPE family ORF, Rv2430c, induces a strong B-cell response in infected subjects.
MATERIALS AND METHODS
In silico analysis of Rv2430c.
In silico pattern search analysis of the PPE family was carried out to classify the products of the various ORFs into three subgroups. Products of ORFs with ≤200 amino acids and belonging to the third subgroup of the PPE family were further analyzed using protein analysis software (Protean version 4.0, Lasergene Navigator; DNASTAR Inc., Madison, Wis.) to calculate their antigenic indexes.
RNA extraction and reverse transcriptase (RT) PCR.
RNA was extracted from 109 H37Rv cells, cultured in vitro in Middlebrook 7H9 medium supplemented with albumin-dextrose complex according to the Qiaquick total RNA extraction kit (Qiagen Inc.) instructions, dissolved in 50 μl of nuclease-free water, and stored at −70°C until further use. First-strand synthesis was carried out using avian myeloblastosis virus reverse transcriptase. This was followed by heat denaturation to inactivate the enzyme. Subsequent second-strand synthesis was performed using Tfl polymerase. The PCR product was visualized by electrophoresis in a 1% agarose gel.
Expression and purification of the recombinant protein encoded by Rv2430c.
Genomic DNA of H37Rv was extracted using the Genome Extraction kit provided by Epicentre Technologies, as described earlier (26). The Rv2430c gene was PCR amplified from the genomic DNA of H37Rv using upstream (5′-GGATCCATGCATTTCGAAGCGTAC-3′) and downstream (5′-AAGCTTCTAAGTGTCTGTACGCGATGA-3′) primers. BamHI and HindIII sites were incorporated in the 5′ and 3′ ends of the primers, respectively. The purified fragment was ligated into the pGEMT-Easy vector (Promega Inc.), and the recombinant clone carrying the Rv2430c insert was confirmed by DNA sequencing (ABI Prism 377 DNA Sequencer; PE Biosystems). The insert was then subcloned as a BamHI and HindIII fragment into the PQE30 expression vector (Qiagen Inc.) to generate the plasmid construct PQERv2430c carrying Rv2430c as an N-terminal histidine-tagged fusion. PQERv2430c was transformed into the host M15pREP4 strain of Escherichia coli. A single colony of E. coli strain M15pREP4 harboring PQERv2430c was inoculated into 5 ml of Luria-Bertani broth with the appropriate antibiotics (100 μg of ampicillin/ml and 25 μg of kanamycin/ml) and grown overnight at 37°C with constant agitation; 100 μl of this overnight culture was inoculated into 5 ml of Luria-Bertani broth with the appropriate antibiotics and grown to an optical density at 590 nm of 0.6, at which time expression was induced with 1 mM IPTG. A separate aliquot of uninduced culture was kept as a control. Cells were harvested 3 h postinduction, suspended in 1× SDS sample buffer, and denatured by being heated at 100°C for 10 min. The recombinant protein was purified to homogeneity with the QIA Expressionist kit (Qiagen Inc.).
Serological characterization of the recombinant protein.
ELISAs were performed in 96-well microtiter plates (Corning Costar) coated with the recombinant Rv2430c protein. After overnight incubation at 4°C, the plates were washed three times with phosphate-buffered saline (PBS) buffer and blocked with 200 μl of blocking buffer (PBS containing 1% bovine serum albumin) for 1 h at 37°C. The plates were then washed three times with PBS-Tween wash buffer (0.05% Tween 20 in 1× PBS, pH 8.0) and incubated for 1 h at 37°C with human sera (1: 200 dilution in blocking buffer). The plates were washed with PBS-Tween and further incubated with either anti-human immunoglobulin G (IgG)-horseradish peroxidase (HRP) or anti-human IgM-HRP (Sigma). HRP activity was detected using a chromogenic substance, o-phenylenediamine tetrahydrochloride (Sigma), in citrate-phosphate buffer (pH 5.4) and 1 μl of H2O2 (Qualigens, India)/ml. The reactions were terminated using 1 N H2SO4, and the absorbance values were measured at 492 nm in an ELISA reader (Bio-Rad).
Study population.
Serum samples were obtained from 92 TB patients who had reported to the Mahavir Hospital and Research Centre, Hyderabad, India, and 10 clinically healthy donors. The 92 patients belonged to three well-defined categories. Category 1 (n = 32) comprised patients who had contracted the pathogen for the first time and had no history of TB treatment. Category 2 (n = 30) comprised patients with relapsed TB, i.e., who were treated earlier for TB but the symptoms resurfaced after completion of the treatment. Category 3 (n = 30) comprised patients with extrapulmonary TB in which the disease was confirmed by tissue biopsy. In the cases of category 1 and category 2 patients, diagnosis was confirmed by examination of the sputum (acid-fast bacillus smear positive). Clinically healthy donors were vaccinated with Mycobacterium bovis BCG. The study was carried out after approval from the Institute Bioethics Committee.
Statistical analysis.
Student's t test was used for analysis of statistical significance (P value). Graphpad Quickcalcs (online t test calculator [http://www.graphpad.com/quickcalcs/ttest1.cfm]) was used for this purpose.
RESULTS
The product of the hypothetical PPE ORF Rv2430c has a high antigenicity profile score and is expressed at the mRNA level.
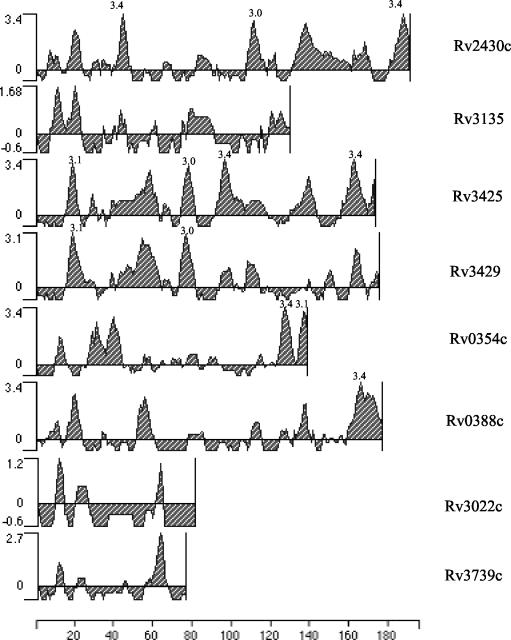
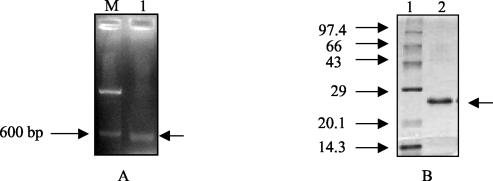
In silico analyses of the PPE ORF products, ≤200 amino acids in length, belonging to subgroup 3 of the PPE family (7) were carried out. Rv2430c and Rv3425 displayed major antigenic stretches (Fig. 1) with peak values of ≥3.0. However, an analysis of the microarray expression data (24) identified Rv2430c as one of the overexpressed genes in an IdeR mutant of M. tuberculosis, and it was thereby implicated in pathogenesis. Rv2430c was thus selected for our study. Rv2430c was further subjected to detailed analysis to predict its likely structure through various algorithms. The Predict Protein server (http://www.embl-heidelberg.de/predictprotein/) gave a very low score for Rv2430c, pointing to the unlikely possibility that this ORF encoded a transmembrane protein (data not shown). To check whether the hypothetical Rv2430c indeed represented a functional gene, the mRNA extracted from in vitro-cultured H37Rv cells was used as a template for reverse transcription followed by PCR. The RT-PCR product was fractionated on a 1% agarose gel. A 597-bp band was observed upon staining with ethidium bromide, indicating that the ORF was expressed at the mRNA level in the liquid cultures of M. tuberculosis (Fig. 2A). The ORF Rv2430c was expressed as a His-tagged fusion protein in E. coli (Fig. 2B) and used for immunological studies.
FIG. 1.
In silico analysis of PPE ORFs. Only the ORFs belonging to subgroup 3 and encoding products ≤200 amino acids in length were subjected to analysis. The presence of stretches of high antigenic index are shown. The x axis indicates the ORF product length in amino acids, while the y axis represents the antigenic index.
FIG. 2.
(A) Transcription of hypothetical PPE ORF Rv2430c. RNA extracted from the virulent H37Rv laboratory strain of M. tuberculosis was used in an RT-PCR. A 597-bp RT-PCR product was observed after electrophoresis in a 1% agarose gel (lane 1). Lane M is the 100-bp DNA molecular size marker run alongside. The arrow on the right indicates the position of the 597-bp PCR product. (B) Expression and purification of the recombinant PPE protein. The recombinant protein was expressed in strain M15pREP4 of E. coli and was purified to homogeneity using the NiNTA protein purification kit. Lane 1, marker; lane 2, purified protein. The arrow on the right indicates the position of the 23-kDa protein.
The recombinant PPE protein Rv2430c displays strong B-cell responses during infection with TB.
Having shown that the ORF encoding RV2430c of M. tuberculosis was expressed at the mRNA level, experiments were designed to evaluate the immune response of TB patients to recombinant Rv2430c PPE protein. For this, the recombinant Rv2430c protein was used to screen the TB patient sera by ELISA, using anti-human IgG-HRP and anti-human IgM-HRP as conjugates. The humoral immune responses directed against the recombinant protein by patients with TB and healthy controls were compared. The data (Fig. 3) reveal that the sera of all the infected patients mounted significantly higher antibody responses against Rv2430c than those of the healthy controls (P < 0.0001). Since negligible antibody responses were obtained in the healthy-control group (Fig. 3), it is likely that this protein is expressed during the course of M. tuberculosis infection, and it may be associated with disease manifestation and progression.
FIG. 3.
The recombinant Rv2430c PPE protein elicits strong antibody responses in M. tuberculosis-infected patients as opposed to healthy controls. ELISA reactivities of IgG anti-Rv2430c antibodies were assayed in sera of either M. tuberculosis-infected patients or healthy controls (P < 0.0001). Symbols, patient population.
Immunodominant nature of Rv2430c.
Since all the patients infected with TB revealed strong humoral responses against Rv2430c compared to the healthy controls, it was of interest to compare the antibody responses in various clinical categories and also to evaluate whether Rv2430c is immunodominant. For this, we chose recombinant Hsp10 of M. tuberculosis, which is a well-known immunodominant antigen of M. tuberculosis (28). Figure 4 clearly shows that strong antibody responses were elicited against Rv2430c in all three study groups (category 1, category 2, and category 3). Rv2430c elicited statistically significant immune responses compared to Hsp10 (P < 0.003) in patients with fresh infections (Fig. 4A). Patients belonging to categories 2 and 3 exhibited Rv2430c-specific antibody equivalent to that for Hsp10 (Fig. 4B and C). Since the PPD antigen of M. tuberculosis is also used to diagnose TB infection (2, 15, 29), we compared the immunopotentiality of Rv2430c over PPD only in cases of fresh infection (Fig. 4A). The results clearly indicated that the PPE protein Rv2430c is far more immunogenic and could elicit a stronger B-cell response than PPD. Sera from the fresh-infection category responded better against the Rv2430c antigen than against PPD (P < 0.0001) (Fig. 4A).
FIG. 4.
PPE Rv2430c protein shows strong reactivities to sera from all three patient categories. Reactivities to both recombinant Rv2430c and Hsp10 of M. tuberculosis in the three categories of patients were estimated by ELISA. The patients belonging to categories 2 (B) and 3 (C) displayed similar antibody responses to both antigens. However, the antibody responses of category 1 patients (A) to Rv2430c were higher than those to Hsp10 (P < 0.003) or PPD (P < 0.0001).
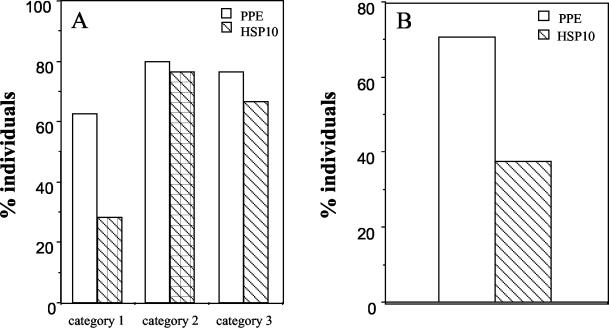
In order to compare the serological sensitivities of Rv2430c and Hsp10, the data presented in Fig. 4 were recalculated as percentages of individuals showing absorbances above 0.65 at 492 nm. Although only 28.12% of individuals with fresh infections mounted strong antibody responses of the IgG type to Hsp10, a very high percentage of individuals (62.5%) recognized Rv2430c (Fig. 5A). Similar conclusions could be drawn when IgM antibodies were assayed (Rv2430c versus Hsp10, 71 versus 37.5%) (Fig. 5B). From these results, it is apparent that Rv2430c shows better reactivity vis-a-vis Hsp10 to sera from patients with fresh infection than to those of relapsed or extrapulmonary-TB patients. Our results, therefore, convincingly demonstrate the immunodominant nature of the product of the hypothetical PPE ORF Rv2430c.
FIG. 5.
Serological sensitivities of PPE Rv2430c and Hsp10 as a function of percentages of individuals. (A) The results shown in Fig. 4 were recalculated as percentages of individuals showing A492 values of >0.65. The anti-IgG responses against Rv2430c or Hsp10 were compared for all three categories of patients studied. A higher percentage of individuals belonging to category 1 showed stronger reactivity to Rv2430c than to Hsp10, but for the other two categories, the values were comparable. (B) Percentage of individuals in category 1 showing anti-IgM antibody A492 of >0.5. A higher percentage of individuals showed anti-IgM antibody A492 of >0.5 against Rv2430c than against Hsp10.
DISCUSSION
The 69 members of the PPE protein family have a conserved N-terminal domain that comprises ∼180 amino acids followed by C-terminal segments that vary markedly in sequence and length (7). Based on our pattern search analysis of the TubercuList database (http://genolist.pasteur.fr/TubercuList/), these proteins were categorized into three groups. Subgroup 1, represented by 20 members, constitutes the MPTR class characterized by the presence of multiple tandem copies of the motif Asn-X-Gly-X-Gly-Asn-X-Gly. The second subgroup, comprising 21 members, contains a characteristic well-conserved motif, Gly-X-X-Ser-Val-Pro-X-X-Trp, around position 350. The proteins of the third subgroup, with 28 members, are unrelated except for the presence of the common PPE domain. That ORF products across the subgroup do not react immunologically was evident from our studies with Rv2608 (unpublished data). Rv2430c belongs to the third subgroup. ORFs belonging to the third subgroup with coding capacities of ≤200 amino acids were shortlisted. This shortlist was further narrowed down based on two very important criteria, namely, the antigenic profile and the association of the ORF with pathological conditions as evident from DNA microarray expression data (24). Rv2430c and Rv3425 were the ORFs with the highest antigenicity indexes. DNA microarray results demonstrated that of these two ORFs, Rv2430c was one of the genes induced in an IdeR mutant of M. tuberculosis (24), pointing to its possible role in pathogenesis. Rv2430c was accordingly shortlisted and evaluated for the role of its product as an antigen in a clinical setting.
The ORF was shown to be RT-PCR positive, pointing to the likelihood that it is expressed during infection. The Rv2430c ORF was expressed in E. coli, and the recombinant protein was purified and tested for its ability to recognize IgG antibody in the sera of TB patients and healthy individuals.
The TB patients used in our study represent a heterogeneous population, including fresh infection cases characterized by patients who contracted the pathogen for the first time (category 1); relapsed cases, in which the disease resurfaced after the completion of treatment (category 2); and extrapulmonary cases, which are mostly sputum negative (category 3). The immune response profiles of Rv2430c in different clinical categories were studied. The PPE protein Rv2430c was found to be recognized by antibodies in the sera of infected patients in ELISA with a serum dilution of 1:200, whereas poorer ELISA reactivity was observed for the sera of all of the healthy individuals. The presence of antibodies to Rv2430c in sera from TB patients (Fig. 3) and their absence in sera from healthy individuals suggests that the protein is expressed in vivo during active infection with M. tuberculosis and that the native molecule is immunogenic.
Several reports have emphasized the observation of a lack of sufficient immune responses in TB patients against many promising serodiagnostic antigens of M. tuberculosis. This fact is more distressing in cases of fresh infection, where in a majority of the cases the immune system is not sufficiently primed to elicit a strong antibody response against most of the M. tuberculosis antigens. The recombinant Rv2430c protein was very strongly recognized by all three categories of patients, including the fresh-infection group (category 1). The members of the heat shock protein family, including Hsp70 (20, 28) and Hsp10 (28), have been known to elicit a strong B-cell response. Surprisingly, in our study, the immunodominant antigen Hsp10, though recognized by category 2 and 3 patient sera, was not sensitive enough to detect the patients with fresh infections (category 1). The picture remained unaltered when we used PPD in place of Hsp10. It is pertinent to note that although several antigens have been tested for use in serodiagnosis, no test with a single antigen has proved able to achieve sensitivity and specificity in a study population that is suitably large and heterogeneous (3, 5, 9, 11, 13, 14, 16, 17, 18, 19, 21, 22). The factors responsible for this include (i) the stage of the disease, (ii) the location of the infection, and (iii) the genetic background. Our results show that the PPE protein Rv2430c, which lacks a transmembrane domain and is therefore likely to be cytosolic or secretory in localization, is an immunodominant B-cell target antigen with apparent diagnostic potential. It is also interesting to speculate on the use of Rv2430c, along with other immunodominant antigens (10), for vaccine development.
Acknowledgments
Recombinant Hsp10 was a kind gift from S. C Mande, Structural Biology Laboratory, CDFD. We thank the National Mycobacterial Repository, Central Jalma Institute for Leprosy, Agra, India, for providing us with liquid and Lowenstein-Jensen slant cultures of H37Rv.
This study was supported by research grants from the Department of Biotechnology, Government of India, to S.E.H. R.K.C and P.C. thank the Council of Scientific and Industrial Research, Government of India, for the award of Senior Research Fellowships.
Editor: J. M. Mansfield
REFERENCES
- 1.Abou-Zeid, C., T. Garbe, R. Lathigra, H. G. Wiker, M. Harboe, G. A. Rook, and D. B. Young. 1991. Genetic and immunological analysis of Mycobacterium tuberculosis fibronectin-binding proteins. Infect. Immun. 59:2712-2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Almeida, L. M., M. A. Barbieri, A. C. Da Paixao, and L. E. Cuevas. 2001. Use of purified protein derivative to assess the risk of infection in children in close contact with adults with tuberculosis in a population with high Calmette-Guerin bacillus coverage. Pediatr. Infect. Dis. J. 20:1061-1065. [DOI] [PubMed] [Google Scholar]
- 3.Amara, R. R., S. Shanti, and V. Satchidanandam. 1998. Characterization of novel immunodominant antigens of Mycobacterium tuberculosis. Microbiology 144:1197-1203. [DOI] [PubMed] [Google Scholar]
- 4.Banu, S., N. Honore, B. Saint-Joanis, D. Philpott, M. C. Prevost, and S. T. Cole. 2002. Are the PE-PGRS proteins of Mycobacterium tuberculosis variable surface antigens? Mol. Microbiol. 44:9-19. [DOI] [PubMed] [Google Scholar]
- 5.Batoni, G., D. Bottai, S. Esin, W. Florio, M. Pardini, G. Maisetta, G. Freer, S. Senesi, and M. Campa. 2002. Purification, biochemical characterization and immunogenicity of SA5K, a secretion antigen of Mycobacterium tuberculosis. Scand. J. Immunol. 56:43-51. [DOI] [PubMed] [Google Scholar]
- 6.Brennan, M. J., G. Delogu, Y. Chen, S. Bardarov, J. Kriakov, M. Alavi, and W. R. Jacobs, Jr. 2001. Evidence that mycobacterial PE_PGRS proteins are cell surface constituents that influence interactions with other cells. Infect. Immun. 69:7326-7333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cole, S. T., et al. 1998. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393:537-544. [DOI] [PubMed] [Google Scholar]
- 8.Delogu, G., and M. J. Brennan. 2001. Comparative immune response to PE and PE_PGRS antigens of Mycobacterium tuberculosis. Infect. Immun. 69:5606-5611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Devi, K. R., K. S. Kumar, B. Ramalingam, and R. Alamelu. 2002. Purification and characterization of three immunodominant proteins (38, 30, and 16 kDa) of Mycobacterium tuberculosis. Protein Expr. Purif. 24:188-195. [DOI] [PubMed] [Google Scholar]
- 10.Dhar, N., V. Rao, and A. K. Tyagi. 2000. Recombinant BCG approach for development of vaccines: cloning and expression of immunodominant antigens of Mycobacterium tuberculosis. FEMS Microbiol. Lett. 190:309-316. [DOI] [PubMed] [Google Scholar]
- 11.Dillon, D. C., M. R. Alderson, C. H. Day, T. Bement, A. Campos-Neto, Y. A. Skeiky, T. Vedvick, R. Badaro, S. G. Reed, and R. L. Houghton. 2000. Molecular and immunological characterization of Mycobacterium tuberculosis CFP-10, an immunodiagnostic antigen missing in Mycobacterium bovis BCG. J. Clin. Microbiol. 38:3285-3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Espitia, C., J. P. Laclette, M. Mondragon-Palomino, A. Amador, J. Campuzano, A. Martens, M. Singh, R. Cicero, Y. Zhang, and C. Moreno. 1999. The PE-PGRS glycine-rich proteins of Mycobacterium tuberculosis: a new family of fibronectin-binding proteins? Microbiology 145:3487-3495. [DOI] [PubMed] [Google Scholar]
- 13.Florio, W., D. Bottai, G. Batoni, S. Esin, M. Pardini, G. Maisetta, and M. Campa. 2002. Identification, molecular cloning, and evaluation of potential use of isocitrate dehydrogenase II of Mycobacterium bovis BCG in serodiagnosis of tuberculosis. Clin. Diagn. Lab. Immunol. 9:846-851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Houghton, R. L., M. J. Lodes, D. C. Dillon, L. D. Reynolds, C. H. Day, P. D. McNeill, R. C. Hendrickson, Y. A. Skeiky, D. P. Sampaio, R. Badaro, K. P. Lyashchenko, and S. G. Reed. 2002. Use of multiepitope polyproteins in serodiagnosis of active tuberculosis. Clin. Diagn. Lab. Immunol. 9:883-891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kalish, S. B., R. C. Radin, J. P. Phair, D. Levitz, C. R. Zeiss, and E. Metzger. 1983. Use of an enzyme-linked immunosorbent assay technique in the differential diagnosis of active pulmonary tuberculosis in humans. J. Infect. Dis. 147:523-530. [DOI] [PubMed] [Google Scholar]
- 16.Laal, S., K. M. Samanich, M. G. Sonnenberg, S. Zolla-Pazner, J. M. Phadtare, and J. T. Belisle. 1997. Human humoral responses to antigens of Mycobacterium tuberculosis: immunodominance of high-molecular-mass antigens. Clin. Diagn. Lab. Immunol. 4:49-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lim, R. L., L. K. Tan, W. F. Lau, M. C. Ming, R. Dunn, H. P. Too, and L. Chan. 2000. Cloning and expression of immunoreactive antigens from Mycobacterium tuberculosis. Clin. Diagn. Lab. Immunol. 7:600-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ljungqvist, L., A. B. Andersen, P. Andersen, K. Haslov, A. Worsaae, J. Bennedsen, and I. Heron. 1990. Affinity purification, biological characterization and serological evaluation of defined antigens from Mycobacterium tuberculosis. Trop. Med. Parasitol. 41:333-335. [PubMed] [Google Scholar]
- 19.Lodes, M. J., D. C. Dillon, R. Mohamath, C. H. Day, D. R. Benson, L. D. Reynolds, P. McNeill, D. P. Sampaio, Y. A. Skeiky, R. Badaro, D. H. Persing, S. G. Reed, and R. L. Houghton. 2001. Serological expression cloning and immunological evaluation of MTB48, a novel Mycobacterium tuberculosis antigen. J. Clin. Microbiol. 39:2485-2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mehlert, A., and D. B. Young. 1989. Biochemical and antigenic characterization of the Mycobacterium tuberculosis 71kD antigen, a member of the 70kD heat-shock protein family. Mol. Microbiol. 3:125-130. [DOI] [PubMed] [Google Scholar]
- 21.Mustafa, A. S., P. J. Cockle, F. Shaban, R. G. Hewinson, and H. M. Vordermeier. 2002. Immunogenicity of Mycobacterium tuberculosis RD1 region gene products in infected cattle. Clin. Exp. Immunol. 130:37-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mustafa, A. S. 2002. Development of new vaccines and diagnostic reagents against tuberculosis. Mol. Immunol. 39:113-119. [DOI] [PubMed] [Google Scholar]
- 23.Ramakrishnan, L., N. A. Federspiel, and S. Falkow. 2000. Granuloma-specific expression of Mycobacterium virulence proteins from the glycine-rich PE-PGRS family. Science 288:1436-1439. [DOI] [PubMed] [Google Scholar]
- 24.Rodriguez, G. M., M. I. Voskuil, B. Gold, G. K. Schoolnik, and I. Smith. 2002. IdeR, An essential gene in Mycobacterium tuberculosis: role of IdeR in iron-dependent gene expression, iron metabolism, and oxidative stress response. Infect. Immun. 70:3371-3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sampson, S. L., P. Lukey, R. M. Warren, P. D. van Helden, M. Richardson, and M. J. Everett. 2001. Expression, characterization and subcellular localization of the Mycobacterium tuberculosis PPE gene Rv1917c. Tuberculosis 81:305-317. [DOI] [PubMed] [Google Scholar]
- 26.Siddiqi, N., M. Shamim, S. Hussain, R. K. Choudhary, N. Ahmed, Prachee, S. Banerjee, G. R. Savithri, M. Alam, N. Pathak, A. Amin, M. Hanief, V. M. Katoch, S. K. Sharma, and S. E. Hasnain. 2002. Molecular characterization of multidrug-resistant isolates of Mycobacterium tuberculosis from patients in North India. Antimicrob. Agents Chemother. 46:443-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singh, K. K., X. Zhang, A. S. Patibandla, P. Chien, Jr., and S. Laal. 2001. Antigens of Mycobacterium tuberculosis expressed during preclinical tuberculosis: serological immunodominance of proteins with repetitive amino acid sequences. Infect. Immun. 69:4185-4191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Young, D. B., and T. R. Garbe. 1991. Heat shock proteins and antigens of Mycobacterium tuberculosis. Infect. Immun. 59:3086-3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zeiss, C. R., S. B. Kalish, K. S. Erlich, D. Levitz, E. Metzger, R. Radin, and J. P. Phair. 1984. IgG antibody to purified protein derivative by enzyme-linked immunosorbent assay in the diagnosis of pulmonary tuberculosis. Am. Rev. Respir. Dis. 130:845-848. [DOI] [PubMed] [Google Scholar]